Abstract
The present study investigated the effects of conditional deletion of ephrinB1 in osteoprogenitor cells driven by the Osterix (Osx) promoter, on skeletal integrity in a murine model of ovariectomy-induced (OVX) osteoporosis. Histomorphometric and μCT analyses revealed that loss of ephrinB1 in sham Osx:cre-ephrinB1fl/fl mice caused a reduction in trabecular bone comparable to OVX Osx:Cre mice, which was associated with a significant reduction in bone formation rates and decrease in osteoblast numbers. Interestingly, these observations were not exacerbated in OVX Osx:cre-ephrinB1fl/fl mice. Furthermore, sham Osx:cre-ephrinB1fl/fl mice displayed significantly higher osteoclast numbers and circulating degraded collagen type 1 compared to OVX Osx:Cre mice. Confirmation studies found that cultured monocytes expressing EphB2 formed fewer TRAP+ multinucleated osteoclasts and exhibited lower resorption activity in the presence of soluble ephrinB1-Fc compared to IgG control. This inhibition of osteoclast formation and function induced by ephrinB1-Fc was reversed in the presence of an EphB2 chemical inhibitor. Collectively, these observations suggest that ephrinB1, expressed by osteoprogenitors, influences bone loss during the development of osteoporosis, by regulating both osteoblast and osteoclast formation and function, leading to a loss of skeletal integrity.
Introduction
The Eph/ephrin molecules originally identified in the hematopoietic system1 have subsequently been implicated in numerous developmental and pathophysiological processes2–5. Importantly, members of both subclasses have been associated with skeletal development and bone homeostasis, with particular focus on the signalling between EphB4/ephrinB2 and EphB2/ephrinB1 interacting pairs6–12. Pioneering studies in mouse proposed that bi-directional signalling between EphB4 expressing osteoblasts and ephrinB2 expressing osteoclast precursors promoted mineral formation, while inhibiting osteoclast formation and function, respectively6,9. Importantly, these EphB/ephrinB interactions are not limited to the communication between osteoblasts and osteoclasts, as ephrinB ligands are also expressed by osteoblasts and osteocytes6; and can act on neighbouring osteogenic cells, to influence osteoblast formation and function7,10,11. In vitro studies assessing human mesenchymal stem cell populations demonstrated the expression of multiple Eph/ephrin molecules, and identified EphB2/ephrinB interactions as important promoters of mineral formation4,5,8. Other studies have demonstrated that biomechanical loading caused an elevation in EphB2 expression in wild type mice when compared to un-loaded conditions13,14, with similar observations reported for ephrinB1 over-expressing transgenic mice15. Notably, EphB4 expression was unchanged in these studies, suggesting that in situations of active bone remodelling, mineralisation may occur through EphB2/ephrinB1 interactions independently of EphB4/ephrinB2.
Human mutations of ephrinB1 cause skeletal defects, of both the axial and appendicular skeleton, particularly coronal craniosynostosis and frontonasal dysplasia, in addition to asymmetrical lower limb shortness16–18. In mouse, global knockout19,20 of ephrinB1 or deletion within osteogenic populations, under the control of collagen type 1 promoter21 or Osterix promoter12, also result in skeletal defects similar to the human phenotype. Conversely, ephrinB1-overexpressing transgenic mice demonstrate an elevation in bone mass and strength15. Collectively these observations suggest that ephrinB1 has an important role skeletal formation, where the loss of ephrinB1 in osteoprogenitors during skeletal development perturbed osteoblast formation and function, and osteoclast formation12, by an undetermined mechanism. This observation suggests that, while ephrinB1 may be communicating with adjacent EphB expressing osteogenic cells, direct or indirect communication may occurs between osteoblasts and osteoclasts, to influence bone homeostasis. The importance of ephrinB1 in skeletal homeostasis is also evident in pathological situations. This molecule has been associated with the pathogenesis of osteosarcoma, where ephrinB1 expressed by osteosarcoma cells and blood vessels, associated with a poorer prognosis22. Furthermore, during lactation-induced maternal bone loss, ephrinB1 expression was upregulated, while EphB4 and ephrinB2 expression remained unaltered23.
Collectively, these studies suggest that ephrinB1 may play an important role in bone disease and could act as an important regulator in maintaining bone homeostasis. However, to date, little is known about the role of ephrinB1 in the context of dysregulated bone homeostasis, as occurs with osteoporosis. The present study investigated the importance of ephrinB1 expressed by the osteogenic cell lineage to maintain skeletal integrity in mature mice following ovariectomy-induced osteoporosis.
Materials and Methods
All methods were performed in accordance with the relevant Institutional (Adelaide University) and Australian Federal Government guidelines and regulations. Animal breeding and experiments approved by the SA Pathology (BC BC01/11 and 23/11) and the University of Adelaide Animal Ethics Committee (M-2013-144). Human blood samples were isolated from normal healthy donors with informed consent, in accordance to the guidelines and regulations of the Royal Adelaide Hospital Human Ethics Committee (protocol No. 940911a).
Animal Breeding and surgery
Animal breeding was approved by the SA Pathology (BC BC01/11) Animal Ethics Committee. All animal experiments and analyses were approved by both SA Pathology (23/11) and the University of Adelaide (M-2013-144) Animal Ethics Committees. The tTA:Osx1-GFP:Cre (hereafter referred to as Osx:Cre) mice24 were used to conditionally delete ephrinB1 in osteogenic progenitors25, where Osx:Cre targets stromal cells, adipocytes and perivascular cells within the bone marrow. External to the skeletal system Osx:Cre has also been shown to target a subset of cells within the gastric and intestinal epithelium and the olfactory bulb26,27. To delete ephirnB1 in osteogenic progenitors, breeding was performed as previously described12. Briefly, 129S-Efnb1tm1Sor/J (EfnB1fl/fl) female mice (JAX Laboratories, Bar Harbor, Me, USA) that had been backcrossed for 10 generations to a C57BL/6 genetic background, were bred with Osx:Cre males to obtain Osx:cre-EfnB1fl/0 males. These males were bred with EfnB1fl/fl females to obtain homozygote Osx:cre-EfnB1fl/fl females in the osteoblast lineage (hereafter referred to as EfnB1OB−/−). Twelve week old C57BL/6 female Osx:Cre or EfnB1OB−/− mice were anesthetised, there ovaries were either sited (sham control) or were ovariectomised (OVX). The animals had their dorsal skin sutured and were allowed to recover under normal housing conditions and food and water were provided ad libitum. Calcein (20 mg/kg) was administered by intraperitoneal injection 10 and 3 days prior to tissue collection. Samples were analysed at 12 weeks post-surgery.
Micro-CT (μCT) scanning and analysis
Three-dimensional micro computed tomography (µCT) was performed as previously outlined of the femur12 and the fifth lumbar vertebrae28 using Micro-CT (femur -Skyscan 1076× -ray Microtomography SkyScan, Vertebrae - Skyscan 1176× -ray Microtomography SkyScan Bruker Microct, Kontich, Belgium). Briefly, femora isolated from sham and OVX mice were scanned at 9 µm resolution, Aluminium 0.5 mm filter, Excitation 5890 ms, Voltage 48 kV, Current 110 mA, rotation step 0.6 and two frame averaging. Vertebrae were scanned at 9 µm resolution, Aluminium 0.5 mm filter, Excitation 1100 ms, Voltage 75 kV, Current 196 mA, rotation step 0.8 and one frame averaging. NRecon software was used to reconstruct the femora and vertebrae, parameters were set as follows: smoothing of 1, selection of ring artefact reduction and beam-hardening of 30%, Threshold was set to 0–0.10 (femur) and 0–0.05 (vertebrae). Reconstructed bones were aligned in the same orientation using DataViewer software and then analysed using CTAn software. The femur were analysed by avoiding the primary spongiosa (0.43 mm below the growth plate), the trabecular region (1.73 mm) of the distal femora was traced as the region of interest (ROI) and analysed. The vertebral body of the fifth lumbar vertebrae was analysed as previously described28. Briefly, the transverse orientation was acquired, the entire vertebral body was traced within 0.3 mm below and above the end-plates of the vertebrae. The thresholding was consistent between samples. CTVol Software (SkyScan) was used to generate 3D models.
