Abstract
The differentiation of endometrial stromal cells into decidual cells, termed decidualization, is an integral step in the establishment of pregnancy. The mitogen-activated protein kinase homolog, WNK lysine deficient protein kinase 1 (WNK1), is activated downstream of epidermal growth factor receptor during decidualization. Primary human endometrial stromal cells (HESCs) were subjected to small interfering RNA knockdown of WNK1 followed by in vitro decidualization. This abrogated expression of the decidual marker genes, insulin like growth factor binding protein 1 (IGFBP1) and prolactin (PRL), and prevented adoption of decidual cell morphology. Analysis of the WNK1-dependent transcriptome by RNA-Seq demonstrated that WNK1 regulates the expression of 1858 genes during decidualization. Gene ontology and upstream regulator pathway analysis showed that WNK1 regulates cell migration, differentiation, and proliferation. WNK1 was required for many of the gene expression changes that drive decidualization, including the induction of the inflammatory cytokines, C-C motif chemokine ligand 8 (CCL8), interleukin 1 beta (IL1B), and interleukin 15 (IL15), and the repression of transforming growth factor-beta (TGF-beta) pathway genes, including early growth response 2 (EGR2), SMAD family member 3 (SMAD3), integrin subunit alpha 2 (ITGA2), integrin subunit alpha 4 (ITGA4), and integrin subunit beta 3 (ITGB3). In addition to abrogating decidualization, WNK1 knockdown decreased the migration and proliferation of HESCs. Furthermore, mitogen-activated protein kinase 7 (MAPK7), a known downstream target of WNK1, was activated during decidualization in a WNK1-dependent manner. Small interfering RNA knockdown of MAPK7 demonstrated that MAPK7 regulates a subset of WNK1-regulated genes and controls the migration and proliferation of HESCs. These results indicate that WNK1 and MAPK7 promote migration and proliferation during decidualization and regulate the expression of inflammatory cytokines and TGF-beta pathway genes in HESCs.
Keywords: kinases, signal transduction, cytokines, extracellular matrix, endometrium, cell culture
WNK1 and MAPK7 signaling are required for multiple decidual cell functions, including proliferation, migration, induction of inflammatory cytokines, and repression of TGF-beta pathway genes.
Introduction
Decidualization, the process by which endometrial stromal cells differentiate into specialized decidual cells, is a critical step in the establishment of pregnancy. In humans, decidualization begins with the proliferative expansion of endometrial stromal cells during the mid-secretory phase of the menstrual cycle [1]. After undergoing mitotic expansion, these cells differentiate into decidual cells, which serve multiple functions during early pregnancy, including supporting the developing embryo, modulating maternal immunity, and regulating trophoblast invasion [2]. The process of decidualization involves a morphological transformation, as elongated stromal cells become epithelioid, adopting the characteristic cobblestone appearance of decidual cells [3]. This transformation is a hallmark of decidual cell differentiation and correlates with the induction of decidual cell secretory functions [4]. Decidual cells are highly secretory, producing a variety of growth factors, cytokines, and hormones that regulate maternal and embryonic processes. During early decidualization, endometrial stromal cells secrete an assortment of cytokines that contribute to the recruitment of uterine natural killer (uNK) cells, while during later pregnancy the secretions of these cells are predominantly anti-inflammatory [5]. Decidual cells play a dual role in the regulation of trophoblast invasion, as they both promote trophoblast migration and prevent excessive invasion of trophoblast into maternal tissue [6]. Furthermore, decidual cells display migratory and invasive behavior, which is enhanced near implantation sites and contributes to the organization of multicellular branches of decidual and trophoblast cells [7,8]. The successful execution of these functions is critical for maternal and fetal health throughout the course of pregnancy. Early defects in decidualization can contribute to pathologies during later stages of pregnancy, including preeclampsia, intrauterine growth restriction, and placenta accreta [2]. As such, understanding the molecular signaling pathways that facilitate successful decidualization is critical to promoting healthy pregnancies.
Kinase signaling regulates many of the molecular changes that occur during decidualization. Several signaling kinases, such as protein kinase B (PKB/AKT) and extracellular-signal-regulated kinase 1/2 (ERK1/2), have been shown to coordinate cellular functions during the process of decidualization [9,10]. In addition, receptor tyrosine kinases act as mediators that facilitate the complex cell–cell communications that occur during the peri-implantation period. For instance, epidermal growth factor receptor (EGFR; previously known as ERBB) is required for stromal cell decidualization and activates multiple downstream signaling pathways in endometrial stromal cells [11]. One of the downstream targets of EGFR signaling in stromal cells is the mitogen-activated protein kinase (MAPK) homolog, WNK lysine deficient protein kinase 1 (WNK1; previously known as HSN2, PRKWNK1), which is activated by phosphorylation downstream of EGFR [11].
WNK1, a member of the WNK kinase family, is a uniquely structured kinase known for its roles in regulating ion homeostasis, proliferation, and cell migration [12]. Similar to other MAPK pathway proteins, WNK1 can be activated downstream of receptor tyrosine kinases. In human embryonic kidney cells, WNK1 is activated downstream of AKT in the EGFR and insulin like growth factor 1 (IGF1) signaling pathways [13,14]. WNK1 is a positive regulator of proliferation in multiple cell types, including prostate carcinoma and neural progenitor cells [15,16]. In addition to promoting proliferation, WNK1 regulates cell migration and invasiveness in a variety of contexts. WNK1 positively regulates tumor cell migration in triple-negative breast cancer, glioma, and pancreatic ductal adenocarcinoma cell lines and promotes metastasis in xenograft models of hepatocellular carcinoma and prostate adenocarcinoma [15,17–20]. In vivo, WNK1 plays a critical role in angiogenic processes such as vascular remodeling and endothelial sprouting [21]. WNK1 is expressed throughout the cardiovascular system during murine embryogenesis, including in the developing heart, as well as in the placenta and yolk sac [22]. In the mouse, Wnk1 deletion results in embryonic lethality due to defects in the development of the cardiovascular system [21]. While it is known that WNK1 regulates diverse cellular functions, including proliferation, ion channel expression and activity, and immune cell migration, its role in the endometrium has not been explored [15,23].
Several of the downstream effects of WNK1 are the result of its participation in MAPK signaling cascades. Notably, WNK1 activates mitogen-activated protein kinase 7 (MAPK7, also known as ERK5) in a variety of cell types [13,16]. In many cases, MAPK7 has been found to mediate the proproliferative and promigratory effects of WNK1. MAPK7 promotes migration and invasiveness in multiple cancer cell lines, including osteosarcoma, mesothelioma, and prostate cancer [24–26]. WNK1/MAPK7 signaling also promotes tumor growth and metastasis in vivo in prostate cancer xenograft models [15,24]. Similar to WNK1, MAPK7 has been shown to regulate angiogenesis in the mouse [27]. Mapk7 knockout results in embryonic lethality at day 10.5, with obvious defects in placentation and angiogenesis [27]. In addition, MAPK7 has been implicated in promoting angiogenesis in human umbilical vein endothelial cells, suggesting conservation of this function in human systems [28]. Given the role of WNK1/MAPK7 signaling in regulating cellular proliferation, migration, and angiogenesis in other systems, it is possible that this pathway controls similar functions during the decidualization of endometrial stromal cells.
To define the role of WNK1 in stromal cell decidualization, we investigated the effect of small-interfering RNA (siRNA) knockdown of WNK1 on the ability of primary human endometrial stromal cells (HESCs) to decidualize in vitro. WNK1 was required for the decidualization of HESCs, and RNA sequencing (RNA-Seq) demonstrated that WNK1 regulates inflammation and transforming growth factor-beta (TGF-beta) signaling in decidualizing stromal cells. In addition, MAPK7 was activated during decidualization in a WNK1-dependent manner. MAPK7 regulated HESC proliferation and migration and modulated the expression of a subset of WNK1-regulated genes, suggesting that the WNK1/MAPK7 signaling axis regulates multiple decidual cell functions.
Materials and methods
Primary human endometrial stromal cell culture
HESCs were isolated from proliferative phase endometrial biopsies obtained from healthy volunteers of reproductive age with regular menstrual cycles and no history of gynecological malignancy, according to a human subjects protocol approved by the Institutional Review Board of Baylor College of Medicine. HESC isolation was performed as previously described [29]. Briefly, endometrial biopsies were washed with Hanks balanced salt solution containing 100 U/mL penicillin and 100 μg/mL streptomycin. Biopsy samples were mechanically digested for 20 min, and then subjected to further digestion by incubation with 25 mg collagenase (C-130; Sigma) and 5 mg deoxyribonuclease I (DN25; Sigma) and filtration through a 0.2 μm filter for 90 min. Stromal cells were isolated by filtering digested samples through a 40 μm filter. Isolated stromal cells were cultured in HESC medium (DMEM/F12 supplemented with 10% fetal bovine serum and penicillin/streptomycin). All experiments were conducted in HESC cultures of less than 10 passages and repeated in cell cultures derived from three individual patients.
