Abstract
In single-channel recordings, the rabbit cardiac Ca2+ release channel (RyR2) is converted to a fully open subconductance state with about 50% of full conductance by micromolar concentrations of ryanodine. At +30 mV, corresponding to a luminal to cytoplasmic cation current, the probability of opening (Po) of ryanodine-modified channels was only marginally altered at pCa 10 (pCa = −log10 Ca concentration). However, at −30 mV, the Po was highly sensitive to Ca2+ added to the cis (cytoplasmic) side and, at pCa 10, was reduced to less than 0.27. The EC50 value for channel opening was about pCa 8. No significant Ca2+ inactivation was observed for ryanodine-modified channels at either −30 mV or +30 mV. The opening of unmodified Ca2+ channels is Ca2+ sensitive, with an EC50 value of about pCa 6 (two orders of magnitude less sensitive than ryanodine-modified channels) and IC50 values of pCa 2.2 at −30 mV and 2.5 at +30 mV. Mg2+ decreased the Po of ryanodine-modified channels at low Ca2+ concentrations at both −30 and +30 mV. Caffeine, ATP, and ruthenium red were modulators of the Po of ryanodine-modified channels. In a [3H]ryanodine binding assay, [3H]ryanodine dissociation from the high-affinity binding site was found to be Ca2+ sensitive, with an IC50 of pCa 7.1. High concentrations of unlabeled ryanodine prevented [3H]ryanodine dissociation, but ruthenium red accelerated dissociation. These results suggest that ryanodine sensitizes Ca2+ activation of the Ca2+ release channel and desensitizes Ca2+ inactivation through an allosteric interaction. [3H]Ryanodine dissociates from the high-affinity site when the channel is closed by removal of Ca2+, implying that high-affinity ryanodine and Ca2+ binding sites are linked through either short- or long-range interactions, probably involving conformational changes.
Ryanodine, a neutral plant alkaloid, was of early interest to physiologists because of its inhibitory effects on cardiac and skeletal muscle function (1). The specific, high-affinity binding of [3H]ryanodine to a class of Ca2+ release channels made possible their purification from skeletal and cardiac muscle sarcoplasmic reticulum and accounts for the name, ryanodine receptor (RyR) (2–5). More recently, the property of binding to RyR Ca2+ release channels only in the open state (6–11) has made [3H]ryanodine binding a useful functional probe of the opening of Ca2+ release channels by other ligands.
High-affinity ryanodine binding in vitro is influenced by temperature, pH, ion concentration, and ligands (see review in ref. 12). Among known ligands, Ca2+ is considered to be a prerequisite for channel opening and thus for ryanodine binding. Ryanodine bound to the high-affinity site in RyRs dissociates very slowly. At higher concentrations, ryanodine slows the rate of dissociation of ryanodine bound to the high-affinity site of RyR1 by occupation of the low-affinity sites (6, 13–15). Ruthenium red, a channel blocker (6), and Ca2+, a channel activator, also delay dissociation of bound ryanodine (14). It is not known why these compounds, which have such different effects on RyR channel function, affect the dissociation of ryanodine from its high-affinity sites in a similar way.
Ryanodine activates Ca2+ release from the sarcoplasmic reticulum, but prolonged exposure of microsomes to micromolar concentrations of ryanodine inhibits Ca2+ release (2, 16–19). Single-channel recordings have revealed that ryanodine activates Ca2+ release channels at nanomolar concentrations and stabilizes four discrete channel states at higher concentrations (20). A common finding is that micromolar concentrations of ryanodine convert Ca2+ release channels to a subconductance state at about 50% of full conductance and a probability of opening (Po) very close to unity (21). Alterations in conductance by ryanodine have been attributed mainly to modified ion handling, a consequence of allosterically determined conformational changes (22). The mechanism of action of ryanodine on Ca2+ release channels, particularly its ability to alter channel gating, is still poorly understood.
In this study, we used single-channel recordings across planar lipid bilayers to examine possible mechanisms for the ryanodine modification of rabbit cardiac muscle RyR (RyR2 isoform) Ca2+ release channels. We found that ryanodine-modified channels were much more sensitive to Ca2+ than normal channels and were modulated by Mg2+, caffeine, and ATP in the presence of 1 mM EGTA. In addition, the dissociation of bound [3H]ryanodine from the high-affinity site of RyR2 was observed when the channel was closed by decreased Ca2+, but not by the addition of ruthenium red.
Materials and Methods
Preparation of Sarcoplasmic Reticulum Microsomes.
Heavy sarcoplasmic reticulum microsomes were isolated from rabbit heart muscle as described (23). Microsomes were suspended in 0.25 M sucrose, 125 mM KCl, and 10 mM Tris⋅HCl (pH 8.0) and stored at −70°C.
Partial Purification of RyR2 Protein and Single-Channel Recording.
