Abstract
Background
This study examined the effects of supervised and home-based exercise interventions on change in metabolic syndrome (MetS) according to breast cancer risk (high vs low) in Black women enrolled in the Focused Intervention on Exercise to Reduce CancEr (FIERCE) trial.
Methods
Postmenopausal obese metabolically unhealthy 45–65 years-old Black women were randomized to supervised aerobic exercise (73 women), home-based walking-based exercise (69 women), or control arm (71 women). Participants in the exercise arms underwent a 6-month intervention with study assessments conducted at baseline and 6 months. Primary outcome measures were MetS (fasting glucose, waist circumference, blood pressure, serum triglycerides and HDL). The intervention effect on MetS stratified by breast cancer risk as measured by family history of breast cancer and model-based projected breast cancer risk were examined by intent-to-treat analyses using generalized estimating equation models.
Results
Among women with a family history of breast cancer, exercise arms had lower mean MetS Z-scores, suggesting an improvement in the metabolic profile, than controls at 6-months (controls +0.55; home-based −0.97, P < 0.01; supervised −0.89, P < 0.01). Stratified analyses by projected breast cancer risk suggested similar but statistically non-significant findings, with those at high risk having more favorable changes in the MetS Z score in the exercise arms compared to control arm. These changes were primarily attributable to changes in blood pressure, triglycerides, and HDL.
Conclusion
Short-term aerobic activity regimens may improve the metabolic profile thereby reducing breast cancer risk in obese, metabolically unhealthy Black women at high risk of cancer.
Keywords: Metabolic syndrome, Breast Cancer, Exercise, Clinical Trial, Black
INTRODUCTION
Metabolic syndrome (MetS), characterized by abdominal obesity, high blood glucose levels, impaired glucose tolerance, dyslipidemia, and hypertension, has been suggested to play a role in breast carcinogenesis.1–4 Black women are 20% more likely to have metabolic syndrome compared to White women, and have a higher prevalence of certain MetS components, such as abdominal obesity, elevated fasting glucose, and hypertension. 4–6 Being obese and metabolically unhealthy is associated with the highest risk for breast cancer.7 This risk is mediated through interrelated pathways, such as those involving insulin, estrogen, cytokines, and growth factors.1–3, 7
Breast cancer is the most common cancer among Black women with an incidence and mortality of 125.5 and 29.5 cases per 100,000, respectively. Incidence in Black women has continued to rise,7 from around 90 per 100,000 in the 1970s to more than 120 per 100,000 in the 2010–2014 period, in part due to the rising prevalence of obesity and MetS in this group.8 As a result, numerous expert panel guidelines have recommended lifestyle modifications, such as increased physical activity, as an effective intervention to reduce risk of breast cancer 9, 10. These approaches targeted to obese metabolically unhealthy women at high risk of breast cancer due to family history or other hormonal risk factors, such as those determined by the National Cancer Institute’s breast cancer risk assessment tool for Black women developed using data from the Women’s Contraceptive and Reproductive Experiences study (CARE model),11 could lead to the most gains in breast cancer prevention. Although a recent meta-analysis of trials of aerobic exercise among metabolically unhealthy women reported an exercise-associated improved metabolic profile, data from controlled trials of exercise among Black women at high risk of breast cancer are not available.12 In addition, most of the interventions studied have been facility-based and/or intensive and it is unclear whether unsupervised home-based interventions might have similar outcomes to facility-based supervised interventions.
In the current randomized controlled trial, we investigated the effects of a short-term supervised and a home-based aerobic exercise intervention on change in MetS and their individual components according to family history of breast cancer and predicted risk of breast cancer utilizing the CARE model, in obese metabolically unhealthy Black women who were enrolled in the Focused Intervention on Exercise to Reduce CancEr (FIERCE) study. To our knowledge, this is the first study to investigate the effect of an exercise intervention on MetS by breast cancer risk in obese, postmenopausal Black women.
MATERIALS AND METHODS
The FIERCE study is a 6-month, 3-arm randomized controlled trial (RCT) of two moderate-intensity exercise interventions compared to a control group, among obese (waist circumference > 35 inches), physically inactive, postmenopausal Black women ages 45 – 65 years. The RCT was conducted between 2012–2016 at the community-based office of the Georgetown-Lombardi Comprehensive Cancer Center, with approval of the Institutional Review Board. Written informed consent was obtained from each participant. The FIERCE study is described in detail elsewhere 13. Briefly, 213 eligible participants were randomly assigned to one of three clinical trial arms: (1) a supervised facility-based aerobic exercise intervention with a goal of 150 min/week of moderate intensity exercise (three 50 minute sessions per week) at the community-based office (N = 73); (2) home-based exercise intervention with a goal of 7,000–10,000 steps per day (equivalent to 150min/week of moderate intensity activity)14 as measured by a pedometer through brisk walking or slow jogging (N = 69); and (3) wait-listed control group whose participants were asked to maintain their baseline daily activities for the duration of the study (N = 71). Participants in the supervised group exercised at a heart rate range within 45–65% of their VO2max, as determined by baseline treadmill testing. Participants wore heart rate monitors to ensure moderate exercise intensity. Participants in the home -based group exercised at an exercise intensity in the range of 11–14 (moderate) on the 20-point Rating of Perceived Exertion (RPE) scale. Participants were taught how determine moderate exercise intensity using the RPE during the baseline visit.
