Abstract
Objective
To evaluate cerebrospinal fluid (CSF) cytokine profiles in myelin oligodendrocyte glycoprotein IgG-positive (MOG-IgG+) disease in adult and paediatric patients.
Methods
In this cross-sectional study, we measured 27 cytokines in the CSF of MOG-IgG+ disease in acute phase before treatment (n=29). The data were directly compared with those in aquaporin-4 antibody-positive (AQP4-IgG+) neuromyelitis optica spectrum disorder (NMOSD) (n=20), multiple sclerosis (MS) (n=20) and non-inflammatory controls (n=14).
Results
In MOG-IgG+ disease, there was no female preponderance and the ages were younger (mean 18 years, range 3–68; 15 were below 18 years) relative to AQP4-IgG+ NMOSD (41, 15–77) and MS (34, 17–48). CSF cell counts were higher and oligoclonal IgG bands were mostly negative in MOG-IgG+ disease and AQP4-IgG+ NMOSD compared with MS. MOG-IgG+ disease had significantly elevated levels of interleukin (IL)-6, IL-8, granulocyte-colony stimulating factor and granulocyte macrophage-colony stimulating factor, interferon-γ, IL-10, IL-1 receptor antagonist, monocyte chemotactic protein-1 and macrophage inflammatory protein-1α as compared with MS. No cytokine in MOG-IgG+ disease was significantly different from AQP4-IgG+ NMOSD. Moreover many elevated cytokines were correlated with each other in MOG-IgG+ disease and AQP4-IgG+ NMOSD but not in MS. No difference in the data was seen between adult and paediatric MOG-IgG+ cases.
Conclusions
The CSF cytokine profile in the acute phase of MOG-IgG+ disease is characterised by coordinated upregulation of T helper 17 (Th17) and other cytokines including some Th1-related and regulatory T cells-related ones in adults and children, which is similar to AQP4-IgG+ NMOSD but clearly different from MS. The results suggest that as with AQP4-IgG+ NMOSD, some disease-modifying drugs for MS may be ineffective in MOG-IgG+ disease while they may provide potential therapeutic targets.
Keywords: myelin oligodendrocyte glycoprotein-IgG, cytokine profile, demyelinating disease, aquaporin-4-IgG, neuromyelitis optica spectrum disorders, multiple sclerosis
Introduction
Myelin oligodendrocyte glycoprotein (MOG) is a myelin protein localised at the surface of myelin sheath and is a potential target of demyelinating diseases.1 In fact, experimental autoimmune encephalomyelitis immunised by MOG peptides is an established animal model for human demyelinating disorders of the central nervous system (CNS), including multiple sclerosis (MS).2 3 In humans, there have been controversial results in early studies on antibody against MOG (MOG-IgG) using western blot and ELISA mainly due to low specificity.4
Recently, cell-based assay (CBA) using live cells transfected with human MOG has successfully detected conformational-sensitive MOG-IgG. With CBA, MOG-IgG is usually negative in prototypic MS but is detected in some cases of acute disseminated encephalomyelitis (ADEM), multiphasic disseminated encephalomyelitis, optic neuritis (ON) and aquaporin-4 antibody (AQP4-IgG)-seronegative neuromyelitis optica spectrum disorder (NMOSD).4–12 MOG-IgG+ disease is likely an inflammatory CNS demyelinating disease, but its clinical features are different from MS.13 Moreover, MOG-IgG+ disease and AQP4-IgG+ NMOSD share some clinical features,14 15 but MOG-IgG+ disease does not induce astrocyte injury like AQP4-IgG+ NMOSD.16–18
In inflammatory CNS diseases, profiles of cytokine, chemokine and related molecule in the cerebrospinal fluid (CSF) may reflect unique immunopathological processes, and such profiles might have therapeutic implications. AQP4-IgG+ NMOSD was reported to have a T helper 17 (Th17)-dominant cytokine profile which is different from MS,19 but the previously reported cytokine profiles in MOG-IgG+ cases are limited.20 21 In this study, we evaluated levels of cytokines, chemokines and related molecules in CSF samples from MOG-IgG+ disease in the acute phase of both adult and paediatric cases, and compared the results with AQP4-IgG+ NMOSD, MS and non-inflammatory disease controls. Such a direct comparison of profiles of CSF cytokines, chemokines and related molecules in the four disease groups and a comparison in adult and paediatric MOG-IgG+ cases have not been done before.
Materials and methods
Materials
In this cross-sectional study, we collected 136 paired CSF and sera from patients with idiopathic inflammatory CNS diseases, obtained from December 2011 to December 2015. The samples were gathered in various hospitals in Japan and Brazil and sent to our laboratory to examine the status of MOG-IgG and AQP4-IgG. Among them, 47 patients were excluded due to the following reasons: lacking clinical or laboratory information (n=18), samples were collected after acute-phase treatment (n=16), inadequate amount of samples (n=10) and previous use of disease-modifying drugs such as interferon (IFN)-ß or fingolimod (n=3).
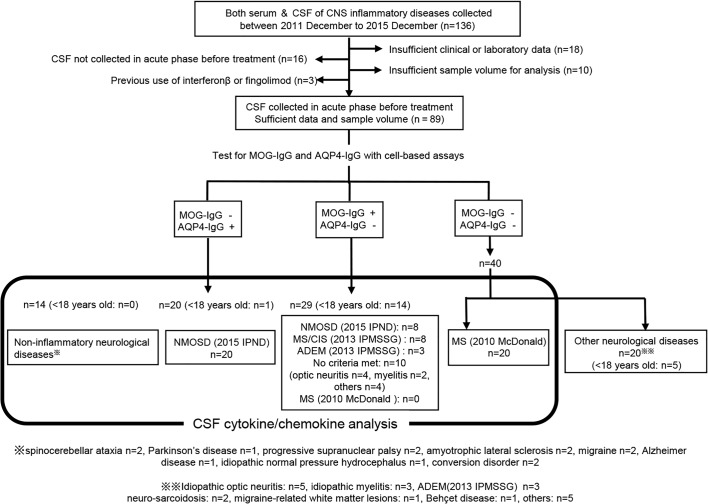
Then, we examined MOG-IgG and AQP4-IgG using CBAs as previously reported.14 22 As a result, 29 were positive for MOG-IgG (28 Japanese and 1 Caucasian living in Japan) and 20 were positive for AQP4-IgG (13 Japanese and 7 Brazilians) fulfilling the 2015 criteria of the International Panel on Neuromyelitis Optica Diagnosis for AQP4-IgG-seropositive NMOSD.23 We checked the remaining 40 patients without MOG-IgG or AQP4-IgG as to whether they met the McDonald criteria 2010 for MS, and 20 patients were diagnosed with MS.24 The diagnostic process is shown in a flow chart (figure 1).
Figure 1.
Flow chart of the present study of CSF cytokines/chemokines profiles in MOG-IgG+ disease, AQP4-IgG+ NMOSD, MS and controls. This figure shows how we collected the patients in MOG-IgG+ disease, AQP4-IgG+ NMOSD, MS and control groups in the present study. ADEM, acute disseminated encephalomyelitis; AQP4-IgG, aquaporin-4-IgG; CIS, clinically isolated syndrome; CNS, central nervous system; CSF, cerebrospinal fluid; IPMSSG, International Pediatric Multiple Sclerosis Study Group; IPND, International Panel on Neuromyelitis Optica Diagnosis; McDonald, McDonald criteria; MOG-IgG, myelin oligodendrocyte glycoprotein-IgG; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders.
