Abstract
The timing of the occurrence of a swallow in a respiratory cycle is critical for safe swallowing, and changes with infant development. Infants with damage to the recurrent laryngeal nerve, which receives sensory information from the larynx and supplies the intrinsic muscles of the larynx, experience a significant incidence of dysphagia. Using our validated infant pig model, ewe determined the interaction between this nerve damage and the coordination between respiration and swallowing during postnatal development. We recorded 23 infant pigs at two ages (neonatal and older, pre-weaning) feeding on milk with barium using simultaneous highspeed videofluroscopy and measurements of thoracic movement. With a complete linear model, we tested for changes with maturation, and whether these changes are the same in control and lesioned individuals. We found (1) the timing of swallowing and respiration coordination changes with maturation; (2) no overall effect of RLN lesion on the timing of coordination, but (3) a greater magnitude of maturational change occurs with RLN injury. We also determined that animals with no surgical intervention did not differ from animals that had surgery for marker placement and a sham procedure for nerve lesion. The coordination between respiration and swallowing changes in normal, intact individuals to provide increased airway protection prior to weaning. Further, in animals with an RLN lesion, the maturation process has a larger effect. Finally, these results suggest a high level of brainstem sensorimotor interactions with respect to these two functions.
Keywords: Deglutition, Respiration, recurrent laryngeal nerve, sensorimotor, infant, development, animal model
Introduction
Swallowing is an intricate process that requires coordination with respiration since both functions use the oropharynx. At birth all mammals, including humans, have a high larynx that projects into the space where the oropharynx and laryngopharynx meet [25]. This configuration helps ensure that penetration or aspiration, i.e., liquid entering the upper or lower airway, does not occur. As the newborn transitions from being a neonate into infancy, deglutition must continue to be efficient and safe while anatomical and functional changes occur. Human oropharyngeal anatomy begins to resemble adult anatomy by 5 months as the larynx descends [70], with additional changes to the vocal folds and the mucosa covering them [59]. In addition to anatomical changes, both human and non-human mammalian infants begin utilizing faster and more rhythmic sucking rates and have more stable suck-swallow rhythms [22, 29].
Mechanisms of airway protection also change. The human infant swallows around rather than over the larynx, and the epiglottis remains stationary. The timing of respiration must also be controlled to prevent penetration and aspiration. In infants, abrupt closure of the vocal cords in both phases of respiration can be triggered by esophageal mechanostimulation, which in turn is caused by esophageal stretching from a bolus [40]. In adults, swallowing occurs during the expiratory phase with respiration resuming with continued expiration [53].
Problems in this coordination are well documented, due to specific disease entities or craniofacial birth defects, esophageal atresia, metabolic disorders, and iatrogenic causes. Pathophysiology of the coordination between respiration and deglutition can lead to acute issues, such as aspiration pneumonia. It may also result in life-long complications such as dysphagia, lung injury, aspiration pneumonia, and nutrition compromise [10, 51]. Yet little is known about the intricacies of the coordination of deglutition and respiration and the physiological relationship in the context of postnatal maturation.
A further, but not infrequent, complication in the relationship between swallowing and respiration occurs as a result of recurrent laryngeal nerve (RLN) injury. Iatrogenic RLN damage results in variable degrees of dysphagia severity and recovery [12, 16, 45, 54, 57]. In neonates in particular, RLN damage occurs subsequent to patent ductus arteriosus (PDA) ligation surgery [50, 52]. Our previous work demonstrated that despite a controlled injury to the RLN, variability in the extent and degree of dysphagia existed among individuals [32, 33].
Ethical constraints associated with using human infants as test subjects limits direct evidence of the relationship between swallowing and respiration under the setting of a recurrent laryngeal nerve (RLN) injury. We used a translational neonatal pig model that has been validated for evaluation of mammalian deglutition, notably infant suckling and swallowing [11, 24] to study the relationship between the physiologic processes of respiration and deglutition. Because of similar oropharyngeal anatomy, tongue movements and head positioning, neonatal pigs are a good model for human infant function [14, 15, 33, 39, 68]. Pigs mature at a faster rate than humans which allows for studying developmental changes more efficiently [17, 25, 27, 28]
Our study aims to provide insight into the relationship between deglutition and respiration during infancy maturation, and the impact of an RLN injury on that coordination. We measured the precise timing of a swallow within a respiratory cycle in infant pigs at different ages, and with and without a controlled RLN lesion. We collected data to test the following hypotheses:
H1: The timing of inspiration relative to swallow changes with maturation of the neonate to a more mature, but pre-weaning infant pig.
H2: An RLN injury will cause a difference in the timing of respiration relative to a swallow in both neonates and more mature infant pigs.
In addition, we tested the interaction between age and treatment with an additional specific hypothesis:
H3: The developmental change in timing of respiration differs between the Lesioned animals and the Controls, that is, early RLN damage impacts the normal maturational changes in swallowing/respiration coordination.
Finally, we tested the impact of our surgical manipulations. Lesioned and control individuals experience a standard surgery [32], for the placement of radio-opaque markers that are used for kinematics studies [33]. Thus our standard “controls” are experimental shams. For one arm of this project, we used animals that had no oral or pharyngeal surgeries, and compared these no-surgery control individuals to the sham surgery individuals. We included an additional hypothesis to determine if placement of markers, including surgical identification of the RLN, impacts our measurements of swallowing and respiration:
H4: No difference exists between the Controls/Surgery group and the No Surgery group at either age, such that the developmental changes are equivalent in these groups.
