Abstract
Objective
Research on the cognitive sequelae of mild traumatic brain injury (mTBI) suggests that despite generally rapid recovery, difficulties may persist in the domain of cognitive control. The goal of this study was to examine whether individuals with chronic blast-related mTBI show behavioral or neural alterations associated with cognitive control.
Method
We collected event-related functional magnetic resonance imaging (fMRI) data during a flanker task in 17 individuals with blast-related mTBI and 16 individuals with blast-exposure without TBI (control).
Results
Groups did not significantly differ in behavioral measures of cognitive control. Relative to the control group, the mTBI group showed greater deactivation of regions associated with the default mode network during the processing of errors. Additionally, error processing in the mTBI group was associated with enhanced negative coupling between the default mode network and the dorsal anterior cingulate cortex as well as the dorsolateral prefrontal cortex, regions of the salience and central executive networks that are associated with cognitive control.
Conclusions
These results suggest that deactivation of default mode network regions and associated enhancements of connectivity with cognitive control regions may act as a compensatory mechanism for successful cognitive control task performance in mTBI.
Keywords: mTBI, task fMRI, default mode network, salience network, central executive network, OEF/OIF
Introduction
After mild traumatic brain injury (mTBI), neuropsychological functioning typically recovers to pre-injury levels (Belanger & Vanderploeg, 2005; Frencham, Fox, & Maybery, 2005; McCrea, 2008). One notable exception, however, is the observation of residual behavioral (Bonnelle et al., 2012; Pontifex, O’Connor, Broglio, & Hillman, 2009; Seignourel et al., 2005) and neural (Mayer et al., 2012; Pontifex et al., 2009) alterations in cognitive control, the processes that allow for the flexible modulation of information processing in the service of goal-directed behavior (Botvinick, Braver, Barch, Carter, & Cohen, 2001). Cognitive control involves detecting salient events and errors as well as adjustment of attention in response to such events. Notably, neural alterations in cognitive control have been reported even in the absence of observable behavioral impairment in mTBI (Broglio, Pontifex, O’Connor, & Hillman, 2009; Mayer et al., 2012; Mayer et al., 2015), suggesting that even when cognitive control is intact, its neural implementation may be altered by mTBI.
Although early work focused on residual cognitive control deficits in civilian mTBI, recent studies of blast-related mTBI have also shown neural alterations during the performance of cognitive control tasks, albeit in the absence of behavioral impairment. Scheibel and colleagues (2012) compared functional activation in a group of individuals with chronic blast-related mTBI and individuals without blast exposure or TBI in the context of a stimulus-response compatibility task. They found that mTBI was associated with increased activation in anterior regions such as the anterior cingulate cortex, insula, and medial frontal cortex as well as in posterior regions involved in visual processing. Fischer and colleagues (2014) administered a response inhibition task, and found that mTBI was associated with reduced activation during successful inhibition in regions including the medial frontal gyrus, middle frontal gyrus, middle temporal gyrus, and precuneus.
The apparent inconsistency in these findings (i.e., increased vs. decreased activation in mTBI) can be understood with reference to the involvement of distinct functional brain networks in cognitive control. Cognitive control strongly relies on several prefrontal areas, including the dorsal anterior cingulate cortex and dorsolateraleral prefrontal cortex, as well as the insula and posterior parietal cortex (Bunge, Hazeltine, Scanlon, Rosen, & Gabrieli, 2002; Kerns et al., 2004; MacDonald, Cohen, Stenger, & Carter, 2000). The insula and dorsal anterior cingulate cortex are two core hubs of the salience network, whereas the dorsolateral prefrontal cortex and posterior parietal cortex are key hubs of the central executive network (Seeley et al., 2007). These networks typically co-activate during cognitive control tasks (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Nee, Wager, & Jonides, 2007). Successful cognitive control also relies on deactivation of the default mode network (Bonnelle et al., 2012; Kelly, Uddin, Biswal, Castellanos, & Milham, 2008; Singh & Fawcett, 2008), a network that includes the posterior cingulate cortex/precuneus, medial frontal cortex, and temporal cortex and is typically deactivated during tasks. Thus, the results of Scheibel et al. (2012), who reported increased activation in salience network regions in mTBI, and Fischer et al. (2014), who reported decreased activation in default mode network regions in mTBI, are consistent with the notion that cognitive control is associated with both heightened salience network activity and reduced default mode network activity1. In both studies, the pattern observed in mTBI represents an amplification of the pattern seen in control participants.
Moreover, cognitive control depends not only on these functional networks independently, but also on their interaction. Sridharan and colleagues (2008) demonstrated that the salience network switches between internally- and externally-directed cognitive processing by initiating control signals that upregulate the central executive network and deactivate the default mode network. Further, disruption of the structural integrity of the salience network predicts reduced default mode network deactivation during a stop-signal task in moderate-severe TBI (Bonnelle et al., 2012). However, it is unknown how mTBI impacts the interaction of these networks during cognitive control. In the present study, we extend previous work on cognitive control in blast-related mTBI by assessing the functioning of these networks as well as their interactions. To do this, we administered a functional magnetic resonance imaging (fMRI) flanker paradigm to Operation Enduring Freedom/Operation Iraqi Freedom (OEF/OIF) blast-exposed veterans with and without mTBI and used psychophysiological interaction (PPI) analysis (Friston et al., 1997; O’Reilly, Woolrich, Behrens, Smith, & Johansen-Berg, 2012) to assess task-based functional connectivity. Based on previous studies in blast-related mTBI, we hypothesized that mTBI would be associated with greater activation in salience network regions and/or greater deactivation in default mode network regions during increased demands on cognitive control. Further, we hypothesized that the interaction between these networks would be altered in mTBI.
The flanker task requires participants to respond to a center arrow that is flanked by arrows in the same direction (congruent trials) or in the opposite direction (incongruent trials). Incongruent trials pose high response conflict because of the discrepancy in the direction of the flanking arrows compared to the center arrow, and require inhibition of task irrelevant information (i.e., flanking arrows) in order to correctly respond to task relevant stimuli (i.e., center arrow). The difference in response latency to incongruent versus congruent trials thus constitutes a measure of cognitive control. Additionally, processing during error trials can be examined as a measure of cognitive control, as error trials yield a response conflict resulting from competition between a correct response and a strong response tendency for an incorrect response. Moreover, a number of studies suggest that error processing concerns not only the identification of the error but also the correction of differences between the intended task goal and executed response (Menon, Adleman, White, Glover, & Reiss, 2001; Taylor, Stern, & Gehring, 2007; Ullsperger & von Cramon, 2001). Thus, the difference in response latency on trials immediately following an error (post-error) and trials immediately following a correct response (post-correct) constitutes an additional behavioral measure of cognitive control (i.e., post-error slowing). Using these measures, we examined behavioral and neural alterations in cognitive control associated with blast-related mTBI.