Exclusion criteria included a history of cancer, except non-melanoma skin cancer; diabetes; <1.40% projected risk of breast cancer as determined by the CARE model; current regular exercise; current enrollment in another clinical trial or on weight loss program; and inability to commit to the intervention schedule. Prior to randomization, all participants completed a physical activity readiness medical examination (PARmed-X) 15. Random assignment was generated by the study statistician (KM) using a computer-based random number generator. Investigators and staff were blinded to the assignment. All study measures were collected at baseline and 6-month time points by trained personnel.
Blood sample collection and processing
Fasting baseline venous blood samples were drawn in the morning. During follow-up visits, fasting morning blood samples were drawn 16–24 h after the most recent exercise session for participants on the intervention arms to minimize measurement of acute effects of exercise.16 Samples were processed within two hours and stored at −70°C.
Metabolic variables and assays
Waist circumference was measured at the level of the navel with a measuring tape and recorded in centimeters. Fasting glucose was analyzed on the stored serum samples using the Ortho Clinical Diagnostics Vitros 5.1 FS Chemistry Systems’ glucose oxidase slide method. Intra- and inter-assay coefficient of variation (CVs) for serum glucose were 1.2–1.5%. Systolic and diastolic blood pressure were measured after 10 minutes of rest with the participant in a seated position by trained personnel, and repeated again after 10 minutes. The average of the two measures was used in the analysis. Mean arterial pressure (MAP) was calculated as {(systolic BP) + (Disatolic BP *2)}/3. Serum lipids were analyzed on the Ortho Clinical Diagnostics Vitros 5.1 FS Chemistry Systems platform using a multilayered, enzymatic, slide method. Intra- and inter-assay CVs were 1.5–1.8% for total cholesterol, 2.9–3.0% for HDL-cholesterol, 0.9–1.4% for triglycerides, and 1.8–3.7% for calculated LDL.
Metabolic syndrome criteria and metabolic syndrome z-score
Based on the US National Cholesterol Education Program Adult Treatment Panel III (ATPIII) definition,17 metabolic syndrome was defined as ≥3 of the following 5 factors: waist circumference ≥ 88 cm, fasting glucose ≥ 100 mg/dL; systolic blood pressure ≥ 130 mm Hg or ≥85 mm Hg diastolic blood pressure; triglycerides ≥ 150 mg/dL; and HDL cholesterol < 50.
In addition to the dichotomous MetS, a continuous MetS z-score was also calculated based on equations described previously.18–21 The modified z-score was calculated using individual subject data, the Adult Treatment Panel (ATP) III criteria, and standard deviations for each MetS component using data from all participants at baseline. The equation used to calculate the MetS z score was as follows: {z-Score = [(waist circumference − 88)/13.72] + [(fasting blood glucose − 100)/13.04] + [(mean arterial pressure − 100)/11.26] + [(TG − 150)/61.17] + [(50 − HDL)/18.22].
Family History of Breast Cancer
Family history of breast cancer in a first degree relative (i.e. mothers, daughters, sisters) was assessed through self-report.
CARE Model
Five-year individual invasive breast cancer risk was assessed using the “CARE” model. The CARE model was used to project absolute risk of breast cancer because it has been shown to perform better among Black women as compared to the Gail model.11, 22 The CARE model uses data on current age, race, age at menarche, number of first degree relatives with breast cancer, number of breast biopsies, and atypical hyperplasia on biopsy to determine breast cancer risk.11 Based on prior publications, a 5-year risk of 1.67% or more was considered “high risk”.
Covariates
Information on demographic and lifestyle factors, including smoking, was collected through self-reported questionnaires. Exercise behavior was assessed using the International Physical Activity Questionnaire (IPAQ), a structured interview that measures a person’s time spent engaging in exercise over a 7-day period.23 A metabolic equivalent task (MET) score was assigned to each activity based on its energy cost.24 To calculate the amount of energy expended, the time spent at each activity in hours per week was multiplied by its MET score then summed over all activities to yield total MET-hours/week. The usual dietary intake was assessed using the Block 2005 Food Frequency Questionnaires (FFQ) 25. Anthropometric measures of height, weight, and waist and hip circumference were also collected.
Statistical analysis
Intention to treat (ITT) analysis was performed for all participants who were enrolled and randomly allocated to one of the clinical trials arms to which they were randomized. The intervention effects were evaluated with generalized estimating equations (GEE) models considering the metabolic syndrome components and the z-score as repeated measures. The models included main effects of intervention and time, as well as their interaction term, and were adjusted for baseline values of the outcome studied. All statistical tests were two-sided, with a level of significance set at P = .05. Statistical analyses were performed using SAS software (Version 9.3; SAS Institute, Cary, NC).