Cardiac microsomes were solubilized and partially purified by density sucrose gradient centrifugation, and single-channel properties were recorded in a planar lipid bilayer system, as described (24, 25). Free Ca2+ was calculated with the use of the apparent binding constants described by Fabiato (26).
[3H]Ryanodine Dissociation Assay.
A previous protocol (27) was modified to determine dissociation of bound [3H]ryanodine to cardiac microsomes. In brief, cardiac microsomes were incubated with 10 nM [3H]ryanodine in a buffer containing 0.5 mM KCl, 10 or 100 μM free Ca2+, 0.2 mM EGTA, and 25 mM Hepes (pH 7.2) for 90 min at 37°C to reach equilibrium. At that point, additional agents, taken from highly concentrated stock to minimize changes in volume, were added, and the incubation proceeded. At different time points, duplicate 0.2-ml samples, containing about 50 μg protein, were filtered on Whatman GF/B membrane filters, presoaked with a washing buffer composed of 25 mM Hepes (pH 7.4) and 0.25 M KCl, and the filters were washed with 12 ml of washing buffer. Background binding was measured in the presence of 10 μM unlabeled ryanodine (1,000-fold excess). [3H]Ryanodine bound to the filter was quantified by liquid scintillation counting.
Results
Ryanodine Sensitizes Ca2+ Activation of RyR2.
In the absence of EGTA in the 1-ml cis chamber, the addition of 3 μl of the purified Ca2+ release channel, which was suspended in 100 μM Ca2+, would raise the Ca2+ concentration of the cis chamber to about pCa 6.5 (pCa = −log10 Ca concentration). Under these conditions, a K+ current of about 21 pA with an open probability (Po) of about 0.10 could be observed with a single RyR2 channel incorporated into a planar lipid bilayer at a holding potential of either +30 mV or −30 mV in symmetrical 250 mM KCl (not shown). The addition of 1–10 μM ryanodine to the cis chamber locked the channel in a subconductance state with ≈50% conductance of about 12 pA and full Po (Fig. 1A). These are the archetypal properties for modulation of Ca2+ release channels by ryanodine (21).
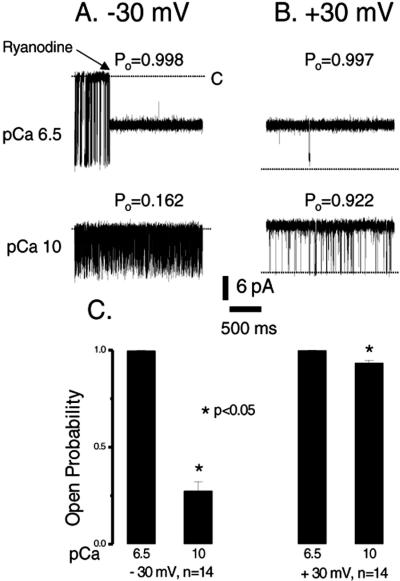
Figure 1.
Effect of EGTA on ryanodine-modified Ca2+ release channels. Single-channel currents are shown as downward (A) or upward (B) inflections from the closed level at −30 mV or +30 mV, indicated by a dotted line in each trace. Tracings were recorded in symmetrical 250 mM KCl solution. The upper traces in both A and B were recorded in the absence of EGTA to give a pCa of 6.5, and the lower traces were recorded in the presence of 1 mM EGTA, which lowered the Ca2+ concentration to a pCa of 10. Modulation of the channel by ryanodine is shown in the upper trace at −30 mV (A). The ryanodine-modified channel had about half the conductance of the normal channel. The Po shown above each trace is for the ryanodine-modified channel. Traces were filtered at 1 kHz and digitized at 10 kHz. The averaged Po for the ryanodine-modified channels measured in A and B before and after the addition of 1 mM EGTA is shown in C.
The question of how ryanodine increases the probability of channel opening has not yet been answered. In our experiment, only ryanodine and Ca2+ were present as possible activators of Ca2+ channel opening. Thus, changes in channel gating could result from either a direct or an indirect action of ryanodine. To test the hypothesis that the high Po for ryanodine-modified Ca2+ release channels is mediated through an alteration in Ca2+ sensitivity, we lowered the Ca2+ concentration to pCa 10 by the addition of 1 mM EGTA to the cis chamber in the same single-channel recording as that shown at pCa 6.5 in Fig. 1A. At −30 mV, where the current flows from the cytoplasmic side to the luminal side, the Po of ryanodine-modified Ca2+ release channels was reduced from nearly 1.0 at pCa 6.5 to less than 0.27 at pCa 10 (Fig. 1 A and C). At +30 mV and pCa 10, however, the Po was reduced from 0.997, observed at pCa 6.5, by a small, but significant extent, to about 0.922 (Fig. 1 B and C). These values did not change when 1 mM EGTA was also added to the trans chamber. Under these conditions, the channel remained in a subconductance state, the 350-pS conductance for K+ being about 50% of the control. These results demonstrate that ryanodine alone is not capable of opening the channel fully: ryanodine sensitizes the channel to Ca2+-induced opening. This sensitization is more readily evident at −30 mV.