In addition, we also collected 14 pairs of CSF and sera of non-inflammatory disorders as disease controls: spinocerebellar ataxia (n=2), idiopathic Parkinson’s disease (n=2), progressive supranuclear palsy (n=2), amyotrophic lateral sclerosis (n=2), migraine (n=2), Alzheimer’s disease (n=1), idiopathic normal pressure hydrocephalus (n=1) and conversion disorder (n=2). All the samples were centrifuged immediately after collection, stored at −80°C, sent to our laboratory and kept frozen until analysis.
Methods
We reviewed gender, age at sample collection, clinical diagnosis, lesion distributions, clinical course (onset or relapse), medical history, serum antinuclear antibody (ANA), anti-Sjögren syndrome A and anti-Sjögren syndrome B. We also reviewed CSF cell count, protein level and oligoclonal IgG band (OCB). We measured levels of cytokines, chemokines, and related molecules, glial fibrillar acidic protein (GFAP) and myelin basic protein (MBP) in CSF and made correlation analyses.
Measurements of CSF cytokines, chemokines and related molecules
Levels of cytokines, chemokines and related molecules in the CSF were determined using commercially available beads-based immunoassays (Bio-Plex Pro Human Cytokine GI 27-Plex Panel #M500KCAF0Y; Bio-Rad, Richmond, California), which can measure the following 27 cytokines, chemokines and related molecules simultaneously: interleukin (IL)-1 receptor antagonist (IL-1ra), IL-2, IL-4, IL-5, IL-6, IL-7, IL-8 (CXCL-8), IL-9, IL-10, IL-12 (p70), IL-13, IL-15, IL-17A, eotaxin (CCL11), fibroblast growth factor basic (FGF basic), granulocyte-colony stimulating factor (G-CSF), granulocyte macrophage colony stimulating factor (GM-CSF), IFN-γ, IFN-gamma-inducible protein-10 (IP-10, CXCL-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein (MIP)-1α (CCL2), MIP-1β (CCL4), platelet-derived growth factor (PDGF-BB), tumour necrosis factor-alpha (TNF-α), and vascular endothelial growth factor (VEGF). In this manufacturer’s kit, we added diluted magnetic beads coated with primary antibody against each cytokine, chemokine and related molecule into washed wells of filter plate; added undiluted CSF samples or fourfold standard dilution series to each well; incubated at room temperature on shaker at 850 round per minute for 30 min with protection from light; and washed each well three times. We added detection antibodies to each well; incubated for 30 min as described; and washed each well again three times. We added streptavidin-phycoerythrin conjugate; incubated for 10 min as described; and washed each well three times. Finally, we added resuspend beads buffer; measured fluorescence intensity by Bio-Plex MAGPIX Reader (Bio-Rad); and calculated the cytokine concentrations using the standard curve generated by five-parameter logistic regression method (the 27 cytokines were measured in duplicate in three kits, and in each we determined the values with a standard curve). The recovery rates were over 93%. In samples where cytokines were undetectable, the values of detection limit were used for analyses.
CSF-GFAP and MBP measurements
Levels of GFAP and MBP were measured using commercially available ELISA kits purchased from Cosmic Corporation (Tokyo, Japan) and SPI Bio (Montigny-le-Bretonneux, France), respectively. The optical densities were measured at 450 nm and the lower detection limits were 0.0035 pg/mL for GFAP and 31.3 ng/mL for MBP. The experiments were done according to the manufacturers’ protocol.
MOG-IgG and AQP4-IgG detection assays
MOG-IgG and AQP4-IgG detections were performed by CBA with full-length human MOG- or M23 isoform of AQP4-transfected human embryonic kidneys cell 293 and a goat antihuman Fc-specific IgG cross-adsorbed secondary antibody (Pierce Biotechnology, Rockford, Illinois, USA) to reduce the risk of light chain cross-reactivity from other immunoglobulin subclasses. CSF antibodies were measured without dilution. Antibody titre was measured semiquantitatively using endpoint serial dilutions.14 22
Statistical analyses
For group comparison, we used Fisher’s exact test for categorical data. Continuous variables were analysed using non-parametric tests (Mann-Whitney U test). We considered two-tailed p<0.05 as statistically significant and made Bonferroni correction when we consider the difference of cytokine levels. In Bonferroni correction, 0.05 divided by 27 is p=0.0019. But we applied a slightly more stringent p value (p<0.001 or p<0.0001). Correlation rank was evaluated by Spearman’s rank correlation tests. These analyses were done using GraphPad Prism V.7.0 software.
Results
Clinical and laboratory findings
Clinical and laboratory profiles were summarised in table 1. No patients with MOG-IgG or AQP4-IgG fulfil the McDonald criteria 2010. Female predominance was apparent in AQP4-IgG+ NMOSD (75.0%) and MS (85%) but not in MOG-IgG+ cases (48.3%) (p=0.0149 and p=0.0094, respectively). The median age at sampling was significantly younger in MOG-IgG+ disease (median 18 (3–68) years old) compared with AQP4-IgG+ NMOSD (median 41 (15–77); p=0.0006) and MS (median 34 (17–48); p=0.0217), and patients with MS were significantly younger than those with AQP4-IgG+ NMOSD (p=0.0247). Fourteen MOG-IgG+ cases (48.3%) and one AQP4-IgG+ NMOSD (5%) were below 18 years old. The clinical diagnosis in MOG-IgG+ cases was NMOSD [(n=8, 27.6%). All of them had ON and long myelitis (>3 vertebral segments)], paediatric clinically isolated syndrome/MS (n=8, 27.6%) and ADEM (n=3, 10.3%). The main sites of the lesion at CSF sampling for MOG-IgG+ cases were the optic nerve (n=7, 24.1%), brain (n=7, 24.1%), simultaneous optic nerve and spinal cord (n=6, 20.7%), and spinal cord (n=4, 13.8%). For AQP4-IgG+ NMOSD, the main site of the lesion was the spinal cord (15/20, 75%). There was no difference in time from onset to sample collection among the groups. Samples of 15 MOG-IgG+ patients and 15 AQP4-IgG+ NMOSD patients were collected at relapse.
Table 1.
