Abstract
Purpose:
Several observational studies suggest that metformin reduces incidence cancer risk; however, many of these studies suffer from time-related biases and several cancer outcomes have not been investigated due to small sample sizes.
Methods:
We constructed a propensity score-matched retrospective cohort of 84,434 veterans newly prescribed metformin or a sulfonylurea as monotherapy. We used Cox proportional hazard regression to assess the association between metformin use compared to sulfonylurea use and incidence cancer risk for 10 solid tumors. We adjusted for clinical covariates including hemoglobin A1C, anti-hypertensive and lipid lowering medications, and body mass index. Incidence cancers were defined by ICD-9-CM codes.
Results:
Among 42,217 new metformin users and 42,217 matched-new sulfonylurea users, we identified 2,575 incidence cancers. Metformin was inversely associated with liver cancer (adjusted hazard ratio [aHR] = 0.44, 95% CI 0.31, 0.64) compared to sulfonylurea. We found no association between metformin use and risk of incidence bladder, breast, colorectal, esophageal, gastric, lung, pancreatic, prostate, or renal cancer when compared to sulfonylurea use.
Conclusions:
In this large cohort study that accounted for time related-biases, we observed no association between the use of metformin and most cancers; however, we found a strong inverse association between metformin and liver cancer. Randomized trials of metformin for prevention of liver cancer would be useful to verify these observations.
Keywords: Diabetes mellitus, Metformin, Cancer, Sulfonylureas
Introduction
Metformin, a biguanide whose glycemic lowering effects are not entirely understood, is indicated for the treatment and prevention of type 2 diabetes. The primary known effect of metformin is to decrease hepatic glucose output and increase glucose utilization in muscle [1]. Metformin also decreases circulating free fatty acids [2], activates AMP-activated protein kinase [3] and inhibits the phosphoinositide 3-kinase/Akt/ mammalian target of rapamycin (mTOR) signaling pathway which reduces cell proliferation and promotes apoptosis [4]. Additionally, metformin reduces the mitochondrial production of adenosine triphosphate through binding to complex I of the electron transport chain in hepatocytes [5]. These direct cellular effects, coupled with the known ability of metformin to reduce circulating insulin levels support a plausible mechanism for metformin’s cancer inhibitory actions.
Several observational studies have investigated the impact of metformin use on cancer incidence. Meta-analyses of observational studies have been conducted for colorectal [6,7], hepatocellular [8,7,9,10], breast [11], lung [7,12,13], pancreatic [14,15], prostate [16] and all cancer events combined [17,7,18–20]. In many of these studies, metformin use was associated with cancer risk reductions, sometimes as high as 94%. Nevertheless, several of these previous studies have serious methodological flaws resulting from time-related biases [21]. A recent meta-analysis which included the 8 published studies that were free of time-related biases reported a summary risk estimate of 0.90 (95% CI 0.89–0.91) for the association between metformin and any cancer incidence [19]. For individual cancers, the number of studies that accounted for time-related biases was smaller ranging from only three studies of lung cancer to six for breast and prostate cancer.
Given the uncertainty regarding the impact of metformin use on cancer incidence rates and the paucity of studies that have not incurred time-related biases and accounted for important confounders such as body mass index and glycemic control, we used a large retrospective cohort of patients with diabetes cared for within the Veterans Health Administration who initiated treatment with metformin or a sulfonylurea to determine the association between metformin and the incidence of 10 solid tumors.
Materials and Methods
Study Design and Data Sources
We constructed a retrospective cohort of veterans initiating an oral anti-diabetes drug between October 1, 2001 and September 30, 2008 using national Veterans Health Administration (VHA) databases. Details on this cohort have been published previously [22–24]. Briefly, these data included outpatient and inpatient healthcare encounters (coded using International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] and Current Procedural Terminology [CPT] codes), pharmacy files, vital sign data including weight and height, and laboratory testing values. The VHA pharmacy datasets included data on medication, date filled, days supply, pills dispensed and prescribed dosage. Laboratory results were collected from standard clinical sources. Data on vital signs included all outpatient measures of height, weight, and blood pressure. Dates of death were obtained from National Death Index. For Medicare or Medicaid eligible veterans, we obtained supplemental data on medication use, healthcare encounters and race from the Centers for Medicare & Medicaid Services through an interagency exchange agreement. The institutional review boards of Vanderbilt University and the VA Tennessee Valley Healthcare System approved this study.
Study Population
The study population included veterans who were aged 18 years or older, active users of the VHA health system (defined as at least two clinical encounters or prescription fill occurring over the past 730 days), and had a new prescription dispensed for either metformin, or a sulfonylurea (glyburide, glipizide, glimepiride). This new prescription was the first prescription dispensed after 180 days without prescriptions filled for any hypoglycemic medication. The date of the new prescription represents the cohort entry date.
For this study, we excluded individual who were not prescribed either metformin or sulfonylurea monotherapies, had a baseline cancer diagnosis, or had a creatinine level greater than or equal to 132.6 μmol/L (1.5 mg/dL) (Figure 1). During the study period metformin was not recommended for patients with a creatinine exceeding this threshold. To ensure we were correctly classifying exposure time, we excluded patients who were not persistent with their initial monotherapy regimen within the first 12 months of observation.
Figure 1:
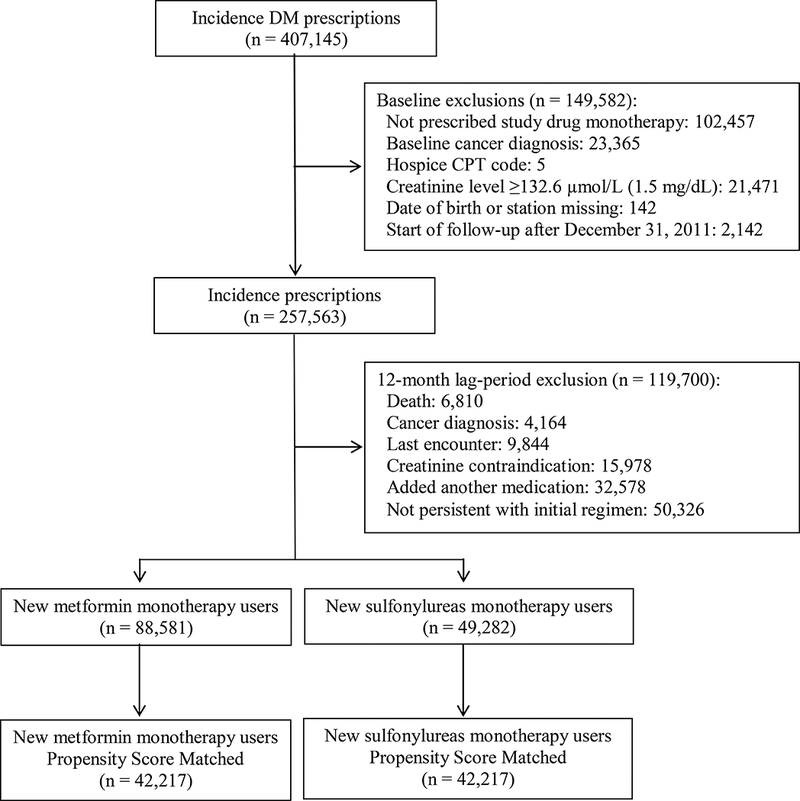
Study Flow Diagram
Exposures
The primary exposure was persistent use of metformin or sulfonylureas (glyburide, glipizide, glimepiride). We used pharmacy information of pills dispensed and prescribed days’ supply to determine individual patients’ drug exposure. Persistent use of a medication was defined as continuous use with no gaps in medication use greater than 90 days.
