Abstract
Fear extinction depends on N-methyl-D-aspartate glutamate receptors (NMDARs) and brain-derived neurotrophic factor (BDNF) activation in the limbic system. However, postsynaptic density-95 (PSD-95) and neuronal nitric oxide synthase (nNOS) coupling, the downstream signaling of NMDARs activation, obstructs the BDNF signaling transduction. Thus, we wondered distinct roles of NMDAR activation and PSD-95-nNOS coupling on fear extinction. To explore the mechanisms, we detected protein-protein interaction using coimmunoprecipitation and measured protein expression by western blot. Contextual fear extinction induced a shift from PSD-95-nNOS to PSD-95-TrkB association in the dorsal hippocampus and c-Fos expression in the dorsal CA3. Disrupting PSD-95-nNOS coupling in the dorsal CA3 up-regulated phosphorylation of extracellular signal-regulates kinase (ERK) and BDNF, enhanced the association of BDNF-TrkB signaling with PSD-95, and promoted contextual fear extinction. Conversely, blocking NMDARs in the dorsal CA3 down-regulated BDNF expression and hindered contextual fear extinction. NMDARs activation and PSD-95-nNOS coupling play different roles in modulating contextual fear extinction in the hippocampus. Because inhibitors of PSD-95-nNOS interaction produce antidepressant and anxiolytic effect without NMDAR-induced side effects, PSD-95-nNOS could be a valuable target for PTSD treatment.
Introduction
Learning about potential dangers in the environment is critical for adaptive function, but the fear learning for emotional disorders, post-traumatic stress disorders (PTSD) in particular, can be maladaptive, resulting in excessive fear and anxiety1. PTSD is extraordinarily robust and difficult to treat, because of enhanced fear learning, impaired extinction or inability to modulate fear expression using contextual information2. Extinction, the learned inhibition of retrieval, is widely used in the treatment of PTSD, often under the term “exposure therapy”3. The extinction learning involves new learning of an inhibitory signal that competes with the previously learned fear memory4. Contexts, a set of circumstances around an event, are essential for abstracting situationally informed meaning from the world. Contextual processing deficits are at the core of PTSD pathophysiology1,2. Hippocampus has a crucial role in tasks involving learning and remembering contexts1. Therefore, understanding molecular pathways mediating contextual extinction learning in the hippocampus is particularly important to treat the disorder.
Convergent evidence from animal and human studies suggests that extinction of recently and remotely acquired fear depends on N-methyl-D-aspartate glutamate receptor (NMDAR) activation in the hippocampus, basolateral amygdala and ventromedial prefrontal cortex5–7. Each NMDAR is a calcium-permeable tetrameric ionotropic receptor complex consisting of two obligatory GluN1 subunits and two GluN2 (A-D) or GluN3 (A, B) subunits8. In the adult forebrain regions, GluN2A and GluN2B subunits are the main subunits available in excitatory synapses for receptor complex formation9. GluN2B-containing receptor has a preferential role in the induction of synaptic plasticity critical for the extinction of fear memories10. The carboxyl terminus of each subunit binds important intracellular signaling complexes, allowing for their efficient and selective activation by calcium influx through the opening of NMDAR channels11. One of the well-characterized intracellular signaling complexes of GluN2B is the PSD-95-nNOS complex, in which, the protein postsynaptic density-95 (PSD-95) is a scaffolding protein that links GluN2B carboxyl terminus to neuronal nitric oxide synthase (nNOS) at excitatory synapses12. Activation of nNOS depends on its association with PSD-95 and on NMDAR-mediated calcium influx13. We recently found that the PSD-95-nNOS signaling complex impairs neuroplasticity, including neurogenesis, spine growth and dendrite development14, which is clearly different from the role of NMDAR activation. Given that neuroplasticity is crucial for memory extinction15, we hypothesized that NMDAR activation and PSD-95-nNOS coupling may play different role in the modulation of contextual fear extinction in the hippocampus.
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family identified as a critical factor that mediates synaptic plasticity associated with learning and memory, specifically in fear learning and extinction16. The functions of BDNF are mediated by the receptor tyrosine kinase TrkB, which is present in the fraction of postsynaptic density in the adult rat brain17. Upon NMDARs activation, PSD-95 not only interacts with nNOS to form PSD-95-nNOS complex13, but also with TrkB to form PSD-95-TrkB complex17,18 at excitatory synapses. Based on previous reports, we speculated that nNOS and TrkB may compete with each other to form complexes with PSD-95, thus playing an important role in fear extinction.
Extracellular regulated protein kinase (ERK) regulates hippocampal histone following contextual fear conditioning19. NO produced from nNOS in the presence of L-arginine is a potent inhibitor of Ca2+-mediated ERK activation20. Therefore, ERK activation may contribute to the role of PSD-95-nNOS in regulating BDNF expression.
In general, we hypothesized that disassociating PSD-95-nNOS coupling in the hippocampus may up-regulate BDNF expression via inhibiting ERK activation, enhanced the association of BDNF-TrkB signaling with PSD-95, and promoted contextual fear extinction. Conversely, blocking NMDARs down-regulated BDNF expression and hindered contextual fear extinction. NMDARs activation and PSD-95-nNOS coupling play distinct roles in modulating contextual fear extinction.
Results
Contextual Fear Extinction Induces a Shift from PSD-95-nNOS to PSD-95-TrkB Coupling in the Hippocampus
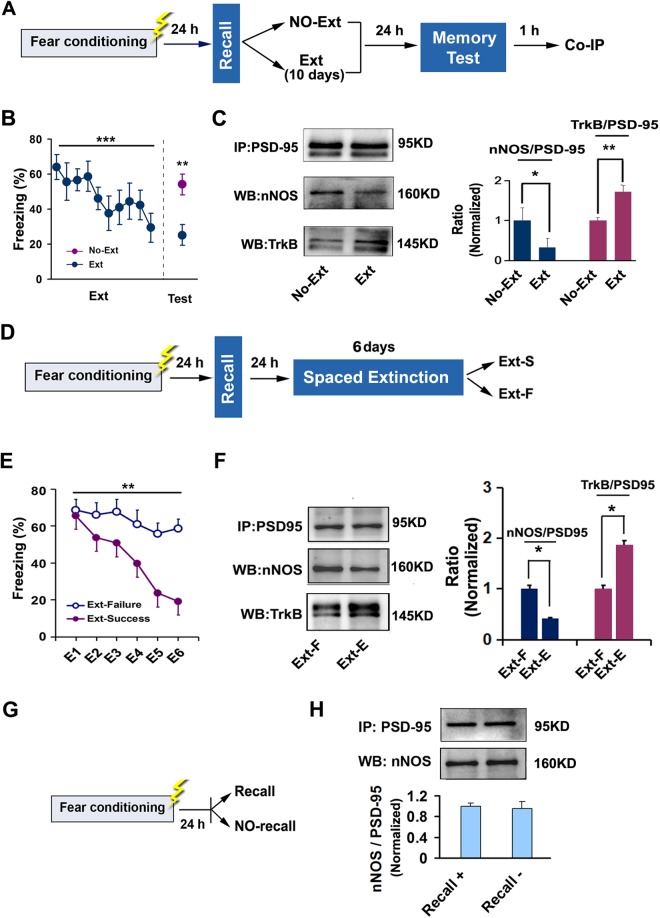
To test whether contextual fear extinction affects the interactions of PSD-95 with nNOS and TrkB in the hippocampus, we trained mice in the contextual fear-conditioning procedure as previously described21. All animals acquired contextual conditioned fear to the same extent as indicated by high freezing levels in response to the context at the end of the conditioning session and in the fear recall session performed 24 h later (data not shown). Next day after recall, the mice were randomly divided into extinction and no-extinction groups. Mice in extinction group were subjected to a daily extinction trial for 10 consecutive days, while no-extinction animals stayed in the home cages. Each extinction trial consisted of a 3 min re-exposure to the conditioned context without presenting the foot shock again. Extinction memory test was performed 24 h after the end of extinction trial, and 1 h later, protein-protein interaction in the dorsal hippocampus was detected (Fig. 1A). Extinction trial significantly reduced freezing level (extinction, F(1,10) = 72.41, p < 0.001; extinction memory test, F(1,10) = 11.92, p = 0.006) (Fig. 1B), substantially decreased PSD-95-nNOS complex level (F(1,8) = 7.99, p = 0.018) and increased PSD-95-TrkB level (F(1,8) = 23.00, p = 0.001) (Fig. 1C) in the dorsal hippocampus.
