Abstract
The inhibitory kappa B kinases (IKKs) and IKK related kinases are crucial regulators of the pro-inflammatory transcription factor, nuclear factor kappa B (NF-κB). The dysregulation in the activities of these kinases has been reported in several cancer types. These kinases are known to regulate survival, proliferation, invasion, angiogenesis, and metastasis of cancer cells. Thus, IKK and IKK related kinases have emerged as an attractive target for the development of cancer therapeutics. Several IKK inhibitors have been developed, few of which have advanced to the clinic. These inhibitors target IKK either directly or indirectly by modulating the activities of other signaling molecules. Some inhibitors suppress IKK activity by disrupting the protein-protein interaction in the IKK complex. The inhibition of IKK has also been shown to enhance the efficacy of conventional chemotherapeutic agents. Because IKK and NF-κB are the key components of innate immunity, suppressing IKK is associated with the risk of immune suppression. Furthermore, IKK inhibitors may hit other signaling molecules and thus may produce off-target effects. Recent studies suggest that multiple cytoplasmic and nuclear proteins distinct from NF-κB and inhibitory κB are also substrates of IKK. In this review, we discuss the utility of IKK inhibitors for cancer therapy. The limitations associated with the intervention of IKK are also discussed.
Keywords: Cancer therapy, Inhibitory kappa B kinase, IKK inhibitor, IKK related kinase, Nuclear factor kappa B
1. Introduction
The nuclear factor-κB (NF-κB) is an evolutionarily conserved proinflammatory transcription factor that was first identified by Sen and Baltimore in response to pathogens and viruses [1]. It plays a crucial role during development, differentiation, and various pathological conditions. Accumulating evidence suggests that NF-κB is a link between inflammation and cancer [1–10]. NF-κB is known to regulate inflammatory molecules such as adhesion molecules, chemokines, cyclooxygenase (COX)-2, interleukin (IL)-1, IL-6, 5-lipooxygenase (5-LOX), matrix metalloproteinases (MMPs), tumor necrosis factor (TNF), and vascular endothelial growth factor (VEGF), all of which are involved in tumor development. This has provided a molecular basis for the role of inflammation in cancer. In mammals, NF-κB is comprised of five subunits: NF-κB1 (p105/p50), NF-κB2 (p100/p52), c-Rel, RelA (p65), and RelB [11]. These subunits associate to form heterodimers or homodimers and regulate the expression of NF-κB dependent target genes [8,9]. Under normal conditions, NF-κB subunits reside in the cytoplasm in association with inhibitory κB (IκB) proteins (IκBα, IκBβ, IκBε, IκBNS) that control NF-κB activation [11–14].
Although more than 15 pathways have been reported for NF-κB activation, two most common pathways are canonical (classical) and noncanonical (alternative) pathways (Fig. 1) [15]. The canonical pathway is initiated by p105/p50, while the noncanonical pathway is initiated by p100/p52. The canonical pathway depends on IKKβ and the inhibitory subunit IκBs, whereas the alternative pathway depends on IKKα homodimers and NF-κB inducing kinase (NIK) [16–18]. In the canonical pathway, IKKβ specifically phosphorylates IκBα on two conserved N-terminal serine residues (Ser32/36). IκBα phosphorylation triggers the polyubiquitination (by E2- and E3-ligases) and subsequent degradation (by 26S proteasome) of IκBα [19]. IκBα degradation releases NF-κB dimers (p65/p50) in the cytoplasm. Subsequently, the dimer is translocated to the nucleus where it regulates the expression of NF-κB dependent target genes. The p65 subunit of NF-κB undergoes a series of posttranslational modifications including phosphorylation at Ser536 by IKK that is required for NF-κB’s full activity [20]. Conversely, p65 phosphorylation at Ser536 can also suppress NF-κB signaling leading to harmful inflammation as observed in mice model [21]. The activation of the alternative pathway, which is commonly associated with RelB, results in regulated processing of the p100 precursor protein to p52 and subsequent translocation of p52-RelB heterodimers to the nucleus [17]. Because of its central role in the NF-κB signaling pathway, IKK has been targeted by various means for cancer therapy. In the following sections, we discuss the IKK signaling pathway and the potential strategies for its intervention in cancer cells.
Fig. 1.
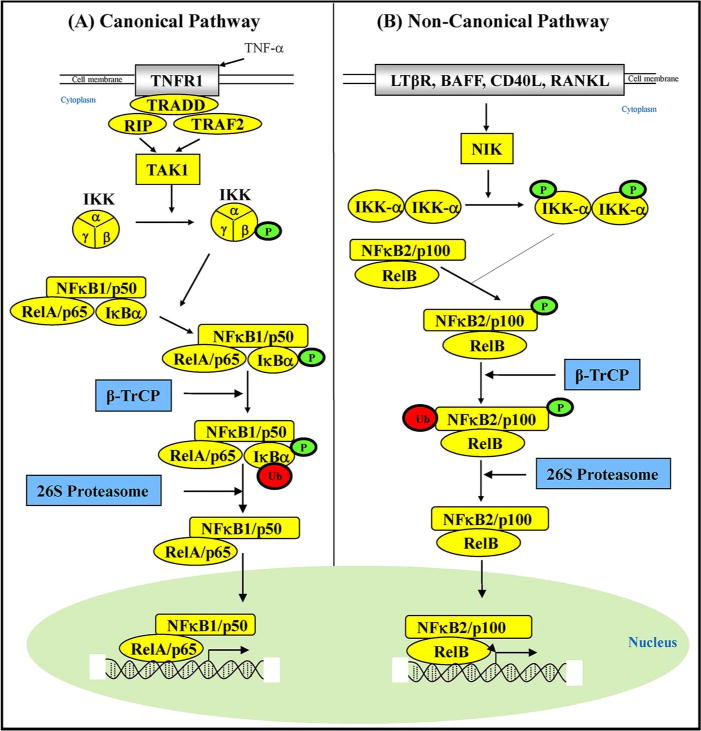
Common pathways of NF-κB activation.
2. IKK signaling
IKK is a trimeric complex consisting of IKKα, IKKβ, and NF-κB essential modulator (NEMO) or IKKγ [22–24]. Whereas IKKα and IKKβ subunits possess kinase activities, IKKγ is the regulatory subunit. An additional substrate-targeting subunit called ELKS may also be present in the IKK complex [25]. IKKβ is required for rapid degradation of NF-κB-bound IκBs. IKKα controls the processing of p100, leading to activation of p52: RelB dimers [26]. Both IKKα and IKKβ were identified as constituents of a 700- to 900-kD protein complex that exhibits TNFα-induced IKK activity [22,27]. In general, IKK can be activated through (i) direct phosphorylation of one of its catalytic subunits, (ii) trans-autophosphorylation; and (iii) conformational change induced by post-translational modification of its subunits [28]. IKKα and IKKβ were purified and cloned by their ability to phosphorylate IκB proteins in response to tumor necrosis factor-α (TNF-α) [27,29]. Although both IKKα and IKKβ possess considerable similarities, they have largely nonoverlapping functions because of the difference in their substrate specificity. The IKKβ-dependent pathway is essential for activation of innate immunity, whereas IKKα is important for regulation of adaptive immunity and lymphoid organogenesis [26].
Two other IKK-related kinases called IKKε (IKK-i) [30,31] and TBK1 [NF-κB-activated kinase (NAK) or TRAF2-associated kinase (T2 K)] have been identified [32–34]. Although both IKKε and TBK1 possess sequence similarity to IKKα and IKKβ, none of these kinases are part of the classical IKKγ-containing IKK complex [30,31,35]. IKK related kinases are known to play a crucial role in promoting transformation, survival, and proliferation of cancer cells [36–42]. IKKε is also a potential target for cancer immunotherapy [43]
The role of IKK in regulating cancer pathogenesis is reported from several lines of evidence. First, IKK is a regulator of NF-κB that controls various aspects of tumor development such as transformation, survival, proliferation, invasion, angiogenesis and metastasis [44,45] [46]. Second, the activation of the IKKs has been observed in both solid tumors and hematological malignancies such as breast cancer, prostate cancer, colorectal cancer, leukemia, lymphoma and multiple myeloma [47–50]. Third, epidemiological studies employing gene expression analyzes has revealed that IKK is a mediator of clear cell renal cell carcinoma overall survival [51]. Fourth, gene silencing of IKK subunits by siRNA or by pharmacological inhibitors is known to promote cell death and sensitize cancer cells to chemotherapeutic agents [47,52]. Fifth, IKKs may also regulate cell cycle progression independent of NF-κB [52]. Sixth, IKK is known to regulate cancer stem cell growth through TAK1 mediated TGF-β signaling [53]. Seventh, a mouse model of prostate cancer expressing an inactive form of IKKα showed delayed cancer onset, decreased distant-site metastases and an increase in the survival of mice [54]. The implication of all these suggests that IKK could be a potential target for cancer therapy [55,56]. We discuss the strategies used for IKK inhibition in the following section. The potential of IKK inhibitors for cancer therapy as evident from in vitro, in vivo and human studies, are discussed.
3. IKK inhibitors
Because IKK and IKK related kinases play a crucial role in the NF-κB activation pathway, several inhibitors have been developed against these kinases for cancer therapy. Since IKKβ is the primary regulator of NF-κB, most inhibitors are based on the modulation of the activity of this kinase. The efficacy of IKK inhibitors has been examined mostly in preclinical studies; a few have advanced to the clinic (Table 1). Although various IKK inhibitors have been developed, we discuss only important inhibitors in this review. These inhibitors are of varying nature such as natural products, proteasome inhibitors, viral components, peptides, synthetic agents, and many others (Fig. 2). Furthermore, IKK inhibitors are structurally diverse (Fig. 3).
Table 1.
IKK inhibitors and their potential in cancer therapy.
Specific IKK inhibitors
|
Natural products
|
Non-cancer agents
|
Peptides/Bio-molecules
|
Direct inhibitors
|
Combination therapy
|
Clinical studies
|
Miscellaneous
|
Fig. 2.
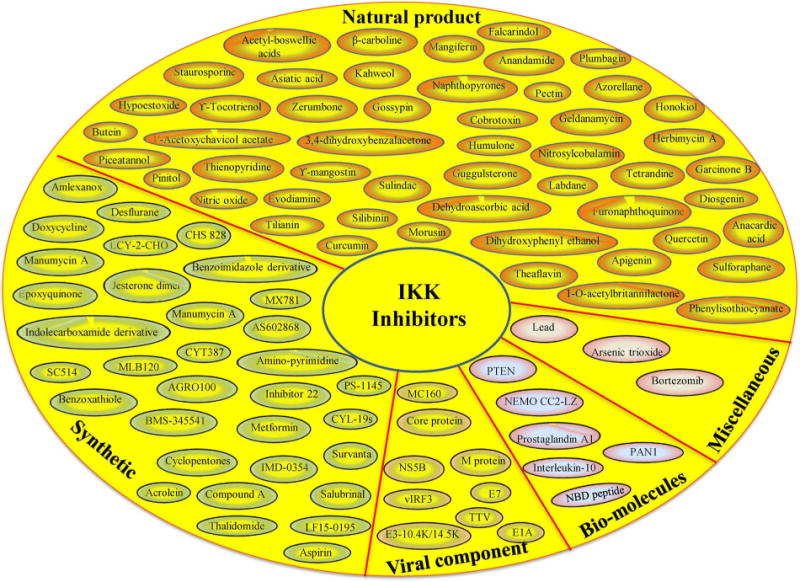
Common IKK inhibitors studied in cancer models. Abbreviations: AGRO100: G-quadruplexoligodeoxynucleotide; BMS-345541: N-(1,8-Dimethylimidazo[1,2-a]quinoxalin-4-yl)-1,2-ethanediamine; Compound A: fluoromethyl-2,2-difluoro-1-[trifluoromethyl]vinylether; CHS828: 2-[6-(4-chlorophenoxy)hexyl]-1-cyano-3-pyridin-4-ylguanidine; CYL-19s: α-methylenegamma-butyrolactone derivatives; CYT387: N-(cyanomethyl)-4-[2-(4-morpholin-4-ylanilino)pyrimidin-4-yl] benzamide; E7: human papillomavirus; IMD-0354: N-[3,5-bis(trifluoromethyl)phenyl]-5-chloro-2-hydroxybenzamide; LCY-2-CHO: 9-(2-chlorobenzyl)-9H-carbazole-3-carbaldehyde; LF15-0195: 2-[6-(diaminomethylideneamino)hexylamino]-2-oxoethyl] N-[4-[[(3R)-3-aminobutyl]amino]butyl]carbamate; MC160: Molluscum contagiosum 160; ML120B: N-(6-chloro-7-methoxy-9H-pyrido[3,4-b]indol-8-yl)-2-methylpyridine-3-carboxamide; NBD peptide: NEMO-binding domain peptide; NS5B: nonstructural protein 5B; PS-1145: N-(6-Chloro-9H-pyrido[3,4-b]indol-8-yl)-3-pyridinecarboxamide; PTEN: phosphatase and tensin homolog; SC-514: 3-Amino-5-thiophen-3-ylthiophene-2-carboxamide; TTV: torque teno virus; vIRF3: viral interferon regulatory factor 3.
