Abstract
Thrombosis and bleeding are devastating adverse events in patients supported with blood contacting medical devices (BCMDs). In this study, we delineated that high non-physiological shear stress (NPSS) caused platelet dysfunction that may contribute to both thrombosis and bleeding. Human blood was subjected to NPSSwith short exposure time. Levels of platelet surface GPIbα and GPVIreceptors as well as activation level of GPIIb/IIIa in NPSS-sheared blood were examined with flow cytometry. Adhesion of sheared platelets on fibrinogen, von Willibrand factor (VWF) and collagen was quantified with fluorescent microscopy. Ristocetin- and collagen-induced platelet aggregation was characterized by aggregometry. NPSS activated platelets in a shear and exposuretime dependent manner. The number of activated plateletsincreased with increasing levels of NPSS and exposure time, which corresponded well with increased adhesion of sheared platelets on fibrinogen. Concurrently, NPSS caused shedding of GPIbα and GPVI in a manner dependent of shear and exposuretime. The loss of intact GPIbα and GPVI increased with increasing levels of NPSS and exposuretime. The number of platelets adhered on VWF and collagen decreased with increasing levels of NPSS and exposure time, respectively. The decrease in the number of platelets adhered on VWF and collagen corresponded well with the loss in GPIbα and GPVI on platelet surface. Both ristocetin- and collagen-induced platelet aggregation in sheared blood decreased with increasing levels of NPSS and exposure time.The study clearly demonstrated that high NPSS causes simultaneous platelet activation and receptor shedding, resulting in a paradoxical effect on platelet function via two distinct mechanisms. The results from the study suggested that the NPSS could induce the concurrent propensity for both thrombosis and bleeding in patients.
Keywords: blood contacting medical devices, non-physiological shear stress, platelet dysfunction, thrombosis, bleeding
Introduction
Blood contacting medical devices (BCMDs) are frequently used in device-assisted circulation to treat or replace diseased organs in human cardiovascular, pulmonary and renal systems [1]. Implantable ventricular assist devices (VADs) have emerged as a standard therapy for patients with advanced heart failure as a bridge to heart transplant or myocardial recovery and permanent support [2]. In recent years, there has also been a rapid growth with the use of extracorporeal membrane oxygenation (ECMO) in patients suffering from respiratory and/or cardiac failure [3]. Hemodialysis is a routine treatment for patients with kidney failure.Although BCMDs are accepted therapyin these standard clinical practices, BCMD-related complications remain a significant challenge for patient management and are associated with increased morbidity and mortality. Thromboembolism and bleeding are the most common adverse events associated with BCMD-assisted circulation [4–14]. Bleeding is often attributed to excessive anticoagulation.
High non-physiological shear stress (NPSS) commonly exists in some regions within BCMDs. For example, the levelof shear stress in the blade tip regions of continuous flow VADs and in the hinge regions of mechanical heart valves is well above 100 Pascal (Pa) and even reaches 500 Pa, which is much higher than physiological shear stress level.Blood in patients supported with BCMDs isinevitably subjected to this high level NPSS [15, 16].It is generally believed that thrombotic complications are initiated by non-physiological flow shear conditions and/or artificial surfaces of BCMDs. NPSS generated by BCMDs can induce activation of platelets and coagulation factors while proteins tend to deposit on artificial surfaces. These two conditions both increase the propensity of platelet aggregation and adhesion to artificial surfaces, leading to thrombus formation. Thus, a complex anticoagulant drug regimen is often prescribed to mitigate the thrombotic risk associated with artificial surfaces and device-induced activation of platelets and coagulation factors.With every anticoagulation approach to reduce thrombotic complications, however, there is an accompanying risk of increasing bleeding complications.Despite anticoagulation, the concurrent risk of thrombosis and bleeding has been reported in patients on VAD, cardiopulmonary bypass and ECMO[2, 9, 12, 17].
Platelets play a vital role in initiating normal hemostasis and pathophysiological thrombosis. The significance of shear stress in regulating platelet function and key signaling pathwaysin hemostasis and thrombosis has been extensively investigated[18, 19]. The shear-mediated surface interaction of platelet GPIbα with VWF(A1domain) immobilized on exposed subendothelial extracellular matrix at sites of vascular injury initiates a sequence of signaling events in platelets, resulting in the activation of the ligand binding function of the integrin GPIIb/IIIa. This interaction mediates stable platelet adhesion, aggregation, and thrombus formation [20–23]. NPSS alone in the absence of VWF in suspension can also induce platelet activation [24]. Several studies reported that pathological shear stress modulates a direct VWF-GPIIb/IIIa interaction, resulting in platelet aggregation, independent of VWF/GPIbα/GPIIb/IIIa axis [19, 25–28]. In the case of the BCMD-assisted circulation, platelets are exposed to both high NPSS and artificial surface concurrently. Although the precise mechanisms of BCMD-induced platelet activation and thrombosis are unknown, thrombi formed in stagnant flow areas within a BCMD and circulating activated platelets are common phenomena, which present the increased risk of thrombotic events in patients.
The past emphasis has been placed on anticoagulant-induced bleeding and shear-induced platelet activation towards increased propensity of thrombosis. Recent studies showed that NPSS can also cause shedding of two key platelet adhesion receptors (GPIbα, GPVI)[29–31]. The loss of GPIbα and GPVI receptors on the platelet surface may result in adiminishedhemostatic capacity for platelet adhesion and aggregation to VWF and collagen, leading the opposite effectsof normal hemostasis and increased risk of bleeding. In the present study, we examined the impact of high NPSS on platelet functionality towards the two opposite hemostatic phenomena: thrombosis and bleeding. Human citrated blood was subjected to NPSS (up to 300 Pa)with short exposure time (<1.5 sec) in a specially designed flow-through Couette-type viscometer. The shear conditions mimic the environment which blood cells would encounter in device-assisted circulation. Levels of platelet activation (GPIIb/IIIa) and shedding of platelet receptors (GPIbα, GPVI) in the sheared blood were quantified. Ristocetin- and collagen-induced aggregation capacities of the sheared blood were characterized. Platelet adhesion of the sheared blood on biologically relevant substrates (fibrinogen, VWF and collagen) was examined by perfusing the sheared blood through fibrinogen, VWF and collagen-coated capillary tubes.
