Abstract
Background
Accountable care organizations (ACOs) have been shown to reduce prostate cancer treatment among men unlikely to benefit due to competing risks (i.e., potential overtreatment). We assessed whether the level of engagement in ACOs by urologists affected rates of treatment, overtreatment, and spending.
Methods
We used a 20% sample of national Medicare data to identify men diagnosed with prostate cancer between 2012 and 2014. The extent of urologist engagement in an ACO, as measured by the proportion of patients in an ACO managed by an ACO-participating urologist, served as the exposure. We modeled the use of treatment, potential overtreatment (i.e., treatment in men with ≥75% risk of 10-year non-cancer mortality) and average payments in the year after diagnosis for each ACO.
Results
Among 2,822 men with newly diagnosed prostate cancer, median rates of treatment and potential overtreatment by an ACO were 71.3% (range 23.6% to 79.5%) and 53.6% (range 12.4% to 76.9%), respectively. Average Medicare payments among ACOs in the year after diagnosis ranged from $16,523.52 to $34,766.33. Stronger urologist-ACO engagement was not associated with treatment (OR 0.87, 95%CI 0.6-1.2, p=0.4) or spending (9.7% decrease in spending, p=0.08). However, urologist engagement was associated with a lower likelihood of potential overtreatment (OR 0.29, 95%CI 0.1-0.86, p=0.03).
Conclusions
ACOs vary widely in treatment, potential overtreatment, and spending for prostate cancer. ACOs with stronger urologist engagement were less likely to treat men with a high risk of non-cancer mortality suggesting that organizations that better engage specialists may be able to improve the value of specialty care.
Keywords: prostate cancer, treatment, accountable care organizations, overtreatment, spending
Introduction
Prostate cancer is a common and expensive disease, with an anticipated 164,690 new cases in 2018 and spending approaching $12 billion.1-4 Recently, the understanding that many prostate cancers are slow growing and do not require treatment5 has led to changes in the screening and diagnosis of prostate cancer.6,7 However, despite decreased screening and fewer diagnoses, treatment and potential overtreatment of men diagnosed with prostate cancer has remained common.8 Financial incentives embedded in the fee-for-service payment system that favor treatment have the potential to influence this trend.
Policies that align financial incentives with evidenced-based management (i.e., those that improve “value”), have the potential to affect the treatment of prostate cancer. Accountable care organizations (ACOs) are emblematic of such a policy. These integrated health systems aim to improve value by enhancing quality and reducing spending.9,10 For example, it is well established that a man with significant competing risk of death from non-cancer causes is unlikely to benefit from treatment for prostate cancer (i.e., potential overtreatment).11 By limiting the use of treatment in these men (i.e., reducing potential overtreatment), ACOs could provide higher quality care and reduce overall spending. In fact, prior work has demonstrated that ACOs are associated with lower rates of potential overtreatment.12 However, ACOs are organized around the primary care physician, and it is unclear whether the mission critical philosophy of improving value trickles down to associated specialists. Indeed, a minority of surgeons13 and urologists14 participate in ACOs, which may limit the ability of these organizations to influence care delivery in certain specialist-oriented clinical contexts, such as prostate cancer.
We hypothesized that variation among ACOs in the care of men with prostate cancer may be due to differing levels of engagement with urologists, who most commonly make a new diagnosis. We proposed that ACOs better integrate with participating urologists would be able to constrain the use of lower value prostate cancer treatment and therefore have lower rates of potential overtreatment and reduced spending. To address this question, we examined Medicare Shared Savings Program ACOs using national Medicare claims. In particular, we aimed to characterize the variation among ACOs in initial treatment, potential overtreatment, and average spending patterns for newly diagnosed men with prostate cancer.
Methods
Data and study population
Using a 20% sample of national Medicare claims, we performed a retrospective cohort study of fee-for-service beneficiaries with of men diagnosed with prostate cancer between 2012 and 2014. All men were followed through December 31, 2015. Incident prostate cancer was identified using a previously validated algorithm, which has a specificity of 99.8% and a positive predictive value of 88.7%.15 We limited the cohort to men 66 years of age and older to allow for health status assessment in the year preceding the cancer diagnosis.16 Only men with continuous enrollment in Medicare Parts A and B for one year before and after the new diagnosis were included. Men participating in Medicare managed care plans were excluded as they were not eligible to participate in ACOs per Centers for Medicare and Medicaid regulations.