RESULTS
Participants
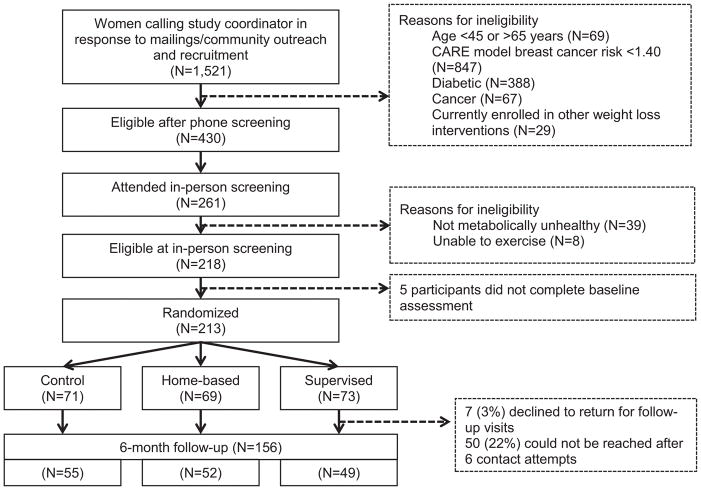
Depicted in Figure 1 is the Consort diagram for the FIERCE Study. A total of 1,521 Black women were screened of which, 430 (28%) were initially eligible for the office visit screening. The study comprised a total of 213 women randomized to the three arms of the study; Supervised, n = 73; Home-based, n = 69; and Control, n= 71). At the end of 6 months of follow-up, the retention rate was 73%. Adherence to the interventions, defined as completing 80% or more of the exercise target set at baseline, was similar in both exercise arms (supervised: 65%, home-based: 68%). Adherence to interventions did not differ by family history or projected breast cancer risk in either arm.
Figure 1.
CONSORT diagram for the FIERCE trial
Baseline characteristics were similar between the 3 arms and are shown in Table 1. The mean age of participants was 58.3 years with more than 90% having a high school or higher education. Participants had low mean exercise levels at baseline (~ 4 MET-hours/week) and had a high daily caloric intake (~ 2,000 kcal). Family history of breast cancer in a first degree relative was reported by 40% of the participants, and the average projected 5-year absolute risk of breast cancer based on the CARE model in this group was 1.85.
Table 1.
Baseline Characteristics of FIERCE Study Participants by Intervention Arm (N=213)
| Characteristic | Control (N=71) | Home-Based Exercise (N=69) | Supervised Exercise (N=73) |
|---|---|---|---|
| Age, mean(SD) | 58.39(5.31) | 58.27(4.69) | 58.11(5.10) |
| Education level, n(%) | |||
| ≤ High school | 6(9) | 4(6) | 7(10) |
| High school/some college | 35(50) | 41(59) | 33(45) |
| ≥ College | 29(41) | 24(35) | 33(45) |
| Marital status, n(%) | |||
| Single/Never married | 23 (33) | 17 (25) | 24 (33) |
| Married/Living with partner | 18 (25) | 20 (29) | 18 (25) |
| Divorced/Separated/Widowed | 30 (42) | 32 (46) | 31 (42) |
| Smoking, n(%) | |||
| Current smoker | 11 (16) | 14 (20) | 5 (7) |
| Former smoker | 20 (28) | 21 (31) | 30 (41) |
| Never smoker | 40 (56) | 34 (49) | 38 (52) |
| Body mass index, in kg/m2, mean (SD) | 35.9 (7.6) | 36.1 (7.2) | 35.2 (6.1) |
| MET-hours per week, mean (SD) | 4.12 (4.38) | 4.30 (4.85) | 4.49 (4.54) |
| Total energy intake, in kcal, mean (SD) | 1,847 (1,004) | 1,852 (1,030) | 1,900 (1,300) |
| Family history of breast cancer in first degree relatives, n (%) | 24 (34) | 32 (46) | 29 (40) |
| Absolute 5-year risk of breast cancer, mean (SD) | 1.81 (0.61) | 1.85 (0.48) | 1.92 (0.78) |
| Mammography screening (ever), n (%) | 64 (91) | 63 (91) | 67 (92) |
Exercise intervention effects on MetS
In intention to treat analyses, proportion of participants with MetS was lower at the 6-month intervention than baseline in the exercise arms, but not the control arm. Compared to the 8% increase in MetS among participants in the control arm at 6 months, those in the supervised group and home-based group had 4% (p = 0.04) and 23% (p < 0.01) decrease in proportion with MetS.