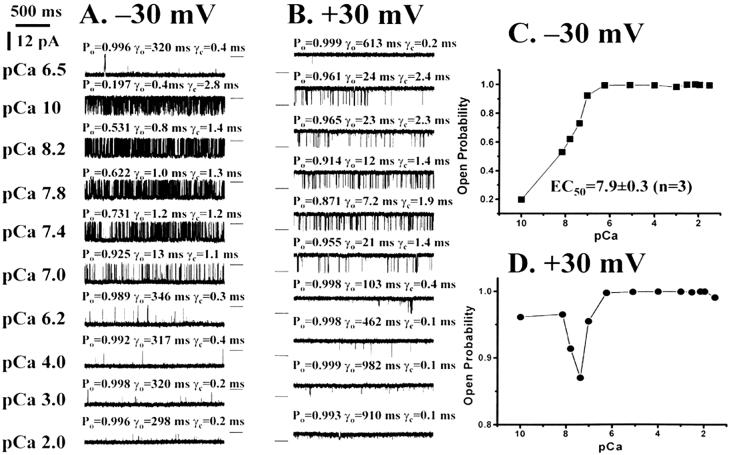
The Ca2+ dependence of channel opening in ryanodine-modified Ca2+ release channels was measured. At −30 mV, incremental additions of Ca2+ to the cis chamber, beginning at pCa 10, induced incremental increases in Po (Fig. 2 A and C). Plots of Po against pCa at −30 mV show a smooth curve for Ca2+ dependence of Po, rising from 0.197 to 0.992 between pCa 10 and pCa 6.2, with a plateau above pCa 6 (Fig. 2 A and C). The EC50 value, calculated from the dose–response curve for Ca2+ activation at −30 mV, was pCa 7.9 ± 0.3 (n = 3). In contrast, there was limited inhibition of channel opening in the presence of 1 mM EGTA at +30 mV (Figs. 1 and 2 B and C). The Po increased after incremental additions of Ca2+ to the cis chamber, dipped slightly as Ca2+ concentrations were raised to pCa 7.4 in all three recordings, and then increased again to the fully open state at Ca2+ concentrations above pCa 6 (Fig. 2 B and D). The lifetime of the open state (γo) was increased, and the lifetime of the closed state (γc) was decreased in parallel with the elevation of Ca2+ (Fig. 2 B and D). There was no evidence of inactivation of channel opening by concentrations of Ca2+ up to 10 mM for ryanodine-modified Ca2+ release channels at either negative or positive potentials (Fig. 2).
Figure 2.
Ca2+ activation of ryanodine-modified Ca2+ release channels. Single-channel currents, at −30 mV or +30 mV, are shown as downward (A) or upward (B) inflections from the closed level, which is indicated by a short line to the right (A) or left (B) of each trace. Currents were recorded in symmetrical 250 mM KCl and 1 mM EGTA at various free Ca2+ concentrations, indicated on the left of A. Po, open-state lifetime (γo), and closed-state lifetime (γc) are shown above each trace. Traces were filtered at 1 kHz and digitized at 10 kHz. Resulting Ca2+ activation curves are shown in C for −30 mV and D for +30 mV. (Please note the change in scale between C and D.)
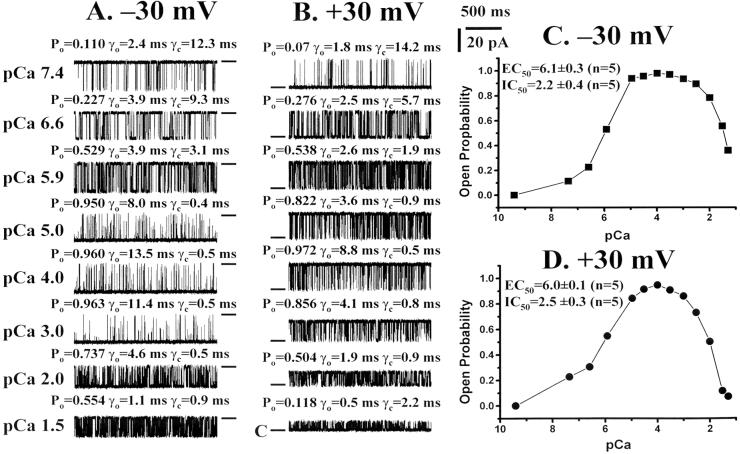
The Ca2+ dependence of channel opening and closing was also measured in normal channels (Fig. 3). The results were not nearly as pronounced as those seen with ryanodine-modified Ca2+ release channels, inasmuch as a strong Ca2+ dependence of channel opening and closing was observed at both −30 mV and +30 mV. As the Ca2+ concentration was elevated in the cis chamber, Po was increased at both +30 and −30 mV (Fig. 3). Dose–response curves (Fig. 3 C and D) yielded EC50 values of 6.1 ± 0.3 and 6.0 ± 0.1 (n = 5) for −30 and +30 mV, respectively. These curves illustrate that the Ca2+ sensitivity at −30 mV (pCa 7.9 vs. pCa 6.1) is two orders of magnitude higher in ryanodine-modified Ca2+ release channels and, at +30 mV (pCa >10 vs. 6.0), is more than four orders of magnitude higher. The average of five independent experiments showed a decrease in Po at pCa values between 3 and 1.5, so that the IC50 value was 2.2 ± 0.4 at +30 mV and 2.5 ± 0.3 at −30 mV (n = 5) (Fig. 3 A and B). At −30 mV, the increase in Po, which reached a maximum at approximately pCa 5, was associated with an increase in γo and a decrease in γc. The pattern was qualitatively similar at +30 mV.