All men with prostate cancer were assigned to their primary care physician implementing the methodology used by the Medicare Shared Savings Program.17 Physicians were then aligned with Medicare Shared Savings Program ACOs using the Provider-level Research Identifiable File provided by the Centers for Medicare and Medicaid Services. This study only included men who were attributed to an ACO at the time of prostate cancer diagnosis.
Exposure
The primary exposure was the extent of urologist engagement by the beneficiary’s ACO. Previous studies have found that only 10% of urologists participate in Medicare Shared Savings Program ACOs and only 50% of ACOs include a urologist.14 We postulated that in order for an ACO to affect prostate cancer care it would have to both include participating urologists and also preferentially direct referrals to those urologists. Therefore, we constructed a variable to characterize the strength of each ACO’s engagement with participating urologists. Urologist engagement was defined as the proportion of prostate cancer patients in each ACO managed by an ACO-participating urologist. For the purposes of this measure, the urologist could participate in any Shared Savings Program ACO to be counted as an ACO-participant and need not participate in the same ACO as the primary care provider. Each beneficiary’s primary urologist was defined using previously described methods.18 If the urologist participated in any Medicare Shared Savings Program ACO from 2012-2014, he or she was considered an ACO-participating urologist for the purposes of creating the urologist engagement variable.
Outcomes
The primary outcome of this study was use of curative treatment for prostate cancer within 12 months of diagnosis and was measured at the beneficiary level. Treatment was ascertained from the Medicare Provider Analysis and Review, Carrier, and Outpatient files using Healthcare Common Procedure Coding System codes for external beam radiation therapy, surgery, cryotherapy, and brachytherapy. Patients managed without treatment within 12 months of diagnosis or with primary androgen deprivation therapy (i.e., without contemporaneous surgery or radiation therapy) were classified as undergoing observation.
We also measured two secondary outcomes likely to be impacted by ACOs. First, we assessed the use of potential overtreatment (i.e., treatment in men with ≥75% chance of 10-year mortality). Treatment is generally not recommended for men expected to live less than 10 years after diagnosis due to the slow-growing nature of most prostate cancers.19 Therefore, ACOs that aim to reduce low-value prostate cancer care would be expected to constrain potential overtreatment. Using established methods,8,12,20 we identified beneficiaries with the highest predicted risk (≥75%) of non-cancer death within 10 years and modeled the use of treatment in this subset of patients.8 Second, we determined total price standardized payments for the 12-month period after diagnosis. A primary goal of ACOs is to reduce spending, in part by improving care coordination and reducing waste. We used this comprehensive measure of spending to capture all claims (e.g., visits, complications, readmissions) related to prostate cancer management. Price standardization was used to control for differences in payments related to geography and facility characteristics (Supplemental appendix).
Analysis
We compared patient characteristics between those who underwent treatment and those who did not using Pearson’s chi-squared test. We fit multivariable mixed-effects models with a logit link to estimate patient-level treatment and potential overtreatment. A similar approach was implemented for a model to estimate spending differences, although a log link was used. All models were adjusted using patient age, race, comorbidity,16 socioeconomic class at the zip code level,21 and degree of urbanization of the beneficiary place of residence (i.e., urban vs. rural). We used these models to generate plots of treatment and spending at the ACO level by averaging individual-level best linear unbiased predictions for beneficiaries in each ACO. Because we identified different numbers of prostate cancer patients among ACOs, all models were reliability adjusted using empirical Bayes techniques to reduce statistical noise.22,23 This technique adjusts the point estimate for an outcome in each ACO toward the overall mean, with the degree of adjustment proportional to the precision of the point estimate.