Materials and Methods
Collection of pigs by caesarian section and postnatal care
All animal work was done in accordance with NEOMED IACUC approval, protocol #17-04-071. Two Yorkshire/Landrace cross pregnant sows were used to obtain neonatal pigs at 114 days of gestation, one day prior to term (Shoup Farms, Wooster, Ohio). The two groups of experimental pigs and all subsequent data collection were done at different times, approximately 6 months apart. Delivering pigs by Cesarean section provides consistency and is relevant to studies using preterm infant pigs at an age where induction for vaginal delivery will not be possible. With the ultimate goal of comparing term infant pigs with preterm infant pigs in future studies, all animals for the study were be delivered by C-section. Although much of the postnatal infant care and design followed previous studies [13, 32, 38], the procedures involving C-section described below established a new protocol for this lab, and are described here for the first time.
The sow was sedated with Telazol (10ml IM), placed on a surgical table, and anesthesia was induced and maintained with isoflurane. Standard aseptic procedures were followed during the C-section. The right mid abdominal flank was shaved and a sterile surgical field was prepared using betadine followed by isopropyl alcohol 70%. The site of the incision was injected with lidocaine (15 ml, SC), sterile drapes were placed, and a 12 cm incision was made to expose the uterus. The neonatal pigs were removed individually by making an incision in the uterus. After the umbilical cord was clamped and cut the pigs were wrapped in a warm towel, fluid in the airways was allowed to drain with additional fluid removed by aspiration. The newborn pigs were placed in a warmed incubator (38–39° C). Pigs with slowed breathing had the chest rubbed and were paired with strong breathers to encourage spontaneous ventilation. Body temperature was maintained between 38 to 40° C. After delivery of the entire litter, the mother was euthanized. We largely followed the protocols previously developed and validated [60, 63]. Within two hours, the neonatal pigs were fed infant pig formula Solustart Pig Milk Replacement, Land o’ Lakes, Arden Mills, MN) from a bottle fitted with a specially designed nipple. Subsequent care followed validated and standard care for infant pigs, which supports normal growth and development [24, 26, 65, 67]. This formula is standard for infant pigs, both in research and agricultural settings, and promotes normal growth [36].
Marker Placement and RLN Surgery
The pigs were assigned to one of three groups: control with surgery (C), control with no surgery (NS) and lesion (L) groups. The control group had surgery for placement of thyroid and hyoid markers, as well as oral markers placed in in the tongue, palate and epiglottis [18, 20, 65]. The recurrent laryngeal nerve was exposed, but not cut. The no surgery group was a control for surgical manipulations. This group had no markers or any other procedure prior to recording data and was used to test if the surgery for marker placement and nerve identification had an impact on subsequent feeding or respiration. The lesion group had markers placed during the same surgery in which the RLN was lesioned, as described below. The right RLN was selected for consistency with data collected previously [32–34]. In the no-lesion group, the nerve was only identified. In the lesion group a 2mm portion of the nerve was ligated and removed. The free ends of the nerve were clipped and displaced to prevent reinnervation [32, 33].
Videofluoroscopy Protocol and Data Recording
At postnatal day 7, swallowing was assessed under videofluoroscopy while measuring respiration using procedures outlined in [32, 33]. The pigs had a respiratory inductance plethysmograph (Powerlab, ADInstruments) placed around the thorax to monitor ventilation during videofluoroscopy to image drinking of pig formula (Solustart Pig Milk Replacement, Land o’ Lakes, Arden Mills, MN) mixed with barium. The fluoroscope (GE9400 C-Arm, 80 kV, 4MA) digitally recorded images at 100 fps (XC 1 M digital video camera, XCitex, Cambridge, MA). The XCitex system is a computer driven digital system that records all fluoroscopic images which are subsequently stored for analysis. The plethysmograph signal, together with a synchronization signal from the digital video system was recorded on an ADInstruments Powerlab 30/16 (Colorado Springs, CO) at 10 kHz. Pigs were allowed to feed, unrestrained, until satiation. An entire sequence video sequence lasted from 40 to 600 seconds, with anywhere from 50 to 1000 swallows. All recording was done with standard XROMM (X-ray Reconstruction of Moving Morphology, http://www.xromm.org/) protocol, including recording the distortion grid and three-dimensional scaling before and after every recording session of animals [3, 8, 30] so that these data could be used for future kinematic studies [33, 34]. After the feeding sessions, images were assessed for quality and the exact frame of each swallow noted for the entire feeding session.
Data for this paper consisted of feeding records from 7 and 17 days. These days were chosen for both practical and clinical reasons. Infant pigs have an accelerated developmental schedule relative to humans, but one with similar scaling [63]. Pigs wean between 21 and 35 days after birth [36], which is equivalent to 18.2–30.5% of the 115 days of gestation. This is similar to the 20–40% of gestation when human infants wean and shorter than the 70–90% for rodents [61]. A seven day old pig can feed from a bottle and nipple, but does not have post-canine teeth. Seven days is our earliest recording because that was the point at which the animals could maintain a stable temperature, and be moved from the housing facility to the XROMM/videofluroscopy suite. At 17 days, the animals are approaching weaning status. Premolars have erupted, and at this age, pigs show interest in solid food [69]. We chose an age prior to weaning, but when the animals are more efficient at feeding. The animals were recorded subsequent to 17 days, but such data did not appear different from that extracted at 17 days. Future studies will include analysis of the data up to 25 days of age.
Data Extraction
Video recordings and plethysmograph signals were examined for quality of image and signal, including an adequate synchronization signal to identify and coordinate the frames between the videofluoroscopy and plethysmograph data. The initial five seconds of feeding were not used as this has been shown to be a different rate than subsequent feeding [31]. The first 10 seconds of swallows and respiration that contained adequate synchronization were selected for analysis.