Clinical and laboratory profiles in MOG-IgG+ disease, AQP4-IgG+ NMOSD and MS
| MOG-IgG+ (n=29) | AQP4-IgG+ (n=20) | MS (n=20) | |
| Gender (male:female) | 15:14* | 5:15 | 3:17‡ |
| Age at sampling, years (range) | 18 (3–68)†††** <18: n=14 |
41 (15–77)‡‡‡* <18: n=1 |
34 (17–48)‡‡† <18: n=0 |
| Clinical diagnosis on diagnostic criteria | NMOSD (2015 IPND), n=8 Paediatric CIS/MS (2013 IPMSSG), n=8 ADEM (2013 IPMSSG), n=3 No criteria met, n=10 |
NMOSD (2015 IPND), n=20 | MS (McDonald 2010), n=20 |
| Clinical site of the lesion at CSF sampling | Optic nerve, n=7 Brain, n=7 Optic nerve+spinal cord, n=6 Spinal cord, n=4 Brain+spinal cord, n=3 Brainstem, n=1 Optic nerve+brain, n=1 |
Spinal cord, n=15 Optic nerve, n=3 Optic nerve+spinal cord, n=1 Brainstem, n=1 |
Spinal cord, n=12 Brainstem, n=5 Optic nerve, n=3 |
| Onset/Relapse | 14/15 | 5/15 | 13/7 |
| Preceding infection/vaccination | (Infection)†** Influenza, n=3 Mycoplasma, n=1 Group A streptococcus, n=1 Varicella, n=1 Flu-like symptom, n=3 (Vaccination) Influenza, n=1 |
(Infection)‡ Flu-like symptom, n=1 (Vaccination) None |
None‡‡ |
| History of immune-mediated disease | 2/29 (6.9%) Idiopathic thrombocytopaenic purpura, n=1 Kawasaki disease, n=1 |
5/20 (25%)* Systemic lupus erythematosus, n=2 Sjögren syndrome, n=1 Basedow disease, n=1 Rheumatoid arthritis, n=1 |
0/20 (0%)‡ None |
| Other medical history | Mental retardation, n=2 Malignant lymphoma, n=1 Ovarian tumour, n=1 HCV infection, n=1 Cerebrovascular disease, n=1 Diabetes mellitus, n=1 |
Atopic dermatitis, n=1 Meniere’s disease, n=1 Fatty liver, n=1 Anaplastic anaemia, n=1 Gallstone, n=1 |
Atopic dermatitis, n=1 Ovarian tumour, n=1 Depression, n=1 |
| Autoantibody | ANA, n=3; SS-A, n=0; SS-B, n=0 | ANA, n=7; SS-A, n=3; SS-B, n=0 | ANA, n=3; SS-A, n=0; SS-B, n=0; not examined, n=2 |
| CSF cell count (x109/L), mean±SD | 0.0528±0.0609*** | 0.0358±0.0620* | 0.0075±0.0143‡‡‡† |
| CSF protein (mg/dL), mean±SD | 46.6±27.8* | 61.6±43.1** | 32.3±12.3‡†† |
| Oligoclonal IgG band positivity | 2/26 (7.7%)*** Not examined, n=1 |
1/10 (10%)*** Not examined, n=8 |
13/16 (81.3%)‡‡‡††† Not examined, n=4 |
Versus MS: *p<0.05, **p<0.01, ***p<0.001.
Versus AQP4: †p<0.05, ††p<0.01, †††p<0.001.
Versus MOG: ‡p<0.05, ‡‡p<0.01, ‡‡‡p<0.001.
ADEM, acute disseminated encephalomyelitis; ANA, antinuclear antibody; AQP4, aquaporin-4; CIS, clinically isolated syndrome; CSF, cerebrospinal fluid; IPMSSG, International Pediatric Multiple Sclerosis Study Group; IPND, International Panel on Neuromyelitis Optica Diagnosis; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders; SS-A, Sjögren syndrome A; SS-B, Sjögren syndrome B.
As for preceding infection/vaccination or immune-mediated diseases, in MOG-IgG+ cases, one patient had a preceding history of influenza vaccination, five had preceding infections within a month of neurological attacks (three with influenza, one with mycoplasma pneumonia, one with group A Streptococcus pyogenes infection and one with varicella), but only two patients with MOG-IgG (6.9%) had a history of immune-mediated diseases (idiopathic thrombocytopaenic purpura and Kawasaki disease). However, they had already recovered and were not receiving any treatment for those diseases during the neurological attacks. On the other hand, in AQP4-IgG+ NMOSD, only one patient had preceding infection (flu-like symptom) and no patient had history of vaccination, but five (20%) had history of immune-mediated diseases (two with systemic lupus erythematosus, one with Sjögren syndrome, one with Basedow disease and one with rheumatoid arthritis). Frequency of preceding infections was significantly higher in MOG-IgG+ disease than in AQP4-IgG+ NMOSD (p=0.0336) and MS (p=0.0067), whereas frequency of immune-mediated disease was significantly higher in AQP4-IgG+ NMOSD than in MS (p=0.0471).
Regarding laboratory data, serum ANAs were positive in 3/27 (11.1%) in MOG-IgG+ disease, 3/18 (16.6%) in MS and 7/20 (35%) of AQP4-IgG+ NMOSD. In routine CSF analysis, cell counts in MOG-IgG+ cases and AQP4-IgG+ NMOSD were significantly higher than in MS (MOG-IgG+ cases vs MS: p<0.0001; AQP4-IgG+ NMOSD vs MS: p=0.0195) or non-inflammatory controls. In the cases with CSF cell count >0.01 x 109/L, polymorphic cells were also observed more than 10% in 9/15 of MOG-IgG+ disease and 3/7 in AQP4-IgG+ NMOSD, but no such CSF pleocytosis was seen in MS. CSF protein was also significantly elevated in MOG-IgG+ disease and AQP4-IgG+ NMOSD than in MS (MOG-IgG+ cases vs MS: p<0.0416; AQP4-IgG+ NMOSD vs MS: p=0.0036) and control. OCB positivity was low in MOG-IgG+ disease (2/26, 7.7%) and AQP4-IgG+ NMOSD (1/10, 10%), but high in MS (13/16, 81.3%).