Small-interfering RNA knockdown and in vitro decidualization
HESCs were transfected with 60 nM nontargeting siRNA (siNT), siRNA targeting WNK1 (siWNK1), or siRNA targeting MAPK7 (siMAPK7) (ON-TARGETplus SMARTpool; Dharmacon). Transfection was performed using Lipofectamine RNAiMax (Invitrogen) according to the manufacturer's instructions. Following 48-h transfection, cells were cultured in OPTI-MEM supplemented with 2% charcoal-stripped fetal bovine serum and penicillin/streptomycin and treated with control vehicle (Veh treatment) or 10 nM 17 beta-estradiol (E1024; Sigma), 1 μM medroxyprogesterone acetate (MPA) (M1629; Sigma), and 100 μM 2’-O-dibutyryladenosine-3’, cAMP (db-cAMP) (D0627; Sigma) to induce decidualization (EPC treatment). HESCs were subjected to Veh or EPC treatment for 3 or 6 days, with media and hormone replacement every 48 h.
RNA sequencing
RNA isolation was performed using the Qiagen RNeasy Mini kit as per manufacturer's instructions, and cDNA libraries were generated using the TruSeq RNA library prep kit v2 (Illumina) with polyA selection per manufacturer's instructions. RNA sequencing was performed on EPC siNT- and EPC siWNK1-treated cells from three individual patients, with EPC siNT- and EPC siWNK1-treated samples from the same patient treated as paired samples for statistical analysis. Raw reads were trimmed and resultant pair-ended reads were mapped to the human genome (hg19) using TopHat [30]. Read duplicates were removed using Picard tools (https://broadinstitute.github.io/picard/) to account for PCR biases, and HTseq was used to quantify reads falling in known genes [31]. Differential gene expression was determined using edgeR using a false discovery rate (FDR)-adjusted P-value cutoff of <0.01 [32]. An FDR-adjusted P-value cutoff of <0.01 was similarly applied to an RNA-Seq dataset comparing Veh- and EPC-treated HESCs to identify overlapping genes with the WNK1 RNA-Seq dataset [33]. Differentially expressed genes were analyzed using DAVID to identify clusters of enriched gene ontology (GO) terms [34,35]. Ingenuity Pathway Analysis (IPA) was utilized to identify enrichments for upstream regulator pathways. Fold change values from the WNK1 RNA-Seq dataset were used as input for determination of activation Z-scores by IPA. Activation Z-score indicates that regulatory pathways are activated (positive Z-score) or inhibited (negative Z-Score) by WNK1 knockdown [36].
Quantitative reverse transcriptase PCR
Gene expression changes identified by RNA-Seq were validated by reverse transcription real-time quantitative PCR (RT-qPCR). Following siRNA transfection and EPC treatment, RNA was extracted using TriZOL (Invitrogen) according to the manufacturer's instructions and cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (Invitrogen) as per the manufacturer's instructions. Messenger RNA (mRNA) expression was determined by RT-qPCR using SYBR Green master mix (Roche Diagnostics) and oligonucleotide primers synthesized by Sigma-Aldrich (Supplementary Table S1). Relative mRNA expression was determined using the delta delta CT method and normalized to expression of 18S rRNA [37]. Expression data were analyzed by one-way analysis of variance (ANOVA) with Tukey's multiple comparisons adjustment (MCA) using the GraphPad Prism software.
Western blot
Following siRNA transfection and EPC treatment, cells were washed and lysed in protein lysis buffer containing 10 mM Tris, pH 7.4; 150 mM NaCl; 2.5 mM EDTA; and Nonidet P-40 supplemented with cOmplete Mini EDTA free protease inhibitor cocktail (Roche Diagnostics) and PhosSTOP phosphatase inhibitor cocktail (Roche Diagnostics). Denatured protein samples were loaded on Bis-Tris NuPAGE 4%-12% gels (Invitrogen) and separated by electrophoresis. Protein bands were transferred to polyvinylidene difluoride membranes (BioRad) in transfer buffer containing 25 mM Tris, 192 mM glycine, and 20% methanol (BioRad). Membranes were blocked for 1 h at room temperature in 5% blotting grade nonfat milk (BioRad) in PBS containing 0.1% Tween 20 (PBS-T). Membranes were incubated with primary antibody suspended in 5% blotting grade nonfat milk in PBS-T overnight at 4°C (Supplementary Table S2). Membranes were washed in PBS-T and then incubated with secondary antibody (antirabbit or antigoat peroxidase) for 1 h at room temperature. Membranes were washed in PBS-T, followed by PBS, and treated with Amersham ECL Western blotting system reagents (GE Healthcare) according to the manufacturer's instructions for luminol-based band detection. Quantification of protein bands was performed using the Image J software to measure band density. Relative phosphorylation of MAPK7 was calculated as the ratio of phospho-MAPK7 to total MAPK7, normalized to beta-actin, and relative to the level of MAPK7 phosphorylation observed in Veh siNT-treated cells.
Migration assay
HESC migration was assessed by in vitro scratch assay [38]. Briefly, cultured cells were transfected with siRNA for 48 h. Following the transfection period, a pipette tip was used to create a scratch across the surface of the culture plate. After formation of the scratch, cells were washed in culture medium to remove debris and subsequently cultured in EPC treatment medium. The scratch area was photographed at 0 and 24 h, and the distance by which the leading edge of cells migrated into the scratch area was used to quantify cell migration. Results were analyzed by two-tailed t-test using the GraphPad Prism software.
Proliferation assay
HESC proliferation was assessed using the CellTiter 96 AQueous One Solution cell proliferation assay (Promega) according to the manufacturer's instructions. Briefly, cells underwent siRNA transfection for 48 h. Following transfection, cells were plated in 96-well culture plates at a concentration of 1000 cells per well. Cells were allowed to adhere for 24 h, and then treated with EPC treatment medium for 6 days. Absorbance at 490 nm was corrected and normalized to EPC siNT-treated cells to determine the relative number of viable cells at each time point. Results at each time point were analyzed by two-tailed t-test using the GraphPad Prism software.
Microscopy
HESC morphology was assessed by fluorescence microscopy using CellMask Deep Red Plasma membrane Stain (ThermoFisher Scientific). HESCs were cultured in 12-well culture plates containing poly D-Lysine-coated coverslips. Cells were subjected to 48-h siRNA transfection followed by 3-day treatment with vehicle control or EPC medium. At the conclusion of treatment, the coverslips were removed and stained with CellMask according to the manufacturer's instructions. Briefly, coverslips were submerged in CellMask staining solution for 10 min at 37°C, fixed in 3.75% formaldehyde for 10 min at 37°C, and imaged immediately.
Results
WNK1 is required for human endometrial stromal cell decidualization
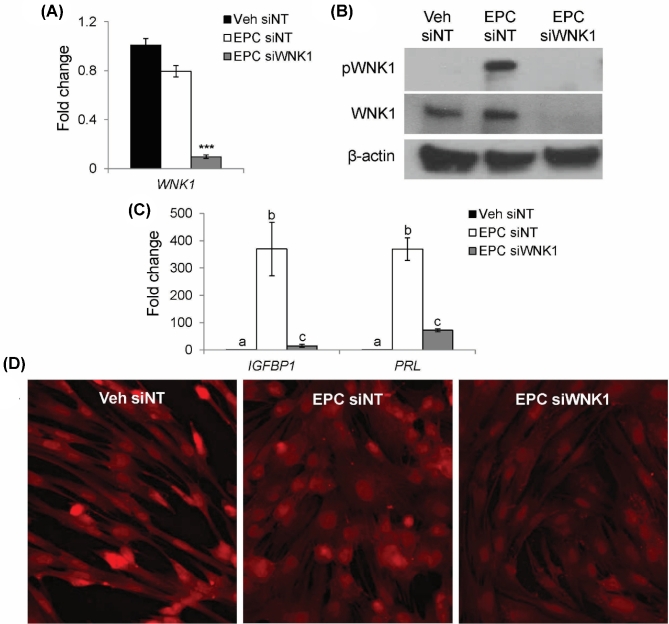
The role of WNK1 in stromal cell decidualization was investigated by examining the effects of WNK1 knockdown on cultured primary HESCs. HESCs were transfected with siNT or siWNK1. Following 48-h siRNA transfection, HESCs were treated with a combination of 17 beta-estradiol, MPA, and db-cAMP (EPC) for 72 h to induce decidualization; vehicle-treated (Veh) cells were used as a nondecidualized control. As demonstrated in Figure 1A, WNK1 was expressed in both Veh- and EPC-treated HESCs. Transfection with siWNK1 resulted in decreased expression of WNK1 mRNA. The expression of total WNK1 protein was similar in both Veh siNT- and EPC siNT-treated HESCs (Figure 1B). Phosphorylation of WNK1 was induced by EPC treatment, indicating activation of WNK1 during decidualization. Expression of WNK1 protein was abolished in HESCs transfected with siWNK1.
Figure 1.
WNK1 knockdown inhibits expression of decidual marker genes and prevents adoption of decidual cell morphology. (A) WNK1 mRNA relative to Veh siNT. Bars indicate mean values and SEM. *** P < 0.001, ANOVA with Tukey's MCA. (B) Western blot for total and phosphorylated WNK1 proteins. (C) Relative mRNA expression of decidual marker genes, IGFBP1 and PRL. Different letters denote significantly different means (P < 0.05, ANOVA with Tukey's MCA). (D) CellMask staining (red) of HESC plasma membranes.