Figure 1.
Contextual fear extinction induces a shift from PSD-95-nNOS to PSD-95-TrkB coupling in the dorsal hippocampus. (A) Design of the experiments for (B–D). (B) Freezing behavior measured during extinction trial and retrieval of extinction memory (n = 6). (C) PSD-95-nNOS and PSD-95-TrkB complex levels in the dorsal hippocampus after contextual fear extinction. (PSD-95-nNOS: n = 5; PSD-95-TrkB: n = 5). (D) Design of the experiments for (E and F). (E) Freezing behavior measured during extinction trial from the mice with successful extinction and failured extinction (n = 5–6). (F) PSD-95-nNOS and PSD-95-TrkB complex level in the dorsal hippocampus after contextual fear extinction (PSD-95-nNOS: n = 5–6; PSD-95-TrkB: n = 5–6). (G) Design of the experiments for (H). (H) PSD-95-nNOS complex level in the dorsal hippocampus after recall (n = 3). Ext: extinction. Ext-S: extinction success. Ext-F: extinction failure.
Using the procedure above, we subjected mice to a daily extinction trial for 6 consecutive days (Fig. 1D), and detected PSD-95-nNOS and PSD-95-TrkB complex level in the dorsal hippocampus from the mice of extinction-success and extinction-failure (F(5,10) = 23.165, p = 0.001) (Fig. 1E). The mice with successful extinction displayed significantly reduced PSD-95-nNOS complex level (F(1,8) = 7.43, p = 0.026) and increased PSD-95-TrkB complex level (F(1,8) = 6.26, p = 0.036), compared to the mice with failed extinction (Fig. 1F). However, retrieval of contextual fear did not affect the interaction of PSD-95 with nNOS, because the mice performed recall and no recall had similar PSD-95-nNOS level (F(1,4) = 0.01, p = 0.910) (Fig. 1H). These data suggest that contextual fear extinction leads to a shift from PSD-95-nNOS to PSD-95‒TrkB coupling in the hippocampus.
PSD-95-nNOS Coupling in the CA3 Regulates Contextual Fear Extinction
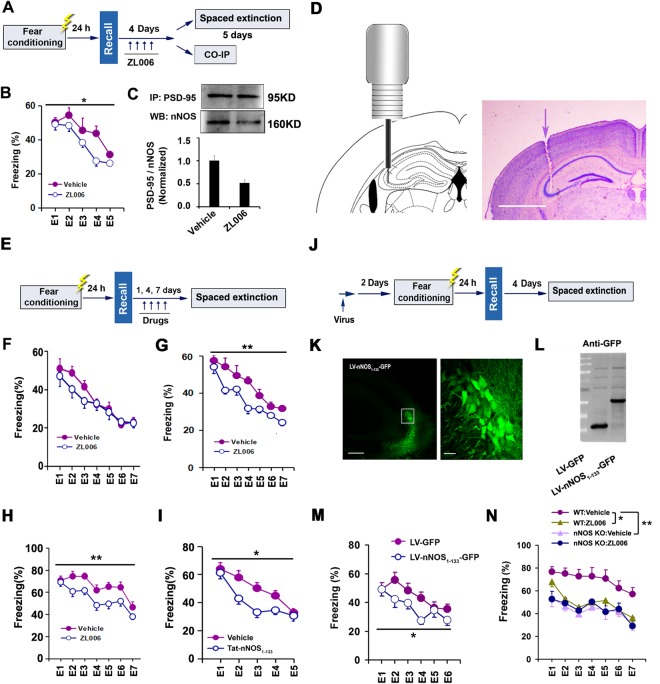
To test whether PSD-95-nNOS coupling regulates contextual fear extinction, we subjected mice to contextual fear conditioning as above. Two hours after fear recall, mice were treated with ZL006 (20 mg/kg/d, i.p.), a small molecular compound blocking PSD-95-nNOS binding13, or vehicle for 4 consecutive days. Twenty-four hours later, the mice were subjected to daily extinction for 5 consecutive days in the conditioning context (Fig. 2A). Mice treated by systemic ZL006 displayed significantly decreased levels of freezing (main group effect: F(1,16) = 4.96, p = 0.019), compared to vehicle, suggesting increased contextual fear extinction (Fig. 2B). Moreover, we measured PSD-95-nNOS complex level in the dorsal hippocampus 1 h after the last ZL006 injection and found that the drug significantly reduced amount of PSD-95-nNOS complex (F(1,8) = 9.84, p = 0.014) (Fig. 2C).
Figure 2.
PSD-95-nNOS coupling in the CA3 regulates contextual fear extinction. (A) Design of the experiments for (B,C). (B) Effect of systemic ZL006 on fear extinction (n = 9). (C) PSD-95-nNOS complex level in the dorsal hippocampus (n = 5). (D) The representative images of micro-injection site by cresyl violet staining after every behavioural test (scale bar, 1000 μm). (E) Design of the experiments for (F–I). (F) Effect of intra-CA3 ZL006 on fear extinction (n = 8–10). (F) Effect of intra-CA3 ZL006 for 1 day on fear extinction (n = 13). (G) Effect of intra-CA3 ZL006 for 4 days on fear extinction (n = 8–10). (H) Effect of intra-CA3 ZL006 for 7 days on fear extinction (n = 13). (I) Effect of intra-CA3 Tat-nNOS1–133 on fear extinction (n = 10). (J) Design of the experiments for (K–M). (K) A representative fluorescence image showing the LV-nNOS1–133-GFP-infected CA3 (left, scale bar, 200 μm) and a high-magnification image from a selected area in the leftward image (right, scale bar, 20 μm). (L) Immunoblots showing nNOS1–133 and GFP expression in the LV-nNOS1–133-GFP- or LV-GFP-infected CA3. (M) Effect of LV-nNOS1–133-GFP in the CA3 on fear extinction (n = 12). (N) Effect of intra-CA3 ZL006 (using the procedure in (E)) on fear extinction in nNOS KO and WT mice (n = 11).