A swallow was identified at the frame from the high-speed videofluorscopic images when the bolus was condensed in the supraglottic space and had not yet crossed the epiglottis. We justified this type of identification for swallowing initiation from previous work illustrating the maturation of infant pig epiglottis movement [11]. The epiglottis does not always fold during an infant swallow; thus, identification of epiglottis movement was not always possible in the video sequence. All swallows were identified by two people. Intra and inter-rater reliability was determined using a two-step process. Intra rater reliability was assessed by having each rater score three repeats of the same blinded sequences. Inter-rater reliability was assessed by having the two raters score the same sequence. In both cases correlations greater than 95% were achieved. We extracted the entire 10 seconds of signal from the plethysmograph, downsampling to 1kHz. The time of the swallow was identified in this stream, and a 200ms window around the swallow was extracted. The time of the swallow was set as 0.0. We measured the onset of inspiration following the swallow as the delay from time zero to the local minimum of the plethysmograph signal (Fig 1).
Figure 1.
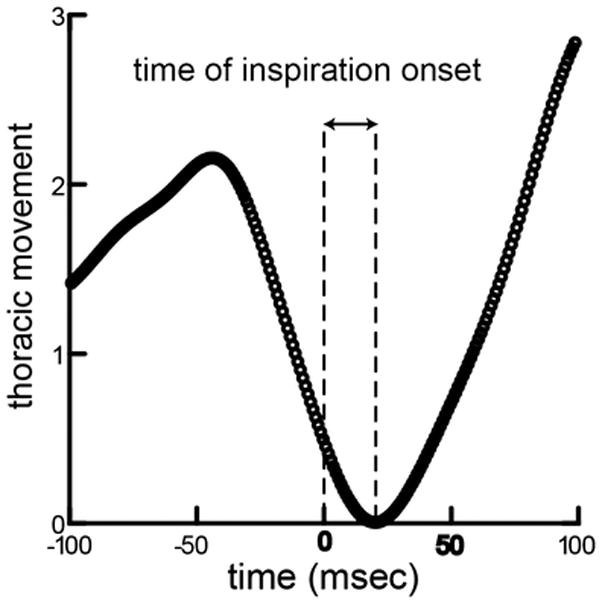
Measurement of time of inspiration onset. The trace shows thoracic movement over time, with time = 0 set to the time milk transits over the laryngeal opening, the precise timing of the swallow. The amount of thoracic movement is idiosyncratic to the animal, depending on their size, and the values are scaled to maximum movement in the sequence. The lag from the swallow to the start of thoracic expansion was measured for each swallow in the data set.
Sample Size and Design of Analysis
The data consisted of two sets of littermates from unrelated sows from the same herd. Within a litter, individuals were randomly assigned to treatment at birth. Originally, there were 28 animals, however some individuals did not survive the first week: 3 individuals in the first litter and 2 in the second litter. This resulted in unequal samples among the treatments, and a total of 23 animals in the final analyses. The first litter included seven C (control), three L (lesion) and four NS (no surgery) surviving piglets. The second litter included four control and five lesion individuals so there were a total of fifteen control and nine lesion animals. There was an overall paired design in the sample of these data, a set of swallows from each animal at two ages, 7 and 17 days. Thus, the unit of analysis was a swallow, with multiple swallows for each animal-age nested within a treatment. There were a total of 700 swallows, 383 control and 317 lesion, with a range of 11 to 40 with a median of 20 swallows in each animal-age group.
We performed two main analyses of the data. To test for age and treatment effect, we used the control and lesion data, from both litters. However, because only the first litter contained a NS (no surgery) group, we used only the C and NS groups for a second analysis to test for a surgery/no surgery effect. This was the most conservative design, and permitted both analyses to include the appropriate random factor.
Statistical Analysis
In each 10 second window (Fig 2.), we measured rates of respiration during feeding. In this case, the unit of analysis was a sequence, and there were 26 sequences. We tested for differences due to age or to treatment using a complete linear model. For all tests, P<0.05 was accepted as the critical level of significance.
Figure 2.
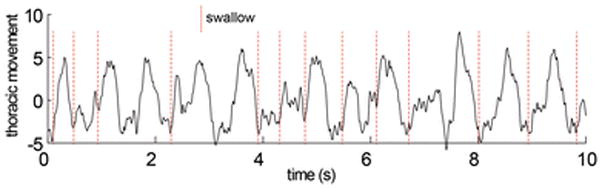
Thoracic movement over a portion of a feeding sequence with swallows indicated by dotted lines. All data consisted of complete sequences of more than 50 swallows. 10–20 swallows per sequence were identified and selected from the XROMM visual data. The time equivalent data from the respiratory data were then extracted using the synchronization signal recorded on both data traces. In this example, neither respiration nor swallowing were regular.
The main response variable was the timing of onset of inspiration following the swallow. There were two predictor variables or main effects, each with two levels: age of animal (7 or 17 days) and treatment group: (C or L). We used a complete ANOVA model, which included two main factors (age and surgical treatment), two random factors (individual nested within litter and experiment/litter), and one interaction between the two main factors. Using this model, we could test the following null hypotheses:
H1: there is no main effect/difference due to age
H2: there is no main effect/difference due to treatment (C, L)
We included two factors that contributed to random variation: individual nested within litter as a random factor and experiment/litter. We also wished to test potential interactions between treatment and age and included an interaction term in the main model. A significant interaction would indicate that the two treatments matured differently with respect to the response variable we measured. A significant interaction specifically tested whether the change in the onset of inspiration from 7 to 17 days was the same in the C and L groups (H3). For each hypothesis, we calculated Cohen’s d as a measure of effect size.
Finally, to determine if surgery had an effect, we compared the C and NS groups using a similar model. In this case, animal was a random factor, and age and treatment were fixed factors, and we included an interaction term.
Results
The thoracic movement at the time of a swallow, movement of milk out of the valleculae across the laryngeal opening, was relatively consistent within a set of swallows. There was usually apnea around the time of swallow for all ages.
