Abstract
Resistance of bacteria to antibiotics is a public health concern worldwide due to the increasing failure of standard antibiotic therapies. Antimicrobial photodynamic inactivation (aPDI) is a promising non-antibiotic alternative for treating localized bacterial infections that uses non-toxic photosensitizers and harmless visible light to produce reactive oxygen species and kill microbes. Phenothiazinium photosensitizers like methylene blue (MB) and toluidine blue O are hydrophobic cations that are naturally expelled from bacterial cells by multidrug efflux pumps, which reduces their effectiveness. We recently reported the discovery of a NorA efflux pump inhibitor-methylene blue (EPI-MB) hybrid compound INF55-(Ac)en-MB that shows enhanced photodynamic inactivation of the Gram-positive bacterium methicillin-resistant Staphylococcus aureus (MRSA) relative to MB, both in vitro and in vivo. Here, we report the surprising observation that INF55-(Ac)en-MB and two related hybrids bearing the NorA efflux pump inhibitors INF55 and INF271 also show enhanced aPDI activity in vitro (relative to MB) against the Gram-negative bacteria Escherichia coli and Acinetobacter baumannii, despite neither species expressing the NorA pump. Two of the hybrids showed superior effects to MB in murine aPDI infection models. The findings motivate wider exploration of aPDI with EPI-MB hybrids against Gram-negative pathogens and more detailed studies into the molecular mechanisms underpinning their activity.
Keywords: Antimicrobial Photodynamic Inactivation, Efflux Pump Inhibitor, MethyleneBlue, Escherichia coli, Acinetobacter baumannii
Graphical abstract

Antibiotic resistance has emerged as a significant threat to global public health,1-3 with the diminishing treatment options for several infections leading to commentary that we are approaching the end of the ‘golden-age’ of antibiotics.4-6 Resistance in the Gram-positive bacteria methicillin resistant Staphylococcus aureus (MRSA) is extensive in US hospitals and healthcare facilities,7 where it accounts for more than 60% of S. aureus isolates and kills 23,000 patients each year.8
Drug resistant Gram-negative bacteria like Escherichia coli and Acinetobacter baumannii are increasingly causing life-threatening infections in hospitals,6, 9, 10 with an estimated 12% of critical infections caused by E. coli alone.11 Data from the Centres for Disease Control and Prevention (CDC) shows that Acinetobacter baumanii causes 2% of all nosocomial infections and 7% of infections in critically ill patients on mechanical ventilators.12 It has been estimated that 63% of the 12,000 annual Acinetobacter infections are multidrug resistant and cause 500 deaths annually.
Antimicrobial photodynamic inactivation (aPDI) is an emerging non-antibiotic alternative for treating localized infections and countering microbial resistance.14, 15 In this approach, photosensitizing dyes (PS) like methylene blue (MB) and toluidine blue O (TBO) (Figure 1) are illuminated with red light to produce reactive oxygen species (ROS) (e.g. singlet oxygen, 1O2 and hydroxyl radicals, •OH) that kill microbes.16, 17 The approach is used routinely in dentistry18, 19 and in some dermatological treatments.20, 21
Figure 1.

Structures of phenothiazinium photosensitisers methylene blue (MB) and toluidine blue O (TBO) and efflux pump inhibitor-MB hybrids 1-3. Structures of the NorA efflux pump inhibitors INF55 and INF27113 are also shown.
Over the past ten years the powerful killing effect of aPDI has been demonstrated against a wide variety of Gram-positive and Gram-negative bacteria,22, 23 with MRSA being the focus of several studies.24-26 One of the limitations when using phenothiazinium salts in aPDI is that as hydrophobic cations, these photosensitizers are natural substrates for bacterial multi-drug efflux pumps, which serve to rapidly expel the compounds from cells and reduce aPDI effectiveness,27 presumably by lowering the concentration of intracellular ROS. It was shown that aPDI with phenothiazinium salts can be enhanced in S. aureus when used in combination with NorA efflux pump inhibitors (EPI).28 Based on these observations, we postulated that covalently linking NorA inhibitors to a phenothiazinium PS to form a single EPI-MB hybrid compound might have similar effects, and we recently prepared sixteen such hybrids and reported their aPDI activities against S. aureus.29 Two of the hybrids incorporating the NorA EPI INF55 (1 and 2) and one containing the NorA EPI INF271 3 showed the highest in vitro aPDI of MRSA in vitro. The most potent hybrid 2 (denoted INF55-(Ac)en-MB) showed enhanced aPDI activity and wound healing effects (relative to MB) in a murine MRSA wound infection model. In the current study, we examined the in vitro and in vivo aPDI activities of EPI-MB hybrids 1-3 against two representative Gram-negative bacteria, E. coli and A. baumannii.