Histology
Sample preparation and staining
Femora were fixed in 10% buffered formalin for 48 hours, infiltrated and embedded in methyl methacrylate (Merk Millipore, Vic, AUS). Samples were sectioned (5 μm) as previously described9 and mounted on gelatine coated super frost plus slides. Longitudinal sections were stained with either Toluidine blue or Tartrate Resistant Acid Phosphatase (TRAP) and counterstained with fast green, as previously described9. Paraffin embedded tibial 5μm sections were rehydrated and mounted in Faramount aqueous mounting media (DAKO). Collagen fibres were visualised using multiphoton and second harmonic generation imaging (Leica TCS SP8MP multiphoton microscope), with an excitation of 880 nm and emission at 440 nm, as previously described29. Images were processed using Fiji/Image J software30.
Histomorphometric Analysis
OsteoMeasure V3.3.02 (Osteometric, Decatur, GA) software with an Olympus DP72 Microscope (Notting Hill, Victoria, AUS) was used for histomorphometric analyses of longitudinal sections. The trabecular bone 40x magnification images below the primary spongiosa and avoiding regions in contact with the cortical bone were analysed, in a 0.72 mm2 region for Calcein, and 0.96 mm2 region for Toluidine Blue and TRAP+ osteoclasts analysis. Bone formation rate was determined by measuring the distance between the two mineralisation fronts that were labelled with Calcein and analysed with OsteoMeasure software.
Flow cytometric analysis
Flow cytometric analysis of osteogenic populations was performed as previously described31–33. Briefly, compact bone was obtained by crushing the femora and tibiae, washed with PBS + 2%FCS, and then crushed fractions were cut into small fragments and enzymatically digested with collagenase type I (400 U/mL) and DNase I (50 U/mL) for 45 min at 37 °C, while shaking. Cells were washed, filtered through a 70 μm strainer and centrifuged for 10 min at 800 g. Resuspended cells together with flushed bone marrow were incubated in mouse gamma globulin, washed and stained with biotinylated lineage-specific antibodies. Flow cytometric analysis was performed on BD LSR Fortessa X20 (BD Biosciences). The frequency of osteoblasts (Lin−CD45−CD31−Sca-1−CD51+), and osteoprogenitor (Lin−CD45−CD31−Sca-1+CD51+) was determined using FlowJo software (University of Washington, WA) as previously described(26–28).
Analysis of bone turnover serum markers
Mice were anesthetised and blood serum samples collected following cardiac puncture. The levels of circulating CTX-1 was analysed using RatLaps CTX-I EIA ELISA (AC-06F1; ids) as recommended by the manufacturer (Immunodiagnostic Systems, Boldon Business Park, UK).
RNA isolation
RNA isolation, cDNA synthesis and real time PCR were conducted as previously described32, using β-actin and human-specific B subclass Eph primers4,5; and Cathepsin K (NC_018912.2 Fwd: 5′-GGCCAACTCAAGAAGAAAACTG-3′, Rev: 5′-TCTCTGTACCCTCTGCATTTAGC-3′); c-fms (NM_005211 Fwd: 5′-AGCTTGGCATGGTCAGGGAA-3′, Rev: 5′-GAAGGTAGCGTTGTTGGTGC-3′); RANK (NM_003839 Fwd: 5′-GCTGTAACAAATGTGAACCAGGA-3′, Rev: 5′-GCCTTGCCTGTATCACAAACT-3′); CXCR4 (NM_003467 Fwd: 5′-CAGCAGGTAGCAAAGTGACG-3′, Rev: 5′-GTAGATGGTGGGCAGGAAGA-3′); ephrinB1 (Fwd 5′ AACAAGCCACACCAGGAAAT 3′, Rev 5′ GCTCCCATTGGACGTTGAT 3′).
Osteoclast differentiation and resorption assays
Osteoclast Differentiation In Vitro: Human peripheral blood mononuclear cells (hPBMNC) were isolated from buffy coats from normal healthy donors. All experiments were performed with informed consent from each donor, in accordance with relevant guidelines and regulations of the Royal Adelaide Hospital Human Ethics Committee (protocol No. 940911a) as previously described34. The cells were seeded (0.5 × 106 per 0.32 cm2) and cultured overnight in osteoclast basal media (Minimum Essential Medium Alpha Modified (Sigma), 10% foetal calf serum (FCS), 1% penicillin streptomycin, 1% l-glutamine) and 25 ng/ml macrophage colony stimulating factor (M-CSF) (Miltenyi Biotec, NSW, AUS) (Day -1) in 96-well plates (TRAP staining and viability analysis) or Corning Osteo Assay Surface Muliple Well plates (Sigma) (resorption analysis). The following day, the Human IgG-Fc or ephrinB1-Fc at 1 µg/ml (R&D systems) were pre-clustered with biotinylated anti-goat IgG (Jackson ImmunoReseaarch, West Grove, PA, 2.4 mg/ml) in osteoclast media (osteoclast base media supplemented with 25 ng/ml M-CSF, 10−8M dexamethasone (Hosporia Aust. Mulgave, Vic, Aust.), and 10−8M 1α,25-dihydroxyvitaminD3 (Cerilliant Corporation, TX) for 1 hour at room temperature prior to being added to osteoclast cultures. Media was changed three-times per week. From day 7, osteoclast media was also supplemented with 50 ng/ml RANKL (Miltenyi Biotec). On day 14, wells were processed for either RNA isolation or TRAP staining using the Sigma Acid Phosphate, Leukocyte (TRAP) kit (Sigma).
Bone marrow (BM) cells flushed from the femur and tibia of mice were cultured overnight, then non-adherent cells were collected and seeded (3.1 × 105 cells/cm2) in α-MEM supplemented with 75 ng/ml of M-CSF (GF053; Merck Millipore) and RANKL (GF091; Merck Millipore). Medium was replaced every 3 days. On day 6, RNA was isolated, cDNA synthesised, and qPCR performed.
EphB2 Inhibitor Assays: Human hPBMNC were incubated for 1 hour in half the final volume of osteoclast media (outlined above) containing 100 µM blocking peptide specific for EphB2 (SNEWILPRLPQH) or control peptide (RTVAHHGGLYHTNAEVK) (Mimotopes, Vic, Aust.). During this 1-hour incubation 2 µg/ml of ephrinB1-Fc (final concentration, 1 µg/ml) was clustered (outlined above) in the other half of the final volume of osteoclast media. The remaining 100 µM blocking peptide (SNEW or RTVA) was then combined with the clustered ephrinB1-Fc. This ephrinB1-Fc-blocking peptide osteoclast media was immediately added to hPBMNC that had been incubated with the blocking peptide. Media changes with ephrinB1-Fc and blocking peptides were carried out as outlined above.
