Abstract
Objectives
To assess the prevalence of antiepileptic drug (AED) exposure in pregnant women and the comparative risk of terminations of pregnancy (TOPs), spontaneous abortions, stillbirths, major birth defects (MBDs), neonatal distress and small for gestational age (SGA) infants following intrauterine AED exposure in the Emilia Romagna region, Italy (4 459 246 inhabitants on 31 December 2011).
Methods
We identified all deliveries and hospitalised abortions in Emilia Romagna in the period 2009–2011 from the certificate of delivery assistance registry (Certificato di Assistenza al Parto— CedAP) and the hospital discharge card registry, exposure to AEDs from the reimbursed drug prescription registries, MBDs from the regional registry of congenital malformations, and Apgar scores and cases of SGA from the CedAP. Records from different registries were linked.
Results
We identified 145 243 pregnancies: 111 284 deliveries, 16 408 spontaneous abortions and 17 551 TOPs. Six hundred and eleven pregnancies (0.42%; 95% Cl 0.39 to 0.46) were exposed to AEDs. In the AED-exposed group 21% of pregnancies ended in TOPs vs 12% in the non-exposed women (OR: 2.24; 95% CI 1.41 to 3.56). Rates of spontaneous abortions, stillbirths, neonatal distress and SGA were comparable. Three hundred and fifty-three babies (0.31%; 95% CI 0.28 to 0.35) were exposed to AEDs during the first trimester. MBD rates were 2.3% in the exposed vs 2.0% in the non-exposed pregnancies (OR: 1.12, 95% CI 0.55 to 2.55).
Conclusion
The Emilia Romagna prevalence of AED exposure in pregnancy was 0.42%, comparable with previous European studies. Rates of spontaneous abortions, stillbirths, neonatal distress, SGA and MBDs following AED exposure were not significantly increased. The rate of TOPs was significantly higher in the AED-exposed women.
Keywords: epilepsy, anticonvulsants, clinical neurology, pharmacology, neuropharmacology
Introduction
The reported rate of antiepileptic drug (AED) use in pregnant women is 0.2%–2.2%, with a possible rising trend due to the increasing use of these medications for indications other than epilepsy.1–7 Exposure to older generation AEDs during the first trimester has been consistently associated with an approximately twofold to threefold increased risk of major birth defects (MBDs),8 whereas data on most of the newer AEDs are still insufficient. A slightly higher risk of being small for gestational age (SGA) or low birth weight has been reported in AED-exposed babies, but not consistently.5 9–15 Although several studies have reported no increase in spontaneous abortions in women with epilepsy,16 17 others have found a higher frequency associated with AED therapy and a family history of MBDs.18 19 Data on stillbirths in women with epilepsy range from an incidence comparable with that of the general population20 to a twofold increase.21 A population study found that preterm deliveries were more common in women using AEDs, but only for indications other than epilepsy.5 Although the risk of adverse fetal events appears to be linked primarily to the use of AEDs, the indication of use could therefore play a role.22 Few data are available on induced abortions in women with epilepsy or exposed to AEDs.17
Only very rarely can therapy be withdrawn for pregnancy in epilepsy and psychiatric disorders, making it essential to choose the safest substances for the woman and her offspring. Currently the main sources of data on AED safety in pregnancy are pregnancy registries. Although these registries have led to a vast increase in the information available on the topic, they have several selection biases, being based on voluntary enrolment and including mainly women with epilepsy, mostly lacking comparison with an AED-unexposed population. Population-based studies can address many of these limits, offering a major complementary source of information. Since 2010 several population-based studies have been published mainly from northern Europe, while data from other countries are scant.
We performed a retrospective observational population-based study in an Italian region to assess AED exposure and safety in pregnancy.
Methods
Ethical requirements
To protect patient privacy, all data were anonymised at regional level by the data owner in accordance with Italian legislation.
Study population
We identified all women residents in Emilia Romagna, a northern Italian region of 4 459 246 inhabitants on 31 December 2011,23 who had a delivery or underwent an abortion in a hospital in Emilia Romagna, between 1 January 2009 and 31 December 2011. The women were identified using two administrative databases: the certificate of delivery assistance (Certificato di Assistenza al Parto—CedAP) and the hospital discharge card (Scheda di Dimissione Ospedaliera (SDO)). The CedAP collects all deliveries including stillbirths, defined as fetal deaths after the 23rd week of gestation, and covered >99% of births during the studied period. The SDO collects the International Classification of Diseases (ICD-9) codes of all discharge diagnoses from hospitals, including spontaneous abortion, covering approximately all abortions occurring in inpatients and terminations of pregnancy (TOPs), covering 64.6% of TOPs (the incomplete coverage being due to adjustment to a new national privacy policy during the study period). Both databases identify the subject by a unique anonymous code allowing the information to be linked with the other regional administrative databases.