Neuronal activity causes the rapid expression of immediate early genes, such as c-Fos22. Accordingly, we measured c-Fos expression in the dorsal hippocampus 90 min after contextual fear recall and extinction. The number of c-Fos positive cells was increased in the CA3 and CA1, but not in the DG region among homcage, fear recall and fear extinction groups. Moreover, c-Fos induction was higher in the CA3 than in the CA1 region with extinction exposure (% increase of c-Fos-positive cells = (number in extinction group - number in homecage group)/number in homecage group × 100%, CA3 = 304.4%, CA1 = 209%) and CA1 receives the projection of CA3, suggesting dorsal CA3 may be more important in the contextual fear extinction (Supplemental Fig. S1). Furthermore, the rapid encoding of contextual memory requires the CA3 region of the hippocampus23. Thus, we focused on the role of PSD-95-nNOS coupling in the dorsal CA3.
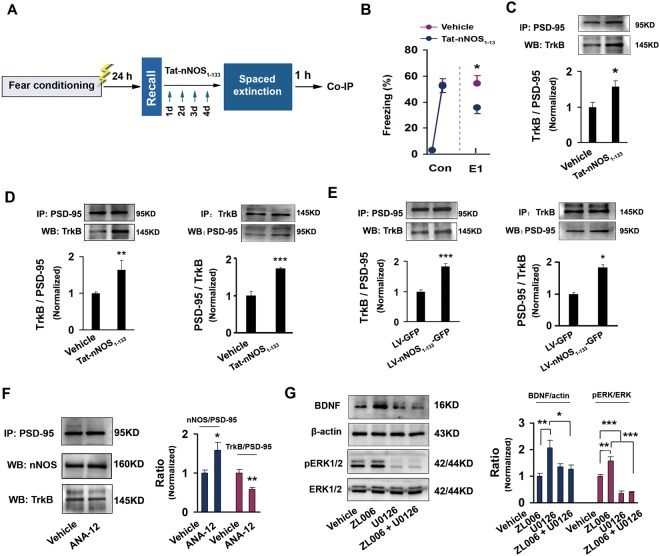
Accordingly, we implanted microcannula into the dorsal CA3 in mice (Fig. 2D), and 7 days later, the mice were subjected to contextual fear conditioning. Two hours after fear recall, we infused ZL006 or vehicle through the microcannula into the CA3 of conscious mice for 1, 4 and 7 days after fear recall (Fig. 2E). From next day, the mice were subjected to a spaced extinction. Contextual fear extinction was not accelerated in mice treated with ZL006 for 1 day (main group effect: F(6,26) = 4.96, p = 0.376) (Fig. 2F), but significantly facilitated in mice treated with ZL006 for 4 and 7 days (Fig. 2G: main group effect: F(1,16) = 11.171, p = 0.004; Fig. 2H: main group effect: F(1,24) = 20.177, p < 0.001) (Fig. 2G,H). Based on the results of preliminary experiments, we employ the 4 days treatment scheme in the following experiments to investigate the effects of PSD-95-nNOS coupling on contextual fear extinction. Furthermore, the dosage scheme was based on LC-MS/MS analysis after 24 hours of intra-hippocampus infusions. The result showed that 5.935 μg/g ZL006 was detected in the dorsal hippocampus tissues after 24 hours injection (Supplemental Fig. S2). According to previous reports13, 0.5 μg/g ZL006 in brain tissue was sufficient to disassociate PSD-95-nNOS coupling. So the residual dosage of ZL006 after 24 hours was able to uncouple PSD-95-nNOS complex. Using the same contextual fear conditioning procedure, we infused Tat-nNOS1–133, a peptide blocking PSD-95-nNOS binding13, or vehicle through the microcannula into the CA3 after fear recall. From next day, the mice were subjected to a spaced extinction (Fig. 2E). Similarly, Intra-CA3 microinjection of Tat-nNOS1–133 significantly promoted contextual fear extinction too (main group effect: F(1,25) = 5.268, p = 0.030) (Fig. 2I). Additionally, ZL006 and Tat-nNOS1–133 had no effect on the spontaneous activity of mice in the open-field test (Supplemental Fig. S3A–C). To further address the role of PSD-95-nNOS coupling in the CA3, we generated a lentiviral vector that selectively expresses nNOS-N1–133, a region crucial for PSD-95-nNOS interaction13, and named it LV-nNOS1–133-GFP. LV-nNOS1–133-GFP or its control LV-GFP was microinjected into the dorsal CA3 of mice through microcannula, and two days later, the mice were subjected to contextual fear conditioning as above. Four days after fear recall, the mice received a spaced extinction (Fig. 2J). The recombinant virus effectively infected the CA3 (Fig. 2K), produced considerable nNOS-N1-133 peptide (Fig. 2L). As ZL006 and Tat-nNOS1–133 did, LV-nNOS1–133-GFP significantly promoted contextual fear extinction (main group effect: F(5,22) = 4.444, p = 0.047) (Fig. 2M).
To determine whether effect of drug on contextual fear extinction depends on the dissociation of PSD-95-nNOS coupling, we subjected nNOS gene knock-out (nNOS KO) or WT mice to contextual fear conditioning. Two hours after fear recall, we infused ZL006 or vehicle through implanted microcannula into the dorsal CA3 for 4 consecutive days. From next day, the mice were subjected to a spaced extinction. Although ZL006 significantly facilitated contextual fear extinction in WT mice, it had no effect on nNOS KO mice (main group effect: F(5,22) = 7.004, p = 0.001; post hoc comparisons, WT Vehicle vs WT ZL006, p = 0.022; WT Vehicle vs nNOS KO Vehicle, p = 0.001; nNOS KO Vehicle vs nNOS KO ZL006, p = 0.997) (Fig. 2N), suggesting requirement of PSD-95-nNOS coupling for behavioral effect of ZL006.
Next, we used same contextual fear conditioning procedure as the study in the CA3 and investigated the role of PSD-95-nNOS coupling in the CA1 and DG of dorsal hippocampus. Two hours after fear recall, we daily infused Tat-nNOS1–133 into the dorsal CA1 or DG through the microcannula for 4 consecutive days (Supplemental Fig. S4A). Surprisingly, Tat-nNOS1–133 did not affect fear extinction in the CA1 (main group effect: F(5,22) = 0.009, p = 0.923) (Supplemental Fig. S4B), although contextual fear recall and fear extinction induced rare c-Fos expression in the CA1. And Tat-nNOS1–133 did not affect fear extinction in the DG either (main group effect: F(6,21) = 0.002, p = 0.969) (Supplemental Fig. S4C). These results suggesting that PSD-95-nNOS coupling in the DG and CA1 is not implicated in the modulation of contextual fear extinction.
NMDARs Activation in the CA3 Promotes Contextual Fear Extinction
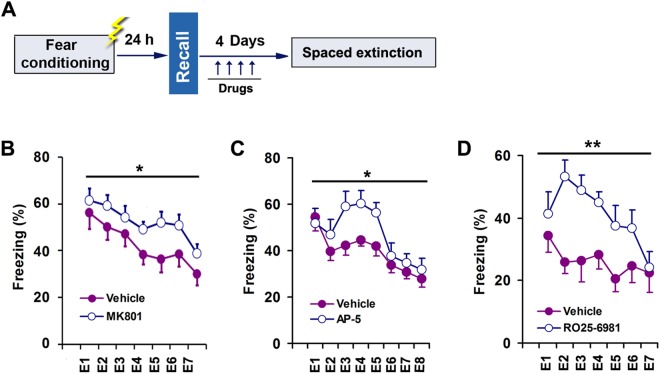
To determine whether NMDARs activation in the CA3 is necessary for contextual fear extinction, we observed effects of MK801, a potent non-competitive antagonist of NMDARs that use-dependently blocks the channel in the open (glutamate-bound) state. We used same contextual fear conditioning procedure as above, and 2 h after fear recall, we infused MK801 into the dorsal CA3 of conscious mice through the implanted microcannula for 4 consecutive days (Fig. 3A). As expected, mice treated with MK801 displayed significantly enhanced freezing levels, compared with vehicle (Fig. 3B), indicating reduced contextual fear extinction (main group effect: F(6,27) = 4.889, p = 0.036). And there is no changes in locomotor activity after treatment with MK801 in the open-field test (Supplemental Fig. S3A,D).