Cell Viability Assays: On day 3 and 7 following osteoclast induction, viability and metabolism were assessed by WST-1 (Sigma). Briefly, WST-1 was diluted in Pheno-Red free alpha-modification media (Sigma) containing 25 ng/ml M-CSF. Cells and control blank wells were incubated with the diluted WST-1 for 1 hour at 37 °C, after which time the absorbance was read at 450 nm. Experimental values were normalised to the blank. On day 21, cells in the Corning Osteo Assay Surface Multiple Well plates were lysed with 6% sodium hypochlorite, washed with water three times and then left to dry. Whole wells were imaged and analysed with Image J software.
Osteogenic Differentiation In Vitro
Bone derived mesenchymal stromal cells isolated from murine tibia and femora were cultured in αMEM supplemented with 20% (v/v) FCS, 2 mM l-glutamine, 1 mM sodium pyruvate, 10 mM HEPES buffer, 50 U/ml penicillin, 50 μg/ml streptomycin, 100 µM l-ascorbate-2-phosphate (Wako Pure Chemical Industries, Richmond, VA, USA), 10 nM dexamethasone (RAH688A; Royal Adelaide Hospital, Adelaide, SA, Australia), and 4 mM KH2PO4 (Asia Pacific Specialty Chemicals Limited, Seven Hills, NSW, Australia). Cultures were stained with Alizarin red (A5533-25G; Sigma-Aldrich) to identify mineral deposits. Extracellular calcium (Ca2+) was measured by Calcium Arsenazo III (TR29226; Thermo Fisher Scientific) and normalized to DNA per well with a PicoGreen dsNDNA quantitation kit (P11496; Thermo Fisher Scientific). This analysis was conducted in triplicate for each mouse.
Statistical analysis
Microsoft GraphPad Prism 5 (La Jolla, CA) was used for data and statistical analysis. Student t-test, one-way ANOVA with multiple comparisons and two-way ANOVA, using a Tukey’s multiple comparisons post-hoc test was used throughout the study as specified. Statistical significance is represented as p ≤ 0.05.
Results
Confirmation of osteogenic-specific deletion of ephrinB1 in EfnB1OB−/− mice
It has previously been reported that the deletion of ephrinB1 under the control of the Osterix promoter impairs endochondral ossification, compromising skeletal development and integrity12. The study demonstrated that ephrinB1 gene and protein levels were significantly reduced in a heterogeneous population of cultured osteogenic cells isolated from Osx:Cre and EfnB1OB−/− mice12. The present study confirmed that following osteogenic differentiation of osteogenic cells isolated from Osx:Cre and EfnB1OB−/− mice, there was a significant reduction in the formation of mineral nodules and extracellular calcium levels in osteogenic cells isolated from EfnB1OB−/−mice when compared to Osx:Cre controls (Supplementary Fig. 1A,B). This observation correlated with a significant reduction in ephrinB1 gene expression in stromal cells isolated from EfnB1OB−/− mice following osteogenic induction when compared to the Osx:Cre control (Supplementary Fig. 1C). Furthermore, to demonstrate that the Osterix-Cre promoter was active specifically within the stromal population within the skeleton, marrow cells flushed from the long bones were placed under osteoclastic differentiation conditions. Following 6 days in osteoclast inductive conditions, ephrinB1 gene expression levels were found to be comparable in osteoclasts/monocyte populations isolated from Osx:Cre control and EfnB1OB−/− mice (Supplementary Fig. 1D–F). These observations confirmed that ephrinB1 under the control of the Osterix-Cre promotor was regulated in the osteogenic cell lineage and not the haematopoietic population within the bone marrow.
The loss of ephrinB1 in osteoprogenitors is sufficient to induce an osteoporotic bone phenotype
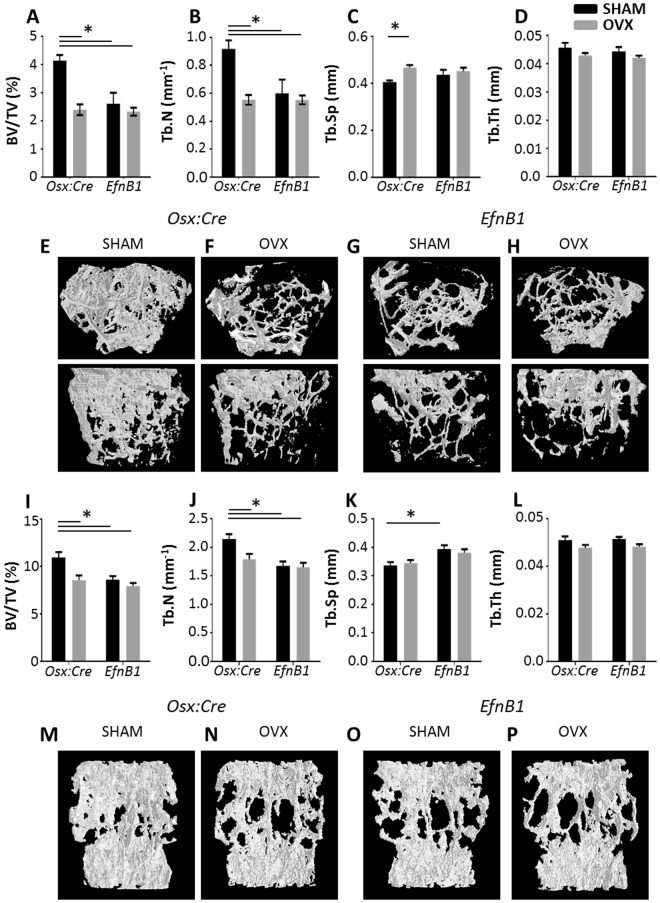
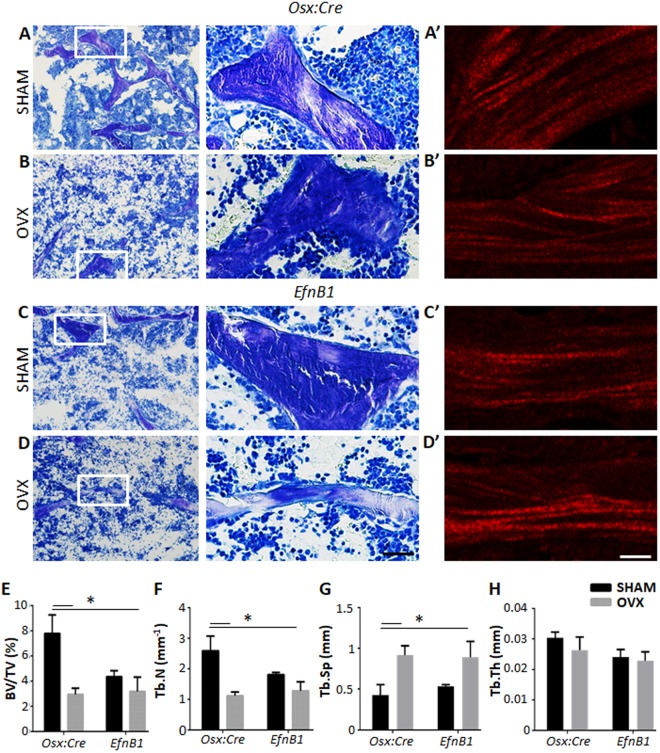
We investigated whether ephrinB1, expressed by cells of the osteogenic lineage contributed to bone remodelling using a model of ovariectomy (OVX)-induced osteoporosis. Ovariectomy or sham surgery was performed on female homozygote EfnB1OB−/− mice, or Osx:Cre control mice and skeletal parameters were assessed at 12 weeks post-surgery. Three dimensional µCT analysis revealed that OVX induced an osteoporotic phenotype, as evidenced by a significant reduction in bone to tissue volume and trabecular number in both the femora (Fig. 1A–F), and vertebra (Fig. 1I–N) in OVX Osx:Cre mice compared to sham Osx:Cre. Interestingly, the loss of ephrinB1 in sham EfnB1OB−/− mice resulted in a skeletal phenotype comparable to the OVX Osx:Cre mice, which was not further exacerbated following OVX in EfnB1OB−/− mice, that was observed in both the femora and the vertebra (Fig. 1). A significant increase in trabecular separation was observed in the femora of OVX Osx:Cre mice compared to sham Osx:Cre (Fig. 1C), however, this was not maintained in the vertebrae (Fig. 1K). Notably, a significant increase in trabecular separation within the vertebrae was observed between sham Osx:Cre and sham EfnB1OB−/− mice, while OVX EfnB1OB−/− mice did not reach statistical significance. Furthermore, there was no significant difference observed in the trabecular thickness between any of the cohorts (Fig. 1D,L). Confirmatory 2D histomorphometric analysis of toluidine blue stained sections of trabecular regions within the distal femora, showed a significant reduction in bone to tissue volume, trabecular number and significant increase trabecular separation of OVX-induced Osx:Cre control mice when compared to sham-treated Osx:Cre control mice. Consistent with the µCT analysis, there was no significant difference in trabecular thickness between any of the groups. Furthermore, homozygote sham and OVX EfnB1OB−/− mice displayed a similar phenotype to OVX-induced Osx:Cre mice (Fig. 2). Multiphoton and second harmonic generation imaging demonstrated that the perturbation in trabecular architecture was not due to an abnormal pattern of collagen type I deposition (Fig. 2A’–D’). Taken together, these observations suggest that ephrinB1 deletion in osteogenic cells can recapitulate an osteoporotic phenotype that cannot be further exacerbated by the oestrogen deprivation that accompanies OVX.