In the main model total error variation was 0.000923. The variation due to the random factor individual was 0.000271 and the variation due to experiment was 0.000029, both less than the error variation. Because individual and litter are random factors, there is no significance attached to this value. In the main analysis (Table 1), the test for H1 indicated a significant age effect (p < .0001), but, for H2, no significant treatment effect (p = 0.772). The Cohen’s d measure of effect size for age was 1.07. The timing of inspiration becomes later in the older animals within each treatment (Fig 4), as indicated by the two dotted lines. The value of these differences is, in lesioned animals, 0.049s, with a SE of 0.0048 and in control animals 0.031s with SE of 0.0040. The significant interaction (p < .003), a test for H3, indicates that the change in timing of inspiration relative to the swallow from 7 to 17 days differed between Controls and Lesions. The difference in lengths in the two dotted lines in Figure 4, reflected the significant interaction, indicating that the change in timing is different in the two treatments.
Table 1.
Results from main analysis of full model with age, treatment and their interaction
| Effect | numerator df | denominator df | F-Ratio | p-Value |
|---|---|---|---|---|
| Age | 1 | 443 | 165.407 | <0.0001 |
| treatment | 2 | 443 | 0.084 | 0.772 |
| age * treatment | 2 | 443 | 9.024 | 0.003 |
Figure 4.
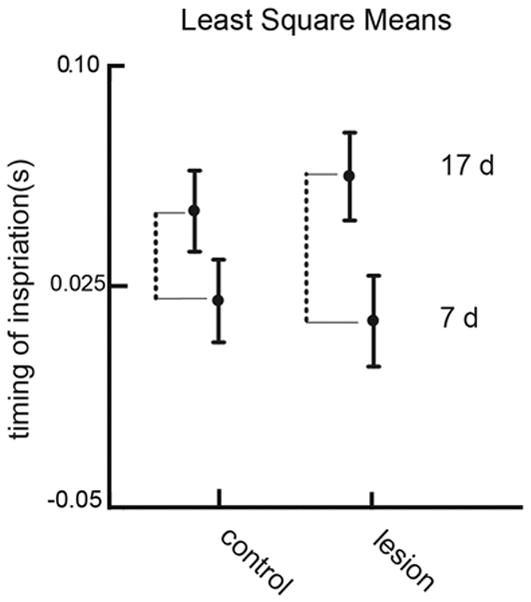
Least square means showing the main effects and the interaction between treatment and age for the timing of inspiration. Error bars represent standard errors of LSM. The dotted line indicates the difference in response due to age within each treatment group. The significant interaction term indicates that this difference in the lesioned group is larger than the difference in the control group.
For H4, the total error variance in the model was 0.002508 and the random effect variation due to individual was 0.000328, nearly an order of magnitude less. The age was a significant main effect but treatment was not (Table 2). There was no significant interaction. This shows that both Controls with surgery and without surgery changed the timing of respiration with age. However, there was no treatment effect or interaction, indicating that there was no effect of the surgery.
Table 2.
Full model results of no-surgery control vs control with surgery
| Effect | numerator df | denominator df | F-Ratio | p-Value |
|---|---|---|---|---|
| Age | 1 | 384 | 12.95 | <0.001 |
| treatment | 1 | 384 | 0.28 | 0.598 |
| age * treatment | 1 | 384 | 0.471 | 0.492 |
Discussion
Validation of Animal Model
The pig model used here has been validated in numerous studies for evaluation of mammalian deglutition[11, 24], as well as testing the impact of sensorimotor deficits in infant oropharyngeal function [14, 15, 33, 39, 68]. Animal studies of fetal, neonatal and postnatal development of respiration, largely experimental in nature, provide more information than human studies, given the ethical limitations of using human infants as test subjects [1, 6, 9, 17, 19]. Understanding of the coordination between respiration and swallowing is limited because of the restrictions of human studies [29, 41, 43], and the difficulty of measuring oropharyngeal function in the neonates of small animal models [60]. The present results also confirm that the surgeries to implant radio-opaque markers, as was done in previous studies [21, 32, 66], as well as the nerve lesion procedure [32–34], do not interfere with normal sensorimotor feedback. Beyond providing a better understanding of the coordination of swallowing and respiration during early postnatal development, our findings contribute to validating the neonatal pig as a translational large animal model for studying coordination of these two functions.
Changes with age in respiration during swallowing
Several anatomical and neurological changes occur while the neonate matures, and deglutition must remain safe and efficient [23, 29, 35, 47–49]. Swallowing frequency and volume, among other feeding parameters, change over neonatal through infant development [2, 46, 49, 61]. The strongest relationship in the data presented here was the increasing delay in the onset of inspiration with age. The difference was significant in our analysis, and the Cohen’s d indicated a large effect size. Yet, the value of this difference was on the order of 50 msec, or 0.05 sec. Because human data is based on more approximate measures (drums attached to hyoid and thorax), the data we collected from our animal model are more precise. In particular we can measure the exact time of the swallow from videofluroscopic images at a time resolution of 100 fps, something that is not possible in human studies. A greater delay between swallow time and onset of inspiration is consistent with airway protection improving with age. However, actual data on occurrence of aspiration at different ages is needed to confirm this link and will form the basis of future studies.
Impact of RLN lesion on coordination of respiration and swallowing
The greater developmental changes in the timing of the swallow that occurred in animals with an RLN lesion, compared to the control animals, suggests a response to the lesion that occurs with development. Such animals are known to change the size of bolus and the kinematics of swallows to generate safe swallows with no aspiration or penetration[34]. Yet, differences in these factors can produce aspiration in similarly aged animals. The next step in these analyses is to examine the variation in safety of these swallows.
The damage to the RLN in human infants frequently occurs at an analogous point in developmental time in the neontal period, usually within the first few weeks of age [58, 62]. It is frequently a result of the need for cardiac surgery, and also associated with preterm birth, and is highly associated with dysphagia or other oropharyngeal problems. What these results speak to are the longitudinal changes that occur post-lesion in this coordination. The lesioned animals received no specific treatment or rehabilitation. Yet, they demonstrated a change in the timing between swallowing and respiration that occurred as a function of development. This suggests that airway concerns have the potential to resolve through maturation.
