Abstract
Purpose:
Various aspects of diet, including specific food items and nutrients, have been shown to modulate inflammation and have been implicated in the etiology of prostate cancer (PrCA). No study examining the role of diet-associated inflammation in PrCA has been conducted in Latin America.
Method:
We examined the association between the dietary inflammatory index (DII®) and PrCA in a population-based case-control study in Córdoba, Argentina. A total of 153 incident cases of PrCA and 309 controls frequency matched on sex, age (± 5 years), and place of residence were recruited from 2008–2015. The DII was developed to determine the inflammatory potential of individuals’ diets and was computed from a validated food frequency questionnaire using nutrients data from diet only. Multi-level logistic regression models were fit to evaluate the association between DII scores and PrCA, adjusting for age, body mass index, energy intake and occupational exposure as first-level covariates and family history of prostate cancer as the second-level variable. Odds ratios were estimated in all subject and stratified by BMI (<30 vs. ≥30kg/m2).
Results:
Men in the most pro-inflammatory group (tertile 3) had 50% higher odds of having PrCA compared to men in the most anti-inflammatory group (tertile 1) (ORtertile3 vs. tertile1 1.50; 95%CI: 1.24 to 1.80). The odds of prostate cancer were higher in obese men (n=109, OR tertile3 vs. tertile1 1.81; 95%CI 1.45 to 2.27), while no association was found among non-obese men (n=375, OR tertile3 vs. tertile1 0.93; 95%CI 0.25 to 3.51).
Conclusions:
A pro-inflammatory diet, reflected by higher DII scores, was positively associated with PrCA occurrence, based on these results and those from other studies, steps should be taken to promote a diet rich in anti-inflammatory foods, in order to reduce risk of PrCA and other chronic diseases. Future studies should explore this association in a prospective setting.
Keywords: dietary inflammatory index, prostate cancer, case-control, Argentina
Introduction:
Prostate cancer (PrCA) is the second most commonly diagnosed non-skin cancer [1]. Data on PrCA incidence in Argentina is sparse; however, reports from GLOBOCAN, 2012 shows that, PrCA is the most frequently diagnosed cancer and it is the third most common cause of cancer death among Argentinean men [2]. Analyses indicating socioeconomic and geographical variation in cancer incidence rates in Córdoba (Argentina) have motivated the study of possible lifestyle and environmental factors involved in the development of PrCA, including diet [3]. Chronic inflammation, a persistent state of low-grade systemic inflammation in which tissue destruction and repair occur simultaneously [4,5] and involving continuous recruitment of pro-inflammatory cytokines (associated with increased blood flow to the injured tissue due to histamine released by damaged mast cells) [6], has been shown to play a major role in the development of PrCA [7–9].
Consistent with the inflammation-PrCA hypothesis, several studies have shown chronic inflammation to be associated with PrCA [10]. Additionally, chronic inflammation, as measured by levels of inflammatory markers at baseline, has been shown to be positively associated with PrCA risk [11,12]. Chronic inflammation increases insulin resistance, which leads to increased circulating levels of insulin that, in turn, have been associated with the development of PrCA by inhibiting apoptosis and stimulating cell proliferation [13].
There is growing evidence that specific dietary components influence both acute and chronic inflammation [14–17]. Although many studies have been conducted to discern the relationship between diet and PrCA, results are inconclusive [18–20]. Research into the role of diet in inflammation and PrCA suggests that diet represents a complicated set of exposures that often interact, and whose cumulative effect modifies both inflammatory responses and health outcomes [21]. The Dietary Inflammatory Index (DII®), a tool developed by researchers at the University of South Carolina’s Cancer Prevention and Control Program, can be used in diverse populations in order to predict levels of inflammatory markers and related health outcomes [22,23]. A higher DII score indicates a pro-inflammatory dietary milieu rich in nutrients like saturated fat and total cholesterol; a lower DII score indicates that diet is more anti-inflammatory, rich in nutrients such as vitamins, minerals and flavonoids [22].
Thus far, the DII has been found to be associated with inflammatory cytokines including C-reactive protein (CRP), interleukin-6 and homocysteine [23–27]. In the first validation study higher DII scores were associated with values of hs-CRP >3 mg/l (OR = 1.08; 95 % CI 1.01, 1.16, P = 0.035) [23]. In another study, conducted in Iran, for every 1-unit increase in DII, there was a corresponding increase in interleukin-6 of 0.15 pg/mL, 95% CI (<0.01, 0.28) [28]. In a cross-sectional study conducted in Belgium, multivariable analyses showed significant positive associations between the DII and the inflammatory markers IL-6 (>1·6 pg/ml) (OR 1.19, 95 % CI 1.04, 1.36) and homocysteine (>15 μmol/l) (OR 1.56, 95 % CI 1.25, 1.94) [29]. The DII also has been associated with the glucose intolerance component of metabolic syndrome [24], increased odds of asthma in an Australian population [25], shiftwork [30], cardiovascular disease [31], colorectal cancer [32–34], gastric cancer [35] and pancreatic cancer [36]. Previous research among Cordoba, Argentinian men showed that those with a higher adherence to the Traditional dietary pattern characterized by high consumption of fatty red meats, offal, processed meat, starchy vegetables, added sugars and sweets, and vegetable oils (OR 2.82, 95%CI: 1.57–5.10) and Carbohydrate pattern (OR 2.14, 95%CI: 1.47–3.13) showed increased odds for PrCA [37].
The DII-PrCA association has been examined in Italy, Jamaica, Iran, Mexico, France and Canada [38–44]. However, this association has not been explored in a Latin American country where the dietary habits are very different from other regions [45–47]. The objective of this case-control study, conducted in Cordoba, Argentina, was to examine if increasing DII scores are associated with increased odds of prostate cancer. Our working hypothesis is that higher DII scores (indicating pro-inflammatory diet) increases odds of developing PrCA in this Argentinian population.
Methods:
Full details regarding the design of this case-control study have been published elsewhere [37]. In brief, this study was conducted within the framework of the Environmental Epidemiology of Cancer in Córdoba (EECC) project. It was conducted from January 2008 to December 2015 in Córdoba, the second most populated Argentinean province (3,067,000 inhabitants, per the 2010 census), located in the center of the country. Cases were men with incident, histologically confirmed PrCA (ICD-10th Edition, ICIE10:C61) with no previous diagnosis of cancer at other sites. Cases were identified in public and private health institutions and registered at the Córdoba Tumor Registry (CTR). Controls were selected based on geographical residence. Two controls per case, frequency matched on age (±5 years), were chosen from blocks randomly selected from the same neighborhoods and time-period as cases, and were included in the study after verifying the absence of any neoplastic diseases. Cases and controls with diseases (e.g., diabetes, cardiovascular diseases, celiac disease, renal insufficiency) that may generate a long-term modification of dietary habits where excluded. A total of 153 men with PrCA aged 48–89 (median age 72) years and 309 controls aged 46–89 (median age 71) years were included (response rate 91% in cases and 89% in controls). Subjects interviewed were from rural (54%) and urban (46%) areas (including the most populated area, Córdoba City, with 1,300,000 inhabitants). This study was conducted per the guidelines laid down in the Declaration of Helsinki and its later amendments. In addition, specific national laws have been observed. All procedures involving human subjects were approved by the Ethical Committee of the Faculty of Medical Sciences, University of Córdoba. Written informed consent was obtained from all subjects.