Materials and methods
Blood Collection
Human blood was collected from six healthy volunteers (5 men and 1 woman with age range between 22 to 27 years) who had not taken any antiplatelet medication 10 days prior to blood donation. All the volunteers had given informed consent. The study protocol was approved by the Institutional Review Board (IRB) in accordance with the declaration of Helsinki. Blood (250 ml) drawn from an antecubital vein of the volunteers was directly collected into a sterilized collection bag mixed with sodium citrate (ratio: 9 to 1). Complete blood counts were assessed with a blood hematology analyzer (ABX Micros 60 CT, Horiba Medical, Irvine, CA) to ascertain thatthe collected blood sample was normal.
Blood-shearing device
Aspecially designed axial flow-through Couette-type device adapted from the adult Jarvik 2000 blood pump (Jarvik Heart, Inc., New York, NY, USA)was used as the blood-shearing device.The shape of the inner rotor was designed to be a spindleand auniform gap of 100 μm was formed between the spindle rotor and the outer housing (Figure 1). The detail of this device can be found in our previous work[32]. For the experiment, the blood was loaded into a syringe placed on a syringe pump (PHD 2000, Harvard Apparatus, Holliston, MA) and pressured to flow through the narrow gap, subjectingthe blood to a well-controlleduniform high shear stress andshort exposure time.
Figure 1.
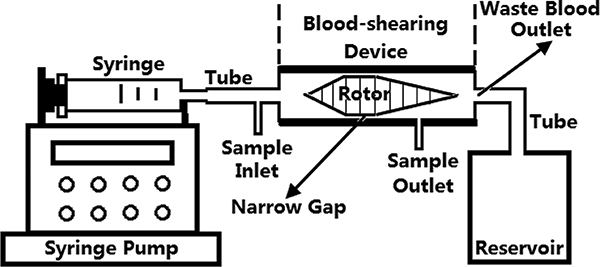
Blood shearing system. It includes a syringe pump, a syringe, a blood-shearing device (axial flow-through Couette-type device), a waste blood reservoir and connecting tubes. The syringe pump can be used to control the flow rate to obtain the desired exposure time.
Experimental procedure of blood-shearing experiments
The blood was measured by using a semi-micro viscometer (Cannon Instrument Company, State College, PA) before the blood shearing experiment. The expected levels of shear stress were generated by adjusting the rotational speed of the spindle rotorbased on the blood viscosity[32]. The desired exposure time was achieved by adjusting the axial flow rate.To mimic high NPSS relevant to by BCMDs and to examine the impact of high NPSS on the hemostatic function of platelets, two levels of high NPSS (150, 300 Pa) and two exposure times(0.5 and 1.5 sec.) were selected. For each combination of shear stress and exposure time, the sheared blood sample was taken from a small port at the sample outlet (Figure 1), locatedclosely to the narrow gap. The unsheared blood sample was takenat the inlet of the shearing device and used as the baseline sample for comparison with the sheared blood samples.
Flow cytometric analysis of platelet activation (GPIIb/IIIa activation) and shedding of platelet GPIbα and GPVI receptors
The activation of GPIIb/IIIa was used as the marker to quantify platelet activation.Antibodies used for measuring platelet activation included phycoerythrin (PE)-labeled anti-human CD41antibody (BioLegend, San Diego, CA) for platelet identification and isothiocyanate (FITC)-labeled anti-human PAC-1 antibody (BD Bioscience, San Jose, CA) for platelet activation. FITC labeled Mouse IgM, κ Isotype Control (BD Bioscience, San Jose, CA) was used as the negative control for PAC-1.
Antibodies used for measuring the shedding of platelet GPIbα and GPVIreceptors included PE-labeled anti-human CD41 antibody for platelet identification, FITC-labeled anti-human CD42bantibody (BioLegend, San Diego, CA) for GPIbα surface expression, and eFluor 660-labeled anti-human GPVIantibody (eBioscience, San Diego, CA) for GPVI surface expression. FITC-labeled Isotype control IgG1K antibody (BioLegend, San Diego, CA) and eFluor 660-labeled Isotype control IgG1K antibody (eBioscience, San Diego, CA) were used as the negative controls for CD42b and GPVI surface expression, respectively.The details ofthe sample preparation steps for flow cytometric analysis were the same with our previously study[33].
Platelet aggregation
Ristocetin- and collagen-induced platelet aggregation capacities of the baseline and sheared blood samples were analyzed using an impedance aggregometer, Multiplate® analyzer(VerumDiagnostica GmbH, Munich, Germany).Ristocetin causes platelet activation and aggregation by enhancing the binding between VWF and platelet surface receptor GPIbα. Collagen can bind directly to the platelet surface integrin α2β1 and GPVI, leading to platelet activation and aggregation.The Multiplate® analyzer is based on the principle that platelets become sticky upon activation, adhere and aggregate onto the metal sensor wires in the Multiplate® test cell, which increases the electrical resistance between the wires. The increase in impedance is expressed in aggregation units (AU) and can be plotted as separate aggregation curves over time. The area under the aggregation curve (AUC, AU*min) was used to indicate ristocetin- or collagen-induced platelet aggregation capacity of the baseline and sheared blood samples. The details ofthe aggregation experiment can be found in Tóth O’s study[34].