The role of the RLN in respiration
The specific efferent and afferent functions of the RLN are known for a number of species, including humans, dogs, pigs and rats [4, 5, 44, 56, 64], and include sensation below the vocal folds and motor inputs to the intrinsic muscles of the larynx, excepting cricothyroid. Yet, the role of the RLN in respiration is more complex and less well understood[4]. While ample clinical [7, 42, 55] and basic [37] evidence document that RLN injury produces swallowing deficits, the exact mechanism of the performance problems remains elusive[34]. These results suggest that with detailed and fine-grained studies that include both respiration and swallowing have the potential to clarify this relationship. These results are a first step towards understanding what changes naturally occur with development, and how interruption of the normal laryngeal signals impact this coordination. As RLN lesion changes the relationship between swallowing and respiration during infant development, future research into the mechanism of airway protection failure should incorporate respiration along with swallowing mechanics.
Implications for clinical interventions
These results document differences in plastic change over the course of development that between normal and infants with nerve damage. The changes seen here were for damage occurring on a similar time scale to human iatrogenic issues, early in the neonatal time period. They also suggest that targeted behavioral therapies may be possible that correct for lesion induced changes if applied early in infant development.
Conclusion
Infant pigs increased the delay in the onset of inspiration, following a swallow, as they became older, enhancing the safety of the breath and swallow. This increase was larger in infants that had received an RLN lesion prior to measurement. Because the RLN does not supply the diaphragm, or receive sensation from fields directly involved in swallowing, these results suggest significant brainstem involvement in the coordination of respiration and swallowing. Furthermore, the developmental differences indicate that targeted behavioral therapies may be possible that correct for lesion induced changes if applied early in infant development.
Figure 3.
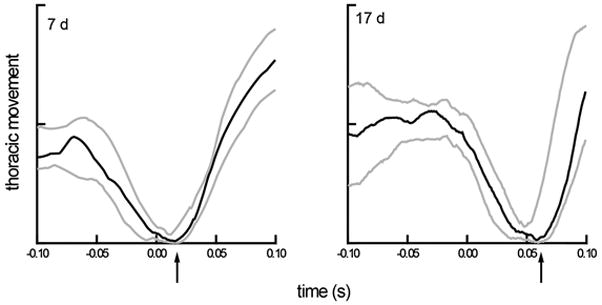
Median thoracic movement and variation (interquartile range) for two sets of 20 swallows, representing two ages, aligned to the time of the swallow (time=0). The solid line is the median value at each time point of 20 respiratory cycles and the grey lines are the upper and lower quartiles from those cycles. The data were collected at 1kHz from the same animal. The value of thoracic movement is idiosyncratic to each animal. The value analyzed is the time of the start of inspiration after the swallow, indicated by the arrow on the x-axis.
Acknowledgments
We thank the CMU at NEOMED for their extensive support of this project.
Footnotes
Compliance with Ethical Standards
This study was funded by NIH 1R01HD088561 to RZG. All authors declare they have no Conflicts of Interest. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed, and was done in accordance with NEOMED IACUC approval, protocol #17-04-071.
Literature Cited
- 1.Abu-Shaweesh JM. Maturation of respiratory reflex responses in the fetus and neonate. Semin Neonatol. 2004;9(3):169–80. doi: 10.1016/j.siny.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Amaizu N, Shulman R, Schanler R, Lau C. Maturation of oral feeding skills in preterm infants. Acta paediatrica (Oslo, Norway: 1992) 2008;97(1):61–7. doi: 10.1111/j.1651-2227.2007.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baier DB, Gatesy SM, Dial KP. Three-dimensional, high-resolution skeletal kinematics of the avian wing and shoulder during ascending flapping flight and uphill flap-running. PLoS One. 2013;8(5):e63982. doi: 10.1371/journal.pone.0063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bautista TG, Xing T, Fong AY, Pilowsky PM. Recurrent laryngeal nerve activity exhibits a 5-HT-mediated long-term facilitation and enhanced response to hypoxia following acute intermittent hypoxia in rat. J Appl Physiol (1985) 2012;112(7):1144–56. doi: 10.1152/japplphysiol.01356.2011. [DOI] [PubMed] [Google Scholar]
- 5.Bautista TG, Dutschmann M. Ponto-medullary nuclei involved in the generation of sequential pharyngeal swallowing and concomitant protective laryngeal adduction in situ. The Journal of physiology. 2014;592(Pt 12):2605–23. doi: 10.1113/jphysiol.2014.272468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bautista TG, Fong AY, Dutschmann M. Spontaneous swallowing occurs during autoresuscitation in the in situ brainstem preparation of rat. Respiratory physiology & neurobiology. 2014;202C:35–43. doi: 10.1016/j.resp.2014.07.015. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin JR, Smith PB, Cotten CM, Jaggers J, Goldstein RF, Malcolm WF. Long-term morbidities associated with vocal cord paralysis after surgical closure of a patent ductus arteriosus in extremely low birth weight infants. J Perinatol. 2010;30(6):408–13. doi: 10.1038/jp.2009.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brainerd EL, Baier DB, Gatesy SM, Hedrick TL, Metzger KA, Gilbert SL, Crisco JJ. X-ray reconstruction of moving morphology (XROMM): precision, accuracy and applications in comparative biomechanics research. J Exp Zool A Ecol Genet Physiol. 2010;313(5):262–79. doi: 10.1002/jez.589. [DOI] [PubMed] [Google Scholar]