Subject Information
All participants were interviewed at home by centrally trained and routinely supervised nutritionists. A structured questionnaire was completed including information about sociodemographic characteristics, occupational history, smoking habits, alcohol consumption, self-reported anthropometric characteristics, physical activity, medical insurance, personal medical history, and family history of cancer. To assess dietary exposure, a validated food frequency questionnaire (FFQ) of 127 items [48] was completed. Subjects were asked about their dietary intake over the 5 years prior to diagnosis (cases) or interview (controls). The FFQ was coupled with a validated photographical atlas based on standard portion sizes in Argentina [49]. The seasonal pattern of consumption of each vegetable or fruit also was taken into account by averaging across all days of the year foods reported to have been consumed in a particular season. Physical activity was measured by means of the International Physical Activity Questionnaire [50]. Frequency and duration of physical activity were then expressed as metabolic equivalent of tasks (METs). Frequency, duration and intensity of physical activity were then expressed as metabolic equivalent of tasks (METs). Subsequently, METS were categorized into low (<600 METs), moderate (600–1500 METs), and high (>1500 METs) categories of physical activity intensity.
Dietary Inflammatory Index (DII®)
The development [22] and validation [23]of the DII has been explained elsewhere. Through evaluation of peer-reviewed literature published from 1950 up to 2010, the score is based on 1943 articles that identified 45 individual nutrient, food or flavonoid intake parameters in relation to these six established inflammatory biomarkers: IL-1b, IL-4, IL-6, IL-10, tumour necrosis factor-α (TNF-α) and CRP. Points were assigned to each of these parameters according to whether they increased (+1), decreased (−1) or had no (0) effect on the six established inflammatory biomarkers. The score for each of the DII components was weighted according to the study designs and total number of research articles. Overall parameter-specific inflammatory effect scores were then calculated based on the ratio of the total weighted number of articles to the weighted pro- and anti-inflammatory articles for each parameter followed by subtracting the anti- from the pro-inflammatory fraction. Parameters which had a robust pool of literature, i.e., greater than the median number of 236 weighted articles, were assigned the full value of that score. Parameters with a number of weighted articles less than 236 were adjusted according to the distance of their number from this median.
Actual dietary intake data from the FFQ were adjusted against a reference global daily mean and standard deviation intake for each parameter to obtain a Z-score. These, in turn, were converted to proportions (i.e., with values from 0 to 1) to prevent problems with right skewing of the data (as often occurs with dietary data). They were then centered on zero by doubling and subtracting 1. The global intake data was based on consumption data from 11 countries in different parts of the world. The centred percentile for each intake parameter was multiplied by its respective parameter-specific inflammatory effect score. All of the DII component-specific DII scores were then summed to create the overall DII score for each participant in the study, DII=b1*n1+b2*n2+...+b22*n22, where bi (i=1,...,22) refers to the literature-derived inflammatory effect scores for each of the evaluable DII components and ni refers to the DII component-specific centered percentiles, which were computed from the FFQ-derived dietary data. DII density scores, which adjust for energy intake and use an energy-adjusted global comparison database, also were calculated; prior to standardisation with the global intakes the parameters were each converted to a per 1000 kcal consumption value and the parameter of energy intake was excluded. The steps involved in DII calculation are shown in Figure 1.
Figure 1.
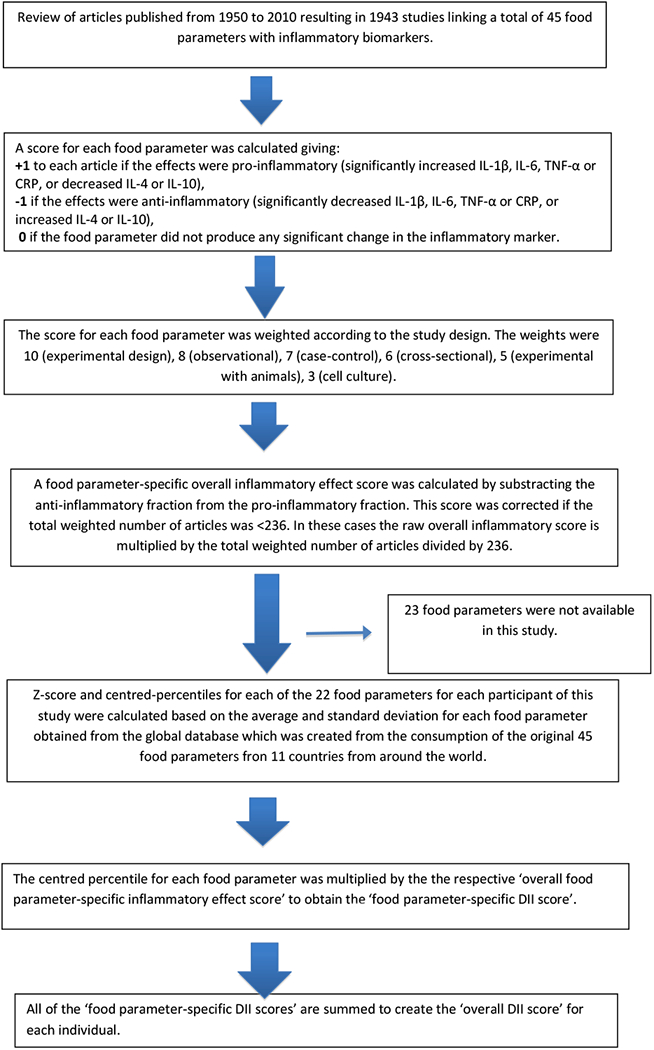
Sequence of steps in creating the dietary inflammatory index in the Argentinian prostate cancer case-control study
For the current study, data on 22 of the 45 DII components could be derived from the FFQ and were thus used for calculating the DII scores. These include: pro-inflammatory components (carbohydrate, protein, fat, saturated fat, iron, cholesterol) and anti-inflammatory components (alcohol, fiber, mono-unsaturated fat, poly-unsaturated fat, omega-3, omega-6, niacin, thiamin, riboflavin, magnesium, zinc, vitamin A, vitamin C, vitamin E, garlic and onions). Data on these nutrients were derived from diet only.