All analyses were carried out using SAS 9.4 (Cary, NC) and Stata 14 (College Station, TX). All tests were two-sided with probability of Type 1 error (alpha) set at 0.05. This study protocol was deemed exempt from review by the University of Michigan institutional review board.
Results
Treatment and Overtreatment
We identified 2,822 beneficiaries with newly diagnosed prostate cancer who were assigned to one of 296 ACOs (Table 1). The median rate of treatment among all ACOs was 71.3% and ranged from 23.6% to 79.5% (Figure 1). Among men newly diagnosed with prostate cancer in an ACO, age was significantly associated with the use of treatment (p<0.001). No significant associations were noted between the use of treatment and race, comorbidity, socioeconomic status, or urban place of residence (Table 2). Of the 2,822 men with newly diagnosed prostate cancer in an ACO, 255 had ≥75% chance of non-cancer mortality within 10 years (i.e., those subject to potential overtreatment) and were attributed to 137 ACOs. In these ACOs, the median rate of potential overtreatment was 53.6% and ranged from 12.4% to 76.9%. Younger age was associated with potential overtreatment (p=0.003). No significant associations were noted between the use of potential overtreatment and race, comorbidity, socioeconomic status, or urban place of residence (Table 2).
Table 1.
Patient characteristics by receipt of treatment.
| No curative treatment (%) | Curative treatment (%) | p-value | |
|---|---|---|---|
| No. patients | 849 | 1973 | – |
| Age: | <0.001 | ||
| 66-69 | 214 (25.2) | 692 (35.1) | |
| 70-74 | 262 (30.9) | 708 (35.9) | |
| 75-79 | 164 (19.3) | 430 (21.8) | |
| 80-84 | 137 (16.1) | 119 (6.0) | |
| 85+ | 72 (8.5) | 24 (1.2) | |
| Race/ethnicity: | 0.62 | ||
| White | 749 (88.2) | 1743 (88.3) | |
| Black | 72 (8.5) | 175 (8.9) | |
| Other/unknown | 28 (3.4) | 55 (2.8) | |
| Comorbidity: | 0.093 | ||
| 0 | 449 (52.9) | 1132 (57.4) | |
| 1 | 206 (24.3) | 450 (22.8) | |
| 2 | 100 (11.8) | 219 (11.1) | |
| 3+ | 94 (11.1) | 172 (8.7) | |
| Socioeconomic status: | 0.24 | ||
| Low | 221 (26.0) | 466 (23.6) | |
| Medium | 306 (36.0) | 699 (35.4) | |
| High | 322 (37.9) | 808 (41.0) | |
| Residential area: | 0.38 | ||
| ≥1 million metropolitan county | 464 (54.7) | 1119 (56.7) | |
| <1 million metropolitan county | 281 (33.1) | 616 (31.2) | |
| Non-metropolitan rural or urban population* | 104 (12.2) | 228 (11.6%) | |
| Urologists per 100k: | 0.59 | ||
| Low (<3) | 287 (33.8) | 676 (34.3) | |
| Intermediate | 271 (31.9) | 658 (33.4) | |
| High (>23) | 291 (34.3) | 639 (32.4) | |
| Radiation oncologists per 100k: | 0.36 | ||
| Low (<17) | 291 (34.3) | 651 (33.0) | |
| Intermediate | 296 (34.9) | 659 (33.4) | |
| High (>33) | 262 (30.9) | 663 (33.6) | |
| Hospital beds per 100k: | 0.9 | ||
| Low (<3589) | 285 (33.6) | 656 (33.2) | |
| Intermediate | 292 (34.4) | 668 (33.9) | |
| High (>6556) | 272 (32.0) | 649 (32.9) |
Categories have been combined due to small cell size.
Figure 1.