Exposure to AEDs
In Italy, AEDs are reimbursed by the National Health Service. All prescriptions are recorded in prescription databases, which in Emilia Romagna have had complete coverage since 2009. All prescriptions for the following AEDs were selected: phenobarbital, primidone, phenytoin, ethosuximide, clonazepam, carbamazepine, oxcarbazepine, rufinamide, valproic acid, vigabatrin, tiagabine, lamotrigine, felbamate, topiramate, gabapentin, levetiracetam, zonisamide, pregabalin and lacosamide. For the deliveries cohort, two exposure periods were considered based on the date of delivery and gestational age: the pregnancy period and the first trimester. For the abortions cohort, the trimester preceding the event was fixed by consensus among the authors as the AED exposure period in pregnancy, the gestational age being unavailable. For each woman, the average daily dose was calculated as the ratio between the total amount of drug prescribed during the observation period and the number of observed days.
Stratification according to clinical indication
As the prescription databases in Italy do not contain information on clinical indications, this was assessed using an algorithm designed and validated ad hoc for AED prescriptions (methods described elsewhere).24 This tool distinguishes between women receiving AEDs for epilepsy and those taking AEDs for psychiatric disorders, by far the most common indications for the use of AEDs in pregnancy, using exclusively AED prescriptions.
Identification of MBDs
We identified all the MBDs reported to the Emilia Romagna Registry of Congenital Malformations (IMER), which covers >95% of all births including stillbirths of 26 weeks or more. Reporting is made by paediatricians during the first week of the infant’s life. A further contribution to the registry, consisting of MBDs detected during the first year of life, was taken directly from the hospital discharge cards. Each case is coded using a British Paediatric Association modification of the WHO’s ICD system.25 IMER records were linked to the CedAP, and consequently to all the other records of the mothers, using the following information: place of birth, child’s date of birth, maternal date of birth and maternal residence.
Infant health
Cases of SGA infants and Apgar scores were taken from the CedAP. SGA was defined as birth weight below the 10th percentile of the gender-specific birth weight for gestational age reference curves of the Italian population.26 An Apgar score between 1 and 3 was considered to indicate severe fetal distress, while an Apgar score between 4 and 6 indicated moderate distress. The exposure period considered was the entire pregnancy.
Identification of confounding factors
The following factors were considered confounders for TOPs: maternal age (drawn from the SDO), and prescriptions for drugs belonging to the Food and Drug Administration pregnancy categories X (contraindicated in pregnancy) and D (positive evidence of risk) (from the prescription registries). Educational level and smoking habit were unavailable for this population.
The following factors were considered confounders for MBDs: maternal age, educational level, smoking habit (from the CedAP), and maternal and fetal diseases that could affect pregnancy outcome (from diagnosis recorded in SDO database or prescriptions as disease proxies: hypertension, eclampsia and pre-eclampsia, diabetes, familial genetic disease, presumed fetal lesion from viral or other kind of maternal disease, and all antihypertensive and antidiabetic drugs).
The following factors were considered confounders for SGA: maternal age and smoking habit, twin pregnancy (from the CedAP), hypertension and diabetes.
Statistical analysis
Daily dose of AED was presented as mean (SD) and median (IQR). Categorical variables were presented as absolute (n) and relative frequency (%).
Fisher’s exact test was use to evaluate the relation between potential confounders, exposures and outcomes (data not shown). Univariate and multivariate logistic regression analyses, adjusted for confounders, were performed to study the association between outcomes (TOPs, MBDs, SGA, Apgar score) and exposure to AEDs in two periods (pregnancy and first trimester), using the variable as dichotomic and categorical (no AED, one AED, more AEDs). The results are presented as OR and 95% CI. Statistical analysis was performed using the statistical package Stata SE V.14.2.
Results
Population characteristics
Our study included 145 243 pregnancies: 111 284 deliveries (278 stillbirths), 16 408 spontaneous abortions and 17 551 TOPs. Out of all deliveries, 2516 (2.2%), corresponding to 2555 newborns, were excluded because of non-univocal registry linkage.
Exposure to AEDs
Six hundred and eleven women (0.42%; 95% CI 0.39 to 0.46) were exposed to AEDs during pregnancy: 537 to one AED, 74 to more than one. The average daily doses of the active substances are listed in table 1. No woman was exposed to rufinamide, tiagabine, felbamate or lacosamide. The mean age was 32.5 years (range 12–54) vs 31.9 (16–48) of the non-exposed.
Table 1.