To confirm previous observations that WNK1 is required for the induction of insulin like growth factor binding protein 1 (IGFBP1; previously known as IBP1) and prolactin (PRL), HESC decidualization was evaluated by examining expression of the decidual marker genes, IGFBP1 and PRL [11,39,40]. IGFBP1 and PRL were robustly induced following 3-day EPC treatment in siNT-treated cells (Figure 1C). In contrast, HESCs transfected with siWNK1 displayed decreased induction of IGFBP1 and PRL despite EPC treatment. These results supported previous findings that WNK1 is required for the induction of IGFBP1 and PRL [11]. To further evaluate the effect of WNK1 knockdown on decidualization, CellMask staining of plasma membranes was used to visualize the morphological transformation of decidual cells following EPC treatment (Figure 1D). Veh-treated cells retained the elongated, fibroblastic morphology of nondecidualized stromal cells, while EPC treatment induced a morphological change to the polygonal, cobblestone appearance that is characteristic of decidual cells. In contrast, HESCs transfected with siWNK1 failed to adopt the cobblestone morphology of decidual cells despite EPC treatment. These findings demonstrated that WNK1 is critical for the decidual cell transformation, evidenced by the loss of induction of decidual markers and failure to adopt the cobblestone morphology characteristic of decidual cells following WNK1 knockdown.
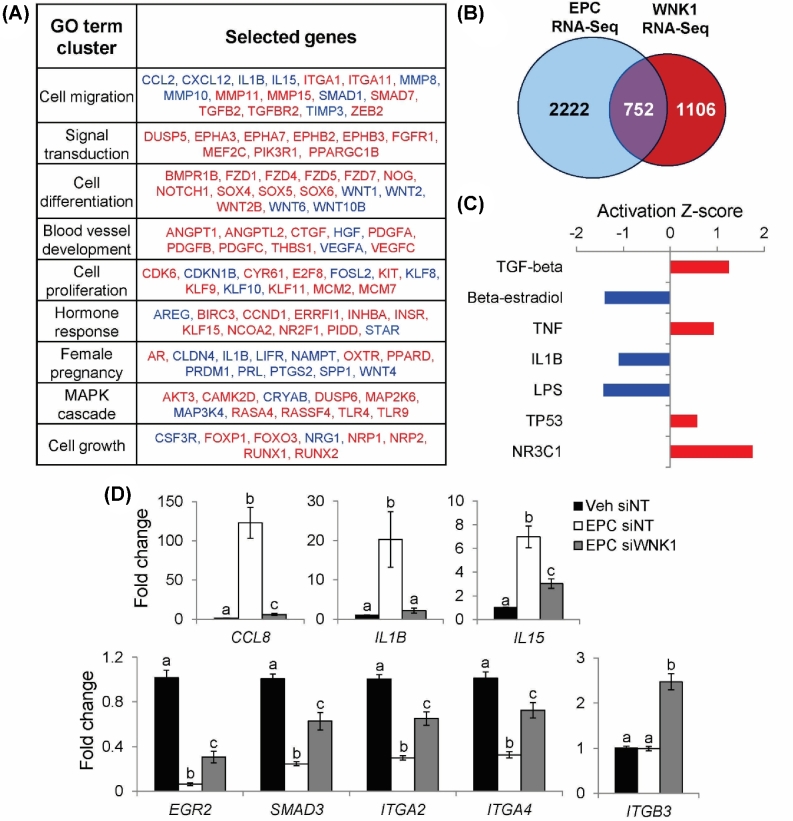
WNK1 regulates 1858 genes during decidualization
To identify WNK1-dependent transformations in decidual cells, an unbiased analysis of gene expression was performed using RNA-Seq following WNK1 knockdown in EPC-treated HESCs. EPC siNT-treated cells were compared to EPC siWNK1-treated cells to identify WNK1-dependent changes in gene expression during decidualization. WNK1 knockdown altered the expression of 1858 genes in decidualizing HESCs, leading to upregulation of 1208 genes and downregulation of 650 genes (Supplementary Table S3). The 1858 genes regulated by WNK1 were categorized into clusters of enriched GO terms using DAVID (Figure 2A). Enrichment statistics for all GO terms in each cluster and complete lists of WNK1-regulated genes belonging to each GO term are detailed in Supplementary Tables S4 and 5. Notably, the top WNK1-regulated gene ontologies included cell migration, signal transduction, cell differentiation, cell proliferation, hormone response, and female pregnancy. To highlight gene expression changes that are relevant to the process of decidualization, the WNK1 RNA-Seq dataset was compared to an RNA-Seq dataset examining gene expression changes between Veh- and EPC-treated HESCs [33]. Overlaying the 1858 genes regulated by WNK1 (WNK1 RNA-Seq) with the 2974 genes regulated by EPC treatment (EPC RNA-Seq) produced 752 genes that are regulated by both WNK1 and decidualization (Figure 2B). Analysis of these 752 genes demonstrated that WNK1 knockdown reversed the direction of gene expression change in 80% of genes, indicating that siWNK1-treated cells failed to undergo many of the gene expression changes that define decidualization (Supplementary Table S6). This dataset was further analyzed using IPA to identify upstream regulator pathways that are regulated by WNK1 during decidualization (Figure 2C, Supplementary Table S7). IPA demonstrated that WNK1 regulates genes involved in a multitude of signaling pathways, including TGF-beta, beta-estradiol, tumor necrosis factor (TNF), and interleukin 1 beta (IL1B). Notably, WNK1 knockdown led to upregulation of the TGF-beta, TNF, tumor protein p53 (TP53), and glucocorticoid receptor (NR3C1) pathways, and downregulation of the beta-estradiol, IL1B, and lipopolysaccharide (LPS) pathways.
Figure 2.
Analysis of WNK1-regulated functions and signaling pathways by RNA-Seq. (A) Identification of enriched GO terms among 1858 WNK1-regulated genes using DAVID. Top GO term clusters (ranked by enrichment P-value) are shown along with WNK1 target genes belonging to each ontological cluster. Genes in red are upregulated by WNK1 knockdown; genes in blue are downregulated by WNK1 knockdown. (B) Overlap between EPC RNA-Seq (comparing Veh- and EPC-treated HESCs) and WNK1 RNA-Seq. (C) IPA of 752 genes regulated by WNK1 and decidualization to identify enrichments for upstream regulator pathways. Activation Z-score indicates that regulatory pathways are activated (positive Z-score, red) or inhibited (negative Z-Score, blue) by WNK1 knockdown. (D) Relative mRNA expression of CCL8, IL1B, IL15, EGR2, SMAD3, ITGA2, ITGA4, and ITGB3. Bars indicate mean values and SEM. Different letters denote means that are significantly different (P < 0.05, ANOVA with Tukey's MCA).
The effect of decidualization and WNK1 knockdown on expression of WNK1 target genes was validated by RT-qPCR. IPA indicated that WNK1 knockdown led to downregulation of the inflammatory IL1B and LPS pathways (Figure 2C). To evaluate the effect of WNK1 knockdown on expression of inflammation pathway-associated genes, RT-qPCR was used to validate the expression changes of the cytokines, C-C motif chemokine ligand 8 (CCL8; previously known as SCYA8), IL1B, and interleukin 15 (IL15). In siNT-treated cells, EPC treatment induced the expression of CCL8, IL1B, and IL15 (Figure 2D). In contrast, the induction of CCL8 and IL15 was significantly reduced in siWNK1-treated cells. Induction of IL1B was abolished in siWNK1-treated cells, with expression levels equivalent to those of Veh siNT-treated cells. These results supported the results of RNA-Seq and suggested that WNK1 signaling is required for the induction of inflammatory cytokines during decidualization.
In addition, RNA-Seq analysis indicated that WNK1 regulates genes involved in TGF-beta signaling, which may regulate the proliferation and differentiation of decidualizing stromal cells. The expression of several TGF-beta pathway genes, including early growth response 2 (EGR2; previously known as KROX20), integrin subunit alpha 2 (ITGA2; previously known as CD49B), integrin subunit alpha 4 (ITGA4; previously known as CD49D), integrin subunit beta 3 (ITGB3; previously known as GP3A), and SMAD family member 3 (SMAD3; previously known as MADH3), was validated by RT-qPCR. EPC treatment of siNT-treated cells led to repression of several TGF-beta pathway genes, including EGR2, SMAD3, and the integrin subunits ITGA2 and ITGA4 (Figure 2D). WNK1 knockdown reversed the repressive effect of EPC treatment on the expression of EGR2, SMAD3, ITGA2, and ITGA4. In addition, siWNK1 treatment upregulated the expression of ITGB3, an integrin family member that is unaltered during normal decidualization. Taken together, these results suggest that WNK1 expression is required for the repression of multiple TGF-beta pathway genes during decidualization.
