Abstract
We report a case of a 33-year-old man with a background of longstanding ileo-colonic Crohn’s disease and primary sclerosing cholangitis. Following a trip to India he developed diarrhoea which was treated as an exacerbation of Crohn’s disease. Liver tests became chronically deranged after increasing immunosuppression, which comprised mercaptopurine, adalimumab and prednisolone. Chronic genotype 1 hepatitis E was diagnosed and successfully treated with reduction of immunosuppression followed by a 24-week course of ribavirin. We believe that this is the first reported case of chronic hepatitis E in genotype 1. Deranged liver tests should prompt testing for hepatitis E infection in the context of immunosuppression for inflammatory bowel disease.
CASE REPORT
A 33-year-old gentleman was diagnosed with ileo-colonic Crohn’s disease in 2003. Initial treatment comprised azathioprine and methotrexate which was escalated to infliximab in 2004 due to poor treatment efficacy. His disease was stable on infliximab until 2007 when it was stopped to allow for travelling, with prednisolone used on an intermittent basis for acute disease exacerbations. Poor disease control necessitated introduction of adalimumab therapy in 2012, which stabilized his symptoms.
Cholestatic liver biochemistry was attributed to primary sclerosing cholangitis (PSC) following liver biopsy and MRI cholangiography in February 2013 (Fig. 1). By this time, maintenance treatment comprised mercaptopurine (6-MP) 100 mg daily, prednisolone 5 mg daily and adalimumab 40 µg every 10 days. Over Christmas 2015 he travelled to India and reported increased frequency of bowel movements. He self-treated this with a higher dose of prednisolone and his symptoms resolved.
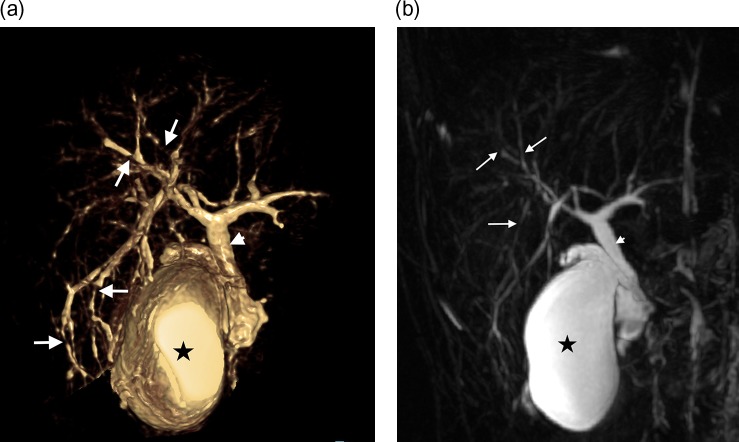
Figure 1:
(a) Volume-rendered magnetic resonance cholangiopancreatogram (VRT MRCP) image (anterior view) demonstrates multifocal stricturing, irregularity and mild dilatation of intrahepatic ducts, predominantly with a peripheral location (arrows). Gallbladder (asterisk) and common hepatic duct (arrowhead) indicated for orientation. (b) Coronal maximum intensity projection (MIP) MRCP image demonstrates subtle structuring and irregularity of peripheral intrahepatic ducts. Gallbladder (asterisk) and common hepatic duct (arrowhead) indicated.
In April 2016, a significant transaminitis developed (ALT 710 U/L). Mercaptopurine was stopped and a higher dose of steroids initiated. A serological liver screen was performed which excluded hepatitis A, B and C, epstein barr virus, cytomegalovirus and herpes simplex. An autoantibody profile was negative, but IgG elevated at 16 g/L. Alkaline phosphatase was mildly raised throughout (peak 142 U/L) but bilirubin remained normal. Given the background diagnosis of PSC, a liver biopsy was undertaken which demonstrated a moderately active portal and lobular hepatitis with possible causes including drugs, virus and autoimmune hepatitis. The biopsy prompted testing for hepatitis E, which returned positive (HEV IgM positive and HEV RNA 23 CT). Further testing has confirmed genotype 1 suggesting the infection was contracted in India at the time of the diarrhoeal illness.