Exercise intervention effects on MetS by family history of breast cancer
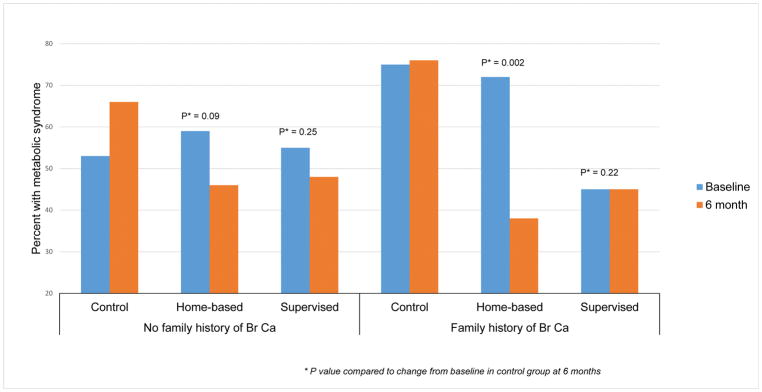
Six-month changes from baseline in levels of MetS components and MetS Z-score for the three study arms by family history of breast cancer are presented in Table 2. Among women with a family history of breast cancer, women in the supervised and the home-based arms had lower mean Mets Z-scores, suggesting an improvement in the metabolic profile, compared to the control arm which had higher Z-scores at the 6 moth follow-up than baseline (controls +0.55; home-based −0.97, P < 0.01; supervised −0.89, P < 0.01). These changes were primarily driven by statistically significant decreases in mean arterial pressure and increases in serum HDL in the supervised arm; and by changes in serum triglycerides and serum HDL in the home-based arm, compared to the control arm in whom mean arterial pressure and triglycerides increased, and HDL decreased over the 6-month follow-up. None of the changes in the Mets components or the Z-score at 6-month follow-up were significantly different between the three groups in women without a family history of breast cancer. Changes in proportion of women with MetS from baseline was also significantly lower in the supervised and home-based arms compared to the control among women with a family history of breast cancer (control +1%; supervised 0%, P = 0.22; home-based −34%, P < 0.01) but not in those without a family history (control +13%; supervised −7%, P = 0.25; home-based −7%, P = 0.09). (Figure 2)
Table 2.
Six- month changes in metabolic syndrome components and metabolic syndrome in the FIERCE trial by family history of breast cancer in first degree relatives
| No family history of breast cancer (N=128) | Family history of breast cancer (N=85+) | |||||
|---|---|---|---|---|---|---|
| Outcome | Baseline Mean (SD) |
6-month mean change (SE) | P value* | Baseline Mean (SD) |
6-month mean change (SE) | P value* |
| Waist circumference (in cm) | ||||||
| Control (C) | 107.79 (12.90) | +0.13 (1.29) | PC-H = 0.94 PC-S = 0.81 PH-S = 0.75 |
114.29 (14.29) | −1.53 (1.52) | PC-H = 0.98 PC-S = 0.52 PH-S = 0.52 |
| Home-based (HB) | 108.08 (12.23) | +0.29 (1.35) | 111.66 (14.25) | −1.46 (1.41) | ||
| Supervised (S) | 106.55 (12.53) | −0.21 (1.35) | 109.90 (16.71) | −3.05 (2.11) | ||
| Fasting glucose (in mg/dL) | ||||||
| Control (C) | 100.47 (12.77) | +2.13 (2.01) | PC-H = 0.98 PC-S = 0.50 PH-S = 0.53 |
107.42 (11.03) | −3.29 (2.80) | PC-H = 0.60 PC-S = 0.78 PH-S = 0.54 |
| Home-based (HB) | 101.08 (12.08) | +1.54 (3.05) | 105.50 (14.27) | −3.00 (3.11) | ||
| Supervised (S) | 104.09 (13.22) | −2.62 (2.89) | 102.66 (14.02) | +0.45 (5.16) | ||
| Mean arterial pressure (in mmHg) | ||||||
| Control (C) | 101.09 (12.59) | −0.75 (2.04) | PC-H = 0.57 PC-S = 0.60 PH-S = 0.90 |
98.39 (11.91) | +5.57 (2.11) | PC-H = 0.10 PC-S = <0.01 PH-S = 0.02 |
| Home-based (HB) | 102.20 (11.65) | −1.74 (2.09) | 98.86 (11.58) | −0.06 (2.41) | ||
| Supervised (S) | 94.41 (8.49) | +3.23 (1.56) | 95.72 (9.27) | −5.85 (2.75) | ||
| Serum triglycerides (in mg/dL) | ||||||
| Control (C) | 112.69 (65.87) | −2.25 (14.71) | PC-H = 0.42 PC-S = 0.77 PH-S = 0.57 |
126.38 (64.26) | +22.09 (19.02) | PC-H = 0.04 PC-S = 0.19 PH-S = 0.59 |
| Home-based (HB) | 108.97 (51.63) | +1.36 (17.00) | 121.20 (59.16) | −17.59 (15.44) | ||
| Supervised (S) | 109.15 (64.94) | +2.48 (14.80) | 101.13 (60.06) | +8.28 (16.68) | ||
| Serum HDL (in mg/dL) | ||||||
| Control (C) | 64.66 (20.18) | +3.21 (1.42) | PC-H = 0.79 PC-S = 0.72 PH-S = 0.62 |
53.08 (12.72) | −1.06 (1.38) | PC-H = 0.02 PC-S = 0.01 PH-S = 0.86 |
| Home-based (HB) | 61.08 (17.62) | +2.36 (3.45) | 57.59 (20.12) | +6.21 (3.00) | ||
| Supervised (S) | 59.64 (17.64) | +4.55 (2.81) | 58.83 (17.31) | +5.80 (2.33) | ||
| Metabolic syndrome Z-Score | ||||||
| Control (C) | 0.16 (2.80) | −0.11 (0.38) | PC-H = 0.62 PC-S = 0.73 PH-S = 0.42 |
1.79 (0.51) | +0.55 (0.53) | PC-H = <0.01 PC-S = <0.01 PH-S = 0.81 |
| Home-based (HB) | 0.46 (1.89) | +0.11 (0.42) | 1.16 (2.41) | −0.97 (0.43) | ||
| Supervised (S) | −0.27 (2.42) | −0.14 (0.47) | 0.14 (3.22) | −0.89 (0.59) | ||
P values for comparing the changes adjusting for baseline values of the outcomes; PC-H: home-based vs. control group; PC-S: supervised vs. control group; PH-S: home-based vs. supervised group
Figure 2.