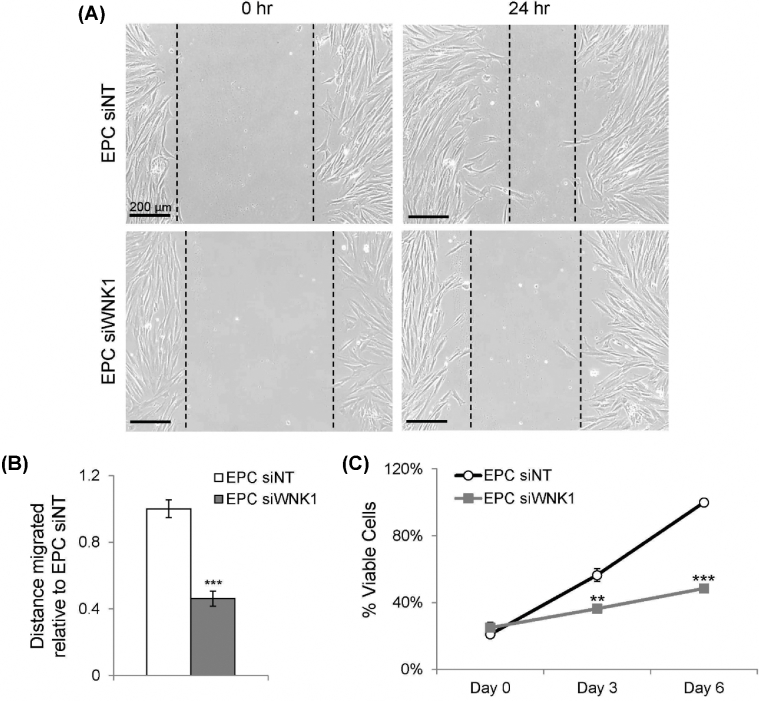
WNK1 regulates human endometrial stromal cell migration and proliferation
Unbiased ontological analysis of WNK1-dependent gene expression changes demonstrated that WNK1 regulates cell migration and proliferation in decidual cells (Figure 2A). The effect of WNK1 knockdown on these cellular functions was evaluated by examining the migratory and proliferative capacities of decidual cells. HESC migration was evaluated by scratch migration assay, in which wells of cultured cells were scratched with a pipette tip and monitored for subsequent migration into the scratch area. Cell movement into the scratch area was monitored over 24 h and quantified as distance migrated into the scratch area. WNK1 knockdown decreased migration of HESCs into the scratch area over a 24-h period (Figure 3A and B). Cell proliferation was examined by the MTS assay, in which the metabolic activity of viable cells was measured at 0, 3, and 6 days of EPC treatment. WNK1 knockdown decreased proliferation during decidualization at both 3 and 6 days of EPC treatment (Figure 3C). These results indicated that WNK1 is required for optimal migration and proliferation of HESCs during decidualization.
Figure 3.
WNK1 promotes HESC migration and proliferation during decidualization. (A) Cell migration into the scratch area at 0 and 24 h. Leading edges (dotted lines) were identified as the most medial line intersecting at least three cells. (B) Quantification of distance migrated into the scratch area relative to EPC siNT-treated cells. Bars indicate mean values and SEM. *** P < 0.001, two-tailed T-test. (C) Relative proliferation of HESCs measured by the MTS assay. Points indicate mean values and SEM. ** P < 0.01, *** P < 0.001, two-tailed t-test.
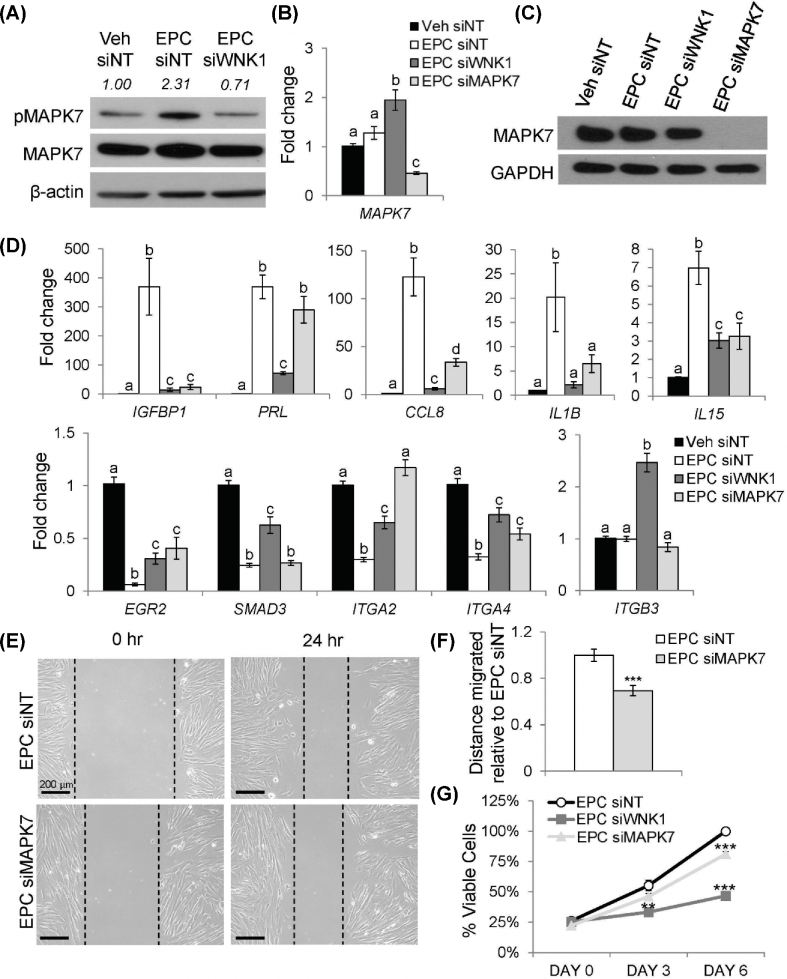
MAPK7 is activated downstream of WNK1
Given that WNK1 promotes cell migration and proliferation by activating MAPK7 in multiple contexts, MAPK7 was hypothesized as a downstream mediator that drives the transcriptional effects of WNK1 in decidual cells [15,16]. MAPK7 is activated by dual phosphorylation at Thr218 and Tyr220 [41]. To determine if MAPK7 is activated during decidualization, the expression and phosphorylation status of MAPK7 were examined by western blot (Figure 4A). In siNT-treated HESCs, EPC treatment induced phosphorylation of MAPK7 protein relative to Veh-treated cells, resulting in a 2.3-fold increase in MAPK7 phosphorylation. Importantly, the induction of MAPK7 phosphorylation did not occur in siWNK1-treated cells, despite the expression of total MAPK7 protein at a level similar to that of siNT-treated cells. WNK1 knockdown resulted in a 3.3-fold decrease in MAPK7 phosphorylation relative to EPC siNT-treated cells. Furthermore, the level of MAPK7 phosphorylation was slightly lower than that observed in Veh siNT-treated cells, suggesting that WNK1 may contribute to the baseline phosphorylation of MAPK7 in addition to driving MAPK7 phosphorylation during decidualization. The loss of MAPK7 phosphorylation in siWNK1-treated cells despite EPC treatment suggested that MAPK7 phosphorylation is WNK1 dependent. This indicated that WNK1 induces phosphorylation of the downstream mediator MAPK7 during decidualization, consistent with observations in other cell types [13,15,16].
Figure 4.
MAPK7 regulates a subset of WNK1-regulated genes and promotes HESC migration and proliferation. (A) Western blot for total (MAPK7) and phosphorylated (pMAPK7) MAPK7 proteins. Quantification of MAPK7 phosphorylation is listed above the pMAPK7 band, expressed as the ratio of pMAPK7 to total MAPK7, normalized to beta-actin and relative to Veh siNT-treated cells. (B) Relative expression of MAPK7 mRNA. (C) Western blot for MAPK7 protein. (D) Relative mRNA expression of IGFBP1, PRL, CCL8, IL1B, IL15, EGR2, SMAD3, ITGA2, ITGA4, and ITGB3. Bars indicate mean values and SEM. Different letters denote means that are significantly different (P < 0.05, ANOVA with Tukey's MCA). (E) Cell migration into the scratch area at 0 and 24 h. (F) Quantification of distance migrated into the scratch area relative to EPC siNT-treated cells. Bars indicate mean values and SEM. *** P < 0.001, two-tailed t-test. (G) Relative proliferation of HESCs measured by the MTS assay. Points indicate mean values and SEM. ** P < 0.01, *** P < 0.001, two-tailed t-test.
To elucidate the role of MAPK7 in mediating the effects of WNK1 signaling in the context of decidualization, siMAPK7 was utilized to knock down MAPK7 expression during the decidualization of HESCs. MAPK7 mRNA was expressed at similar levels in both Veh- and EPC-treated stromal cells that were transfected with siNT (Figure 4B). MAPK7 mRNA expression was slightly upregulated in siWNK1-treated cells, although this did not translate to an increase in MAPK7 protein expression (Figure 4C). Treatment with siMAPK7 resulted in reduction of MAPK7 mRNA expression and expression of MAPK7 protein was undetectable by western blot. RT-qPCR was used to evaluate the role of MAPK7 in mediating WNK1-dependent changes in gene expression. Similar to WNK1 knockdown, MAPK7 knockdown resulted in decreased induction of the decidual marker gene, IGFBP1 (Figure 4D). Knockdown of MAPK7 did not have an effect on the expression of PRL, suggesting that WNK1 regulates PRL expression via an alternative mechanism. Induction of the inflammatory cytokines, CCL8, IL1B, and IL15, was diminished by MAPK7 knockdown, as seen in siWNK1-treated HESCs. Similarly, MAPK7 was required for decidualization-dependent changes in the expression TGF-beta pathway genes, as MAPK7 knockdown caused decreased repression of EGR2, ITGA2, and ITGA4. MAPK7 knockdown did not have an effect on the expression of SMAD3 or ITGB3, suggesting that WNK1 represses these genes via an alternate signaling pathway. Together, these results indicated that WNK1 induces phosphorylation of MAPK7 during decidualization and that MAPK7 drives a subset of the WNK1-regulated transcriptional changes in decidual cells.