Cytokine, chemokine and related molecule levels in the CSF
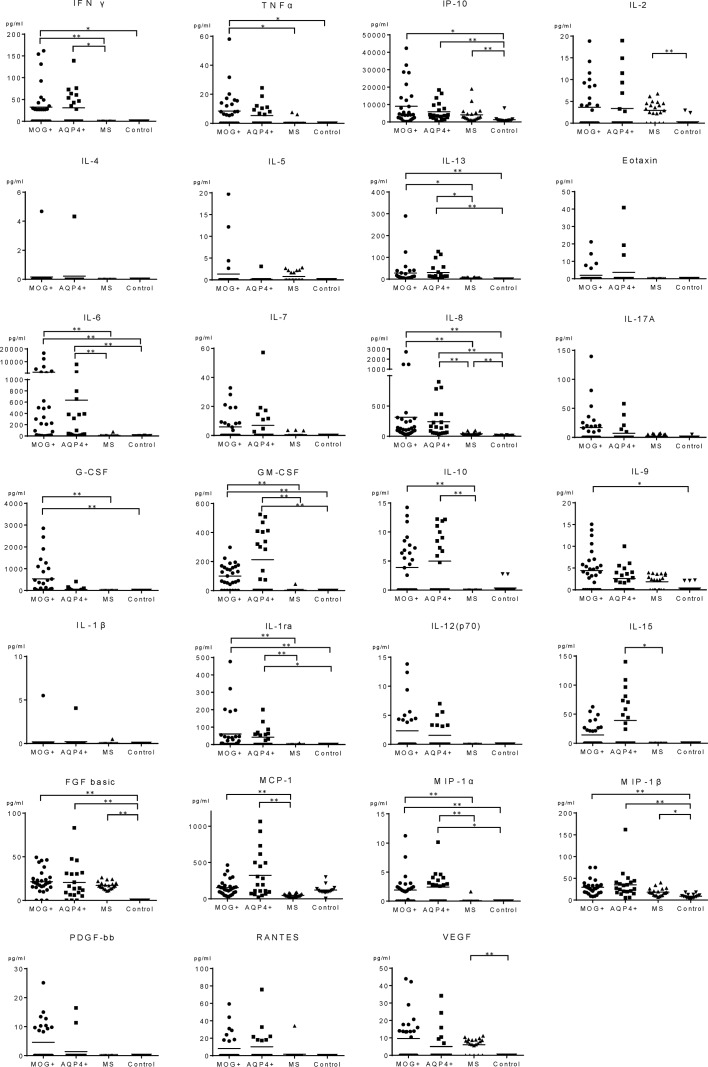
The dot plots of individual CSF cytokines, chemokines and related molecule levels are shown in figure 2 and the values are summarised in online supplementary e-Table 1. The significantly different results in the groups are summarised in table 2. In non-inflammatory controls, most cytokines, chemokines and related molecules were below detection limit. Some Th1 (IP-10, IL-2), Th17 (IL-8), other cytokines, chemokines and related molecules (FGF basic, VEGF and others) in MS were significantly elevated than in controls, but in MOG-IgG+ and AQP4-IgG+ cases, more cytokines, chemokines and related molecules including the ones associated with Th17 (IL-6, IL-8, GM-CSF), Th1 (IP-10), Th2 (IL-13) and other pathways (IL-1ra, MIP-1β and others) were significantly higher than in controls. There was no significant difference in data between patients whose sample were collected at onset or those in relapse, or between adult and paediatric MOG-IgG+ cases (online supplementary e-Figure 1). In comparison of the two autoantibody-associated diseases versus MS, cytokines, chemokines and related molecules associated with Th17 (IL-6, GM-CSF), Th1 (IFN-γ) and regulatory T cells (Treg) (IL-10) were significantly elevated in the autoantibody-associated diseases. Among AQP4-IgG+ cases, the data of Japanese and Brazilians were not significantly different. In a direct comparison of MOG-IgG+ and AQP4-IgG+ diseases, no cytokine or chemokine or related molecule was significantly different.
Figure 2.
CSF cytokine/chemokine levels in MOG-IgG+ disease, AQP4-IgG+ NMOSD, MS and controls. Dots represent CSF cytokine/chemokine levels in individual patients in MOG-IgG+ disease, AQP4-IgG+ NMOSD, MS and control groups. AQP4-IgG, aquaporin-4-IgG; CSF, cerebrospinal fluid; FGF, fibroblast growth factor; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte monocyte-colony stimulating factor; IFN, interferon; IL, interleukin; IP, IFN-γ inducible protein; MOG-IgG, myelin oligodendrocyte glycoprotein-IgG; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders; PDGF-BB, platelet-derived growth factor-BB; RANTES, regulated on activation normal T cell expressed and secreted; TNF, tumour necrosis factor; VEGF, vascular endothelial growth factor. *P<0.001, **p<0.0001.
Table 2.
Summary of CSF cytokine/chemokine profiles in patient groups
| Cytokines/Chemokines | MOG-IgG vs AQP4-IgG |
MOG-IgG vs MS |
AQP4-IgG | MOG-IgG vs control |
AQP4-IgG | MS v vs control |
|
| vs MS | vs control | ||||||
| Th1 | IFN-γ | – | >> | > | > | – | – |
| TNF-α | – | > | – | > | – | – | |
| IP-10 | – | – | – | > | >> | >> | |
| IL-2 | – | – | – | – | – | >> | |
| Th2 | IL-4 | – | – | – | – | – | – |
| IL-5 | – | – | – | – | – | – | |
| IL-13 | – | > | > | >> | >> | – | |
| Eotaxin | – | – | – | – | – | – | |
| Th17 | IL-6 | – | >> | >> | >> | >> | – |
| IL-7 | – | – | – | – | – | – | |
| IL-8 | – | >> | >> | >> | >> | >> | |
| IL-17A | – | – | – | – | – | – | |
| G-CSF | – | >> | – | >> | – | – | |
| GM-CSF | – | >> | >> | >> | >> | – | |
| Treg | IL-10 | – | >> | >> | – | – | – |
| Th9 | IL-9 | – | – | – | > | – | – |
| Others | IL-1β | – | – | – | – | – | – |
| IL-1ra | – | >> | >> | >> | > | – | |
| IL-12 (p70) | – | – | – | – | – | – | |
| IL-15 | – | – | > | – | – | – | |
| FGF basic | – | – | – | >> | >> | >> | |
| MCP-1 | – | >> | >> | – | – | – | |
| MIP-1α | – | >> | >> | >> | > | – | |
| MIP-1β | – | – | – | >> | >> | > | |
| PDGF-BB | – | – | – | – | – | – | |
| RANTES | – | – | – | – | – | – | |
| VEGF | – | – | – | – | – | >> |
‘ –’ means no significant difference, ‘>’ means significant difference at p<0.001 and ‘>>’ means p<0.0001 in the analysis of non-parametric tests (Mann-Whitney U test).
AQP4, aquaporin4; CSF, cerebrospinal fluid; FGF, fibroblast growth factor; G-CSF, granulocyte-colony stimulating factor; GM-CSF, granulocyte monocyte-colony stimulating factor; IFN, interferon; IL, interleukin; IP, IFN-γ inducible protein; MCP, monocyte chemotactic protein; MIP, macrophage inflammatory protein; MOG, myelin oligodendrocyte glycoprotein; MS, multiple sclerosis; PDGF-BB, platelet-derived growth factor-BB; RANTES, regulated on activation normal T cell expressed and secreted; Th, T helper cell; TNF, tumour necrosis factor; Treg, regulatory T cells; VEGF, vascular endothelial growth factor.