Methods
Participants
Of 69 OEF/OIF veterans initially contacted for this study, 38 agreed to participate. Three were not enrolled because of exclusionary criteria (see below), leaving 35 participants who completed the protocol. The study sample consisted of 18 veterans with blast-related mTBI, as defined by the American Congress Rehabilitation Medicine (1993) criteria, and 17 blast-exposed veterans who reported no TBI from any mechanism of injury during deployment (control). TBI assessment was based on an extensive clinical interview described in detail in Verfaellie, Lafleche, Spiro, Tun, and Bousquet (2013). In brief, participants were queried about their blast exposure(s) to determine the index event, which they were then asked to describe in detail. Two investigators evaluated the interviews and sought consensus as to whether mTBI criteria had been met and whether any reported disorientation was the result of mTBI. The mTBI group consisted of seven individuals with loss of consciousness (LOC) and 11 without LOC. Study procedures were approved by the VA Boston Institutional Review Board and all participants provided written informed consent consistent with the Declaration of Helsinki.
Participants were excluded from the study if they reported a history of pre-deployment TBI with LOC or with symptoms persisting longer than three months post-injury, demonstrated questionable effort with raw scores below 45 on the retention trial of the Test of Memory Malingering (TOMM; Tombaugh & Tombaugh, 1996), had structural brain abnormalities (e.g., hemorrhages, hematomas) on T2-FLAIR, susceptibility weighted imaging (SWI), or T1-weighted sequences as determined by a board-certified neuroradiologist, showed evidence of excessive alcohol use as reflected by scores above 20 on the Alcohol Use Disorder Identification Test (AUDIT), or reported a diagnosis of attention deficit hyperactivity disorder (ADHD) or medication use consistent with its treatment.
Two participants (one mTBI, one control) were unable to stay awake during the task, thus yielding a final sample of 33 participants. A summary of demographic characteristics can be found in Table 1.
Table 1.
Summary of demographic and clinical characteristics of participants.
| Control (n = 16) | mTBI (n = 17) | Group Comparison | |
|---|---|---|---|
| Age in years, M (SD) | 33.1 (5.6) | 31.7 (6.8) | t(31) = 0.7, P = 0.5 |
| Males, no. (%) | 14 (87.5) | 17 (100.0) | χ2(1) = 2.3, P = 0.1 |
| Education in years, M (SD) | 15.6 (2.4) | 14.7 (1.6) | t(31) = 1.2, P = 0.2 |
| Blast exposure or TBI to scan interval in months, M (SD) | 93.3 (34.6) | 81.0 (40.4) | t(31) = 0.9, P = 0.4 |
| PCL-M score, M (SD) | 37.6 (12.6) | 40.2 (13.2) | t(31) = −0.6, P = 0.6 |
| Individuals with LOC, no. (%) | 7 (41.2) | ||
| Pre-deployment TBIs, M (SD) | 0.9 (2.1) | 0.7 (2.0) | t(31) = 0.2, P = 0.8 |
Note: For the control group, the scan interval indicates the time from blast exposure to scan, whereas for the mTBI group, the scan interval indicates the time from blast-related mTBI to scan. mTBI=mild traumatic brain injury; PCL-M=PTSD Checklist-Military version.
Clinical Assessment
The posttraumatic stress disorder (PTSD) Checklist – Military Version (PCL-M; Weathers, Huska, & Keane, 1991) was used to measure PTSD symptom severity within the last month preceding testing. The PCL has good convergent validity with the Clinician-Administered PTSD Scale (Wilkins, Lang, & Norman, 2011), which is the gold standard for PTSD assessment (Blake et al., 1995).
Flanker Task
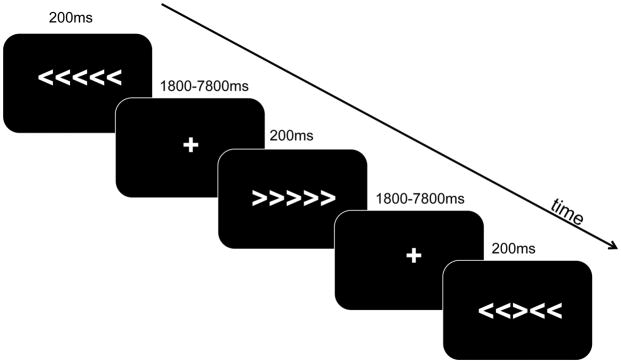
The flanker task was administered in the scanner as an event-related fMRI paradigm. Visual stimuli were presented with E-Prime 2.10 Software (Psychology Software Tools, Pittsburg, PA) and were projected to a screen at the back of the scanner, which participants viewed with a mirror attached to the head coil. On each trial, participants viewed a string of arrows that was presented for 200 ms and was immediately followed by a crosshair that was randomly jittered between 1800 ms and 7800 ms (mean = 4300 ms; see Figure 1). Participants’ task was to respond to the direction of the center arrow, which was surrounded by flanking arrows on either side. On half the trials, the flanking arrows pointed in the same direction as the center arrow (congruent condition), whereas on the other half of trials, the flanking arrows pointed in the opposite direction from the center arrow (incongruent condition). Direction of the center arrow was counterbalanced across trials. The order of stimulus presentation was pseudo-randomized to ensure that no more than three incongruent trials or no more than three trials with the center arrow pointing in the same direction appeared in a row. Responses were made with the index and middle fingers of the right hand. Responses were collected up to 2000 ms after stimulus onset2. There were four runs with 80 trials per run, equaling a total of 320 trials. The order of runs was counterbalanced across participants. Instructions and practice were given outside the scanner a half hour before scanning took place. During fMRI data acquisition, response accuracy, onset time, and reaction time were recorded for each stimulus and only correct trials were included in analyses of response latency.
Figure 1.
Flanker Task. Stimuli were presented for 200ms followed immediately by a cross hair with presentation duration randomly jittered between 1800ms–7800ms.