To further understand the role of MAPK7 as a downstream mediator of WNK1 during decidualization, HESC migration and proliferation were examined following MAPK7 knockdown. Similar to WNK1 knockdown, MAPK7 knockdown led to decreased migration on scratch migration assay (Figure 4E and F). Small-interfering RNA targeting MAPK7 treatment also decreased proliferation of HESCs during decidualization (Figure 4G). However, decreased proliferation in siMAPK7-treated cells was not observed at 3 days of EPC treatment. In addition, the reduction of proliferation in siMAPK7-treated cells was less pronounced than the decreased proliferation observed in siWNK1-treated cells, suggesting that alternative downstream mediators may contribute to the proproliferative effects of WNK1 in decidual cells. Nonetheless, these results indicated that WNK1 promotes the migration and proliferation of HESCs in part through MAPK7. Coupled with the WNK1-dependent activation of MAPK7 during decidualization, these results suggested that MAPK7 acts as a downstream mediator of WNK1 in decidual cells and that WNK1/MAPK7 signaling regulates decidual cell migration, proliferation, cytokine induction, and TGF-beta signaling pathway gene repression.
Discussion
Multiple decidual cell functions, including proliferation, migration, and cytokine induction, are dependent on the expression of WNK1 and MAPK7. Importantly, MAPK7 was activated during decidualization in a WNK1-dependent manner, suggesting that the WNK1/MAPK7 signaling axis that has been described in other cell types is active in endometrial stromal cells [13,16]. Knockdown of either WNK1 or MAPK7 resulted in decreased HESC migration and proliferation, highlighting the role of these proteins in supporting these functions during decidualization. Furthermore, MAPK7 regulated the expression of a subset of WNK1-regulated genes, including IGFBP1, CCL8, IL1B, IL15, EGR2, ITGA2, and ITGA4. MAPK7 likely acts as a downstream mediator of WNK1 in driving these changes in gene expression during decidualization. Like other MAPK family proteins, MAPK7 can induce transcriptional changes by phosphorylating and modulating the activity of nuclear transcription factors. Previous studies have demonstrated that MAPK7 regulates the activity of several transcription factors, including myocyte enhancer factor family member proteins, MYC proto-oncogene (c-Myc), and cAMP response element binding protein [42–46]. In addition, MAPK7 can directly regulate transcription via a transcriptional activation domain [47,48]. Thus, some of the WNK1-dependent changes in decidual gene expression may be directly regulated by MAPK7. It would be informative to study the mechanism by which MAPK7 regulates gene expression during decidualization by examining its interactions with other transcription factors and investigating its DNA-binding activity. Fully elucidating the targets of MAPK7 in endometrial stromal cells and the mechanism by which MAPK7 exerts control over these targets would further inform our understanding of the complex signaling pathways that orchestrate decidualization.
Global gene expression analysis by RNA-Seq highlighted the critical role of WNK1 in regulating diverse signaling pathways during stromal cell decidualization. Interestingly, knockdown of WNK1 resulted in dysregulation of 752 genes that are specific to the decidualization of endometrial stromal cells. WNK1 knockdown reversed the direction of change in gene expression for 80% of these overlapping genes, indicating that siWNK1-treated cells failed to undergo many of the gene expression changes that define decidualization. The regulation by WNK1 of 25% of all genes altered during decidualization further underscores the importance of WNK1 in driving the differentiation of decidual cells. Ontological analyses indicated that WNK1 regulates the expression of genes involved in diverse signaling pathways. In particular, WNK1 was required for the induction of multiple inflammatory cytokines and for the repression of several TGF-beta pathway genes.
The induction of cytokines downstream of WNK1/MAPK7 could be of critical importance during decidualization and the maintenance of pregnancy. Previous studies have demonstrated the role of secreted cytokines in promoting decidualization and modulating the recruitment of immune cells, including uNK cells and macrophages, to the decidua [5]. In particular, IL15 is known to promote the proliferation of uNK cells [49]. Knockout of Il15 in female mice leads to a lack of uNK cells and failure of spiral artery remodeling, underscoring the importance of this cytokine in the maintenance of pregnancy [50,51]. IL1B is secreted by decidual cells and preimplantation embryos and promotes both embryo implantation and stromal cell decidualization [3,52]. In baboons, IL1B promotes the secretion of matrix metalloproteases (MMPs) from the decidua, suggesting a possible role in extracellular matrix remodeling and trophoblast invasion [53]. Furthermore, IL1B promotes the expression of additional cytokines, including C-C motif chemokine ligand 2 (CCL8; previously known as SCYA2), C-C motif chemokine ligand 5 (CCL5; previously known as D17S136E, RANTES, SCYA5), C-X-C chemokine ligand 2 (CXCL2; previously known as GRO2), C-X-C chemokine ligand 3 (CXCL3; previously known as GRO3), and C-X-C chemokine ligand 8 (CXCL8; previously known as IL8), which enhance macrophage recruitment [54]. Thus, the impaired induction of these cytokines following WNK1 knockdown could result in abnormal immune cell recruitment in addition to contributing to defects in stromal cell decidualization.
The induction of TGF-beta target genes following WNK1 knockdown is consistent with previous findings [55]. WNK1 depletion in HeLa cells leads to increased SMAD2/3-dependent transcriptional activity, suggesting that WNK1 plays an inhibitory role in TGF-beta/SMAD signaling in multiple cell types [55]. In the mouse, genetically engineered models have suggested that intact TGF-beta signaling is required for uterine function during the establishment and maintenance of pregnancy [56]. Uterine deletion of transforming growth factor beta receptor 1 (Tgfbr1) in the mouse causes abnormal implantation, in addition to defects in uNK cell recruitment and spiral artery remodeling [57]. In addition, deletion of the TGF-beta pathway mediator, Smad3, leads to compromised decidualization in mice [58]. Studies utilizing human cells have demonstrated a more complex role for TGF-beta signaling in decidualization that remains to be fully elucidated. Multiple studies have shown upregulation of transforming growth factor beta 1 (TGFB1) during stromal cell decidualization [59,60]. However, treatment of human decidual cells with TGFB1 leads to repression of the progesterone receptor and attenuation of the production and secretion of IGFBP1 and PRL [61,62]. The repression of TGF-beta signaling by WNK1 could serve several functions in decidual cells, as TGF-beta regulates diverse cellular processes, including proliferation, differentiation, migration, and cell death. TGB-beta signaling antagonizes cellular proliferation in a multitude of cell types, including first trimester trophoblast cells [63,64]. The TGF-beta pathway may also repress proliferation during decidualization, which could contribute to the decreased proliferation observed following WNK1 knockdown. TGF-beta signaling may also influence the activity of immune cells in the decidua, as TGF-beta signaling can induce the differentiation of uNK cells [65].
The TGB-beta pathway is also known as a global regulator of extracellular matrix proteins. Overexpression of TGF-beta can lead to fibrosis in multiple tissue types; in the uterus, increased TGF-beta signaling has been associated with excessive extracellular matrix production in uterine fibroids [66,67]. TGF-beta signaling can regulate the expression of integrins, which are heterodimer proteins that mediate cellular adhesion by binding to ligands and proteins within the extracellular matrix. These proteins likely mediate the interaction between the implanting embryo and the decidua, and their expression is therefore tightly regulated during the window of implantation [68]. Integrin alpha-V beta-3, considered a marker of uterine receptivity, is expressed in both the endometrial epithelium and stroma [69–72]. Integrin alpha-V beta-3 is upregulated during the window of receptivity, and expression of the beta-3 component is reduced in secretory phase endometrium of women with unexplained infertility [69–72]. ITGA4 is also expressed in the endometrium and demonstrates variations in expression with the menstrual cycle [72,73]. Similar to ITGB3, decreased expression of ITGA4 has been found in women with fertility problems [69]. In trophoblast cells, siRNA knockdown of ITGA4 leads to decreased trophoblast invasion activity [74]. In vivo models also suggest a role for ITGA4 in pregnancy, as treatment with an anti-ITGA4 immunoglobulin antibody leads to decreased fertility in female guinea pigs [75]. While these studies have examined the effects of deficient integrin signaling on reproductive function, the effects of excessive integrin expression on the endometrium have yet to be investigated. Given the tight control of integrin expression that is displayed during the menstrual cycle and early pregnancy, the overexpression of multiple integrin family members following WNK1 or MAPK7 knockdown could lead to adverse effects on maternal–embryo adhesion.