Daily doses of antiepileptic drugs during the first trimester or trimester preceding abortion (mg/day)
| Active substance | Mean | SD | Median | First quartile | Third quartile | n |
| Phenobarbital | 97.2 | 43.4 | 94.4 | 66.7 | 133.3 | 44 |
| Clonazepam | 1.1 | 1.4 | 0.6 | 0.3 | 1.4 | 85 |
| Carbamazepine | 540.6 | 370.2 | 444.4 | 266.7 | 800.0 | 120 |
| Oxcarbazepine | 639.8 | 443.3 | 500.0 | 333.3 | 666.7 | 31 |
| Valproate | 508.8 | 385.4 | 366.7 | 200.0 | 666.7 | 116 |
| Valpromide | 800.0 | 565.7 | 800.0 | 400.0 | 1200 | 2 |
| Lamotrigine | 214.1 | 222.3 | 124.5 | 62.2 | 248.9 | 99 |
| Topiramate | 136.7 | 188.1 | 66.7 | 33.3 | 133.3 | 30 |
| Levetiracetam | 1556 | 1026 | 1333 | 1000 | 2000 | 33 |
| Zonisamide | 118.6 | 68.8 | 132.2 | 73.9 | 163.3 | 4 |
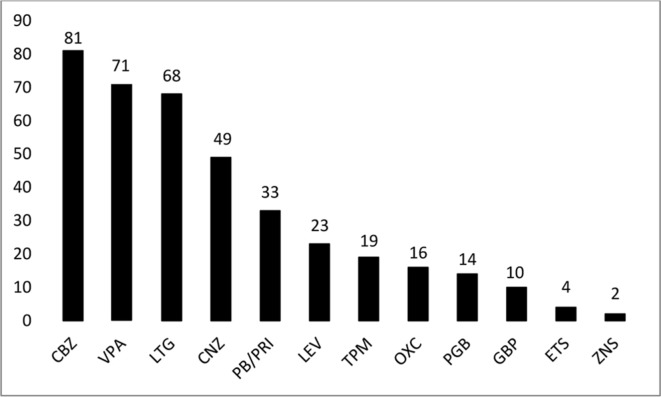
Three hundred and fifty three babies (0.31%; 95% CI 0.28% to 0.35%) were exposed to AEDs during the first trimester: 321 to one AED, 32 to more than one. The proportion of newborns exposed to each active substance, in mono or polytherapy, is shown in figure 1. According to the algorithm, the maternal indication for AEDs was epilepsy in 295 cases (83.6%).
Figure 1.
Exposure to antiepileptic drugs in the first trimester (on the top of each column the number of exposed newborns is reported). CBZ, carbamazepine; CNZ, clonazepam; ETS, ethosuximide; GBP, gabapentin; LEV, levetiracetam; LTG, lamotrigine; OXC, oxcarbazepine; PB/PRI, phenobarbital/primidone; PGB, pregabalin; TPM, topiramate; VPA, valproic acid; ZNS, zonisamide.
Pregnancy outcomes
Table 2 shows the outcome of the 145 243 pregnancies in the AED-exposed and non-exposed women.
Table 2.
Antiepileptic drug exposure and pregnancy outcome
| Non-exposed | Exposed | |||
| n | % | n | % | |
| Delivery | 110 871 | 76.7 | 413 | 67.6 |
| Stillbirth | 278 | 0.2 | 1 | 0.3 |
| Spontaneous abortion | 16 338 | 11.3 | 70 | 11.5 |
| Terminations of pregnancy | 17 423 | 12.0 | 128 | 20.9 |
| Total | 144 632 | 100 | 611 | 100 |
The risk of TOP was significantly increased after exposure to AEDs. When spontaneous abortions were excluded from the analysis, pregnancies ended in TOP in 23.7% of the AED-exposed women vs 13.6% of the unexposed (OR 1.97; CI 1.62 to 2.40) with a positive relation to the number of AEDs taken, as shown in table 3. According to the algorithm among the 128 women exposed to AEDs, 57 had epilepsy (44.5%), 39 a psychiatric disorder (30.4%) while for 32 (25%) the algorithm was not applicable (no exposure from 12 to 6 months before the event).24
Table 3.
Risk of induced abortion per number of AEDs
| AEDs (n) | n | Events | % | Crude OR | Adjusted OR* |
| 0 | 128 294 | 17 423 | 13.6 | 1 | 1 |
| 1 | 479 | 110 | 23.0 | 1.90 (CI 1.53 to 2.34) | 1.55 (CI 1.24 to 1.93) |
| >1 | 62 | 18 | 29.0 | 2.60 (CI 1.50 to 4.50) | 2.08 (CI 1.19 to 3.64) |
*Adjusted for maternal age, drugs belonging to X and D Food and Drug Administration pregnancy categories, antipsychotics, lithium and antidepressants.
AEDs, antiepileptic drugs.
Major birth defects
During the 3-year observational period, 2302 cases of newborns with MBDs were reported: 1928 from paediatricians’ reports during the first week and 374 from hospital discharge cards during the first year of life. Eight babies with MBDs proved to have been exposed to AEDs during the first trimester of pregnancy. The incidence of MBDs was 2.3% in the newborns from exposed mothers (first trimester) and 2.0% in the newborns from non-exposed mothers (OR 1.12; CI 0.55 to 2.55). The risk of MBDs slightly increased when the mother was exposed to more than one AED; however, this was not significant, as reported in table 4.