Platelet adhesion on biologically relevant proteins (fibrinogen, VWF and collagen)
The adhesion capacity of the baseline and sheared blood on biologically relevant proteins(VWF, collagen and fibrinogen) was examined using a flow-through perfusion assay system (Figure 2).Before the adhesion experiment, the glass tubes were coated with the proteins overnight in a humid environment at 4°C. The glass tubes were thenblocked with 5% BSA in phosphate buffer saline (PBS) at room temperature (RT) for 2 hours [35].To quantify the platelet adhesion on the proteins, the platelets inthe blood samples (baseline and sheared) after the shearing experiments werefluorescently labeled by incubation with mepacrine (quinacrine dihydrochloride,Sigma, St. Louis, MO). The blood sampleswere loaded into the syringe andperfused to flow through the three coated glass tubesunder pressure by using the syringe pump at a constant flow rate (0.4ml/min) for 5 minutes in the dark. The corresponding shear rate was 500 s−1at this flow rate, which is within the normal physiological range [36]. After the perfusion, PBS was used to wash the tubes gently and 3.7% paraformaldehyde solution (PFA) was used to fix the adherent platelets. Adhered platelets onthe protein substrates werevisualized using a fluorescent microscope(IX71, Olympus) equipped with an Olympus DP80 digital camera. Digital images wererecorded on the hard drive of a personal computer using the Olympus cellSens software (Olympus, Shinjuku-ku, Tokyo Japan).Tenimages with 2mm interval along the center axis of each glass tube were taken. The mean area coverage of adheredplatelets on each tube was quantified from the 10 images with custom-written software in MATLAB (Natick, MA,USA).
Figure 2.
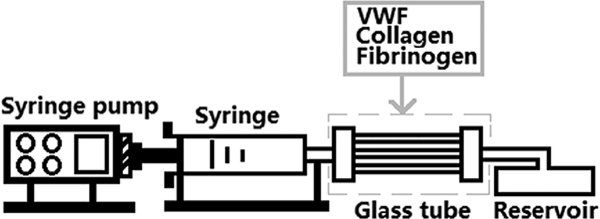
Platelet adhesion system. It includes a syringe pump which can be used to control the shear rate during the platelet adhesion, a syringe, three glass tubes (0.2mm x 2mm x 25mm, VitroCom, Mountain Lakes, NJ) coated with VWF (100μg/ml, EMD Millipore, Billerica, MA), collagen (1mg/ml, Chrono-log, Havertown, PA) and fibrinogen (1mg/ml, EMD Millipore, Billerica, MAf) respectively and a waste blood reservoir.
Statistical analysis
The data are presented as mean ± SE (standard Error of Mean)(n=6). Statistical differences were determined by using Student’s t-test. P< 0.05 was considered statistically significant.
Results
High NPSS with a short exposure time induces platelet activation leading to enhanced platelet adhesion on fibrinogen
In most early studies on shear-induced platelet activation, blood is often subjected to shear stresses less than 100 Pa for a duration (a few seconds to minutes)much longer than that in relevant clinical conditions. In contrary, NPSS encountered in BCMDs is often well above 100 Pa and the time of blood exposed to NPSS at hot spots is less than one second. To examine the effects of the high NPSS with a short exposure time on platelet activation, whole blood was subjected to two levels of high NPSS (150 Pa, 300 Pa) for 0.5 sec and 1.5 sec, respectively. The flow cytometric analysis of the sheared and baseline blood for platelet GPIIb/IIIa activation showed that high NPSS (>150 Pa) even with a short exposure time (<1.5 sec) could induce platelet activation (Figure 3A). The percentage of platelets with activated GPIIb/IIIa increased with increasing boththe levels of high NPSS and exposure time. For the high NPSS of 150Pa, the percentage of activated plateletsin the sheared blood became significantly different from that in the baseline blood when the exposure time was 1.5 sec.For the high NPSS of 300 Pa, the percentage of activated platelets in the sheared bloodincreased from 5.71% ± 0.65% to 21.85% ± 3.05% for the exposure times of 0.5 sec and 1.5 sec. After exposed to this level of high NPSS, platelet activation was significantly different from that in the baseline blood, even when the exposure time was 0.5 sec.
Figure 3.
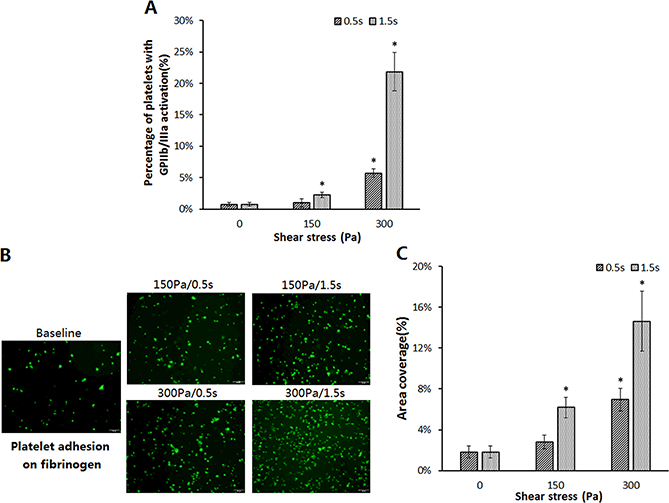
Comparison of platelet activation and platelet adhesion on fibrinogenin the baseline sample and sheared samples. (A)The percentage of activated platelets indicated by the GP IIb/IIIa activation in the baseline and sheared blood samples (n=6, *P<0.05);(B)Representative fluorescence images of platelet adhesion on fibrinogen from the baseline and four sheared samples (magnification, X400). All scale bars = 20μm; (C) The comparison of the area coverage (ten images taken from the each glass tube) of adherent platelets on fibrinogen for the baseline and four sheared samples (n=6, *P<0.05).