Neuroimaging Acquisition
Data were acquired with a 32-channel head coil on a 3-Tesla Siemens Trio whole-body MRI scanner located at the VA Boston Healthcare System, Jamaica Plain campus. An auto align scout scan was acquired first. One T1-weighted three-dimensional magnetization-prepared rapid gradient-echo imaging (MP RAGE) scan was collected for each participant (FOV=256, Matrix=256 × 256 × 176 slices, 1 × 1 × 1 mm voxels, TR=2530 ms, TE=3.32 ms, flip angle=7°). A T2-FLAIR image was also collected (FOV=256, Matrix=512 × 512 × 160 slices, 0.49 × 0.49 × 1 mm voxels, TR=6000ms, TE=388ms, flip angle=120°) for each participant. Four blood oxygen level dependent (BOLD) T2*-weighted echo-planar imaging runs were acquired parallel to the anterior commissure-posterior commissure plane (FOV=192, TR=2000 ms, TE=30 ms, voxel size=2.67 × 2.67 × 3.75 mm, slice order=interleaved, flip angle=90°, matrix=722, volumes=185). The first five volumes of each run collected before stimulus presentation began were discarded to allow for signal magnetization equilibrium.
Behavioral Analysis
Statistical analyses were performed with SPSS, version 19 (IBM Corp., Armonk, NY). Demographic data were analyzed with independent samples t-tests for linear variables and chi square tests for categorical variables. Congruency effects were analyzed using 2 (group: mTBI, control) × 2 (condition: incongruent, congruent) repeated measures analyses of covariance (ANCOVA) with reaction time and accuracy as the dependent measures, respectively. Effects of error processing were analyzed using an ANCOVA with post-error slowing scores as the dependent measure and group as the independent measure. PCL-M scores, number of pre-deployment TBIs, and age were entered as covariates in all analyses. However, because these variables did not contribute significant variance, they are not reported. Assumptions for ANCOVA were checked including normality, linear relationships between covariates for each group, outliers, homogeneity for regression slopes, homogeneity of covariance (Box’s M Test), and homogeneity of variance (Levene’s Test). No assumptions were violated, justifying the use of ANCOVA.
Neuroimaging Analysis
All preprocessing procedures and analyses were carried out using The Oxford Centre for FMRIB FSL software package (version 4.15; http://www.fmrib.ox.ac.uk/fsl). FMRI data were processed using fMRI Expert Analysis Tool (FEAT; Version 5.98). Data were preprocessed with the following pre-statistics: motion correction using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002), slice-timing correction using Fourier-space time-series phase-shifting, non-brain removal using BET (Smith, 2002), spatial smoothing using a Gaussian kernel of full-width/half-max (FWHM) 5mm, and grand-mean intensity normalization of the entire 4D dataset by a single multiplicative factor. To remove head motion artifact, we used a data-driven independent component analysis (ICA) method to identify and remove motion-related components from the data (ICA-based strategy for Automatic Removal of Motion Artifact [ICA-AROMA]; Pruim et al., 2015). After removing these motion components, we removed signal from white matter and cerebral spinal fluid using nuisance regression in order to further minimize noise-related artifact in the functional data (Pruim, Mennes, Buitelaar, & Beckmann, 2015). Next, we applied linear detrending and a highpass temporal filter (σ= 45.0s). Registration to high-resolution structural and standard space images was carried out using FMRIB’s Linear Image Registration Tool (FLIRT) and further refined using FMRIB’s Nonlinear Image Registration Tool (FNIRT).
Functional analysis of incongruent trial processing was performed using an incongruent > congruent contrast for correct trials. The processing of error trials was analyzed using an incorrect > correct contrast for congruent and incongruent trials combined. Higher-level analyses were carried out using FMRIB’s Local Analysis of Mixed Effects (FLAME) stage 1 (Beckmann, Jenkinson, & Smith, 2003; Woolrich, 2008; Woolrich, Behrens, Beckmann, Jenkinson, & Smith, 2004). To examine each contrast, runs were combined for each participant. To determine activation differences across groups, group level activation maps were generated for each contrast using FLAME stage 1. Age, number of pre-deployment TBIs, and PTSD symptom severity were entered into the model as regressors. In order to examine group differences at the significance level of P = 0.005, Z statistic images were thresholded using clusters determined by Z > 2.6 and a corrected cluster significance threshold of P = 0.05.
To examine the implications of observed group activation differences within the context of larger networks, we performed a PPI analysis (Friston et al., 1997; O’Reilly et al., 2012). PPI analysis involves a psychological regressor (i.e., incongruent vs. congruent or incorrect vs. correct responses), a physiological regressor, which is the time course of the seed region of interest (ROI), and the PPI term, which is the interaction between the psychological and physiological regressors. Group level PPI maps were generated to determine group differences in brain regions that were modulated by the interaction of the task and activation of the seed ROI. We extracted the time course of the seed region, defined as the significant region from the functional group analysis, from all participants. Higher-level analyses for the PPI regressor were carried out using FLAME stage 1. Runs were combined for all participants. To determine group differences, age, number of pre-deployment TBIs, and PTSD symptom severity were entered into the model as regressors and group PPI maps were generated. Z statistic images were thresholded using clusters determined by Z > 2.6 and a corrected cluster significance threshold of P = 0.05. The maps of the PPI analysis represent the effects of the interaction that are over and above the main effects of the BOLD response to the task contrast and correlations with the seed region.
Finally, to identify brain regions that were associated with behavior, we performed a mixed effects (FLAME stage 1) group-level analysis using behavior as a regressor. Because significant group differences in neural activation were found only when examining the processing of errors, we performed this analysis for error-related performance only. We calculated a post-error slowing score, reflecting the difference in reaction time on trials immediately following an error (post-error) and trials immediately following a correct response (post-correct). Next, we identified brain regions that were positively and/or negatively associated with post-error slowing within the incorrect > correct contrast, by using the post-error slowing score as a regressor in the analysis. Additionally, to determine whether these brain-behavior associations differed across groups, we conducted a continuous covariate interaction analysis. The post-error slowing score was entered as a separate regressor for each group. Age, number of pre-deployment TBIs, and PTSD symptom severity were entered as regressors in all analyses. Z statistic images were thresholded using clusters determined by Z > 2.6 and a corrected cluster significance threshold of P = 0.05.