jnnp-2018-317969supp001.pdf (116.2KB, pdf)
jnnp-2018-317969supp002.pdf (53.2KB, pdf)
Correlation among levels of cytokines, chemokines and related molecules
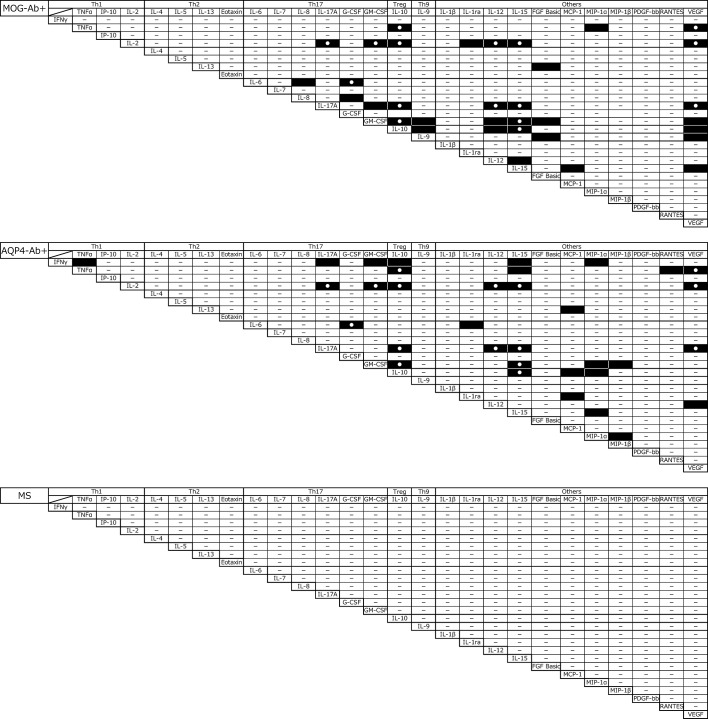
Correlations between levels of cytokines, chemokines and related molecules are shown in figure 3. In MOG-IgG+ disease, there were 34 significant correlations, mainly in Th17-related, Th9-related and Treg-related cytokines, chemokine and related molecules, such as IL-6–G-CSF (p<0.0001, r=0.7249), IL-17A–GM-CSF (p<0.0001, r=0.8392), IL-10–IL17A (p<0.0001, r=0.9136), IL-10–GM-CSF (p<0.0001, r=0.8352) and IL-9–GM-CSF (p<0.0001, r=0.8300). In AQP4-IgG+ NMOSD, the same number (34) of significant correlations in the levels of cytokine, chemokine and related molecule and 13 (IL-10–TNF-α, IL-10–IL-2, IL-17A–IL-2, IL-6–G-CSF, IL-10–GM-CSF, IL-15–GM-CSF, IL-10–IL-15, IL-15–IL-17A, GM-CSF–IL-2, IL-12–IL-2, IL-12–IL-17A, IL-15–IL-2, IL-17–VEGF, VEGF–TNF-α and VEGF–IL-2) of which were shared by MOG-IgG+ disease were seen, while no such significant correlations of cytokines, chemokines and related molecule levels were observed in MS.
Figure 3.
Correlation matrices of CSF cytokines/chemokines in MOG-IgG+ disease, AQP4-IgG+ NMOSD and MS. Significant correlations in pairs of CSF cytokines/chemokines are shown. Black rectangles represent p<0.0001 and white circles represent significant correlations in both MOG-IgG+ disease and AQP4-IgG+ NMOSD. AQP4-IgG, aquaporin-4-IgG; CSF, cerebrospinal fluids; MOG-IgG, myelin oligodendrocyte glycoprotein-IgG; MS, multiple sclerosis; NMOSD, neuromyelitis optica spectrum disorders.
MBP and GFAP levels in the CSF
The MBP levels were significantly elevated in both MOG-IgG+ disease (249 pg/mL (31.3–5837 pg/mL)) and AQP4-IgG+ NMOSD (490.5 pg/mL (31.3–3760 pg/mL)) than in MS (31.3 pg/mL (31.3–872.2 pg/mL)) (MOG vs MS: p=0.0005; AQP4 vs MS: p=0.0002) and in the control (31.3 pg/mL (31.3–46 pg/mL)). The GFAP levels were remarkably elevated in AQP4-IgG+ NMOSD (604.2 ng/mL (0.45–57906 ng/mL)) than in MOG-IgG+ disease (0.0035 ng/mL (0.0035–6.86 ng/mL)), MS (0.54 ng/mL (0.25–1.59 ng/mL)) and in the control (0.77 ng/mL (0–2.83 ng/mL)) (MOG vs AQP4: p<0.0001; MOG vs MS: p=0.0069; AQP4 vs MS p<0.0001) (online supplementary e-Figure 2). In AQP4-IgG+ NMOSD, IL-6 level correlated well with GFAP level, CSF cell count and MBP level (online supplementary e-Table 2). In contrast, in MOG-IgG+ disease, IL-6 and G-CSF weakly correlated with CSF-MOG-IgG titre but not with serum MOG-IgG titre (online supplementary e-Table 2). IL-6 level did not correlate with CSF cell count or MBP level. As for correlations between the cell damage markers and levels of cytokine, chemokine and related molecule in MOG-IgG+ disease, MBP correlated with IP-10 (p<0.0001, r=0.5796) and MIP1a (p=0.0003, r=0.6225), while no correlations were seen in GFAP and levels of cytokines, chemokines and related molecules.
jnnp-2018-317969supp003.pdf (35.5KB, pdf)
jnnp-2018-317969supp004.pdf (35.8KB, pdf)
Discussion
In this study, we investigated the levels of cytokines, chemokines and related molecules in the CSF in the acute phase of MOG-IgG+ disease, AQP4-IgG+ NMOSD and MS and compared them with controls. Such a comparative study has not been done before. We also compared the data in adult and paediatric MOG-IgG+ cases.
As a result, some Th1 (IL-2 and IP-10) and other cytokines, chemokines and related molecules (IL-8, IL-15, FGF basic and VEGF) were high in MS. The Th1 polarisation seen in MS is essentially in agreement with previous reports.25 However, MOG-IgG+ disease showed more significant upregulation of Th17-related cytokines (IL-6, IL-8, G-CSF and GM-CSF), Th1-related cytokines (IFN-γ and TNF-α), Treg (IL-10) as well as other cytokines (IL-1ra, MCP-1 and MIP-1ß) in comparison with MS. The data in adult and paediatric MOG-IgG+ cases were not significantly different. In MOG-IgG+ disease, many patients without elevated cytokines had ON. On the other hand, AQP4-IgG+ NMOSD showed a largely similar pattern to MOG-IgG+ disease, and no cytokine level was significantly different in the two autoantibody-associated CNS diseases. Moreover, higher CSF cell counts, mainly mononuclear cells in many cases but polymorphonuclear pleocytosis in some, were evident in MOG-IgG+ disease and AQP4-IgG+ NMOSD. Additionally, 34 significant correlations among levels in cytokines, chemokines and related molecules were seen in MOG-IgG+ disease and AQP4-IgG+ NMOSD, which were not observed in MS. These data imply a coordinated upregulation of Th17 and other cytokines, chemokines and related molecules in MOG-IgG+ disease and AQP4-IgG+ NMOSD compared with MS. Low OCB positivity (10% or less) in MOG-IgG+ disease and AQP4-IgG+ NMOSD was also a distinguishing laboratory feature from MS (over 80%).
A previous study demonstrated elevations of B cell-related (CXCL13, APRIL, BAFF and CCL19), Th17-related (IL6 and G-CSF) and neutrophil-related (IL8, G-CSF) cytokines in the CSF in 10 MOG-IgG+ children with ADEM.20 In another study, CSF-IL-6 and serum MOG-IgG titres were mildly correlated in 12 paediatric MOG-IgG+ patients with acquired demyelinating syndrome.21 Our study is larger in the number of MOG-IgG+ cases (n=29) and confirmed upregulation of Th17-related cytokine in the CSF of MOG-IgG+ diseases. We also compared the data with those in AQP4-IgG+ NMOSD, MS and controls and analysed both MOG-IgG+ adults and children with various clinical phenotypes including ADEM. Levels of cytokines, chemokines and related molecules in CSF may vary in studies with different MOG-IgG+ samples and methods (eg, CSF-IL-6 levels were 171 (5–745.4) pg/mL (bead-based assay, Millipore),20 66.3±23.7 pg/mL (bead-based assay, BD Bioscience),21 1649.3±3836.2 (bead-based assay, Bio-Rad) in the present study, and 9460 pg/mL (ELISA) in a single case report by Amano H, et al, BMC Neurol, 2014). It may be important to know whether the levels of CSF cytokines, chemokines and related molecules during attacks are different in adults and children from the viewpoint of age-related modification of disease, but we found no significant differences of CSF cytokine levels in the two age groups of MOG-IgG+ disease.