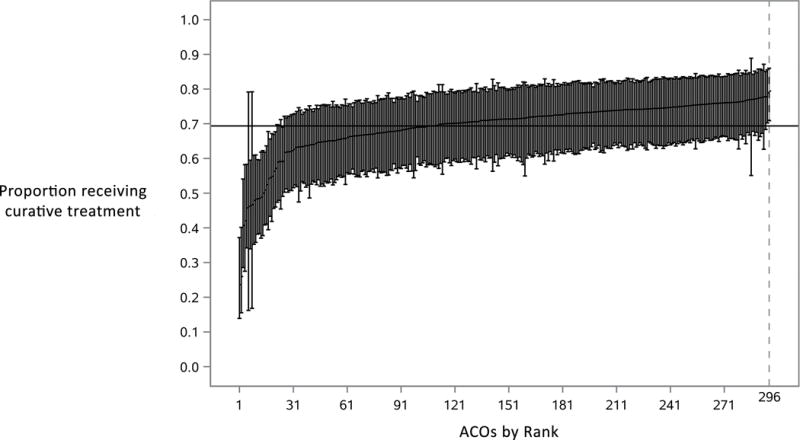
ACOs ranked by proportion of men with prostate cancer receiving treatment. ACO-level estimates were generated by averaging best linear unbiased predictions from our mixed-effects model. This model estimated treatment after adjusting for age, race, comorbidity score, socioeconomic status, and place of residence. These ACO-level averages were then reliability-adjusted using empirical Bayes techniques and then ordered from lowest to highest proportion of men treated.
Table 2.
Models estimating treatment, overtreatment, and spending among men with prostate cancer in a Medicare Shared Savings Program ACO.
| Treatment (n=2822) | Potential overtreatment (n=255) | Spending (n=2822) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| aOR | 95% CI | P-value | aOR | 95% CI | P-value | IRR | SE | P-value | |
| Age: | <0.001 | 0.003 | 0.001 | ||||||
| 66-69 | ref | * | ref | ||||||
| 70-74 | 0.84 | 0.68-1.04 | ref | 1.07 | 1.04 | ||||
| 75-79 | 0.81 | 0.64-1.03 | 1.00 | 0.37-2.70 | 1.18 | 1.04 | |||
| 80-84 | 0.27 | 0.20-0.36 | 0.68 | 0.24-1.93 | 1.08 | 1.06 | |||
| 85+ | 0.10 | 0.06-0.16 | 0.13 | 0.03-0.50 | 0.97 | 1.09 | |||
| Race/ethnicity: | 0.27 | 0.89 | 0.64 | ||||||
| White | ref | ref | ref | ||||||
| Black | 1.04 | 0.76-1.41 | 0.81 | 0.34-1.92 | 0.96 | 1.06 | |||
| Comorbidity: | 0.49 | 0.85 | <0.001 | ||||||
| 0 | ref | ref | ref | ||||||
| 1 | 0.91 | 0.74-1.12 | 0.66 | 0.19-2.26 | 1.07 | 1.04 | |||
| 2 | 0.95 | 0.72-1.26 | 0.62 | 0.18-2.15 | 1.19 | 1.05 | |||
| 3+ | 0.81 | 0.60-1.08 | 0.56 | 0.15-2.05 | 1.59 | 1.05 | |||
| Socioeconomic status: | 0.39 | 0.23 | 0.83 | ||||||
| Low | ref | ref | ref | ||||||
| Medium | 1.11 | 0.88-1.39 | 1.33 | 0.65-2.71 | 1.00 | 1.04 | |||
| High | 1.18 | 0.93-1.50 | 2.02 | 0.89-4.56 | 1.02 | 1.04 | |||
| Residential area: | 0.28 | 0.47 | 0.04 | ||||||
| ≥1 million metropolitan county | ref | ref | ref | ||||||
| <1 million metropolitan county | 0.88 | 0.72-1.08 | 1.08 | 0.55-2.13 | 0.94 | 1.04 | |||
| Urban population | 0.97 | 0.72-1.31 | 0.67 | 0.27-1.67 | 0.92 | 1.05 | |||
| Rural population | 1.96 | 0.76-5.08 | 4.95 | 0.35-69.27 | 1.29 | 1.15 | |||
| ACO urologist engagement (0 to 1) | 0.87 | 0.63-1.21 | 0.4 | 0.29 | 0.10-0.86 | 0.03 | 0.90 | 1.06 | 0.08 |
no patients in this category. aOR -adjusted odds ratio; IRR - incident rate ratio; SE - standard error.