To address potential latency-bias, we included a 12-month lag-period to remove patients who developed cancer (see below) or died within the first year after initiating metformin or a sulfonylurea. Thus, follow-up began 12 months after the initial antidiabetic prescription date, and continued through the 181st day of no medical contact (inpatient, outpatient or pharmacy use); non-persistence on the original monotherapy (addition of another antidiabetic medication; or the 91st day without drug available); a study outcome; reaching a serum creatinine level of 132.6 μmol/L (1.5 mg/dL), death, or end of study.
Cancer Outcomes
The primary outcome was a new diagnosis of a study cancer. Cancer diagnoses outcomes were defined by ICD-9-CM codes. Definitions included: bladder (ICD-9-CM codes 188.x); breast (ICD-9-CM codes 174x, 175.x), colorectal (ICD-9-CM codes 153.x, 154.x); esophageal (ICD-9-CM codes 150.x); gastric (ICD-9-CM codes 151.x); liver (ICD-9-CM codes 155.x); lung (ICD-9-CM codes 162.x); pancreas (ICD-9-CM codes 157.x); prostate (ICD-9-CM codes 185.x); and renal (ICD-9-CM codes 189.x)[25].
Covariates
Study covariates were collected in the 730 days prior to medication initiation. Covariates included but were not limited to age, sex, race (white, black, other), date of cohort entry, body mass index, blood pressure, glomerular filtration rate, hemoglobin A1c (HbA1c), low-density lipoprotein levels, smoking status, select medications (statins, aspirin, anti-hypertensives, anti-coagulants, antiarrhythmic, diuretics, antipsychotics, glucocorticoids), co-morbid illnesses (cardiovascular disease, severe mental illness, cardiac valvar disease, arrhythmias, Parkinson’s disease, chronic obstructive pulmonary disease, liver disease) number of medications, and number of outpatient visits. Liver diseases were further characterized by individual conditions including acute/subacute necrosis of the liver, chronic liver disease/cirrhosis, liver abscess and sequelae, and other liver diseases. Covariate definitions are presented in Online Resource Table 1.
Statistical Analysis
The primary analysis was time to each individual cancer diagnosis in a propensity score-matched cohort. We constructed separate Cox proportional hazard regression models to estimate the adjusted hazard ratios and 95% two-sided confidence intervals for the association of metformin compared to sulfonylureas and specific incidence cancers. The Cox proportional hazard regression models were estimated using the cph fitting function in the rms [26] package in R (available at: http://www.r-project.org). For each model, the outcome was a specific incidence cancer, and the occurrence of other cancers did not end follow-up. The covariates used in the regression are described in Online Resource Table 1 and Online Resource Table 2. They were selected based on prior knowledge and previous studies exploring the use of metformin and sulfonylureas in a cohort of U.S. veterans [22,24,23]. Continuous covariates were modeled using restricted cubic splines (rcs) with three knots to account for nonlinearity. Missing covariates were handled with multiple imputations using predictive mean matching with bootstrapping [27]. All covariates from the adjusted analysis as well as an indicator for each Veterans Integrated Service Networks were included in twenty imputation models to compute final estimates. The hazard function for the Cox proportional hazards regression model is given in Online Resource Figure 5.
The propensity score modeled the probability of metformin use given baseline study covariates. Visual inspection of the propensity score distributions between metformin and sulfonylurea users showed good overlap. Propensity score distributions are presented in Online Resource Figure 1. Metformin and sulfonylurea observations were matched using an 8-to-1 digit matching algorithm, yielding 84,434 propensity score-matched observations (42,217 in each exposure group). Odds ratios for being prescribed metformin are presented in Online Resource Table 2.
Sensitivity and Subgroup Analysis
In an approach similar to the intention-to-treat analysis used in clinical trials, we conducted a sensitivity analysis ignoring subsequent changes to the medication regimen (persistent exposure not required-[PENR]). To evaluate for dose effects we determined the daily dose amount of metformin users in our cohort and the beginning of the follow-up and divided this by the defined daily dose (DDD) of metformin (2 gram/day) [28]. The DDD factors were separated based on the cohort distribution and categorized as less than 0.5 of the DDD (32.1%, n = 9943), equal to 0.5 of the DDD (48.4%, n = 20,424) and greater than 0.5 of the DDD (28%, n = 11,850). We conducted additional adjusted analyses comparing the metformin DDD level to sulfonylurea users.
Furthermore, given that prior studies have suggested that statins use could impact the risk of cancer [29,30] we conducted additional stratified analyses by statin use at baseline (yes or no), and baseline cirrhosis as defined by ICD-9-CM codes (Online Resource Table 1) (yes or no). We formally tested for an interaction between statin medication use and cirrhosis, and metformin use by including cross-product terms in our regression models.
In addition, we conducted a sensitivity to unmeasured confounding analysis, quantifying the required prevalence difference between exposures that a hypothetical unmeasured binary confounder would need to tip statistically significant results into non-significant results [31]. In all models sulfonylurea users were the reference group. Statistical analyses were conducted using R (available at: http://www.r-project.org.) and SAS for Windows 9.2 (SAS Institute, Cary, NC).
Results
In the full, unmatched cohort, we identified 257,563 incidence prescriptions for metformin or a sulfonylurea monotherapy. During the 12-month lag period, we excluded 6,810 (2.6%) patients due to death, 4,164 (1.6%) with a new cancer diagnosis; 9,844 (3.8%) lost to follow-up; 82,904 (32.2%) who were non-persistent on the initial regimen or who added a new medication, and 15,978 (6.2%) who developed a creatinine level greater than 132.6 μmol/L (1.5 mg/dL) (Figure 1). Our full cohort included 88,581 new metformin users and 49,282 new sulfonylurea users; after propensity score-matching, our study included 42,217 patients in each exposure group.