Results
Behavioral Performance
We examined congruency effects in both accuracy and reaction time data (see Table 2). A 2×2 repeated measures ANCOVA of accuracy data with group as the between subjects factor and congruency as the within subjects factor revealed that accuracy did not significantly differ as a function of group (F(1,28) < 1, P > 0.4) or congruency (F(1,28) < 1, P > 0.9). Moreover, the group by congruency interaction was also not significant (F(1,28) < 1, P > 0.8). As a follow-up analysis of accuracy, we performed a signal detection analysis of discriminability and bias in which correct responses on congruent trials were considered hits and incorrect responses on incongruent trials were considered false alarms. Results revealed that neither discriminability nor response bias significantly differed as a function of group (d′: F(1,28) = 1.4, P > 0.2; beta: F(1,28) < 1, P > 0.4). Analysis of latency data revealed that performance again did not significantly differ as a function of group (F(1,28) < 1, P > 0.5). Performance was numerically slower in the incongruent condition than congruent condition, but the difference was not significant (F(1,28) = 2.1, P > 0.1). The group by congruency interaction was also not significant (F(1,28) < 1, P > 0.6).
Table 2.
Performance as a function of congruency and post-error slowing.
| Control (n=16) | mTBI (n=17) | |
|---|---|---|
| Congruency | ||
| Congruent accuracy, M (SD) | 92.3 (9.2) | 94.0 (6.0) |
| Incongruent accuracy, M (SD) | 83.0 (10.8) | 85.1 (8.8) |
| Congruent RT, M (SD) | 590.3 (122.5) | 559.0 (117.6) |
| Incongruent RT, M (SD) | 697.4 (133.6) | 649.8 (159.8) |
| D′, M (SD) | −0.3 (1.8) | 0.3 (1.3) |
| Beta, M (SD) | 1.5 (1.2) | 1.2 (0.5) |
|
| ||
| Post-error slowing | ||
| Post-correct RT, M (SD) | 637.7 (116.0) | 599.5 (130.8) |
| Post-error RT, M (SD) | 661.4 (121.1) | 622.8 (140.4) |
| Post-error slowing score, M (SD) | 23.7 (61.1) | 23.3 (47.4) |
Note: Accuracy is reported as a percent. Reaction times are in milliseconds. mTBI=mild traumatic brain injury; RT=reaction time.
We next examined the behavioral measure of cognitive control as indexed by post-error slowing. An ANCOVA revealed that groups did not significantly differ in the magnitude of post-error slowing (F(1,28) < 1, P > 0.9).
Neuroimaging Results
Processing of incongruent trials
The incongruent > congruent contrast was examined to determine if there were significant group differences in brain activation associated with the processing of incongruent information. There were no significant group differences in any brain region in the incongruent > congruent contrast. When we examined group maps separately, the control group showed increased activation in the right superior parietal lobe; the mTBI group showed increased activation in the left superior parietal lobe, left dorsal anterior cingulate cortex, right supramarginal gyrus, and right lateral occipital cortex (see Table S1). Covariate effects are reported in the supplemental materials.
Processing of error trials
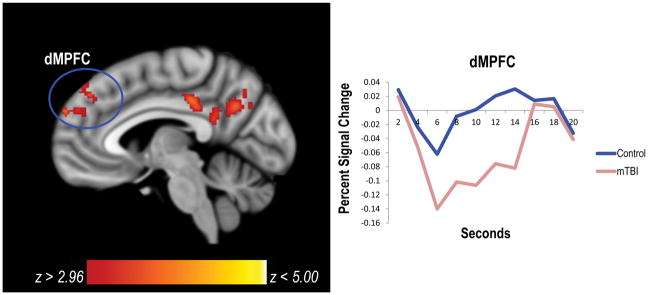
The incorrect > correct contrast was examined to determine if there were significant group differences in brain activation associated with the processing of errors. Both groups showed increased activation in the insula and dorsolateral prefrontal cortex (see Table S2), regions that are part of the salience network and central executive network, respectively. There were no group differences in activation in these regions. By contrast, compared to controls, individuals with mTBI showed greater deactivation in areas of the default mode network including the left dorsomedial prefrontal cortex (dMPFC) and left posterior cingulate cortex (PCC)/precuneus (see Table 3, Figure 2). Covariate effects are reported in the supplemental materials.
Table 3.
Significant group differences in brain regions for the incorrect > correct contrast.
| Brain Region | Cluster Size | Peak Voxel (MNI coordinates) | Z-statistic |
|---|---|---|---|
| Control > mTBI for Incorrect > Correct | |||
| Left PCC/Precuneus* | 676 | −4 −30 36 | 3.88 |
| Left Lateral Occipital Cortex/Precuneus* | 494 | −36 −70 40 | 4.16 |
| Left Middle Frontal Gyrus/dMPFC* | 398 | −28 26 50 | 4.19 |
| Right Cerebellum | 340 | 40 −76 −50 | 3.71 |
| Left dMPFC* | 261 | −4 60 28 | 3.78 |
| Right Lateral Occipital Cortex/Precuneus* | 198 | −54 22 18 | 4.13 |
| Left Inferior Frontal Gyrus | 177 | −54 22 18 | 4.13 |
|
| |||
| mTBI > Control for Incorrect > Correct | |||
| Left Occipital Cortex | 531 | −4 −92 12 | 3.86 |
Note:
for these regions, Control > mTBI for Incorrect > Correct represents greater deactivation in mTBI.
Cluster size is number of voxels. Only clusters are reported; sub-clusters are not reported. dMPFC=dorsomedial prefrontal cortex; mTBI=mild traumatic brain injury; PCC=posterior cingulate cortex.
Figure 2.
Compared to controls, the mTBI group had significant deactivation in default mode network regions for the incorrect > correct contrast. In particular, the mTBI group had greater deactivation in the left dMPFC and left PCC. The hemodynamic response function for the dMPFC is plotted to the right of the figure. dMPFC=dorsomedial prefrontal cortex; mTBI=mild traumatic brain injury; PCC=posterior cingulate cortex.