Once activated, GPIIb/IIIareceptorson platelet surface are capable of binding fibrinogen. It would be expected that the platelets in the sheared blood samples could be more readily adhere on the immobilized fibrinogen compared with the platelets in the baseline blood. To examine the adhesion capacity of the sheared platelets by high NPSS, the sheared blood was perfused over immobilized fibrinogen in the glass tube. Platelets adhered to fibrinogen were quantified.Figure 3B shows representative fluorescent images of platelets adhered on fibrinogen surface after perfusing the baseline blood sample and the four sheared blood samples over immobilized fibrinogen. It can be clearly seen from these images that much more platelets adhered on fibrinogen from the sheared blood samples compared to the baseline blood sample. The percentage of area coverage of platelet adhesion on fibrinogen was 1.8%±0.6% for the baseline sample. It increased to2.8%±0.7%, 6.2%±1.0%, 7.0%±1.1%, 14.6%±2.9% for the sheared blood sample under the shear conditions of 150Pa/0.5s, 150Pa/1.5s, 300Pa/0.5s, 300Pa/1.5s, respectively (Figure 3C). The area coverage of platelet adhesion on fibrinogen increased with increasing the levels of high NPSS and exposure time. The area coverage percentage of the sheared blood under the shear conditions of 150Pa/1.5s, 300Pa/0.5s and 300Pa/1.5s became significantly highercompared to that of the baseline blood.
High NPSS with a short exposure time induces the shedding of GPIbα and GPVI receptors on platelet surface leading to diminished platelet adhesion capacity on VWF and collagen
To examine the effect of the high NPSS with a short exposure time on the structural integrity of platelet surface GPIbα and GPVI receptors, flow cytometric assay was performed for the sheared and baseline blood samples. The flow cytometric analysis of the platelet GPIbα and GPVI receptors indicated that there was a significant reduction in the expression levels of both platelet GPIbα and GPVIreceptors in the sheared blood samples compared with the baseline sample. The peaks of the histograms of the channel fluorescence intensity for the two platelet receptors of the sheared blood samples shifted leftward (Figures 4A and4B). The higher the NPSS and the longer the exposure time were, the farther to the left the shift of the histogram peaks was. Correspondingly, the mean fluorescence intensity (MFI) for the surface expression of platelet GPIbα and GPVI receptors significantlydecreased in the sheared blood samples compared to the baseline blood sample (Figures 4C and4D). TheMFI reduction for GPIbα and GPVIindicated that there were less GPIbα and GPVIreceptors on the surface of platelets in the sheared blood, suggesting the loss of these two receptors on the platelet surface after the blood was exposed to high NPSS even for a short exposure time (<0.5 sec).
Figure 4.
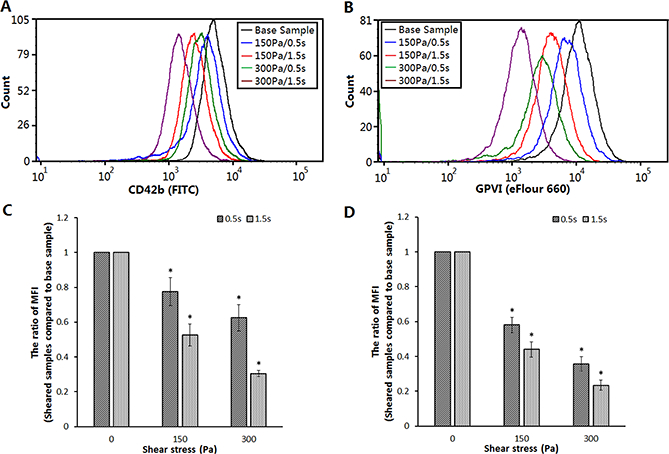
Comparison of GPIbα and GPVIsurface expressionin the baseline sample and sheared samples. (A) Typical histograms of channel fluorescence of the platelet GPIbα surface expression; (B) Typical histograms of channel fluorescence of the platelet GPVI surface expression; (C) The quantification of the platelet GPIbα surface expression in the baseline sample and sheared samples.The ratio of mean fluorescence intensity (MFI) using sheared samples to compare with baseline sample was taken to perform quantitative comparison (n=6, *P<0.05); (D) The quantification of the platelet GPVI surface expression in the baseline sample and sheared samples (n=6, *P<0.05).
The platelet GPIbα and GPVIreceptors are involved in the initial hemostatic response through their binding VWF and collagen, respectively. Their structural integrity is essential in the hemostatic process. To examine the impact of the high NPSS-induced loss of these two platelet receptors on platelet adhesion capacity on VWF and collagen, the sheared and baseline blood samples were perfused over VWF and collagen coated on the glass tubes. Then platelets adhered to VWF and collagen were quantified. Figures 5A and5C show representative fluorescent images of platelets adhered on VWF and collagen after the baseline blood sample and the four sheared blood samples were perfused over immobilized VWF and collagen. Unlike the platelet adhesion to fibrinogen, the numbers of platelets adhered to VWF and collagen decreased in the sheared blood samples compared to the baseline blood sample. For the baseline blood sample, the percentage of the platelet area coverage on VWF was 18.8%±1.9% (Figure 5B). It decreased to 13.7%±2.1%, 9.2%±1.3%, 12.2%±1.4% and 6.8%±1.3% after subjected to the shear conditions of 150 Pa/0.5s, 150Pa/1.5s, 300Pa/0.5s and 300Pa/1.5s. For the baseline blood sample, the percentage of the platelet area coverage on collagen was 19.9%±1.2%. It decreased to 15.2%±1%, 13.8%±1.2%, 13.6%±1.6% and 7.9%±0.7% after subjected to the four high NPSS conditions (Figure 5D). The decreased platelet adhesion capacity of the sheared blood was dependent on both the levels of high NPSS and exposure time. The decreases in the platelet adhesion capacity on VWF and collagen corresponded well to the decreases in the reduced surface levels of the platelet GPIbα and GPVI receptors in the sheared blood samples.