Figure 3.
Ca2+ activation and inactivation of ryanodine-modified Ca2+ release channels. Single-channel currents, at −30 mV or +30 mV, are shown as downward (A) or upward (B) inflections from the closed level, which is indicated by a short line to the right (A) or left (B) of each trace. Currents were recorded in symmetrical 250 mM KCl and 50 μM EGTA at various free Ca2+ concentrations, indicated on the left of A. Po, open-state lifetime (γo), and closed state lifetime (γc) are shown above each trace. Traces were filtered at 1 kHz and digitized at 10 kHz. Resulting Ca2+ dose–response curves are shown in C for −30 mV and D for +30 mV.
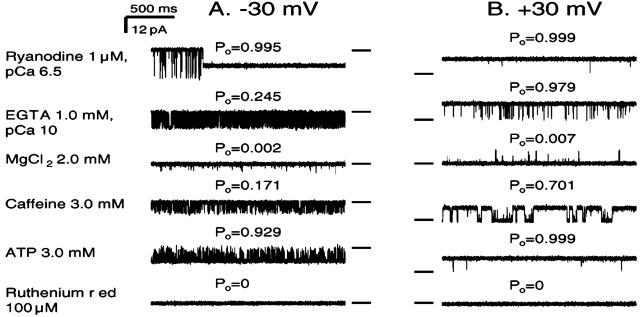
Ryanodine-modified Ca2+ release channels were tested for modulation by other compounds. In the sequential traces shown in Fig. 4, the presence of 1 mM EGTA (pCa 10) in the cis chamber reduced Po from 0.995 to 0.245 at −30 mV, but to only 0.979 at +30 mV. After the addition of 2 mM MgCl2 to the cis chamber, the Po was decreased at both holding potentials, from 0.245 to 0.002 at −30 mV and from 0.979 to 0.007 at +30 mV. At pCa 5, however, the Po for the ryanodine-modified Ca2+ release channels was close to 1 at both −30 and +30 mV, and this Po was unchanged, even when up to 10 mM MgCl2 was added to the cis side (data not shown).
Figure 4.
Modulation of ryanodine-modified Ca2+ release channels by Mg2+, ATP, caffeine, and ruthenium red. Single-channel currents, shown as downward (A) or upward (B) inflections from the closed level at −30 mV or +30 mV, indicated by a short line to the right (A) or left (B) of each trace, were recorded in symmetrical 250 mM KCl solution. The top traces in both A and B were recorded at pCa 6.5, and the lower traces are selected from continued recordings in the presence of agents, labeled at the left of A. Modulation of the channel by ryanodine is shown in the top trace at −30 mV (A). The ryanodine-modified channel exhibited about half of the conductance of the normal channel. Po, shown above each trace, is for the ryanodine-modified channel. Traces were filtered at 1 kHz and digitized at 10 kHz.
In the presence of 2 mM MgCl2 in the cis chamber, Ca2+ activation curves showed that the EC50 was shifted from pCa ≈7.9 to pCa ≈6.0 for ryanodine-modified Ca2+ release channels and from pCa ≈6.0 to pCa ≈4.0 for normal channels, a shift in Ca2+ sensitivity of about two orders of magnitude for both normal and modified channels at both −30 and +30 mV. These observations illustrate that Mg2+ is a powerful inhibitor of both normal and ryanodine-modified Ca2+ release channels at both positive and negative voltages. The fact that miniscule Ca2+ concentrations can overcome Mg2+ inhibition, however, shows that the Ca2+ affinity for the site of Ca2+ activation of the channel is much higher than Mg2+ affinity in ryanodine-modified Ca2+ release channels.