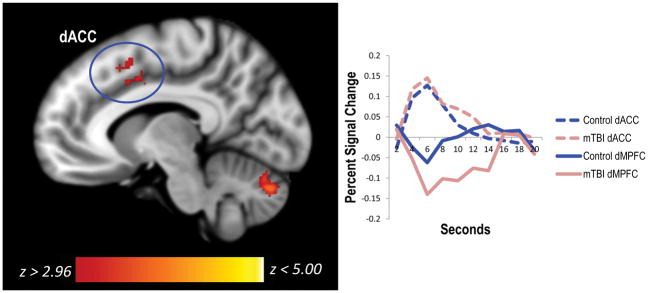
We next examined whether there were regions where functional connectivity with the default mode network for incorrect (vs. correct) trials differed across groups. To do this, we extracted PCC and dMPFC ROIs based on significant group differences in the incorrect > correct analysis and used the PCC (peak MNI coordinates = −4 −30 36) and the dMPFC (peak MNI coordinates = −4 60 28) ROIs as seeds in two separate PPI analyses. Using the PCC as a seed, there were no significant group differences in the functional connectivity between the PCC and any brain region for incorrect (vs. correct) trials. Using the dMPFC as a seed, we found that functional coupling with the left dorsal anterior cingulate cortex for incorrect vs. correct trials was greater in the mTBI group than the control group. Upon further inspection, results showed that there was increased negative coupling between these regions in the mTBI group (see Table 4, Figure 3). There were no regions where the control group showed greater differential functional connectivity for incorrect vs. correct trials than the mTBI group.
Table 4.
Significant group differences in functional connectivity with dMPFC seed region in the incorrect > correct contrast as determined by PPI analysis.
| Brain Region | Cluster Size | Peak Voxel (MNI coordinates) | Z-statistic |
|---|---|---|---|
| Control > mTBI for Incorrect > Correct | |||
| --- | |||
|
| |||
| mTBI > Control for Incorrect > Correct | |||
| Left dACC | 222 | −6 10 50 | 3.42 |
| Left Cerebellum | 180 | −8 −80 −30 | 3.87 |
Note: Cluster size is number of voxels. Only clusters are reported; sub-clusters are not reported. dACC=dorsal anterior cingulate cortex; dMPFC=dorsomedial prefrontal cortex; mTBI=mild traumatic brain injury.
Figure 3.
The mTBI group had enhanced functional connectivity between the dMPFC of the default mode network and a region of the salience network for the incorrect > correct contrast. Specifically, negative functional connectivity between the left dMPFC and left dorsal anterior cingulate cortex for incorrect vs. correct trials was greater for the mTBI group than the control group. The hemodynamic response function is plotted to the right of the figure to show coupling. mTBI=mild traumatic brain injury; dACC=dorsal anterior cingulate cortex; dMPFC=dorsomedial prefrontal cortex.
In a follow-up analysis, we examined the connectivity with the dMPFC for incorrect (vs. correct) trials within each group separately. Results revealed that within the mTBI group, the dMPFC was functionally coupled with the right insula and left postcentral gyrus, extending into the dorsal anterior cingulate cortex, as well as the right dorsolateral prefrontal cortex and right superior parietal lobe, regions of the salience and central executive networks, for incorrect (vs. correct) trials (see Table 5). This coupling was negative such that these regions are active when the dMPFC is deactivated. In the control group, there were no regions that showed greater coupling with the dMPFC for incorrect (vs. correct) trials.
Table 5.
Significant brain regions for PPI analysis of incorrect > correct contrast in each group.
| Brain Region | Cluster Size | Peak Voxel (MNI coordinates) | Z-statistic |
|---|---|---|---|
| Control group map | |||
| --- | |||
|
| |||
| mTBI group map | |||
| Left Temporal Pole/Frontal Operculum Cortex | 908 | −54 12 −8 | 3.96 |
| Right Middle Frontal Gyrus/DLPFC | 755 | 48 34 10 | 3.92 |
| Left Postcentral Gyrus | 504 | −42 −18 50 | 3.8 |
| Right Insula | 387 | 38 24 −4 | 4.01 |
| Right Superior Parietal Lobe/Supramarginal Gyrus | 285 | 58 −26 44 | 3.9 |
| Right Superior Parietal Lobe | 179 | 36 −54 52 | 3.37 |
| Left Cerebellum | 166 | −10 −78 −30 | 3.88 |
Note: Cluster size is number of voxels. Only clusters are reported; sub-clusters are not reported.
dACC=dorsal anterior cingulate cortex; DLPFC=dorsolateral prefrontal cortex; mTBI=mild traumatic brain injury.
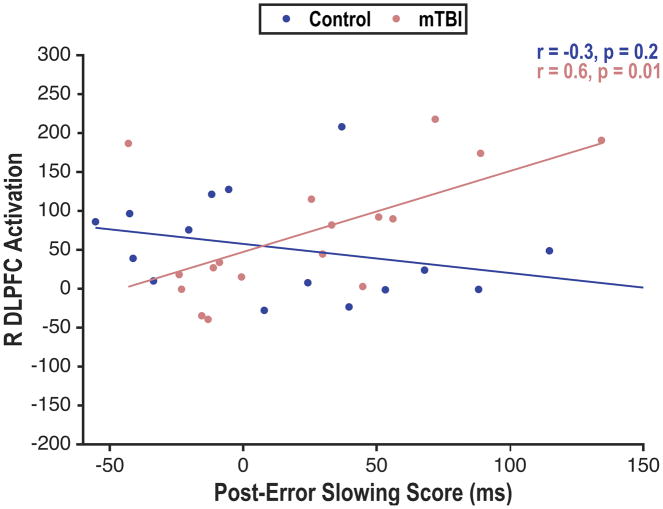
Finally, to examine the functional significance of these neural alterations in cognitive control, we performed a whole-brain imaging analysis to determine whether brain activation associated with error processing was associated with a behavioral measure of cognitive control, as indexed by post-error slowing. The analysis revealed no significant associations between post-error slowing and activation in any brain region. However, group moderated the association between post-error slowing and activation in the right dorsolateral prefrontal cortex (ZMax = 3.69; peak MNI coordinates: 46 38 26). Greater post-error slowing was associated with greater recruitment of the right dorsolateral prefrontal cortex in individuals with mTBI, but not in controls (Figure 4).
Figure 4.
Increased post-error slowing was significantly associated with greater activity in the right dorsolateral prefrontal cortex, a central executive network region, in mTBI. There was no significant association in the control group. Activation is represented as contrast of parameter estimate values. DLPFC=dorsolateral prefrontal cortex; mTBI=mild traumatic brain injury; R= right.
Discussion
We examined behavioral and neural indices of cognitive control in OEF/OIF veterans with blast-related mTBI in the context of a flanker task. Behavioral performance did not differ in individuals with and without mTBI, but the neural signature of cognitive control was amplified in the mTBI group. That is, with increased demands on cognitive control processes, the mTBI group showed greater deactivation of default mode network regions than the control group. Furthermore, there was enhanced negative connectivity between the dMPFC, a region within the default mode network, and regions of the salience network and central executive network. Taken together, these findings suggest that mTBI did not affect the ability to engage in cognitive control, but altered how such control was neurally implemented.