- 9.Caminita F, van der Merwe M, Hance B, Krishnan R, Miller S, Buddington K, Buddington RK. A preterm pig model of lung immaturity and spontaneous infant respiratory distress syndrome. Am J Physiol Lung Cell Mol Physiol. 2015;308(2):L118–29. doi: 10.1152/ajplung.00173.2014. [DOI] [PubMed] [Google Scholar]
- 10.Collaco JM, Aherrera AD, Au Yeung KJ, Lefton-Greif MA, Hoch J, Skinner ML. Interdisciplinary pediatric aerodigestive care and reduction in health care costs and burden. JAMA Otolaryngol Head Neck Surg. 2015;141(2):101–5. doi: 10.1001/jamaoto.2014.3057. [DOI] [PubMed] [Google Scholar]
- 11.Crompton AW, Thexton AJ, German RZ. Development of the movement of the epiglottis in infant and juvenile pigs. Zoology. 2008;111:339–349. doi: 10.1016/j.zool.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daya H, Hosni A, Bejar-Solar I, Evans JN, Bailey CM. Pediatric vocal fold paralysis: a long-term retrospective study. Arch Otolaryngol Head Neck Surg. 2000;126(1):21–25. doi: 10.1001/archotol.126.1.21. [DOI] [PubMed] [Google Scholar]
- 13.Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Fukuhara T, Gierbolini-Norat EM, Thexton AJ, German RZ. Unilateral Superior Laryngeal Nerve Lesion in an Animal Model of Dysphagia and Its Effect on Sucking and Swallowing. Dysphagia. 2013 doi: 10.1007/s00455-013-9448-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ding P, Campbell-Malone R, Holman SD, Lukasik SL, Thexton AJ, German RZ. The effect of unilateral superior laryngeal nerve lesion on swallowing threshold volume. The Laryngoscope. 2013;123(8):1942–7. doi: 10.1002/lary.24051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding P, Fung GS, Lin M, Holman SD, German RZ. The effect of bilateral superior laryngeal nerve lesion on swallowing: a novel method to quantitate aspirated volume and pharyngeal threshold in videofluoroscopy. Dysphagia. 2015;30(1):47–56. doi: 10.1007/s00455-014-9572-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans N. Preterm patent ductus arteriosus: A continuing conundrum for the neonatologist? Semin Fetal Neonatal Med. 2015;20(4):272–7. doi: 10.1016/j.siny.2015.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Feldman JL. Chapter 14--looking forward to breathing. Progress in brain research. 2011;188:213–8. doi: 10.1016/B978-0-444-53825-3.00019-X. [DOI] [PubMed] [Google Scholar]
- 18.Franks HA, German RZ, Crompton AW, Hiiemae KM. Mechanism of intra-oral transport in a herbivore, the hyrax (Procavia syriacus) Archives of Oral Biology. 1985;30(7):539–44. doi: 10.1016/0003-9969(85)90054-8. [DOI] [PubMed] [Google Scholar]
- 19.Fregosi RF, Ludlow CL. Activation of upper airway muscles during breathing and swallowing. J Appl Physiol (1985) 2014;116(3):291–301. doi: 10.1152/japplphysiol.00670.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.German RZ, Franks HA. Timing in the movement of jaws, tongue, and hyoid during feeding in the hyrax, Procavia syriacus. Journal of Experimental Zoology. 1991;257(1):34–42. doi: 10.1002/jez.1402570105. [DOI] [PubMed] [Google Scholar]
- 21.German RZ, Crompton AW, Levitch LC, Thexton AJ. The mechanism of suckling in two species of infant mammal: miniature pigs and long-tailed macaques. J Exp Zool. 1992;261:322–330. doi: 10.1002/jez.1402610311. [DOI] [PubMed] [Google Scholar]
- 22.German RZ, Crompton AW. Ontogeny of suckling mechanisms in opossums (Didelphis virginiana) Brain, behavior and evolution. 1996;48(3):157–64. doi: 10.1159/000113194. [DOI] [PubMed] [Google Scholar]
- 23.German RZ, Crompton AW, McCluskey C, Thexton AJ. Coordination between respiration and deglutition in a preterm infant mammal, Sus scrofa. Archives of Oral Biology. 1996;41(6):619–622. doi: 10.1016/0003-9969(96)00023-4. [DOI] [PubMed] [Google Scholar]
- 24.German RZ, Crompton AW, Thexton AJ. The coordination and interaction between respiration and deglutition in young pigs. Journal of Comparative Physiology (A) 1998;182:539–547. doi: 10.1007/s003590050201. [DOI] [PubMed] [Google Scholar]
- 25.German RZ, Crompton AW. The Ontogeny of Feeding in Mammals. In: Schwenk K, editor. Feeding: Form, Function and Evolution in Tetrapod Vertebrates. Academic Press; San Diego: 2000. pp. 449–457. [Google Scholar]
- 26.German RZ, Crompton AW, Thexton AJ. Integration of the reflex pharyngeal swallow into rhythmic oral activity in a neurologically intact pig model. J Neurophysiol. 2009;102(2):1017–25. doi: 10.1152/jn.00100.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.German RZ, Campbell-Malone R, Crompton AW, Ding P, Holman S, Konow N, Thexton AJ. The concept of hyoid posture. Dysphagia. 2011;26(2):97–8. doi: 10.1007/s00455-011-9339-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.German RZ, Crompton AW, Gould FD, Thexton AJ. Animal Models for Dysphagia Studies: What Have We Learnt So Far. Dysphagia. 2017;32(1):73–77. doi: 10.1007/s00455-016-9778-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gewolb IH, Vice FL. Maturational changes in the rhythms, patterning, and coordination of respiration and swallow during feeding in preterm and term infants. Developmental medicine and child neurology. 2006;48(7):589–94. doi: 10.1017/S001216220600123X. [DOI] [PubMed] [Google Scholar]