Fig. 3.
Chemical structure of common IKK inhibitors.
3.1. Pre-clinical studies
3.1.1. Specific IKK inhibitors
Some specific inhibitors of IKK include BMS-345541, PS-1145, Bay 11-7085, IMD-0354, AS602868, and ACHP. BMS-345541 inhibits IKK activity selectively by targeting an allosteric site of IKKα (IC50: 4 μmol/L) and IKKβ (IC50: 0.3 μmol/L) [57]. Furthermore, this agent produces no inhibitory effects on a panel of 15 other kinases [57]. Interestingly, the agent was found to be safe in mice [58]. BMS-345541 can also suppress breast tumorigenesis and metastases by targeting GD2+ cancer stem cells [59]. In another study, BMS-345541 was found to reduce proliferation and induce apoptosis in PC-3 cells [60]. Furthermore, the level of phospho-IκBα and nuclear p65 was also suppressed by BMS-345541. This inhibitor also down-regulated mesenchymal markers, such as N-cadherin, Snail, Slug, and Twist, while up-regulating epithelial markers such as E-cadherin and phospho-NDRG1. The changes in molecular markers were associated with reduced invasion and metastasis of PC-3 cells. The authors of this study concluded that IKK plays a key role in inducing epithelial-to-mesenchymal transition (EMT) and apoptosis in prostate cancer cells. Moreover, BMS-345541 can reverse EMT phenotype in prostate cancer cells. BMS-345541 is also known to inhibit constitutive IKK activity and melanoma cell survival both by in vitro and in vivo studies [61]. Furthermore, BMS-345541 induced mitochondria-mediated apoptosis in melanoma cells [61].
PS-1145 is a derivative of β-carboline alkaloid with the potential of inhibiting IKKβ activity (IC50: 150 nM) without any effect on PKA, PKC and CKII activity. It can also suppress TNFα-induced IκBα phosphorylation and degradation and thereby inhibit NF-κB activation [62]. PS-1145 also induces multiple myeloma cell toxicity in the presence of TNFα [63]. The agent was selectively toxic to activated B-cell-like subgroup of diffuse large B-cell lymphoma [64]. BAY 11-7085, a specific IKK inhibitor was found to inhibit EMT and invasiveness in pancreatic cancer mice model [65]. This inhibitor can also significantly reduce the proliferation of ovarian cancer cells [66]. IMD-0354, a selective inhibitor of IKKβ is known to suppress neoplastic proliferation of human mast cells with constitutively active c-kit receptors [67]. It also induces G0/G1 cell cycle arrest and apoptosis in breast cancer cells [68].
Visfatin is a 52-kDa adipokine originally found in the visceral fat [69,70]. An increase in visfatin expression is positively correlated with tumorigenesis and/or metastasis of many cancer types [71]. This adi-pokine can discriminate between post-menopausal breast cancer patients at an early cancer stage and those at a late stage [72]. ACHP is a selective inhibitor of IKKα (IC50: 8.5 nM) and IKKβ (IC50: 250 nM). It inhibits NF-κB DNA binding activity, and induces cell growth arrest and apoptosis in multiple myeloma cell lines [73]. It was also found to block visfatin-induced NF-κB activation and up-regulation of matrix me-talloproteinase (MMP)-2 and MMP-9 in non-small cell lung cancer (NSCLC) [74]. Amlexanox is a selective inhibitor of TANK-binding ki-nase 1 (TBK1) and IKKε (IC50: ∼1–2 μM) [75]. Amlexanox interacts with human S100A4 protein [76], reduces proliferation, and induces G1-phase cell cycle arrest of melanoma cells [77]. Amlexanox is also known to selectively inhibit the viability of NSCLC cells with EGFR mutations [78]. In addition, some other specific IKK inhibitors include GSK 319347A for IKKε, and BI 605906 (IC50: 380 nM) [79], PF 184 (IC50: 37 nM) [80] and SC 514 (IC50: 3–12 μM) [81] for IKKβ.
It is clear from the above that the different subunits of IKK can be targeted in a specific manner. The specific IKK inhibitors exhibit activities at nanomolar to micromolar range. However, the evidence for the activities of these inhibitors is based on preclinical studies in animals and cell lines. Future studies should be focused to examine the clinical efficacy of these inhibitors.
3.1.2. Potential of natural products as IKK inhibitors
Because of their safety, affordability and being used since ancient time, agents derived from mother nature are preferred. These agents are called ‘nutraceutical’ (nutrition + pharmaceutical) and possess potential to inhibit IKK activity. Curcumin, a polyphenol derived from the golden spice (turmeric) is one of the most widely studied nutraceuticals. This polyphenol is known to exhibit activities against several cancer types [82]. Curcumin’s anti-inflammatory activity through modulation of NF-κB activation pathway was first reported in 1995 [83]. Furthermore, the polyphenol inhibits IKKβ activity by inducing its S-nitrosylation [84]. Artemisinin is a sesquiterpene lactone derived from a medicinal plant Artemisia annua. For its discovery, Dr. Tu Youyou shared Nobel Prize in Medicine in 2015. Artemisinin and its derivatives are now considered standard therapy for malaria. One study was aimed to explore the anti-inflammatory activity of artemisinin [85]. The sesquiterpene exhibited anti-inflammatory activities in 12-O-tetra-decanoylphorbol-13-acetate (TPA)-induced skin inflammation in mice. Mechanistically, artemisinin inhibited TNFα induced NF-κB reporter gene expression, phosphorylation and degradation of IκBα, and p65 nuclear translocation. Artemisinin treatment also inhibited the kinases upstream to IKK such as TNF receptor-associated factor 2 (TRAF2) and receptor interacting protein 1 (RIP1). Furthermore, artemisinin suppressed TNFα-induced NF-κB target genes involved in cell survival (c-IAP1, Bcl-2, FLIP), proliferation (COX-2 and cyclinD1), invasion (MMP-9), and angiogenesis (VEGF). Additionally, major inflammatory cyto-kines (TNF-α, iNOS, MCP1) were also suppressed by artemisinin treatment. Sesquiterpene also potentiated TNF-α-induced apoptosis. Moreover, reactive oxygen species (ROS) production and phosphorylation of p38 and ERK were significantly suppressed by artemisinin. However, the phosphorylation of JNK was unaffected. Collectively, these results suggest that artemisinin may be a potential therapeutic agent against inflammation, that is associated with cancer [85].
Mangiferin is a naturally occurring glucosyl xanthone derived from several folk medicines and foods including mango [72]. One study was aimed to evaluate the efficacy of mangiferin against tumor growth and metastasis in a mouse model of melanoma [86]. Mangiferin inhibited spontaneous metastasis and tumor growth, p65 nuclear translocation, and activation of the NF-κB-inducing kinase (NIK) and IKK. Additionally, mangiferin inhibited the expression of matrix metalloproteinases (MMPs) and very late antigens (VLAs) in vivo. Mangiferin also induced the cleavage of caspase-3 and poly ADP ribose polymerase (PARP). Furthermore, an up-regulation in p53 up-regulated modulator of apoptosis (PUMA), p53, and phosphorylated p53 proteins, along with down-regulation of survivin and Bcl-xL was observed after mangiferin treatment. The authors of this study concluded that mangiferin could be a potential therapeutic agent against NIK for the treatment of metastatic melanoma [86]. Betulinic acid, a triterpenoid was found to induce apoptosis in prostate cancer cells that was mediated through decreased phosphorylation of IKKα and IκBα, and inhibition of NF-κB p65 nuclear translocation [87]. In another study, betulinic acid inhibited lipopoly-saccharide (LPS)-triggered phosphorylation of IKK in CRC cells that contributed to its anti-cancer activities [88]. In addition to purified components, crude extract and powder from natural sources have also been shown to inhibit IKK activation pathways. For example, colorant powder from a perennial vine, Basella alba was found to suppress IKK activation induced by LPS in macrophages [89].
3.1.3. IKK inhibition by non-cancer agents
A potential strategy for cancer drug development is to evaluate the effectiveness of drugs approved for diseases other than cancer. This process called ‘drug repurposing’ is based on the fact that most human diseases share common pathways and thus one drug can be useful for more than one disease [90]. Doxycycline is an antibiotic used for the treatment of bacterial and protozoan infections. Originally used as an antibiotic, doxycycline can also inhibit LPS-induced IKKβ and NF-κB activation, and the expression of NF-κB dependent tumorigenic proteins in PC3 human prostate cancer cells [91]. Aspirin (acetylsalicylic acid) is one of the most commonly used non-steroidal anti-inflammatory drugs (NSAIDs). Originally used as an analgesic and as an anti-pyretic, aspirin has now been found to possess anti-cancer activities, particularly for colorectal cancer [90]. In a recent study, aspirin was found to directly inhibit IKK activity in cancer cells [92]. Aspirin can also suppress prostate cancer cell invasion by decreasing IKK-β-induced NF-κB activation that leads to a reduction in MMP-9 activity and uPA expression [93]. Arsenic has been used for more than 2400 years for some human ailments including ulcers, plague, and malaria [94]. It is now used for the treatment of acute promyelocytic leukemia [95]. In one study, arsenic was found to rapidly down-regulate constitutive IKK/NF-κB activity in Hodgkin/Reed-Sternberg (HRS) cell lines with functional IκB proteins [96]. This was concomitant with an increased apoptosis in HRS cell lines. Additionally, administration of arsenic trioxide dramatically reduced tumor formation in NOD/SCID mice xenotransplanted with L540Cy Hodgkin tumors.
3.1.4. IKK inhibition by peptides/bio-molecules
Another approach for suppressing IKK activity is by disrupting protein-protein interactions in the IKK complex. IKKγ (NEMO) interacts at the carboxyl-terminal of IKKα and IKKβ through NEMO-binding domain (NBD) that contains a conserved hexapeptide sequence (LDWSWL) [97]. Cell permeable peptides have been shown to disrupt protein-protein interactions in the IKK complex. For example, the cell permeable NBD peptide inhibited the association of NEMO with the IKK complex [97], and suppressed cytokine-induced NF-κB activation and its target gene expression. Furthermore, in mouse models of acute inflammation, NBD blocked TNFα- and PMA-induced NF-κB activation without inhibiting the constitutive NF-κB activity. NBD peptide can also inhibit osteoclastogenesis, which is a pathological hallmark of several chronic diseases including cancer [98].
Apart from peptides, other bio-molecules have also been used to inhibit protein-protein interaction in the IKK complex. For example, the protein pVHL is a product of the von Hippel-Lindau (VHL) tumor suppressor and functions as an adaptor of E3-ligase [99]. In one study, pVHL was found to inhibit NF-κB activation through K63-ubiquitination of IKKβ [74]. This lead to an inhibition of TAK1 binding and inhibition of IKKβ phosphorylation. Furthermore, prolyl hydroxylase activities were essential for pVHL-mediated K63-linked ubiquitination of IKKβ. Overall, these results suggest a function for pVHL in which it regulates IKK/NF-κB signaling by mediating IKKβ K63-ubiquitination. MC159 protein of Molluscum contagiosum virus (MCV) interacts with the NEMO [100]. This prevented the interaction of NEMO with the cIAP1 E3 ubiquitin ligase and thus inhibition of NEMO polyubiquitination and NF-κB activation. The inhibition of cIAP1-NEMO interactions could be used as a strategy to minimize IKK activation and to maximize anticancer activity.