Figure 3.
NMDARs activation in the CA3 promotes contextual fear extinction. (A) Design of the experiments for (B–D). (B) Effect of intra-CA3 MK801 on fear extinction (n = 14–15). (C) Effect of intra-CA3 AP-5 on fear extinction (n = 13–14). (D) Effect of intra-CA3 RO25-6981 on fear extinction (n = 10–11).
Next, we investigated effect of AP-5, a competitive antagonist of NMDARs, on contextual fear extinction. Two hours after fear recall, we infused AP-5 into the dorsal CA3 of conscious mice through the implanted microcannula for 4 consecutive days (Fig. 3A). Similar to MK801, AP-5 significantly reduced fear extinction (main group effect: F(7,24) = 4.286, p = 0.049) (Fig. 3C).
Based on the importance of GluN2B in the extinction of fear memories10, we specifically investigated the role of GluN2B-containing receptors in the CA3 in contextual fear extinction. Two hours after fear recall, we daily infused Ro25-6981, a selective GluN2B antagonist, into the dorsal CA3 of conscious mice through the implanted microcannula for 4 consecutive days (Fig. 3A). As MK801 and AP-5 did, Ro25-6981 significantly inhibited contextual fear extinction (main group effect: F(6,25) = 8.229, p = 0.008) (Fig. 3D). The treatment of RO25-6981 did not alter the basic movement in the open-field test (Supplemental Fig. S3A,E). Thus, NMDARs activation and PSD-95-nNOS coupling in the dorsal CA3 have completely different roles for contextual fear extinction.
BDNF-TrkB Signaling Is Responsible for the Different Regulation of Fear Extinction by NMDARs Activation and PSD-95-nNOS Association
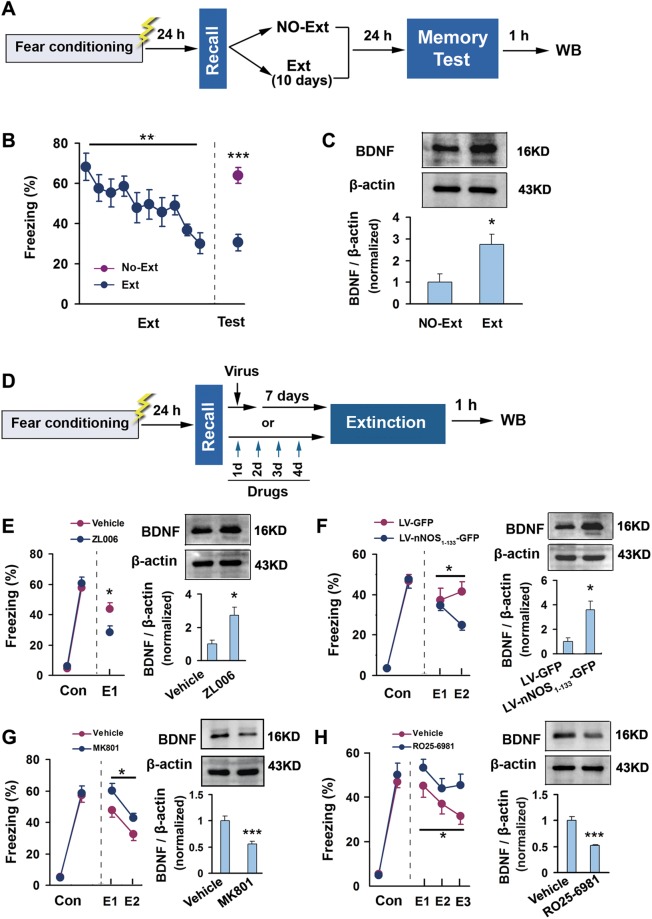
NMDARs activation induces gene expression of BDNF24, a neurotrophic factor is crucial for fear extinction16. To investigate whether BDNF is responsible for the different role of NMDARs activation and it-mediated PSD-95-nNOS binding in contextual fear extinction, we examined BDNF expression in the dorsal CA3. After fear recall, mice were subjected to spaced extinction trial or not (Fig. 4A). We found that the mice with extinction (F(1,14) = 34.78, p < 0.001) showed significantly increased BDNF level (F(1,6) = 8.29, p = 0.028) (Fig. 4B,C). We next infused ZL006, Ro25-6981 or MK801 for 4 consecutive days, or infused LV-nNOS1–133-GFP on time into the dorsal CA3 through the implanted microcannula beginning 2 h after fear recall, and detected BDNF level in the dorsal hippocampus when extinctions were significantly different between groups (Fig. 4D). Disrupting PSD-95-nNOS binding in the CA3 with ZL006 (E1, F(1,23) = 6.45, p = 0.018) or LV-nNOS1–133-GFP (main group effect: F(1,10) = 5.369, p = 0.043) significantly increased BDNF expression (Fig. 4E: F(1,6) = 9.12, p = 0.023; Fig. 4F: F(1,4) = 10.95, p = 0.030) (Fig. 4E,F), whereas blocking NMDARs in the CA3 with MK801 (main group effect: F(1,22) = 4.728, p = 0.041) or Ro25–6981 (main group effect: F(1,21) = 4.606, p = 0.044) caused a significant decrease in BDNF level (Fig. 4G: F(1,11) = 37.76, p < 0.001; Fig. 4H: F(1,10) = 37.40, p < 0.001) (Fig. 4G,H). Thus, PSD-95-nNOS blockers and NMDARs antagonists oppositely regulate BDNF expression.
Figure 4.
PSD-95-nNOS blockers and NMDARs antagonists oppositely regulate BDNF expression. (A) Design of the experiments for (B,C). (B) Freezing behavior measured during extinction trial and retrial of extinction memory (n = 8). (C) Immunoblots showing BDNF expression in the dorsal CA3 after contextual fear extinction (n = 4). (D) Design of the experiments for (E–H). (E) Effect of intra-CA3 ZL006 on fear extinction (n = 12–13) and BDNF expression in the dorsal CA3 (n = 4). (F) Effect of LV-nNOS1–133-GFP in the CA3 on fear extinction (n = 6) and BDNF expression in the dorsal CA3 (n = 3). (G) Effect of intra-CA3 MK801 on fear extinction (n = 12–14) and BDNF expression in the dorsal CA3 (n = 6–7). (H) Effect of intra-CA3 RO25-6981 on fear extinction (n = 12) and BDNF expression in the dorsal CA3 (n = 6). Ext: extinction. Con: fear conditioning.