Table 4.
Number of AEDs and MBDs
| Unadjusted | Adjusted* | ||||||||
| AEDs (n) | n | MBDs | % | OR | 95% CI | OR | 95% CI | ||
| 0 | 112 771 | 2294 | 2.0 | 1 | – | 1 | – | ||
| 1 | 321 | 7 | 2.2 | 1.07 | 0.51 to 2.27 | 1.00 | 0.47 to 2.12 | ||
| >1 | 32 | 1 | 3.1 | 1.56 | 0.21 to 11.39 | 1.44 | 0.20 to 10.59 | ||
*OR adjusted for maternal age, education, smoking habit (5256—4.6% missing), diabetes, prescription of drugs belonging to X and D Food and Drug Administration pregnancy categories and fetal confounding pathologies of different aetiologies.
AED, antiepileptic drug; MBD, major birth defects.
The specific MBDs reported in the AED-exposed group are listed in table 5. All eight MBDs were reported by paediatricians during the first week of life. The only abnormality to occur in more than one baby was ventricular septal defect, which had a prevalence of 84.98 per 10 000 liveborns in the exposed population vs 33.81 per 10 000 liveborns in the non-exposed. The drug to which these babies were exposed was gabapentin in two cases and pregabalin in the third. Table 4 also reports the supposed diagnosis according to the algorithm.
Table 5.
Types of MBDs in children from AED-exposed mothers
| Type of MBD | Affected babies (n) | AEDs | Supposed diagnosis |
| Ventricular septal defect | 3 | GBP; GBP; PGB | Epi; Epi; Psy |
| Atrial septal defect, tricuspid insufficiency | 1 | LTG | Epi |
| Bilateral postaxial polydactyly | 1 | CBZ | Epi |
| Ectopic kidney | 1 | CNZ | Psy |
| Congenital diaphragmatic hernia | 1 | CBZ | Epi |
| Multiple congenital anomalies+hypospadias | 1 | PB+ETS | Epi |
AED, antiepileptic drug; CBZ, carbamazepine; CNZ, clonazepam; Epi, epilepsy; ETS, ethosuximide; GBP, gabapentin; LTG, lamotrigine; MBD, major birth defects; PB, phenobarbital; PGB, pregabalin; Psy, psychiatric disorder.
Infant health
There were 34 cases of SGA (8.2%) in exposed infants and 7899 (7%) in non-exposed babies (OR 1.18; CI 0.83 to 1.68). Even considering polytherapy the rate was not significantly increased; indeed it was 6.8% (OR 0.97; CI 0.30 to 3.14).
The only single drug for which a significant increase in SGA was found was gabapentin, as we have reported elsewhere.27 Confounding factors, that is, maternal age (<20 years and >37 years) and smoking habit, twin pregnancy, hypertension and diabetes, did not affect the result.
The Apgar scores did not differ significantly in AED-exposed and non-exposed infants: an Apgar score between 1 and 3 (severe fetal distress) was attributed to none of the exposed and to 0.1% of the non-exposed babies, an Apgar score between 4 and 6 (moderate distress) was attributed to 1% of the exposed and 0.5% of the non-exposed infants, and an Apgar score between 7 and 10 (normal) was attributed to 99% of the exposed and 99.4% of the non-exposed babies (P=0.317, Fisher’s exact test).
Discussion
To our knowledge, this is the first study in a southern European country to explore the use of AEDs in pregnancy and their possible adverse consequences.
The frequency of AED use in the first trimester of pregnancy in our population, 4 per 1000 pregnancies, or 3.1 per 1000 deliveries, is comparable with the data reported elsewhere.1–3 5 7
Carbamazepine was the most used anticonvulsant in Emilia Romagna during the study period, being taken in 23% of all the exposed pregnancies. This differed from reports from Denmark and the USA,5 7 where lamotrigine was the most prescribed drug in pregnancy during the same period. Levetiracetam was much less used in our population (5.9% of the total used AEDs) than in studies referring to similar periods of time in other European countries and the USA.5 7 28 29 A striking finding of our study is that approximately one in five AED users was taking valproate, a drug currently considered the most hazardous anticonvulsant in pregnancy due to its higher teratogenic risk and possible detrimental cognitive and developmental effects.30–33 One possible explanation for the unexpected over-representation of valproate is that it is used both in epilepsy and in psychiatric disorders. Indeed, one study investigating trends of use of antiepileptic agents in pregnant women showed that over time valproate use decreased overall, but not in patients with a psychiatric disorder.7 According to our algorithm, 30% of the valproate-exposed patients suffered from psychiatric disorders. Moreover, taking into account the limitations of the ascertainment methods, the mean dose of valproate in our population (approximately 500 mg/day) should be considered low, associated with a lower risk.30 No MBDs were reported in these patients, but the cohort is too small to draw any conclusion. Lastly, our data refer to a period preceding the strict recommendations on valproate use in women of childbearing potential.34 35
In agreement with most of the literature data, the rates of spontaneous abortions and stillbirths were not increased in the antiepileptic-exposed women.16 17 20
One limitation of our study is that due to the current Italian privacy policy, the ascertainment of pregnancy terminations did not cover all the hospitals in Emilia Romagna. Compared with data from the Italian National Institute for Statistics (ISTAT), to which all TOP figures were transmitted anonymously and not linkable to the other registries, the coverage was 64.6%.36 We consider that the incomplete coverage of terminations did not significantly affect our results as there is no reason to assume that in some hospitals women taking anticonvulsants were more represented among women undergoing an abortion.