Statistical analysis
A Chi-square test was used to compare categorical variables. In order to evaluate associations between DII and PrCA, odds ratios and 95% confidence intervals (OR; 95% CI) were estimated using multi-level logistic regression (MLR) models (31) for the binary response (1 if a PrCA case, 0 if a control). On this basis, a hierarchical structure in the data was assumed. Subjects were included in a first level, in order to capture the inter-individual variability and to assess individual-level variable effects such as the DII score in relation to the outcome. These were then nested into a second level, the family history of cancer, defined according three categories, first- or second-degree relatives with PrCA, first- or second-degree relatives with any other cancer, or no family history of cancer. Family history of cancer was considered as an underlying or latent variable referring to the contextual (family) dimension. Although it depicts a random variable whose realizations are hidden for us, its approximation could be represented with a self-reported scale, like the one used in the questionnaire used here. Including latent variables, typically referred to as random effects, in statistical models is a common way of taking unobserved heterogeneity into account. Then, we assumed fixed effects for all covariates (DII score, age, BMI, etc.) and a random variable to control for underlying clustering information coming from the family history of cancer. This model combined the classical logistic model with a variance component in order to capture variance coming from a latent variable.
DII score (as continuous variable or categorized into tertiles, based on controls cutpoints), age (as continuous variable), body mass index [BMI=weight(kg)/height(m)2, as continuous variable], energy intake (as continuous variable) and occupational exposure (two or more years of industrial exposure to chemical contaminants recognized by IARC as carcinogens -i.e., dyes, paints, textiles, plastics, rubber, leather, herbicides, automotive, chemical, coal-) were included as first-level covariates. The risk measures were estimated in all subjects and stratified by BMI (<30 vs. ≥30kg/m2) as well. SAS® 9.3 was used for DII calculation and Stata 14.2 software was used for statistical analysis. α<0.05 was used as the criterion for assessing statistical significance.
Results:
A summary of the characteristics of PrCA cases and controls is presented in Table 1. Cases and controls had a similar distribution of socioeconomic status, occupational exposure, smoking habits, physical activity, BMI and energy intake. Compared to controls, cases had more frequently a lower educational level (p=0.049) and a family history of PrCA (p<0.001) (Table 1).
Table 1-.
Characteristics of cases and control subjects in the in the EECC case-control study of prostate cancer, Cordoba, Argentina (2008–2015)
| Cases (n= 163) Subjects (%) | Controls (n= 324) Subjects (%) | |
|---|---|---|
| Age (years) | ||
| ≤50 | 1 (0.61) | 4 (1.23) |
| 51–60 | 14 (8.59) | 32 (9.88) |
| 61–70 | 49 (30.06) | 112 (34.57) |
| >70 | 99 (60.74) | 176 (54.32) |
| Socioeconomic status | ||
| Low | 37 (22.70) | 84 (25.93) |
| Middle | 55 (33.74) | 106 (32.72) |
| High | 65 (39.88) | 124 (38.27) |
| Unknown | 6(3.86) | 10 (3.09) |
| Educational level* | ||
| Low | 36 (22.09) | 51 (15.74) |
| Middle | 75 (46.01) | 149 (45.99) |
| High | 46 (28.22) | 111 (34.26) |
| Unkwon | 6 (3.68) | 13 (4.01) |
| Family history of PC* | ||
| No | 137 (84.05) | 307 (94.75) |
| Yes | 26 (15.95) | 17 (5.25) |
| Unknown | - | - |
| Occupational exposurea | ||
| No | 107 (68.15) | 227 (72.52) |
| Yes | 50 (31.85) | 86 (27.48) |
| Smoking habits | ||
| No | 55 (33.74) | 101 (31.17) |
| Yes | 108 (66.26) | 223(68.83) |
| Lifetime PA | ||
| Low | 70 (42.94) | 128 (39.51) |
| Middle | 37 (22.70) | 88 (27.16) |
| High | 56 (34.36) | 108 (33.33) |
| BMI | ||
| ≤24.9 | 36 (22.09) | 91 (28.09) |
| 25–29.9 | 90 (55.21) | 158 (48.77) |
| ≥30 | 36 (22.09) | 73 (22.53) |
| Unknown | 1 (0.61) | 2 (0.62) |
| Energy intakeb | ||
| Low | 45 (27.61) | 108 (33.33) |
| Middle | 55 (33.74) | 108 (33.33) |
| High | 63 (38.65) | 108 (33.33) |
NSAID nonsteroidal anti-inflammatory drugs, PCprostate cancer, BMI body mass index.
Exposure to chemical contaminants for 2 years or longer.
Categories based on tertiles of intake in controls.
p<0.05as level of significance.
The mean value of the DII score was +1.47 (SD 1.13) indicating a slightly pro-inflammatory diet overall in this study compared to the mean DII in the previous study in Argentina which looked at the association between DII and colorectal cancer where the mean DII was +1.24 (SD 1.26);[51] and scores ranged from −1.94 (most anti-inflammatory score) to 3.82 (most pro-inflammatory score). Participant characteristics by DII categories are provided in Table 2. Reported intake of energy, fat, red meat and alcohol increased across tertiles of DII and the proportion of men with family history of PrCA is higher in tertiles II and III (Table 2).
Table 2-.
Characteristics of all subjects by tertiles of dietary inflammatory index, in the EECC case-control study of prostate cancer, Cordoba, Argentina (2008–2015)
| Tertiles of dietary inflammatory index | |||
|---|---|---|---|
| I (DII <0.98) n=154 | II (DII 0.99–1.96) n=161 | III (DII >1.96) n=172 | |
| Age (years)a | 71.89 (8.46) | 69.85 (8.12) | 69.31 (8.43) |
| Current BMI (kg/m2)a | 27.04 (3.13) | 27.49 (3.85) | 27.69 (3.75) |
| Usual BM (kg/m2)a | 27.11 (3.46) | 27.42 (3.91) | 27.71 (4.04) |
| Smoker (%) | 64.29 | 68.32 | 70.93 |
| Family history of PC (%) | 6.49 | 10.56 | 9.30 |
| Occupational exposureb (%) | 30.00 | 32.68 | 24.55 |
| Vigorous or moderate PAc(%) | 51.30 | 53.42 | 63.37 |
| Energy intake (Kcal)a | 3049.78 (987.89) | 3379.89 (1021.50) | 4297.96 (1532.72) |
| Fat intake (g) a | 113.49 (56.75) | 135.86 (57.92) | 197.86 (95.99) |
| Red meat intake (g)a | 176.85 (114.70) | 224.77 (130.03) | 325.78 (194.62) |
| Alcohol intake (g) a | 21.76 (22.88) | 23.53 (25.38) | 29.53 (43.25) |
DII, dietary inflammatory index; BMI, body mass index; PC, prostate cancer; PA, physical activity.
Mean (standard deviation).
Exposure to chemical contaminants for 2 years or longer.
Subjects who performed regular physical activity reaching at least 600 METs by minutes/week.