Figure 5.
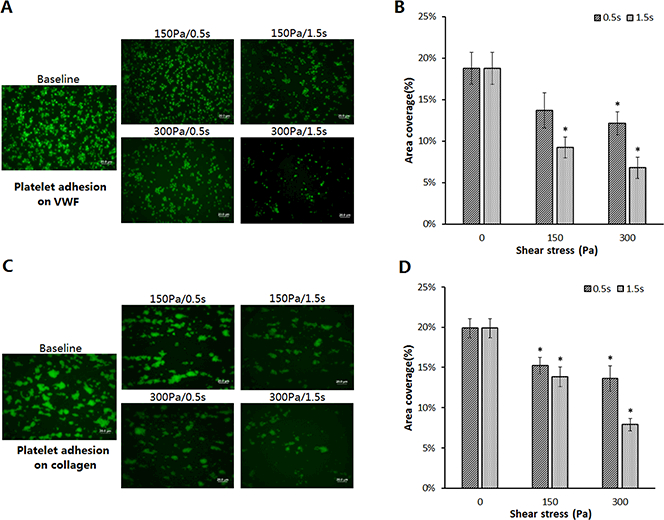
Comparison of platelet adhesion on VWF and collagenin the baseline sample and sheared samples. (A) Representative fluorescence images of adherent platelets on VWF in the baseline and four sheared samples (magnification, X400). All scale bars = 20μm; (B) The comparison of the area coverage (ten images of each glass tube) of adherent platelets on VWF for the baseline and four sheared samples (n=6, *P<0.05); (C) Representative fluorescence images of adherent platelets on collagen in the baseline and four sheared samples (magnification, X400). All scale bars = 20μm; (D) The comparison of the area coverage (ten images of each glass tube) of adherent platelets on collagen for the baseline and four sheared samples (n=6, *P<0.05).
High NPSS with a short exposure timecauses the reduction ofristocetin- and collagen-induced platelet aggregation
The loss of the platelet GPIbα and GPVIreceptors induced by high NPSS would also have an impact on the platelet aggregation capacity stimulated by GPIbα and GPVIagonists. Theristocetin- and collagen-induced platelet aggregation of the sheared and baseline bloodsamples wereexamined. By comparing the sheared blood sampleswith the baseline blood sample,the high NPSS (150 Pa and 300 Pa)with short exposure times (0.5 sec and 1.5 sec) causeda reduction inboth ristocetin- and collagen-induced platelet aggregation. Representative impedance change curves over time are shown in Figures 6A and 6C. These curves indicated that platelets in the sheared blood samples adhered and aggregated to the sensor wires slower than those in the baseline blood samples during the 6-minute duration after the blood samples were stimulated by ristocetin or collagen. The quantitative comparison of the ristocetin- and collagen-induced aggregation (AUC) between the baseline and sheared blood samples are shown in Figures 6B and 6D. The reductionin the AUC units for the ristocetin- and collagen-induced platelet aggregation of the sheared blood samples increased withincreasing the levels of high NPSS and exposure time.
Figure 6.
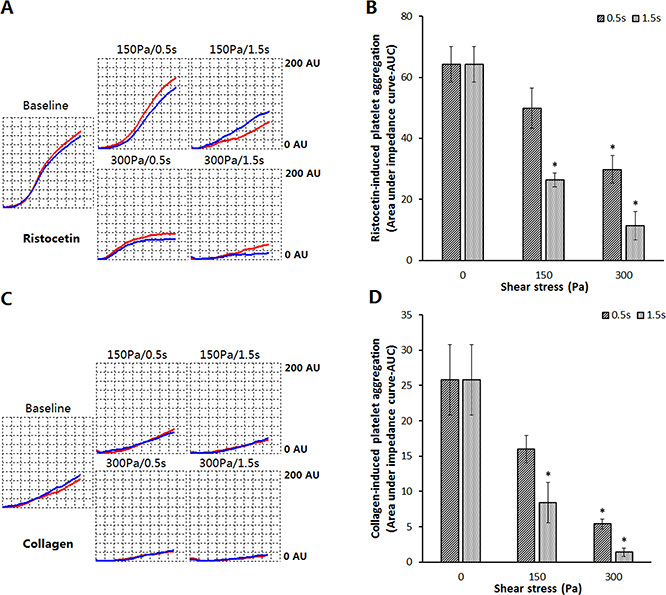
Comparison of platelet aggregation induced by ristocetin and collagen in the baseline sample and sheared samples. In this aggregation test, 300 μlof whole blood was mixed with 300 μl of saline solution with or without 3mM CaCl2intest cells and 50 μl of ristocetin (final concentration of 0.77mg/ml) or 25 μl of collagen (final concentration of 3.2ug/ml) was used to induce platelet aggregation. (A) The impedance curves of ristocetin-induced platelet aggregation of the baseline and four sheared samples; (B) The quantitative comparison of ristocetin-induced platelet aggregation of the baseline and sheared blood samples (n=6, *P<0.05); (C) The impedance curves of collagen-induced platelet aggregation of the baseline and four sheared blood samples; (D) The quantitative comparison of collagen-induced platelet aggregation of the baseline and sheared samples (n=6, *P<0.05).