Although we evaluated activation associated with the processing of both incongruent information and errors as neural measures of cognitive control, group differences emerged for error processing only. The question arises whether the failure to observe group differences in the incongruent-congruent comparison may be due to the fact that this comparison does not provide a pure index of cognitive control. That is, it could be argued that this comparison reflects a combination of priming effects associated with the presence of congruent flankers and demands on cognitive control associated with the presence of incongruent flankers. However, studies that have included a baseline condition to disentangle these two effects have shown that reaction times for congruent and baseline trials do not differ, suggesting that priming effects are negligible and that differences between incongruent and congruent trials are largely due to demands on inhibitory cognitive control processes associated with incongruent trials (Bunge et al., 2002; Hazeltine, Bunge, Scanlon, & Gabrieli, 2003). Such findings argue that the difference between incongruent and congruent trials is an appropriate measure of cognitive control. Thus, the fact that we did not observe group differences in the incongruent-congruent comparison is unlikely to reflect a measurement problem. Rather, the greater sensitivity of error processing in the current study may be due to the fact that the task was relatively easy, and cognitive control was more strongly taxed during the processing of errors than during the processing of incongruent flankers.
In the context of incorrect relative to correct responses and in comparison to controls, mTBI was associated with greater deactivation in the PCC and dMPFC, two regions within the default mode network. The default mode network is a well-established resting state network that is most active at rest and is involved in autobiographical memory retrieval, mind-wandering, and other self-generated thought (Gusnard, Akbudak, Shulman, & Raichle, 2001; McKiernan, Kaufman, Kucera-Thompson, & Binder, 2003; Raichle et al., 2001). The default mode network is deactivated during tasks that require externally oriented attention (Fox et al., 2005; Fransson, 2006; Uddin, Kelly, Biswal, Castellanos, & Milham, 2009). Previous work by Fischer et al. (2014) has shown that default mode network deactivation is exaggerated in blast-related mTBI, a finding that is replicated in the current study.
Using PPI analysis to examine functional connectivity, our findings go beyond Fischer et al. (2014) by demonstrating that during the processing of errors, individuals with mTBI, in comparison to controls, show enhanced negative coupling between the dMPFC and the dorsal anterior cingulate cortex, a region of the salience network. When we examined the groups separately, we found that the mTBI group in particular displayed significantly enhanced negative coupling between the dMPFC and regions of both the salience and central executive networks associated with error processing. Previous studies suggest that this coupling between the default mode, salience, and central executive networks facilitates successful task performance (Fransson, 2006; McKiernan et al., 2003). The salience network has been hypothesized to be a “switching” network in cognitive control and may be especially important in switching between the default mode and central executive networks to accomplish task goals (Menon & Uddin, 2010). Accordingly, Sridharan and colleagues (2008) showed in healthy individuals that with increased demands on cognitive control, the salience network is engaged to suppress default mode network regions and to amplify the response of central executive network regions. In the present study, these network dynamics were up-regulated in the mTBI group. Given that this occurred in the context of intact behavioral performance in the mTBI group, we postulate that the enhanced negative coupling between the default mode network and salience and central executive networks in mTBI serves as a compensatory mechanism for successful task performance. Consistent with this notion, we found an association between post-error slowing and dorsolateral prefrontal cortex recruitment in mTBI, suggesting that recruitment of the central executive network may facilitate the implementation of cognitive control and contribute to adjustments in behavioral performance after an error.
By focusing not only on activation in distinct brain regions, but also on the interaction of the networks they are a part of, our study sheds light on the findings of Scheibel et al. (2012) and Fischer et al. (2014) and suggests that the neural alterations reported in those studies can be understood with reference to larger network dynamics. These findings point to the importance of examining networks and their interactions in cognitive control and emphasize that cognitive control is not a singular process but instead relies on the modulation of multiple processes through network interactions.
Altered communication between the functional networks involved in cognitive control has also been observed in civilian TBI, albeit in the context of more severe injury that led to behavioral impairment (Bonnelle et al., 2012). In a recent study focused on cognitive control in civilian mTBI, enhanced activation in the mTBI group was also interpreted as reflecting a compensatory mechanism, but this activation was observed in inferior parietal cortex, a region not part of the functional networks discussed in the current study (Mayer et al., 2015). Moreover, this study did not examine functional network interactions. Thus, it is unknown whether our findings of compensatory functional network interactions would generalize to civilian mTBI, especially given that the pathology in blast and non-blast mTBI is somewhat different. For example, white matter alterations associated with blast-related mTBI tend to be spatially variable (Hayes, Miller, Lafleche, Salat, & Verfaellie, 2015; Miller, Hayes, Lafleche, Salat, & Verfaellie, 2016), which is in contrast to the more consistent findings of white matter alterations in specific long fiber pathways in non-blast mTBI (Aoki, Inokuchi, Gunshin, Yahagi, & Suwa, 2012; Hayes, Bigler, & Verfaellie, 2016). Thus, it remains an open question whether the mechanism of injury impacts how cognitive control is implemented in mTBI.
Given that the focus of this study was on changes in cognitive control associated with blast-related mTBI, we included a control group of individuals who had been exposed to blast, but did not suffer TBI. The inclusion of a blast-exposed control group in this study thus helped to isolate the contribution of mTBI. However, recent reports suggest that blast exposure itself is associated with neural changes (Robinson et al., 2015; Taber et al., 2015), leaving open the possibility that blast-exposure might be associated with altered dynamics in the functional networks mediating cognitive control. Future studies examining the effects of blast exposure on cognitive control are needed to evaluate this possibility.
The results reported in this study should be considered within the context of the limitation that mTBI group assignment was based on self-report. However, mTBI assessment was conducted with an in-depth structured clinical interview, which is currently the gold standard of diagnosis (Corrigan & Bogner, 2007). Another limitation is the small sample size of the control and patient groups of this study. The exclusion of individuals with ADHD as well as those with current alcohol abuse limited potential enrollment in this study. It will be important to replicate these findings in studies with larger samples. A third limitation is the inability to examine LOC-associated effects on cognitive control. As shown in Matthews, Simmons, and Strigo (2011), LOC may moderate the neural changes associated with cognitive control in blast-related mTBI, but our sample size was too small to allow for a direct comparison between individuals who suffered mTBI with and without LOC. Future studies will need to examine whether there are differences in cognitive control in individuals with mTBI as a function of presence of LOC.