Odds ratios (OR) and 95% confidence intervals (CI) for having PrCA according to DII score are shown in Table 3. Increasing DII score (as a continuous variable) corresponding to 17% increase in DII in the current study showed significant positive associations with PrCA (OR 1.12; 95%CI 1.06 to 1.95) after adjusting for age, BMI, energy intake and occupational exposure at the individual level, and fitting the family history of any cancer as the clustering variable. When the DII score was categorized into tertiles, the third tertile showed a significant effect, increasing odds of PrCA (OR 1.50; 95%CI 1.24 to 1.80) compared to the first tertile (Table 3).
Table 3-.
Associations between the dietary inflammatory index and prostate cancer from multilevel logistic modeling in the EECC case-control study of colorectal cancer, Cordoba, Argentina (2008–2015).
| Subjects | Crude OR (CI 95%) | Adjusteda OR (CI 95%) | |
|---|---|---|---|
| Dietary inflammatory index | |||
| Continuous | 1.11 (0.939–1.319) | 1.12 (1.061–1.195)** | |
| Tertile I | 46/108 | l (ref) | l (ref) |
| Tertile II | 53/108 | 1.15 (0.715–1.855) | 1.10 (0.613–1.964) |
| Tertile III | 64/108 | 1.39 (0.875–2.211) | 1.50 (1.244–1.804)** |
OR, odds ratio; CI, confidence interval
Age, usual BMI, energy intake and Occupational Exposure were included as covariates at first level, and Family history of cancer at second level.
p<0.05
p<0.001 as level of significance.
Results obtained within strata of BMI (<30 vs. ≥30kg/m2) are presented in Table 4. No association was found among men with BMI<30kg/m2 (ORcontinuous 0.90; 95%CI 0.61 to 1.34 and OR tertile3 vs. tertile1 0.93; 95%CI 0.25 to 3.51), while higher DII scores, were positively associated with PrCA occurrence in obese men (BMI ≥30kg/m2), with higher ORs than in all subjects without stratifying (ORcontinuous 1.22; 95%CI 1.08 to 1.38 and OR tertile3 vs.tertile1 1.81 95%CI 1.45 to 2.26).
Table 4-.
Associations between the dietary inflammatory index and prostate cancer stratified by body mass index (<30 vs. >30kg/m2) from multilevel logistic modeling in the EECC case-control study of colorectal cancer, Córdoba, Argentina (2008–2015).
| Subjects | Crude OR (CI 95%) | Adjusted OR (CI 95%) | |
|---|---|---|---|
| Dietary inflammatory index | |||
| BMI <30 kg/m2 | |||
| Continuous | 0.92 (0.66–1.29) | 0.90 (0.61–1.34) | |
| Tertile I | 12/28 | 1 | 1 |
| Tertile II | 11/31 | 0.83 (0.32–2.17) | 0.73 (0.51–1.06) |
| Tertile III | 13/32 | 0.95 (0.37–2.41) | 0.93 (0.25–3.51) |
| BMI ≥30 kg/m2 | |||
| Continuous | 1.19 (0.97–1.45) | 1.22 (1.08–1.38)* | |
| Tertile I | 34/80 | 1 | 1 |
| Tertile II | 42/77 | 1.28 (0.74–2.22) | 1.25 (0.66–2.38) |
| Tertile III | 51/76 | 1.57 (0.92–2.67) | 1.81 (1.45–2.27)** |
OR, odds ratio; CI, confidence interval; BMI, body mass index.
Age, energy intake, body mass index and Occupational Exposure were included as covariates at first level, and Family history of cancer at second level.
p<0.005
p<0.001 as levels of significance.
Discussion:
In this case-control study of Argentinian males, consuming a more pro-inflammatory diet, as reflected in higher DII scores, was associated with increased odds of PrCA. This effect was more pronounced among obese men. Hence, these results confirm our hypothesis and are consistent with findings from other studies. For example, in a large case-control study in Italy, participants in quartiles 3 and 4 relative to quartile 1 of the DII were at higher odds of having PrCA (ORQuartile3vs1 1.32, 95% CI 1.03–1.69 and ORQuartile4vs1 1.33, 95% CI 1.01–1.76; Ptrend=0.04)[38]. In another case-control study in Jamaica, men in the highest quartile of the DII were at higher odds of having PrCA (ORQuartile4vs1 2.39; 95% CI 1.14–5.04) [39]. In an Iranian case control study men with higher DII score (>0.23) were at higher odds of having PrCA (OR 3.96; 95% CI =1.29–12.16) compared to men with lower DII scores (≤0.23) [40]. A positive association of similar magnitude was observed in a prospective study in France (HRQuartile4vs1 2.08, 95% CI 1.06–4.09) [42]. These results show that the DII can be applied to widely varying populations, using any competent dietary assessment tool, including different types of validated FFQs, to predict PrCA. Furthermore, results obtained are very consistent across widely varying populations. We observed stronger association among obese men; this is along expected lines, because obesity itself is both a risk factor for PrCA [52] and is a pro-inflammatory condition [53]. However, it also is conceivable that the effect of obesity could countervail that of diet and it simply did not do so in this study.
Previous results from this same study found that two different patterns increased odds of having PrCA. The Traditional pattern (fatty red meats, offal, processed meat, starchy vegetables, added sugars and sweets, candies, fats, and vegetable oils) nearly tripled the odds (OR 2.82, 95%CI: 1.57–5.10); and the Carbohydrate pattern (sodas/juices and bakery products) more than doubled the odds (OR 2.14, 95%CI: 1.47–3.13) [37].
Other studies conducted to examine the association between various dietary patterns and PrCA have shown increased risk with unhealthy patterns of intake [54,55]. In a systematic review of the epidemiological studies on diet and risk of PrCA focusing on those carried out in South America, suggested that dairy products, red meat, processed meat, α-linolenic fatty acids, as well as dietary patterns characterized by higher intakes of red and processed meat, eggs, and grains may play a role in the development of PrCA [46].
One of the possible mechanisms responsible for the association observed in this study is the effect of a pro-inflammatory diet on systemic inflammation and insulin resistance [56,57]. Consumption of food items such as meat and butter have been shown to increase levels of high-sensitivity C-reactive protein, E-selectin and soluble vascular cell adhesion molecule-that, in turn, increase systemic inflammation [56], which then is responsible for increasing insulin resistance [57]. Increasing insulin resistance leads to increased circulating levels of insulin, which has been demonstrated to play a role in the development of PrCA by inhibiting apoptosis and stimulating cell proliferation [13].
A normal human diet consists of both pro-inflammatory and anti-inflammatory DII components. Hence, the DII, which takes into account the full spectrum of inflammation-influencing food components as of the year 2010 when the latest literature search was done, more accurately reflects the relationship of diet in relation to cancer risk than would individual nutrients.
In keeping with the intention of accounting for heterogeneity of responses coming from a second level of aggregation of data, we used a modeling approach that included the family history of cancer as a clustering variable. Family history of cancer was selected based on the known heritability of this disease derived from either genetic susceptibility [58] or exposure to common environmental factors [59], or both. In accordance, the results showed a dependence on the PrCA risk linked to this clustering, and the multilevel model improved the precision of the individual effect estimations. This approach confers a statistical and interpretative advantage as it proposes a theoretical construct for addressing diet-cancer relationship that takes into account an important way in which humans organize their work and residential environments [60].