In ryanodine-modified Ca2+ release channels, both caffeine and ATP increased Po that had been depressed by 1 mM EGTA and MgCl2. These different ligands are both believed to act through Ca2+, by increasing the sensitivity of the channel to Ca2+ activation. Po was reduced to 0 by the addition of ruthenium red at both +30 and −30 mV (Fig. 4), in a manner similar to that observed with unmodified channels.
Dissociation of [3H]Ryanodine Associated with Closure of Channel.
If ryanodine binds only to open channels, and if these channels close, then it is reasonable to assume that ryanodine might dissociate from the channel to allow it to close. To test this hypothesis, we attempted to detect the dissociation of [3H]ryanodine bound to the high-affinity site of RyR2 by depletion of Ca2+.
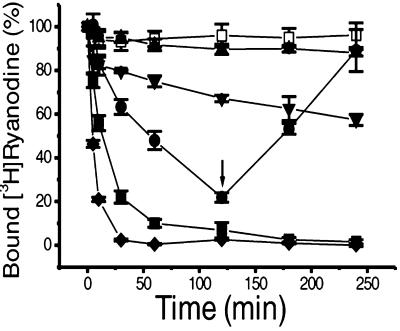
Fig. 5 shows time courses for the dissociation of bound [3H]ryanodine under different experimental conditions. Cardiac muscle microsomes were incubated for 90 min at 37°C in the binding buffer (pCa 4) before different agents were added and incubation was continued. The addition of 1 mM EGTA (pCa 7.7) initiated dissociation of bound [3H]ryanodine in a time-dependent manner, whereas the control was unchanged. The time course could be fitted to a single-exponential equation with a dissociation rate constant of 74.9 ± 6.3 min−1 (n = 4). The addition of 1 mM Ca2+ at 120 min led to full recovery of [3H]ryanodine binding after 60 min. The addition of 2 mM EGTA, reducing the pCa to 8.0, produced a deeper curve than that for pCa 7.7, with a dissociation rate constant of 19.1 ± 1.4 min−1 (n = 4, P < 0.001). Because the equilibrium state for [3H]ryanodine binding was not altered under these conditions, the dissociation behavior can be attributed mainly to the lowering of Ca2+ by EGTA, which closes the channels.
Figure 5.
Dissociation of [3H]ryanodine from the high-affinity binding site of cardiac muscle RyR2. Microsomes were incubated with 10 nM [3H]ryanodine in a binding buffer containing 100 μM free Ca2+ for 90 min at 37°C to reach equilibrium. Incubation was then continued after the addition of different agents: □, no addition (control); ●, 1 mM EGTA; ■, 2 mM EGTA; ⧫, 2 mM EGTA plus 100 μM ruthenium red; ▴, 2 mM EGTA plus 100 μM ryanodine; ▾, 100 μM ruthenium red. At different time points, duplicate 0.2-ml aliquots were filtered. The arrow indicates the addition of 1 mM CaCl2 at a time point of 120 min for the 1 mM EGTA curve. The data are normalized to the values obtained after the first 90-min incubation, which is shown as time 0.
Because occupation of low-affinity binding sites by ryanodine delays dissociation of [3H]ryanodine bound to high-affinity sites (6, 13–15), the effect of 100 μM ryanodine on the dissociation initiated by lowered Ca2+ was tested. In the presence of 2 mM EGTA and 100 μM ryanodine, no significant dissociation of [3H]ryanodine was observed up to 240 min of incubation at 37°C when compared with the control (Fig. 5). There was also no significant dissociation when only 100 μM ryanodine was present (data not shown). This finding suggests that occupation of low-affinity sites by higher concentrations of ryanodine maintained the binding of [3H]ryanodine to high-affinity sites, even in the presence of Ca2+ concentrations so low that channels should have been closed.
Ruthenium red is a powerful blocker of Ca2+ release channels that acts by binding to sites located in the conduction pore of the channel (28). Its mode of action is obviously distinct from that of channel closure induced by Ca2+ depletion. Ruthenium red also delays dissociation of ryanodine bound to the high-affinity site in a nonequilibrium state (6). Accordingly, we attempted to determine whether ruthenium red affects the dissociation of [3H]ryanodine bound to the high-affinity site of the channel. In the presence of 100 μM ruthenium red, a very slow dissociation of [3H]ryanodine with a rate constant of 363.1 ± 36.5 min−1 (n = 3) was observed when compared with the control (Fig. 5). These results suggest that ruthenium red has little effect on high-affinity [3H]ryanodine binding, even though it blocks the Ca2+ release channels. When added together with 2 mM EGTA, however, 100 μM ruthenium red speeded dissociation of [3H]ryanodine bound to the high-affinity site, with a rate constant of 6.7 ± 0.3 min−1 (n = 4, P < 0.001 when compared with the rate in the presence of 2 mM EGTA).
