Abstract
Although the histologically diverse classification of salivary gland tumors can be successfully applied to the epithelial lacrimal gland neoplasms, it is not clear whether the molecular makeup differs between these two different tumor types. Adenocarcinomas have known to have driver mutations in non-small cell lung cancer, however, besides HER2 expression not much is known regarding molecular drivers in lacrimal tumors. Androgen receptor (AR) expression and deprivation combined with checkpoint inhibition (CPI) have not been described before in lacrimal gland adenocarcinoma. To our knowledge, this is the first case report describing a prolonged response to CPI and AR inhibitors.
BACKGROUND
Carcinoma of the lacrimal gland is a rare malignancy with an estimated incidence of less than one case per million per year with no standard guidelines on optimal treatment of advanced disease [1]. It carries a dismal prognosis with fatality ranging from 50% to 80% [2, 3].
More than 30 different subtypes were described in the 2005 WHO Classification of Tumours [4, 5]. Most of the molecular information about epithelial lacrimal neoplasms comes from the study of salivary gland neoplasms, just by virtue of their greater numbers [6].
CASE REPORT
A 55-year-diabetic male was diagnosed with adenocarcinoma of the lacrimal gland underwent left orbital surgical resection, ethmoidectomy, maxillectomy, sphenoidectomy and left neck dissection followed by adjuvant chemoradiation in February 2010. He developed a local recurrence after 4 years in September 2014 in his left cheek which was excised with negative margins. He had two more recurrences over the next 2 years in the left parotid gland requiring a parotidectomy while involving the left facial nerve. In November 2016, an indwelling catheter was placed for a large right malignant pleural effusion. Three months later in February 2017, a solitary 6 mm left parietal lobe brain metastasis developed that was irradiated with stereotactic body radiation therapy. A left orbital wall, paranasal sinus recurrence developed with cavernous sinus extension and bilateral loculated malignant pleural effusion requiring CT tube drainage. Bone scan showed extensive bony metastases. In early March 2017, patient was started on everolimus 10 mg. CT scan in May showed a partial response. His chest tube drainage eventually decreased. While on everolimus, the patient developed extensive dental abscess that led to orbital oral fistula and necrotic orbital abscess requiring IV antibiotic, IV fungals for Actinomyces, Candida albicans. Everolimus was stopped between July and August 2017.
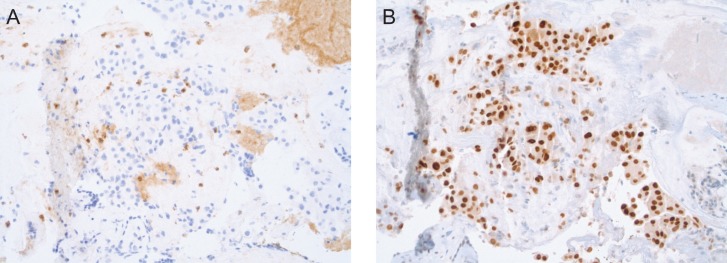
Sequencing the tumor revealed an intermediate tumor mutational load (TML) (eight mutations per megabase), stable MSI, AR highly expressed (90%), HER2 negative, PD-L1 >1% (Fig. 1).
Figure 1:
(A) PD-L1 expression by IHC. (B) AR overexpression (90%) by IHC.
In November 2017, PET/CT showed progressive pleural, pericardial metastatic disease and peritoneal disease with increased glycolytic activity involving the fundus of the stomach. Severe normocytic anemia developed (Hb 6.6) with mild thrombocytopenia requiring multiple blood transfusions. Bone marrow showed involvement with adenocarcinoma.
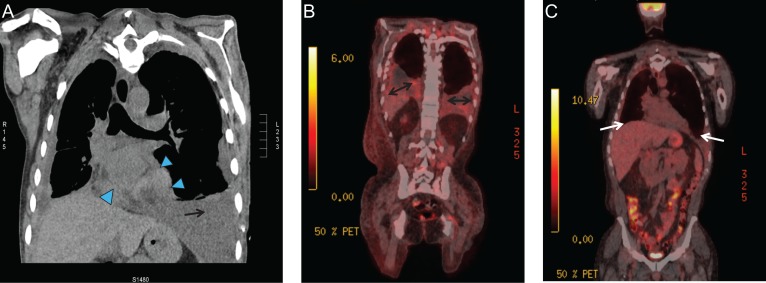
In December 2017, nivolumab was started along with biclutamide for androgen deprivation achieving near castrate levels. The patient has now had partial response on two successive scans over 6 months in February and May 2018. There is stable mild loculated pleural effusion inferiorly in the right hemithorax. Loculated pleural effusion in the base of the left hemithorax is smaller in comparison with November 2017. Left pleural drain was removed while the pericardial effusion decreased from moderate to mild with stable bony metastases (Fig. 2).