Patient Characteristics
Demographic characteristics of the full and propensity-matched cohort are presented in Table 1. After propensity score-matching, metformin and sulfonylurea baseline characteristics were similar (Table 1) and standardized differences in the proportion with these characteristics were negligible (Online Resource Figure 2).
Table 1:
Baseline Characteristics of Metformin and Sulfonylurea Users in Full and Propensity Score-Matched Cohort
| Characteristic | Full Cohort before Matchinga | Propensity Matched Cohort | ||
|---|---|---|---|---|
| Metformin (n = 88,581) |
Sulfonylurea (n = 49,282) |
Metformin (n = 42,217) |
Sulfonylurea (n = 42,217) |
|
| Age, y, median (IQR) | 62 (56, 71) | 67 (58, 76) | 66.2 (57.6 to 74.7) | 65.4 (57.3 to 74.6) |
| Female, No (%) | 4,314 (4.9) | 1,226 (2.5) | 1,176 (2.8) | 1,142 (2.8) |
| Race/ethnicity, No (%) | ||||
| White | 68,464 (78) | 39,116 (80) | 33,343 (79) | 33,396 (79) |
| Black | 9,954 (11) | 5,988 (12) | 5,050 (12) | 5,114 (12) |
| Hispanic/other | 3,745 (4.2) | 2,183 (4.4) | 1,851 (4.4) | 1,836 (4.3) |
| Missing data, No (%) | 6,503(7.3) | 2,060 (4.2) | 2,027 (4.8) | 2,007 (4.8) |
| HbA1c, median (IQR), | 6.8 (6.2, 7.4) | 6.9 (6.3, 7.7) | 6.8 (6.3 to 7.5) | 6.9 (6.3 to 7.7) |
| Missing data, No (%) | 16,606 (19) | 10,447 (21) | 8,627 (20) | 8,679 (21) |
| Body mass index, median (IQR) | 31.9 (28.5 to 36.1) | 30.4 (27.1 to 34.3) | 30.7 (27.5, 34.7) | 30.7 (27.5, 34.7) |
| Missing data, No (%) | 2,465 (2.8) | 2,021 (4.1) | 1,534 (3.6) | 1,553 (3.7) |
| Low-density lipoprotein, median (IQR) | 98 (78, 122) | 98 (77, 122) | 97 (77.6, 121) | 98 (78, 122) |
| Missing data, No (%) | 21,520 (24) | 14,853 (30) | 12,107 (29) | 12,014 (28) |
| Systolic blood pressure, median (IQR) | 134 (124, 146) | 136 (124, 148) | 136 (124, 148) | 136 (124, 147) |
| Diastolic blood pressure, median (IQR) | 77 (70, 84) | 76 (68, 83) | 76 (68, 83) | 76 (68, 83) |
| Missing data, No (%) | 1,655 (1.9) | 1,313 (2.7) | 1,017 (2.4) | 1,027 (2.4) |
| Glomerular filtration rate, median (IQR) | 84 (72, 99) | 78 (65, 94) | 79.6 (67.6, 96.0) | 80.0 (67.2, 96.4) |
| Missing data, No (%) | 12,102 (14) | 9,011 (18) | 7,336 (17) | 7,215 (17) |
| Baseline comorbid conditions, No (%) | ||||
| Liver disease | 628 (0.7) | 800(1.6) | 487 (1.1) | 511 (1.2) |
| Smoking | 10,321 (12) | 5,323 (11) | 4,615 (11) | 4,657 (11) |
| Cardiovascular Disease | 19,747 (22) | 13,828 (28) | 10,877 (26) | 10,963 (26) |
| Chronic obstructive pulmonary disease | 10,223 (12) | 6,687 (14) | 5,544 (13) | 5,505(13) |
| Serious mental illness, | 15,318 (17) | 7,796 (16) | 6,772 (16) | 6,832 (16) |
| Cardiac valve disease | 1004 (1.1) | 1,022 (2.1) | 657 (1.6) | 670 (1.6) |
| Arrhythmia | 5,467 (6.2) | 5,188 (11) | 3,576 (8.5) | 3,540 (8.4) |
| Congestive heart failure | 3,065 (3.5) | 4,393 (8.9) | 2,478 (5.9) | 2,464 (5.8) |
| Parkinson’s Disease | 415 (0.47) | 393 (0.80) | 281 (0.7) | 290 (0.7) |
| HIV | 297 (0.34) | 274 (0.56) | 202 (0.5) | 207 (0.5) |
| Use of Medication, No (%) | ||||
| Statin | 60,723(69) | 30,606(62) | 26,978 (62) | 26,937 (62) |
| Beta blockers | 35,444 (40) | 21,635 (44) | 17,959 (43) | 17,892 (42) |
| Ace inhibitor | 47,127 (53) | 26,805 (54) | 22,769 (54) | 22,738 (54) |
| Angiotensin receptor blockers | 7,002 (7.9) | 3,599 (7.3) | 3,178 (7.5) | 3,101 (7.3) |
| Calcium channel blockers | 21,426 (24) | 13,168 (27) | 11,068 (26) | 11,029 (26) |
| Aspirin | 15,292 (17) | 8,689 (18) | 7,267 (17) | 7,326 (17) |
| Antipsychotic | 7,052 (8.0) | 3,608 (7.3) | 3,159 (7.5) | 3, 152 (7.5) |
| Antiarrhythmic | 1,284 (1.4) | 1,080 (2.2) | 769 (1.8) | 770 (1.8) |
| Nonselective alpha blockers | 12,686 (14) | 7,777 (16) | 6,546 (16) | 6,434 (15) |
| Other anti-hypertensives | 20,941 (24) | 12,556 (25) | 10,594 (25) | 10,562 (25) |
| Thiazide diuretics | 29,390 (33) | 15,572 (32) | 13,351 (32) | 13,471 (32) |
| Loop diuretics | 8,743 (10) | 8,669 (18) | 5,880 (14) | 5,821 (14) |
| Anticoagulants | 4,611 (5.2) | 3,875 (7.9) | 2,793 (6.6) | 2,799 (6.6) |
| Nitrates | 9,850 (11) | 7,416 (15) | 5,796 (14) | 5,732 (14) |
| Glucocorticoids | 9,185 (10) | 5,689 (12) | 4,632 (11) | 4,660 (11) |