Change in proportion of participants with metabolic syndrome in the FIERCE study from baseline to 6-month follow-up by family history of breast cancer
Exercise intervention effects on MetS by projected breast cancer risk
Stratified analyses by projected breast cancer risk using the CARE model (“high risk” ≥ 1.67; “low risk” < 1.67) suggested findings similar to stratification by family history, with those at high risk having more favorable changes in the MetS Z score in the exercise arms compared to control arm than those with a risk score below 1.67 (Supplementary table 1). However, these changes for Z-scores were not statistically significant between the control and exercise intervention arms (controls −0.03; home-based −0.78, P = 0.11; supervised −0.80, P = 0.08).
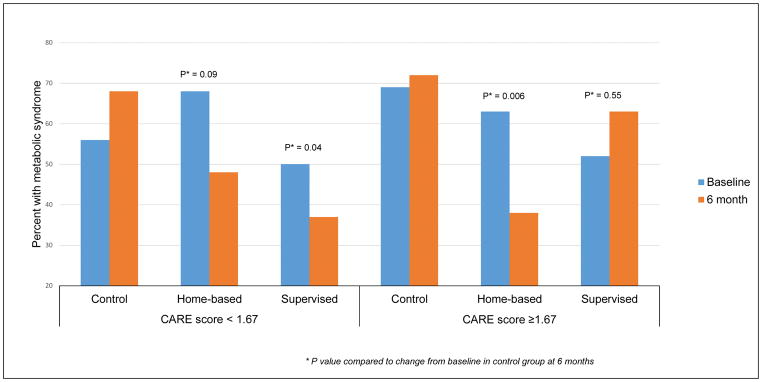
When MetS was analyzed as a dichotomous variable, among participants with low projected breast cancer risk score at baseline, women in both exercise arms had lower proportion of MetS compared to controls (control +12%, supervised −13%, P = 0.04; home-based −20%, P = 0.09). However, in the high projected breast cancer risk group, women in home-based arm had lower proportions of MetS at 6 months compared to baseline but not women in supervised or control groups (control +3%, supervised +11%, P = 0.55; home-based −25%, P < 0.01). (Figure 3)
Figure 3.
Change in proportion of participants with metabolic syndrome in the FIERCE study from baseline to 6-month follow-up by 5-year projected breast cancer risk assessment score (CARE model)
DISCUSSION
This community-based exercise intervention trial among postmenopausal obese metabolically unhealthy Black women examined the effectiveness of a supervised exercise and a home-based exercise intervention compared to controls on metabolic syndrome outcomes by breast cancer risk. Women in the home-based intervention arm who had a family history of breast cancer or were at high risk (CARE score > 1.67) had statistically significant improvements in their metabolic syndrome profiles compared to those with no family history and in low risk group. Furthermore, the changes in metabolic profile were primarily attributed to reductions in blood pressure and triglycerides at 6 months.
It has been suggested that weight loss and exercise intervention are most successful when focused on medically at-risk populations 26. However, exercise or lifestyle change trials among medically at-risk Black women or those with co-morbidities are limited.27, 28, 28, 29, 29 Additionally, none of the trials have focused on obese metabolically unhealthy Black women at high risk of cancer, and none have focused exclusively on exercise. To the best of our knowledge, this is the first trial to suggest that short-term moderate aerobic exercise might reduce the risk of breast cancer by altering the metabolic profile among Black women at high-, but not low-risk of breast cancer. Our results seemed to suggest a greater improvement in MetS among home-based participants than supervised group participants. Reasons for this finding are unclear but it is possible they might be related to longer duration of moderate intensity activity for home-group (7,000 to 10,000 steps per day) versus supervised group participants (150 minutes of supervised aerobic exercise per week) for the same effective dose of aerobic activity. However, the role of chance in these findings cannot be ruled out and replication in future studies is warranted.