Figure 2:
Radiological progression and response to therapy. (A) November 2017: progressive pleural thickening (arrow), pericardial involvement (arrowhead) and peritoneal disease (not shown). (B) February 2018: Progressive loculated pleural effusion (double arrows), pericardial metastatic disease (not shown) and peritoneal disease with increased glycolytic activity involving the fundus of the stomach (not shown). (C) May 2018: resolving pleural effusion and near resolution of pericardial and peritoneal disease (white arrow).
CONCLUSION
Cases describing Her-2/neu overexpression, phosphatase and tensin homolog point mutation have previously been described in lacrimal gland carcinoma [7, 8]. Adenoid cystic carcinoma (ACC) is the most common lacrimal neoplasm, followed by carcinoma ex-pleomorphic adenoma [3, 5]. Adenocarcinoma accounts for 4–13% in a recent series [5]. Oncogenic mutations are present in more than half of the cases of ACC, with KRAS mutations in 10 of 24 patients in one series, suggesting potential benefit with everolimus targeting the EGFR–RAS–RAF cascade [9]. Besides Her2 expression not much is known regarding primary drivers in salivary and lacrimal tumors [3].
Androgen expression has been noted in adenocarcinomas [10]. AR and its mRNA have been located in human lacrimal gland acinar cell nuclei and lacrimal gland [11]. Whether immunotherapy would be beneficial in these tumors (by evaluating TML, PDL1 and microsatellite instability) is not clear and requires further study. To our knowledge, this case report is the first to suggest the activity of checkpoint inhibitors and possible role of androgen receptors in lacrimal gland tumors.
ACKNOWLEDGEMENTS
Joanne Xiu for providing IHC images.
CONFLICT OF INTEREST STATEMENT
Dr Bulbul has served advisory boards for AstraZeneca and Pfizer.
REFERENCES
- 1. von Holstein SL, Coupland SE, Briscoe D, Le Tourneau C, Heegaard S. Epithelial tumours of the lacrimal gland: a clinical, histopathological, surgical and oncological survey. Acta Ophthalmol 2013;91:195–206. [DOI] [PubMed] [Google Scholar]
- 2. Rootman J, White VA. Changes in the 7th edition of the AJCC TNM classification and recommendations for pathologic analysis of lacrimal gland tumors. Arch Pathol Lab Med 2009;133:1268–71. [DOI] [PubMed] [Google Scholar]
- 3. Vidal L, Tsao MS, Pond GR, Cohen EE, Cohen RB, Chen EX, et al. Fluorescence in situ hybridization gene amplification analysis of EGFR and HER2 in patients with malignant salivary gland tumors treated with lapatinib. Head Neck 2009;31:1006–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zimmerman LE, Sobin LH. Histological Typing of Tumours of the Eye and its Adnexa Geneva: WHO, 1980, 23–7. [Google Scholar]
- 5. Weis E, Rootman J, Joly TJ, Berean KW, Al-Katan HM, Pasternak S, et al. Epithelial lacrimal gland tumors: pathologic classification and current understanding. Arch Ophthalmol 2009;127:1016–28. [DOI] [PubMed] [Google Scholar]
- 6. White VA. Update on lacrimal gland neoplasms: molecular pathology of interest. Saudi J Ophthalmol 2012;26:133–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dennie T. Metastatic, Her-2 amplified lacrimal gland carcinoma with response to lapatinib treatment. Case Reports in Oncological Medicine 2015;2015:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andreasen S, Grauslund M, Heegaard S. Lacrimal gland ductal carcinomas: clinical, morphological and genetic characterization and implications for targeted treatment. Acta Ophthalmol 2017;95:299–306. [DOI] [PubMed] [Google Scholar]
- 9. Woo KI, Kim YD, Sa HS, Esmaeli B. Current treatment of lacrimal gland carcinoma. Curr Opin Ophthalmol 2016;27:449–56. [DOI] [PubMed] [Google Scholar]
- 10. Tihan T, Harmon JW, Wan X, Younes Z, Nass P, Duncan KL, et al. Evidence of androgen receptor expression in squamous and adenocarcinoma of the esophagus. Anticancer Res 2001;21:3107–14. [PubMed] [Google Scholar]
- 11. Smith RE, Taylor CR, Rao NA, Young LL, Rife LL. Immunohistochemical identification of androgen receptors in human lacrimal glands. Curr Eye Res 1999;18:300–9. [DOI] [PubMed] [Google Scholar]