The activation of BDNF-TrkB signaling by NMDARs recruits more PSD-95 to synapses via the association of TrkB with PSD-9518,25 and supports extinction learning26, which raises a possibility that uncoupling PSD-95-nNOS leads to an increase in association of TrkB with PSD-95 and thereby enhances BDNF-mediated extinction. To address this notion, we infused Tat-nNOS1–133 or vehicle through microcannula into the dorsal CA3 of mice for 4 consecutive days beginning 2 h after fear recall, and detected PSD-95-TrkB complex levels in the dorsal CA3 when extinctions were significantly different between groups (E1, F(1,23) = 6.65, p = 0.017) (Fig. 5A,B). Disrupting PSD-95-nNOS binding in the CA3 significantly increased association of PSD-95 with TrkB (F(1,8) = 9.59, p = 0.015) (Fig. 5C). To further address this, cultured hippocampal neurons were exposed to Tat-nNOS1–133 for 24 h or LV- nNOS1–133-GFP for 7 d. Similar with the findings in in vivo, blocking PSD-95-nNOS in in vitro significantly increased PSD-95-TrkB complex levels also (Fig. 5D, Left: F(1,11) = 10.53, p = 0.008; Right: F(1,11) = 44.62, p < 0.001; Fig. 5E, Left: F(1,16) = 33.22, p < 0.001; Right: F(1,14) = 8.42, p = 0.012) (Fig. 5D,E). Next, we infused ANA-12, a TrkB receptor antagonist, into the dorsal CA3 of mice, and 24 h later, detected PSD-95-nNOS and PSD-95-TrkB complexes. The drug significantly decreased PSD-95-TrkB complex (F(1,10) = 20.78, p = 0.001) and increased PSD-95-nNOS complex (F(1,10) = 8.47, p = 0.016) (Fig. 5F). Collectively, these findings suggest a competition between nNOS and TrkB for binding to PSD-95. Thus, NMDARs activation and PSD-95-nNOS binding have distinct role in regulating BDNF-TrkB signaling.
Figure 5.
Mechanisms underlying the role of PSD-95-nNOS in regulating BDNF-TrkB signalling. (A) Design of the experiments for (B,C). (B) Effect of intra-CA3 Tat-nNOS1–133 on fear extinction (n = 12–13). (C) PSD-95-TrkB complex level in the dorsal CA3 after extinction (n = 4–6). (D,E) PSD-95-TrkB complex level in the cultured hippocampal neurons treated by Tat-nNOS1–133 ((D) Left: n = 6–7; Right: n = 6–7) or infected with LV- nNOS1–133-GFP ((E) Left: n = 9; Right: n = 7–9). (F) Effect of intra-CA3 ANA-12 on PSD-95-nNOS and PSD-95-TrkB complex levels in the dorsal CA3 (PSD-95-nNOS: n = 6; PSD-95-TrkB). (G) ERK inhibitor reverses the effect of ZL006 on BDNF expression and ERK phosphorylation (n = 6).
To address this, we treated cultured hippocampal neurons with ZL006, U0126 (an ERK inhibitor) or combination of two drugs for 24 h, and measured BDNF and phosporylated ERK (pERK) levels. Indeed, blocking PSD-95-nNOS by ZL006 significantly increased pERK level and BDNF expression, ERK inhibitor reversed the effects of ZL006 (BDNF/β-actin: F(3,20) = 6.09, vehicle vs ZL006, p = 0.006; ZL006 vs ZL006 + U0126, p = 0.048. pERK/ERK: n = 6, F(3,20) = 34.97, vehicle vs ZL006, p = 0.005; vehicle vs U0126, p = 0.001; ZL006 vs ZL006 + U0126, p < 0.001) (Fig. 5G). NMDARs activation enhances BDNF production through ERK phosporylation27. Therefore, NMDARs activation and PSD-95-nNOS coupling may oppositely regulate ERK phosphorylation and thereby leading to a bidirectional regulation of BDNF expression.
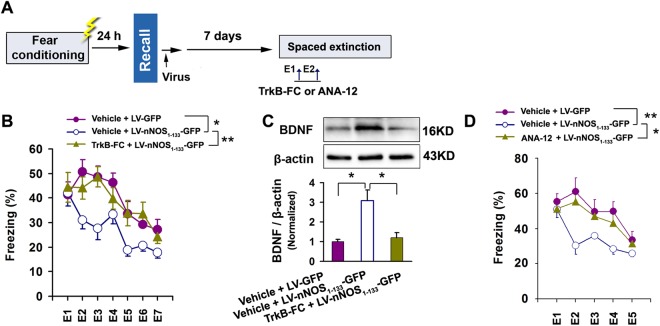
To determine whether BDNF is requirement for the role of PSD-95-nNOS in contextual fear extinction, we infused LV-nNOS1–133-GFP or its control LV-GFP into the dorsal CA3 of mice through the implanted microcannula after fear recall. Seven days later, the mice were subjected to contextual fear extinction for 7 consecutive days (E1-E7), and we infused TrkB-FC, a BDNF scavenger, into the dorsal CA3 after E1 and E2 to remove local BDNF (Fig. 6A). Fear levels after recall were similar between groups. LV-nNOS1–133-GFP significantly reduced freezing behavior (F(2,36) = 6.15, p = 0.008, p = 0.018) and up-regulated BDNF expression in the dorsal CA3, compared to LV-GFP, and TrkB-FC completely reversed the effects of LV-nNOS1–133-GFP (main group effect: F(2,32) = 5.933, p = 0.006; post hoc comparisons, vehicle + LV-GFP vs vehicle + LV-nNOS1–133-GFP, p = 0.027; vehicle + LV-nNOS1–133-GFP vs TrkB-FC + LV-nNOS1–133-GFP, p = 0.009) (Fig. 6B,C). Next, we infused ANA-12, a TrkB receptor antagonist, into the dorsal CA3 after E1 and E2 to block BDNF receptor (Fig. 6A). With similar fear levels between groups after recall, ANA-12 completely reversed the effect of LV-nNOS1–133-GFP on contextual fear extinction (main group effect: F(2,30) = 8.580, p = 0.004; post hoc comparisons, vehicle + LV-GFP vs vehicle + LV-nNOS1–133-GFP, p = 0.001; vehicle + LV-nNOS1–133-GFP vs ANA-12 + LV-nNOS1–133-GFP, p = 0.017) (Fig. 6D). Together, these data suggest that the role of PSD-95-nNOS on extinction is BDNF-dependent.
Figure 6.
The role of PSD-95-nNOS in regulating contextual fear extinction is BDNF-dependent. (A) Design of the experiments for (B–D). (B) BDNF scavenger TrkB-FC reversed the effect of LV-nNOS1–133-GFP on fear extinction (n = 13). (C) BDNF scavenger TrkB-FC reversed the effect of LV-nNOS1–133-GFP on BDNF expression (n = 4–5). (D) TrkB receptor antagonist ANA-12 reversed the effect of LV-nNOS1–133-GFP on fear extinction (n = 11).