In vitro aPDI
E. coli wild-type (K-12) cells and an isogenic TolC efflux pump knock-out strain JW5503-1 (TolC-) were incubated with MB and hybrids 1-3 over the concentration range 1-20 μM and illuminated with red light (652 nm) at 6 J/cm2. CFUs were counted from serially diluted aliquots and the results plotted as survival fractions verses compound concentration (Figure 2). MB and the hybrids showed no killing effect against either strain in the dark (Supplementary Data Figure S1 and S2). For the wild-type strain, illumination in the presence of MB produced a 2log10 kill at 10 μM, which increased to 2.5log10 at 20 μM. MB showed similar killing at 10 μM against the TolC mutant strain with higher killing (3.5 log10) at 20 μM. The increased susceptibility of the TolC- mutant was consistent with MB serving as a TolC efflux substrate.30 Hybrid 1 produced a 2log10 kill against the wild-type strain at 10 μM and a 4log10 kill at 20 μM. Against the TolC- strain, hybrid 1 produced a 2log10 kill at 10 μM that increased to 7log10 at 20 μM. For hybrid 2, a 4log10 kill was observed against the wild-type strain at 10 μM, which increased to 6log10 at 20 μM. Exceptional potency was seen with 2 against the TolC- strain, where a 6log10 kill was observed at 10 μM and almost complete eradication was achieved at 20 μM. Hybrid 3 produced a 3log10 kill at the highest concentration (20 μM) against the wild-type strain and 4.5log10 against the TolC- mutant. The increased activity of all three hybrids against the TolC- strain relative to the wild-type suggests they may be substrates for this pump.
Figure 2.

aPDI of E. coli wild-type (WT, K-12) and TolC knockout (TolC-, JW5503-1) strains using: (a) MB, (b) 1, (c) 2 and (d) 3. Cells were illuminated with 100 mW/cm2 red light (652 nm, 6 J/cm2) and survival fractions determined. Data represent the mean ± SEM from three independent experiments.
aPDI of A. baumannii was examined in vitro using the wild-type strain AB007. MB and the three hybrids showed no killing of AB007 in the dark over the concentration range 1-20 μM (Figure 3). Following illumination, hybrids 2 and 3 showed similar aPDI potency to MB at 20 μM (∼4log10 kill), with hybrid 1 producing an extra log10 kill at this concentration.
Figure 3.

aPDI of Acinetobacter baumannii AB007 (and dark controls) using: (a) MB, (b) 1, (c) 2 and (d) 3 over the concentration range 1-20 μM against. Data represent the mean ± SEM from three independent experiments.
In vivo aPDI of E. coli with hybrid 2
Having shown the highest aPDI potency against the two E. coli strains in vitro, hybrid 2 was evaluated alongside MB for in vivo aPDI efficacy using a mouse full-thickness third-degree burn E. coli infection model.31 A pathogenic variant of bioluminescent enteropathogenic E. coli (EPEC, WS2572) was inoculated into burns on the shaved dorsal surfaces of mice and the infected areas were treated with solutions containing MB or hybrid 2. The areas were illuminated with 652 nm red light and luminescence images captured. The normalized luminescence emanating from wounds (relative luminescence units, RLU) during the ‘light treatment’ phase of the experiment was plotted as a function of applied light fluence (Figure 4(a)).
Figure 4.