Figure 1.
The loss of EfnB1 in osteoprogenitors alone induces an osteoporotic bone phenotype. (A–P) µCT analysis of the trabecular region of the (A–D) distal femoral and (I–L) fifth lumbar vertebral body of Osx:Cre (Osx) and EfnB1OB−/− (EfnB1) mice that had undergone sham or ovariectomy (OVX) surgery; represented as (A,I) Bone to tissue volume (BV/TV), (B,J) trabecular number (Tb.N), (C,K) trabecular separation (Tb.Sp) and (D,L) trabecular thickness (Tb.Th). (E–H,M–P) Representative images of 3D µCT from the (E–H) femur (in cross-sectional (top) and longitudinal (bottom) orientation) and (M–P) vertebral body of (E,G,M,O) sham and (F,H,N,P) ovariectomised (OVX) mice. All statistical analysis performed by 2-way ANOVA, multiple comparisons, n = 6–9 mice/condition/strain, *p < 0.05.
Figure 2.
The loss of EfnB1 by osteoprogenitors alone impairs trabecular bone architecture similar to ovariectomised mice. (A–D) Representative toluidine blue stained sections of (A,B) Osx:Cre (Osx) control and (C,D) EfnB1OB−/− (EfnB1) femora, 12 weeks following (A–C) sham and (B–D) ovariectomy (OVX) surgery. (A’–D’) Representative images of collagen type I fibres. (E–H) Histomorphometric analysis of the trabecular region of the distal femora post-surgery represented as (E) percentage of bone to tissue volume, (F) trabecular number, (G) trabecular separation, and (H) trabecular thickness. All statistical analysis performed by 2-way ANOVA, multiple comparisons, *p < 0.05, n = 3–5 samples/strain/condition. Magnification A-D and A’–D’ = 10 µM.
Osteoblast maturation and function is compromised by the loss of ephrinB1
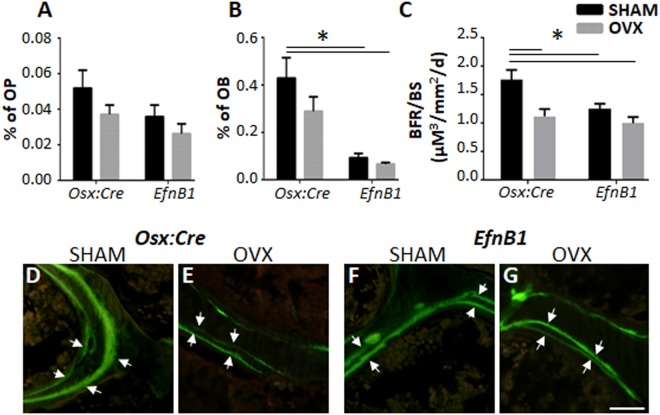
Flow cytometric analysis was performed as previously described31–33, to enumerate the incidence of cells of the osteogenic lineage in the femora and tibiae of sham EfnB1OB−/− homozygote females and sham and OVX Osx:Cre mice (Fig. 3A–C). While there was a reduction in the osteogenic progenitors (Sca-1+/Lin−/CD31−/CD45−/CD51+) population between Osx:Cre and EfnB1OB−/− samples, this did not reach significance (Fig. 3A). However, there was a significant reduction in the proportion of osteoblasts (Sca-1−/Lin−/CD31−/CD45−/CD51+) between sham Osx:Cre mice and both sham and OVX EfnB1OB−/− mice (Fig. 3B). These findings were consistent with an observed reduction in the bone formation rate of OVX Osx:Cre mice, compared to sham Osx:Cre mice, and compared to both sham and OVX EfnB1OB−/− mice (Fig. 3C–G). These observations suggest that the cell-autonomous loss of ephrinB1 in osteogenic progenitors inhibits osteogenic maturation and function.
Figure 3.
The loss of EfnB1 by osteoprogenitors inhibits osteoblast maturation and bone formation rate. (A,B) Fluorescent cytometric analysis of the percentage of (A) osteoprogenitors (OP, Sca-1+/Lin−/CD31−/CD45−/CD51+), and (B) osteoblasts (OB, Sca-1−/Lin−/CD31−/CD45−/CD51+), isolated from flushed and digested femora and tibia of Osx:Cre and EfnB1OB−/− sham and ovariectomised (OVX) mice 12 weeks post-surgery (n = 5–6 samples/strain/condition). (C) Bone formation rate (BFR) was analysed by Calcein labelling (n = 3–5 samples/strain/condition) (D–G) Representative double labelled Calcein images. All statistical analysis performed by 2-way ANOVA, multiple comparisons, *p ≤ 0.05. Magnification D–G = 5 µM.