The contribution of other potential downstream mediators of WNK1 signaling in endometrial stromal cells also merits further study. In functional assays, the decreases in cellular proliferation and migration following MAPK7 knockdown did not fully explain the more dramatic decreases in proliferation and migration observed following WNK1 knockdown. This observation suggests that WNK1 may activate other downstream targets in addition to MAPK7 during decidualization, which could contribute to the proproliferative and promigratory effects of WNK1. For instance, oxidative stress responsive 1 (OXSR1; previously known as OSR1) has been shown to regulate cell migration downstream of WNK1 in human umbilical vein endothelial cells [76]. Thus, it is possible that WNK1 activates both MAPK7 and OXSR1 in endometrial stromal cells to promote cell migration. Similarly, WNK1 activates the prosurvival protein, serum/glucocorticoid regulated kinase 1 (SGK1; previously known as SGK), in HEK293 cells via a phosphorylation-independent mechanism [77]. Loss of activation of SGK1, which promotes cell survival and inhibits apoptosis, might contribute to the severe decrease in proliferation observed following WNK1 knockdown [78]. Another possibility is that WNK1 may directly impact cell proliferation by regulating mitosis. In HeLa cells, WNK1 localizes to the mitotic spindle and is required for successful mitosis, indicating that WNK1 may play a direct role in permitting stromal cell proliferation [79]. In addition, the changes in PRL and SMAD3 expression that occur downstream of WNK1 are not MAPK7 dependent, suggesting that WNK1 may interact with other cellular signaling pathways to drive these transcriptional responses. The transcriptional regulation of PRL expression during decidualization has been well studied and suggests many potential nodes through which WNK1 signaling might modulate transcriptional activity. Importantly, PRL induction involves the integration of cAMP and progesterone signals to culminate in the expression of this decidual marker gene [3]. PRL is induced downstream of protein kinase A (PKA) signaling, in response to CCAAT/enhancer binding protein (C/EBP) activity [80]. Progesterone agonists act synergistically to enhance PRL expression in the context of elevated cAMP, as unliganded PR transcriptionally represses PRL [29]. The induction of PRL requires additional transcription factors, including homeobox A11 (HOXA11; previously known as HOX1, HOX1I) and forkhead box O1 (FOXO1; previously known as FKHR, FOXO1A). HOXA11, which represses PRL transcription in the absence of the decidual stimulus, becomes a PRL inducer when co-expressed with FOXO1 [81]. This complex regulatory system suggests multiple signaling nodes that might be modulated downstream of WNK1 signaling, independent of MAPK7. For instance, SGK1, a downstream target of WNK1 in HEK293 cells, is known to phosphorylate and induce nuclear export of FOXO1 [82]. However, overexpression of SGK1 represses the induction of PRL as a consequence of the nuclear export of FOXO1 [82]. Thus, this signaling pathway cannot explain the abrogation of PRL induction following WNK1 knockdown. It is possible that WNK1 might activate an intermediate involved in the transduction of PKA signaling, or modulate the activities of the C/EBP family, HOXA11, or FOXO1 transcription factors, although these possibilities remain to be investigated. Interactions between WNK1 signaling and these transcription factors would likely have profound effects on decidualization and female fertility, underscoring the importance of fully elucidating the complex signaling pathways that regulate stromal cell decidualization.
The functions of WNK1 and MAPK7 on female fertility and the maintenance of pregnancy also warrant further investigation. The interaction between WNK1/MAPK7 signaling in decidual cells and signaling pathways in other tissue compartments could inform our understanding of the molecular regulators that orchestrate embryo implantation, decidualization, and placentation. In particular, WNK1 and MAPK7 signaling may contribute to the regulation of trophoblast invasion and maternal vascular remodeling by decidual cells. WNK1 and MAPK7 were required for the induction of multiple cytokines that are known to regulate trophoblast invasion in vitro. IL1B enhances the invasion of cultured trophoblasts by inducing the expression of MMPs. In addition, IL-15 induces matrix metallopeptidase 1 (MMP1) expression in human choriocarcinoma cell lines and exhibits aberrant expression in patients with recurrent miscarriage [6]. Failure to induce these cytokines could also lead to reduced recruitment of uNK cells and subsequent defects in spiral artery remodeling. Furthermore, the aberrant expression of integrins in the absence of WNK1 signaling could lead to abnormal adhesion between the implanting embryo and maternal decidua. These alterations in the extracellular matrix of the decidua could also contribute to aberrant invasion by trophoblast cells. The decreased migratory capacity of decidual cells following WNK1 or MAPK7 knockdown might prove detrimental to the establishment of pregnancy and subsequent trophoblast invasion. Decidual cells are actively invasive and exhibit increased migration in response to blastocyst implantation, suggesting a possible role for decidual cells in surrounding the implanting blastocyst [7]. In co-culture models, decidual cells contribute to the formation of multicellular bridges of decidual and trophoblast cells, indicating that the migratory capacity of decidual cells may play a role in trophoblast invasion [8]. Thus, WNK1 and MAPK7 may contribute to the critical role of decidual cells in balancing trophoblast invasion during the establishment of pregnancy.
These findings may also inform our understanding of uterine receptivity and clinical assessments of endometrial function. The critical roles of WNK1 and MAPK7 during decidualization suggest that these proteins are required for proper endometrial function during early pregnancy. In the future, assessments of WNK1 and MAPK7 phosphorylation could be used to evaluate endometrial function. Pharmacological modulation of WNK1 or MAPK7 activity might be employed to enhance or inhibit female fertility. WNK1, in particular, has the potential to be modulated by highly specific activating or inhibitory drugs due to the unique structure of its kinase domain [83]. More generally, this work highlights the importance of kinase signaling in regulating endometrial function. While many studies have characterized the transcriptional signatures that define the window of receptivity, many questions remain regarding the expression and post-translational modification status of important signaling proteins at this critical time period [84–89]. Both WNK1 and MAPK7 do not display alterations at the level of mRNA expression during decidualization despite their essential role in driving this differentiation process. Thus, future studies of the protein expression and post-translational modification status of signaling proteins in the endometrium will be highly informative. In conclusion, the findings that WNK1/MAPK7 signaling regulates decidual cell proliferation, migration, cytokine induction, and TGF-beta pathway gene repression underscore the critical importance of kinase signaling activity in coordinating the decidual transformation and highlight the necessity of investigating kinase signaling pathways as orchestrators of the molecular changes that establish and maintain pregnancy.
Supplementary Material
Acknowledgments
The authors thank Jian Liu, Margeaux Wetendorf, San-pin Wu, and Sylvia Hewitt for technical assistance. This work was made possible by the Genomic and RNA Profiling Core and the Integrated Microscopy Core Lab at Baylor College of Medicine.
Notes
Grant support: This research was supported by the Intramural Research Program of the National Institute of Environmental Health Sciences: Z1AES103311-01 (FJD).
Supplementary data
Supplementary Table S1. Sequences of forward and reverse primers for RT-qPCR. Nucleotide sequences are given 5’ to 3’.
Supplementary Table S2. Primary antibodies used for western blot.
Supplementary Table S3. List of 1858 genes differentially regulated by WNK1 knockdown. Genes are listed with fold change in mRNA expression relative to EPC siNT-treated HESCs (given as log2[fold change]) and FDR-adjusted P-value.
Supplementary Table S4. Enriched GO term clusters generated by DAVID. GO term clusters are listed along with all associated enriched GO terms and enrichment statistics.
Supplementary Table S5. WNK1-regulated genes associated with each enriched GO term listed in Supplementary Table S5.
Supplementary Table S6. List of 752 genes differentially regulated by decidualization (EPC RNA-Seq) and WNK1 knockdown (WNK1 RNA-Seq). Data from EPC RNA-Seq are presented as fold change in mRNA expression relative to Veh siNT-treated HESCs (given as log2[fold change]). Data from WNK1 RNA-Seq are presented as fold change in mRNA expression relative to EPC siNT-treated HESCs (given as log2[fold change]). FDR-adjusted P-values from each dataset are provided.
Supplementary Table S7. WNK1-regulated genes associated with IPA upstream regulator pathways. Upstream regulator pathways are listed with pathway enrichment statistics, activation Z-scores, and associated genes from the list of 752 genes regulated by WNK1 and decidualization.