According to the data we obtained, the Th17-related and Treg-related cytokines may be a prominent part in causing the pathological conditions in the CNS of MOG-IgG+ disease and AQP4-IgG+ NMOSD. In the process of T cell differentiation into its subsets, a network of cytokines, chemokines and related molecules may play a pivotal role. Although the exact mechanisms remain unknown, IL-6 induces a differentiation of CD4+ T cells into Th17 cells. In addition, IL-6 can stimulate plasmablasts as well as B cells, contributing to autoantibody production. CSF-IL-6 may be produced by lymphocytes but further analyses are needed. G-CSF, GM-CSF and IL-17A are mainly derived from Th17 cells. These Th17-related cytokines can recruit inflammatory cells including polymorphonuclear cells like neutrophils in the CNS, and the humoral and cellular immunity may jointly induce CNS inflammation in MOG-IgG+ disease and AQP4-IgG+ NMOSD. Elevated CSF-IL-8 in MOG-IgG+ disease may be associated with CSF-polymorphonuclear pleocytosis. IL-15 upregulated in MOG-IgG+ disease, AQP4-IgG+ NMOSD and MS is a possible trigger of IL17 under certain situations26 and could be an integral part of Th17-associated proinflammatory mechanisms. Th9 is a recently identified T cell population27 28 and is also differentiated from CD4+ T cells and secretes IL-9. IL-9 is reported to promote differentiation of Th17 and Treg.29 In our study, IL-9 correlated well with some Th17-related cytokines and IL-10, and thus Th9+ T cell subset may also contribute to the pathogenesis in MOG-IgG+ disease and AQP4-Ab+ NMOSD, although IL-9 levels in MOG-IgG+ disease and AQP4-IgG+ NMOSD fell short of reaching a statistical significance in comparison with MS. We did not measure serum cytokines, but they may also be important in considering the immunopathogenesis of MOG-IgG+ disease.
Whether MOG-IgG is pathogenic and directly involved in the pathogenesis is not fully understood,10 but some recent studies support MOG-IgG’s pathogenicity. For example, MOG-IgG can induce alteration of oligodendrocyte cytoskeleton organisation in vitro.30 A few reports of biopsied cases showed there were depositions of IgG and complements associated with demyelination in the lesions of cases with MOG-IgG+ disease, which is consistent with the MS pattern II pathology and suggests a pathogenic role for humoral immunity in the lesion formation.31–33 Intracerebral injection of MOG-IgG alone (without complements) into mouse brain induced myelin changes and altered the expression of axonal proteins that are essential for action potential firing, but did not produce inflammation, axonal loss, neuronal or astrocyte death.34 Another recent study demonstrated MOG-IgG and complements could induce callosal demyelination in an animal study, and interestingly the pathology was dependent on type I IFN.35 A remarkable elevation of CSF-MBP without elevation of GFAP in MOG-IgG+ disease confirmed in the present and previous studies indicates that the myelin antibody-associated CNS disease is an inflammatory demyelinating disease.17 18 However, unlike AQP4-IgG+ NMOSD, in which IL-6 level significantly correlated with CSF-GFAP, MBP levels and cell count, such correlations were not observed in MOG-IgG+ disease, suggesting that the relation between proinflammatory cytokines, chemokines and related molecules and cell damages in the two autoantibody-associated CNS diseases may not be similar.
The distinct profiles of CSF cytokines, chemokines and related molecules between the two autoantibody-associated CNS diseases and MS may have therapeutic implications. Since some disease-modifying therapies (DMT) for MS, such as IFN-β, fingolimod and natalizumab, exacerbate AQP4-IgG+ NMOSD,36–38 a similar therapeutic response might occur in MOG-IgG+ disease. Therapeutic evidence in MOG-IgG+ disease is still very limited, but there is a case report of early relapse (ON on day 19 of fingolimod treatment) in MOG-IgG+ case receiving fingolimod.39 A German study on 50 cases of MOG-IgG+ disease suggested that relapses tended to occur in connection with insufficient immunosuppression and that all four patients treated with IFN-β had ongoing or increasing disease activity.9 The failure of IFN-β, a type I IFN, in MOG-IgG+ disease and multiple DMT (including IFN-β) in MOG-IgG+ ‘MS’ cases40 is consistent with the stimulatory influence of type I IFN signalling on MOG-IgG-mediated demyelination in an animal study.35 The unique immunological profiles characterised by the upregulation of Th17 and other cytokines, chemokines and related molecules including some Th1-related and Treg-related ones seen in our study of MOG-IgG+ and AQP4-IgG+ cases may predict poor responses to some DMT for MS.
There are some limitations in our study. First, there might be a selection bias of patients because the samples sent to our laboratory were chosen by neurologists in each institute, which might have influenced the proportion of the clinical phenotypes in the patient groups (eg, 75% of cases with AQP4-IgG+ NMOSD had myelitis), and the timing of lumbar punctures might make an impact on the results of levels of cytokine, chemokine and related molecules. However, there was no clear association between the levels of cytokines, chemokines and related molecules and the site of lesions/clinical phenotypes, and the intervals between attacks and lumbar punctures were not different among the patient groups. Moreover, the data on Japanese and Brazilian AQP4-IgG+ cases were not different. Second, although majority of the samples were from patients whose samples were collected before acute-phase treatment, three patients with MOG-IgG+ relapsing disease had been treated with low-dose prednisolone (5 mg/day or less), which might have an impact on the CSF cytokine analysis. But the levels of cytokine, chemokine and related molecule in those patients were not necessarily low. Third, it is unknown if the levels of CSF cytokines, chemokines and related molecules in individual patients may fluctuate in relapses and are as low as those in non-inflammatory disease controls. Measuring CSF-C5a in MOG-IgG+ cases would also be interesting in terms of the myelin antibody’s complement-mediated cytotoxicity. Larger scale prospective studies are expected to address these issues.
Acknowledgments
The authors thank Yuri Atobe, Sora Bang and Kaori Tobise for technical assistance.
Footnotes
Contributors: KK: contributed to study conception, sample collection, cytokine analysis, interpretation of data, and drafting the manuscript including the figures, tables and references; completion of the work to be submitted; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. DKS: contributed to study conception, cytokine analysis, interpretation of data and revised the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. IN: contributed to study conception, interpretation of data and revised the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. RO: contributed to study conception, interpretation of data and reviewed the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. TA: contributed to interpretation of data especially in statistical analysis, and reviewed the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. YT and TM: contributed to interpretation of data and reviewed the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. SN: contributed to interpretation of data, helped in measuring glial fibrillar acidic protein and myelin basic protein, and reviewed the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. TT: contributed to study conception, sample collection and reviewed the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. HK: contributed to interpretation of data and reviewing the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. ST: contributed to sample collection and reviewing of the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. KN: contributed to sample collection and reviewed the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. YH: contributed to sample collection and reviewed the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. DC: contributed to study conception and reviewed the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. LS: contributed to interpretation of data and revised the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. KF: contributed to study conception, supervision of the acquisition, interpretation of data, revised the manuscript including the figures, tables and references; provided final approval of the version to be published; agreed to be accountable for all aspects of the work. MA: contributed to study conception, supervision of the acquisition, analysis and interpretation of data, and reviewed the manuscript; provided final approval of the version to be published; agreed to be accountable for all aspects of the work.