Discussion
Impairments in fear extinction are thought to be central to the psychopathology of PTSD. Crucial role of NMDARs in the limbic system for fear extinction5–7, especially, the role of GluN2B-containing NMDARs10, offers an inviting strategy to treat PTSD by activating NMDARs. However, NMDARs activation has a completely opposite effect on other affective processes, such as depression- and anxiety-related behaviors28–30. Because PTSD is often complicated by general anxiety and depressed mood31, the treatment targeting on NMDARs for PTSD may pose a challenge, render their use problematic. Activation of GluN2B-containing NMDARs induces the interaction of nNOS with PSD-9512,13. Using different behavioral protocols, we demonstrated that dissociation of nNOS from PSD-95 in the CA3 promoted contextual fear extinction, while NMDARs antagonists impaired contextual fear extinction. To our knowledge, this is the first report revealing the significance of PSD-95-nNOS coupling for fear extinction, and showing opposite roles of NMDARs activation and NMDARs-mediated PSD-95-nNOS coupling in regulating extinction. Because inhibitors of PSD-95-nNOS interaction produce antidepressant and anxiolytic effect without unwanted side effects associated with NMDARs13,32,33, PSD-95-nNOS could be a valuable target for the treatment of PTSD.
The hippocampus is a crucial part of the neuronal circuit mediating fear extinction. Pharmacological inhibition of hippocampal Cdk5 activity facilitates extinction in the contextual fear-conditioning procedure34. DG was reported to be involved in fear extinction learning and adult-born neurons in the DG are integral for the maintenance of remote contextual fear memory35,36. Moreover, inhibition of Rac1 activity in the CA1 has been shown to impair massed extinction of contextual fear37. Here, we provide strong evidence that CA3 is an important hippocampal subregion playing crucial roles in fear extinction.
Because of the extensive recurrent collateral system connecting pyramidal cells of the CA3 area of hippocampus, CA3 is thought to promote the flexible formation and reorganization of information-coding ensembles and serve as an associative memory network38–40. Within the hippocampus, the NMDARs presumably participate in plastic synaptic events in the CA1 and CA3, the principal pyramidal cell fields39. However, the hippocampal subfields CA1 and CA3 are functionally segregated41. Indeed, after contextual fear extinction, immediate early gene c-Fos is abundantly expressed in the CA3 but not in the CA1. Our findings that uncoupling PSD-95-nNOS in the CA3 but not in the CA1 facilitates contextual fear extinction could be a presentation of function difference between CA1 and CA3. Although the number of c-Fos-positive cells in the CA1 was rarely increased after contextual fear recall and contextual fear extinction, this change may be due to the projection from CA3 c-Fos positive cells. However, CA3 and CA1 have different efferent pathway. CA3 projects to the septofimbrial nucleus, throughout the rostral lateral septum, the dorsal medial septum, and the dorsal-most portions of the vertical limb of the diagonal band of Broca. CA1 projects to the septofimbrial nucleus, the ventral portions of the medial septum, as well as the horizontal limb of the diagonal band of Broca42,43. These differential targets of CA3 and CA1 subcortical efferents suggest that there may be functional heterogeneity in the classical fear conditioning44.
For the contextual memory, CA3 NMDARs are required for the rapid formation of a salient contextual representation45, whereas DG NMDARs mediate rapid pattern separation in the hippocampal network46, which may explain our results that uncoupling PSD-95-nNOS in the CA3 but not in the DG promoted contextual fear extinction.
BDNF in the limbic system are crucial for fear extinction3,16,47. NMDARs activation not only induces BDNF gene expression in hippocampal neurons24, but also facilitates BDNF-TrkB signaling to recruit PSD-95 to synapses and promotes extinction learning18,25,26. In contrast, we found that disrupting the association of nNOS with PSD-95, downstream of NMDARs activation, up-regulated BDNF expression and association of BDNF-TrkB signaling with PSD-95. More importantly, BDNF scavenger and TrkB receptor antagonist abolished the effect of LV-nNOS1–133-GFP on fear extinction. Thus, PSD-95-nNOS interaction regulates contextual fear extinction via BDNF-TrkB signaling. Furthermore, we showed that contextual fear extinction induced a shift from PSD-95-nNOS to PSD-95-TrkB coupling. Therefore, different BDNF-TrkB signaling in the CA3 may explain different roles of NMDARs activation and NMDARs-mediated PSD-95-nNOS coupling in regulating extinction.
It is generally believed that synaptic NMDAR conveys the synaptic activity-driven activation of the survival-signaling protein ERK leading to the activation of the transcription factor CREB and the production of the survival-promoting protein BDNF27. Thus, ERK-BDNF signaling pathway may account for the role of NMDARs activation in fear extinction. PSD-95-nNOS interaction facilitates NO production13. nNOS-derived NO is a potent inhibitor of Ca2+-mediated ERK activation20. Here, we found that uncoupling PSD-95-nNOS increased ERK phosphorylation and ERK inhibitor reversed the effect of ZL006 and Tat-nNOS1–133 on BDNF expression. Thus, PSD-95-nNOS interaction may down-regulate BDNF expression via inhibiting ERK activation. However, we can not exclude the possibility that PSD-95-nNOS interaction may have other way to regulate BDNF, such as histone deacetylases (HDACs)-mediated epigenetic modification. HDACs play a negative role in histone H3 and H4 acetylation of BDNF promoters, thereby influence BDNF expression48. It has been reported that nitrosylation of HDAC2 is implicated in the regulation of fear memory15. Moreover, our previous study showed that NO up-regulates HDAC2 level and activity14. Thus, further investigation will be required to test whether HDACs are implicated in the regulation of BDNF expression and consequent fear extinction by PSD-95-nNOS coupling.
Apart from decreased level of PSD95-nNOS coupling (F(1,8) = 9.88, p = 0.010) (Supplemental Fig. S5), directly blocking NMDARs also affect other signaling pathway via Ca2+ influx, such as BDNF-TrkB signalling. But disassociating PSD-95-nNOS coupling, the downstream signalling of NMDAR activation, has no effect on Ca2+ influx. Thus, NMDARs activation and NMDARs-mediated PSD-95-nNOS coupling may play a different role in modulating contextual fear extinction.
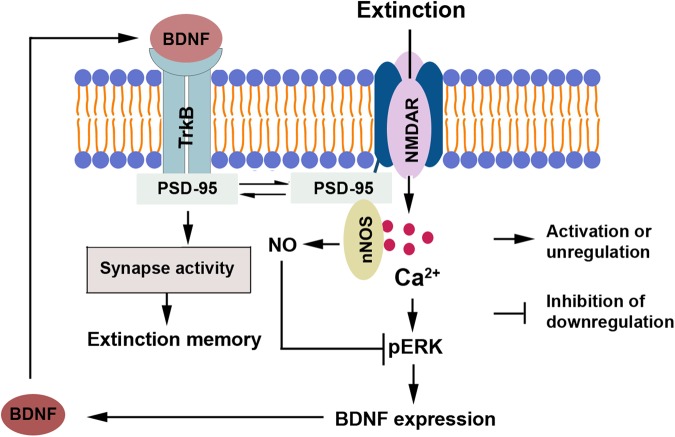
Collectively, NMDARs activation up-regulates BDNF expression via Ca2+-mediated ERK phosphorylation and enhances the association of BDNF-TrkB signaling with PSD-95, subsequently enhances synapse activity, thereby promotes contextual fear extinction. NMDARs-mediated PSD-95-nNOS interaction weakens the role of NMDARs activation via reducing ERK phosphorylation and BDNF-TrkB signaling with PSD-95 (Fig. 7). Thus, blocking PSD-95-nNOS and activating NMDARs produce similar effect on fear extinction. Different from NMDARs activation, however, uncoupling PSD-95-nNOS is beneficial to both fear extinction and mood. Our present work provides an important addition to the knowledge of modulation of extinction, and suggests that PSD-95-nNOS could be a valuable target for the treatment of PTSD.