Taking these limits into account, the most original finding of our study is the clear-cut higher risk of induced abortion in women taking AEDs, with a trend towards an increased risk when more than one drug is used. A possible explanation could be an excess number of birth defects in the exposed fetuses detected by prenatal ultrasound. This would indicate that studies on malformations detected exclusively on delivery are not completely reliable. Unfortunately, no further information was available on the abortions, including the clinical indication. This, however, is unlikely to be the main explanation as second trimester abortions (allowed in Italy only for severe fetal or maternal pathologies) accounted for 3.5% of all terminations during that period in Emilia Romagna (ISTAT36). Accordingly, the only other report to our knowledge on induced abortions in women taking anticonvulsants showed only a slight increase not accounted for by fetal disease.17 According to our algorithm, the rate of women with psychiatric disorders was higher in the TOP population than in the one of women who gave birth to a child. Interestingly, data from northern European population-based studies show that women on antipsychotics and antidepressants had a significantly increased risk of induced abortion, which was associated with mental health rather than fetal malformations, also considering only late terminations.37–39 On the other hand, physicians caring for women with epilepsy, including several members of the ESPEA group (BM, FB, RDA, PT, LL, IN), report that faced with an unplanned pregnancy, a significant number of women opt for TOP very early due to a groundless fear of the pregnancy outcome. These ‘unnecessary’ abortions are the result of a malpractice, which merits investigation. Further studies focused on both physicians’ attitudes and patients’ risk perception are needed to explain the excess number of terminations in this population.
The MBD rate in the antiepileptic-exposed population only slightly exceeded that in the non-exposed women: indeed, after adjusting for confounding factors the OR was 1. The small numbers preclude a discussion of the apparent trend towards a major risk of birth defects on polytherapy as only one patient out of 32 women exposed to polytherapy had a child with an MBD. Caution is needed in speculating on single AED or specific malformations due to the low number of MBDs. However, we found a significantly higher risk of ventricular septal defect, which had a more than twofold higher prevalence in the antiepileptic-exposed population. Interestingly, two out of the three cases involved maternal exposure to gabapentin and in the third to pregabalin, a molecule with a similar chemical and pharmacodynamic profile as we already reported elsewhere.27 In discussing our results on MBDs, several limitations, intrinsic to the methods we used, should be acknowledged. Birth defects are mainly ascertained during the first week of life. After that period, and until the first year of life, further information is added to the malformation registry only for in-hospital cases, as the source of information is the hospital discharge card. Therefore a non-quantifiable, although limited, number of cases is lost. As an additional limit, we did not provide data on folic acid use and consequently on its possible association with the incidence of MBDs. In Italy only the folic acid dosage of 5 mg is reimbursable, while the most used 400 μg can be purchased as an over-the-counter drug and is therefore untraceable. Furthermore, the reimbursable formulation is very inexpensive and therefore is often purchased with untraceable private prescriptions. As a further limit, information on the clinical indication could only be inferred using an algorithm created ad hoc while information on seizures in pregnancy was unavailable.
In agreement with some studies, we did not find a higher risk of SGA in infants exposed to AEDs,10 11 but different substances might have a different impact on fetal growth.14 15 In our population, gabapentin seems to carry a higher risk than other anticonvulsants.27
However, due to the rarity of MBDs and SGA infants, our study was underpowered to provide information on the risks associated with single AEDs. This is a crucial point given that the continuation of antiepileptic therapy during pregnancy is the safest solution in most cases, as seizure relapse during pregnancy is associated with increased maternal morbidity and mortality.40
The need for similar systems to monitor the safety of AEDs in pregnancy in a population-based context is evident, also considering that new anticonvulsants will be marketed in the coming years. For this reason, we suggest that reliable administrative data in existing European registries be pooled to obtain a sufficient statistical power to investigate each single antiepileptic agent.
Acknowledgments
The authors thank Fiorenzo Albani for his valuable contribution to the conception of this study and Anne Collins for editing the English text.