(a) Bioluminescence of E. coli-infected mouse burn wounds during initial ‘light-treatment’ phase of experiment: Group A - light controls (no compound), Group B - MB dark control, Group C - MB with aPDI, Group D - hybrid 2 dark control and Group E - hybrid 2 with aPDI. MB or hybrid (40 μL of 250 μM stock solution) was applied to infection sites. Data represent the mean (± SEM) normalized relative luminescence units (RLU) emanating from the burn wounds of 4-6 mice in each group during application of 0, 12, 36, 84, 108 and 120 J/cm2 red light (652 nm). (b) Fourteen day post-treatment monitoring of burn wound infection site luminescence.
The light only control cohort (Group A) showed a slight (∼10%) reduction in luminescence following application of the highest fluence (120 J/cm2). Application of MB in the absence of light (Group B) produced a ∼25% reduction in luminescence at the same fluence, while aPDI with MB (Group C) resulted in a ∼90% reduction in luminescence. In the absence of light, hybrid 2 (Group D) showed a slightly higher killing than MB (∼40% reduction in luminescence), while aPDI with hybrid 2 (Group E) produced a remarkable ∼80% reduction in bacterial luminescence at low fluence (36 J/cm2) and total loss of the luminescence signal at 84 J/cm2.
Post-treatment monitoring of infection sites by capturing daily bioluminescence images for 14 days (Figure 4(b)) revealed a slight rebound in bacterial load for the controls (Group A) and MB treated groups (Groups B and C) 1 day after infection/treatment. Infections in the control groups A, B and D were all resolved (i.e. no luminescence detected) within 13-14 days, while for the MB aPDI cohort (Group C) infections were resolved after 12 days. aPDI with hybrid 2 (Group E) produced a lower bacterial burden compared to all othergroups throughout the entire monitoring period and the infections were resolved more rapidly (10 days).
In vivo aPDI of A. baumannii with hybrid 1
Hybrid 1 was tested for in vivo aPDI efficacy alongside MB using a mouse needle back-scratch wound abrasion A. baumannii infection model.32 Bioluminescent A. baumannii (strain AB Iraqi 007) was inoculated into needle-scratch wounds on the shaved dorsal surfaces of mice and the infected areas were treated with solutions containing MB or hybrid 1. Control and aPDI treatment groups (Groups A-E) and infection site monitoring were as for the E. coli burn model.
The light only controls (Group A) showed no reduction in the luminescence signal over the 20 min ‘light-treatment’ phase of the experiment, confirming that the A. baumannii infection was stable over this period (Figure 5(a)). The MB (Group B) and hybrid 1 (Group D) dark controls produced slight reductions (<25%) in luminescence during this period. A 50% reduction in the luminescence signal was seen following aPDI with MB (Group C) at 36 J/ cm2 and total loss of the signal occurred at 108 J/cm2. aPDI with hybrid 1 (Group E) showed an impressive 95% reduction in luminescence at low fluence (36 J/cm2) and complete loss of the signal at 84 J/cm2.
Figure 5.
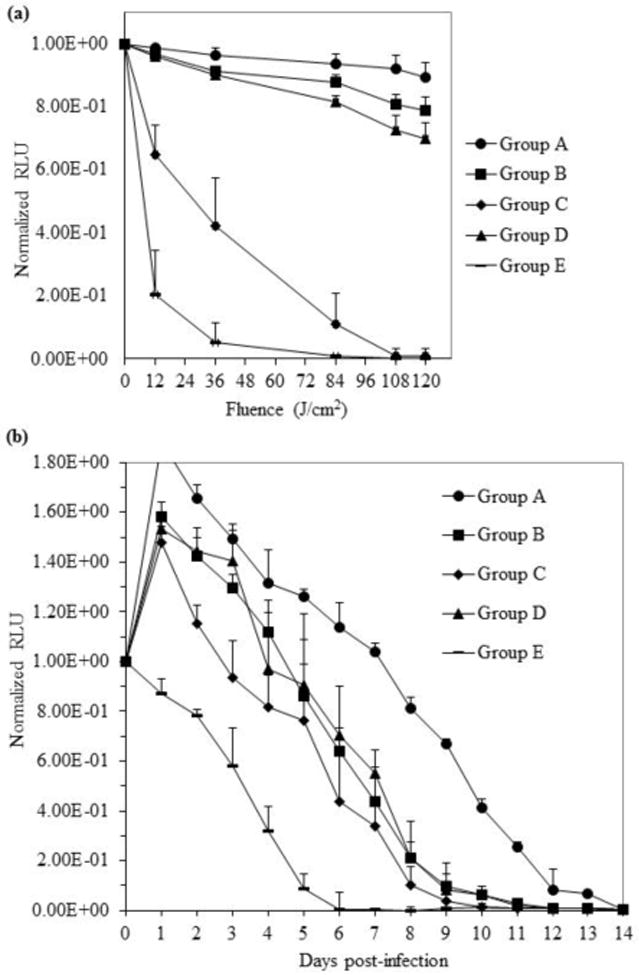
(a) Bioluminescence of A. baumannii-infected mouse back scratch abrasion wounds during initial ‘light-treatment’ phase of experiment: Group A - light control (no compound), Group B - MB dark control, Group C - MB with aPDI, Group D - hybrid 1 dark control and Group E - hybrid 1 with aPDI. MB or hybrid (40 μL of a 250 μM stock solution) was applied to infection sites. Data represent the mean (± SEM) normalized relative luminescence units (RLU) emanating from the scratch wounds of 4-6 mice in each group after application of 0, 12, 36, 84, 108 and 120 J/cm2 red light (652 nm). (b) Fourteen day post-treatment monitoring of wound infection site luminescence.