- 30.Gidmark NJ, Staab KL, Brainerd EL, Hernandez LP. Flexibility in starting posture drives flexibility in kinematic behavior of the kinethmoid-mediated premaxillary protrusion mechanism in a cyprinid fish, Cyprinus carpio. J Exp Biol. 2012;215(Pt 13):2262–72. doi: 10.1242/jeb.070516. [DOI] [PubMed] [Google Scholar]
- 31.Gierbolini-Norat E, Holman S, Ding P, Bakshi S, German R. Variation in the Timing and Frequency of Sucking and Swallowing over an Entire Feeding Session in the Infant Pig Sus scrofa. Dysphagia. 2014:1–8. doi: 10.1007/s00455-014-9532-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gould FD, Lammers AR, Ohlemacher J, Ballester A, Fraley L, Gross A, German RZ. The Physiologic Impact of Unilateral Recurrent Laryngeal Nerve (RLN) Lesion on Infant Oropharyngeal and Esophageal Performance. Dysphagia. 2015;30(6):714–22. doi: 10.1007/s00455-015-9648-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gould FD, Ohlemacher J, Lammers AR, Gross A, Ballester A, Fraley L, German RZ. Central nervous system integration of sensorimotor signals in oral and pharyngeal structures: oropharyngeal kinematics response to recurrent laryngeal nerve lesion. J Appl Physiol (1985) 2016;120(5):495–502. doi: 10.1152/japplphysiol.00946.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gould FDH, Yglesias B, Ohlemacher J, German RZ. Pre-pharyngeal Swallow Effects of Recurrent Laryngeal Nerve Lesion on Bolus Shape and Airway Protection in an Infant Pig Model. Dysphagia. 2017;32(3):362–373. doi: 10.1007/s00455-016-9762-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Greer JJ. Control of breathing activity in the fetus and newborn. Compr Physiol. 2012;2(3):1873–88. doi: 10.1002/cphy.c110006. [DOI] [PubMed] [Google Scholar]
- 36.Helm JW, German RZ. The epigenetic impact of weaning on craniofacial morphology during growth. The Journal of experimental zoology. 1996;276(4):243–53. doi: 10.1002/(SICI)1097-010X(19961101)276:4<243::AID-JEZ1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 37.Hernandez-Morato I, Valderrama-Canales FJ, Berdugo G, Arias G, McHanwell S, Sanudo J, Vazquez T, Pascual-Font A. Reorganization of laryngeal motoneurons after crush injury in the recurrent laryngeal nerve of the rat. Journal of anatomy. 2013;222(4):451–61. doi: 10.1111/joa.12031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holman SD, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Lukasik SL, Waranch DR, German RZ. Swallowing kinematics and airway protection after palatal local anesthesia in infant pigs. The Laryngoscope. 2013 doi: 10.1002/lary.24204. p. doi 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holman SD, Waranch DR, Campbell-Malone R, Ding P, Gierbolini-Norat EM, Lukasik SL, German RZ. Sucking and swallowing rates after palatal anesthesia: an electromyographic study in infant pigs. Journal of neurophysiology. 2013;110(2):387–96. doi: 10.1152/jn.00064.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jadcherla SR, Gupta A, Coley BD, Fernandez S, Shaker R. Esophago-glottal closure reflex in human infants: a novel reflex elicited with concurrent manometry and ultrasonography. The American journal of gastroenterology. 2007;102(10):2286–93. doi: 10.1111/j.1572-0241.2007.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jadcherla SR, Gupta A, Wang M, Coley BD, Fernandez S, Shaker R. Definition and implications of novel pharyngo-glottal reflex in human infants using concurrent manometry ultrasonography. The American journal of gastroenterology. 2009;104(10):2572–82. doi: 10.1038/ajg.2009.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang SL, Samsudin S, Kuruvilla M, Dhelaria A, Kent S, Kelsall WA. Outcome of patent ductus arteriosus ligation in premature infants in the East of England: a prospective cohort study. Cardiol Young. 2013;23(5):711–6. doi: 10.1017/S1047951112001795. [DOI] [PubMed] [Google Scholar]
- 43.Kelly BN, Huckabee M-L, Jones RD, Frampton CMA. The first year of human life: coordinating respiration and nutritive swallowing. Dysphagia. 2007;22:37–43. doi: 10.1007/s00455-006-9038-3. [DOI] [PubMed] [Google Scholar]
- 44.Knight MJ, McDonald SE, Birchall MA. Intrinsic muscles and distribution of the recurrent laryngeal nerve in the pig larynx. Eur Arch Otorhinolaryngol. 2005;262(4):281–5. doi: 10.1007/s00405-004-0803-3. [DOI] [PubMed] [Google Scholar]
- 45.Kupfer RA, Callaghan BC, Hogikyan ND. Neurogenic vocal fold motion impairment after routine intubation for tonsillectomy in a pediatric patient. Journal of voice: official journal of the Voice Foundation. 2014;28(1):112–4. doi: 10.1016/j.jvoice.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 46.Laitman JT, Reidenberg JS. The evolution and development of human swallowing: the most important function we least appreciate. Otolaryngologic clinics of North America. 2013;46(6):923–35. doi: 10.1016/j.otc.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Lau C, Alagugurusamy R, Schanler RJ, Smith EO, Shulman RJ. Characterization of the developmental stages of sucking in preterm infants during bottle feeding. Acta paediatrica (Oslo, Norway: 1992) 2000;89(7):846–52. [PubMed] [Google Scholar]
- 48.Lau C, Smith EO, Schanler RJ. Coordination of suck-swallow and swallow respiration in preterm infants. Acta paediatrica (Oslo, Norway: 1992) 2003;92(6):721–7. [PubMed] [Google Scholar]
- 49.Lau C, Geddes D, Mizuno K, Schaal B. The development of oral feeding skills in infants. Int J Pediatr. 2012;2012:572341. doi: 10.1155/2012/572341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lefton-Greif MA, Loughlin GM. Specialized studies in pediatric dysphagia. Semin Speech Lang. 1996;17(4):311–29. doi: 10.1055/s-2008-1064106. quiz 330. [DOI] [PubMed] [Google Scholar]