The main strength of this study is that it is the first investigation regarding diet quality in relation to inflammatory potential and PrCA in Argentina, which has a high incidence of PrCA among men and unusual dietary patterns. These unique characteristics enabled us to explore the association between the inflammatory properties of diet, using the DII, in relation to incident PrCA. It is one of the few studies that have looked at the association between diet as a whole and PrCA. Even with a relatively small sample size we have observed significant results which indicate the importance of consuming anti-inflammatory diet in protecting against PrCA.
As with any case-control study of diet and health outcomes, this investigation shares weaknesses of such designs. Most notably these include information bias related to knowledge of disease state and selection bias. To avoid potentially important bias due to confounders, similar distribution of age and place of residence in cases and controls was sought, and both groups were interviewed in the same period. Controls came from the same geographical “catchment area” as the cases and mostly the same interview setting (the home) were used for both groups, and there was almost complete participation (about 90% participation rate). All of these procedures contribute to minimizing both selection and information biases. Community controls obviate some of the problems of selection bias that may occur among hospital controls. With reference to information bias, a classification bias caused by “rumination” in cases regarding the possible causes of their disease must be considered.
The kind of biases that exist in this Argentinian population may be quite different that what would be expected in other studies of the DII in relation to PrCA conducted in Europe and North America. Indeed, we anticipate that the bias in recalling food intake by cases should be small, given the limited knowledge and attention paid in this population at the time of the study to the possible relationship between diet and cancer (including PrCA). Indeed, the sensitivity analysis performed previously [37] based on the possibility of the systematic errors mentioned, showed no major evidence of influence of bias. A further limitation of our study is that we could derive DII score from only 22 of the potential 45 food and nutrient items that theoretically can be used to compute this index. The DII components that were missing from this study were saffron, turmeric, thyme/oregano, ginger, rosemary, eugenol, beta-carotene, pepper, tea, anthocyanidins, flavan3ol, flavones, flavonols, flavonones, isoflavones, magnesium, vitamin-D, B1, B3, B12, folic acid and trans-fat. However, other published studies also derive DII scores from a sub-optimal number of items, and the ability to still detect significant associations suggests that this has only led to a potential underestimate of the association between DII and PrCA risk. Additionally, some of the missing DII components such as saffron, ginger and turmeric are consumed infrequently in this population; so, non-availability of these parameters may not have exerted a major impact. However, availability of information on DII components like vitamin B1, B3, B12 and magnesium, which are present in several of the food items consumed in Argentina, might have modified the results. Further to this issue of calculating DII from fewer DII components, we have previously demonstrated in the SEASONS study that DII scores calculated from 44 DII components using the 24-hour recalls and DII scores calculated from 27 DII components using 7-day dietary recall resulted in virtually identical ORs, where CRP (>3 mg/l) was the study outcome [22]. The components present in this study to calculate the DII are all nutrients which are present in almost all of our previous DII and PrCA publications. The values of these components are what determines the DII score and this is what makes the DII different for different populations. For example, the mean DII among controls for a study in Canada [43], where DII was derived from almost similar number of DII components, was +0.023 whereas the mean DII among control in the current Argentinian study was −0.35. So, from this we can discern that the diet in the Canadian study is slightly more pro-inflammatory than the diet among controls in the current Argentinian study. It must be emphasized that the list of 45 DII components was not specified a priori. A search was first done to see which dietary components have been studied the most in relation to the six inflammatory biomarkers. Thus, the list is the result of this search. This search was first done in 2007 and later in 2011. An updated search may be done in the future to see if any other component can be added to this list or if the estimates of effect might have changed.
The influence of diet on cancer is difficult to measure precisely, and challenges in dietary exposure assessment are greatest in case-control studies. Although we used a validated FFQ for assessing dietary intake, measurement errors that might distort associations were inevitable. Even though diet in mid-life may be more important than the diet later in life, the long time that had passed from the patients’ mid-life restricts our ability to evaluate that time period. No data were available on inflammatory markers in this study; hence, the DII could not be validated with inflammation in this case-control study. Because most of the parameters used to calculate the DII are nutrients, non-availability of supplement data can be considered a limitation. In previous studies that have examined DII with and without supplements [61–63], improved results were seen with DII with supplements, this is because with supplements more data or information is added to the DII calculation, so the overall mean DII tends to be lower, though the range of the DII scores was larger compared to DII with diet only [61–63]. So, because of the increased contrast, the most pro-inflammatory category of the DII with supplements appeared to have a larger effect on health outcomes; the logical corollary is that the most anti-inflammatory category of the DII with supplements had a more protective association with health outcomes [61–63].
Small sample size is another limitation that might result in unstable risk estimates with wide confidence intervals. Epidemiological and statistical literature provide asymptotic formulas for the computation of case-control sample sizes required for odds ratios, whether unadjusted or adjusted for a confounder [64]. However, all these recommendations take into account only for fixed effects of covariates, including the intercept. The limited number of parameters fit in the model and the constraint on the sources of variability (a variance component to quantify the intraclass correlation), constitute a reasonable effort to compensate for the small size of our study. It also should be kept in mind that the average DII score in this population was generally higher (more pro-inflammatory) than in other populations (i.e., around 1.5 vs. 0) and the range was narrower than we normally see (around 5 vs. 9). Finally, in this study there is no data on lycopene intake and PrCA screening, two variables that have been shown to be associated with PrCA. In Argentina, there are no population-level data on lycopene intake. Also, the prevalence of prostate cancer screening in Argentina is unknown. According to the results from few available studies, the mean intake of lycopene range from 3000 to 5000 µg per day in adult men [65,66]. Related to the prevalence of prostate cancer screening, that practice is not widely available in Argentina. Moreover, there are no official screening programs for PrCA.
Notwithstanding the design limitations of case-control studies in general, we believe that our findings of a positive association between DII with prostate cancer are plausible and could be related to immune and hormonal factors [67,68,13]. They also are consistent with prior investigations of the DII in relation to PrCA.
The logical next step would be to use DII scores to predict incidence of other cancers and serum levels of inflammatory markers in Argentina and other Latin American countries in prospective studies and examine other outcomes which are related to diet and inflammation like cardiovascular diseases.
In conclusion, this study implicates diet-associated inflammation in the etiology of PrCA, a finding that it consistent with both biological mechanisms of action and prior results implicating DII scores in PrCA. However, there is a need for other studies to be conducted in different populations and with prospective cohorts to more firmly establish cause and effect.