For the high NPSS of 150Pa with the exposure time of 0.5 s, the AUC value for ristocetin-induced platelet aggregation decreased from 64.3±5.8 AU*min for to the baseline blood sample to 49.9±6.6AU*min. But the reduction did not reach statistical significance. At the exposure time of 1.5s, the platelet aggregation decreased significantlyto 26.4±2.2 AU*min. For the high NPSS of 300Pa, the AUCvalue forristocetin-induced platelet aggregation was 29.8±4.6 AU*min for 0.5s and 11.4±6.9 AU*min for 1.5s, respectively, which was significantly different from the AUC value for the baseline sample. Because ristocetin induces platelet aggregation by stimulating the binding of platelet GPIbα and VWF, a potential reason forthe reducedristocetin-induced platelet aggregation in the sheared samples may be the NPSS-induced shedding of the platelet GPIbα receptors.
For collagen-induced platelet aggregation, the AUC value of the baseline blood sample was 25.8±5.0 AU*min. The AUC values for collagen-induced platelet aggregation decreased to 16.0±1.9 AU*min and 8.4±2.9 AU*min for the shear conditions of 150Pa/0.5s and 150Pa/1.5s, respectively. The decrease was significant for the shear condition of 150Pa/1.5s compared to the baseline blood sample. For the shear conditions with 300Pa/0.5s and 300Pa/1.5s, the AUC values for collagen-induced platelet aggregation were 5.4±0.7 AU*min and 1.4±0.6 AU*min, respectively, which were statisticallysignificant reductions compared to the baseline value (Figure 6D). Since collagen can bind with the platelet GPVI to induce platelet aggregation, the shear-induced shedding of GPVI from the platelet surface may be the potential reason for the reduced collagen-induce platelet aggregation in the sheared blood samples. Theristocetin- and collagen-induced platelet aggregationfurther confirmed the shear-induced shedding of the platelet GPIbα and GPVI receptors.
Discussion
Thrombosis and bleeding are two clinically-significantcomplications in patients supported with BCMDs. Despites the use of anticoagulants in these patients, the concurrent occurrence of these two opposite hemostatic complications often happens. The mechanisms for the concurrent thrombosis and bleeding are not well understood and in critical need for effective patient management strategies. Since platelets play a critical role in pathophysiological thrombosis and hemostasis, the high NPSS-induced platelet dysfunction (activation andshedding of receptors) within BCMDs may be a key factor associated with these adverse events. To test this hypothesis, blood samples obtained fromhealthy donors wereexposed to the two levels of NPSS (150Pa and 300Pa) with two short exposure times (0.5s and 1.5s).These shear conditions mimic those generated by BCMDs implanted in patients. Our results show that the high NPSS at the level of 150 Pa even with a short exposure time can induce platelet activation.By perfusing the sheared and unsheared blood samples through fibrinogen-coated microcapillary tubes, it was found that the platelet adhesion to fibrinogen was higher in the sheared blood sample compared to the unsheared sample. Asthe high NPSS level and exposure time were increased, the number of activated platelets increased, subsequently resulting in more platelets adheredto fibrinogen.This phenomenon is logical because platelets with activated GPIIb/IIIa had high affinity with fibrinogen. The more plateletsbecame activated, the greater the number of plateletsadhered on fibrinogen.
Interestingly, the experimental results showed that high NPSS with a short exposure time could simultaneously induce the shedding of platelet GPIbα and GPVIreceptors. The reduction of GPIbα and GPVIreceptors on the platelet surface increased withincreasing the levels of NPSS and exposuretime. By perfusing the sheared and unsheared blood through VWF- and collagen-coated microcapillary tubes, the number of platelets adhered toVWF and collagen decreased with increasingthe high NPSS and exposure time. The decrease in platelet adhesion on these two surfaces corresponded well with the loss of GPIbα and GPVI on the platelet surface.The aggregation evaluation of the sheared blood further showed that both the ristocetin- and collagen-induced platelet aggregation in the sheared blood sample decreased with increasing the NPSS and exposure time. This further confirmed the high NPSS-induced loss of GPIbα and GPVI on the platelet surface.
Platelet adhesion to biologically-relevant substrates and aggregation are the essential function of platelets in physiological hemostasis and pathological thrombosis.Studies continue to showthe impact of high shear stresson platelet functionality. Most of early studies have focused on investigating platelet adhesion to hemostasis-related proteins (VWF, collagen, fibrinogen) under low shear conditions [37–40]. However, the high NPSS caused by BCMDs appears to have a much different effect on platelet function as platelets become activated or impaired and either adhere to the artificial surface or exit the BCMD and then flow to the microvasculature to fulfill their hemostatic function. The platelet adhesion occurs at the site of vascular injury under normal physiological range shear condition.The present study was designed to mimic this in vivo situation in which plateletsare subjected toNPSS generated by BCMDs implanted in patients and sheared platelets continue to circulate in the body vasculature. Our study demonstrated that high NPSS can induce the platelet activation and increase the platelet adhesion on fibrinogen. Clinically, platelets become activated after passing through the BCMDs and these platelets are more likely to adhere to fibrinogen deposited on the artificial surfaces of BCMDs or in stagnant regions in the circulation, such as bifurcation of blood vessels or stenosis. This phenomenon explains, in part, the increased risk of thrombus and occurrence of thrombotic events in BCMD patients. Simultaneously, the high NPSS can induce the loss of platelet GPIbα and GPVI receptors, leading to reducedplatelet adhesion capacity with VWF and collagen, respectively. For the in vivo condition of BCMD patients, the shedding of platelet adhesive receptorsmay happen because of the high NPSS created by BCMDs. The platelet adhesion capacity for normal hemostasiswasdiminished due to the receptor shedding of platelets. If there are minor injuries in the vasculature, such as gastrointestinal tract or surgical sites, the reduced hemostatic capacity of platelets may increase the risk of bleeding in BCMD patients. Therefore, in addition to the anticoagulant-induced bleeding, the NPSS-induced shedding of platelet adhesion receptors may be another important reason for the incidence of bleeding events in patients with BCMDs.