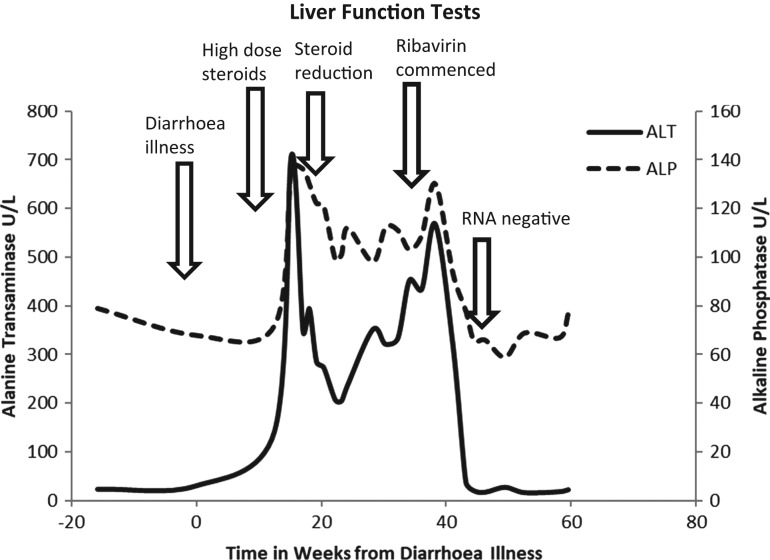
Initial management was with reduction of immunosuppression. However, given Crohn’s disease severity, prednisolone reduction was the only possible strategy but at five months from initial presentation there was no improvement in ALT or viral load. A decision was made to commence ribavirin, which was tolerated at 600 mg twice daily for a complete course of 24 weeks. At the start of treatment, the HEV was 35 CT and ALT 316 U/L. By Week 4 he was HEV RNA negative and ALT had normalised (ALT 21 U/L). HEV remained negative throughout treatment and at 12 and 24 weeks after cessation (Fig. 2).
Figure 2:
Showing change in alanine transaminase and alkaline phosphatase over time in relation to important clinical events. Bilirubin remained normal throughout.
DISCUSSION
We present a case of chronic hepatitis E complicating immunosuppression for Crohn’s disease. Modest reduction in immunosuppression had no effect on host viral control and so ribavirin was used with good efficacy. In retrospect hepatitis E virus should have been tested at the point of the acute liver screen, thus avoiding liver biopsy. At the time the biopsy was justified as the focus was on a likely autoimmune hepatitis given a background of PSC and Crohn’s disease. The inadvertent increase in steroid dose may have contributed to the chronicity of hepatitis E infection. Subsequent analysis has confirmed genotype 1 hepatitis E which was likely contracted in India, due to the known genotype distribution of the virus [1].
The incidence of hepatitis E is increasing in the UK with 1243 cases reported in 2016 [2]. Hepatitis E virus is a single-stranded RNA virus with faeco-oral and zoonotic transmission. In developed countries this is usually spread via undercooked meat whilst in developing countries outbreaks are seen in areas of poor hygiene [3]. There are four genotypes, G1 and G2 are limited to humans and seen during outbreaks in developing countries. G3 and G4 are seen worldwide and carried by other animals including pigs and sheep as well as humans [1]. An acute hepatitis is seen with acute infection which seems clinically more severe in the developing world [4]. Infection in the elderly and immunocompromised can lead to a prolonged or chronic form of infection [5].
Hepatitis E usually causes a self-resolving infection. Chronic infection may develop in the immunosuppressed. Reported cases of chronic infection to date are in G3 and G4, but not G1 as in our case [5]. It has been reported following renal [6], heart and lung transplantation [7] and blood products for solid organ transplant recipients are now required to be hepatitis E negative in the UK [8]. HIV is also a risk factor [9].
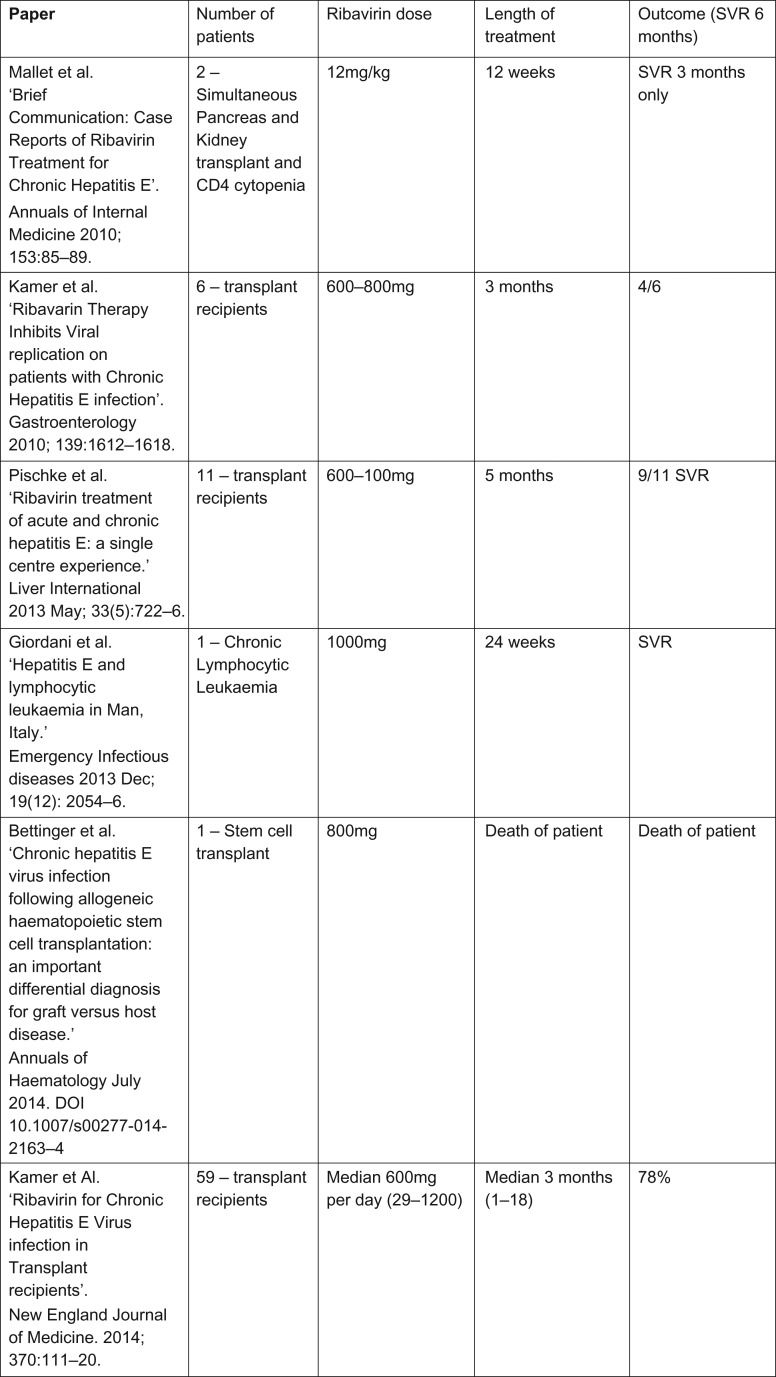