The adenovirus E1A, a tumor suppressor, was found to inhibit TNFα-induced IKKβ activity that in turn leads to inhibition of IκBα degradation and NF-κB activation in cancer cells [101]. E1A has also been shown to inhibit radiation-induced NF-κB activation and to sensitize multiple cancer types to TNFα [102]. Viral IL-10 (vIL-10) is an Epstein-Barr virus homolog of human IL-10 (hIL-10). One study examined whether vIL-10 inhibits components of antigen processing machinery (HLA-I, LMP-2, LMP-7, TAP-1, and TAP-2) through the NF-κB signaling pathway in nasopharyngeal carcinoma cells [103]. An inhibition of NF-κB activation was observed by vIL-10 that was mediated through suppression of IKK phosphorylation. While TNF-α treatment led to a substantial translocation of NF-κB p65, pretreatment with vIL-10 blocked this translocation. vIL-10 also inhibited TNF-α-induced DNA-binding of NF-κB p65 in the nucleus. Chromatin immunoprecipitation (ChIP) assay demonstrated that NF-κB p65 could bind to the TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I gene promoters. Furthermore, vIL-10 was found to induce NF-κB-mediated down-regulation of TAP-1, TAP-2, LMP-2, LMP-7 and HLA-I transcription in nasopharyngeal carcinoma cells. It was concluded that the inhibition of NF-κB activity might be an important mechanism for vIL-10 suppression of transcription of the antigen processing machinery (HLA-I, LMP-2, LMP-7, TAP-1, and TAP-2) in nasopharyngeal carcinoma cells.
TNF receptor-associated factor 6 (TRAF6) is implicated in poly-ubiquitin-mediated IL1R/TLR signaling through activation of IKK to regulate NF-κB and JNK signaling pathways. In one study, higher expression of TRAF6 was observed in bone marrow mononuclear cells from patients with multiple myeloma [104]. TRAF6 was significantly elevated in BMMCs from patients with progressive disease. Furthermore, a reduction in the activation of IKK and cellular growth, and an increase in the apoptosis of multiple myeloma tumor cells was observed by the use of TRAF6 dominant-negative (TRAF6dn) peptides. Finally, TRAF6dn peptide dose-dependently inhibited RANKL- and mCSF-induced osteoclast formation from CD14+ monocytes and markedly reduced bone resorption in dentin pits. Collectively, these data demonstrate that blocking TRAF6 signaling is associated with anti-multiple myeloma effects and reduces bone loss. Thus, targeting TRAF6 signaling and associated pathways could be a promising approach for multiple myeloma therapy.
One study assessed the role of IKKα in melanoma patients [105]. The expression of IKKα and overall activation of NF-κB were heterogeneous. Furthermore, IKKα-specific p100/p52 processing was detected in a small subset of melanoma patients as well as in melanoma cell lines. Down-regulation of IKKα by siRNA was associated with a reduction in doxorubicin-induced NF-κB activation. Furthermore, constitutive and TNFα-stimulated expression of CXCL8 and ICAM-1, and cell migration was also reduced by IKKα gene silencing. In contrast, overexpression of IKKα in melanoma cell lines did not significantly affect progression-related functions. The authors of this study concluded that IKKα may be a potential target only in some individuals but not for general melanoma therapy.
3.1.5. Direct inhibitors of IKK
A cysteine residue at position 179 in the activation loop is critical for IKKβ activity. Some agents are known to inhibit IKK activity by directly targeting this residue. Epoxyquinone A monomer (EqM) is a synthetic derivative of epoxyquinol A. EqM is a potent inhibitor of TNF-α-induced NF-κB activation. EqM targets Cys179 of IKKβ to inhibit IκBα phosphorylation and degradation. Furthermore, inhibition of NF-κB activity by EqM is associated with its anti-growth effects on colon, kidney, and leukemia cancer cells [106]. Cys179 of IKKβ is targeted by several other agents, such as arsenite [107], prostaglandins [108], nimbolide [15], nitric oxide [109], butein [110], and cobrotoxin [111].
3.1.6. Utility of IKK inhibition in combination therapy
A major obstacle in the cancer therapy is that although in the beginning cancer cells respond to drugs, over time, these cells develop resistance to therapy partly owing to the development of resistance mechanism. For example, 5-fluorouracil (5-FU) is a pyrimidine analog that can induce apoptosis in salivary gland cancer cells through inhibition of IKK activity [112]. Conversely, 5-FU was found to induce IKK-mediated NF-κB activity in colorectal cancer (CRC) cells [70]. The inhibition of IKK can enhance the efficacy of conventional chemotherapeutic agents. For example, AS602868 is a specific inhibitor of IKKβ that can induce apoptosis in human primary AML cells [113]. AS602868 also enhanced the apoptotic effects of chemotherapeutic agents, such as doxorubicin, cytarabine, and etoposide [113]. Similarly, a combination of TRAF6 dominant-negative (TRAF6dn) peptide with bortezomib or carfilzomib is associated with an enhanced anti-multiple myeloma effects [104]. Bay 11-7085, in combination with vorinostat significantly reduced tumor growth in ovarian cancer mice model [114]. Because HDAC inhibitors can activate NF-κB activation pathway [115], use of IKK inhibitors can reverse the effects of HDAC inhibitors. Thus, a combination of IKK and HDAC inhibitors could be an ideal strategy to fight ovarian cancer. However, further animal studies are required to support these conclusions.
TPCA-1 is a potent and selective inhibitor of IKKβ (IC50: 17.9 nM). It exhibits more than 22- and 550-fold selectivity over IKKα and other kinases, respectively [116]. Vesicular stomatitis virus (VSV) based recombinant viruses such as VSV-ΔM51, are effective oncolytic viruses (OVs) against a majority of pancreatic ductal adenocarcinoma (PDAC) cell lines. However, some PDAC cell lines are highly resistant to VSV-ΔM51. TPCA-1 can break the resistance of PDAC cells to VSV-ΔM51 [117,118]. In combination with tyrosine kinase inhibitors (TKI), TPCA-1 produces synergistic effects on non-small cell lung cancer (NSCLC) [119]. IKK-related kinases promote KRAS-driven tumorigenesis. CYT387, a potent JAK/TBK1/IKKε inhibitor is known to impair KRAS-driven murine lung cancer growth [120]. CYT387 in combination with mitogen-activated protein kinase (MAPK) inhibition regresses aggressive KRAS mutant and p53 null lung adenocarcinomas in mice. These observations suggest that simultaneous inhibition of TBK1/IKKε, JAK, and MEK signaling could be an effective treatment in oncogenic KRAS-driven lung adenocarcinoma [120]. Mucoepidermoid carcinoma (MEC) is the most common type of salivary gland cancer [121,122]. MEC is associated with low survival rates and high morbidity. Although ionizing radiation (IR) is the first line option for MEC, patients frequently develop resistance to IR over time. In one study, pharmacological inhibition of IKK-β/IκBα/NFκB axis, using a single dose of emetine was found to sensitize MEC cells to IR [123]. Similarly, combining amlexanox with MEK inhibitor AZD6244 was found to significantly inhibit the xenograft tumor growth of NSCLC cells harboring EGFR mutations [78]. Thus, inhibition of IKK could be a novel approach to overcome radioresistance in MEC cells.
Overall, studies mentioned above suggest that the inhibition of IKK can be helpful to combat chemoresistance. However, comprehensive pre-clinical studies are warranted before this strategy can proceed for human clinical trials.
3.2. Clinical studies
Although several agents have shown efficacy against IKK by preclinical studies, the efficacy of IKK inhibitors in human is unclear. Only few IKK inhibitors have been tested in clinical trials for human diseases. Bortezomib (Velcade/PS-341) is one of the most successful inhibitor approved by United States Food and Drug Administration (FDA) for the treatment of relapsed and newly diagnosed multiple myeloma (MM) patients [124–129]. An inhibitor of 20S proteasome, bortezomib is known to suppress NF-κB activation in an indirect manner. Bortezomib is also useful in patients with mantle cell lymphoma [130], acting at least in part by inhibiting NF-κB activation [131,132]. Because IκBα subunit of NF-κB is a substrate of the proteasome, the initial rationale for the use of bortezomib in MM patients was through its inhibition of IκBα degradation. Indeed, bortezomib has been reported to inhibit inducible NF-κB activation [133,134]. However, in MM cell lines and primary tumor cells from MM patients, bortezomib significantly down-regulated IκBα expression and induced NF-κB activation [135]. Furthermore, bortezomib was found to trigger phosphorylation of IKKβ and its upstream receptor-interacting protein 2. The use of MLN120B, an IKKβ inhibitor, was found to abrogate IκBα down-regulation and NF-κB activation induced by bortezomib [135]. Moreover, IKKβ inhibitor enhanced bortezomib-induced cytotoxicity [135]. A phase II clinical trial was designed to examine the effectiveness of bortezomib in treating patients with metastatic or recurrent colorectal cancer (www.clinicaltrials.gov). A total of 21–41 patients within 2–4 months were recruited in the study. As of 18th Feb 2018, the study was completed. However, the results are yet to be published, to our knowledge. Overall, these studies suggest that depending on the cellular context, bortezomib can inhibit as well as induce NF-κB activation in MM cells.
Denosumab is a monoclonal antibody against RANKL. It is the first RANKL inhibitor to be approved by FDA for use in postmenopausal women with risk of osteoporosis [136]. Denosumab has also been approved for the prevention of skeleton-related events in patients with bone metastases from solid tumors. It has been shown to indirectly inhibit IKK pathway through modulation of RANKL/RANK pathway [137,138]. Brentuximab vedotin is an antibody-drug conjugate (ADC) with a potential to target CD30, which is one of the surface antigens expressed in lymphoma cells. Brentuximab vedotin is approved for the treatment of relapsed or refractory Hodgkin lymphoma (HL) and systemic anaplastic large cell lymphoma (ALCL). A member of the TNFR superfamily, CD30 is involved in the activation of the NF-κB pathway [139]. Thus, Brentuximab vedotin could also indirectly inhibit the IKK/NF-κB activation pathway. However, whether these agents modulate IKK activity in cancer patients remains unexplored.
3.3. Miscellaneous studies
BX-795 is a pharmacological inhibitor identified against IKKε/TBK1 [140]. BX-795 can also target Aurora B and phosphoinositide-dependent kinase-1 (PDK1). To our knowledge, the anti-tumor activity of BX-795 has not been explored. A derivative of 6-Aminopyrazolopyrimidine was identified as an inhibitor of TBK1 kinase activity from a library of 250,000 compounds [141]. This pharmacological inhibitor is reported toxic to TBK1-dependent cancer cell lines. However, the efficacy of this inhibitor has not yet been explored in vivo. Overall, these studies suggest that non-canonical IKKs could be targeted for cancer therapy. However, non-canonical IKKs are known to regulate the production of Type 1 interferons during bacterial and viral infection [140] Thus, systemic inhibition of IKKε/TBK1 may lead to compromised anti-bacterial and anti-viral responses.
IKKα shuttles between the cytoplasm and the nucleus. The nuclear IKKα is known to regulate the expression of several genes including Maspin (mammary serine protease inhibitor or SerpinB5), a tumor suppressor whose expression is negatively regulated by nuclear IKKα [54]. One study explored the ability of a glucosamine derivative, 2-(N-carbobenzyloxy)l-phenylalanylamido-2-deoxy-β-D-glucose (NCPA) to stimulate the production of Maspin in osteosarcoma cells [142]. NCPA inhibited IKKα nuclear translocation and stimulated Maspin production. Furthermore, Maspin production was accompanied by down-regulation in the expression of β1 integrin, MMP-9, MMP-13, and reduced cell migration. In summary, these results suggest that inhibition of IKKα by NCPA contribute to its activities against osteosarcoma.