Conclusion
High NPSS causes platelet dysfunction that may lead to theconcurrent propensity for both thrombosis and bleeding via two distinct mechanisms. On the one side, high NPSS induces platelet activation and increases the platelet adhesion with fibrinogen, which mayincrease the thrombotic propensity. On the other side, high NPSS induces the shedding of platelet adhesion receptors (GPIbαand GPVI), resulting in the decreased capacity of platelet adhesion on VWF and collagen, which reduces the potential for normal hemostasis leading to the increased risk of bleeding events. This is a paradoxical phenomenon, which may explain why thromboembolic and bleeding events appear simultaneously in patients implanted with BCMDs.
Acknowledgments
The authors thank Jun Ding for assistance in the initial platelet adhesion experiment.
This work was funded in part by the National Institutes of Health (Grant number: R01HL124170).
Footnotes
Declaration of Interest statement
All of the authors declared no conflicts of interests.
References
- [1].Torregrossa G, Anyanwu A, Zucchetta F, Gerosa G. SynCardia: the total artificial heart. Ann CardiothoracSurg 2014;3:612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Baldwin JT, Young JB. Fifth INTERMACS Annual Report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. J Heart Lung Transplant. 2013;32:141–156. [DOI] [PubMed] [Google Scholar]
- [3].Raman L, Dalton HJ.Year in Review 2015: Extracorporeal Membrane Oxygenation. Respir Care 2016;61:986–91. [DOI] [PubMed] [Google Scholar]
- [4].Aagaard J The Carbomedics aortic heart valve prosthesis: a review. J CardiovascSurg (Torino) 2004;45:531–4. [PubMed] [Google Scholar]
- [5].Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, Song HK, Clough ER, Shore-Lesserson LJ, Goodnough LT, Mazer CD, Shander A, Stafford-Smith M, Waters J, Baker RA, Dickinson TA, FitzGerald DJ, Likosky DS, Shann KG. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann ThoracSurg 2011;91:944–82. [DOI] [PubMed] [Google Scholar]
- [6].Gupta P, McDonald R, Chipman CW, Stroud M, Gossett JM, Imamura M, Bhutta AT. 20-year experience of prolonged extracorporeal membrane oxygenation in critically ill children with cardiac or pulmonary failure. Ann ThoracSurg 2012;93:1584–90. [DOI] [PubMed] [Google Scholar]
- [7].Hering D, Piper C, Bergemann R, Hillenbach C, Dahm M, Huth C, Horstkotte D. Thromboembolic and bleeding complications following St. Jude Medical valve replacement: results of the German Experience With Low-Intensity Anticoagulation Study. Chest 2005;127:53–9. [DOI] [PubMed] [Google Scholar]
- [8].Himmelfarb J, Nelson S, McMonagle E, Holbrook D, Benoit SE, Michelson AD, Ault K. Elevated plasma glycocalicin levels and decreased ristocetin-induced platelet agglutination in hemodialysis patients. Am J Kidney Dis 1998;32:132–8. [DOI] [PubMed] [Google Scholar]
- [9].Lazar DA, Cass DL, Olutoye OO, Kim ES, Welty SE, Fernandes CJ, Lee TC . Venovenouscannulation for extracorporeal membrane oxygenation using a bicaval dual-lumen catheter in neonates. J PediatrSurg 2012;47:430–4. [DOI] [PubMed] [Google Scholar]
- [10].Mankad S Management of prosthetic heart valve complications. Curr Treat Options Cardiovasc Med 2012;14:608–21. [DOI] [PubMed] [Google Scholar]
- [11].Najjar SS, Slaughter MS, Pagani FD, Starling RC, McGee EC, Eckman P, Tatooles AJ, Moazami N, Kormos RL, Hathaway DR, Najarian KB, Bhat G, Aaronson KD, Boyce SW. An analysis of pump thrombus events in patients in the HeartWare ADVANCE bridge to transplant and continued access protocol trial. J Heart Lung Transplant 2014;33:23–34. [DOI] [PubMed] [Google Scholar]
- [12].Goldstein D, John R, Salerno C Increase in left ventricular assist device thrombosis. N Engl J Med 2014;370:1465–6. [DOI] [PubMed] [Google Scholar]
- [13].Eckman PM, John R. Bleeding and thrombosis in patients with continuous-flow ventricular assist devices. Circulation 2012;125:3038–47. [DOI] [PubMed] [Google Scholar]
- [14].Torregrossa G, Morshuis M, Varghese R, Hosseinian L, Vida V, Tarzia V, Loforte A, Duveau D, Arabia F, Leprince P, Kasirajan V, Beyersdorf F, Musumeci F, Hetzer R, Krabatsch T, Gummert J, Copeland J, Gerosa G. Results with SynCardia total artificial heart beyond 1 year. ASAIO J 2014;60:626–34. [DOI] [PubMed] [Google Scholar]
- [15].Fraser KH, Zhang T, Taskin ME, Griffith BP, Wu ZJ: A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. Journal of biomechanical engineering 134: 081002, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Anderson JB, Wood HG, Allaire PE, McDaniel JC, Olsen DB, Bearnson G: Numerical Studies of blood shear and washing in a continuous flow ventricular assist device. ASAIO J 46(4): 486–94, 2000. [DOI] [PubMed] [Google Scholar]
- [17].Ranucci M,Baryshnikova E,Castelvecchio S, Pelissero G Major bleeding, transfusions, and anemia: the deadly triad of cardiac surgery. Ann ThoracSurg 2013;96:478–85. [DOI] [PubMed] [Google Scholar]