Osteoclast formation and function is affected by EphB2 forward signalling
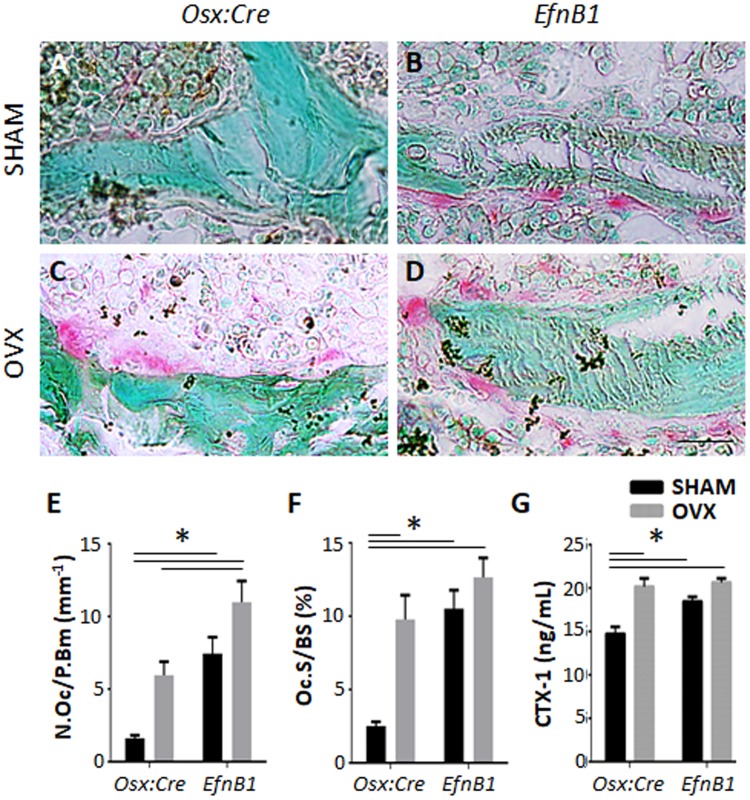
To determine if osteoclast formation and activity is EphB2 dependent, and therefore affected in response to the loss of ephrinB1 by osteoprogenitors and mature osteogenic populations, methacrylate embedded sections were stained with TRAP, and the number of TRAP + multinucleated osteoclasts were enumerated within the trabecular region of the distal femora of sham and OVX mice (Fig. 4A–F). Twelve weeks post-surgery, OVX Osx:Cre mice presented with significantly greater numbers of TRAP+ osteoclasts per bone perimeter compared to sham Osx:Cre mice (Fig. 4E). Similarly, sham EfnB1OB−/− mice displayed comparable numbers of TRAP + osteoclasts to OVX Osx:Cre mice, while OVX EfnB1OB−/− mice displayed significantly more TRAP + osteoclasts when compared to OVX Osx:Cre mice (Fig. 4E). The proportion of osteoclast surface to bone surface was significantly greater in OVX Osx:Cre mice compared to sham Osx:Cre mice. However, the osteoclast surface to bone surface was comparable between sham and OVX EfnB1OB−/− mice; and OVX Osx:Cre mice (Fig. 4F). These data suggest that the loss of ephrinB1 within osteoprogenitor and mature osteogenic populations influences osteoclast formation. To determine if osteoclast function was also altered due to the loss of ephrinB1, serum samples were used to investigate circulating levels of degraded collagen type 1. A significant increase in circulating degraded collagen type 1 (CTX-1) was identified in OVX Osx:Cre mice, and both sham and OVX EfnB1OB−/− mice, when compared to sham Osx:Cre control mice (Fig. 4G). Importantly, ephrinB1 gene expression was similar between EfnB1OB−/− and Osx:Cre mice (Supplementary Fig. 1D). These findings suggest that ephrinB1 expressed by the osteogenic population affects osteoclast formation and function rather than in a cell-autonomous manner.
Figure 4.
The loss of EfnB1 by osteoprogenitors influences osteoclast formation and function. (A–D) Representative methacrylate embedded sections of the distal femora from (A,B) sham and (C,D) ovariectomised (OVX) (A,C) Osx:Cre (Osx) control and (B,D) EfnB1OB−/− (EfnB1) mice 12 weeks post-surgery stained with TRAP. (E,F) Histomorphometric analysis quantitating (E) the number of TRAP+ osteoclasts lining the bone perimeter, and (F) the percentage of osteoclast surface to bone surface within the trabecular region of the distal femora of Osx:Cre and EfnB1OB−/− sham and OVX mice (n = 4–5 samples/strain/condition). (G) Circulating collagen type I was measured by ELISA (n = 5–8 samples/strain/condition). All statistical analysis performed by 2-way ANOVA, multiple comparisons, *p ≤ 0.05. Magnification A–D = 10 µM.
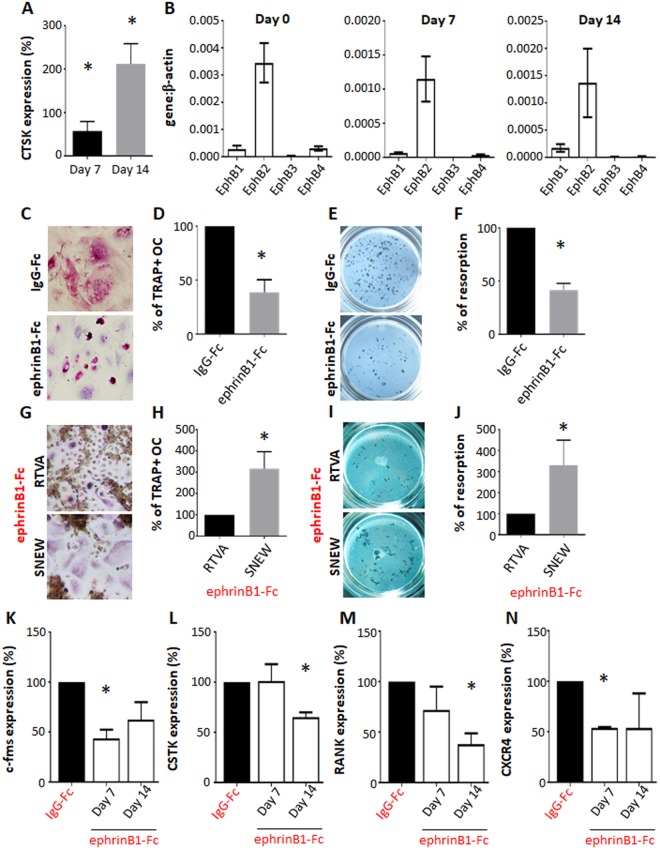
In vitro osteoclastogenesis studies were conducted to determine the direct role of ephrinB1 during human osteoclast formation. Gene expression analysis confirmed osteoclast differentiation of Human peripheral blood mononuclear cells (hPBMNC) with increasing expression levels of CathepsinK during osteoclastogenesis (Fig. 5A). Moreover, profiling of the Eph receptors identified that the cognate ephrinB1 receptor, EphB2, was the most highly expressed EphB receptor identified on hPBMNC during osteoclast differentiation (Fig. 5B). As the mouse studies demonstrated that loss of ephrinB1 by osteoprogenitors enhanced osteoclast formation, functional studies were conducted to confirm whether stimulation of hPBMNC with ephrinB1 alone, could inhibit osteoclast differentiation. Human PBMNC were cultured under osteoclastogenic conditions in the presence of either soluble ephrinB1-Fc or the human IgG control. The data showed that ephrinB1-Fc significantly inhibited TRAP+ multinucleated osteoclast formation (Fig. 5C,D). Consistent with these findings, osteoclast bone resorption activity was significantly inhibited, in the presence of soluble ephrinB1-Fc (Fig. 5E,F). Importantly, the presence of ephrinB1-Fc did not influence cell viability or metabolism during osteoclast formation, as demonstrated by no change in amount of formazan dye formed following incubation with tetrazolium salt, WST-1 (Supplementary Fig. 2).
Figure 5.