Funding: This study was partially supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI), and the Health and Labour Sciences Research Grant on Intractable Diseases (neuroimmunological diseases) from the Ministry of Health, Labour and Welfare of Japan. This study was not industry-sponsored.
Competing interests: DKS has received a scholarship from the Ministry of Education, Culture, Sports, Science and Technology (MEXT) of Japan, Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (KAKENHI 15K19472), research support from CAPES/Brazil (CSF-PAJT—88887.091277/ 2014-00) and speaker honoraria from Novartis. IN has received funding for travel and received speaker honoraria from Mitsubishi Tanabe Pharma Corporation and has received research funding from LSI Medience Corporation and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. YT has received research support from Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. SN has received research support from Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. TM has received speaker honoraria from Bayer Schering Pharma, Biogen Idec Japan, Mitsubishi Tanabe Pharma Corporation, Asahi Kasei Medical and Astellas Pharma, and has received research support from Bayer Schering Pharma, Biogen Idec Japan, Asahi Kasei Kuraray Medical, Chemo-Sero-Therapeutic Research Institute, Teva Pharmaceutical KK, Mitsubishi Tanabe Pharma Corporation, Teijin Pharma, and Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare of Japan. HK has received research support from Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan. KN reports personal fees from Mitsubishi Tanabe Pharma Corporation, personal fees from Astellas Pharma, personal fees from Chemo-Sero-Therapeutic Research Institute and personal fees from Teijin Pharma. LS served on the scientific advisory board for Novartis, Receptos, Atreca, Tolerion and Teva, received travel funding and/or speaker honoraria from Biogen, Bayhill, Bayer, Celgene and Receptos, is on the editorial board for Multiple Sclerosis Journal and Proceedings of the National Academy of Science, holds patents for antigen-specific tolerance, has a patent pending for cytokines and type 1 interferons, is on the speakers' bureau for EMD Serono, received research support from NIH, has stock options and board membership in Tolerion, and is on the board of directors for BioAtla. KF serves on the scientific advisory boards for Bayer Schering Pharma, Biogen Idec, Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical, Merck Serono, Alexion Pharmaceuticals, MedImmune and Medical Review; has received funding for travel and speaker honoraria from Bayer Schering Pharma, Biogen Idec, Eisai, Mitsubishi Tanabe Pharma Corporation, Novartis Pharma, Astellas Pharma, Takeda Pharmaceutical Company, Asahi Kasei Medical, Daiichi Sankyo and Nihon Pharmaceutical; serves as an editorial board member of Clinical and Experimental Neuroimmunology (2009–present) and an advisory board member of Sri Lanka Journal of Neurology; has received research support from Bayer Schering Pharma, Biogen Idec Japan, Asahi Kasei Medical, Chemo-Sero-Therapeutic Research Institute, Teva Pharmaceutical, Mitsubishi Tanabe Pharma, Teijin Pharma, Chugai Pharmaceutical, Ono Pharmaceutical, Nihon Pharmaceutical and Genzyme Japan; is funded by the Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (#22229008, 2010-2015; #26293205, 2014-2016) and by the Grants-in-Aid for Scientific Research from the Ministry of Health, Welfare and Labour of Japan (2010–present). MA has received research support from Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, and the Ministry of Health, Labour and Welfare of Japan.
Patient consent: Obtained.
Ethics approval: Ethics approval was granted by the Ethics Committee of Tohoku University Graduate School of Medicine, Sendai, Japan. All the patients gave informed consent for their participation.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: Since this article was published online first changes have been made to the headings in table two.
References
- 1. Johns TG, Bernard CC. The structure and function of myelin oligodendrocyte glycoprotein. J Neurochem 1999;72:1–9. 10.1046/j.1471-4159.1999.0720001.x [DOI] [PubMed] [Google Scholar]
- 2. Linington C, Bradl M, Lassmann H, et al. . Augmentation of demyelination in rat acute allergic encephalomyelitis by circulating mouse monoclonal antibodies directed against a myelin/oligodendrocyte glycoprotein. Am J Pathol 1988;130:443–54. [PMC free article] [PubMed] [Google Scholar]
- 3. Johns TG, Kerlero de Rosbo N, Menon KK, et al. . Myelin oligodendrocyte glycoprotein induces a demyelinating encephalomyelitis resembling multiple sclerosis. J Immunol 1995;154:5536–41. [PubMed] [Google Scholar]
- 4. Ramanathan S, Dale RC, Brilot F. Anti-MOG antibody: The history, clinical phenotype, and pathogenicity of a serum biomarker for demyelination. Autoimmun Rev 2016;15:307–24. 10.1016/j.autrev.2015.12.004 [DOI] [PubMed] [Google Scholar]
- 5. Brilot F, Dale RC, Selter RC, et al. . Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol 2009;66:833–42. 10.1002/ana.21916 [DOI] [PubMed] [Google Scholar]
- 6. Pröbstel AK, Dornmair K, Bittner R, et al. . Antibodies to MOG are transient in childhood acute disseminated encephalomyelitis. Neurology 2011;77:580–8. 10.1212/WNL.0b013e318228c0b1 [DOI] [PubMed] [Google Scholar]
- 7. Mader S, Gredler V, Schanda K, et al. . Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflammation 2011;8:184 10.1186/1742-2094-8-184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hacohen Y, Absoud M, Deiva K, et al. . Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol Neuroimmunol Neuroinflamm 2015;2:e81 10.1212/NXI.0000000000000081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jarius S, Ruprecht K, Kleiter I, et al. . MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation 2016;13:280 10.1186/s12974-016-0718-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Peschl P, Bradl M, Höftberger R, et al. . Myelin Oligodendrocyte Glycoprotein: Deciphering a Target in Inflammatory Demyelinating Diseases. Front Immunol 2017;8:529 10.3389/fimmu.2017.00529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ramanathan S, Mohammad S, Tantsis E, et al. . Clinical course, therapeutic responses and outcomes in relapsing MOG antibody-associated demyelination. J Neurol Neurosurg Psychiatry 2018;89:127–37. 10.1136/jnnp-2017-316880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jurynczyk M, Messina S, Woodhall MR, et al. . Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain 2017;140:3128–38. 10.1093/brain/awx276 [DOI] [PubMed] [Google Scholar]