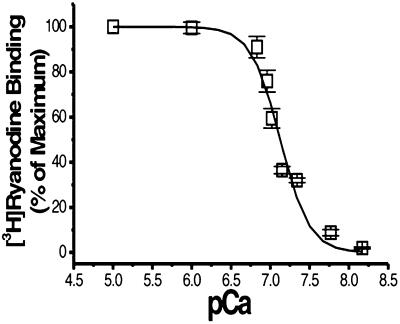
In the presence of different concentrations of Ca2+, dissociation of [3H]ryanodine from the high-affinity site of RyR2 was increased as Ca2+ concentrations decreased (Fig. 6), showing a typical curve of Ca2+ dependence. The IC50 value obtained by fitting the curve with a sigmoid equation was 7.12 ± 0.02 pCa units (n = 4), and the slope was 2.3 ± 0.3.
Figure 6.
Ca2+-dependent dissociation of [3H]ryanodine from the high-affinity binding site of RyR2. Microsomes were incubated with 10 nM [3H]ryanodine in a binding buffer containing 10 μM free Ca2+ for 90 min at 37°C to reach equilibrium. The reaction mixes were then incubated for 2 h with different concentrations of EGTA to set the desired pCa values. Duplicate 0.2-ml aliquots were filtered at the time points indicated. The data are normalized to the values obtained after the first 90-min incubation.
Discussion
Increased Asymmetric Ca2+ Sensitivity of Ryanodine-Modified Ca2+ Release Channels.
In this study, we explored the effect of ryanodine binding to RyR2 on Ca2+ sensitivity at both positive and negative voltages. We found that ryanodine binding induces a higher sensitivity to Ca2+ when channel function is measured at positive voltages. At −30 mV, the current flows from the cytoplasmic side to the luminal side, whereas, at +30 mV, the current flows from the luminal side to the cytoplasmic side, the direction of Ca2+ flow that is physiologically relevant. Single-channel recordings at +30 mV show that ryanodine-modified Ca2+ release channels retain a Po close to unity in the presence of 1 mM EGTA (pCa 10), and at −30 mV the Po is about 0.2. Under these conditions, unmodified channels have a Po of 0.
In earlier studies (1), cardiac and skeletal muscle function was shown to be modulated by ryanodine. Since then ryanodine has been shown to increase Ca2+ permeability from sarcoplasmic reticulum and, at higher concentrations, to inhibit Ca2+ release (refs. 2, 16, 29, 30, and see reviews in refs. 10 and 11). Ryanodine blocks the channel in a subconductance state at about 50% of full conductance and a Po near unity (21). This action has been regarded as a direct effect of ryanodine. Our results help to clarify the action of ryanodine on Ca2+ release by showing that increased Ca2+ permeability is due to an indirect effect of ryanodine on Ca2+ sensitivity.
The binding of ryanodine, through allosteric interactions, could convert Ca2+ release channels to a specific conformation, associated with a 50% reduction of the conductance (31). Altered ion handling resulting from conformation changes induced by ryanodine has been considered to be a major reason for alterations in conductance (22). Evidence presented in this study shows that the high Po induced by ryanodine is likely to be caused by increased Ca2+ sensitivity. In earlier studies of the effects of site-directed mutagenesis of residues lining the pore, ryanodine restored or enhanced the response of some mutant channels to caffeine activation. This effect of ryanodine may have occurred through a ryanodine-induced increase in Ca2+ sensitivity, because caffeine can sensitize Ca2+ activation (25, 32). Thus, the high-affinity binding of ryanodine to Ca2+ release channels is likely to induce a conformational change that alters not only conductance but also the affinity of Ca2+ for Ca2+ activation.
Inhibition by Mg2+ of Ryanodine-Modified Ca2+ Release Channels.
The increased Ca2+ sensitivity of ryanodine-modified Ca2+ release channels was asymmetric, inasmuch as higher Ca2+ sensitivity was observed when the current flowed from the lumen to the cytoplasm. When Mg2+ was added at pCa 10, however, the asymmetry was lost and channel opening was inhibited to an equal extent when current flowed in either direction. The inhibited channel was then activated in either direction by subsequent addition of caffeine and ATP (Fig. 4) or Ca2+ (data not shown).
Mg2+ competes with Ca2+ at both the activation and inactivation sites (33, 34). In this study, Ca2+ bound to low-affinity sites did not inhibit channel opening of ryanodine-modified Ca2+ release channels. This lack of inhibition of channel opening by bound Ca2+ suggests that the inhibition by Mg2+ of channel opening is due to the competitive binding of Mg2+ to the high-affinity Ca2+ binding site. Mg2+ is considered to be an important regulator in controlling Ca2+ release from the sarcoplasmic reticulum of skeletal muscle, even though it has low binding affinity (35). Our results, together with those of others (33, 34), suggest that Mg2+ competition with the high-affinity Ca2+ sites could be equally important in controlling Ca2+ release under both physiological and pathological conditions and in both cardiac and skeletal muscles.