(E)-3-(3,5-dimethoxyphenyl)-1-(2-methoxyphenyl)prop-2-en-1-one [DPP23], the derivative of a synthetic polyphenol induces apoptosis in cancer cells through the unfolded protein response in a selective manner. One study evaluated the potential of DPP23 on tumor invasion and metastasis [143]. This polyphenol inhibited TNF-α-induced motility, F-actin formation, and the invasive capability of MDA-MB-231 cells. The polyphenol also inhibited NF-κB-dependent MMP-9 expression at the transcriptional level. Furthermore, DPP23 inhibited IKK and AKT activation; the gene silencing of AKT2 significantly suppressed TNF-α-induced IKK phosphorylation. Collectively, these results suggested that DPP23 can prevent TNF-α-induced invasiveness of MDA-MB-231 breast cancer cells by inhibiting AKT-IKK-NF-κB-mediated MMP-9 expression. In addition, DPP23 attenuated liver metastasis in syngeneic mice model bearing 4T1 mammary carcinoma cells. The authors of this study concluded that DPP23 possess the potential for the prevention of invasion and metastasis or as an adjuvant for breast cancer chemo/radiotherapy. However, further studies are required to support these claims.
An analysis performed in 288 human colorectal cancer (CRC) samples revealed a significant association between nuclear IKK and malignancy [144]. Importantly, tumor cells were characterized by the presence of an active truncated nuclear IKKα with a predicted molecular weight of 45 kDa (p45-IKKα). The active nuclear p45-IKKα was found to possess kinase domain but lacked several regulatory regions. Furthermore, nuclear p45-IKKα formed a complex with nonactive IKKα and NEMO that mediates phosphorylation of SMRT and histone H3. Interestingly, proteolytic cleavage of full-length IKKα into p45-IKKα was associated with suppressed apoptosis of CRC cells in vitro and sustained tumor growth in vivo. Overall, these results identified a potential drug target for treating patients with advanced refractory CRC. In a subsequent study, activation of p45-IKKα was observed downstream of mutant KRAS and BRAF proteins [145]. Interestingly, activation of p45-IKKα was independent of the NF-κB pathway but was dependent on the endosomal compartment. Accordingly, chloroquine or bafilomycin A1 (inhibitors of endosomal acidification) completely suppressed p45-IKKα phosphorylation without affecting the activity of the NF-κB pathway. Using orthotopic xenografts of CRC, an enhancement in the anti-tumor effects of conventional chemotherapy (irinotecan, 5-azacytidine) was observed by bafilomycin A1 and chloroquine treatment. Most notably, the metastatic capacity of CRC cells was completely suppressed by the combined treatment. Overall, these results suggest that NF-κB-independent functions of IKK can be targeted for therapeutic intervention. Moreover, other endosomal-dependent pathways such as Notch [146] and Wnt [147] can also be inhibited by blocking the endosomal functions.
CHS 828 is a pyridyl cyanoguanidine known to exert antitumor activities both by in vitro and in vivo studies [148]. This cyanoguanidine is in phase I/II clinical trials [148,149]. CHS 828 was found to inhibit LPS-induced nuclear localization as well as the transcriptional activity of NF-κB in human THP-1 leukemia cells [50]. Furthermore, CHS 828 inhibited IKK activity (IC50: 8 nM) that correlated with its anti-proliferative effect in lung cancer cells.
In general, agents with the potential to inhibit IKK possess anticancer activities. However, some anti-cancer agents are known to activate IKK. For example, UV and ionizing radiation, used commonly in cancer therapy can activate the NF-κB pathway in an IKKβ-dependent manner [150]. This relies on the nuclear translocation, sumoylation and ataxia telangiectasia mutated (ATM) mediated phosphorylation of NEMO scaffolding protein [151]. The de-sumoylation and ubiquitylation of NEMO can facilitate the nuclear export of an NEMO-ATM complex and activation of the IKK complex, chiefly IKKβ [151]. However, it is unknown if IKK is activated in response to all forms of radiation in all cancer types. It is also not known if IKKβ is the predominant kinase activated in response to UV and ionizing radiation. However, the non-canonical NF-κB activation pathway has been implicated in mediating survival of endometrial carcinoma cells in response to ionizing radiation [152]. Curdlan is a 1,3-β-D-glucan produced by the bacterium Alcaligenes faecalis. Curdlan is approved by United States FDA for use in foods as a formulation aid, processing aid, stabilizer, thickener, or texturizer. Despite having antitumor activities [153], curdlan is also known to induce phosphorylation of IKK in dendritic cells [154].
4. Conclusions
IKK plays a crucial role in NF-κB activation pathway and cancer pathogenesis and thus represents a potential target for therapeutic intervention. Several inhibitors have been developed based on the IKK-NF-κB pathway, few of which have advanced to the clinic. These inhibitors target IKK directly or indirectly. Some inhibitors suppress IKK activity by disrupting protein-protein interaction in the IKK complex. IKK inhibitors have also been shown to enhance the efficacy of conventional chemotherapeutic agents. Although IKK inhibitors have shown promise for cancer therapy, there are certain limitations associated with the intervention of IKK that deserve attention.
First, although several inhibitors have been developed, only a few have been tested in the clinic. Future studies should be focused on evaluating the clinical efficacy of more IKK inhibitors. Second, cancer cells are intelligent and can compensate for the loss of IKK by adapting to alternate survival signaling pathways. This may lead to the attenuation of the efficacy of kinase inhibition. For example, loss of TBK1, an IKK related kinase leads to compensatory activation of receptor tyrosine kinases in lung cancer cells [155]. Thus, a co-targeting strategy could be helpful in these situations. Geldanomycin is an antibiotic with ability to block both IKKα/β and EGFR pathways [156]. This antibiotic is reported to be more active than IKKβ-specific inhibitors in suppressing NF-κB activation and proliferation and inducing cell death in head and neck cancer [156]. Similarly, targeting IKKα and the proteasome can increase efficacy of bortezomib in androgen-independent prostate cancer cells [157]. Third, IKK and NF-κB are the key components of innate immunity; the inhibition of these pathways may lead to compromised immune response. For example, systemic inhibition of IKKε/TBK1 may lead to compromised anti-viral responses. Future efforts should be focused on targeting IKK in a cancer-specific manner. Fourth, although much is known about IKK signaling, more and more IKK-associated signaling molecules continue to emerge that leads to a challenge in targeting this pathway. Fifth, several NF-κB independent substrates of IKK such as p53, TSC1, and FOXO3a, have been identified. In fact, IKK is also known to limit tumor progression and inflammation by targeting proteins other than IκBα [158]. IKKα/β can control biliary homeostasis and hepatocarcinogenesis in mice by phosphorylating the cell-death mediator receptor-interacting protein kinase 1 (RIPK1) [159].
In conclusion, several inhibitors have demonstrated potential against IKK by preclinical studies. However, none has been reported to directly inhibit IKK in humans. Several factors such as dual nature of IKK, lack of reliable pharmacokinetics/pharmacodynamics data for inhibitors, and redundancy in the kinase pathways may contribute to the failure of IKK inhibitors in humans. It is our hope that ongoing studies across the world will help to produce novel IKK inhibitors for human use in the coming years.
Acknowledgments
Authors wish to thank Miss Shruti Mishra for critically reading the article. Dr. Gupta’s laboratory is supported by Science and Engineering Research Board (ECR/2016/000034), University Grants Commission [No. F. 30-112/2015 (BSR)], and Design and Innovation Center (DIC-BHU/Project Approval/2015-16/608). Dr. Challagundlas’s laboratory is supported in whole or part from the National Institutes of Health (NIH) grant 1K22CA197074-01; the Nebraska State Department of Health & Human Services (LB506); an Institutional Development Award (IDeA) from the NIGMS/NIH (P30 GM106397); and the Biochemistry and Molecular Biology Department and the Fred and Pamela Buffet Cancer Center at University of Nebraska Medical Center. Dr. Chauhan’s laboratory is supported by grants from the National Institute of Health (R01CA210192, R01CA206069, R01CA204552) and from Kosten Foundation for pancreatic cancer research (UT 14-0558).
Abbreviations
- ACHP
(2-amino-6-[2(cyclopropylmethoxy)-6-hydroxyphenyl]4-piperidin-4-yl nicotinonitrile
- Bay11-7085
N-(6-Chloro-9H-pyrido[3,4-b]indol-8-yl)-3-pyridinecarbox-amide
- BMS-345541
N′-(1,8-dimethylimidazo[1,2-a]quinoxalin-4-yl)ethane-1,2-diamine
- BX-795
N-(3-(5-Iodo-4-(3-(thiophene-2-carboxamido)propylamino)pyrimidin-2-ylamino) phenyl)pyrrolidine-1-carboxamide
- CHS828
N-(6-(4-chlorophenoxy)hexyl)-N′-cyano-N″-4-pyridyl guanidine)
- CYT387
N-(cyanomethyl)-4-[2-(4-morpholin-4-ylanilino)pyrimidin-4-yl] benzamide
- CKII
casein kinase II
- CRC
colorectal cancer
- CXCL8
C-X-C motif chemokine ligand 8
- DPP23
(E)-3-(3,5-dimethoxyphenyl)-1-(2-methoxyphenyl)prop-2-en-1-one
- EGFR
epidermal growth factor receptor
- EMT
epithelial-to-mesenchymal transition
- EqM
epoxyquinone A monomer (EqM)
- HDAC
histone deacetylase
- HLA
human leukocyte antigen
- ICAM-1
intercellular adhesion molecule-1
- IKK
IκB kinase
- IMD-0354
N-[3,5-bis(trifluoromethyl)phenyl]-5-chloro-2-hydroxybenzamide
- IκB
inhibitor of kappa B
- IR
infrared
- LMP
latent membrane protein
- LPS
lipopolysaccharide
- MAPK
mitogen-activated protein kinase
- MCV
Molluscum contagiosum virus
- MMP
matrix metalloproteinase
- NBD
NEMO-binding domain
- NCPA
2-(N-Carbobenzyloxy)l-phenylalanylamido-2-deoxy-β-D-glucose
- NF-κB
nuclear factor kappa B
- NOD/SCID
non-obese diabetic/severe combined immunodeficiency
- NSCLC
non-small-cell lung carcinoma
- PKA
protein kinase A
- PKC
protein kinase C
- PS-1145
N-(6-chloro-9H-pyrido[3,4-b]indol-8-yl)pyridine-3-carboxamide
- pVHL
product of the von Hippel-Lindau
- RANKL
receptor activator of nuclear factor kappa B ligand
- RIP1
receptor-interacting protein 1
- TAP
transporter associated with antigen processing
- TBK1
TANK binding kinase 1
- TPCA-1
2-[(Aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide
- TNFα
tumor necrosis factor alpha
- TRAF
TNF receptor-associated factor
- vIL-10
viral IL-10
- uPA
urokinase type plasminogen activator
- VSV
vesicular stomatitis virus
Footnotes
Conflict of interest
None.