A rebound in bacterial load (50% or greater) was observed 1 day after infection/treatment in all cohorts except Group E (aPDI with 1) (Figure 5(b)). Low level luminescence remained in control Group A at Day 14, while the signal disappeared after 12 days in the presence of MB in the dark (Group B). aPDI with MB (Group C) produced slightly lower bacterial loads throughout the monitoring period and the luminescence signal had disappeared after 10 days. Treatment with hybrid 1 in the dark (Group D) reduced the bacterial load at a rate that paralleled the MB dark control, with infections resolving within 12 days. aPDI with 1 (Group E) showed lower bacterial loads than all other cohorts throughout the entire monitoring period and the infections were resolved within 6 days. Bioluminescence images captured from representative animals in Groups A-E during the first 6 days of the monitoring period are provided in the Supplementary Data (Figure S3).
Previous work by Tegos et al. showed that phenothiazinium-based photosensitizers such as MB are substrates for bacterial MDR efflux pumps and that these pumps can expel photosensitizers from cells leading to reduced aPDI effectiveness.27 They also showed that aPDI with MB against the Gram-positive bacterium S. aureus is enhanced by the co-presence of inhibitors of the major facilitator efflux pump NorA.27 We recently reported that synthetic hybrids formed by covalently attaching NorA pump inhibitors to MB also enhance aPDI of S. aureus,29 with three leading hybrids 1-3 showing significant ROS generation upon illumination, potent aPDI of S. aureus in vitro and higher intracellular accumulation in S. aureus cells than MB. When hybrid 2 (INF55-(Ac)en-MB) was advanced to in vivo aPDI studies it outperformed MB by all measures in a murine back-scratch S. aureus infection model.
Buoyed by these findings, we chose to study the aPDI effects of 1-3 against two representative Gram-negative bacteria, i.e. E. coli and A. baumannii, despite neither having previously been shown to express membrane efflux pumps that are inhibited by INF55 or INF271. Indeed these two EPIs to date have only been shown to inhibit the NorA pump in S. aureus.33 Nevertheless, Gram-negative bacteria are known to be less susceptible to extracellular singlet oxygen than Gram-positive species,34,35 suggesting that a hybrid approach that could increase intracellular ROS was worth investigating.
The major efflux pump in E. coli comprises the outer membrane channel protein TolC and two other proteins AcrA and AcrB, which together form the tripartite efflux system AcrAB-TolC; a member of the resistance nodulation division (RND) superfamily.36 Several reports have shown that phenothiazinium salts are efflux substrates in E. coli.27,37,38 When tested against E. coli wild-type cells and a TolC knockout mutant, all three hybrids showed greater aPDI than MB against both strains. When the most potent hybrid 2 was evaluated for aPDI efficacy in a murine E. coli burn infection model it showed greater aPDI than MB during the ‘light-treatment’ phase of the experiment, lower bacterial counts throughout the post-treatment monitoring period and more rapid resolution of the infection. These results suggest that hybrid 2 is either a poorer substrate for E. coli pumps than MB or it acts directly as a pump inhibitor, both of which would lead to higher intracellular concentrations of the hybrid and higher intracellular ROS during aPDI. Further experiments are ultimately required, however, to confirm which (if any) E. coli pumps are targeted by 2, how they are affected and whether these effects play a dominant role in the enhanced E. coli aPDI seen with 2.