Mechanism of osteoclast function in human in vitro osteoclast differentiation assays. (A) Gene expression of Cathepsin K (CTSK) expressed as a fold increase compared to day 0 (n = 9 human donors, *p < 0.05, one-way ANOVA, multiple comparisons). (B) Expression profile of EphB receptors identified on human PBMNC prior to (Day 0) and under osteoclastogenic conditions (Day 7 and 14). (C–F) Human PBMNC placed under osteoclastic conditions in the presence of human IgG-Fc control or ephrinB1-Fc for 14 days were (C) stained for TRAP+ osteoclasts, (D) enumerated and represented as a percentage of TRAP+ multi-nucleated osteoclasts (OC) relative to the IgG-Fc control (n = 6 independent donors, *p < 0.05, Student t-test). (E,F) Samples were cultured for 21 days on Corning Osteo Assay Surface Muliple Well plates and (E) imaged. (F) The proportion of resorbed surface area was quantitated and represented as a percentage relative to the human IgG-Fc control (n = 3 human donors, *p < 0.05, Student t-test). (G–J) Samples cultured under osteoclastic conditions with ephrinB1-Fc in the presence of either the scramble peptide control (RTVA) or the EphB2-specific blocking peptide (SNEW). (G–H) On Day 14 samples were (G) stained for TRAP+ osteoclasts, (H) quantitated and represented as a percentage of TRAP+ OC relative to RTVA (n = 3 independent human donors, *p < 0.05, Student t-test). Resorptive function was assessed on day 21 of culture (I–J). Corning Osteo Assay plates were (I) imaged, (J) quantitated and represented as a percentage of resorption capacity relative to RTVA (n = 3 independent human donors, *p < 0.05, Student t-test). (K–N) Gene expression of (K) c-fms (L) Cathepsin K (CSTK), (M) RANK and (N) CXCR4 were analysed 7 or 14 days following osteoclast induction and represented as a percentage of gene expression when cultured in the presence of ephrinB1-Fc relative to human IgG (n = 2 human donors, *p < 0.05, Student t-test).
To confirm that ephrinB1 was blocking osteoclast formation and function through the EphB2 receptor, inhibitor studies were conducted utilising the EphB2 specific blocking peptide (SNEW). SNEW specifically binds to the ligand binding cleft of EphB2, competing with ephrin ligand binding35. Human PBMNC cultured under osteoclastogenic conditions were incubated with SNEW or a scrambled peptide control (RTVA) prior to culturing with ephrinB1-Fc. The competitive binding of SNEW to EphB2 in the presence of ephrinB1 significantly ameliorated TRAP+ osteoclast formation (Fig. 5G,H) and resorption (Fig. 5I,J) when compared to the scrambled control peptide (RTVA). This observation confirms that ephrinB1 interaction with EphB2 expressing osteoclast precursors inhibits osteoclast formation and subsequently function. Furthermore, supportive gene expression studies showed that transcript levels of osteoclast associated factors, Cathepsin K, c-fms, RANK and CXCR4 were significantly decreased during osteoclastogenesis in the presence of soluble ephrinB1 (Fig. 5K–N). These observations suggest that ephrinB1 binding, through the EphB2 receptor, inhibits osteoclast formation.
Discussion
In the present study, targeted deletion of ephrinB1, under the control of the osterix promoter, induced an osteoporotic phenotype in sham treated EfnB1OB−/− mice, which was comparable to that observed in OVX Osx:cre control mice. Notably, the osteoporotic phenotype was not exacerbated in EfnB1OB−/− mice following OVX. We observed a significant reduction in trabecular bone between OVX Osx:Cre mice compared to sham Osx:Cre controls. The loss of ephrinB1 in sham operated EfnB1OB−/− mice resulted in a similar trabecular architecture to OVX induced Osx:Cre and EfnB1OB−/− mice, as demonstrated by μCT analysis of two independent sites, the femur and the fifth lumbar vertebrae, and histomorphometric analyses of the femur. The formation of type I collagen fibres is essential for skeletal architecture and integrity, where interactions of Eph family members with the extracellular matrix can influence extracellular matrix organisation36,37. However, the present study failed to observe any noticeable differences in the patterns of collagen deposition between Osx:Cre controls and EfnB1OB−/− mice. Additionally, measurement of oestrogen levels in serum failed to detect any changes in oestrogen levels (data not shown) to explain the difference observed in skeletal architecture between sham EfnB1OB−/− mice. However, the reduction in trabecular bone observed between OVX Osx:Cre mice, OVX EfnB1OB−/− mice and sham EfnB1OB−/− mice was associated with a significant reduction in bone formation rate within the femur. As the Osx:Cre mouse line has been reported to exhibit changes in skeletal parameters, including cortical bone augmentation and accrual38, the present study utilized Osx:Cre mice rather than EfnB1fl/fl mice as the control. Therefore, the osteoporotic-like phenotype observed in sham EfnB1OB−/− mice did not appear to be a consequence of the Osx:Cre background, but rather due to deletion of ephrinB1 in osteogenic cells, which could not be exacerbated following OVX.
The importance of ephrinB1 in skeletal formation can be observed in individuals who display cranial and skeletal defects and harbour functional mutations in the ephrinB1 gene16–18. Similar skeletal abnormalities have been reported in global and targeted deletion of ephrinB1 during murine development12,19–21. At the molecular level, ephrinB1 mediates mineral formation by activating Osterix expression21. EphB2 activation of ephrinB1 reverse signalling results in the dephosphorylation of TAZ from its complex with the PDZ domain of ephrinB1, PTPN13 and NHERF1. The dephosphorylated TAZ translocates to the nucleus and induces osterix expression, osteogenic differentiation and subsequently mineral production21. Consistent with these observations, human studies have shown that ephrinB1 is the most highly expressed EphB ligand on human BMSC, and activation of ephrinB1 promotes BMSC osteo/chondrogenic differentiation in vitro5. Flow cytometric analysis found a trend of lower numbers of osteoprogenitors but significantly reduced osteoblast numbers in both the sham and OVX treated EfnB1OB−/− mice, compared to sham Osx:cre control mice. Given the reported redundancy between EphB/ ephrinB family members, where ephrinB2/ EphB4 signaling within the osteogenic lineage has previously been shown to be important in the late stages of osteoblast differentiation10. Mice lacking ephrinB2 in osteoblasts exhibit delayed bone mineralization, significant reduction in bone stiffness, and a reduction in osteoblast differentiation in the presence of parathyroid hormone39. Therefore, compensation by ephrinB2 reverse signalling in mature bone cells may be a contributing factor in preventing an exacerbated osteoporotic phenotype in OVX EfnB1OB−/− mice as has been suggested in other systems6,40,41. However, this is hypothesis requires furthermore investigation.
In the present study, both sham and OXV induced EfnB1OB−/− mice displayed higher osteoclast numbers compared to Osx:cre OVX-induced mice. These data are consistent with previous studies demonstrating that the loss of ephrinB1 by osteoprogenitors stimulated an increase in the number of osteoclasts during skeletal development12. The findings presented here suggest that ephrinB1 activation may act as a contact-dependent regulator of osteoclast differentiation. Importantly, histomorphometric analysis of the distal femur identified that OVX-induced EfnB1OB−/− mice exhibited a two-fold increase in the number of osteoclasts compared to OVX Osx:cre mice, and a significant increase in resorption activity when compared to the sham Osx:cre mice. It is therefore plausible that ephrinB1, expressed by osteoblasts, interacts with an Eph receptor expressed by monocytes/osteoclast progenitors to inhibit osteoclast formation and activity. While Zhao and colleagues have reported that ephrinB1 and ephrinB2 are expressed by osteoclasts, but lack EphB expression6, other studies have reported that low levels of EphB receptors are expressed by murine osteoclasts42. However, it has been shown that EphB4 indirectly suppresses osteoclast differentiation by inhibiting RANKL production on osteoblasts10. Importantly, the study showed that the direct delivery of EphB4 inhibitor (sEphB4) did not influence osteoclast production, demonstrating that EphB4 was not required for osteoclast formation. Rather administration of sEphB4, during co-culture of osteoblasts and osteoclast precursors was required to inhibit EphB4 on osteoblasts; which subsequently enhanced RANKL production and therefore osteoclast formation10. However, these studies have been conducted on mouse primary cells or cell lines.