Dissociation of [3H]Ryanodine Bound to RyR2 Ca2+ Release Channels Is Associated with Closure of Channels.
In this study, we have modified a protocol (27) to detect dissociation of [3H]ryanodine bound to the high-affinity site of RyR2 by lowering Ca2+ under equilibrium binding conditions. It is known that [3H]ryanodine binds to the high-affinity site only when the channel is in the open state, with a Ca2+ EC50 value of about pCa 6 for Ca2+ association. Results from this study suggest that closing of the channel by removal of Ca2+ resulted in dissociation of bound [3H]ryanodine with a Ca2+ IC50 value for dissociation of about pCa 7.1—one order of magnitude higher than the pCa value for Ca2+ association. Thus both [3H]ryanodine association and [3H]ryanodine dissociation are primarily Ca2+ dependent, reflecting a close relationship between Ca2+ and ryanodine binding sites. This relationship is particularly shown to be true when we consider that ryanodine binding to the high-affinity site increases Ca2+ sensitivity of Ca2+ release channels and that Ca2+ is a basic activator of RyR channels. Although a direct interaction between these two sites is possible, most observations are consistent with the view that the high-affinity site for ryanodine binding is exposed or formed by conformational changes resulting from the opening of the channel by Ca2+. When the channel is closed by removal of Ca2+, the ryanodine-binding site is either cryptic or disrupted (6, 13–15).
In an earlier report, Chu et al. (6) showed that ruthenium red, at high concentrations, slowed the rate of dissociation of [3H]ryanodine from the high-affinity site. In contrast, we found that ruthenium red accelerated the dissociation of [3H]ryanodine that was induced by removal of Ca2+. Ruthenium red, at 100 μM, closed RyR channels, but it induced only a very slow dissociation of [3H]ryanodine bound to the high-affinity site under equilibrium conditions. Removal of Ca2+ also closed channels but led to the complete dissociation of [3H]ryanodine. It is believed that ruthenium red blocks Ca2+ release channels by binding to sites located in the conduction pore (28) or inhibits the channels by binding to cytosolic and luminal sites (36). Our results demonstrate that ruthenium red and removal of Ca2+ have very different effects on the dissociation of [3H]ryanodine from the high-affinity binding site, even though their actions are cooperative.
The protocol for measuring ryanodine dissociation may become a useful tool for distinguishing among the actions of different compounds. It may also become a useful tool for measurement of channel function, because Ca2+-dependent closure of the channel, detected by [3H]ryanodine dissociation from its high-affinity binding site, provides the converse measure to Ca2+-dependent opening of the channel, which is measured by [3H]ryanodine association.
Acknowledgments
This work was supported by Grant MT-3399 from the Canadian Institutes for Health Research (to D.H.M.). G.G.D. was a postdoctoral fellow of the Heart and Stroke Foundation of Canada.
Abbreviations
- RyR
ryanodine receptor
- RyR2
the cardiac muscle ryanodine receptor isoform
- Po
channel open probability
- pCa
−log10 Ca concentration
References
- 1.Jenden D J, Fairhurst A S. Pharmacol Rev. 1969;21:1–25. [PubMed] [Google Scholar]
- 2.Fleischer S, Ogunbunmi E M, Dixon M C, Fleer E A. Proc Natl Acad Sci USA. 1985;82:7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pessah I N, Waterhouse A L, Casida J E. Biochem Biophys Res Commun. 1985;128:449–456. doi: 10.1016/0006-291x(85)91699-7. [DOI] [PubMed] [Google Scholar]
- 4.Inui M, Saito A, Fleischer S. J Biol Chem. 1987;262:1740–1747. [PubMed] [Google Scholar]
- 5.Lai F A, Erickson H P, Rousseau E, Liu Q Y, Meissner G. Nature (London) 1988;331:315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- 6.Chu A, Diaz-Munoz M, Hawkes M J, Brush K, Hamilton S L. Mol Pharmacol. 1990;37:735–741. [PubMed] [Google Scholar]