| Healthcare utilization, No (%) | ||||
| Nursing home encounters in past year | 38 (0.04) | 26 (0.05) | 20 (0.1) | 20 (0.1) |
| Hospitalized in past year | ||||
| VA only | 4,765 (5.4) | 3,510 (7.1) | 2,671 (6.3) | 2,704 (6.4) |
| Medicare only | 4,500 (5.1) | 4,825 (9.8) | 3,220 (7.6) | 3,213 (7.6) |
| Medicaid only | 161 (0.18) | 136 (0.28) | 94 (0.2) | 103 (0.2) |
| Medicare use in past year | 22,699 (26) | 16,539 (34) | 13,264 (31) | 13,065 (31) |
| Medicaid use in past year | 7,237 (8.2) | 6,690 (14) | 4,759 (11) | 4,744 (11) |
| Outpatient visits in past year, median (IQR) |
5 (3, 8) | 5 (3, 9) | 5 (3, 8) | 5 (3, 9) |
| Number of unique medication, median (IQR) | 9.0 (6.0, 14) | 10.0 (7, 14) | 10 (6, 14) | 10 (6, 14) |
| Year, No (%) | ||||
| 2002–2003 | 15,408 (17) | 12,982 (26) | 10,396 (25) | 10,063 (24) |
| 2004 | 14,999 (17) | 10,186 (21) | 8,053 (19) | 8,634 (20) |
| 2005 | 18,879 (21) | 10,383 (21) | 8,582 (20) | 9,134 (22) |
| 2006 | 19,909(22) | 8,524 (17) | 8,075 (19) | 7,726 (18) |
| 2007 | 14,456(16) | 4,764 (9.7) | 5,393 (13) | 4,383 (10) |
| 2008 | 2,195 (2.5) | 1,152 (2.3) | 816 (1.9) | 1,056 (2.5) |
| 2009 | 1,562(1.8) | 750 (1.5) | 532 (1.3) | 714 (1.7) |
| 2010 | 1,169 (1.3) | 529 (1.1) | 366 (0.9) | 503 (1.2) |
Full cohort descriptive information is presented for comparison purposes and the propensity-match cohort was used for all analysis
Primary Outcome: Individual Cancer Outcomes
Event counts, person-years, event rates, and unadjusted and adjusted hazard ratios for each cancer outcome are presented in Table 2 and Figure 2. In the primary analysis, requiring persistent medication exposure, we identified 43 incidence liver cancers during 83,290 person-years of follow-up among metformin users and 83 during 69,319 person-years of follow-up among sulfonylurea users for an event rate of 0.5 (95% CI 0.4, 0.7) and 1.2 (95% CI 1.0, 1.5) per 1000 person-years, respectively. Seventy-five percent (94) were hepatocellular carcinomas, 10% (12) were cholangiocarcinoma, 16% (20) were primary liver cancers NOS. The adjusted hazard ratio for the association with liver cancer in metformin users compared to sulfonylureas users was 0.44 (95% CI 0.31, 0.65). We found no evidence of an association between metformin use and bladder, breast, colorectal, esophageal, gastric, lung, pancreatic, prostate or renal cancers when compared to sulfonylurea users (Figure 2).
Table 2:
Incidence Rates and Hazard Ratios among Propensity Score-Matched Cohorts of New Users of Metformin Compared with Sulfonylureas
| Propensity Score-Matched Cohort |
||||
|---|---|---|---|---|
| Persistent Exposure Required | Persistent Exposure Not Required | |||
| Sulfonylureas | Metformin | Sulfonylureas | Metformin | |
| Bladder cancer events, n | 97 | 122 | 300 | 327 |
| Person-years | 69,243 | 83140 | 195,572 | 201,182 |
| Events/1000 person-years (95% CI) | 1.4 (1.1, 1.7) | 1.5 (1.2, 1.8) | 1.5 (1.4, 1.7) | 1.6 (1.5, 1.8) |
| Unadjusted hazard ratio (95% CI) | 1.00 | 1.01 (0.77, 1.32) | 1.00 | 1.06 (0.90, 1.24) |
| Adjusted hazard ratio (95% CI) | 1.00 | 1.02 (0.78, 1.34) | 1.00 | 1.04 (0.89, 1.21) |
| Breast cancer events, n | 9 | 13 | 30 | 31 |
| Person-years | 69,347 | 83,293 | 196,060 | 201,842 |
| Events/1000 person-years (95% CI) | 0.1 (0.1, 0.2) | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.2) | 0.2 (0.1, 0.2) |
| Unadjusted hazard ratio (95% CI) | 1.00 | 1.11 (0.47, 2.60) | 1.00 | 1.00 (0.61, 1.66) |
| Adjusted hazard ratio (95% CI) | 1.00 | 1.20 (0.47, 2.66) | 1.00 | 1.00 (0.61, 1.67) |
| Colorectal cancer events, n | 150 | 157 | 415 | 377 |
| Person-years | 69,202 | 83,131 | 195,527 | 201,113 |
| Events/1000 person-years (95% CI) | 2.2 (1.8, 2.5) | 1.9 (1.6, 2.2) | 2.1 (1.9, 2.3) | 1.9 (1.7, 2.1) |
| Unadjusted hazard ratio (95% CI) | 1.00 | 0.87 (0.69, 1.09) | 1.00 | 0.88 (0.77, 1.01) |
| Adjusted hazard ratio (95% CI) | 1.00 | 0.89 (0.71, 1.12) | 1.00 | 0.86 (0.75, 0.99) |
| Esophageal cancer events, n | 35 | 42 | 115 | 103 |
| Person-years | 69,342 | 83,288 | 196,038 | 201,791 |
| Events/1000 person-years (95% CI) | 0.5 (0.4, 0.7) | 0.5 (0.4, 0.7) | 0.6 (0.5, 0.7) | 0.5 (0.4, 0.6) |