References
- 1. Wang W, Li Q, Bagchi IC, Bagchi MK. The CCAAT/enhancer binding protein beta is a critical regulator of steroid-induced mitotic expansion of uterine stromal cells during decidualization. Endocrinology 2010; 151:3929–3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med 2012; 18:1754–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gellersen B, Brosens JJ. Cyclic decidualization of the human endometrium in reproductive health and failure. Endocr Rev 2014; 35:851–905. [DOI] [PubMed] [Google Scholar]
- 4. Lane B, Oxberry W, Mazella J, Tseng L. Decidualization of human endometrial stromal cells in vitro: effects of progestin and relaxin on the ultrastructure and production of decidual secretory proteins. Hum Reprod 1994; 9:259–266. [DOI] [PubMed] [Google Scholar]
- 5. Vinketova K, Mourdjeva M, Oreshkova T. Human decidual stromal cells as a component of the implantation niche and a modulator of maternal immunity. J Pregnancy 2016; 2016:8689436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharma S, Godbole G, Modi D. Decidual control of trophoblast invasion. Am J Reprod Immunol 2016; 75:341–350. [DOI] [PubMed] [Google Scholar]
- 7. Grewal S, Carver JG, Ridley AJ, Mardon HJ. Implantation of the human embryo requires Rac1-dependent endometrial stromal cell migration. Proc Natl Acad Sci USA 2008; 105:16189–16194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gellersen B, Reimann K, Samalecos A, Aupers S, Bamberger AM. Invasiveness of human endometrial stromal cells is promoted by decidualization and by trophoblast-derived signals. Hum Reprod 2010; 25:862–873. [DOI] [PubMed] [Google Scholar]
- 9. Fabi F, Asselin E. Expression, activation, and role of AKT isoforms in the uterus. Reproduction 2014; 148:R85–R95. [DOI] [PubMed] [Google Scholar]
- 10. Lee CH, Kim TH, Lee JH, Oh SJ, Yoo JY, Kwon HS, Kim YI, Ferguson SD, Ahn JY, Ku BJ, Fazleabas AT, Lim JM et al. . Extracellular signal-regulated kinase 1/2 signaling pathway is required for endometrial decidualization in mice and human. PLoS One 2013; 8:e75282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Large MJ, Wetendorf M, Lanz RB, Hartig SM, Creighton CJ, Mancini MA, Kovanci E, Lee KF, Threadgill DW, Lydon JP, Jeong JW, DeMayo FJ. The epidermal growth factor receptor critically regulates endometrial function during early pregnancy. PLoS Genet 2014; 10:e1004451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu B, English JM, Wilsbacher JL, Stippec S, Goldsmith EJ, Cobb MH. WNK1, a novel mammalian serine/threonine protein kinase lacking the catalytic lysine in subdomain II. J Biol Chem 2000; 275:16795–16801. [DOI] [PubMed] [Google Scholar]
- 13. Xu BE, Stippec S, Lenertz L, Lee BH, Zhang W, Lee YK, Cobb MH. WNK1 activates ERK5 by an MEKK2/3-dependent mechanism. J Biol Chem 2004; 279:7826–7831. [DOI] [PubMed] [Google Scholar]
- 14. Cheng CJ, Huang CL. Activation of PI3-kinase stimulates endocytosis of ROMK via Akt1/SGK1-dependent phosphorylation of WNK1. J Am Soc Nephrol 2011; 22:460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fulford L, Milewski D, Ustiyan V, Ravishankar N, Cai Y, Le T, Masineni S, Kasper S, Aronow B, Kalinichenko VV, Kalin TV. The transcription factor FOXF1 promotes prostate cancer by stimulating the mitogen-activated protein kinase ERK5. Sci Signal 2016; 9:ra48. [DOI] [PubMed] [Google Scholar]
- 16. Sun X, Gao L, Yu RK, Zeng G. Down-regulation of WNK1 protein kinase in neural progenitor cells suppresses cell proliferation and migration. J Neurochem 2006; 99:1114–1121. [DOI] [PubMed] [Google Scholar]
- 17. Shyamasundar S, Lim JP, Bay BH. miR-93 inhibits the invasive potential of triple-negative breast cancer cells in vitro via protein kinase WNK1. Int J Oncol 2016; 49:2629–2636. [DOI] [PubMed] [Google Scholar]
- 18. Tsuboi M, Taniuchi K, Furihata M, Naganuma S, Kimura M, Watanabe R, Shimizu T, Saito M, Dabanaka K, Hanazaki K, Saibara T. Vav3 is linked to poor prognosis of pancreatic cancers and promotes the motility and invasiveness of pancreatic cancer cells. Pancreatology 2016; 16:905–916. [DOI] [PubMed] [Google Scholar]
- 19. Ruan HY, Yang C, Tao XM, He J, Wang T, Wang H, Wang C, Jin GZ, Jin HJ, Qin WX. Downregulation of ACSM3 promotes metastasis and predicts poor prognosis in hepatocellular carcinoma. Am J Cancer Res 2017; 7:543–553. [PMC free article] [PubMed] [Google Scholar]
- 20. Zhu W, Begum G, Pointer K, Clark PA, Yang SS, Lin SH, Kahle KT, Kuo JS, Sun D. WNK1-OSR1 kinase-mediated phospho-activation of Na+-K+-2Cl- cotransporter facilitates glioma migration. Mol Cancer 2014; 13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xie J, Wu T, Xu K, Huang IK, Cleaver O, Huang CL. Endothelial-specific expression of WNK1 kinase is essential for angiogenesis and heart development in mice. Am J Pathol 2009; 175:1315–1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Delaloy C, Hadchouel J, Imbert-Teboul M, Clemessy M, Houot AM, Jeunemaitre X. Cardiovascular expression of the mouse WNK1 gene during development and adulthood revealed by a BAC reporter assay. Am J Pathol 2006; 169:105–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kochl R, Thelen F, Vanes L, Brazao TF, Fountain K, Xie J, Huang CL, Lyck R, Stein JV, Tybulewicz VL. WNK1 kinase balances T cell adhesion versus migration in vivo. Nat Immunol 2016; 17:1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ramsay AK, McCracken SR, Soofi M, Fleming J, Yu AX, Ahmad I, Morland R, Machesky L, Nixon C, Edwards DR, Nuttall RK, Seywright M et al. . ERK5 signalling in prostate cancer promotes an invasive phenotype. Br J Cancer 2011; 104:664–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim SM, Lee H, Park YS, Lee Y, Seo SW. ERK5 regulates invasiveness of osteosarcoma by inducing MMP-9. J Orthop Res 2012; 30:1040–1044. [DOI] [PubMed] [Google Scholar]
- 26. Shukla A, Miller JM, Cason C, Sayan M, MacPherson MB, Beuschel SL, Hillegass J, Vacek PM, Pass HI, Mossman BT. Extracellular signal-regulated kinase 5: a potential therapeutic target for malignant mesotheliomas. Clin Cancer Res 2013; 19:2071–2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yan L, Carr J, Ashby PR, Murry-Tait V, Thompson C, Arthur JS. Knockout of ERK5 causes multiple defects in placental and embryonic development. BMC Dev Biol 2003; 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jiang S, Zhang D, Huang H, Lei Y, Han Y, Han W. Extracellular signal-regulated kinase 5 is required for low-concentration H2O2-induced angiogenesis of human umbilical vein endothelial cells. Biomed Res Int 2017; 2017:6895730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brosens JJ, Hayashi N, White JO. Progesterone receptor regulates decidual prolactin expression in differentiating human endometrial stromal cells. Endocrinology 1999; 140:4809–4820. [DOI] [PubMed] [Google Scholar]
- 30. Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 2009; 25:1105–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Anders S, Pyl PT, Huber W. HTSeq–a Python framework to work with high-throughput sequencing data. Bioinformatics 2015; 31:166–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010; 26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mazur EC, Vasquez YM, Li X, Kommagani R, Jiang L, Chen R, Lanz RB, Kovanci E, Gibbons WE, DeMayo FJ. Progesterone receptor transcriptome and cistrome in decidualized human endometrial stromal cells. Endocrinology 2015; 156:2239–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 2009; 4:44–57. [DOI] [PubMed] [Google Scholar]
- 35. Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 2009; 37:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kramer A, Green J, Pollard J Jr, Tugendreich S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014; 30:523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001; 25:402–408. [DOI] [PubMed] [Google Scholar]
- 38. Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc 2007; 2:329–333. [DOI] [PubMed] [Google Scholar]
- 39. Daly DC, Maslar IA, Riddick DH. Prolactin production during in vitro decidualization of proliferative endometrium. Am J Obstet Gynecol 1983; 145:672–678. [DOI] [PubMed] [Google Scholar]
- 40. Giudice LC, Dsupin BA, Irwin JC. Steroid and peptide regulation of insulin-like growth factor-binding proteins secreted by human endometrial stromal cells is dependent on stromal differentiation. J Clin Endocrinol Metab 1992; 75:1235–1241. [DOI] [PubMed] [Google Scholar]
- 41. Kondoh K, Terasawa K, Morimoto H, Nishida E. Regulation of nuclear translocation of extracellular signal-regulated kinase 5 by active nuclear import and export mechanisms. Mol Cell Biol 2006; 26:1679–1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kato Y, Kravchenko VV, Tapping RI, Han JH, Ulevitch RJ, Lee JD. BMK1/ERK5 regulates serum-induced early gene expression through transcription factor MEF2C. EMBO J 1997; 16:7054–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kato Y, Zhao M, Morikawa A, Sugiyama T, Chakravortty D, Koide N, Yoshida T, Tapping RI, Yang Y, Yokochi T, Lee JD. Big mitogen-activated kinase regulates multiple members of the MEF2 protein family. J Biol Chem 2000; 275:18534–18540. [DOI] [PubMed] [Google Scholar]
- 44. Yang CC, Ornatsky OI, McDermott JC, Cruz TF, Prody CA. Interaction of myocyte enhancer factor 2 (MEF2) with a mitogen-activated protein kinase, ERK5/BMK1. Nucleic Acids Res 1998; 26:4771–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. English JM, Pearson G, Baer R, Cobb MH. Identification of substrates and regulators of the mitogen-activated protein kinase ERK5 using chimeric protein kinases. J Biol Chem 1998; 273:3854–3860. [DOI] [PubMed] [Google Scholar]