Acknowledgements:
We are grateful to all field investigators, staffs and participants of the present study. Drs. Shivappa and Hébert were supported by grant number R44DK103377 from the United States National Institute of Diabetes and Digestive and Kidney Diseases. Also, we would like to thank the Science and Technology National Agency, FONCyT grant PICT 2012–1019 for financial support of this study in Argentina and the National Scientific and Technical Research Council (CONICET) for CN, JBC and MDR fellowships.
Footnotes
Conflict of interest:
Dr. James R. Hébert owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII®) from the University of South Carolina in order to develop computer and smart phone applications for patient counselling and dietary intervention in clinical settings. Dr Nitin Shivappa is an employee of CHI.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61 (2):69–90. doi: 10.3322/caac.20107 [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J SI, Ervik M, Dikshit R, Eser S, Mathers C, et al. (2013) GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Available at http://globocan.iarc.fr, accessed June 2016 Lyon, France [Google Scholar]
- 3.Diaz Mdel P, Osella AR, Aballay LR, Munoz SE, Lantieri MJ, Butinof M, Paz RM, Pou S, Eynard AR, La Vecchia C (2009) Cancer incidence pattern in Cordoba, Argentina. European journal of cancer prevention : the official journal of the European Cancer Prevention Organisation 18 (4):259–266. doi: 10.1097/CEJ.0b013e3283152030 [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420 (6917):860–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Philip M, Rowley DA, Schreiber H (2004) Inflammation as a tumor promoter in cancer induction. Semin Cancer Biol 14 (6):433–439 [DOI] [PubMed] [Google Scholar]
- 6.Keibel A, Singh V, Sharma MC (2009) Inflammation, microenvironment, and the immune system in cancer progression. Curr Pharm Des 15 (17):1949–1955 [DOI] [PubMed] [Google Scholar]
- 7.Kopp TI, Friis S, Christensen J, Tjonneland A, Vogel U (2013) Polymorphisms in genes related to inflammation, NSAID use, and the risk of prostate cancer among Danish men. Cancer Genet 20 (13):00084–00087 [DOI] [PubMed] [Google Scholar]
- 8.Cross AJ, Peters U, Kirsh VA, Andriole GL, Reding D, Hayes RB, Sinha R (2005) A prospective study of meat and meat mutagens and prostate cancer risk. Cancer Res 65 (24):11779–11784 [DOI] [PubMed] [Google Scholar]
- 9.Nakai Y, Nonomura N (2013) Inflammation and prostate carcinogenesis. Int J Urol 20 (2):150–160 [DOI] [PubMed] [Google Scholar]
- 10.De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schroder F, Sciarra A, Tubaro A (2011) The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. European urology 60 (1):106–117. doi: 10.1016/j.eururo.2011.03.055 [DOI] [PubMed] [Google Scholar]
- 11.Guo YZ, Pan L, Du CJ, Ren DQ, Xie XM (2013) Association between C-reactive protein and risk of cancer: a meta-analysis of prospective cohort studies. Asian Pac J Cancer Prev 14 (1):243–248 [DOI] [PubMed] [Google Scholar]
- 12.Toriola AT, Laukkanen JA, Kurl S, Nyyssonen K, Ronkainen K, Kauhanen J (2013) Prediagnostic circulating markers of inflammation and risk of prostate cancer. International journal of cancer Journal international du cancer 133 (12):2961–2967. doi: 10.1002/ijc.28313 [DOI] [PubMed] [Google Scholar]
- 13.Kaaks R, Lukanova A (2001) Energy balance and cancer: the role of insulin and insulin-like growth factor-I. Proc Nutr Soc 60 (1):91–106 [DOI] [PubMed] [Google Scholar]
- 14.de Mello VD, Schwab U, Kolehmainen M, Koenig W, Siloaho M, Poutanen K, Mykkanen H, Uusitupa M (2011) A diet high in fatty fish, bilberries and wholegrain products improves markers of endothelial function and inflammation in individuals with impaired glucose metabolism in a randomised controlled trial: the Sysdimet study. Diabetologia 54 (11):2755–2767 [DOI] [PubMed] [Google Scholar]
- 15.Khoo J, Piantadosi C, Duncan R, Worthley SG, Jenkins A, Noakes M, Worthley MI, Lange K, Wittert GA (2011) Comparing effects of a low-energy diet and a high-protein low-fat diet on sexual and endothelial function, urinary tract symptoms, and inflammation in obese diabetic men. J Sex Med 8 (10):2868–2875 [DOI] [PubMed] [Google Scholar]
- 16.Luciano M, Mottus R, Starr JM, McNeill G, Jia X, Craig LC, Deary IJ (2012) Depressive symptoms and diet: their effects on prospective inflammation levels in the elderly. Brain Behav Immun 26 (5):717–720 [DOI] [PubMed] [Google Scholar]
- 17.Michaud DS, Fuchs CS, Liu S, Willett WC, Colditz GA, Giovannucci E (2005) Dietary glycemic load, carbohydrate, sugar, and colorectal cancer risk in men and women. Cancer Epidemiol Biomarkers Prev 14 (1):138–147. doi:14/1/138 [pii] [PubMed] [Google Scholar]
- 18.Lin PH, Aronson W, Freedland SJ (2015) Nutrition, dietary interventions and prostate cancer: the latest evidence. BMC medicine 13:3. doi: 10.1186/s12916-014-0234-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Labbe DP, Zadra G, Ebot EM, Mucci LA, Kantoff PW, Loda M, Brown M (2015) Role of diet in prostate cancer: the epigenetic link. Oncogene 34 (36):4683–4691. doi: 10.1038/onc.2014.422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel VH (2014) Nutrition and prostate cancer: an overview. Expert review of anticancer therapy 14 (11):1295–1304. doi: 10.1586/14737140.2014.972946 [DOI] [PubMed] [Google Scholar]
- 21.Second Expert Report Food, Nutrition, Physical Activity and the Prevention of Cancer: a Global Perspective (2007). vol http://www.dietandcancerreport.org/cancer_resource_center/downloads/Second_Expert_Report_full.pdf World Cancer Research Fund / American Institute for Cancer Research.,
- 22.Shivappa N, Steck SE, Hurley TG, Hussey JR, Hebert JR (2014) Designing and developing a literature-derived, population-based dietary inflammatory index. Public health nutrition 17 (8):1689–1696. doi: 10.1017/S1368980013002115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shivappa N, Steck SE, Hurley TG, Hussey JR, Ma Y, Ockene IS, Tabung F, Hebert JR (2014) A population-based dietary inflammatory index predicts levels of C-reactive protein in the Seasonal Variation of Blood Cholesterol Study (SEASONS). Public health nutrition 17 (8):1825–1833. doi: 10.1017/S1368980013002565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wirth MD, Burch J, Shivappa N, Violanti JM, Burchfiel CM, Fekedulegn D, Andrew ME, Hartley TA, Miller DB, Mnatsakanova A, Charles LE, Steck SE, Hurley TG, Vena JE, Hebert JR (2014) Association of a dietary inflammatory index with inflammatory indices and metabolic syndrome among police officers. J Occup Environ Med 56 (9):986–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wood L, Shivappa N, Berthon BS,Gibson PG,Hebert JR (2014) Dietary inflammatory index is related to asthma risk, lung function and systemic inflammation in asthma. Clinical & Experimental Allergy:n/a-n/a. doi: 10.1111/cea.12323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, Hingle M, Hou L, Hurley TG, Jiao L, Martin LW, Millen AE, Park HL, Rosal MC, Shikany JM, Shivappa N, Ockene JK, Hebert JR (2015) Construct validation of the dietary inflammatory index among postmenopausal women. Annals of epidemiology 25 (6):398–405. doi: 10.1016/j.annepidem.2015.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vahid F, Shivappa N, Faghfoori Z, Khodabakhshi A, Zayeri F, Hebert JR, Davoodi SH (2018) Validation of a Dietary Inflammatory Index (DII) and Association with Risk of Gastric Cancer: a Case-Control Study. Asian Pac J Cancer Prev 19 (6):1471–1477. doi: 10.22034/APJCP.2018.19.6.1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vahid F, Shivappa N, Hekmatdoost A, Hebert JR, Davoodi SH, Sadeghi M (2017) Association between Maternal Dietary Inflammatory Index (DII) and abortion in Iranian women and validation of DII with serum concentration of inflammatory factors: case-control study. Applied physiology, nutrition, and metabolism = Physiologie appliquee, nutrition et metabolisme 42 (5):511–516. doi: 10.1139/apnm-2016-0274 [DOI] [PubMed] [Google Scholar]