Footnotes
Contributors: BM contributed to the conception and design of the study, drafted the study protocol, coordinated the collection and analysis of data, and drafted the paper. FB contributed to the conception and design of the study and to the writing of the study protocol. EP contributed to the design and writing of the study protocol, and to the analysis of data on prescriptions. GC contributed to the writing of the study protocol and analysed data on fetal adverse outcomes. CP contributed to the analysis of data on prescriptions and to the database construction. AC and GS contributed to the analysis of data on abortions. GA linked data from the registries of birth defects to the other regional registries. NR contributed to the writing of the study protocol and to the analysis of data on abortions. CZ contributed to the statistical analysis. RDA contributed to the conception and design of the study, to the writing of the study protocol, and monitored the study methods and the statistical analysis. PT contributed to the conception, design and writing of the study protocol, coordinated the study group. All authors contributed to the interpretation of the results, revised the text of this article and approved it.
Funding: This study was funded by the Italian Ministry of Health (RF 2010-2315893).
Competing interests: BM and FB received a personal fee for an advisory board from EISAI.
Patient consent: Detail has been removed from this case description/these case descriptions to ensure anonymity. The editors and reviewers have seen the detailed information available and are satisfied that the information backs up the case the authors are making.
Ethics approval: Local ethical committee (AUSL of Bologna) and Hospital Management Executive.
Provenance and peer review: Not commissioned; externally peer reviewed.
Collaborators: Gabriele Accetta (IRCCS Institute of Neurological Sciences of Bologna, Bologna, Italy), Gianni Astolfi (IMER Registry - Department of Biomedical and Specialty Surgical Sciences, University ofFerrara, Ferrara, Italy), Cosimo Ippazio Antonazzo(Department of Medical and Surgical Sciences DIMEC, University of Bologna, Bologna, Italy) Sergio Battaglia (Regione Emilia Romagna, SISePS, Italy), Francesca Bisulli (IRCCS Institute of Neurological Sciences of Bologna, Bologna, Italy; Department of Biomedical and Neuromotor Sciences DIBINEM, University of Bologna, Bologna, Italy), Guido Cocchi, Letizia Conti (Department of Medical and Surgical Sciences DIMEC, Division of Neonatology, St Orsola Malpighi Hospital, University of Bologna, Bologna, Italy), Alessandra Curti (Department of Medical and Surgical Sciences DIMEC, Division of Prenatal Medicine, St Orsola Malpighi Hospital, University of Bologna, Bologna, Italy), Roberto D’Alessandro (IRCCS Institute of Neurological Sciences of Bologna, Bologna, Italy), Laura Licchetta (IRCCS Institute of Neurological Sciences of Bologna, Bologna, Italy; Department of Biomedical and Neuromotor Sciences DIBINEM, University of Bologna, Bologna, Italy), Camilla Lupi (Regione Emilia Romagna, SISePS, Italy), Barbara Mostacci (IRCCS Institute of Neurological Sciences of Bologna, Bologna, Italy), Ilaria Naldi, Carlo Piccinni, Elisabetta Poluzzi (Department of Medical and Surgical Sciences DIMEC, University of Bologna, Bologna, Italy), Nicola Rizzo, Giuliana Simonazzi (Department of Medical and Surgical Sciences DIMEC, Division of Prenatal Medicine, St Orsola Malpighi Hospital, University of Bologna, Bologna, Italy), Paolo Tinuper (IRCCS Institute of Neurological Sciences of Bologna, Bologna, Italy; Department of Biomedical and Neuromotor Sciences DIBINEM, University of Bologna, Bologna, Italy), Corrado Zenesini (IRCCS Institute of Neurological Sciences of Bologna, Bologna, Italy).
Author note: This work was part of BM’s PhD thesis in specialised medical sciences at Alma Mater Studiorum-University of Bologna.