- 46. Finegan KG, Wang X, Lee EJ, Robinson AC, Tournier C. Regulation of neuronal survival by the extracellular signal-regulated protein kinase 5. Cell Death Differ 2009; 16:674–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kasler HG, Victoria J, Duramad O, Winoto A. ERK5 is a novel type of mitogen-activated protein kinase containing a transcriptional activation domain. Mol Cell Biol 2000; 20:8382–8389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morimoto H, Kondoh K, Nishimoto S, Terasawa K, Nishida E. Activation of a C-terminal transcriptional activation domain of ERK5 by autophosphorylation. J Biol Chem 2007; 282:35449–35456. [DOI] [PubMed] [Google Scholar]
- 49. Kitaya K, Yasuda J, Yagi I, Tada Y, Fushiki S, Honjo H. IL-15 expression at human endometrium and decidua. Biol Reprod 2000; 63:683–687. [DOI] [PubMed] [Google Scholar]
- 50. Ashkar AA, Black GP, Wei Q, He H, Liang L, Head JR, Croy BA. Assessment of requirements for IL-15 and IFN regulatory factors in uterine NK cell differentiation and function during pregnancy. J Immunol 2003; 171:2937–2944. [DOI] [PubMed] [Google Scholar]
- 51. Barber EM, Pollard JW. The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J Immunol 2003; 171:37–46. [DOI] [PubMed] [Google Scholar]
- 52. Tierney EP, Tulac S, Huang ST, Giudice LC. Activation of the protein kinase A pathway in human endometrial stromal cells reveals sequential categorical gene regulation. Physiol Genomics 2003; 16:47–66. [DOI] [PubMed] [Google Scholar]
- 53. Mori M, Bogdan A, Balassa T, Csabai T, Szekeres-Bartho J. The decidua-the maternal bed embracing the embryo-maintains the pregnancy. Semin Immunopathol 2016; 38:635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Krikun G, Lockwood CJ. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol 2006; 72:60–73. [DOI] [PubMed] [Google Scholar]
- 55. Lee BH, Chen W, Stippec S, Cobb MH. Biological cross-talk between WNK1 and the transforming growth factor beta-Smad signaling pathway. J Biol Chem 2007; 282:17985–17996. [DOI] [PubMed] [Google Scholar]
- 56. Monsivais D, Matzuk MM, Pangas SA. The TGF-beta family in the reproductive tract. Cold Spring Harb Perspect Biol 2017. Advance online publication. doi:10.1101/cshperspect.a022251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Peng J, Monsivais D, You R, Zhong H, Pangas SA, Matzuk MM. Uterine activin receptor-like kinase 5 is crucial for blastocyst implantation and placental development. Proc Natl Acad Sci USA 2015; 112:E5098–E5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Zhao KQ, Lin HY, Zhu C, Yang X, Wang H. Maternal Smad3 deficiency compromises decidualization in mice. J Cell Biochem 2012; 113:3266–3275. [DOI] [PubMed] [Google Scholar]
- 59. Popovici RM, Kao LC, Giudice LC. Discovery of new inducible genes in in vitro decidualized human endometrial stromal cells using microarray technology. Endocrinology 2000; 141:3510–3513. [DOI] [PubMed] [Google Scholar]
- 60. Stoikos CJ, Harrison CA, Salamonsen LA, Dimitriadis E. A distinct cohort of the TGFbeta superfamily members expressed in human endometrium regulate decidualization. Hum Reprod 2008; 23:1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kane NM, Jones M, Brosens JJ, Kelly RW, Saunders PT, Critchley HO. TGFbeta1 attenuates expression of prolactin and IGFBP-1 in decidualized endometrial stromal cells by both SMAD-dependent and SMAD-independent pathways. PLoS One 2010; 5:e12970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kane N, Jones M, Brosens JJ, Saunders PT, Kelly RW, Critchley HO. Transforming growth factor-beta1 attenuates expression of both the progesterone receptor and Dickkopf in differentiated human endometrial stromal cells. Mol Endocrinol 2008; 22:716–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Graham CH, Lysiak JJ, McCrae KR, Lala PK. Localization of transforming growth factor-β at the human fetal-maternal interface: role in trophoblast growth and differentiation1. Biol Reprod 1992; 46:561–572. [DOI] [PubMed] [Google Scholar]
- 64. Massagué J, Blain SW, Lo RS. TGFβ signaling in growth control, cancer, and heritable disorders. Cell 2000; 103:295–309. [DOI] [PubMed] [Google Scholar]
- 65. Keskin DB, Allan DS, Rybalov B, Andzelm MM, Stern JN, Kopcow HD, Koopman LA, Strominger JL. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16- NK cells with similarities to decidual NK cells. Proc Natl Acad Sci USA 2007; 104:3378–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Arici A, Sozen I. Transforming growth factor-β3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril 2000; 73:1006–1011. [DOI] [PubMed] [Google Scholar]
- 67. Norian JM, Malik M, Parker CY, Joseph D, Leppert PC, Segars JH, Catherino WH. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci 2009; 16:1153–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Sueoka K, Shiokawa S, Miyazaki T, Kuji N, Tanaka M, Yoshimura Y. Integrins and reproductive physiology: expression and modulation in fertilization, embryogenesis, and implantation. Fertil Steril 1997; 67:799–811. [DOI] [PubMed] [Google Scholar]
- 69. Gonzalez RR, Palomino A, Boric A, Vega M, Devoto L. A quantitative evaluation of α1, α4, αV and β3 endometrial integrins of fertile and unexplained infertile women during the menstrual cycle. A flow cytometric appraisal. Hum Reprod 1999; 14:2485–2492. [DOI] [PubMed] [Google Scholar]
- 70. Lessey BA, Castelbaum AJ, Sawin SW, Sun J. Integrins as markers of uterine receptivity in women with primary unexplained infertility. Fertil Steril 1995; 63:535–542. [PubMed] [Google Scholar]
- 71. Germeyer A, Savaris RF, Jauckus J, Lessey B. Endometrial beta3 integrin profile reflects endometrial receptivity defects in women with unexplained recurrent pregnancy loss. Reprod Biol Endocrinol 2014; 12:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Lessey BA, Castelbaum AJ, Buck CA, Lei Y, Yowell CW, Sun J. Further characterization of endometrial integrins during the menstrual cycle and in pregnancy. Fertil Steril 1994; 62:497–506. [PubMed] [Google Scholar]
- 73. Tabibzadeh S. Patterns of expression of integrin molecules in human endometrium throughout the menstrual cycle. Hum Reprod 1992; 7:876–882. [DOI] [PubMed] [Google Scholar]
- 74. Na KH, Lee HJ, Choi JH, Eun JW, Nam SW, Yoon TK, Kim GJ. Dynamic alterations in integrin alpha4 expression by hypoxia are involved in trophoblast invasion during early implantation. J Cell Biochem 2012; 113:685–694. [DOI] [PubMed] [Google Scholar]
- 75. Wehner NG, Skov M, Shopp G, Rocca MS, Clarke J. Effects of natalizumab, an alpha4 integrin inhibitor, on fertility in male and female guinea pigs. Birth Defects Res B Dev Reprod Toxicol 2009; 86:108–116. [DOI] [PubMed] [Google Scholar]
- 76. Dbouk HA, Weil LM, Perera GK, Dellinger MT, Pearson G, Brekken RA, Cobb MH. Actions of the protein kinase WNK1 on endothelial cells are differentially mediated by its substrate kinases OSR1 and SPAK. Proc Natl Acad Sci USA 2014; 111:15999–16004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xu BE, Stippec S, Lazrak A, Huang CL, Cobb MH. WNK1 activates SGK1 by a phosphatidylinositol 3-kinase-dependent and non-catalytic mechanism. J Biol Chem 2005; 280:34218–34223. [DOI] [PubMed] [Google Scholar]
- 78. Brunet A, Park J, Tran H, Hu LS, Hemmings BA, Greenberg ME. Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol Cell Biol 2001; 21:952–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tu SW, Bugde A, Luby-Phelps K, Cobb MH. WNK1 is required for mitosis and abscission. Proc Natl Acad Sci USA 2011; 108:1385–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Telgmann R, Gellersen B. Marker genes of decidualization: activation of the decidual prolactin gene. Hum Reprod Update 1998; 4:472–479. [DOI] [PubMed] [Google Scholar]
- 81. Lynch VJ, Brayer K, Gellersen B, Wagner GP. HoxA-11 and FOXO1A cooperate to regulate decidual prolactin expression: towards inferring the core transcriptional regulators of decidual genes. PLoS One 2009; 4:e6845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Feroze-Zaidi F, Fusi L, Takano M, Higham J, Salker MS, Goto T, Edassery S, Klingel K, Boini KM, Palmada M, Kamps R, Groothuis PG et al. . Role and regulation of the serum- and glucocorticoid-regulated kinase 1 in fertile and infertile human endometrium. Endocrinology 2007; 148:5020–5029. [DOI] [PubMed] [Google Scholar]
- 83. McCormick JA, Ellison DH. The WNKs: atypical protein kinases with pleiotropic actions. Physiol Rev 2011; 91:177–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Carson DD, Lagow E, Thathiah A, Al-Shami R, Farach-Carson MC, Vernon M, Yuan L, Fritz MA, Lessey B. Changes in gene expression during the early to mid-luteal (receptive phase) transition in human endometrium detected by high-density microarray screening. Mol Hum Reprod 2002; 8:871–879. [DOI] [PubMed] [Google Scholar]
- 85. Riesewijk A, Martin J, van Os R, Horcajadas JA, Polman J, Pellicer A, Mosselman S, Simon C. Gene expression profiling of human endometrial receptivity on days LH+2 versus LH+7 by microarray technology. Mol Hum Reprod 2003; 9:253–264. [DOI] [PubMed] [Google Scholar]
- 86. Horcajadas JA, Riesewijk A, Martin J, Cervero A, Mosselman S, Pellicer A, Simon C. Global gene expression profiling of human endometrial receptivity. J Reprod Immunol 2004; 63:41–49. [DOI] [PubMed] [Google Scholar]
- 87. Ponnampalam AP, Weston GC, Susil B, Rogers PA. Molecular profiling of human endometrium during the menstrual cycle. Aust N Z J Obstet Gynaecol 2006; 46:154–158. [DOI] [PubMed] [Google Scholar]
- 88. Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, Nayak NR, Giudice LC. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology 2006; 147:1097–1121. [DOI] [PubMed] [Google Scholar]
- 89. Garrido-Gomez T, Ruiz-Alonso M, Blesa D, Diaz-Gimeno P, Vilella F, Simon C. Profiling the gene signature of endometrial receptivity: clinical results. Fertil Steril 2013; 99:1078–1085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.