Spending
We then evaluated total Medicare spending in the first year after diagnosis among men in ACOs. Average price-adjusted spending among ACOs (Figure 2) was $21,152.35 (SD $2,589.40) per beneficiary and ranged from $16,523.52 to $34,766.33. Younger age and fewer comorbidities were associated with lower average spending. Residence in a large county (≥1 million population) was associated with higher spending than residence in smaller counties. No significant associations between spending and race or socioeconomic status were noted (Table 2).
Figure 2.

ACOs ranked according to average spending for men with prostate cancer. ACO-level estimates were generated by averaging best linear unbiased predictions from our mixed-effects model. This model estimated spending after adjusting for age, race, comorbidity score, socioeconomic status, and place of residence. These ACO-level averages were then reliability-adjusted using empirical Bayes techniques and then ordered from lowest to highest average spending among men with prostate cancer.
ACO-urologist engagement
Strength of ACO-urologist engagement ranged among ACOs from 0% (no prostate cancer patients managed by ACO-participating urologists) to 100% (all prostate cancer patients managed by ACO-participating urologists). Among the 2282 patients, the median strength of ACO-urologist engagement was 0.14 (interquartile range 0.43). We noted small, statistically significant differences in patient characteristics across quartiles of ACO-urologist engagement (Supplemental Table 1). Across quartiles of urologist engagement, we noted small differences in treatment among all men with prostate cancer but larger differences in treatment among men with a high risk of death (Supplemental Figure 1). ACO-urologist engagement was not associated with use of treatment (OR 0.87, 95% CI 0.6-1.2, p=0.4) or Medicare spending (9.7% decrease in spending, p=0.08) after adjustment for covariates. However, in the subset of patients with a high risk of 10-year mortality, stronger ACO-urologist engagement was independently associated was with lower odds of potential overtreatment (OR 0.29, 95% CI 0.1-0.9, p=0.03).
Discussion
We found that Medicare Shared Savings Program ACOs vary widely in treatment, potential overtreatment and spending for men with newly diagnosed prostate cancer. The use of treatment in these men varied more than three-fold among Medicare Shared Savings Program ACOs during the study period, ranging from 23.6% to 79.5%. Average spending varied by over $18,000 between the highest and lowest spending ACOs. ACO-urologist engagement was not significantly associated with treatment rate or overall spending. However, among men with a high risk of non-cancer mortality, greater ACO-urologist engagement was associated with reduced use of potential overtreatment.
It is well accepted that men with significant medical conditions that limit their life expectancy are particularly unlikely to benefit from prostate cancer treatment.24,25 While ACOs as a whole have been shown to reduce the overtreatment of these men,12 we found that not all ACOs constrain overtreatment to the same extent. The considerable variation in overtreatment among ACOs is surprising because there is consensus about the value of treatment in men likely to succumb to competing risks within 10 years of a prostate cancer diagnosis.5,24 That such variation exists among ACOs, whose conceptual underpinnings aim to improve both population health and the value of healthcare delivered, suggests a significant opportunity for improvement.9,10
How ACOs can improve value by enhancing population health and reduce spending in the context of conditions traditionally handled by specialists, such as prostate cancer, is unclear. ACOs are defined around the delivery of primary care. In order to be eligible to share in savings, ACOs must meet both quality and spending benchmarks.26 Reducing “low value” healthcare is one mechanism by which ACOs can improve quality and lower spending. In the context of prostate cancer, we posited that ACOs with stronger ties to urologists, as measured by our engagement variable, would lead to lower potential overtreatment and per beneficiary spending. Indeed, we found that ACOs with the highest levels of engagement with urologists were less likely to treat men unlikely to benefit (i.e., those with a high risk of non-cancer mortality within 10 years of their diagnosis). However, urologist engagement was not associated with overall rates of treatment nor Medicare spending in the first year after diagnosis.