Contributor Information
the ESPEA Study Group:
Gabriele Accetta, Gianni Astolfi, Sergio Battaglia, Francesca Bisulli, Guido Cocchi, Letizia Conti, Alessandra Curti, Roberto D’alessandro, Cosimo Ippazio, Laura Licchetta, Camilla Lupi, Barbara Mostacci, Ilaria Naldi, Carlo Piccinni, Elisabetta Poluzzi, Nicola Rizzo, Giuliana Simonazzi, Paolo Tinuper, and Corrado Zenesini
References
- 1. Czeizel AE, Bod M, Halász P. Evaluation of anticonvulsant drugs during pregnancy in a population-based Hungarian study. Eur J Epidemiol 1992;8:122–7. [DOI] [PubMed] [Google Scholar]
- 2. Malm H, Martikainen J, Klaukka T, et al. . Finnish Register-Based Study Prescription drugs during pregnancy and lactation-a Finnish register-based study. Eur J Clin Pharmacol 2003;59:127–33. [DOI] [PubMed] [Google Scholar]
- 3. Wide K, Winbladh B, Källén B. Major malformations in infants exposed to antiepileptic drugs in utero, with emphasis on carbamazepine and valproic acid: a nation-wide, population-based register study. Acta Paediatr 2004;93:174–6. 10.1111/j.1651-2227.2004.tb00701.x [DOI] [PubMed] [Google Scholar]
- 4. Bobo WV, Davis RL, Toh S, et al. . Trends in the use of antiepileptic drugs among pregnant women in the US, 2001-2007: a medication exposure in pregnancy risk evaluation program study. Paediatr Perinat Epidemiol 2012;26:578–88. 10.1111/ppe.12004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kilic D, Pedersen H, Kjaersgaard MI, et al. . Birth outcomes after prenatal exposure to antiepileptic drugs--a population-based study. Epilepsia 2014;55:1714–21. 10.1111/epi.12758 [DOI] [PubMed] [Google Scholar]
- 6. Charlton R, Garne E, Wang H, et al. . Antiepileptic drug prescribing before, during and after pregnancy: a study in seven European regions. Pharmacoepidemiol Drug Saf 2015;24:1144–54. 10.1002/pds.3847 [DOI] [PubMed] [Google Scholar]
- 7. Wen X, Meador KJ, Hartzema A. Antiepileptic drug use by pregnant women enrolled in Florida Medicaid. Neurology 2015;84:944–50. 10.1212/WNL.0000000000001304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perucca E. Birth defects after prenatal exposure to antiepileptic drugs. Lancet Neurol 2005;4:781–6. 10.1016/S1474-4422(05)70224-6 [DOI] [PubMed] [Google Scholar]
- 9. Hvas CL, Henriksen TB, Østergaard JR. Birth weight in offspring of women with epilepsy. Epidemiol Rev 2000;22:275–82. 10.1093/oxfordjournals.epirev.a018039 [DOI] [PubMed] [Google Scholar]
- 10. Lin HL, Chen YH, Lin HC, et al. . No increase in adverse pregnancy outcomes for women receiving antiepileptic drugs. J Neurol 2009;256:1742–9. 10.1007/s00415-009-5222-3 [DOI] [PubMed] [Google Scholar]
- 11. Mawer G, Briggs M, Baker GA, et al. . Liverpool & Manchester Neurodevelopment Group. Pregnancy with epilepsy: obstetric and neonatal outcome of a controlled study. Seizure 2010;19:112–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wide K, Winbladh B, Tomson T, et al. . Body dimensions of infants exposed to antiepileptic drugs in utero: observations spanning 25 years. Epilepsia 2000;41:854–61. 10.1111/j.1528-1157.2000.tb00253.x [DOI] [PubMed] [Google Scholar]
- 13. Artama M, Gissler M, Malm H, et al. . Effects of maternal epilepsy and antiepileptic drug use during pregnancy on perinatal health in offspring: nationwide, retrospective cohort study in Finland. Drug Saf 2013;36:359–69. 10.1007/s40264-013-0052-8 [DOI] [PubMed] [Google Scholar]
- 14. Veiby G, Daltveit AK, Engelsen BA, et al. . Fetal growth restriction and birth defects with newer and older antiepileptic drugs during pregnancy. J Neurol 2014;261:579–88. 10.1007/s00415-013-7239-x [DOI] [PubMed] [Google Scholar]
- 15. Farmen AH, Grundt J, Tomson T, et al. . Intrauterine growth retardation in foetuses of women with epilepsy. Seizure 2015;28:76–80. 10.1016/j.seizure.2015.02.026 [DOI] [PubMed] [Google Scholar]
- 16. Harden CL, Hopp J, Ting TY, et al. . Management issues for women with epilepsy-Focus on pregnancy (an evidence-based review): I. Obstetrical complications and change in seizure frequency: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia 2009;50:1229–36. 10.1111/j.1528-1167.2009.02128.x [DOI] [PubMed] [Google Scholar]
- 17. Bech BH, Kjaersgaard MIS, Pedersen HS, et al. . Use of antiepileptic drugs during pregnancy and risk of spontaneous abortion and stillbirth: population based cohort study. BMJ 2014;349:g5159 10.1136/bmj.g5159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tomson T, Battino D, Bonizzoni E, et al. . Antiepileptic drugs and intrauterine death: A prospective observational study from EURAP. Neurology 2015;85:580–8. [DOI] [PubMed] [Google Scholar]
- 19. Vajda FJE, O’Brien TJ, Graham JE, et al. . Antiepileptic drugs, foetal malformations and spontaneous abortions. Acta Neurol Scand 2017;135:360–5. 10.1111/ane.12672 [DOI] [PubMed] [Google Scholar]
- 20. Olafsson E, Hallgrimsson JT, Hauser WA, et al. . Pregnancies of women with epilepsy: a population-based study in Iceland. Epilepsia 1998;39:887–92. 10.1111/j.1528-1157.1998.tb01186.x [DOI] [PubMed] [Google Scholar]
- 21. Richmond JR, Krishnamoorthy P, Andermann E, et al. . Epilepsy and pregnancy: an obstetric perspective. Am J Obstet Gynecol 2004;190:371–9. 10.1016/j.ajog.2003.09.020 [DOI] [PubMed] [Google Scholar]
- 22. Morrow J, et al. . Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 2006;77:193–8. 10.1136/jnnp.2005.074203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Statistica. Popolazione per sesso ed età. Ammontare. http://statistica.regione.emilia-romagna.it/servizi-online/statistica-self-service-1/popolazione/popolazione-per-eta-e-sesso/pop_eta_ammontare (accessed 24 Nov 2017).