The present study, sought to determine whether the role of EphB/ephrinB1 signaling during osteoclast differentiation was similar between human samples, and that observed in the ephrinB1 mouse knockout studies. Our observations showed that EphB receptors were expressed by human peripheral blood mononuclear cells and differentiated osteoclasts. The expression of EphB2, the high affinity receptor for ephrinB1, was down-regulated during osteoclast differentiation. Functional studies found that stimulation of human peripheral blood monocytes (osteoclast precursors) with soluble ephrinB1-Fc, inhibited TRAP+ multinucleated osteoclast formation, function and associated gene expression, compared to the human-IgG control. To confirm that ephrinB1 mediated its inhibitory function through EphB2 expressing osteoclast precursors, an EphB2-specific blocking peptide was utilised. This peptide functions by competing with the ephrin ligand to bind to the ligand binding domain of EphB235. The addition of the EphB2 blocking peptide reversed the inhibition of osteoclast formation in the presence of ephrinB1-Fc. As previously mentioned, osteoclasts and their precursors also express ephrinB ligands6, and it is therefore plausible to suggest that osteoclast precursors may interact with each other to mediate EphB2-ephrinB1 signalling. However, when hPBMNC were cultured under osteoclast conditions in the presence of the EphB2 blocking peptide but in the absence of ephrinB1-Fc, there was no significant difference in the number of TRAP + osteoclasts enumerated when compared to the scramble peptide control (data not shown). This observation is consist with previous studies investigating ephrinB2-EphB4 communication between osteoclasts using the EphB4 inhibitor10. Collectively, these observations suggest that the communication between EphB2-ephrinB1 within the osteoclast precursor population is not sufficient to activate EphB2 signalling. Rather, this study proposes that ephrinB1 expressing osteogenic cells directly interact with and activate EphB2 expressing osteoclast precursors to inhibit osteoclast differentiation. The findings confirm that EphB2/ephrinB1 signaling during osteoclast differentiation is conserved between mouse and human. Where the loss of ephrinB1 by osteoblasts in EfnB1OB−/− mice, enhanced osteoclast function, while the administration of ephrinB1 during osteoclast differentiation of human PBMNC inhibited osteoclast formation and function. Therefore, ephrinB1 appears to act as a negative regulator of osteoclast formation via EphB2 forward signaling. This is consistent with the reported role of EphA4, which negatively regulates osteoclast resorption activity43. In contrast, interactions between EphA2/ephrinA2, have been reported to enhance osteoclast differentiation, while suppressing osteoblast differentiation44. Therefore, different Eph/ ephrin pairings may provide alternate mechanisms to “fine tune” different phases of bone remodelling during normal bone homeostasis or under pathological conditions.
Collectively, these findings demonstrate the importance of ephrinB1 signalling in maintaining healthy bone, where loss of ephrinB1 in osteoprogenitors not only perturbs osteoblast function, but also affects osteoclast formation. Future studies are required to identify the downstream signaling pathways and mechanism activating osteoclast formation and function in response to EphB2-ephrinB1 signalling between the osteoclastic-osteogenic populations.
Electronic supplementary material
Acknowledgements
This work was supported by NHMRC project grant APP1083804, fellowship APP1042677 and the Mary Overton Research Fellowship, Royal Adelaide Hospital Research Committee.
Author Contributions
Authors responsible for the integrity of the data analysis: A.A., S.G. Authors’ roles: Study design: A.A., A.Z. and S.G. Study conduct: A.A., T.N., S.P. and A.K. Data collection: A.A., T.N., S.P. and A.K. Data analysis: A.A., T.N., A.K. and S.G. Data interpretation and preparation of Figures: A.A., A.Z. and S.G. Drafting manuscript: A.A. and S.G. Revising manuscript content: A.A., A.Z. and S.G. Approving final version of manuscript: A.A., T.N., S.P., A.K., A.Z. and S.G.
Data Availability Statement
All data generated or analysed during this study are included in this published article.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-31190-2.
References
- 1.Hirai H, Maru Y, Hagiwara K, Nishida J, Takaku F. A novel putative tyrosine kinase receptor encoded by the eph gene. Science. 1987;238:1717–1720. doi: 10.1126/science.2825356. [DOI] [PubMed] [Google Scholar]
- 2.Kania A, Klein R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nature reviews. Molecular cell biology. 2016;17:240–256. doi: 10.1038/nrm.2015.16. [DOI] [PubMed] [Google Scholar]
- 3.Boyd AW, Bartlett PF, Lackmann M. Therapeutic targeting of EPH receptors and their ligands. Nat Rev Drug Discov. 2014;13:39–62. doi: 10.1038/nrd4175. [DOI] [PubMed] [Google Scholar]
- 4.Stokowski A, et al. EphB/ephrin-B interaction mediates adult stem cell attachment, spreading, and migration: implications for dental tissue repair. Stem cells. 2007;25:156–164. doi: 10.1634/stemcells.2006-0373. [DOI] [PubMed] [Google Scholar]
- 5.Arthur A, et al. EphB/ephrin-B interactions mediate human MSC attachment, migration and osteochondral differentiation. Bone. 2011;48:533–542. doi: 10.1016/j.bone.2010.10.180. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, et al. Bidirectional ephrinB2-EphB4 signaling controls bone homeostasis. Cell metabolism. 2006;4:111–121. doi: 10.1016/j.cmet.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 7.Allan EH, et al. EphrinB2 regulation by PTH and PTHrP revealed by molecular profiling in differentiating osteoblasts. J Bone Miner Res. 2008;23:1170–1181. doi: 10.1359/jbmr.080324. [DOI] [PubMed] [Google Scholar]
- 8.Arthur A, Koblar S, Shi S, Gronthos S. Eph/ephrinB mediate dental pulp stem cell mobilization and function. Journal of dental research. 2009;88:829–834. doi: 10.1177/0022034509342363. [DOI] [PubMed] [Google Scholar]
- 9.Arthur A, et al. EphB4 enhances the process of endochondral ossification and inhibits remodeling during bone fracture repair. J Bone Miner Res. 2013;28:926–935. doi: 10.1002/jbmr.1821. [DOI] [PubMed] [Google Scholar]
- 10.Takyar FM, et al. EphrinB2/EphB4 inhibition in the osteoblast lineage modifies the anabolic response to parathyroid hormone. J Bone Miner Res. 2013;28:912–925. doi: 10.1002/jbmr.1820. [DOI] [PubMed] [Google Scholar]
- 11.Tonna S, Sims NA. Talking among ourselves: paracrine control of bone formation within the osteoblast lineage. Calcified tissue international. 2014;94:35–45. doi: 10.1007/s00223-013-9738-2. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen, T. M. et al. Loss of ephrinB1 in osteogenic progenitor cells impedes endochondral ossification and compromises bone strength integrity during skeletal development. Bone93 (2016). [DOI] [PubMed]
- 13.Xing W, et al. Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. Journal of cellular biochemistry. 2005;96:1049–1060. doi: 10.1002/jcb.20606. [DOI] [PubMed] [Google Scholar]