Our finding that strength of urologist engagement is associated with decreased overtreatment of prostate cancer has significant implications for primary care providers and urologists. One potential explanation for these findings is that ACOs with the strongest urologist engagement (i.e., those with the highest proportion of patients managed by a urologist participating in an ACO) might influence specialty care by directing referrals toward urologists whose practice patterns align with the goals of the ACO.27 While a urologist’s ACO participation does not necessarily imply a focus on minimizing low-value care, our results suggest that, on average, these urologists may be less likely to treat men unlikely to benefit. Though speculative, potential reasons for this might be that urologists participating in ACOs may be more conscious of population health, more likely to participate in value-based quality improvement efforts or may have different financial incentives than those not participating in ACOs. Most patients who were treated by ACO-participating urologists were treated by urologists in their ACO (72%). However, more than a quarter of these patients were managed by a urologist in a different ACO. In a sensitivity analysis, the association between ACO-urologist engagement and overtreatment persisted with varying definitions of ACO-urologist engagement that considered urologists in the same or different ACO as patient and primary care physician. This finding is not surprising as patients are attributed to ACOs retrospectively and treating physicians cannot know any given patient’s ACO attribution at the time of treatment.
Despite an association with less overtreatment, stronger urologist engagement had no impact on overall treatment or spending in our study. A possible explanation for this is that men with a high risk of non-cancer mortality comprise a small proportion of all ACO patients with newly diagnosed prostate cancer. As a result, overall average spending may be a product of treatment decisions in general and not necessarily decisions in cases of potential overtreatment. Alternatively, physicians in an ACO that provide less treatment to men with a high chance of 10-year non-cancer mortality may increase the use of treatment, and thus average spending, for the remainder of their patients.
Despite the increasing acceptance of observation and active surveillance for prostate cancer28, recent evidence suggests that the rate of treatment among men diagnosed with prostate cancer has decreased only modestly.8 Differences in the use of treatment among ACOs suggests persistent uncertainty, or disagreement, about the role of therapy for some men with newly diagnosed prostate cancer. A comparison of the use of prostate cancer treatment in patients managed within and outside of ACOs demonstrated that ACOs, while not impacting the rate of prostate cancer treatment overall, did constrain the use of potential overtreatment.12 Our results in the present study build on this finding. An ACO’s ability to constrain overtreatment is associated with the strength of its engagement with ACO-participating urologists. However, despite this impact on potential overtreatment, participation in an ACO with strong urologist engagement had no significant effect on prostate cancer treatment or overall spending.
These results must be interpreted with several limitations in mind. First, ACO-urologist engagement is an imperfect measure. We characterized ACO-urologist engagement based on treatment of ACO patients by urologists who formally participated in any Medicare Shared Savings Program ACO from 2012 to 2014. However, our analysis did not capture urologists who may be engaged in improving value without formal ACO participation or by participating in commercial or other Medicare ACO programs. We did not distinguish between urologists who participated in the same ACO as a patient or a different ACO. Additionally, our classification of ACO-urologist engagement is limited by the relatively small number of prostate cancer patients per unique ACO, which could reduce the reliability of this measure. All of these limitations would result in random error in the classification of ACO-urologist engagement. This “noise” in the definition of ACO-urologist engagement would be expected to attenuate the measured association and lead to a conservative estimate of the relationship between ACO-urologist engagement and prostate cancer treatment, potential overtreatment, and spending. Second, Medicare data lacks information about cancer severity, which is a major factor in determining the value of therapy for the individual diagnosed with prostate cancer. While we would not expect population-level differences in cancer severity among ACO-enrolled patients, ACOs may vary with respect to their screening and diagnostic intensity. While such differences have not been empirically demonstrated, such differences could lead to variations in prostate cancer stage and grade as well as comorbidity diagnoses among ACOs and potentially confound the results of this analysis. An ideal analysis would incorporate prostate cancer stage and grade and such data would be available in datasets with clinical registry information linked with Medicare data (e.g., SEER-Medicare). However, given the geographic locations of SEER regions, a large proportion of ACOs and attributed patients would be excluded, rendering a meaningful analysis of ACOs impossible. In order to evaluate Medicare ACOs, this limitation cannot be overcome using available data. Third, while we demonstrated an association between urologist engagement and overtreatment of men with prostate cancer, we cannot infer a causal relationship from this observation. It is possible that ACOs in which patients are less likely to be overtreated more often refer patients to ACO-participating urologists. However, as patients usually make decisions about prostate cancer treatment after consultation with their urologists, it is likely that the choice of urologist plays a significant role in this effect. Finally, our analysis is restricted to men diagnosed with prostate cancer in Medicare Shared Savings Program ACOs from 2012 to 2014. Our findings may not apply to Medicare Pioneer ACOs and commercial ACO programs.