- 24. Naldi I, Piccinni C, Mostacci B, et al. . ESPEA (Emilia-Romagna Study on Pregnancy and Exposure to Antiepileptic drugs) Group. Prescription patterns of antiepileptic drugs in young women: development of a tool to distinguish between epilepsy and psychiatric disorders. Pharmacoepidemiol Drug Saf 2016;25:763–9. [DOI] [PubMed] [Google Scholar]
- 25. British Paediatric Association. British Paediatric Association Classification of Diseases, (successor to the Cardiff Diagnostic Classification): a paediatric supplement compatible with the ninth revision of the WHO International Classification of Diseases, 1977. London: British Paediatric Association, 1979. [Google Scholar]
- 26. Bertino E, Spada E, Occhi L, et al. . Neonatal Anthropometric Charts: The Italian neonatal study compared with other European studies. JPGN 2010;51:353–61. [DOI] [PubMed] [Google Scholar]
- 27. Mostacci B, Poluzzi E, D’Alessandro R, et al. . Adverse pregnancy outcomes in women exposed to gabapentin and pregabalin: data from a population-based study. J Neurol Neurosurg Psychiatry 2018;89:223–4. 10.1136/jnnp-2017-316143 [DOI] [PubMed] [Google Scholar]
- 28. Meador KJ, Penovich P, Baker GA, et al. . Antiepileptic drug use in women of childbearing age. Epilepsy & Behavior 2009;15:339–43. 10.1016/j.yebeh.2009.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mølgaard-Nielsen D, Hviid A. Newer-generation antiepileptic drugs and the risk of major birth defects. JAMA 2011;305:1996–2002. 10.1001/jama.2011.624 [DOI] [PubMed] [Google Scholar]
- 30. Tomson T, Battino D, Bonizzoni E, et al. . Dose-dependent risk of malformations with antiepileptic drugs: an analysis of data from the EURAP epilepsy and pregnancy registry. Lancet Neurol 2011;10:609–17. 10.1016/S1474-4422(11)70107-7 [DOI] [PubMed] [Google Scholar]
- 31. Hernandez-Diaz S, Smith CR, Shen A, et al. . North American AED Pregnancy Registry; North American AED Pregnancy Registry. Comparative safety of antiepileptic drugs during pregnancy. Neurology 2012;78:1692–9. [DOI] [PubMed] [Google Scholar]
- 32. Campbell E, Kennedy F, Russell A, et al. . Malformation risks of antiepileptic drug monotherapies in pregnancy: updated results from the UK and Ireland Epilepsy and Pregnancy Registers. Journal of Neurology, Neurosurgery & Psychiatry 2014;85:1029–34. 10.1136/jnnp-2013-306318 [DOI] [PubMed] [Google Scholar]
- 33. Velez-Ruiz NJ, Meador KJ. Neurodevelopmental Effects of Fetal Antiepileptic Drug Exposure. Drug Saf 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. European Medicines Agency. PRAC recommends strengthening the restrictions on the use of valproate in women and girls. http://www.ema.europa.eu/docs/en_GB/document_library/Press_release/2014/10/WC500175208.pdf (accessed 24 Nov 2017).
- 35. Tomson T, Marson A, Boon P, et al. . Valproate in the treatment of epilepsy in girls and women of childbearing potential. Epilepsia 2015;56:1006–19. 10.1111/epi.13021 [DOI] [PubMed] [Google Scholar]
- http://dati.istat.it/ :36.
- 37. Boden R, Lundgren M, Brandt L, et al. . Risks of adverse pregnancy and birth outcomes in women treated or not treated with mood stabilisers for bipolar disorder: population based cohort study. BMJ 2012;345:e7085 10.1136/bmj.e7085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gissler M, Artama M, Ritvanen A, et al. . Use of psychotropic drugs before pregnancy and the risk for induced abortion: population-based register-data from Finland 1996-2006. BMC Public Health 2010;10:383 10.1186/1471-2458-10-383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kieler H, Malm H, Artama M, et al. . Use of antidepressants and association with elective termination of pregnancy: population based case-control study. BJOG: An International Journal of Obstetrics & Gynaecology 2015;122:1618–24. 10.1111/1471-0528.13164 [DOI] [PubMed] [Google Scholar]
- 40. Edey S, Moran N, Nashef L. SUDEP and epilepsy-related mortality in pregnancy. Epilepsia 2014;55:e72–4. 10.1111/epi.12621 [DOI] [PubMed] [Google Scholar]