| Unadjusted hazard ratio (95% CI) | 1.00 | 0.97 (0.62, 1.52) | 1.00 | 0.87 (0.67, 1.13) |
| Adjusted hazard ratio (95% CI) | 1.00 | 0.99 (0.63, 1.55) | 1.00 | 0.85 (0.65, 1.10) |
| Gastric cancer events, n | 31 | 28 | 76 | 83 |
| Person-years | 69,342 | 83,296 | 196,075 | 201,813 |
| Events/1000 person-years (95% CI) | 0.4 (0.3, 0.6) | 0.3 (0.2, 0.5) | 0.4 (0.3, 0.5) | 0.4 (0.3, 0.5) |
| Unadjusted hazard ratio (95% CI) | 1.00 | 0.72 (0.43, 1.21) | 1.00 | 1.06 (0.78, 1.44) |
| Adjusted hazard ratio (95% CI) | 1.00 | 0.74 (0.44, 1.23) | 1.00 | 1.03 (0.75, 1.40) |
| Liver cancer events, n | 83 | 43 | 269 | 126 |
| Person-years | 69,319 | 83,290 | 195,935 | 201,790 |
| Events/1000 person-years (95% CI) | 1.2 (1.0, 1.5) | 0.5 (0.4, 0.7) | 1.4 (1.2, 1.5) | 0.6 (0.5, 0.7) |
| Unadjusted hazard ratio (95% CI) | 1.00 | 0.43 (0.30, 0.63) | 1.00 | 0.45 (0.37, 0.56) |
| Adjusted hazard ratio (95% CI) | 1.00 | 0.44 (0.31, 0.65) | 1.00 | 0.46 (0.37, 0.57) |
| Lung cancer events, n | 316 | 336 | 929 | 860 |
| Person-years | 69,193 | 83,096 | 195,282 | 201,087 |
| Events/1000 person-years (95% CI) | 4.6 (4.1, 5.1) | 4.0 (3.6, 4.5) | 4.8 (4.5, 5.1) | 4.3 (4.0, 4.8) |
| Unadjusted hazard ratio (95% CI) | 1.00 | 0.88 (0.76, 1.03) | 1.00 | 0.90 (0.82, 0.99) |
| Adjusted hazard ratio (95% CI) | 1.00 | 0.89 (0.76, 1.04) | 1.00 | 0.87 (0.79, 0.96) |
| Pancreatic cancer events, n | 49 | 47 | 179 | 189 |
| Person-years | 69,334 | 83,293 | 196,025 | 201,772 |
| Events/1000 person-years (95% CI) | 0.7 (0.5, 0.9) | 0.6 (0.4, 0.8) | 0.9 (0.8, 1.1) | 0.9 (0.8, 1.1) |
| Unadjusted hazard ratio (95% CI) | 1.00 | 0.83 (0.56, 1.24) | 1.00 | 1.02 (0.83, 1.26) |
| Adjusted hazard ratio (95% CI) | 1.00 | 0.85 (0.57, 1.27) | 1.00 | 1.02 (0.83, 1.25) |
| Prostate cancer events, n | 410 | 474 | 1131 | 1190 |
| Person-years | 68,749 | 82,585 | 193,053 | 198,678 |
| Events/1000 person-years (95% CI) | 6.0 (5.4, 6.6) | 5.7 (5.2, 6.3) | 5.9 (5.5, 6.2) | 6.1 (5.7, 6.3) |
| Unadjusted hazard ratio (95% CI) | 1.00 | 0.96 (0.84, 1.10) | 1.00 | 1.02 (0.94, 1.10) |
| Adjusted hazard ratio (95% CI) | 1.00 | 0.97 (0.85, 1.11) | 1.00 | 1.00 (0.93, 1.10) |
| Renal cancer events, n | 67 | 66 | 196 | 197 |
| Person-years | 69,302 | 83,275 | 195,680 | 201,489 |
| Events/1000 person-years (95% CI) | 1.0 (0.8, 1.2) | 0.8 (0.6, 1.0) | 1.0 (0.9, 1.2) | 1.0 (0.9, 1.2) |
| Unadjusted hazard ratio (95% CI) | 1.00 | 0.80 (0.57, 1.13) | 1.00 | 0.97 (0.80, 1.19) |
| Adjusted hazard ratio (95% CI) | 1.00 | 0.81 (0.58, 1.14) | 1.00 | 0.96 (0.79, 1.17) |
Figure 2:
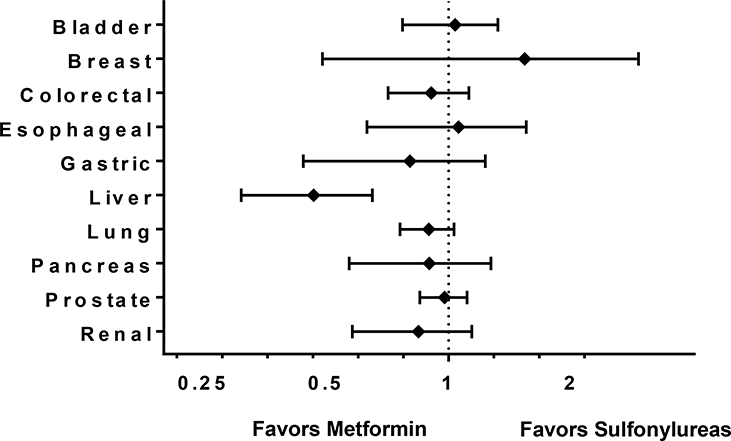
Propensity-Score Matched Hazard Ratio and 95 % Confidence Intervals for the Association between New-User Metformin Monotherapy Users and New-Users Sulfonylureas Monotherapy Users and Cancer Risk, Persistent Exposure Required
Sensitivity Analyses
We conducted a sensitivity analysis removing the requirement for patients to be adherent to their initially prescribed medication (i.e. persistent exposure not required). This resulted in an additional 4,548 cancer outcomes, including 269 liver cancers. In this analysis, the association remained significant for liver cancer (aHR 0.46; 95% CI 0.37, 0.57). Allowing additional outcomes, we also found statistically significant associations between metformin compared to sulfonylurea and lung cancer (aHR 0.87; 95% CI 0.79, 0.96) and colorectal cancer (aHR 0.86; 95% CI 0.75, 0.99). However, we found no evidence of an association between metformin compared to sulfonylurea use and bladder, breast, esophageal, gastric, pancreatic, prostate, and renal cancer (Table 2). The cumulative incidence plots for the cancer outcomes (Online Resource Figure 3) show a clear separation between metformin and sulfonylurea users for liver cancer outcomes in analyses that did and did not require persistent exposure. When stratified by dose, metformin users when compared to sulfonylurea users had a lower risk of liver cancer without any clear evidence of a dose effect (less than 0.5 of the DDD [aHR 0.56; 95% CI 0.32, 0.99]; equal to 0.5 of the DDD [aHR 0.39; 95% CI 0.24, 0.65]; and greater than 0.5 DDD [aHR0.44; 95% CI 0.22, 0.85]).