Conclusions
Medicare Shared Savings Program ACOs vary considerably in how they treat men with newly diagnosed prostate cancer and in average spending in the year after diagnosis. This variation is representative of the difficulties ACOs may have in effecting changes in specialty care. The ability of an ACO to engage urologists is associated with how often it provides prostate cancer treatment to men who are unlikely to benefit. Further research is needed to understand how ACOs can better engage urologists, whether by improved care coordination, directed referral patterns, or modifying financial incentives.
Supplementary Material
Acknowledgments
Funding: This study was supported by T32CA180984 (PKM, TB), R01CA174768 (DCM), R01HS024525 and R01HS024728 (JMH), R01HS257007 (BKH, VBS) and R01AG048071 (BKH). The views expressed in this article do not reflect the views of the federal government.
Footnotes
Conflict of interest: No authors have any relevant conflicts of interest to disclose.
Author contributions: Parth K. Modi: conceptualization, formal analysis, writing – original draft. Samuel R. Kaufman: formal analysis, data curation. Tudor Borza: conceptualization, supervision, writing – review and editing. Phyllis Yan: formal analysis, data curation. David C. Miller: funding acquisition, supervision, writing – review and editing. Ted A. Skolarus: supervision, writing – review and editing. John M. Hollingsworth: funding acquisition, supervision, writing – review and editing. Edward C. Norton – formal analysis, supervision, data curation. Vahakn B. Shahinian: funding acquisition, conceptualization, supervision, formal analysis. Brent K. Hollenbeck: conceptualization, formal analysis, writing – review and editing, funding acquisition, supervision.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Stokes ME, Ishak J, Proskorovsky I, Black LK, Huang Y. Lifetime economic burden of prostate cancer. BMC Health Serv Res. 2011;11:349. doi: 10.1186/1472-6963-11-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mariotto AB, Yabroff KR, Shao Y, Feuer EJ, Brown ML. Projections of the cost of cancer care in the United States: 2010-2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roehrig C, Miller G, Lake C, Bryant J. National health spending by medical condition, 1996-2005. Health Aff (Millwood) 2009;28(2):w358–367. doi: 10.1377/hlthaff.28.2.w358. [DOI] [PubMed] [Google Scholar]
- 5.Bill-Axelson A, Holmberg L, Garmo H, et al. Radical prostatectomy or watchful waiting in early prostate cancer. N Engl J Med. 2014;370(10):932–942. doi: 10.1056/NEJMoa1311593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zavaski ME, Meyer CP, Sammon JD, et al. Differences in Prostate-Specific Antigen Testing Among Urologists and Primary Care Physicians Following the 2012 USPSTF Recommendations. JAMA Intern Med. 2016;176(4):546–547. doi: 10.1001/jamainternmed.2015.7901. [DOI] [PubMed] [Google Scholar]
- 7.Barocas DA, Mallin K, Graves AJ, et al. Effect of the USPSTF Grade D Recommendation against Screening for Prostate Cancer on Incident Prostate Cancer Diagnoses in the United States. J Urol. 2015;194(6):1587–1593. doi: 10.1016/j.juro.2015.06.075. [DOI] [PubMed] [Google Scholar]
- 8.Borza T, Kaufman SR, Shahinian VB, et al. Sharp Decline In Prostate Cancer Treatment Among Men In The General Population, But Not Among Diagnosed Men. Health Aff (Millwood) 2017;36(1):108–115. doi: 10.1377/hlthaff.2016.0739. [DOI] [PubMed] [Google Scholar]
- 9.Fisher ES, Shortell SM. Accountable care organizations: accountable for what, to whom, and how. JAMA. 2010;304(15):1715–1716. doi: 10.1001/jama.2010.1513. [DOI] [PubMed] [Google Scholar]