- 7.Meissner G, el-Hashem A. Mol Cell Biochem. 1992;114:119–123. doi: 10.1007/BF00240306. [DOI] [PubMed] [Google Scholar]
- 8.Hawkes M J, Nelson T E, Hamilton S L. J Biol Chem. 1992;267:6702–6709. [PubMed] [Google Scholar]
- 9.Du G G, Imredy J P, MacLennan D H. J Biol Chem. 1998;273:33259–33266. doi: 10.1074/jbc.273.50.33259. [DOI] [PubMed] [Google Scholar]
- 10.Zucchi R, Ronca-Testoni S. Pharmacol Rev. 1997;49:53–98. [PubMed] [Google Scholar]
- 11.Sutko J L, Airey J A, Welch W, Ruest L. Pharmacol Rev. 1997;49:53–98. [PubMed] [Google Scholar]
- 12.Coronado R, Morrissette J, Sukhareva M, Vaughan D M. Am J Physiol. 1994;266:C1485–C1504. doi: 10.1152/ajpcell.1994.266.6.C1485. [DOI] [PubMed] [Google Scholar]
- 13.McGrew S G, Wolleben C, Siegl P, Inui M, Fleischer S. Biochemistry. 1989;28:1686–1691. doi: 10.1021/bi00430a039. [DOI] [PubMed] [Google Scholar]
- 14.Lai F A, Misra M, Xu L, Smith H A, Meissner G. J Biol Chem. 1989;264:16776–16785. [PubMed] [Google Scholar]
- 15.Wang J P, Needleman D H, Hamilton S L. J Biol Chem. 1993;268:20974–20982. [PubMed] [Google Scholar]
- 16.Meissner G. J Biol Chem. 1986;261:6300–6306. [PubMed] [Google Scholar]
- 17.Lattanzio F A, Jr, Schlatterer R G, Nicar M, Campbell K P, Sutko J L. J Biol Chem. 1987;262:2711–2718. [PubMed] [Google Scholar]
- 18.Pessah I N, Zimanyi I. Mol Pharmacol. 1991;39:679–689. [PubMed] [Google Scholar]
- 19.Kasai M, Kawasaki T. J Biochem (Tokyo) 1993;113:327–333. doi: 10.1093/oxfordjournals.jbchem.a124047. [DOI] [PubMed] [Google Scholar]
- 20.Buck E, Zimanyi I, Abramson J J, Pessah I N. J Biol Chem. 1992;267:23560–23567. [PubMed] [Google Scholar]
- 21.Rousseau E, Smith J S, Meissner G. Am J Physiol. 1987;253:C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- 22.Lindsay A R, Tinker A, Williams A J. J Gen Physiol. 1994;104:425–447. doi: 10.1085/jgp.104.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell K P, MacLennan D H. J Biol Chem. 1981;256:4626–4632. [PubMed] [Google Scholar]
- 24.Chen S R, Vaughan D M, Airey J A, Coronado R, MacLennan D H. Biochemistry. 1993;32:3743–3753. doi: 10.1021/bi00065a029. [DOI] [PubMed] [Google Scholar]
- 25.Du G G, Guo X, Khanna V K, MacLennan D H. J Biol Chem. 2001;276:31760–31771. doi: 10.1074/jbc.M102751200. [DOI] [PubMed] [Google Scholar]
- 26.Fabiato A. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- 27.Pessah I N, Stambuk R A, Casida J E. Mol Pharmacol. 1987;31:232–238. [PubMed] [Google Scholar]
- 28.Ma J. J Gen Physiol. 1993;102:1031–1056. doi: 10.1085/jgp.102.6.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones L R, Besch H R, Jr, Sutko J L, Willerson J T. J Pharmacol Exp Ther. 1979;209:48–55. [PubMed] [Google Scholar]
- 30.Sutko J L, Willerson J T, Templeton G H, Jones L R, Besch H R., Jr J Pharmacol Exp Ther. 1979;209:37–47. [PubMed] [Google Scholar]
- 31.Welch W, Williams A J, Tinker A, Mitchell K E, Deslongchamps P, Lamothe J, Gerzon K, Bidasee K R, Besch H R, Jr, Airey J A, et al. Biochemistry. 1997;36:2939–2950. doi: 10.1021/bi9623901. [DOI] [PubMed] [Google Scholar]
- 32.Fessenden J D, Chen L, Wang Y, Paolini C, Franzini-Armstrong C, Allen P D, Pessah I N. Proc Natl Acad Sci USA. 2001;98:2865–2870. doi: 10.1073/pnas.041608898. . (First published February 13, 2001; 10.1073/pnas.041608898) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meissner G, Darling E, Eveleth J. Biochemistry. 1986;25:236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- 34.Laver D R, Baynes T M, Dulhunty A F. J Membr Biol. 1997;156:213–229. doi: 10.1007/s002329900202. [DOI] [PubMed] [Google Scholar]
- 35.Lamb G D. Clin Exp Pharmacol Physiol. 2000;27:216–224. doi: 10.1046/j.1440-1681.2000.03224.x. [DOI] [PubMed] [Google Scholar]
- 36.Xu L, Tripathy A, Pasek D A, Meissner G. J Biol Chem. 1999;274:32680–32691. doi: 10.1074/jbc.274.46.32680. [DOI] [PubMed] [Google Scholar]