When the propensity score matched cohort was stratified by statin use at baseline, the unadjusted hazard ratio of metformin with liver cancer risk was 0.61 (95% 0.31, 1.19) among patients with statin use at baseline versus 0.38 (95% CI 0.24, 0.59) in individuals without statin prescriptions at baseline (Online Resource Figure 4). We found no evidence of an interaction between baseline statin use and metformin on any cancer outcomes when persistence with treatment was required (Online Resource Table 3). In subgroup analysis stratified by cirrhosis at baseline, metformin compared to sulfonylurea was associated with liver cancer both in both patients with (HR 0.32, 95% CI 0.12, 0.89) and without (HR 0.46, 95% CI 0.31, 0.68) baseline cirrhosis (Online Resource Table 4).
We estimated the distribution of a hypothetical unmeasured confounder that could explain the statistically significant association observed between metformin and liver cancer. We found that an unmeasured binary confounder that increased ones risk of cancer with a hazard ratio of 2 would need a 54% prevalence difference, e.g. it occurs in 54% of the sulfonylurea users and none of the metformin users, to render our main findings statistically nonsignificant. In the persistent exposure not required analysis, the prevalence difference would need to be 75%.
Discussion
We found a strong inverse association between metformin use compared to sulfonylurea use and incidence liver cancer. In contrast, metformin compared to sulfonylurea use was not associated with incidence bladder, breast, colorectal, esophageal, gastric, lung, pancreas, prostate, or renal cancer in the primary analysis. The protective association observed with liver cancer was consistently observed in patients with and without baseline liver diseases and in patients with and without baseline use of statins medications.
Our findings are important as only a limited number of pharmacoepidemiological studies have been conducted that are both free of time-related biases and able to adjust for important confounders such as BMI and glycemic control [21,32]. Gandini et al. conducted a meta-analysis including a sub-analysis restricted to the eight studies they considered to be free of time-related biases [19]. Three of those studies included risk ratios for hepatocellular cancer. In the analyses that used sulfonylurea medications as the comparator group, the summary risk ratio (SRR) for hepatocellular cancer was 0.65 (95% CI 0.39, 1.08). These studies did not account for body mass index, glycemic control, or statin use. Similar to our study, no association was found between metformin use and prostate or pancreatic cancer. Gandini et al. also found significant associations between metformin and colorectal cancer (SRR = 0.92, 95% CI 0.85, 0.98) and lung cancer (SRR = 0.88, 95% CI 0.81, 0.95), similar to our findings in the secondary analyses that did not require persistent exposure.
Tsilidis et al. recently published a study including 95,820 participants in the Clinical Practice Research Datalink that examined the association of metformin use compared to sulfonylurea use and cancer risk [33]. They included a similar lag-period and employed a new-user design to avoid time-related biases and did not require persistent exposure to the original regimen, similar to our secondary analysis which did not require persistent exposure. However, they attempted to account for variable adherence to the original study regimens during the follow-up period. In that study no significant association with metformin and any cancer evaluated was found. Similar, albeit non-statistically significant HR were found with lung cancer (HR = 0.85; 95% CI 0.68, 1.07; total cases = 468) and colorectal cancer (HR = 0.92; 95% 0.76, 1.13; total cases = 599). There was a weak non-statistically significant association with liver cancer (HR = 0.85; 95% CI 0.49, 1.48) based on only 74 liver cancer cases, compared to 395 cases in our similar analysis. This cohort had a much higher percentage of women than our study which could contribute to the differences in study results.
We found a very strong inverse association between metformin use and liver cancer. Although our findings might be explained by residual confounding, we made extensive efforts to minimize this concern. Prescribers may avoid metformin in persons with liver disease, which may in turn increase the risk of liver cancer. Indeed, in the unmatched cohort, liver disease was more common in sulfonylurea users than metformin users. Our propensity score-matching strategy included a comprehensive list of relevant covariates, and specifically assured that measured liver disease was similar in both exposure groups. Analyses stratified by baseline liver disease yielded consistent inverse associations in both strata. Furthermore, in our analysis that evaluated the sensitivity of our findings to a potential unmeasured confounder, we estimated that the observed effect size could only be explained by an unmeasured confounder moderately associated with the outcome, but with a very different prevalence in the two comparison groups.
The precise explanation for the observed selective inverse association between metformin use and incidence liver cancer is unclear[34]. However, the major target of metformin is the liver, with liver tissue being one of the few sites that expresses OCT1, the major transporter associated with metformin uptake [35]. In addition, current clinically used dosages of metformin are expected to yield higher drug concentrations in the portal circulation compared to the systemic circulation. The increased uptake and portal circulation exposure might allow the liver might to achieve higher drug concentrations than other tissues. Nevertheless, indirect cancer-inhibitory effects of metformin, such as reducing insulin sensitivity or weight reduction, could also be beneficial for tumor prevention.
Our study has several strengths. Our new-user design allowed us to minimize time-related biases and is more similar to a clinical trial where drug exposure starts at study entry [21,36]. We applied a number of steps to minimize exposure misclassification in our primary analyses, and focused on identifying incidence cancers during follow-up by considering a lag-period to support the plausibility of the observed associations. Finally, we were able to adjust for important covariates that few prior studies have been able to account for such as body mass index, glycemic control, and use of statin medications. There are several limitations as well. First, our use of administrative codes could have resulted in misclassification of some cancer outcomes as well as some important covariates. We have conducted a validation study of NSAID and tobacco use and found these codes to be quite accurate [37]. An important limitation is that we were unable to adjust for alcohol use. If alcohol consumption influence whether an individuals was initiated on metformin or a sulfonylurea this could have introduced confounding into our analysis. We were also unable to account for the overall duration of diabetes in the cohort. We utilized a new-user design which should result in individuals with similar disease duration being entered into the cohort, however residual confounding by this variable could still persist. In addition, we were unable to investigate the impact of insulin and cancer risk which has been previously associated with cancer risk however the associations have been limited by methodological concerns of the study design [38,39]. Another limitation is that the association with metformin use might represent a result of a protective effect of metformin versus a harmful effect of sulfonylureas. Our overall event rates for all cancers were higher than SEER estimates in men over the age of 50 years which is likely related to the increased prevalence of risk factors in our VHA population (diabetes, obesity, alcohol and tobacco current and former use). Nevertheless, the liver cancer event rate in sulfonylureas users was quite high. Our study is unable to determine the exact contribution towards liver cancer risk made by metformin or sulfonylureas but suggests a relative benefit with metformin compared to sulfonylureas.