- 10.Fisher ES, McClellan MB, Bertko J, et al. Fostering accountable health care: moving forward in medicare. Health Aff (Millwood) 2009;28(2):w219–231. doi: 10.1377/hlthaff.28.2.w219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415–1424. doi: 10.1056/NEJMoa1606220. [DOI] [PubMed] [Google Scholar]
- 12.Borza T, Kaufman SR, Yan P, et al. Early effect of Medicare Shared Savings Program accountable care organization participation on prostate cancer care. Cancer. 2017 doi: 10.1002/cncr.31081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Resnick MJ, Graves AJ, Buntin MB, Richards MR, Penson DF. Surgeon Participation in Early Accountable Care Organizations. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002233. [DOI] [PubMed] [Google Scholar]
- 14.Hawken SR, Herrel LA, Ellimoottil C, Ye Z, Hollenbeck BK, Miller DC. Urologist Participation in Medicare Shared Savings Program Accountable Care Organizations (ACOs) Urology. 2016;90:76–80. doi: 10.1016/j.urology.2015.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollenbeck BK, Bierlein MJ, Kaufman SR, et al. Implications of evolving delivery system reforms for prostate cancer care. Am J Manag Care. 2016;22(9):569–575. [PMC free article] [PubMed] [Google Scholar]
- 16.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258–1267. doi: 10.1016/s0895-4356(00)00256-0. [DOI] [PubMed] [Google Scholar]
- 17.CMS.gov. MSSP Shared Savings and Losses and Assignment Methodology v3 Dec 2014. 2014 [Google Scholar]
- 18.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Determinants of androgen deprivation therapy use for prostate cancer: role of the urologist. J Natl Cancer Inst. 2006;98(12):839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NCCN Guidelines for Patients. Prostate cancer. https://www.nccn.org/patients/guidelines/prostate/index.html.
- 20.Jacobs BL, Zhang Y, Schroeck FR, et al. Use of advanced treatment technologies among men at low risk of dying from prostate cancer. JAMA. 2013;309(24):2587–2595. doi: 10.1001/jama.2013.6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diez Roux AV, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. N Engl J Med. 2001;345(2):99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 22.Dimick JB, Ghaferi AA, Osborne NH, Ko CY, Hall BL. Reliability adjustment for reporting hospital outcomes with surgery. Ann Surg. 2012;255(4):703–707. doi: 10.1097/SLA.0b013e31824b46ff. [DOI] [PubMed] [Google Scholar]
- 23.Dimick JB, Staiger DO, Birkmeyer JD. Ranking hospitals on surgical mortality: the importance of reliability adjustment. Health Serv Res. 2010;45(6 Pt 1):1614–1629. doi: 10.1111/j.1475-6773.2010.01158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol. 2011;29(10):1335–1341. doi: 10.1200/JCO.2010.31.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daskivich TJ, Fan KH, Koyama T, et al. Effect of age, tumor risk, and comorbidity on competing risks for survival in a U.S. population-based cohort of men with prostate cancer. Ann Intern Med. 2013;158(10):709–717. doi: 10.7326/0003-4819-158-10-201305210-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.General USDoHaHSOoI. Medicare Shared Savings Program Accountable Care Organizations Have Shown Potential for Reducing Spending and Improving Quality (OEI-02-15-00450) 2017 [Google Scholar]
- 27.Dupree JM, Patel K, Singer SJ, et al. Attention to surgeons and surgical care is largely missing from early medicare accountable care organizations. Health Aff (Millwood) 2014;33(6):972–979. doi: 10.1377/hlthaff.2013.1300. [DOI] [PubMed] [Google Scholar]
- 28.Ritch CR, Graves AJ, Keegan KA, et al. Increasing use of observation among men at low risk for prostate cancer mortality. J Urol. 2015;193(3):801–806. doi: 10.1016/j.juro.2014.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