Figure 7.
A model of signaling pathway whereby NMDARs activation and it-mediated PSD-95-nNOS interaction differently regulate contextual fear extinction.
Methods and Materials
Detailed and extended data are available in the Supplement.
Animals
Young adult (6- to 7-week-old) male homozygous nNOS-deficient mice (B6;129S4-Nos1tm1Plh, knockout, stock number: 002633) and their wild-type controls of similar genetic background (B6129SF2, WT) (both from Jackson Laboratories, maintained at Model Animal Research Center of Nanjing University, Nanjing, China), and young adult (6–8 weeks) male C57BL/6 mice were used in this study. All procedures were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University. And all experiments were performed in accordance with the approved guidelines and regulations. Every effort was made to minimize the number of animals used and their suffering.
Surgical Procedures and Drug Infusions
For intra-hippocampus infusions, Guide cannulae (26 gauge; Plastics One, RWD Life Science) were unilaterally implanted 1.5 mm above the dorsal hippocampus using coordinates for the CA3: −1.7 mm AP; +1.9 mm ML; −1.9 mm DV; for the CA1: −1.7 mm AP; +1.3 mm ML; −1.8 mm DV; for the DG: −1.7 mm AP; +1.0 mm ML; −2.1 mm DV. Drug infusions at a rate of 0.06 μl/min (Harvard Apparatus, Holliston, MA) included ZL006, Tat-nNOS1–133, LV-nNOS1–133 -GFP, MK801, AP-5, RO25-6981, TrkB-FC and ANA-12.
Contextual Fear Conditioning and Extinction
Extinction and reminder shock procedures were carried out as described previously34. In brief, contextual fear conditioning was performed with a computerized fear conditioning system (CSI Systems). The freezing level was quantified from digitized video images using commercially available software (Freezescan, Clever Systems, Reston, VA). Animals were allowed to explore the training cage for 3 min followed by a mild electric shock (2 s, 0.7 mA). Context-dependent freezing, defined as the absence of movements other than those required for breathing, was assessed 24 h later by re-exposing the mice for 3 min into the conditioning context. Spaced extinction of contextual fear was performed on consecutive days, consisting of re-exposure to the training context in a non-reinforced manner for 3 min. An extinction protocol is composed by time of each extinction trial and the number of extinction trial. A complete extinction is indicated by an appearance of extinction bottom. However, the dynamics of fear extinction can vary among experiments. In other words, the number of extinction trials to get extinction bottom could be different. In our experiments, the number of extinction trials varied from 5 to 10 days, although time of each extinction trial was same. In the experiment about extinction failure and extinction success, “Extinction Failure” and “Extinction Success” were established based on the freezing values in the last extinction trial. Freezing level higher than 65% of the acquisition level was defined “Extinction Failure” and lower than 45% of the acquisition level was defined “Extinction Success”.
Cell Cultures, Recombinant Lentivirus and Fusion Peptide
Culture of hippocampal neurons, the production of recombinant lentivirus, LV-nNOS1–133-GFP or its control LV-GFP, and the preparations of fusion peptide, Tat-nNOS-N1–133, were performed as we previously reported13,14,49 using the procedures detailed in the Supplement.
Western Blot Analysis and Coimmunoprecipitation
The samples of CA3 in the dorsal hippocampus were obtained as follows50: the brains were removed and blocked rapidly over ice into coronal sections. Serial hippocampal sections (200 μm) were made on a vibratome (Leica) in a bath of pre-cold PBS. Then the CA3 of cannulas-implanted side was obtained in the first six pieces of hippocampal sections under a dissecting microscope and placed in the pre-cooling RIPA lysate. Samples from cultured neurons were prepared as described by our previous studies49. Western blot analysis and coimmunoprecipitation used the procedures detailed in the Supplement.
Statistical analysis
In behavioral experiments, freezing data were analyzed with a repeated-measures analysis of variance (ANOVA) followed by Tukey’s post hoc test47. In some cases, with only one factor (Extinction memory test in Fig. 1B) in the experiment, we employed one-way ANOVA (one factor) followed by Tukey’s post hoc test to compare means from two groups at the same time. Student’s t-tests were used for statistical comparison between two groups. Data were presented as the mean ± SEM, and p < 0.05 was considered statistically significant. Investigators were blind to the group allocation when assessing the outcome. For the effects of drugs on fear extinction, animals were randomly assigned into different groups immediately after fear recall.
Electronic supplementary material
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (31530091, 91232304) and National Key Research and Development Program of China (2016YFC1306703), and by the Collaborative Innovation Center for Cardiovascular Disease Translational Medicine.
Author Contributions
Z.D.Y. conceived the project and wrote the manuscript. C.C., C.C.Y., Z.Y. executed the experiments and analyzed the data; H.Z., C.B., Q.C., L.C.X., W.H.Y., C.L., B.X.L., L.H.Y. and T.Y. assisted in some experiments and the data analyses.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Cheng-Yun Cai, Chen Chen and Ying Zhou contributed equally.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-30899-4.
References
- 1.Maren S, Phan KL, Liberzon I. The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci. 2013;14:417–428. doi: 10.1038/nrn3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garfinkel SN, et al. Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study ofextinction retention and fear renewal. J Neurosci. 2014;34:13435–13443. doi: 10.1523/JNEUROSCI.4287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karpova NN, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–1734. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tronson NC, Corcoran KA, Jovasevic V, Radulovic J. Fear conditioning and extinction: emotional states encoded by distinct signaling pathways. Trends Neuorsci. 2012;35:145–155. doi: 10.1016/j.tins.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 6.Quirk GJ, Mueller D. Neural mechanisms of extinction learning and retrieval. Neuropsychopharmacology. 2008;33:56–72. doi: 10.1038/sj.npp.1301555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Kleine RA, Hendriks GJ, Kusters WJ, Broekman TG, van Minnen A. A randomized placebo-controlled trial of D-cycloserine to enhance exposure therapy for posttraumatic stress disorder. Biol Psychiatry. 2012;71:962–968. doi: 10.1016/j.biopsych.2012.02.033. [DOI] [PubMed] [Google Scholar]
- 8.Lee CH, et al. NMDA receptor structures reveal subunit arrangement and pore architecture. Nature. 2014;511:191–197. doi: 10.1038/nature13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monyer H, et al. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- 10.Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacology. 2016;41:58–79. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin HG, Wang YT. Blocking the deadly effects of the NMDA receptor in stroke. Cell. 2010;140:174–176. doi: 10.1016/j.cell.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 12.Aarts M, et al. Treatment of ischemic brain damage by perturbing NMDA receptor- PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 13.Zhou L, et al. Treatment of cerebral ischemia by disrupting ischemiainduced interaction of nNOS with PSD-95. Nat Med. 2010;16:1439–1443. doi: 10.1038/nm.2245. [DOI] [PubMed] [Google Scholar]