- [18].Jurk K,Kehrel BE Platelets: physiology and biochemistry. SeminThrombHemost 2005;31:381–92. [DOI] [PubMed] [Google Scholar]
- [19].Quinn M, Fitzgerald D. Platelet function: assessment, diagnosis and treatment. Totowa, New Jersey: Humana Press; 2005. [Google Scholar]
- [20].Konstanantopoulos K, Wu KK, Udden MM, Banez EI, Shattil SJ, Hellums JD. Flow cytometric studies of platelet responses to shear stress in whole blood. Biorheology 1995;32:73–93. [DOI] [PubMed] [Google Scholar]
- [21].McCrary JK,Nolasco LH,Hellums JD,Kroll MH,Turner NA,Moake JL.Direct demonstration of radiolabeled von Willebrand factor binding to platelet glycoprotein Ib and IIb-IIIa in the presence of shear stress. Ann Biomed Eng 1995;23:787–93. [DOI] [PubMed] [Google Scholar]
- [22].Hellums JD, Peterson DM, Stathopoulos NA, Moake JL, Giorgio TD. Studies on the mechanisms of shear-induced platelet activation in: Hartman A, Kuschinsky W (Eds.) Cerebral Ischemia and Hemorheology Springer-Verlag, New York; 1987:80–89. [Google Scholar]
- [23].Reininger A, Heijnen HF, Schumann H, Specht HM, Schramm W, Ruggeri ZM. Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress. Blood 2006;107:3537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sheriff J, Bluestein D, Girdhar G, Jesty J. High-shear stress sensitizes platelets to subsequent low-shear conditions. Ann Biomed Eng 2010;38:1442–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Feng S, Lu X, Reséndiz JC, Kroll MH. Pathological shear stress directly regulates platelet alphaIIbbeta3 signaling. Am J Physiol Cell Physiol 2006;291:C1346–54. [DOI] [PubMed] [Google Scholar]
- [26].Goto S, Tamura N, Li M, Handa M, Ikeda Y, Handa S, Ruggeri ZM. Different effects of various anti-GPIIb-IIIa agents on shear-induced platelet activation and expression of procoagulant activity. J ThrombHaemost 2003;1:2022–30. [DOI] [PubMed] [Google Scholar]
- [27].Goncalves I, Nesbitt WS, Yuan Y, Jackson SP.Importance of temporal flow gradients and integrin alphaIIbbeta3mechanotransduction for shear activation of platelets. J BiolChem 2005;280(15):15430–7. [DOI] [PubMed] [Google Scholar]
- [28].Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL. Platelets and shear stress. Blood 1996;88:1525–1541. [PubMed] [Google Scholar]
- [29].Leytin V, Allen DJ, Mykhaylov S, Mis L, Lyubimov EV, Garvey B, Freedman J. Pathologic high shear stress induces apoptosis events in human platelets. BiochemBiophys Res Commun 2004;320:303–10. [DOI] [PubMed] [Google Scholar]
- [30].Al-Tamimi M, Tan CW, Qiao J, Pennings GJ, Javadzadegan A, Yong AS, Arthur JF, Davis AK, Jing J, Mu FT, Hamilton JR, Jackson SP, Ludwig A, Berndt MC, Ward CM, Kritharides L, Andrews RK, Gardiner EE. Pathologic shear triggers shedding of vascular receptors: a novel mechanism for down-regulation of platelet glycoprotein VI in stenosed coronary vessels. Blood 2012;119:4311–20. [DOI] [PubMed] [Google Scholar]
- [31].Chen Z, Mondal NK, Ding J, Gao J, Griffith BP, Wu ZJ.Shear-induced platelet receptor shedding by non-physiological high shear stress with short exposure time: Glycoprotein Ibalpha and glycoprotein VI. Thromb Res 2015;135:692–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhang T, Taskin ME, Fang HB, Pampori A, Jarvik R, Griffith BP, Wu ZJ. Study of Flow-Induced Hemolysis Using Novel Couette-Type Blood-Shearing Devices. Artificial organs 2011;35:1180–6. [DOI] [PubMed] [Google Scholar]
- [33].Chen Z, Mondal NK, Ding J, Koenig SC, Slaughter MS, Wu ZJ. Paradoxical Effect of Nonphysiological Shear Stress on Platelets and von Willebrand Factor. Artif Organs 2016;40:659–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Tóth O, Calatzis A, Penz S, Losonczy H, Siess W. Multiple electrode aggregometry: a new device to measure platelet aggregation in whole blood. ThrombHaemost 2006;96:781–8. [PubMed] [Google Scholar]
- [35].Cheng H, Yan R, Li S, Yuan Y, Liu J, Ruan C, Dai K. Shear-induced interaction of platelets with von Willebrand factor results in glycoprotein Ibalpha shedding. Am J Physiol Heart Circ Physiol 2009;297:H2128–35. [DOI] [PubMed] [Google Scholar]
- [36].Malek AM, Alper SL, Izumo S. Hemodynamic shear stress and its role in atherosclerosis. JAMA 1999;282:2035–42. [DOI] [PubMed] [Google Scholar]
- [37].Ruggeri ZM, Dent JA, Saldívar E. Contribution of distinct adhesive interactions to platelet aggregation in flowing blood. Blood 1999;94:172–8. [PubMed] [Google Scholar]
- [38].Reininger AJ, Heijnen HF, Schumann H, Specht HM, Schramm W, Ruggeri ZM. Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress. Blood 2006;107:3537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ruggeri ZM, Orje JN, Habermann R, Federici AB, Reininger AJ. Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 2006;108:1903–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Ruggeri ZM,Mendolicchio GL Adhesion mechanisms in platelet function. Circ Res 2007;100:1673–85. [DOI] [PubMed] [Google Scholar]