- 29.Shivappa N, Hebert JR, Rietzschel ER, De Buyzere ML, Langlois M, Debruyne E, Marcos A, Huybrechts I (2015) Associations between dietary inflammatory index and inflammatory markers in the Asklepios Study. Br J Nutr 113 (4):665–671. doi: 10.1017/S000711451400395X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wirth MD, Burch J, Shivappa N, Steck SE, Hurley TG, Vena JE, Hebert JR (2014) Dietary Inflammatory Index Scores Differ by Shift Work Status: NHANES 2005 to 2010. J Occup Environ Med 56 (2):145–148. doi: 10.1097/JOM.0000000000000088 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, Grosso G (2018) Dietary Inflammatory Index and Cardiovascular Risk and Mortality-A Meta-Analysis. Nutrients 10 (2). doi: 10.3390/nu10020200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tabung FK, Steck SE, Ma Y, Liese AD, Zhang J, Caan B, Hou L, Johnson KC, Mossavar-Rahmani Y, Shivappa N, Wactawski-Wende J, Ockene JK, Hebert JR (2015) The association between dietary inflammatory index and risk of colorectal cancer among postmenopausal women: results from the Women’s Health Initiative. Cancer causes & control : CCC 26 (3):399–408. doi: 10.1007/s10552-014-0515-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wirth MD, Shivappa N, Steck SE, Hurley TG, Hebert JR (2015) The dietary inflammatory index is associated with colorectal cancer in the National Institutes of Health-American Association of Retired Persons Diet and Health Study. Br J Nutr 113 (11):1819–1827. doi: 10.1017/S000711451500104X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shivappa N, Godos J, Hebert JR, Wirth MD, Piuri G, Speciani AF, Grosso G (2017) Dietary Inflammatory Index and Colorectal Cancer Risk-A Meta-Analysis. Nutrients 9 (9). doi: 10.3390/nu9091043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shivappa N, Hebert JR, Ferraroni M, La Vecchia C, Rossi M (2016) Association between Dietary Inflammatory Index and Gastric Cancer Risk in an Italian Case-Control Study. Nutrition and cancer 68 (8):1262–1268. doi: 10.1080/01635581.2016.1224367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shivappa N, Bosetti C, Zucchetto A, Serraino D, La Vecchia C, Hebert JR (2014) Dietary inflammatory index and risk of pancreatic cancer in an Italian case-control study. Br J Nutr:1–7. doi: 10.1017/S0007114514003626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niclis C, Roman MD, Osella AR, Eynard AR, Diaz Mdel P (2015) Traditional Dietary Pattern Increases Risk of Prostate Cancer in Argentina: Results of a Multilevel Modeling and Bias Analysis from a Case-Control Study. Journal of cancer epidemiology 2015:179562. doi: 10.1155/2015/179562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shivappa N, Bosetti C, Zucchetto A, Montella M, Serraino D, La Vecchia C, Hebert JR (2015) Association between dietary inflammatory index and prostate cancer among Italian men. Br J Nutr 113 (2):278–283. doi: 10.1017/S0007114514003572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shivappa N, Jackson MD, Bennett F, Hebert JR (2015) Increased Dietary Inflammatory Index (DII) Is associated with increased risk of prostate cancer in Jamaican Men . Nutr Cancer 67 (6):941–948. doi: 10.1080/01635581.2015.1062117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shivappa N, Hebert JR, Askari F, Kardoust Parizi M, Rashidkhani B (2017) Increased Inflammatory Potential of Diet is Associated with Increased Risk of Prostate Cancer in Iranian Men. International journal for vitamin and nutrition research Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung Journal international de vitaminologie et de nutrition:1–8. doi: 10.1024/0300-9831/a000395 [DOI] [PubMed] [Google Scholar]
- 41.Vazquez-Salas RA, Shivappa N, Galvan-Portillo M, Lopez-Carrillo L, Hebert JR, Torres-Sanchez L (2016) Dietary inflammatory index and prostate cancer risk in a case-control study in Mexico. Br J Nutr 116 (11):1945–1953. doi: 10.1017/S0007114516003986 [DOI] [PubMed] [Google Scholar]
- 42.Graffouillere L, Deschasaux M, Mariotti F, Neufcourt L, Shivappa N, Hebert JR, Wirth MD, Latino-Martel P, Hercberg S, Galan P, Julia C, Kesse-Guyot E, Touvier M (2016) The Dietary Inflammatory Index Is Associated with Prostate Cancer Risk in French Middle-Aged Adults in a Prospective Study. J Nutr. doi: 10.3945/jn.115.225623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shivappa N, Miao Q, Walker M, Hebert J, Aronson K Association Between a Dietary Inflammatory Index and Prostate Cancer Risk in Ontario, Canada. Nutrition and cancer. doi: 10.1080/01635581.2017.1339095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shivappa N, J RH, Jalilpiran Y, Faghih S (2018) Association between Dietary Inflammatory Index and Prostate Cancer in Shiraz Province of Iran. Asian Pac J Cancer Prev 19 (2):415–420. doi: 10.22034/APJCP.2018.19.2.415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bermudez OI, Tucker KL (2003) Trends in dietary patterns of Latin American populations. Cadernos de saude publica 19 Suppl 1:S87–99 [DOI] [PubMed] [Google Scholar]
- 46.Niclis C, Diaz Mdel P, Eynard AR, Roman MD, La Vecchia C (2012) Dietary habits and prostate cancer prevention: a review of observational studies by focusing on South America. Nutrition and cancer 64 (1):23–33. doi: 10.1080/01635581.2012.630163 [DOI] [PubMed] [Google Scholar]
- 47.Navarro A, Munoz SE, Lantieri MJ, del Pilar Diaz M, Cristaldo PE, de Fabro SP, Eynard AR (2004) Meat cooking habits and risk of colorectal cancer in Cordoba, Argentina. Nutrition 20 (10):873–877. doi: 10.1016/j.nut.2004.06.008 [DOI] [PubMed] [Google Scholar]
- 48.Navarro A, Osella A, Guerra V, Muñoz S, Lantieri M, Eynard A (2001) Reproducibility and validity of a food-frequency questionnaire in assessing dietary intakes and food habits in epidemiological cancer studies in Argentina. Journal of experimental & clinical cancer research: CR 20 (3):365–370 [PubMed] [Google Scholar]
- 49.Navarro A, Cristaldo P, Diaz M, Eynard A (1999) Food photography atlas: its suitability for quantifying food and nutrient consumption in nutritional epidemiological research in Cordoba, Argentina. Revista de la Facultad de Ciencias Medicas (Cordoba, Argentina) 57 (1):67–74 [PubMed] [Google Scholar]
- 50.IPAQ Group, International Physical Activity Questionnaire (2012). http://www.ipaq.ki.se.