References
- 1.Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- 2.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 3.Haefner B. NF-kappa B: arresting a major culprit in cancer. Drug Discov Today. 2002;7(12):653–663. doi: 10.1016/s1359-6446(02)02309-7. [DOI] [PubMed] [Google Scholar]
- 4.Richmond A. Nf-kappa B chemokine gene transcription and tumour growth. Nat Rev Immunol. 2002;2(9):664–674. doi: 10.1038/nri887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6(3):203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118(6):671–674. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Balkwill F, Charles KA, Mantovani A. Smoldering and polarized inflammation in the initiation and promotion of malignant disease. Cancer Cell. 2005;7(3):211–217. doi: 10.1016/j.ccr.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 8.Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- 9.Luo JL, Kamata H, Karin M. IKK/NF-kappaB signaling: balancing life and death? a new approach to cancer therapy. J Clin Invest. 2005;115(10):2625–2632. doi: 10.1172/JCI26322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Q, Withoff S, Verma IM. Inflammation-associated cancer: NF-kappaB is the lynchpin. Trends Immunol. 2005;26(6):318–325. doi: 10.1016/j.it.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 11.Hayden MS, Ghosh S. Signaling to NF-kappaB. Genes Dev. 2004;18(18):2195–2224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 12.Fiorini E, Schmitz I, Marissen WE, Osborn SL, Touma M, Sasada T, Reche PA, Tibaldi EV, Hussey RE, Kruisbeek AM, Reinherz EL, Clayton LK. Peptide-induced negative selection of thymocytes activates transcription of an NF-kappa B inhibitor. Mol Cell. 2002;9(3):637–648. doi: 10.1016/s1097-2765(02)00469-0. [DOI] [PubMed] [Google Scholar]
- 13.Kuwata H, Matsumoto M, Atarashi K, Morishita H, Hirotani T, Koga R, Takeda K. IkappaBNS inhibits induction of a subset of Toll-like receptor-dependent genes and limits inflammation. Immunity. 2006;24(1):41–51. doi: 10.1016/j.immuni.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 15.Gupta SC, Prasad S, Reuter S, Kannappan R, Yadav VR, Ravindran J, Hema PS, Chaturvedi MM, Nair M, Aggarwal BB. Modification of cysteine 179 of IkappaBalpha kinase by nimbolide leads to down-regulation of NF-kappaB-regulated cell survival and proliferative proteins and sensitization of tumor cells to chemotherapeutic agents. J Biol Chem. 2010;285(46):35406–35417. doi: 10.1074/jbc.M110.161984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC, Karin M. Activation by IKKalpha of a second evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293(5534):1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 17.Dejardin E, Droin NM, Delhase M, Haas E, Cao Y, Makris C, Li ZW, Karin M, Ware CF, Green DR. The lymphotoxin-beta receptor induces different patterns of gene expression via two NF-kappaB pathways. Immunity. 2002;17(4):525–535. doi: 10.1016/s1074-7613(02)00423-5. [DOI] [PubMed] [Google Scholar]
- 18.Dejardin E. The alternative NF-kappaB pathway from biochemistry to biology: pitfalls and promises for future drug development. Biochem Pharmacol. 2006;72(9):1161–1179. doi: 10.1016/j.bcp.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 20.Chaturvedi MM, Sung B, Yadav VR, Kannappan R, Aggarwal BB. NF-kappaB addiction and its role in cancer: ‘one size does not fit all’. Oncogene. 2011;30(14):1615–1630. doi: 10.1038/onc.2010.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pradère JP, Hernandez C, Koppe C, Friedman RA, Luedde T, Schwabe RF. Negative regulation of NF-κB p65 activity by serine 536 phosphorylation. Sci Signal. 2016;9(442):ra85. doi: 10.1126/scisignal.aab2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercurio F, Zhu H, Murray BW, Shevchenko A, Bennett BL, Li J, Young DB, Barbosa M, Mann M, Manning A, Rao A. IKK-1 and IKK-2: cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science. 1997;278(5339):860–866. doi: 10.1126/science.278.5339.860. [DOI] [PubMed] [Google Scholar]
- 23.Zandi E, Rothwarf DM, Delhase M, Hayakawa M, Karin M. The IkappaB kinase complex (IKK) contains two kinase subunits IKKalpha and IKKbeta, necessary for IkappaB phosphorylation and NF-kappaB activation. Cell. 1997;91(2):243–252. doi: 10.1016/s0092-8674(00)80406-7. [DOI] [PubMed] [Google Scholar]
- 24.Rothwarf DM, Zandi E, Natoli G, Karin M. IKK-gamma is an essential regulatory subunit of the IkappaB kinase complex. Nature. 1998;395(6699):297–300. doi: 10.1038/26261. [DOI] [PubMed] [Google Scholar]
- 25.Ducut Sigala JL, Bottero V, Young DB, Shevchenko A, Mercurio F, Verma IM. Activation of transcription factor NF-kappaB requires ELKS an IkappaB kinase regulatory subunit. Science. 2004;304(5679):1963–1967. doi: 10.1126/science.1098387. [DOI] [PubMed] [Google Scholar]
- 26.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25(6):280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 27.DiDonato JA, Hayakawa M, Rothwarf DM, Zandi E, Karin M. A cytokine-responsive IkappaB kinase that activates the transcription factor NF-kappaB. Nature. 1997;388(6642):548–554. doi: 10.1038/41493. [DOI] [PubMed] [Google Scholar]
- 28.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006(357):re13. doi: 10.1126/stke.3572006re13. [DOI] [PubMed] [Google Scholar]
- 29.Chen ZJ, Parent L, Maniatis T. Site-specific phosphorylation of IkappaBalpha by a novel ubiquitination-dependent protein kinase activity. Cell. 1996;84(6):853–862. doi: 10.1016/s0092-8674(00)81064-8. [DOI] [PubMed] [Google Scholar]
- 30.Shimada T, Kawai T, Takeda K, Matsumoto M, Inoue J, Tatsumi Y, Kanamaru A, Akira S. IKK-i, a novel lipopolysaccharide-inducible kinase that is related to IkappaB kinases. Int Immunol. 1999;11(8):1357–1362. doi: 10.1093/intimm/11.8.1357. [DOI] [PubMed] [Google Scholar]
- 31.Peters RT, Liao SM, Maniatis T. IKKepsilon is part of a novel PMA-inducible IkappaB kinase complex. Mol Cell. 2000;5(3):513–522. doi: 10.1016/s1097-2765(00)80445-1. [DOI] [PubMed] [Google Scholar]
- 32.Pomerantz JL, Baltimore D. NF-kappaB activation by a signaling complex containing TRAF2 TANK and TBK1, a novel IKK-related kinase. EMBO J. 1999;18(23):6694–6704. doi: 10.1093/emboj/18.23.6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bonnard M, Mirtsos C, Suzuki S, Graham K, Huang J, Ng M, Itie A, Wakeham A, Shahinian A, Henzel WJ, Elia AJ, Shillinglaw W, Mak TW, Cao Z, Yeh WC. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J. 2000;19(18):4976–4985. doi: 10.1093/emboj/19.18.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tojima Y, Fujimoto A, Delhase M, Chen Y, Hatakeyama S, Nakayama K-i, Kaneko Y, Nimura Y, Motoyama N, Ikeda K. NAK is an IκB kinase-activating kinase. Nature. 2000;404(6779):778–782. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- 35.Fujita F, Taniguchi Y, Kato T, Narita Y, Furuya A, Ogawa T, Sakurai H, Joh T, Itoh M, Delhase M, Karin M, Nakanishi M. Identification of NAP1 a regulatory subunit of IkappaB kinase-related kinases that potentiates NF-kappaB signaling. Mol Cell Biol. 2003;23(21):7780–7793. doi: 10.1128/MCB.23.21.7780-7793.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim JY, Beg AA, Haura EB. Non-canonical IKKs, IKK and TBK1, as novel therapeutic targets in the treatment of non-small cell lung cancer. Expert Opin Ther Targets. 2013;17(10):1109–1112. doi: 10.1517/14728222.2013.833188. [DOI] [PubMed] [Google Scholar]
- 37.Barbie DA, Tamayo P, Boehm JS, Kim SY, Moody SE, Dunn IF, Schinzel AC, Sandy P, Meylan E, Scholl C, Frohling S, Chan EM, Sos ML, Michel K, Mermel C, Silver SJ, Weir BA, Reiling JH, Sheng Q, Gupta PB, Wadlow RC, Le H, Hoersch S, Wittner BS, Ramaswamy S, Livingston DM, Sabatini DM, Meyerson M, Thomas RK, Lander ES, Mesirov JP, Root DE, Gilliland DG, Jacks T, Hahn WC. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1. Nature. 2009;462(7269):108–112. doi: 10.1038/nature08460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boehm JS, Zhao JJ, Yao J, Kim SY, Firestein R, Dunn IF, Sjostrom SK, Garraway LA, Weremowicz S, Richardson AL. Integrative genomic approaches identify IKBKE as a breast cancer oncogene. Cell. 2007;129(6):1065–1079. doi: 10.1016/j.cell.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 39.Clément JF, Meloche S, Servant MJ. The IKK-related kinases: from innate immunity to oncogenesis. Cell Res. 2008;18(9):889–899. doi: 10.1038/cr.2008.273. [DOI] [PubMed] [Google Scholar]
- 40.Eddy SF, Guo S, Demicco EG, Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Sonenshein GE. Inducible IkappaB kinase/IkappaB kinase epsilon expression is induced by CK2 and promotes aberrant nuclear factor-kappaB activation in breast cancer cells. Cancer Res. 2005;65(24):11375–11383. doi: 10.1158/0008-5472.CAN-05-1602. [DOI] [PubMed] [Google Scholar]
- 41.Li XY, Tang SH, Zhou XC, Ye YH, Xu XQ, Li RZ. Preoperative serum visfatin levels and prognosis of breast cancer among Chinese women. Peptides. 2014;51:86–90. doi: 10.1016/j.peptides.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Muvaffak A, Pan Q, Yan H, Fernandez R, Lim J, Dolinski B, Nguyen TT, Strack P, Wu S, Chung R. Evaluating TBK1 as a therapeutic target in cancers with activated IRF3. Mol Cancer Res. 2014;12(7):1055–1066. doi: 10.1158/1541-7786.MCR-13-0642. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Tian M, Xia Z, Feng P. Roles of IκB kinase ε in the innate immune defense and beyond. Virol Sin. 2016;31(6):457–465. doi: 10.1007/s12250-016-3898-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shukla S, Kanwal R, Shankar E, Datt M, Chance MR, Fu P, MacLennan GT, Gupta S. Apigenin blocks IKKalpha activation and suppresses prostate cancer progression. Oncotarget. 2015;6(31):31216–31232. doi: 10.18632/oncotarget.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li T, Wong VK, Jiang ZH, Jiang SP, Liu Y, Wang TY, Yao XJ, Su XH, Yan FG, Liu J, Leung EL, Yi XQ, Wong YF, Zhou H, Liu L. Mutation of cysteine 46 in IKK-beta increases inflammatory responses. Oncotarget. 2015;6(31):31805–31819. doi: 10.18632/oncotarget.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. 1999;18(49):6853–6866. doi: 10.1038/sj.onc.1203239. [DOI] [PubMed] [Google Scholar]
- 47.Romieu-Mourez R, Landesman-Bollag E, Seldin DC, Traish AM, Mercurio F, Sonenshein GE. Roles of IKK kinases and protein kinase CK2 in activation of nuclear factor-kappaB in breast cancer. Cancer Res. 2001;61(9):3810–3818. [PubMed] [Google Scholar]
- 48.Gasparian AV, Yao YJ, Kowalczyk D, Lyakh LA, Karseladze A, Slaga TJ, Budunova IV. The role of IKK in constitutive activation of NF-kappaB transcription factor in prostate carcinoma cells. J Cell Sci. 2002;15(Pt. 1):141–151. doi: 10.1242/jcs.115.1.141. [DOI] [PubMed] [Google Scholar]