In summary, we report robust neural differences associated with error processing in individuals with chronic blast-related mTBI compared to blast-exposed controls. In particular, individuals with mTBI exhibited increased deactivation in regions of the default mode network. Further, these regions showed greater negative functional connectivity with regions of the salience and central executive networks during the processing of errors. Importantly, these brain changes in mTBI occurred in the context of intact behavioral performance. Taken together, these results suggest that with increased demands on cognitive control, greater deactivation of regions of the default mode network and enhanced negative coupling between the default mode network and regions of the salience and central executive networks may act as a compensatory mechanism for successful task performance in mTBI.
Supplementary Material
Acknowledgments
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The authors would like to thank Michael Esterman, Ph.D. for valuable input on this work. The authors declare no conflicts of interest. This work was supported by the VA Rehabilitation Research & Development Service (M.V., grant number I01RX000216 and J.P.H., grant number I21RX001594); the VA Clinical Science Research and Development Service; and the National Center for PTSD. This work was further supported with resources and the use of facilities at the Neuroimaging Research for Veterans Center, VA Boston Healthcare System.
Footnotes
Notably, the peak of the medial frontal/anterior cingulate cortex cluster in Scheibel et al. (2012) was more lateral and superior than the medial prefrontal hub of the default mode network (Andrews-Hanna, Reidler, Sepulcre, Poulin, & Buckner, 2010), and aligned more closely with the salience network. In contrast, the peak of the medial frontal gyrus cluster reported in Fischer et al. (2014) fell within the default mode network.
For the first 5 participants, responses were recorded only up to 1000ms. Two of these participants were in the mTBI group and three were in the control group. An average of 15 trials (~ 5% of the total number of trials) was lost for each of these participants.
References
- American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. The Journal of Head Trauma Rehabilitation. 1993;8(3):86–87. [Google Scholar]
- Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL. Functional-anatomic fractionation of the brain’s default network. Neuron. 2010;65(4):550–562. doi: 10.1016/j.neuron.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki Y, Inokuchi R, Gunshin M, Yahagi N, Suwa H. Diffusion tensor imaging studies of mild traumatic brain injury: A meta-analysis. Journal of Neurology, Neurosurgery & Psychiatry. 2012;83:870–876. doi: 10.1136/jnnp-2012-302742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. NeuroImage. 2003;20(2):1052–1063. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Belanger HG, Vanderploeg RD. The neuropsychological impact of sports-related concussion: A meta-analysis. Journal of the International Neuropsychological Society. 2005;11(04):345–357. doi: 10.1017/S1355617705050411. [DOI] [PubMed] [Google Scholar]
- Blake DD, Weathers FW, Nagy LM, Kaloupek DG, Gusman FD, Charney DS, Keane TM. The development of a Clinician-Adminstered PTSD Scale. Journal of Traumatic Stress. 1995;8(1):75–90. doi: 10.1007/BF02105408. [DOI] [PubMed] [Google Scholar]
- Bonnelle V, Ham TE, Leech R, Kinnunen KM, Mehta MA, Greenwood RJ, Sharp DJ. Salience network integrity predicts default mode network function after traumatic brain injury. Proc Natl Acad Sci U S A. 2012;109(12):4690–4695. doi: 10.1073/pnas.1113455109. doi:1113455109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychol Rev. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Pontifex MB, O’Connor P, Hillman CH. The persistent effects of concussion on neuroelectric indices of attention. J Neurotrauma. 2009;26(9):1463–1470. doi: 10.1089/neu.2008-0766. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Hazeltine E, Scanlon MD, Rosen AC, Gabrieli JD. Dissociable contributions of prefrontal and parietal cortices to response selection. NeuroImage. 2002;17(3):1562–1571. doi: 10.1006/nimg.2002.1252. S1053811902912528. [DOI] [PubMed] [Google Scholar]
- Corrigan JD, Bogner J. Screening and identification of TBI. Journal of Head Trauma Rehabilitation. 2007;22(6):315–317. doi: 10.1097/01.HTR.0000300227.67748.77. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends in Cognitive Sciences. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer BL, Parsons M, Durgerian S, Reece C, Mourany L, Lowe MJ, … Rao SM. Neural activation during response inhibition differentiates blast from mechanical causes of mild to moderate traumatic brain injury. J Neurotrauma. 2014;31(2):169–179. doi: 10.1089/neu.2013.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci U S A. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. doi:0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fransson P. How default is the default mode of brain function? Further evidence from intrinsic BOLD signal fluctuations. Neuropsychologia. 2006;44(14):2836–2845. doi: 10.1016/j.neuropsychologia.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Frencham KA, Fox AM, Maybery MT. Neuropsychological studies of mild traumatic brain injury: A meta-analytic review of research since 1995. J Clin Exp Neuropsychol. 2005;27(3):334–351. doi: 10.1080/13803390490520328. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Bigler ED, Verfaellie M. Traumatic brain injury as a disorder of brain connectivity. Journal of the International Neuropsychological Society. 2016;22(2):120–137. doi: 10.1017/S1355617715000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JP, Miller DR, Lafleche G, Salat DH, Verfaellie M. The nature of white matter abnormalities in blast-related mild traumatic brain injury. NeuroImage: Clinical. 2015;8:148–156. doi: 10.1016/j.nicl.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazeltine E, Bunge SA, Scanlon MD, Gabrieli JD. Material-dependent and material-independent selection processes in the frontal and parietal lobes: An event-related fMRI investigation of response competition. Neuropsychologia. 2003;41(9):1208–1217. doi: 10.1016/s0028-3932(03)00040-x. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kelly AC, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. Competition between functional brain networks mediates behavioral variability. NeuroImage. 2008;39(1):527–537. doi: 10.1016/j.neuroimage.2007.08.008. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, III, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910303/5660/1023. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, III, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. doi:8537. [DOI] [PubMed] [Google Scholar]