The most prevalent pumps in A. baumannii are members of the RND superfamily and include the AdeABC two-component regulatory system AdeIJK and AdeFGH. Non-RND efflux systems have also been characterised in A. baumannii.39 When tested in vitro for aPDI of A. baumannii AB007, hybrids 2 and 3 showed no increase in activity relative to MB. However, hybrid 1 showed 1log10 greater killing than MB at the highest concentration (20 μM) and was subsequently found to outperform MB in a murine aPDI A. baumannii infection model. These results are consistent with 1 (but not 2 or 3) being a poorer efflux substrate than MB in A. baumannii and possibly a pump inhibitor, although alternative explanations are plausible. For example, the physicochemical properties of 1 may engender higher affinity for A. baumannii cell surface components relative to MB and 2/3, leading to higher cell-localized concentrations of ROS during aPDI and greater lethality. The relationship between higher cell surface affinity of photosensitizers and increased aPDI has been noted.40
In conclusion, this study demonstrates that attaching NorA EPIs to MB can increase aPDI effectiveness against the Gram-negative pathogens E. coli and A. baumannii in vitro and in vivo. Further experiments to establish whether INF55, INF271 and the hybrids are inhibitors of efflux pumps in Gram-negative bacteria will shed light on the underlying mechanisms responsible for the observed increases in aPDI efficacy relative to MB. Hybrids containing MB attached to known inhibitors of Gram-negative efflux pumps (e.g. Phenyl-arginine-beta-naphthylamide, PaβN)41 would be of interest in future studies.
Supplementary Material
Acknowledgments
We thank the University of Wollongong (Wollongong, Australia) and Massachusetts General Hospital (Boston, USA) for supporting this work. The study was partially funded by the US NIH (R01 AI076372 to J.B.B. and M.J.K.; AI050875 to M.R.H.).
Abbreviations
- aPDI
Antimicrobial photodynamic inactivation
- MB
methylene blue
- EPI-MB
efflux pump inhibitor-methylene blue hybrid
- MRSA
methicillin-resistant Staphylococcus aureus
- aPDI
antimicrobial photodynamic inactivation
- PS
photosensitizing dye
- ROS
reactive oxygen species
- •OH
hydroxyl radicals
- EPEC
bioluminescent enteropathogenic E. coli
- BHI
brain heart infusion
- CrEL
Cremophor EL
- CFU
colony forming units
- NIH
national institutes of health
- RLU
relative luminescence units
- RND
resistance nodulation division
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Ciorba V, Odone A, Veronesi L, Pasquarella C, Signorelli C. Antibiotic resistance as a major public health concern: epidemiology and economic impact. Annali Di Igiene: medicina preventiva e di comunita. 2015;27:562. doi: 10.7416/ai.2015.2048. [DOI] [PubMed] [Google Scholar]
- 2.Roca I, Akova M, Baquero F, et al. The global threat of antimicrobial resistance: science for intervention. New Microbes New Infect. 2015;6:22. doi: 10.1016/j.nmni.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prestinaci F, Pezzotti P, Pantosti A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog Glob Health. 2015;109:309. doi: 10.1179/2047773215Y.0000000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spellberg B, Gilbert DN. The future of antibiotics and resistance: A tribute to a career of leadership by John Bartlett. Clin Infect Dis. 2014;59:S71. doi: 10.1093/cid/ciu392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spellberg B. The future of antibiotics. Crit Care. 2014;18:228. doi: 10.1186/cc13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li XZ, Plésiat P, Nikaido H. The challenge of efflux-mediated antibiotic resistance in gram-negative bacteria. Clin Microbiol Rev. 2015;28:337. doi: 10.1128/CMR.00117-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rossolini GM, Arena F, Pecile P, Pollini S. Update on the antibiotic resistance crisis. Curr Opin Pharmacol. 2014;18:56. doi: 10.1016/j.coph.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 8.CDC. [Accessed April 10, 2017];Centers for Disease Control and Prevention, Office of Infectious Disease Antibiotic resistance threats in the United States. 2013 https://www.cdc.gov/drugresistance/threat-report-2013/index.html.