Blacks in weight loss trials tend to lose less weight on average compared to White participants.27, 27, 29, 30, 30 However, when stratified by family history or projected breast cancer risk, Black women at higher risk were more likely to show reductions in waist circumference compared to those at lower risk in the exercise as well as the control groups. The largest reductions were seen for high risk participants in the supervised exercise group even though comparisons between the groups were not statistically significant. Changes in BMI were similar to changes in waist circumference and none of the comparisons between intervention and control arms were statistically significant.
Improvements in metabolic profile in the exercise groups among high-risk participants were primarily driven by reductions in blood pressure and triglyceride levels, and an increase in HDL levels. Although a recent meta-analysis suggested similar changes in blood pressure with exercise as seen in our study,31 the differences between those at high- versus low-risk of breast cancer has not been reported previously. Supervised group participants at high-risk of breast cancer had the greatest reductions in blood pressure compared to the control group. In a meta-analysis of the role of exercise on serum lipids that included 51 studies, Mann et al reported that 12 weeks or more of aerobic exercise resulted in a mean increase in HDL levels by 4.6% and a decrease in triglycerides levels by 3.7%.32 Results from our study are much stronger in high-risk participants randomized to the home-based group for triglycerides (15% decrease) and to both exercise groups for HDL (~ 10% increase) compared to baseline. High-risk control group participants had unfavorable changes at 6 months for both triglycerides and HDL.
Biological mechanisms that underlie our findings of a more favorable metabolic response to exercise among those at high-risk of breast cancer have not been studied but a possible role for hormones, such as insulin-like growth factor-1 (IGF-1) can be hypothesized. Association of IGF-1 with MetS including deregulated lipid metabolism, cardiovascular disease, and diabetes is well-established.33 IGF-1 has multiple physiological effects including effects on tissue growth and development, proliferative, lipid metabolism, pro-survival/anti-aging, anti-inflammatory, and antioxidant effects.34–36 A correlation between IGF-1 and increasing metabolic syndrome markers, including blood pressure, has been demonstrated in the Framingham study.37 There is also ample evidence to support the role of IGF-1 in breast cancer initiation and progression.38 Results from a pooled analysis of seventeen prospective cohort studies has shown a strong association between circulating IGF-1 and hormone-positive breast cancer risk in postmenopausal women.39 IGF-1 levels have also been positively associated with women at increased genetic risk of breast cancer (BRCA gene mutation carriers).40 This suggests a potential biologic rationale for our findings.
Strengths of our study include a fairly large trial of exercise among Black women at high risk of breast cancer – an understudied group in cancer prevention trials. Other advantages of our trial include targeting the trial to obese, metabolically unhealthy women; testing of two different exercise regimens; biomarker-based measurements for MetS; and stratified analyses of trial effects by breast cancer risk status. Limitations include a 27% drop-out rate thereby reducing the effective sample size; lack of adherence in the waitlisted control group, e.g., self-reported exercise in all three groups increased at 6 months compared to baseline and was similar in all three groups (data not shown); and lack of generalizability of study findings to non-obese or metabolically healthy Black women. It is also possible that women in the high risk groups exercised more compared to those in the low-risk groups. However, there was no difference by risk status in adherence within the intervention groups. Moreover, women were eligible only if they had >1.40% projected 5-year absolute risk of breast cancer and were told they were at higher estimated risk of breast cancer than average risk women of their age and race at the start of the study.
The findings from the present study strongly suggest that Black women at increased risk of breast cancer are ideal targets for cancer prevention exercise interventions. Furthermore, moderate, short-term aerobic activity regimens, both home-based and supervised regimens may prove to be impactful in improving the metabolic profile and reducing risk of breast cancer even without weight reduction or changes in abdominal obesity in this high-risk population. Studies on biomarkers of insulin pathway are warranted to determine mechanisms that mediate the effect of exercise on metabolic syndrome among women at high risk of breast cancer.
Supplementary Material
Acknowledgments
This study is supported by grants from NIH NIMHD (1P60MD006920-01). The Georgetown Lombardi Comprehensive Cancer Center Biostatistics & Bioinformatics Shared Resource is partially supported by NIH/NCI grant P30 CA051008 and GHUCCTS grant UL1TR001409. CD is supported by National Cancer Institute training grant K07 CA197112-02 (C. Dash). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health
Footnotes
Conflicts of interest: There are no conflicts of interest to disclose by the authors.