In conclusion, in a large, nationwide cohort of diabetic patients newly initiated on an oral anti-diabetic agent, we found that metformin use was associated with a 56% reduction in liver cancer risk. We did not demonstrate risk reductions for bladder, breast, esophageal, gastric, pancreatic, prostate or renal cancer. Our study design avoided time-related biases. Our findings were consistent across multiple sensitivity analyses, and our analyses suggest that only a very prevalent and strong unmeasured confounder could explain our effect sizes. Given the strong observed association with liver cancer with no similar finding in the other nine solid tumors, our results suggest that this cancer might be the most suitable target for a metformin cancer chemoprevention trial.
Supplementary Material
Acknowledgments:
This was supported under Contract No. 290–05-0042 from the Agency for Healthcare Research and Quality, US Department of Health and Human Services as part of the Developing Evidence to Inform Decisions about Effectiveness (DEcIDE) program. This project was additionally supported through a National Institutes of Health (P20 DK090874–01). This project was supported in part by the by VA Clinical Science research and Development investigator initiated grant CX000570–01 (Roumie). Dr. Murff was supported in part by R01CA143288 and R01CA160938 from the National Cancer Institute. Dr. Roumie was also supported in part by Center for Diabetes Translation Research P30DK092986. Dr. Hung (2–031-09S) was supported by a VA Career Development Award. Dr. Grijalva was supported in part by R01AG043471 from the National Institute on Aging. Support for Veterans Affairs/Centers for Medicare & Medicaid Services data provided by the Department of Veterans Affairs, Veterans Affairs Health Services Research and Development Service, Veterans Affairs Information Resource Center (project numbers SDR 02–237 and 98–004).
Footnotes
Conflict of Interest Disclosure: The authors have no conflicts of interest to disclose.
References
- 1.Boyle JG, Salt IP, McKay GA (2010) Metformin action on AMP-activated protein kinase: a translational research approach to understanding a potential new therapeutic target. Diabet Med [27] (10):1097–1106. doi: 10.1111/j.1464-5491.2010.03098.x [DOI] [PubMed] [Google Scholar]
- 2.Jalving M, Gietema JA, Lefrandt JD, de Jong S, Reyners AK, Gans RO, de Vries EG (2010) Metformin: taking away the candy for cancer? Eur J Cancer 46 (13):2369–2380. doi:S0959-8049(10)00487-9 [pii] 10.1016/j.ejca.2010.06.012 [DOI] [PubMed] [Google Scholar]
- 3.Shaw RJ, Lamia KA, Vasquez D, Koo SH, Bardeesy N, Depinho RA, Montminy M, Cantley LC (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310 (5754):1642–1646. doi:1120781 [pii] 10.1126/science.1120781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ben Sahra I, Le Marchand-Brustel Y, Tanti JF, Bost F (2010) Metformin in cancer therapy: a new perspective for an old antidiabetic drug? Mol Cancer Ther 9 (5):1092–1099. doi:1535-7163.MCT-09-1186 [pii] 10.1158/1535-7163.MCT-09-1186 [DOI] [PubMed] [Google Scholar]
- 5.Bridges HR, Jones AJ, Pollak MN, Hirst J (2014) Effects of metformin and other biguanides on oxidative phosphorylation in mitochondria. Biochem J 462 (3):475–487. doi: 10.1042/BJ20140620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang ZJ, Zheng ZJ, Kan H, Song Y, Cui W, Zhao G, Kip KE (2011) Reduced risk of colorectal cancer with metformin therapy in patients with type 2 diabetes: a meta-analysis. Diabetes Care 34 (10):2323–2328. doi:34/10/2323 [pii] 10.2337/dc11-0512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Noto H, Goto A, Tsujimoto T, Noda M (2012) Cancer risk in diabetic patients treated with metformin: a systematic review and meta-analysis. PLoS One 7 (3):e33411. doi: 10.1371/journal.pone.0033411PONE-D-11-24599 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang ZJ, Zheng ZJ, Shi R, Su Q, Jiang Q, Kip KE (2012) Metformin for liver cancer prevention in patients with type 2 diabetes: a systematic review and meta-analysis. J Clin Endocrinol Metab 97 (7):2347–2353. doi:jc.2012-1267 [pii] 10.1210/jc.2012-1267 [DOI] [PubMed] [Google Scholar]
- 9.Singh S, Singh PP, Singh AG, Murad MH, Sanchez W (2013) Anti-diabetic medications and the risk of hepatocellular cancer: a systematic review and meta-analysis. Am J Gastroenterol 108 (6):881–891; quiz 892. doi: 10.1038/ajg.2013.5 [DOI] [PubMed] [Google Scholar]
- 10.Zhang H, Gao C, Fang L, Zhao HC, Yao SK (2013) Metformin and reduced risk of hepatocellular carcinoma in diabetic patients: a meta-analysis. Scand J Gastroenterol 48 (1):78–87. doi: 10.3109/00365521.2012.719926 [DOI] [PubMed] [Google Scholar]
- 11.Col NF, Ochs L, Springmann V, Aragaki AK, Chlebowski RT (2012) Metformin and breast cancer risk: a meta-analysis and critical literature review. Breast Cancer Res Treat 135 (3):639–646 doi: 10.1007/s10549-012-2170-x [DOI] [PubMed] [Google Scholar]
- 12.Zhang ZJ, Bi Y, Li S, Zhang Q, Zhao G, Guo Y, Song Q (2014) Reduced risk of lung cancer with metformin therapy in diabetic patients: a systematic review and meta-analysis. Am J Epidemiol 180 (1):11–14. doi: 10.1093/aje/kwu124 [DOI] [PubMed] [Google Scholar]
- 13.Nie SP, Chen H, Zhuang MQ, Lu M (2014) Anti-diabetic medications do not influence risk of lung cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Asian Pac J Cancer Prev 15 (16):6863–6869 [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Lai ST, Xie L, Zhao JD, Ma NY, Zhu J, Ren ZG, Jiang GL (2014) Metformin is associated with reduced risk of pancreatic cancer in patients with type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes research and clinical practice 106 (1):19–26. doi: 10.1016/j.diabres.2014.04.007 [DOI] [PubMed] [Google Scholar]
- 15.Singh S, Singh PP, Singh AG, Murad MH, McWilliams RR, Chari ST (2013) Anti-diabetic medications and risk of pancreatic cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Am J Gastroenterol 108 (4):510–519; quiz 520. doi: 10.1038/ajg.2013.7 [DOI] [PubMed] [Google Scholar]