- Matthews S, Simmons A, Strigo I. The effects of loss versus alteration of consciousness on inhibition-related brain activity among individuals with a history of blast-related concussion. Psychiatry Research: Neuroimaging. 2011;191(1):76–79. doi: 10.1016/j.pscychresns.2010.09.013. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Yang Z, Yeo RA, Pena A, Ling JM, Mannell MV, Stippler M, Mojtahed K. A functional MRI study of multimodal selective attention following mild truamatic brain injury. Brain Imaging and Behavior. 2012;6(2):343–354. doi: 10.1007/s11682-012-9178-z. [DOI] [PubMed] [Google Scholar]
- Mayer AR, Hanlon FM, Dodd AB, Ling JM, Klimaj SD, Meier TB. A functional magnetic resonance imaging study of cognitive control and neurosensory deficits in mild traumatic brain injury. Human Brain Mapping. 2015;36(11):4394–4406. doi: 10.1002/hbm.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea MA. Mild Traumatic Brain Injury and Postconcussion Syndrome. New York: Oxford University Press; 2008. [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera-Thompson J, Binder JR. A parametric manipulation of factors affecting task-induced deactivation in functional neuroimaging. J Cogn Neurosci. 2003;15(3):394–408. doi: 10.1162/089892903321593117. [DOI] [PubMed] [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a Go/NoGo response inhibition task. Human Brain Mapping. 2001;12:131–143. doi: 10.1002/1097-0193(200103)12:3<131::AID-HBM1010>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct. 2010;214(5–6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DR, Hayes JP, Lafleche G, Salat DH, Verfaellie M. White matter abnormalities are associated with chronic postconcussion symptoms in blast-related mild traumatic brain injury. Human Brain Mapping. 2016;37(1):220–229. doi: 10.1002/hbm.23022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nee DE, Wager TD, Jonides J. Interference resolution: Insights from a meta-analysis of neuroimaging tasks. Cogn Affect Behav Neurosci. 2007;7(1):1–17. doi: 10.3758/cabn.7.1.1. [DOI] [PubMed] [Google Scholar]
- O’Reilly JX, Woolrich MW, Behrens TE, Smith SM, Johansen-Berg H. Tools of the trade: Psychophysiological interactions and functional connectivity. Soc Cogn Affect Neurosci. 2012;7(5):604–609. doi: 10.1093/scan/nss055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontifex MB, O’Connor PM, Broglio SP, Hillman CH. The association between mild traumatic brain injury history and cognitive control. Neuropsychologia. 2009;47(14):3210–3216. doi: 10.1016/j.neuropsychologia.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Pruim RH, Mennes M, Buitelaar JK, Beckmann CF. Evaluation of ICA-AROMA and alternative strategies for motion artifact removal in resting state fMRI. NeuroImage. 2015;112:278–287. doi: 10.1016/j.neuroimage.2015.02.063. [DOI] [PubMed] [Google Scholar]
- Pruim RHR, Mennes M, van Rooij D, Llera A, Buitelaar JK, Beckmann CF. ICA-AROMA: A robust ICA-based strategy for removing motion artifacts from fMRI data. NeuroImage. 2015;112:267–277. doi: 10.1016/j.neuroimage.2015.02.064. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci U S A. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson ME, Lindemer ER, Fonda JR, Milberg WP, McGlinchey RE, Salat DH. Close-range blast exposure is associated with altered functional connectivity in Veterans independent of concussion symptoms at time of exposure. Hum Brain Mapp. 2015;36(3):911–922. doi: 10.1002/hbm.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Lin X, Steinberg JL, Radaideh M, Levin HS. Altered brain activation in military personnel with one or more traumatic brain injuries following blast. J Int Neuropsychol Soc. 2012;18(1):89–100. doi: 10.1017/S1355617711001433. S1355617711001433. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, … Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. doi:27/9/2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seignourel PJ, Robins DL, Larson MJ, Demery JA, Cole M, Perlstein WM. Cognitive control in closed head injury: Context maintenance dysfunction or prepotent response inhibition deficit? Neuropsychology. 2005;19(5):578–590. doi: 10.1037/0894-4105.19.5.578. doi:2005-11412-003. [DOI] [PubMed] [Google Scholar]
- Singh KD, Fawcett I. Transient and linearly graded deactivation of the human default-mode network by a visual detection task. NeuroImage. 2008;41(1):100–112. doi: 10.1016/j.neuroimage.2008.01.051. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci U S A. 2008;105(34):12569–12574. doi: 10.1073/pnas.0800005105. doi:0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taber KH, Hurley RA, Haswell CC, Rowland JA, Hurt SD, Lamar CD, Morey RA. White matter compromise in veterans exposed to primary blast forces. J Head Trauma Rehabil. 2015;30(1):E15–E25. doi: 10.1097/HTR.0000000000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: Recent findings and theoretical perspectives. Neuroscientist. 2007;13(2):160–172. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Tombaugh TN, Tombaugh PW. Test of Memory Malingering: TOMM. Tonawanda, NY: Multi-Health Systems; 1996. [Google Scholar]
- Uddin LQ, Kelly AM, Biswal BB, Castellanos FX, Milham MP. Functional connectivity of default mode network components: Correlation, anticorrelation, and causality. Hum Brain Mapp. 2009;30(2):625–637. doi: 10.1002/hbm.20531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fMRI and ERPs. NeuroImage. 2001;14(6):1387–1401. doi: 10.1006/nimg.2001.0935S1053-8119(01)90935-8. [DOI] [PubMed] [Google Scholar]
- Verfaellie M, Lafleche G, Spiro A, III, Tun C, Bousquet K. Chronic postconcussion symptoms and functional outcomes in OEF/OIF veterans with self-report of blast exposure. J Int Neuropsychol Soc. 2013;19(1):1–10. doi: 10.1017/S1355617712000902. S1355617712000902. [DOI] [PubMed] [Google Scholar]
- Weathers F, Huska J, Keane T. The PTSD Checklist Military Version (PCL-M) Boston, MA: National Center for PTSD; 1991. [Google Scholar]
- Wilkins KC, Lang AJ, Norman SB. Synthesis of the psychometric properties of the PTSD checklist (PCL) military, civilian, and specific versions. Depression and Anxiety. 2011;28(7):596–606. doi: 10.1002/da.20837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M. Robust group analysis using outlier inference. NeuroImage. 2008;41(2):286–301. doi: 10.1016/j.neuroimage.2008.02.042. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Behrens TE, Beckmann CF, Jenkinson M, Smith SM. Multilevel linear modelling for FMRI group analysis using Bayesian inference. NeuroImage. 2004;21(4):1732–1747. doi: 10.1016/j.neuroimage.2003.12.023. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.