- 14.Kesavan C, Wergedal JE, Lau KH, Mohan S. Conditional disruption of IGF-I gene in type 1alpha collagen-expressing cells shows an essential role of IGF-I in skeletal anabolic response to loading. Am J Physiol Endocrinol Metab. 2011;301:E1191–1197. doi: 10.1152/ajpendo.00440.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng S, et al. Transgenic overexpression of ephrin b1 in bone cells promotes bone formation and an anabolic response to mechanical loading in mice. PloS one. 2013;8:e69051. doi: 10.1371/journal.pone.0069051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Twigg SR, et al. Mutations of ephrin-B1 (EFNB1), a marker of tissue boundary formation, cause craniofrontonasal syndrome. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8652–8657. doi: 10.1073/pnas.0402819101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wieland I, et al. Mutations of the ephrin-B1 gene cause craniofrontonasal syndrome. Am J Hum Genet. 2004;74:1209–1215. doi: 10.1086/421532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van den Elzen ME, et al. Phenotypes of craniofrontonasal syndrome in patients with a pathogenic mutation in EFNB1. European journal of human genetics: EJHG. 2014;22:995–1001. doi: 10.1038/ejhg.2013.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Compagni A, Logan M, Klein R, Adams RH. Control of skeletal patterning by ephrinB1-EphB interactions. Dev Cell. 2003;5:217–230. doi: 10.1016/S1534-5807(03)00198-9. [DOI] [PubMed] [Google Scholar]
- 20.Davy A, Aubin J, Soriano P. Ephrin-B1 forward and reverse signaling are required during mouse development. Genes & development. 2004;18:572–583. doi: 10.1101/gad.1171704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xing W, Kim J, Wergedal J, Chen ST, Mohan S. Ephrin B1 regulates bone marrow stromal cell differentiation and bone formation by influencing TAZ transactivation via complex formation with NHERF1. Molecular and cellular biology. 2010;30:711–721. doi: 10.1128/MCB.00610-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varelias A, Koblar SA, Cowled PA, Carter CD, Clayer M. Human osteosarcoma expresses specific ephrin profiles: implications for tumorigenicity and prognosis. Cancer. 2002;95:862–869. doi: 10.1002/cncr.10749. [DOI] [PubMed] [Google Scholar]
- 23.Wongdee K, Tulalamba W, Thongbunchoo J, Krishnamra N, Charoenphandhu N. Prolactin alters the mRNA expression of osteoblast-derived osteoclastogenic factors in osteoblast-like UMR106 cells. Mol Cell Biochem. 2011;349:195–204. doi: 10.1007/s11010-010-0674-4. [DOI] [PubMed] [Google Scholar]
- 24.Rodda SJ, McMahon AP. Distinct roles for Hedgehog and canonical Wnt signaling in specification, differentiation and maintenance of osteoblast progenitors. Development. 2006;133:3231–3244. doi: 10.1242/dev.02480. [DOI] [PubMed] [Google Scholar]
- 25.Nakashima K, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/S0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 26.Park JS, Baek WY, Kim YH, Kim JE. In vivo expression of Osterix in mature granule cells of adult mouse olfactory bulb. Biochemical and biophysical research communications. 2011;407:842–847. doi: 10.1016/j.bbrc.2011.03.129. [DOI] [PubMed] [Google Scholar]
- 27.Chen J, et al. Osx-Cre targets multiple cell types besides osteoblast lineage in postnatal mice. PloS one. 2014;9:e85161. doi: 10.1371/journal.pone.0085161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glatt V, Canalis E, Stadmeyer L, Bouxsein ML. Age-related changes in trabecular architecture differ in female and male C57BL/6J mice. J Bone Miner Res. 2007;22:1197–1207. doi: 10.1359/jbmr.070507. [DOI] [PubMed] [Google Scholar]
- 29.Monaghan MG, Kroll S, Brucker SY, Schenke-Layland K. Enabling Multiphoton and Second Harmonic Generation Imaging in Paraffin-Embedded and Histologically Stained Sections. Tissue Eng Part C Methods. 2016;22:517–523. doi: 10.1089/ten.tec.2016.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindelin J, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Martin SK, et al. Brief report: the differential roles of mTORC1 and mTORC2 in mesenchymal stem cell differentiation. Stem cells. 2015;33:1359–1365. doi: 10.1002/stem.1931. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen TM, et al. EphB4 Expressing Stromal Cells Exhibit an Enhanced Capacity for Hematopoietic Stem Cell Maintenance. Stem cells. 2015;33:2838–2849. doi: 10.1002/stem.2069. [DOI] [PubMed] [Google Scholar]
- 33.Arthur A, et al. Twist-1 Enhances Bone Marrow Mesenchymal Stromal Cell Support of Hematopoiesis by Modulating CXCL12 Expression. Stem cells. 2016;34:504–509. doi: 10.1002/stem.2265. [DOI] [PubMed] [Google Scholar]
- 34.Atkins GJ, et al. Human trabecular bone-derived osteoblasts support human osteoclast formation in vitro in a defined, serum-free medium. J Cell Physiol. 2005;203:573–582. doi: 10.1002/jcp.20255. [DOI] [PubMed] [Google Scholar]
- 35.Chrencik JE, et al. Three-dimensional structure of the EphB2 receptor in complex with an antagonistic peptide reveals a novel mode of inhibition. J Biol Chem. 2007;282:36505–36513. doi: 10.1074/jbc.M706340200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Genet G, et al. Ephrin-B1 is a novel specific component of the lateral membrane of the cardiomyocyte and is essential for the stability of cardiac tissue architecture cohesion. Circ Res. 2012;110:688–700. doi: 10.1161/CIRCRESAHA.111.262451. [DOI] [PubMed] [Google Scholar]
- 37.Yu M, Wang J, Muller DJ, Helenius J. In PC3 prostate cancer cells ephrin receptors crosstalk to beta1-integrins to strengthen adhesion to collagen type I. Sci Rep. 2015;5:8206. doi: 10.1038/srep08206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Davey RA, et al. Decreased body weight in young Osterix-Cre transgenic mice results in delayed cortical bone expansion and accrual. Transgenic research. 2012;21:885–893. doi: 10.1007/s11248-011-9581-z. [DOI] [PubMed] [Google Scholar]
- 39.Tonna S, et al. EphrinB2 signaling in osteoblasts promotes bone mineralization by preventing apoptosis. FASEB journal. 2014;28:4482–4496. doi: 10.1096/fj.14-254300. [DOI] [PubMed] [Google Scholar]
- 40.McClelland AC, Sheffler-Collins SI, Kayser MS, Dalva MB. Ephrin-B1 and ephrin-B2 mediate EphB-dependent presynaptic development via syntenin-1. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20487–20492. doi: 10.1073/pnas.0811862106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shen LL, et al. Disturbed Expression of EphB4, but Not EphrinB2, Inhibited Bone Regeneration in an In Vivo Inflammatory Microenvironment. Mediators Inflamm. 2016;2016:6430407. doi: 10.1155/2016/6430407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shimizu E, Tamasi J, Partridge NC. Alendronate affects osteoblast functions by crosstalk through EphrinB1-EphB. Journal of dental research. 2012;91:268–274. doi: 10.1177/0022034511432170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stiffel V, Amoui M, Sheng MH, Mohan S, Lau KH. EphA4 receptor is a novel negative regulator of osteoclast activity. J Bone Miner Res. 2014;29:804–819. doi: 10.1002/jbmr.2084. [DOI] [PubMed] [Google Scholar]
- 44.Irie N, et al. Bidirectional signaling through ephrinA2-EphA2 enhances osteoclastogenesis and suppresses osteoblastogenesis. J Biol Chem. 2009;284:14637–14644. doi: 10.1074/jbc.M807598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.