- 51.Lefton-Greif MA, Arvedson JC. Pediatric feeding and swallowing disorders: state of health, population trends, and application of the international classification of functioning, disability, and health. Semin Speech Lang. 2007;28(3):161–5. doi: 10.1055/s-2007-984722. [DOI] [PubMed] [Google Scholar]
- 52.Lefton-Greif MA, Okelo SO, Wright JM, Collaco JM, McGrath-Morrow SA, Eakin MN. Impact of children’s feeding/swallowing problems: validation of a new caregiver instrument. Dysphagia. 2014;29(6):671–7. doi: 10.1007/s00455-014-9560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matsuo K, Palmer JB. Anatomy and physiology of feeding and swallowing: normal and abnormal. Phys Med Rehabil Clin N Am. 2008;19(4):691–707. vii. doi: 10.1016/j.pmr.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mortellaro VE, Pettiford JN, St Peter SD, Fraser JD, Ho B, Wei J. Incidence, diagnosis, and outcomes of vocal fold immobility after esophageal atresia (EA) and/or tracheoesophageal fistula (TEF) repair. Eur J Pediatr Surg. 2011;21(6):386–8. doi: 10.1055/s-0031-1291269. [DOI] [PubMed] [Google Scholar]
- 55.Paniello RC. Vocal fold paralysis: Improved adductor recovery by vincristine blockade of posterior cricoarytenoid. The Laryngoscope. 2014 doi: 10.1002/lary.24951. p. doi 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Paniello RC, Rich JT, Debnath NL. Laryngeal adductor function in experimental models of recurrent laryngeal nerve injury. The Laryngoscope. 2014 doi: 10.1002/lary.24947. p. doi 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.da Pereira KR, Firpo C, Gasparin M, Teixeira AR, Dornelles S, Bacaltchuk T, Levy DS. Evaluation of swallowing in infants with congenital heart defect. Int Arch Otorhinolaryngol. 2015;19(1):55–60. doi: 10.1055/s-0034-1384687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pourmoghadam KK, DeCampli WM, Ruzmetov M, Kosko J, Kishawi S, O’Brien M, Cowden A, Piggott K, Fakioglu H. Recurrent Laryngeal Nerve Injury and Swallowing Dysfunction in Neonatal Aortic Arch Repair. Ann Thorac Surg. 2017;104(5):1611–1618. doi: 10.1016/j.athoracsur.2017.03.080. [DOI] [PubMed] [Google Scholar]
- 59.Prakash M, Johnny JC. Whats special in a child’s larynx? J Pharm Bioallied Sci. 2015;7(Suppl 1):S55–8. doi: 10.4103/0975-7406.155797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rasch S, Sangild PT, Gregersen H, Schmidt M, Omari T, Lau C. The preterm piglet - a model in the study of oesophageal development in preterm neonates. Acta paediatrica (Oslo, Norway: 1992) 2010;99(2):201–8. doi: 10.1111/j.1651-2227.2009.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rommel N, van Wijk M, Boets B, Hebbard G, Haslam R, Davidson G, Omari T. Development of pharyngo-esophageal physiology during swallowing in the preterm infant. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2011;23(10):e401–8. doi: 10.1111/j.1365-2982.2011.01763.x. [DOI] [PubMed] [Google Scholar]
- 62.Sachdeva R, Hussain E, Moss MM, Schmitz ML, Ray RM, Imamura M, Jaquiss RD. Vocal cord dysfunction and feeding difficulties after pediatric cardiovascular surgery. J Pediatr. 2007;151(3):312–5. 315.e1–2. doi: 10.1016/j.jpeds.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 63.Sangild PT, Thymann T, Schmidt M, Stoll B, Burrin DG, Buddington RK. Invited review: the preterm pig as a model in pediatric gastroenterology. J Anim Sci. 2013;91(10):4713–29. doi: 10.2527/jas.2013-6359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tessema B, Roark RM, Pitman MJ, Weissbrod P, Sharma S, Schaefer SD. Observations of recurrent laryngeal nerve injury and recovery using a rat model. The Laryngoscope. 2009;119(8):1644–51. doi: 10.1002/lary.20293. [DOI] [PubMed] [Google Scholar]
- 65.Thexton AJ, Crompton AW, German RZ. Transition from suckling to drinking at weaning: a kinematic and electromyographic study in miniature pigs. Journal of Experimental Zoology. 1998;280(5):327–43. doi: 10.1002/(sici)1097-010x(19980401)280:5<327::aid-jez2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 66.Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Correlation between intraoral pressures and tongue movements in the suckling pig. Archives Oral Biology. 2004;49(7):567–75. doi: 10.1016/j.archoralbio.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 67.Thexton AJ, Crompton AW, German RZ. Electromyographic activity during the reflex pharyngeal swallow in the pig: Doty and Bosma (1956) revisited. Journal of Applied Physiology. 2007;102(2):587–600. doi: 10.1152/japplphysiol.00456.2006. [DOI] [PubMed] [Google Scholar]
- 68.Thexton AJ, Crompton AW, Owerkowicz T, German RZ. Impact of rhythmic oral activity on the timing of muscle activation in the swallow of the decerebrate pig. J Neurophysiol. 2009;101(3):1386–93. doi: 10.1152/jn.90847.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tucker AL, Duncan IJ, Millman ST, Friendship RM, Widowski TM. The effect of dentition on feeding development in piglets and on their growth and behavior after weaning. J Anim Sci. 2010;88(7):2277–88. doi: 10.2527/jas.2009-2404. [DOI] [PubMed] [Google Scholar]
- 70.Tutor JD, Gosa MM. Dysphagia and aspiration in children. Pediatr Pulmonol. 2012;47(4):321–37. doi: 10.1002/ppul.21576. [DOI] [PubMed] [Google Scholar]