- 49.Charalambous MP, Maihofner C, Bhambra U, Lightfoot T, Gooderham NJ, G. Colorectal Cancer Study Upregulation of cyclooxygenase-2 is accompanied by increased expression of nuclear factor-kappa B and I kappa B kinase-alpha in human colorectal cancer epithelial cells. Br J Cancer. 2003;88(10):1598–1604. doi: 10.1038/sj.bjc.6600927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Olsen LS, Hjarnaa PJ, Latini S, Holm PK, Larsson R, Bramm E, Binderup L, Madsen MW. Anticancer agent CHS 828 suppresses nuclear factor-kappa B activity in cancer cells through downregulation of IKK activity. Int J Cancer. 2004;111(2):198–205. doi: 10.1002/ijc.20255. [DOI] [PubMed] [Google Scholar]
- 51.Hildebrandt MA, Tan W, Tamboli P, Huang M, Ye Y, Lin J, Lee JS, Wood CG, Wu X. Kinome expression profiling identifies IKBKE as a predictor of overall survival in clear cell renal cell carcinoma patients. Carcinogenesis. 2012;33(4):799–803. doi: 10.1093/carcin/bgs018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prajapati S, Tu Z, Yamamoto Y, Gaynor RB. IKKalpha regulates the mitotic phase of the cell cycle by modulating Aurora A phosphorylation. ABBV Cell Cycle. 2006;5(20):2371–2380. doi: 10.4161/cc.5.20.3359. [DOI] [PubMed] [Google Scholar]
- 53.Rinkenbaugh AL, Cogswell PC, Calamini B, Dunn DE, Persson AI, Weiss WA, Lo DC, Baldwin AS. IKK/NF-κB signaling contributes to glioblastoma stem cell maintenance. Oncotarget. 2016;7(43):69173. doi: 10.18632/oncotarget.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, Cheresh DA, Karin M. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature. 2007;446(7136):690–694. doi: 10.1038/nature05656. [DOI] [PubMed] [Google Scholar]
- 55.Escarcega RO, Fuentes-Alexandro S, Garcia-Carrasco M, Gatica A, Zamora A. The transcription factor nuclear factor-kappa B and cancer. Clin Oncol (R Coll Radiol) 2007;19(2):154–161. doi: 10.1016/j.clon.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 56.Lee DF, Hung MC. Advances in targeting IKK and IKK-related kinases for cancer therapy. Clin Cancer Res. 2008;14(18):5656–5662. doi: 10.1158/1078-0432.CCR-08-0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Burke JR, Pattoli MA, Gregor KR, Brassil PJ, MacMaster JF, McIntyre KW, Yang X, Iotzova VS, Clarke W, Strnad J, Qiu Y, Zusi FC. BMS-345541 is a highly selective inhibitor of I kappa B kinase that binds at an allosteric site of the enzyme and blocks NF-kappa B-dependent transcription in mice. J Biol Chem. 2003;278(3):1450–1456. doi: 10.1074/jbc.M209677200. [DOI] [PubMed] [Google Scholar]
- 58.McIntosh M, Stone BA, Stanisich VA. Curdlan and other bacterial (1– > 3)-beta-D-glucans. Appl Microbiol Biotechnol. 2005;68(2):163–173. doi: 10.1007/s00253-005-1959-5. [DOI] [PubMed] [Google Scholar]
- 59.Battula V, Piyaranthna B, Nguyen K, Sun J, Jin F, Coarfa C, Nagireddy P, Andreeff M. Abstract P6-02-01: Metabolic Stress Induces GD2 Expression and Cancer Stem Cell Phenotype in Triple Negative Breast Cancer. AACR. 2017 [Google Scholar]
- 60.Ping H, Yang F, Wang M, Niu Y, Xing N. IKK inhibitor suppresses epithelial-mesenchymal transition and induces cell death in prostate cancer. Oncol Rep. 2016;36(3):1658–1664. doi: 10.3892/or.2016.4915. [DOI] [PubMed] [Google Scholar]
- 61.Yang J, Amiri KI, Burke JR, Schmid JA, Richmond A. BMS-345541 targets inhibitor of κB kinase and induces apoptosis in melanoma: involvement of nuclear factor κB and mitochondria pathways. Clin Cancer Res. 2006;12(3):950–960. doi: 10.1158/1078-0432.CCR-05-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castro AC, Dang LC, Soucy F, Grenier L, Mazdiyasni H, Hottelet M, Parent L, Pien C, Palombella V, Adams J. Novel IKK inhibitors: betacarbolines. Bioorg Med Chem Lett. 2003;13(14):2419–2422. doi: 10.1016/s0960-894x(03)00408-6. [DOI] [PubMed] [Google Scholar]
- 63.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, Munshi N, Dang L, Castro A, Palombella V, Adams J, Anderson KC. NF-kappa B as a therapeutic target in multiple myeloma. J Biol Chem. 2002;277(19):16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 64.Lam LT, Davis RE, Pierce J, Hepperle M, Xu Y, Hottelet M, Nong Y, Wen D, Adams J, Dang L. Small molecule inhibitors of IκB kinase are selectively toxic for subgroups of diffuse large B-cell lymphoma defined by gene expression profiling. Clin Cancer Res. 2005;11(1):28–40. [PubMed] [Google Scholar]
- 65.Nomura A, Majumder K, Giri B, Dauer P, Dudeja V, Roy S, Banerjee S, Saluja AK. Inhibition of NF-kappa B pathway leads to deregulation of epithelial–mesenchymal transition and neural invasion in pancreatic cancer. Lab Invest. 2016;96(12):1268–1278. doi: 10.1038/labinvest.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Majidinia M, Alizadeh E, Yousefi B, Akbarzadeh M, Mihanfar A, Rahmati-Yamchi M, Zarghami N. Co-inhibition of Notch and NF-κB Signaling Pathway Decreases Proliferation through Downregulating IκB-α and Hes-1 Expression in Human Ovarian Cancer OVCAR-3Cells. Drug Res. 2017;67(01):13–19. doi: 10.1055/s-0042-115405. [DOI] [PubMed] [Google Scholar]
- 67.Tanaka A, Konno M, Muto S, Kambe N, Morii E, Nakahata T, Itai A, Matsuda H. A novel NF-kappaB inhibitor IMD-0354, suppresses neoplastic proliferation of human mast cells with constitutively activated c-kit receptors. Blood. 2005;105(6):2324–2331. doi: 10.1182/blood-2004-08-3247. [DOI] [PubMed] [Google Scholar]
- 68.Tanaka A, Muto S, Konno M, Itai A, Matsuda H. A new IkappaB kinase beta inhibitor prevents human breast cancer progression through negative regulation of cell cycle transition. Cancer Res. 2006;66(1):419–426. doi: 10.1158/0008-5472.CAN-05-0741. [DOI] [PubMed] [Google Scholar]
- 69.Curat CA, Wegner V, Sengenes C, Miranville A, Tonus C, Busse R, Bouloumie A. Macrophages in human visceral adipose tissue: increased accumulation in obesity and a source of resistin and visfatin. Diabetologia. 2006;49(4):744–747. doi: 10.1007/s00125-006-0173-z. [DOI] [PubMed] [Google Scholar]
- 70.Fukuhara A, Matsuda M, Nishizawa M, Segawa K, Tanaka M, Kishimoto K, Matsuki Y, Murakami M, Ichisaka T, Murakami H, Watanabe E, Takagi T, Akiyoshi M, Ohtsubo T, Kihara S, Yamashita S, Makishima M, Funahashi T, Yamanaka S, Hiramatsu R, Matsuzawa Y, Shimomura I. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307(5708):426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- 71.Bi TQ, Che XM. Nampt/PBEF/visfatin and cancer. Cancer Biol Ther. 2010;10(2):119–125. doi: 10.4161/cbt.10.2.12581. [DOI] [PubMed] [Google Scholar]
- 72.Li H, Huang J, Yang B, Xiang T, Yin X, Peng W, Cheng W, Wan J, Luo F, Li H, Ren G. Mangiferin exerts antitumor activity in breast cancer cells by regulating matrix metalloproteinases epithelial to mesenchymal transition, and beta-catenin signaling pathway. Toxicol Appl Pharmacol. 2013;272(1):180–190. doi: 10.1016/j.taap.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 73.Sanda T, Iida S, Ogura H, Asamitsu K, Murata T, Bacon KB, Ueda R, Okamoto T. Growth inhibition of multiple myeloma cells by a novel IkappaB kinase inhibitor. Clin Cancer Res. 2005;11(5):1974–1982. doi: 10.1158/1078-0432.CCR-04-1936. [DOI] [PubMed] [Google Scholar]
- 74.Wang G, Tian W, Liu Y, Ju Y, Shen Y, Zhao S, Zhang B, Li Y. Visfatin triggers the cell motility of non-Small cell lung cancer via up-regulation of matrix metalloproteinases. Basic Clin Pharmacol Toxicol. 2016;119(6):548–554. doi: 10.1111/bcpt.12623. [DOI] [PubMed] [Google Scholar]
- 75.Reilly SM, Chiang SH, Decker SJ, Chang L, Uhm M, Larsen MJ, Rubin JR, Mowers J, White NM, Hochberg I, Downes M, Yu RT, Liddle C, Evans RM, Oh D, Li P, Olefsky JM, Saltiel AR. An inhibitor of the protein kinases TBK1 and IKK-varepsilon improves obesity-related metabolic dysfunctions in mice. Nat Med. 2013;19(3):313–321. doi: 10.1038/nm.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cho CC, Chou RH, Yu C. Amlexanox blocks the interaction between S100A4 and epidermal growth factor and inhibits cell proliferation. PLoS One. 2016;11(8):e0161663. doi: 10.1371/journal.pone.0161663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Möser CV, Meissner M, Laarmann K, Olbrich K, King-Himmelreich TS, Wolters MC, Geisslinger G, Niederberger E. The protein kinase IKKepsilon contributes to tumour growth and tumour pain in a melanoma model. Biochem Pharmacol. 2016;103:64–73. doi: 10.1016/j.bcp.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 78.Challa S, Guo JP, Ding X, Xu CX, Li Y, Kim D, Smith MA, Cress DW, Coppola D, Haura EB. IKBKE is a substrate of EGFR and a therapeutic target in non–small cell lung cancer with activating mutations of EGFR. Cancer Res. 2016;76(15):4418–4429. doi: 10.1158/0008-5472.CAN-16-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J, Clark K, Lawrence T, Peggie MW, Cohen P. An unexpected twist to the activation of IKKbeta: TAK1 primes IKKbeta for activation by autophosphorylation. Biochem J. 2014;461(3):531–537. doi: 10.1042/BJ20140444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sommers CD, Thompson JM, Guzova JA, Bonar SL, Rader RK, Mathialagan S, Venkatraman N, Holway VW, Kahn LE, Hu G, Garner DS, Huang HC, Chiang PC, Schindler JF, Hu Y, Meyer DM, Kishore NN. Novel tight-binding inhibitory factor-kappaB kinase (IKK-2) inhibitors demonstrate target-specific anti-inflammatory activities in cellular assays and following oral and local delivery in an in vivo model of airway inflammation. J Pharmacol Exp Ther. 2009;330(2):377–388. doi: 10.1124/jpet.108.147538. [DOI] [PubMed] [Google Scholar]
- 81.Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, Huynh K, Bonar S, Mielke C, Albee L, Weier R, Graneto M, Hanau C, Perry T, Tripp CS. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem. 2003;278(35):32861–32871. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- 82.Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J. 2013;15(1):195–218. doi: 10.1208/s12248-012-9432-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh S, Aggarwal BB. Activation of transcription factor NF-kappa B is suppressed by curcumin (diferuloylmethane) [corrected] J Biol Chem. 1995;270(42):24995–25000. doi: 10.1074/jbc.270.42.24995. [DOI] [PubMed] [Google Scholar]
- 84.Kao NJ, Hu JY, Wu CS, Kong ZL. Curcumin represses the activity of inhibitor-(B kinase in dextran sulfate sodium-induced colitis by S-nitrosylation. Int Immunopharmacol. 2016;38:1–7. doi: 10.1016/j.intimp.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 85.Wang KS, Li J, Wang Z, Mi C, Ma J, Piao LX, Xu GH, Li X, Jin X. Artemisinin inhibits inflammatory response via regulating NF-(B and MAPK signaling pathways, Immunopharmacol. Immunotoxicol. 2017;39(1):28–36. doi: 10.1080/08923973.2016.1267744. [DOI] [PubMed] [Google Scholar]