- 9.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehrad B, Clark NM, Zhanel GG, Lynch JP. Antimicrobial resistance in hospital-acquired gram-negative bacterial infections. Chest. 2015;147:1413. doi: 10.1378/chest.14-2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sievert DM, Ricks P, Edwards JR, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2009-2010. Infect Control Hosp Epidemiol. 2013;34:1. doi: 10.1086/668770. [DOI] [PubMed] [Google Scholar]
- 12.CDC. [Accessed April 10, 2017];Acinetobacter in Healthcare Settings. 2010 https://www.cdc.gov/hai/organisms/acinetobacter.html.
- 13.Markham PN, Westhaus E, Klyachko K, Johnson ME, Neyfakh AA. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob Agents Chemother. 1999;43:2404. doi: 10.1128/aac.43.10.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainwright M. Photodynamic antimicrobial chemotherapy (PACT) J Antimicrob Chemother. 1998;42:13. doi: 10.1093/jac/42.1.13. [DOI] [PubMed] [Google Scholar]
- 15.Zeina B, Greenman J, Purcell WM, Das B. Killing of cutaneous microbial species by photodynamic therapy. Br J Dermatol. 2001;144:274. doi: 10.1046/j.1365-2133.2001.04013.x. [DOI] [PubMed] [Google Scholar]
- 16.Dai T, Huang YY, Hamblin MR. Photodynamic therapy for localized infections - state of the art. Photodiagnosis Photodyn Ther. 2009;6:170. doi: 10.1016/j.pdpdt.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castano AP, Demidova TN, Hamblin MR. Mechanisms in photodynamic therapy: part one - photosensitizers, photochemistry and cellular localization. Photodiagnosis Photodyn Ther. 2004;1:279. doi: 10.1016/S1572-1000(05)00007-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wilson M. Bactericidal effect of laser light and its potential use in the treatment of plaque-related diseases. Int Dent J. 1994;44:181. [PubMed] [Google Scholar]
- 19.Wilson M. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal disease. J Appl Bacteriol. 1993;75:299. doi: 10.1111/j.1365-2672.1993.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 20.Sperandio FF, Simoes A, Aranha AC, Correa L, Orsini Machado de Sousa SC. Photodynamic therapy mediated by methylene blue dye in wound healing. Photomed Laser Surg. 2010;28:581. doi: 10.1089/pho.2009.2601. [DOI] [PubMed] [Google Scholar]
- 21.Choudhary S, Nouri K, Elsaie ML. Photodynamic therapy in dermatology: a review. Lasers Med Sci. 2009;24:971. doi: 10.1007/s10103-009-0716-x. [DOI] [PubMed] [Google Scholar]
- 22.Fotinos N, Convert M, Piffaretti JC, Gurny R, Lange N. Effects on gram-negative and gram-positive bacteria mediated by 5-aminolevulinic acid and 5-aminolevulinic acid derivatives. Antimicrob Agents Chemother. 2008;52:1366. doi: 10.1128/AAC.01372-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schastak S, Ziganshyna S, Gitter B, Wiedemann P, Claudepierre T. Efficient photodynamic therapy against gram-positive and gram-negative bacteria using THPTS, a cationic photosensitizer excited by infrared wavelength. PLoS One. 2010;5:e116741. doi: 10.1371/journal.pone.0011674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ferro S, Ricchelli F, Monti D, Mancini G, Jori G. Efficient photoinactivation of methicillin-resistant Staphylococcus aureus by a novel porphyrin incorporated into a poly-cationic liposome. Int J Biochem Cell Biol. 2007;39:1026. doi: 10.1016/j.biocel.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 25.Nitzan Y, Gozhansky S, Malik Z. Effect of photoactivated hematoporphyrin derivative on the viability of Staphylococcus aureus. Curr Microbiol. 1983;8:279. [Google Scholar]