- 51.Niclis C, Pou SA, Shivappa N, Hebert JR, Steck SE, Diaz MDP (2018) Proinflammatory Dietary Intake is Associated with Increased Risk of Colorectal Cancer: Results of a Case-Control Study in Argentina Using a Multilevel Modeling Approach. Nutr Cancer 70 (1):61–68. doi: 10.1080/01635581.2018.1397710 [DOI] [PubMed] [Google Scholar]
- 52.Cao Y, Giovannucci E (2016) Obesity and Prostate Cancer. Recent results in cancer research Fortschritte der Krebsforschung Progres dans les recherches sur le cancer 208:137–153. doi: 10.1007/978-3-319-42542-9_8 [DOI] [PubMed] [Google Scholar]
- 53.Divella R, De Luca R, Abbate I, Naglieri E, Daniele A (2016) Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. Journal of Cancer 7 (15):2346–2359. doi: 10.7150/jca.16884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jackson M, Tulloch-Reid M, Walker S, McFarlane-Anderson N, Bennett F, Francis D, Coard K (2013) Dietary patterns as predictors of prostate cancer in Jamaican men. Nutrition and cancer 65 (3):367–374 [DOI] [PubMed] [Google Scholar]
- 55.Bosire C, Stampfer MJ, Subar AF, Park Y, Kirkpatrick SI, Chiuve SE, Hollenbeck AR, Reedy J (2013) Index-based dietary patterns and the risk of prostate cancer in the NIH-AARP diet and health study. Am J Epidemiol 177 (6):504–513. doi: 10.1093/aje/kws261 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC (2007) Dietary Patterns and Markers of Systemic Inflammation among Iranian Women. The Journal of Nutrition 137 (4):992–998 [DOI] [PubMed] [Google Scholar]
- 57.Festa A, D’Agostino R, Howard G, Mykkänen L, Tracy RP, Haffner SM (2000) Chronic Subclinical Inflammation as Part of the Insulin Resistance Syndrome: The Insulin Resistance Atherosclerosis Study (IRAS). Circulation 102 (1):42–47. doi: 10.1161/01.cir.102.1.42 [DOI] [PubMed] [Google Scholar]
- 58.Whittemore AS, Kolonel LN, Wu AH, John EM, Gallagher RP, Howe GR, Burch JD, Hankin J, Dreon DM, West DW, et al. (1995) Prostate cancer in relation to diet, physical activity, and body size in blacks, whites, and Asians in the United States and Canada. Journal of the National Cancer Institute 87 (9):652–661 [DOI] [PubMed] [Google Scholar]
- 59.Gronberg H (2003) Prostate cancer epidemiology. Lancet 361 (9360):859–864. doi: 10.1016/S0140-6736(03)12713-4 [DOI] [PubMed] [Google Scholar]
- 60.Merlo J (2003) Multilevel analytical approaches in social epidemiology: measures of health variation compared with traditional measures of association. Journal of epidemiology and community health 57 (8):550–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shivappa N, Prizment AE, Blair CK, Jacobs DR Jr., Steck SE, Hebert JR (2014) Dietary inflammatory index and risk of colorectal cancer in the Iowa Women’s Health Study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 23 (11):2383–2392. doi: 10.1158/1055-9965.EPI-14-0537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Peres LC, Bandera EV, Qin B, Guertin KA, Shivappa N, Hebert JR, Abbott SE, Alberg AJ, Barnholtz-Sloan J, Bondy M, Cote ML, Funkhouser E, Moorman PG, Peters ES, Schwartz AG, Terry PD, Camacho F, Wang F, Schildkraut JM (2017) Dietary inflammatory index and risk of epithelial ovarian cancer in African American women. International journal of cancer Journal international du cancer 140 (3):535–543. doi: 10.1002/ijc.30467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zheng J, Tabung FK, Zhang J, Liese AD, Shivappa N, Ockene JK, Caan B, Kroenke CH, Hebert JR, Steck SE (2018) Association between Post-Cancer Diagnosis Dietary Inflammatory Potential and Mortality among Invasive Breast Cancer Survivors in the Women’s Health Initiative. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 27 (4):454–463. doi: 10.1158/1055-9965.EPI-17-0569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.NE B, NE D (1987) Statistical Methods in Cancer Research, Vol. 2: The Design and Analysis of Cohort Studies. IARC Scientific Publications No. 82, vol 2. Lyon, France: IARC; [PubMed] [Google Scholar]
- 65.Lopez Fontana CM, Recalde Rincon GM, Messina Lombino D, Uvilla Recupero AL, Perez Elizalde RF, Lopez Laur JD (2009) [Body mass index and diet affect prostate cancer development]. Actas urologicas espanolas 33 (7):741–746 [DOI] [PubMed] [Google Scholar]
- 66.Messina D, Perez Elizalde R, Soto C, Uvilla A, Lopez Laur JD, Lopez Fontana C (2012) [High intake of lycopene together with low intake of red meat increases the total antioxidant status]. Archivos latinoamericanos de nutricion 62 (1):15–22 [PubMed] [Google Scholar]
- 67.Vykhovanets EV, Shankar E, Vykhovanets OV, Shukla S, Gupta S (2011) High-fat diet increases NF-kappaB signaling in the prostate of reporter mice. Prostate 71 (2):147–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pandey M, Gupta S (2009) Green tea and prostate cancer: from bench to clinic. Front Biosci (Elite Ed) 1:13–25 [DOI] [PMC free article] [PubMed] [Google Scholar]