- 86.Takeda T, Tsubaki M, Sakamoto K, Ichimura E, Enomoto A, Suzuki Y, Itoh T, Imano M, Tanabe G, Muraoka O. Mangiferin, a novel nuclear factor kappa B-inducing kinase inhibitor, suppresses metastasis and tumor growth in a mouse metastatic melanoma model. Toxicol Appl Pharmacol. 2016;306:105–112. doi: 10.1016/j.taap.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 87.Shankar E, Zhang A, Franco D, Gupta S. Betulinic acid-Mediated apoptosis in human prostate cancer cells involves p53 and nuclear factor-Kappa B (NF-kappaB) pathways. Molecules. 2017;22(2) doi: 10.3390/molecules22020264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Su D, Gao Y-q, Dai W-b, Hu Y, Wu Y-f, Mei Q-x. Helicteric acid, oleanic acid, and betulinic acid, three triterpenes from helicteres angustifolia L., inhibit proliferation and induce apoptosis in HT-29 colorectal cancer cells via suppressing NF-κB and STA signaling. Evid Based Complem Altern Med. 2017;2017:T3. doi: 10.1155/2017/5180707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Huang FL, Chiou RYY, Chen WC, Ko HJ, Lai LJ, Lin SM. Dehydrated basella alba fruit juice as a novel natural colorant: pigment stability, In vivo food safety evaluation and anti-inflammatory mechanism characterization. Plant Foods Hum Nutr. 2016;71(3):322–329. doi: 10.1007/s11130-016-0563-4. [DOI] [PubMed] [Google Scholar]
- 90.Gupta SC, Sung B, Prasad S, Webb LJ, Aggarwal BB. Cancer drug discovery by repurposing: teaching new tricks to old dogs. Trends Pharmacol Sci. 2013;34(9):508–517. doi: 10.1016/j.tips.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 91.Ogut D, Reel B, Korkmaz CG, Arun MZ, Micili SC, Ergur BU. Doxycycline down-regulates matrix metalloproteinase expression and inhibits NF-κB signaling in LPS-induced PC3 cells. Folia Histochem Cytobiol. 2016;54(4):171–180. doi: 10.5603/FHC.a2016.0022. [DOI] [PubMed] [Google Scholar]
- 92.Zheng L, Jia J, Dai H, Wan L, Liu J, Hu L, Zhou M, Qiu M, Chen X, Chang L. Triptolide-assisted phosphorylation of p53 suppresses inflammation-induced NF-κB survival pathways in cancer cells. Mol Cell Biol. 2017 doi: 10.1128/MCB.00149-17. 17-00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shi C, Zhang N, Feng Y, Cao J, Chen X, Liu B. Aspirin inhibits IKK-β-mediated prostate cancer cell invasion by targeting matrix metalloproteinase-9 and urokinase-Type plasminogen activator. Cell Physiol Biochem. 2017;41(4):1313–1324. doi: 10.1159/000464434. [DOI] [PubMed] [Google Scholar]
- 94.Waxman S, Anderson KC. History of the development of arsenic derivatives in cancer therapy. Oncologist. 2001;6(Suppl. 2):3–10. doi: 10.1634/theoncologist.6-suppl_2-3. [DOI] [PubMed] [Google Scholar]
- 95.Lo-Coco F, Avvisati G, Vignetti M, Thiede C, Orlando SM, Iacobelli S, Ferrara F, Fazi P, Cicconi L, Di Bona E. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. New Engl J Med. 2013;369(2):111–121. doi: 10.1056/NEJMoa1300874. [DOI] [PubMed] [Google Scholar]
- 96.Mathas S, Lietz A, Janz M, Hinz M, Jundt F, Scheidereit C, Bommert K, Dörken B. Inhibition of NF-κB essentially contributes to arsenic-induced apoptosis. Blood. 2003;102(3):1028–1034. doi: 10.1182/blood-2002-04-1154. [DOI] [PubMed] [Google Scholar]
- 97.May MJ, D’Acquisto F, Madge LA, Glockner J, Pober JS, Ghosh S. Selective inhibition of NF-kappaB activation by a peptide that blocks the interaction of NEMO with the IkappaB kinase complex. Science. 2000;289(5484):1550–1554. doi: 10.1126/science.289.5484.1550. [DOI] [PubMed] [Google Scholar]
- 98.Jimi E, Aoki K, Saito H, D’Acquisto F, May MJ, Nakamura I, Sudo T, Kojima T, Okamoto F, Fukushima H. Selective inhibition of NF-κB blocks osteoclastogenesis and prevents inflammatory bone destruction in vivo. Nat Med. 2004;10(6):617–624. doi: 10.1038/nm1054. [DOI] [PubMed] [Google Scholar]
- 99.Frew IJ, Krek W. pVHL: a multipurpose adaptor protein. Sci Signal. 2008;1(24):pe30. doi: 10.1126/scisignal.124pe30. [DOI] [PubMed] [Google Scholar]
- 100.Biswas S, Shisler JL. Molluscum Contagiosum Virus MC159 abrogates cIAP1—NEMO interactions and inhibits NEMO polyubiquitination. 17-00276. J Virol. 2017 doi: 10.1128/JVI.00276-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shao R, Hu MC, Zhou BP, Lin SY, Chiao PJ, von Lindern RH, Spohn B, Hung MC. E1A sensitizes cells to tumor necrosis factor-induced apoptosis through inhibition of IkappaB kinases and nuclear factor kappaB activities. J Biol Chem. 1999;274(31):21495–21498. doi: 10.1074/jbc.274.31.21495. [DOI] [PubMed] [Google Scholar]
- 102.Shao R, Tsai EM, Wei K, von Lindern R, Chen YH, Makino K, Hung MC. E1A inhibition of radiation-induced NF-kappaB activity through suppression of IKK activity and IkappaB degradation independent of Akt activation. Cancer Res. 2001;61(20):7413–7416. [PubMed] [Google Scholar]
- 103.Ren Y-x, Yang J, Sun R-m, Zhang L-J, Zhao L-F, Li BZ, Li L, Long H-T, Sun Q-M, Huang Y-C. Viral IL-10 down-regulates the MHC-I antigen processing operon through the NF-κB signaling pathway in nasopharyngeal carcinoma cells. Cytotechnology. 2016;68(6):2625–2636. doi: 10.1007/s10616-016-9987-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chen H, Li M, Sanchez E, Wang CS, Lee T, Soof CM, Casas CE, Cao J, Xie C, Udd KA. Combined TRAF6 targeting and proteasome blockade has anti-myeloma and anti-bone resorptive effects. Mol Cancer Res. 2017;15(5):598–609. doi: 10.1158/1541-7786.MCR-16-0293. [DOI] [PubMed] [Google Scholar]
- 105.Dewert N, Amschler K, Lorenz V, Schön MP. The IKKα-dependent non-canonical pathway of NF-κB activation is constitutively active and modulates progression-related functions in a subset of human melanomas. Arch Dermatol Res. 2016;308(10):733–742. doi: 10.1007/s00403-016-1696-x. [DOI] [PubMed] [Google Scholar]
- 106.Liang MC, Bardhan S, Pace EA, Rosman D, Beutler JA, Porco JA, Gilmore TD. Inhibition of transcription factor NF-κB signaling proteins IKKβ and p65 through specific cysteine residues by epoxyquinone A monomer: correlation with its anti-cancer cell growth activity. Biochem Pharmacol. 2006;71(5):634–645. doi: 10.1016/j.bcp.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 107.Kapahi P, Takahashi T, Natoli G, Adams SR, Chen Y, Tsien RY, Karin M. Inhibition of NF-κB activation by arsenite through reaction with a critical cysteine in the activation loop of IκB kinase. J Biol Chem. 2000;275(46):36062–36066. doi: 10.1074/jbc.M007204200. [DOI] [PubMed] [Google Scholar]
- 108.Rossi A, Kapahi P, Natoli G, Takahashi T, Chen Y, Karin M, Santoro MG. Anti-inflammatory cyclopentenone prostaglandins are direct inhibitors of IκB kinase. Nature. 2000;403(6765):103–118. doi: 10.1038/47520. [DOI] [PubMed] [Google Scholar]
- 109.Reynaert NL, Ckless K, Korn SH, Vos N, Guala AS, Wouters EF, van der Vliet A, Janssen-Heininger YM. Nitric oxide represses inhibitory kappaB kinase through S-nitrosylation. Proc Natl Acad Sci U S A. 2004;101(24):8945–8950. doi: 10.1073/pnas.0400588101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pandey MK, Sandur SK, Sung B, Sethi G, Kunnumakkara AB, Aggarwal BB. Butein, a tetrahydroxychalcone, inhibits nuclear factor (NF)-κB and NF-κB-regulated gene expression through direct inhibition of IκBα kinase β on cysteine 179 residue. J Biol Chem. 2007;282(24):17340–17350. doi: 10.1074/jbc.M700890200. [DOI] [PubMed] [Google Scholar]
- 111.Park MH, Song HS, Kim KH, Son DJ, Lee SH, Yoon DY, Kim Y, Park IY, Song S, Hwang BY, Jung JK, Hong JT. Cobrotoxin inhibits NF-kappa B activation and target gene expression through reaction with NF-kappa B signal molecules. Biochemistry. 2005;44(23):8326–8336. doi: 10.1021/bi050156h. [DOI] [PubMed] [Google Scholar]
- 112.Azuma M, Yamashita T, Aota K, Tamatani T, Sato M. 5-Fluorouracil suppression of NF-KappaB is mediated by the inhibition of IKappab kinase activity in human salivary gland cancer cells. Biochem Biophys Res Commun. 2001;282(1):292–296. doi: 10.1006/bbrc.2001.4571. [DOI] [PubMed] [Google Scholar]
- 113.Frelin C, Imbert V, Griessinger E, Peyron AC, Rochet N, Philip P, Dageville C, Sirvent A, Hummelsberger M, Bérard E. Targeting NF-κB activation via pharmacologic inhibition of IKK2-induced apoptosis of human acute myeloid leukemia cells. Blood. 2005;105(2):804–811. doi: 10.1182/blood-2004-04-1463. [DOI] [PubMed] [Google Scholar]
- 114.Gatla HR, Zou Y, Uddin MM, Singha B, Bu P, Vancura A, Vancurova I. Histone deacetylase (HDAC) inhibition induces IκB kinase (IKK)-dependent interleukin-8/CXCL8 expression in ovarian cancer cells. J Biol Chem. 2017;292(12):5043–5054. doi: 10.1074/jbc.M116.771014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bose P, Dai Y, Grant S. Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights. Pharmacol Ther. 2014;143(3):323–336. doi: 10.1016/j.pharmthera.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Podolin PL, Callahan JF, Bolognese BJ, Li YH, Carlson K, Davis TG, Mellor GW, Evans C, Roshak AK. Attenuation of murine collagen-induced arthritis by a novel potent, selective small molecule inhibitor of IkappaB Kinase 2, TPCA-1 (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarbox-amide), occurs via reduction of proinflammatory cytokines and antigen-induced T cell Proliferation. J Pharmacol Exp Ther. 2005;312(1):373–381. doi: 10.1124/jpet.104.074484. [DOI] [PubMed] [Google Scholar]
- 117.Hastie E, Cataldi M, Moerdyk MJ, Felt SA, Steuerwald N, Grdzelishvili VZ. Novel biomarkers of resistance of pancreatic cancer cells to oncolytic vesicular stomatitis virus. Oncotarget. 2016;7(38):61601. doi: 10.18632/oncotarget.11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cataldi M, Shah NR, Felt SA, Grdzelishvili VZ. Breaking resistance of pancreatic cancer cells to an attenuated vesicular stomatitis virus through a novel activity of IKK inhibitor TPCA-1. Virology. 2015;485:340–354. doi: 10.1016/j.virol.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Nan J, Du Y, Chen X, Bai Q, Wang Y, Zhang X, Zhu N, Zhang J, Hou J, Wang Q, Yang J. TPCA-1 is a direct dual inhibitor of STAT3 and NF-kappaB and regresses mutant EGFR-associated human non-small cell lung cancers. Mol Cancer Ther. 2014;13(3):617–629. doi: 10.1158/1535-7163.MCT-13-0464. [DOI] [PubMed] [Google Scholar]
- 120.Zhu Z, Aref AR, Cohoon TJ, Barbie TU, Imamura Y, Yang S, Moody SE, Shen RR, Schinzel AC, Thai TC. Inhibition of KRAS-driven tumorigenicity by interruption of an autocrine cytokine circuit. Cancer Discov. 2014;4(4):452–465. doi: 10.1158/2159-8290.CD-13-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fonseca FP, de V Carvalho M, de Almeida OP, Rangel AL, Takizawa MC, Bueno AG, Vargas PA. Clinicopathologic analysis of 493 cases of salivary gland tumors in a Southern Brazilian population. Oral Surg Oral Med Oral Pathol Oral Radiol. 2012;114(2):230–239. doi: 10.1016/j.oooo.2012.04.008. [DOI] [PubMed] [Google Scholar]