- 26.Fang FuX, Yao YM. Antimicrobial photodynamic therapy for methicillin-resistant Staphylococcus aureus infection. BioMed Res Int. 2013;2013 doi: 10.1155/2013/159157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tegos GP, Hamblin MR. Phenothiazinium antimicrobial photosensitizers are substrates of bacterial multidrug resistance pumps. Antimicrob Agents Chemother. 2006;50:196. doi: 10.1128/AAC.50.1.196-203.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tegos GP, Masago K, Aziz F, Higginbotham A, Stermitz FR, Hamblin MR. Inhibitors of bacterial multidrug efflux pumps potentiate antimicrobial photoinactivation. Antimicrob Agents Chemother. 2008;52:3202. doi: 10.1128/AAC.00006-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rineh A, Dolla NK, Ball AR, et al. Attaching the NorA efflux pump inhibitor INF55 to methylene blue enhances antimicrobial photodynamic inactivation of methicillin-resistant Staphylococcus aureus in vitro and in vivo. ACS Infect Dis. 2017;3:756. doi: 10.1021/acsinfecdis.7b00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yu QZEW. Microbial efflux pumps. Norfolk, UK: Caister Academic Press; 2013. [Google Scholar]
- 31.Huang L, Wang M, Dai T, et al. Nanomedicine. Vol. 9. London, England: 2014. Antimicrobial photodynamic therapy with decacationic monoadducts and bisadducts of [70]fullerene: in vitro and in vivo studies; p. 253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai T, Tegos GP, Zhiyentayev T, Mylonakis E, Hamblin MR. Photodynamic therapy for methicillin-resistant Staphylococcus aureus infection in a mouse skin abrasion model. Lasers Surg Med. 2010;42:38. doi: 10.1002/lsm.20887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tegos G, Stermitz FR, Lomovskaya O, Lewis K. Multidrug pump inhibitors uncover remarkable activity of plant antimicrobials. Antimicrob Agents Chemother. 2002;46:3133. doi: 10.1128/AAC.46.10.3133-3141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang L, Xuan Y, Koide Y, Zhiyentayev T, Tanaka M, Hamblin MR. Type I and Type II mechanisms of antimicrobial photodynamic therapy: An in vitro study on Gram-negative and Gram-positive bacteria. Lasers Surg Med. 2012;44:490. doi: 10.1002/lsm.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valduga G, Bertoloni G, Reddi E, Jori G. Effect of extracellularly generated singlet oxygen on gram-positive and gram-negative bacteria. J Photochem Photobiol B, Biology. 1993;21:81. doi: 10.1016/1011-1344(93)80168-9. [DOI] [PubMed] [Google Scholar]
- 36.Koronakis V. TolC - the bacterial exit duct for proteins and drugs. FEBS Letters. 2003;555:66. doi: 10.1016/s0014-5793(03)01125-6. [DOI] [PubMed] [Google Scholar]
- 37.Minnock A, Vernon DI, Schofield J, Griffiths J, Parish JH, Brown SB. Mechanism of uptake of a cationic water-soluble pyridinium zinc phthalocyanine across the outer membrane of Escherichia coli. Antimicrob Agents Chemother. 2000;44:522. doi: 10.1128/aac.44.3.522-527.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Molnar J, Hever A, Fakla I, Fischer J, Ocsovski I, Aszalos A. Inhibition of the transport function of membrane proteins by some substituted phenothiazines in E. coli and multidrug resistant tumor cells. Anticancer Res. 1997;17:481. [PubMed] [Google Scholar]
- 39.Coyne S, Courvalin P, Perichon B. Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother. 2011;55:947. doi: 10.1128/AAC.01388-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamblin MR, O'Donnell DA, Murthy N, et al. Polycationic photosensitizer conjugates: effects of chain length and gram classification on the photodynamic inactivation of bacteria. J Antimicrob Chemother. 2002;49:941. doi: 10.1093/jac/dkf053. [DOI] [PubMed] [Google Scholar]
- 41.Pannek S, Higgins PG, Steinke P, et al. Multidrug efflux inhibition in Acinetobacter baumannii: comparison between 1-(1-naphthylmethyl)-piperazine and phenyl-arginine-beta-naphthylamide. J Antimicrob Chemother. 2006;57:970. doi: 10.1093/jac/dkl081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