- 14.Luo CX, et al. Interaction of nNOS with PSD-95 negatively controls regenerative repair after stroke. J Neurosci. 2014;34:13535–13548. doi: 10.1523/JNEUROSCI.1305-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gräff J, et al. Epigenetic priming of memory updating during reconsolidation to attenuate remote fear memories. Cell. 2014;156:261–276. doi: 10.1016/j.cell.2013.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Soliman F, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ji Y, Pang PT, Feng L, Lu B. Cyclic AMP controls BDNF-induced TrkB phosphorylation and dendritic spine formation in mature hippocampal neurons. Nat Neurosci. 2005;8:164–172. doi: 10.1038/nn1381. [DOI] [PubMed] [Google Scholar]
- 18.Yoshii A, Constantine-Paton M. BDNF induces transport of PSD-95 to dendrites through PI3K-AKT signaling after NMDA receptor activation. Nat Neurosci. 2007;10:702–711. doi: 10.1038/nn1903. [DOI] [PubMed] [Google Scholar]
- 19.Chwang WB, O’Riordan KJ, Levenson JM, Sweatt JD. ERK/MAPK regulates hippocampal histone phosphorylation following contextual fear conditioning. Learn Mem. 2006;13:322–328. doi: 10.1101/lm.152906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raines KW, et al. Nitric oxide inhibition of ERK1/2 activity in cells expressing neuronal nitric-oxide synthase. J Biol Chem. 2004;279:3933–3940. doi: 10.1074/jbc.M304813200. [DOI] [PubMed] [Google Scholar]
- 21.Guan JS, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60. doi: 10.1038/nature07925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Madabhushi R, et al. Activity-induced DNA breaks govern the expression of neuronal early-response genes. Cell. 2015;161:1592–1605. doi: 10.1016/j.cell.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramamoorthi K, et al. Npas4 regulates a transcriptional program in CA3 required for contextual memory formation. Science. 2011;334:1669–1675. doi: 10.1126/science.1208049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tian F, Marini AM, Lipsky RH. NMDA receptor activation induces differential epigenetic modification of BDNF promoters in hippocampal neurons. Amino Acids. 2011;38:1067–1074. doi: 10.1007/s00726-009-0315-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu X, et al. BDNF-induced increase of PSD-95 in dendritic spines requires dynamic microtubule invasions. J Neurosci. 2011;31:15597–15603. doi: 10.1523/JNEUROSCI.2445-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Otis JM, Fitzgerald MK, Mueller D. Infralimbic BDNF/TrkB enhancement of GluN2B currents facilitates extinction of a cocaine-conditioned place preference. J Neurosci. 2014;34:6057–6064. doi: 10.1523/JNEUROSCI.4980-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karpova A, et al. Encoding and transducing the synaptic or extrasynaptic origin of NMDA receptor signals to the nucleus. Cell. 2013;152:1119–33. doi: 10.1016/j.cell.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Maeng S, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biol Psychiatry. 2008;63:349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 29.Ballard ED, et al. Improvement in suicidal ideation after ketamine infusion: relationship to reductions in depression and anxiety. J Psychiatr Res. 2014;58:161–166. doi: 10.1016/j.jpsychires.2014.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deutschenbaur L, et al. Role of calcium, glutamate and NMDA in major depression and therapeutic application. Prog Neuropsychopharmacol Biol Psychiatry. 2016;64:325–333. doi: 10.1016/j.pnpbp.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 31.Corcoran KA, et al. Regulation of fear extinction versus other effective behaviors by discrete cortical scaffolding complexes associated with NR2B and PKA signaling. Transl Psychiatry. 2015;5:e657. doi: 10.1038/tp.2015.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Doucet MV, Levine H, Dev KK, Harkin A. Small-molecule inhibitors at the PSD-95/nNOS interface have antidepressant-like properties in mice. Neuropsychopharmacology. 2013;38:1575–1584. doi: 10.1038/npp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AE, et al. Source memory in rats is impaired by an NMDA receptor antagonist but not by PSD95-nNOS protein-protein interaction inhibitors. Behav Brain Res. 2016;305:23–29. doi: 10.1016/j.bbr.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sananbenesi F, et al. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci. 2007;10:1012–1019. doi: 10.1038/nn1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernier BE, et al. Dentate gyrus contributes to retrieval as well as encoding: Evidence from context fear conditioning, recall, and extinction. J Neurosci. 2017;37:6359–6371. doi: 10.1523/JNEUROSCI.3029-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pan YW, Storm DR, Xia ZG. Role of adult neurogenesis in hippocampus-dependent memory, contextual fear extinction and remote contextual memory: New insights from ERK5 MAP kinase. Neurobiology of Learning and Memory. 2013;105:81–92. doi: 10.1016/j.nlm.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang, et al. Inhibition of Rac1 activity in the hippocampus impaired extinction of contextual fear. Neuropharmacology. 2016;109:216–222. doi: 10.1016/j.neuropharm.2016.06.017. [DOI] [PubMed] [Google Scholar]
- 38.Nakazawa K, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297:211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gruart A, Muñoz MD, Delgado-Garcia JM. Involvement of the CA3-CA1 synapse in the acquisition of associative learning in behaving mice. J Neurosci. 2006;26:1087–1097. doi: 10.1523/JNEUROSCI.2834-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Makara JK, Magee JC. Variable dendritic intergration in hippocampal CA3 pyramidal neurons. Neuron. 2013;80:1438–1450. doi: 10.1016/j.neuron.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lux V, Atucha E, Kitsukawa T, Sauvage MM. Imaging a memory trace over half a life-time in the medial temporal lobe reveals a time-limited role of CA3 neurons in retrieval. Elife. 2016;5:e11862. doi: 10.7554/eLife.11862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hunsaker MR, Kesner RP. Dissociations across the dorsal-ventral axis of CA3and CA1 for encoding and retrieval of contextual and auditory-cued fear. Neurobiology of Learning and Memory. 2008;89:61–69. doi: 10.1016/j.nlm.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheehan TP, Chambers RA, Russell DS. Regulation of affect by the lateral septum: Implications for neuropsychiatry. Brain Research Reviews. 2006;46:71–117. doi: 10.1016/j.brainresrev.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 44.Hunsaker MR, Tran GT, Kesner RP. A behavioral analysis of the role of CA3 and CA1 subcortical efferents during classical fear conditioning. Behav Neurosci. 2009;123:624–30. doi: 10.1037/a0015455. [DOI] [PubMed] [Google Scholar]
- 45.McHugh TJ, Tonegawa S. CA3 NMDA receptors are required for the rapid formation of a salient contextual representation. Hippocampus. 2009;19:1153–1158. doi: 10.1002/hipo.20684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McHugh TJ, et al. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317:94–99. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- 47.Peters J, Dieppa-Perea LM, Melendez LM, Quirk GJ. Induction of fear extinction with hippocampal-infralimbic BDNF. Science. 2010;328:1288–1290. doi: 10.1126/science.1186909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sen A, Nelson TJ, Alkon DL. ApoE4 and Aβ oligomers reduces BDNF expression via HDAC nuclear translocation. J Neurosci. 2015;35:7538–7551. doi: 10.1523/JNEUROSCI.0260-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Luo CX, et al. Bidirectional regulation of neurogenesis by neuronal nitric oxide synthase derived from neurons and neural stem cells. Stem Cells. 2010;28:2041–2052. doi: 10.1002/stem.522. [DOI] [PubMed] [Google Scholar]
- 50.Powell KL, et al. Decreases in HCN mRNA expression in the hippocampus after kindling and status epilepticus in adult rats. Epilepsia. 2008;49:1686–95. doi: 10.1111/j.1528-1167.2008.01593.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.