Author Contributions:
Chiranjeev Dash: Conceptualization, methodology, project administration, data collection and analysis, writing-original draft
Teletia Taylor: Data collection, writing - review and editing
Kepher Makambi: Statistical analysis, writing - review and editing
Jennifer Hicks: Data collection, writing - review and editing
James Hagberg: Conceptualization, writing - review and editing
Lucile L. Adams-Campbell: Conceptualization, methodology, funding acquisition, writing - review and editing
References
- 1.Vona-Davis L, Howard-McNatt M, Rose DP. Adiposity, type 2 diabetes and the metabolic syndrome in breast cancer. Obes Rev [serial online] 2007;8:395–408. doi: 10.1111/j.1467-789X.2007.00396.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin PJ, Ennis M, Bahl M, et al. High insulin levels in newly diagnosed breast cancer patients reflect underlying insulin resistance and are associated with components of the insulin resistance syndrome. Breast Cancer Res Treat [serial online] 2009;114:517–525. doi: 10.1007/s10549-008-0019-0. [DOI] [PubMed] [Google Scholar]
- 3.Xue F, Michels KB. Diabetes, metabolic syndrome, and breast cancer: A review of the current evidence. Am J Clin Nutr [serial online] 2007;86:s823–35. doi: 10.1093/ajcn/86.3.823S. [DOI] [PubMed] [Google Scholar]
- 4.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: Findings from the third national health and nutrition examination survey. JAMA [serial online] 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 5.Beltran-Sanchez H, Harhay MO, Harhay MM, McElligott S. Prevalence and trends of metabolic syndrome in the adult U.S. population, 1999–2010. J Am Coll Cardiol [serial online] 2013;62:697–703. doi: 10.1016/j.jacc.2013.05.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore JX, Chaudhary N, Akinyemiju T. Metabolic syndrome prevalence by race/ethnicity and sex in the United States, national health and nutrition examination survey, 1988–2012. Prev Chronic Dis [serial online] 2017;14:E24. doi: 10.5888/pcd14.160287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Cancer Society. Breast cancer facts & figures 2017–2018. 2017. [Google Scholar]
- 8.American Cancer Society. Cancer facts & figures for African Americans 2016–2018. 2016. [Google Scholar]
- 9.Food, nutrition, physical activity, and the prevention of cancer: A global perspective. 2007 [Google Scholar]
- 10.Kushi LH, Doyle C, McCullough M, et al. American Cancer Society guidelines on nutrition and physical activity for cancer prevention: Reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin [serial online] 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 11.Gail MH, Costantino JP, Pee D, et al. Projecting individualized absolute invasive breast cancer risk in African American women. J Natl Cancer Inst [serial online] 2007;99:1782–1792. doi: 10.1093/jnci/djm223. [DOI] [PubMed] [Google Scholar]
- 12.Lin X, Zhang X, Guo J, et al. Effects of exercise training on cardiorespiratory fitness and biomarkers of cardiometabolic health: A systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc [serial online] 2015:4. doi: 10.1161/JAHA.115.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dash C, Makambi K, Wallington SF, et al. An exercise trial targeting African-American women with metabolic syndrome and at high risk for breast cancer: Rationale, design, and methods. Contemp Clin Trials [serial online] 2015;43:33–38. doi: 10.1016/j.cct.2015.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tudor-Locke C, Leonardi C, Johnson WD, Katzmarzyk PT, Church TS. Accelerometer steps/day translation of moderate-to-vigorous activity. Prev Med [serial online] 2011;53:31–33. doi: 10.1016/j.ypmed.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 15.Warburton DE, Gledhill N, Jamnik VK, et al. Evidence-based risk assessment and recommendations for physical activity clearance: Consensus document 2011. Appl Physiol Nutr Metab [serial online] 2011;36(Suppl 1):S266–98. doi: 10.1139/h11-062. [DOI] [PubMed] [Google Scholar]
- 16.AbouAssi H, Slentz CA, Mikus CR, et al. The effects of aerobic, resistance, and combination training on insulin sensitivity and secretion in overweight adults from STRRIDE AT/RT: A randomized trial. J Appl Physiol (1985) [serial online] 2015;118:1474–1482. doi: 10.1152/japplphysiol.00509.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grundy SM, Brewer HB, Cleeman jI, Smith SC, Lenfant C. Definition of metabolic syndrome: Report of the national heart, lung, and blood institute/American heart association conference on scientific issues related to definition. 2004 [Google Scholar]