- 122.Jones AV, Craig GT, Speight PM, Franklin CD. The range and demographics of salivary gland tumours diagnosed in a UK population. Oral Oncol. 2008;44(4):407–417. doi: 10.1016/j.oraloncology.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 123.Wagner VP, Martins M, Martins MD, Warner KA, Webber LP, Squarize CH, Nör JE, Castilho RM. Overcoming adaptive resistance in mucoepidermoid carcinoma through inhibition of the IKK-β/IκBα/NFκB axis. Oncotarget. 2016;7(45):73032–73044. doi: 10.18632/oncotarget.12195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Richardson PG, Barlogie B, Berenson J, Singhal S, Jagannath S, Irwin D, Rajkumar SV, Srkalovic G, Alsina M, Alexanian R, Siegel D, Orlowski RZ, Kuter D, Limentani SA, Lee S, Hideshima T, Esseltine DL, Kauffman M, Adams J, Schenkein DP, Anderson KC. A phase 2 study of bortezomib in relapsed refractory myeloma. N Engl J Med. 2003;348(26):2609–2617. doi: 10.1056/NEJMoa030288. [DOI] [PubMed] [Google Scholar]
- 125.Richardson PG, Sonneveld P, Schuster MW, Irwin D, Stadtmauer EA, Facon T, Harousseau JL, Ben-Yehuda D, Lonial S, Goldschmidt H, Reece D, San-Miguel JF, Blade J, Boccadoro M, Cavenagh J, Dalton WS, Boral AL, Esseltine DL, Porter JB, Schenkein D, Anderson KC, I. Assessment of Proteasome Inhibition for Extending Remissions Bortezomib or high-dose dexamethasone for relapsed multiple myeloma. N Engl J Med. 2005;352(24):2487–2498. doi: 10.1056/NEJMoa043445. [DOI] [PubMed] [Google Scholar]
- 126.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos MV, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG, V.T. Investigators Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 127.Hideshima T, Mitsiades C, Akiyama M, Hayashi T, Chauhan D, Richardson P, Schlossman R, Podar K, Munshi NC, Mitsiades N, Anderson KC. Molecular mechanisms mediating antimyeloma activity of proteasome inhibitor PS-341. Blood. 2003;101(4):1530–1534. doi: 10.1182/blood-2002-08-2543. [DOI] [PubMed] [Google Scholar]
- 128.Hideshima T, Richardson P, Chauhan D, Palombella VJ, Elliott PJ, Adams J, Anderson KC. The proteasome inhibitor PS-341 inhibits growth induces apoptosis, and overcomes drug resistance in human multiple myeloma cells. Cancer Res. 2001;61(7):3071–3076. [PubMed] [Google Scholar]
- 129.Mitsiades N, Mitsiades CS, Poulaki V, Chauhan D, Fanourakis G, Gu X, Bailey C, Joseph M, Libermann TA, Treon SP, Munshi NC, Richardson PG, Hideshima T, Anderson KC. Molecular sequelae of proteasome inhibition in human multiple myeloma cells. Proc Natl Acad Sci U S A. 2002;99(22):14374–14379. doi: 10.1073/pnas.202445099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Alinari L, White VL, Earl CT, Ryan TP, Johnston JS, Dalton JT, Ferketich AK, Lai R, Lucas DM, Porcu P, Blum KA, Byrd JC, Baiocchi RA. Combination bortezomib and rituximab treatment affects multiple survival and death pathways to promote apoptosis in mantle cell lymphoma. MAbs. 2009;1(1):31–40. doi: 10.4161/mabs.1.1.7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chiao PJ, Na R, Niu J, Sclabas GM, Dong Q, Curley SA. Role of Rel/NF-kappaB transcription factors in apoptosis of human hepatocellular carcinoma cells. Cancer. 2002;95(8):1696–1705. doi: 10.1002/cncr.10829. [DOI] [PubMed] [Google Scholar]
- 132.Singh S, Shi Q, Bailey ST, Palczewski MJ, Pardee AB, Iglehart JD, Biswas DK. Nuclear factor-kappaB activation: a molecular therapeutic target for estrogen receptor-negative and epidermal growth factor receptor family receptor-positive human breast cancer. Mol Cancer Ther. 2007;6(7):1973–1982. doi: 10.1158/1535-7163.MCT-07-0063. [DOI] [PubMed] [Google Scholar]
- 133.Al-Homsi AS, Lai Z, Roy TS, Al-Malki MM, Kouttab N, Junghans RP. Post-transplant cyclophosphamide and bortezomib inhibit dendritic cell maturation and function and alter their IκB and NFκB. Transpl Immunol. 2014;30(1):40–45. doi: 10.1016/j.trim.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 134.Al-Homsi AS, Lai Z, Roy TS, Al-Malki MM, Kouttab N, Junghans RP. Post-transplant cyclophosphamide and bortezomib inhibit dendritic cell maturation and function and alter their IkappaB and NFkappaB. Transpl Immunol. 2014;30(1):40–45. doi: 10.1016/j.trim.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 135.Hideshima T, Ikeda H, Chauhan D, Okawa Y, Raje N, Podar K, Mitsiades C, Munshi NC, Richardson PG, Carrasco RD, Anderson KC. Bortezomib induces canonical nuclear factor-kappaB activation in multiple myeloma cells. Blood. 2009;114(5):1046–1052. doi: 10.1182/blood-2009-01-199604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Perrone M. FDA clears amgen’s bone-strengthening drug prolia. BioSci Technol. 2010 [Google Scholar]
- 137.Abu-Amer Y. NF-kappaB signaling and bone resorption. Osteoporos Int. 2013;24(9):2377–2386. doi: 10.1007/s00198-013-2313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Noort AR, Tak PP, Tas SW. Non-canonical NF-kappaB signaling in rheumatoid arthritis: Dr Jekyll and Mr Hyde? Arthritis Res Ther. 2015;17:15. doi: 10.1186/s13075-015-0527-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wilcox RA. Cutaneous T-cell lymphoma: 2014 update on diagnosis risk-stratification, and management. Am J Hematol. 2014;89(8):837–851. doi: 10.1002/ajh.23756. [DOI] [PubMed] [Google Scholar]
- 140.Clark K, Plater L, Peggie M, Cohen P. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: a distinct upstream kinase mediates Ser-172 phosphorylation and activation. J Biol Chem. 2009;284(21):14136–14146. doi: 10.1074/jbc.M109.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ou YH, Torres M, Ram R, Formstecher E, Roland C, Cheng T, Brekken R, Wurz R, Tasker A, Polverino T, Tan SL, White MA. TBK1 directly engages Akt/PKB survival signaling to support oncogenic transformation. Mol Cell. 2011;41(4):458–470. doi: 10.1016/j.molcel.2011.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Leopizzi M, Cocchiola R, Milanetti E, Raimondo D, Politi L, Giordano C, Scandurra R, d’Abusco AS. IKKα inibition by a glucosamine derivative enhances Maspin expression in osteosarcoma cell line. Chem Biol Interact. 2017;262:19–28. doi: 10.1016/j.cbi.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 143.Shin SY, Kim CG, Jung YJ, Lim Y, Lee YH. The UPR inducer DPP23 inhibits the metastatic potential of MDA-MB-231 human breast cancer cells by targeting the Akt–IKK–NF-κB–MMP-9 axis. Sci Rep. 2016;6 doi: 10.1038/srep34134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Margalef P, Fernández-Majada V, Villanueva A, Garcia-Carbonell R, Iglesias M, López L, Martínez-Iniesta M, Villà-Freixa J, Mulero MC, Andreu M. A truncated form of IKKα is responsible for specific nuclear IKK activity in colorectal cancer. Cell Rep. 2012;2(4):840–854. doi: 10.1016/j.celrep.2012.08.028. [DOI] [PubMed] [Google Scholar]
- 145.Margalef P, Colomer C, Villanueva A, Montagut C, Iglesias M, Bellosillo B, Salazar R, Martinez-Iniesta M, Bigas A, Espinosa L. BRAF-induced tumorigenesis is IKKalpha-dependent but NF-kappaB-independent. Sci Signal. 2015;8(373):ra38. doi: 10.1126/scisignal.2005886. [DOI] [PubMed] [Google Scholar]
- 146.Vaccari T, Duchi S, Cortese K, Tacchetti C, Bilder D. The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development. 2010;137(11):1825–1832. doi: 10.1242/dev.045484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Taelman VF, Dobrowolski R, Plouhinec JL, Fuentealba LC, Vorwald PP, Gumper I, Sabatini DD, De Robertis EM. Wnt signaling requires sequestration of glycogen synthase kinase 3 inside multivesicular endosomes. Cell. 2010;143(7):1136–1148. doi: 10.1016/j.cell.2010.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hjarnaa PJ, Jonsson E, Latini S, Dhar S, Larsson R, Bramm E, Skov T, Binderup L. CHS 828 a novel pyridyl cyanoguanidine with potent antitumor activity in vitro and in vivo. Cancer Res. 1999;59(22):5751–5757. [PubMed] [Google Scholar]
- 149.von Heideman A, Berglund A, Larsson R, Nygren P. Safety and efficacy of NAD depleting cancer drugs: results of a phase I clinical trial of CHS 828 and overview of published data, Cancer Chemother. Pharmacol. 2010;65(6):1165–1172. doi: 10.1007/s00280-009-1125-3. [DOI] [PubMed] [Google Scholar]
- 150.Li N, Karin M. Ionizing radiation and short wavelength UV activate NF-kappaB through two distinct mechanisms. Proc Natl Acad Sci U S A. 1998;95(22):13012–13017. doi: 10.1073/pnas.95.22.13012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu ZH, Shi Y, Tibbetts RS, Miyamoto S. Molecular linkage between the kinase ATM and NF-kappaB signaling in response to genotoxic stimuli. Science. 2006;311(5764):1141–1146. doi: 10.1126/science.1121513. [DOI] [PubMed] [Google Scholar]
- 152.Wu L, Shao L, An N, Wang J, Pazhanisamy S, Feng W, Hauer-Jensen M, Miyamoto S, Zhou D. IKKbeta regulates the repair of DNA double-strand breaks induced by ionizing radiation in MCF-7 breast cancer cells. PLoS One. 2011;6(4):e18447. doi: 10.1371/journal.pone.0018447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Sasaki T, Abiko N, Sugino Y, Nitta K. Dependence on chain length of antitumor activity of (1 leads to 3)-beta-D-glucan from Alcaligenes faecalis var. myxogenes IFO 13140, and its acid-degraded products. Cancer Res. 1978;38(2):379–383. [PubMed] [Google Scholar]
- 154.Kim HS, Park KH, Lee HK, Kim JS, Kim YG, Lee JH, Kim KH, Yun J, Hwang BY, Hong JT. Curdlan activates dendritic cells through dectin-1 and toll-like receptor 4 signaling. Int Immunopharmacol. 2016;39:71–78. doi: 10.1016/j.intimp.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 155.Kim JY, Welsh EA, Oguz U, Fang B, Bai Y, Kinose F, Bronk C, Remsing Rix LL, Beg AA, Rix U, Eschrich SA, Koomen JM, Haura EB. Dissection of TBK1 signaling via phosphoproteomics in lung cancer cells. Proc Natl Acad Sci U S A. 2013;110(30):12414–12419. doi: 10.1073/pnas.1220674110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Nottingham LK, Yan CH, Yang X, Si H, Coupar J, Bian Y, Cheng TF, Allen C, Arun P, Gius D, Dang L, Van Waes C, Chen Z. Aberrant IKKalpha and IKKbeta cooperatively activate NF-kappaB and induce EGFR/AP1 signaling to promote survival and migration of head and neck cancer. Oncogene. 2014;33(9):1135–1147. doi: 10.1038/onc.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Manna S, Singha B, Phyo SA, Gatla HR, Chang TP, Sanacora S, Ramaswami S, Vancurova I. Proteasome inhibition by bortezomib increases IL-8 expression in androgen-independent prostate cancer cells: the role of IKKalpha. J Immunol. 2013;191(5):2837–2846. doi: 10.4049/jimmunol.1300895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chariot A. The NF-κB-independent functions of IKK subunits in immunity and cancer. Trends Cell Biol. 2009;19(8):404–413. doi: 10.1016/j.tcb.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 159.Koppe C, Verheugd P, Gautheron J, Reisinger F, Kreggenwinkel K, Roderburg C, Quagliata L, Terracciano L, Gassler N, Tolba RH. IκB kinaseα/β control biliary homeostasis and hepatocarcinogenesis in mice by phosphorylating the cell-death mediator receptor-interacting protein kinase 1. Hepatology. 2016;64(4):1217–1231. doi: 10.1002/hep.28723. [DOI] [PubMed] [Google Scholar]