Chronic hepatitis E is usually treated with immunosuppression reduction followed by ribavirin if this strategy fails. European Association for the Study of the Liver guidelines published this year support the treatment with ribavirin [10], however, the optimal ribavirin treatment regimen is not known. Case series suggest a dose between 600 and 1000 mg daily over a varying time of 3–6 months (Fig. 3).
Figure 3:
Table showing current published studies on treatment of HEV with Ribavirin.
We opted for a target ribavirin dose of 600 mg twice daily for 24 weeks, which our patient tolerated well with good efficacy. To our knowledge, this is the first published case of chronic hepatitis E complicating immunosuppression for inflammatory bowel disease. Secondly, we believe that this is the first reported case of chronic hepatitis E caused by G1 infection.
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST STATEMENT
No conflicts of Interest.
CONSENT
Consent was granted for publication of this report.
REFERENCES
- 1. Geng Y, Wang Y. Epidemiology of hepatitis E. Adv Exp Med Biol 2016;948:39–59. [DOI] [PubMed] [Google Scholar]
- 2. Guidance Hepatitis E: Symptoms, Transmission, Treatment and Prevention In: England PH, editor. https://www.gov.uk/government/publications/hepatitis-e-symptoms-transmission-prevention-treatment/hepatitis-e-symptoms-transmission-treatment-and-prevention2018.
- 3. Geng Y, Wang Y. Transmission of hepatitis E Virus. Adv Exp Med Biol 2016;948:89–112. [DOI] [PubMed] [Google Scholar]
- 4. Donnelly M, Scobie L, Crossan C, Dalton H, Hayes PC, Simpson KJ. Review article: Hepatitis E—a concise review of virology, epidemiology, clinical presentation and therapy. Aliment Pharmacol Ther 2017;46:126–41. [DOI] [PubMed] [Google Scholar]
- 5. Kamar N, selves J, Mansuy J, Ouezzani L, Peron J, Guitard J, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 2008;358:811–7. [DOI] [PubMed] [Google Scholar]
- 6. Sridhar S, Chan J, Yap D, Teng J, Huang C, Yip C, et al. Genotype 4 hepatitis E virus is a cause of chronic hepatitis in renal transplant recipients in Hong Kong. J Viral Hepat 2018;25:209–13. [DOI] [PubMed] [Google Scholar]
- 7. Riezebos-Brilman A, Puchhammer-Stockl E, Haagsma E, Jaksch P, Bejvi L, Niesters H, et al. Chronic hepatitis E infection in lung transplant recipients. J Heart Lung Transplant 2013;32:341–6. [DOI] [PubMed] [Google Scholar]
- 8. McPherson S, Elsharkawy A, Ankcorn M, Ijaz S, Powell J, Rowe L, et al. Summary of the British Transplantation Society UK Guidelines for hepatitis E and solid organ transplantation. Transplantation 2018;102:15–20. [DOI] [PubMed] [Google Scholar]
- 9. Zeng H, Wang L, Liu P, Liao L, Wang L, Shao Y. Seroprevalence of hepatitis E virus in HIV-infected patients in China. AIDS 2017;31:2019–21. [DOI] [PubMed] [Google Scholar]
- 10. Dalton H, Kamar N, Baylis S, Moradpour D, Wedemeyer H, Negro F. EASL Clinical Practice Guidelines on hepatitis E infection. J Hepatol 2018;68:1256–71. [DOI] [PubMed] [Google Scholar]