- 18.Thomas GA, Alvarez-Reeves M, Lu L, Yu H, Irwin ML. Effect of exercise on metabolic syndrome variables in breast cancer survivors. Int J Endocrinol [serial online] 2013;2013:168797. doi: 10.1155/2013/168797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacLean PS, Higgins JA, Wyatt HR, et al. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol [serial online] 2009;297:R793–802. doi: 10.1152/ajpregu.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bateman LA, Slentz CA, Willis LH, et al. Comparison of aerobic versus resistance exercise training effects on metabolic syndrome (from the studies of a targeted risk reduction intervention through defined exercise - STRRIDE-AT/RT) Am J Cardiol [serial online] 2011;108:838–844. doi: 10.1016/j.amjcard.2011.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson JL, Slentz CA, Houmard JA, et al. Exercise training amount and intensity effects on metabolic syndrome (from studies of a targeted risk reduction intervention through defined exercise) Am J Cardiol [serial online] 2007;100:1759–1766. doi: 10.1016/j.amjcard.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adams-Campbell LL, Makambi KH, Frederick WA, Gaskins M, Dewitty RL, McCaskill-Stevens W. Breast cancer risk assessments comparing GAIL and CARE models in African-American women. Breast J [serial online] 2009;15(Suppl 1):S72–5. doi: 10.1111/j.1524-4741.2009.00824.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas S, Reading J, Shephard RJ. Revision of the physical activity readiness questionnaire (PAR-Q) Can J Sport Sci [serial online] 1992;17:338–345. [PubMed] [Google Scholar]
- 24.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc [serial online] 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Block G, Woods M, Potosky A, Clifford C. Validation of a self-administered diet history questionnaire using multiple diet records. J Clin Epidemiol [serial online] 1990;43:1327–1335. doi: 10.1016/0895-4356(90)90099-b. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgibbon ML, Tussing-Humphreys LM, Porter JS, Martin IK, Odoms-Young A, Sharp LK. Weight loss and African-American women: A systematic review of the behavioural weight loss intervention literature. Obes Rev [serial online] 2012;13:193–213. doi: 10.1111/j.1467-789X.2011.00945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hollis JF, Gullion CM, Stevens VJ, et al. Weight loss during the intensive intervention phase of the weight-loss maintenance trial. Am J Prev Med [serial online] 2008;35:118–126. doi: 10.1016/j.amepre.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Svetkey LP, Erlinger TP, Vollmer WM, et al. Effect of lifestyle modifications on blood pressure by race, sex, hypertension status, and age. J Hum Hypertens [serial online] 2005;19:21–31. doi: 10.1038/sj.jhh.1001770. [DOI] [PubMed] [Google Scholar]
- 29.West DS, Elaine Prewitt T, Bursac Z, Felix HC. Weight loss of Black, White, and Hispanic men and women in the diabetes prevention program. Obesity (Silver Spring) [serial online] 2008;16:1413–1420. doi: 10.1038/oby.2008.224. [DOI] [PubMed] [Google Scholar]
- 30.Kumanyika SK, Obarzanek E, Stevens VJ, Hebert PR, Whelton PK. Weight-loss experience of Black and White participants in NHLBI-sponsored clinical trials. Am J Clin Nutr [serial online] 1991;53:1631S–1638S. doi: 10.1093/ajcn/53.6.1631S. [DOI] [PubMed] [Google Scholar]
- 31.Cornelissen VA, Smart NA. Exercise training for blood pressure: A systematic review and meta-analysis. J Am Heart Assoc [serial online] 2013;2:e004473. doi: 10.1161/JAHA.112.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mann S, Beedie C, Jimenez A. Differential effects of aerobic exercise, resistance training and combined exercise modalities on cholesterol and the lipid profile: Review, synthesis and recommendations. Sports Med [serial online] 2014;44:211–221. doi: 10.1007/s40279-013-0110-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aguirre GA, De Ita JR, de la Garza RG, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and metabolic syndrome. J Transl Med [serial online] 2016;14 doi: 10.1186/s12967-015-0762-z. 3-015-0762-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perez R, Garcia-Fernandez M, Diaz-Sanchez M, et al. Mitochondrial protection by low doses of insulin-like growth factor- I in experimental cirrhosis. World J Gastroenterol [serial online] 2008;14:2731–2739. doi: 10.3748/wjg.14.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castilla-Cortazar I, Garcia M, Muguerza B, et al. Hepatoprotective effects of insulin-like growth factor I in rats with carbon tetrachloride-induced cirrhosis. Gastroenterology [serial online] 1997;113:1682–1691. doi: 10.1053/gast.1997.v113.pm9352873. [DOI] [PubMed] [Google Scholar]
- 36.Garcia-Fernandez M, Delgado G, Puche JE, Gonzalez-Baron S, Castilla Cortazar I. Low doses of insulin-like growth factor I improve insulin resistance, lipid metabolism, and oxidative damage in aging rats. Endocrinology [serial online] 2008;149:2433–2442. doi: 10.1210/en.2007-1190. [DOI] [PubMed] [Google Scholar]
- 37.Lam CS, Chen MH, Lacey SM, et al. Circulating insulin-like growth factor-1 and its binding protein-3: Metabolic and genetic correlates in the community. Arterioscler Thromb Vasc Biol [serial online] 2010;30:1479–1484. doi: 10.1161/ATVBAHA.110.203943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer [serial online] 2015;14:43-015-0291-7. doi: 10.1186/s12943-015-0291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Endogenous Hormones and Breast Cancer Collaborative Group. Key TJ, Appleby PN, Reeves GK, Roddam AW. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol [serial online] 2010;11:530–542. doi: 10.1016/S1470-2045(10)70095-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pasanisi P, Bruno E, Venturelli E, et al. Serum levels of IGF-I and BRCA penetrance: A case control study in breast cancer families. Fam Cancer [serial online] 2011;10:521–528. doi: 10.1007/s10689-011-9437-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.