- 13. Waters P, Woodhall M, O’Connor KC, et al. . MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol Neuroimmunol Neuroinflamm 2015;2:e89 10.1212/NXI.0000000000000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sato DK, Callegaro D, Lana-Peixoto MA, et al. . Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014;82:474–81. 10.1212/WNL.0000000000000101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kitley J, Waters P, Woodhall M, et al. . Neuromyelitis optica spectrum disorders with aquaporin-4 and myelin-oligodendrocyte glycoprotein antibodies: a comparative study. JAMA Neurol 2014;71:276–83. 10.1001/jamaneurol.2013.5857 [DOI] [PubMed] [Google Scholar]
- 16. Takano R, Misu T, Takahashi T, et al. . Astrocytic damage is far more severe than demyelination in NMO: a clinical CSF biomarker study. Neurology 2010;75:208–16. 10.1212/WNL.0b013e3181e2414b [DOI] [PubMed] [Google Scholar]
- 17. Ikeda K, Kiyota N, Kuroda H, et al. . Severe demyelination but no astrocytopathy in clinically definite neuromyelitis optica with anti-myelin-oligodendrocyte glycoprotein antibody. Mult Scler 2015;21:656–9. 10.1177/1352458514551455 [DOI] [PubMed] [Google Scholar]
- 18. Kaneko K, Sato DK, Nakashima I, et al. . Myelin injury without astrocytopathy in neuroinflammatory disorders with MOG antibodies. J Neurol Neurosurg Psychiatry 2016;87:1257–9. 10.1136/jnnp-2015-312676 [DOI] [PubMed] [Google Scholar]
- 19. Uzawa A, Mori M, Arai K, et al. . Cytokine and chemokine profiles in neuromyelitis optica: significance of interleukin-6. Mult Scler 2010;16:1443–52. 10.1177/1352458510379247 [DOI] [PubMed] [Google Scholar]
- 20. Kothur K, Wienholt L, Tantsis EM, et al. . B Cell, Th17, and Neutrophil Related Cerebrospinal Fluid Cytokine/Chemokines Are Elevated in MOG Antibody Associated Demyelination. PLoS One 2016;11:e0149411 10.1371/journal.pone.0149411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Horellou P, Wang M, Keo V, et al. . Increased interleukin-6 correlates with myelin oligodendrocyte glycoprotein antibodies in pediatric monophasic demyelinating diseases and multiple sclerosis. J Neuroimmunol 2015;289:1–7. 10.1016/j.jneuroim.2015.10.002 [DOI] [PubMed] [Google Scholar]
- 22. Akaishi T, Sato DK, Nakashima I, et al. . MRI and retinal abnormalities in isolated optic neuritis with myelin oligodendrocyte glycoprotein and aquaporin-4 antibodies: a comparative study. J Neurol Neurosurg Psychiatry 2016;87:446–8. 10.1136/jnnp-2014-310206 [DOI] [PubMed] [Google Scholar]
- 23. Wingerchuk DM, Banwell B, Bennett JL, et al. . International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–89. 10.1212/WNL.0000000000001729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Polman CH, Reingold SC, Banwell B, et al. . Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 2011;69:292–302. 10.1002/ana.22366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kothur K, Wienholt L, Brilot F, et al. . CSF cytokines/chemokines as biomarkers in neuroinflammatory CNS disorders: A systematic review. Cytokine 2016;77:227–37. 10.1016/j.cyto.2015.10.001 [DOI] [PubMed] [Google Scholar]
- 26. Ferretti S, Bonneau O, Dubois GR, et al. . IL-17, produced by lymphocytes and neutrophils, is necessary for lipopolysaccharide-induced airway neutrophilia: IL-15 as a possible trigger. J Immunol 2003;170:2106–12. 10.4049/jimmunol.170.4.2106 [DOI] [PubMed] [Google Scholar]
- 27. Veldhoen M, Uyttenhove C, van Snick J, et al. . Transforming growth factor-beta ’reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol 2008;9:1341–6. 10.1038/ni.1659 [DOI] [PubMed] [Google Scholar]
- 28. Dardalhon V, Awasthi A, Kwon H, et al. . IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol 2008;9:1347–55. 10.1038/ni.1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Elyaman W, Bradshaw EM, Uyttenhove C, et al. . IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc Natl Acad Sci U S A 2009;106:12885–90. 10.1073/pnas.0812530106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dale RC, Tantsis EM, Merheb V, et al. . Antibodies to MOG have a demyelination phenotype and affect oligodendrocyte cytoskeleton. Neurol Neuroimmunol Neuroinflamm 2014;1:e12 10.1212/NXI.0000000000000012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Di Pauli F, Höftberger R, Reindl M, et al. . Fulminant demyelinating encephalomyelitis: Insights from antibody studies and neuropathology. Neurol Neuroimmunol Neuroinflamm 2015;2:e175 10.1212/NXI.0000000000000175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spadaro M, Gerdes LA, Mayer MC, et al. . Histopathology and clinical course of MOG-antibody-associated encephalomyelitis. Ann Clin Transl Neurol 2015;2:295–301. 10.1002/acn3.164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jarius S, Metz I, König FB, et al. . Screening for MOG-IgG and 27 other anti-glial and anti-neuronal autoantibodies in ’pattern II multiple sclerosis' and brain biopsy findings in a MOG-IgG-positive case. Mult Scler 2016;22:1541–9. 10.1177/1352458515622986 [DOI] [PubMed] [Google Scholar]
- 34. Saadoun S, Waters P, Owens GP, et al. . Neuromyelitis optica MOG-IgG causes reversible lesions in mouse brain. Acta Neuropathol Commun 2014;2:35 10.1186/2051-5960-2-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Berg CT, Khorooshi R, Asgari N, et al. . Influence of type I IFN signaling on anti-MOG antibody-mediated demyelination. J Neuroinflammation 2017;14:127 10.1186/s12974-017-0899-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shimizu J, Hatanaka Y, Hasegawa M, et al. . IFNβ-1b may severely exacerbate Japanese optic-spinal MS in neuromyelitis optica spectrum. Neurology 2010;75:1423–7. 10.1212/WNL.0b013e3181f8832e [DOI] [PubMed] [Google Scholar]
- 37. Min JH, Kim BJ, Lee KH. Development of extensive brain lesions following fingolimod (FTY720) treatment in a patient with neuromyelitis optica spectrum disorder. Mult Scler 2012;18:113–5. 10.1177/1352458511431973 [DOI] [PubMed] [Google Scholar]
- 38. Gahlen A, Trampe AK, Haupeltshofer S, et al. . Aquaporin-4 antibodies in patients treated with natalizumab for suspected MS. Neurol Neuroimmunol Neuroinflamm 2017;4:e363 10.1212/NXI.0000000000000363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Miyazaki T, Nakajima H, Motomura M, et al. . A case of recurrent optic neuritis associated with cerebral and spinal cord lesions and autoantibodies against myelin oligodendrocyte glycoprotein relapsed after fingolimod therapy. Rinsho Shinkeigaku 2016;56:265–9. 10.5692/clinicalneurol.cn-000756 [DOI] [PubMed] [Google Scholar]
- 40. Spadaro M, Gerdes LA, Krumbholz M, et al. . Autoantibodies to MOG in a distinct subgroup of adult multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2016;3:e257 10.1212/NXI.0000000000000257 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jnnp-2018-317969supp001.pdf (116.2KB, pdf)
jnnp-2018-317969supp002.pdf (53.2KB, pdf)
jnnp-2018-317969supp003.pdf (35.5KB, pdf)
jnnp-2018-317969supp004.pdf (35.8KB, pdf)