- 16.Yu H, Yin L, Jiang X, Sun X, Wu J, Tian H, Gao X, He X (2014) Effect of metformin on cancer risk and treatment outcome of prostate cancer: a meta-analysis of epidemiological observational studies. PLoS One 9 (12):e116327. doi: 10.1371/journal.pone.0116327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decensi A, Puntoni M, Goodwin P, Cazzaniga M, Gennari A, Bonanni B, Gandini S (2010) Metformin and cancer risk in diabetic patients: a systematic review and meta-analysis. Cancer Prev Res (Phila) 3 (11):1451–1461. doi:1940-6207.CAPR-10-0157 [pii] 10.1158/1940-6207.CAPR-10-0157 [DOI] [PubMed] [Google Scholar]
- 18.Zhang P, Li H, Tan X, Chen L, Wang S (2013) Association of metformin use with cancer incidence and mortality: a meta-analysis. Cancer Epidemiol 37 (3):207–218. doi: 10.1016/j.canep.2012.12.009 [DOI] [PubMed] [Google Scholar]
- 19.Gandini S, Puntoni M, Heckman-Stoddard BM, Dunn BK, Ford L, DeCensi A, Szabo E (2014) Metformin and cancer risk and mortality: a systematic review and meta-analysis taking into account biases and confounders. Cancer Prev Res (Phila) 7 (9):867–885. doi: 10.1158/1940-6207.CAPR-13-0424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soranna D, Scotti L, Zambon A, Bosetti C, Grassi G, Catapano A, La Vecchia C, Mancia G, Corrao G (2012) Cancer risk associated with use of metformin and sulfonylurea in type 2 diabetes: a meta-analysis. Oncologist 17 (6):813–822. doi: 10.1634/theoncologist.2011-0462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suissa S, Azoulay L (2012) Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 35 (12):2665–2673. doi: 10.2337/dc12-0788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hung AM, Roumie CL, Greevy RA, Liu X, Grijalva CG, Murff HJ, Ikizler TA, Griffin MR (2012) Comparative effectiveness of incident oral antidiabetic drugs on kidney function. Kidney international 81 (7):698–706. doi: 10.1038/ki.2011.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roumie CL, Hung AM, Greevy RA, Grijalva CG, Liu X, Murff HJ, Elasy TA, Griffin MR (2012) Comparative effectiveness of sulfonylurea and metformin monotherapy on cardiovascular events in type 2 diabetes mellitus: a cohort study. Ann Intern Med 157 (9):601–610. doi: 10.7326/0003-4819-157-9-201211060-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roumie CL, Greevy RA, Grijalva CG, Hung AM, Liu X, Murff HJ, Elasy TA, Griffin MR (2014) Association between intensification of metformin treatment with insulin vs sulfonylureas and cardiovascular events and all-cause mortality among patients with diabetes. JAMA 311 (22):2288–2296. doi: 10.1001/jama.2014.4312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haynes K, Beukelman T, Curtis JR, Newcomb C, Herrinton LJ, Graham DJ, Solomon DH, Griffin MR, Chen L, Liu L, Saag KG, Lewis JD, Collaboration S (2013) Tumor necrosis factor alpha inhibitor therapy and cancer risk in chronic immune-mediated diseases. Arthritis Rheum 65 (1):48–58. doi: 10.1002/art.37740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrell FE (2001) Regression modeling strategies : with applications to linear models, logistic regression, and survival analysis Springer series in statistics. Springer, New York [Google Scholar]
- 27.V S (2002) Flexible Imputation of Missing Data. CRC Press; Taylor and Francis Group, Boca Raton, FL [Google Scholar]
- 28.Drugs and therapeutics committees: A practical guide (2003). World Health Organization: Department of Essential Drugs and Medicine Policy, Geneva, Switzerland [Google Scholar]
- 29.Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM (2005) Statins and cancer prevention. Nat Rev Cancer 5 (12):930–942. doi: 10.1038/nrc1751 [DOI] [PubMed] [Google Scholar]
- 30.Kuoppala J, Lamminpaa A, Pukkala E (2008) Statins and cancer: A systematic review and meta-analysis. Eur J Cancer 44 (15):2122–2132. doi: 10.1016/j.ejca.2008.06.025 [DOI] [PubMed] [Google Scholar]
- 31.Schneeweiss S, Glynn RJ, Tsai EH, Avorn J, Solomon DH (2005) Adjusting for unmeasured confounders in pharmacoepidemiologic claims data using external information: the example of COX2 inhibitors and myocardial infarction. Epidemiology 16 (1):17–24 [DOI] [PubMed] [Google Scholar]
- 32.Suissa S, Azoulay L (2014) Metformin and cancer: mounting evidence against an association. Diabetes Care 37 (7):1786–1788. doi: 10.2337/dc14-0500 [DOI] [PubMed] [Google Scholar]
- 33.Tsilidis KK, Capothanassi D, Allen NE, Rizos EC, Lopez DS, van Veldhoven K, Sacerdote C, Ashby D, Vineis P, Tzoulaki I, Ioannidis JP (2014) Metformin does not affect cancer risk: a cohort study in the U.K. Clinical Practice Research Datalink analyzed like an intention-to-treat trial. Diabetes Care 37 (9):2522–2532. doi: 10.2337/dc14-0584 [DOI] [PubMed] [Google Scholar]
- 34.Ampuero J, Romero-Gomez M (2015) Prevention of hepatocellular carcinoma by correction of metabolic abnormalities: Role of statins and metformin. World J Hepatol 7 (8):1105–1111. doi: 10.4254/wjh.v7.i8.1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pernicova I, Korbonits M (2014) Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol 10 (3):143–156. doi: 10.1038/nrendo.2013.256 [DOI] [PubMed] [Google Scholar]
- 36.Ray WA (2003) Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol 158 (9):915–920 [DOI] [PubMed] [Google Scholar]
- 37.Niesner K, Murff HJ, Griffin MR, Wasserman B, Greevy R, Grijalva CG, Roumie CL (2013) Validation of VA administrative data algorithms for identifying cardiovascular disease hospitalization. Epidemiology 24 (2):334–335. doi: 10.1097/EDE.0b013e3182821e75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu JW, Filion KB, Azoulay L, Doll MK, Suissa S (2016) Effect of Long-Acting Insulin Analogs on the Risk of Cancer: A Systematic Review of Observational Studies. Diabetes Care (39) (3):486–494. doi: 10.2337/dc15-1816 [DOI] [PubMed] [Google Scholar]
- 39.Karlstad O, Starup-Linde J, Vestergaard P, Hjellvik V, Bazelier MT, Schmidt MK, Andersen M, Auvinen A, Haukka J, Furu K, de Vries F, De Bruin ML (2013) Use of insulin and insulin analogs and risk of cancer - systematic review and meta-analysis of observational studies. Curr Drug Saf 8 (5):333–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


