Abstract
Microglia are resident immune cells of the brain that can regulate neural communication and excitability. Any environmental influence on microglial activity has the potential to alter subsequent neural physiology and behavior. Within the prefrontal cortex, several types of stressors have been shown to increase microglial expression of activation markers such as ionized calcium-binding adapter molecule-1 (Iba-1), which suggests altered microglial activity. Recent reports in rodents suggest that exposure to forms of early-life stress such as maternal separation can alter microglial responsivity to subsequent challenges. Several learning paradigms used in rodents require food restriction to provoke motivational states that facilitate approach behaviors. Here, we tested whether food restriction (increasing from 13 g/day-23 g/day in males and 10 g/day-20 g/day in females, which reduced body weight to 72-84% free-fed weight) in adolescent rats is a sufficient challenge to affect microglial Iba-1 expression, and whether previous exposure to postnatal maternal separation influenced microglial outcomes. We measured prefrontal cortex Iba-1 expression and microglial morphology after 20 days of ad libitum or restricted food availability in males and females with or without exposure to maternal separation. Food-restricted animals displayed higher levels of Iba-1 in the prefrontal cortex, with hyper-ramified microglial morphology in maternally separated males and control females, compared to those that were free-fed. Together, our data provide evidence that food restriction paradigms may have unintended effects in some behavioral protocols.
Keywords: maternal separation, prefrontal cortex, early-life stress, food deprivation, adolescence, two-hit hypothesis
Exposure to early-life stress increases vulnerability to psychiatric disorders, including depression, drug abuse and schizophrenia (Teicher, Tomoda, & Andersen, 2006). Since these disorders often first emerge in adolescence (Andersen, 2008; Davey, Yucel , & Allen, 2008), intervening variables found in clinical studies make the role that early-life stress plays in these diseases difficult to interpret. Maternal separation (MS) is a well-characterized animal model of early-life stress that can help clarify the causality of early-life stress effects. Several experimental studies using MS have reported effects in the prefrontal cortex (PFC) (Ganguly and Brenhouse, 2015). The PFC is a relatively late-maturing region (Alexander and Goldman, 1978) and subserves all higher-order cognitive and emotional functions. Notably, most consequences of MS in the PFC typically manifest later in life (Chocyk, Dudys, Przyborowska, Mackowiak, & Wedzony, 2010; Helmeke, Ovtscharoff, Poeggel, & Braun, 2008; Stevenson, Marsden, & Mason, 2008) probably due to its late and protracted development. The late maturation of the PFC is also a purported factor driving the “two-hit” hypothesis of psychiatric illness; a distinct developmental insult can prime an individual for a later event that ultimately leads to onset of the full clinical syndrome (Bayer, Falkai, & Maier, 1999; Deslauriers, Racine, Sarret, & Grignon, 2014).
Experimental studies in animal models have highlighted a link between early-life stress and markers of immune function in the central nervous system—an apparently sexually dimorphic phenomenon with greater effect sizes in male animals (Ganguly and Brenhouse, 2015). For example, early MS was associated with greater synaptic levels of the receptor for the pro-inflammatory cytokine interleukin-1 (IL-1) (Viviani et al., 2013) and greater number and motility of cortical microglial processes (Takatsuru, Nabekura, Ishikawa, Kohsaka, & Koibuchi, 2015). Notably, early-life stress also appears to be associated with greater reactivity to subsequent psychosocial and immune challenges. Several studies have reported potentiated expression of the microglial activation marker Iba-1 during the peripubertal period (Giovanoli et al., 2013) or adulthood (Diz-Chaves, Astiz, Bellini, & Garcia-Segura, 2013) following a subsequent “hit” after an early-life challenge. Increased Iba-1 expression, as well as morphological changes in soma size and branch complexity, is indicative of microglial activation changes (Kozlowski and Weimer, 2012). These findings lend support to the hypothesis that early-life stress primes microglia, leading to a potentiated response to subsequent stress. However, while several stressors have been shown to increase Iba-1 in the PFC, little is known about whether the potentiating effects of early-life stress and a subsequent challenge occur in the PFC (Calcia et al., 2016).
Food restriction is commonly used in animal studies as a motivator for tasks involving approach to a reward. Concurrently, food restriction has also been utilized as a mild stressor in studies investigating effects of stress on behavior (Deroche et al., 1995), and has been shown to increase corticosterone levels in adolescents and adults (Anderson, Bush, & Spear, 2013). Surprisingly, researchers often overlook the potential for food restriction in behavioral studies to have unintended effects, possibly due to its induction of stress. Here, we investigated whether food restriction in adolescence could contribute as a repeated stressor following early life stress, to increase microglial Iba-1 in the PFC. Since MS has differential effects on behavior and neuroimmune development in males and females, we also examined sex differences in microglial changes.
Methods
All experiments were performed in accordance with the 1996 Guide for the Care and Use of Laboratory Animals (NIH) with approval from the Institutional Animal Care and Use Committee at Northeastern University.
Subjects
Twenty gestational day 15 pregnant Sprague-Dawley rats were received from Charles River Laboratories (Wilmington MA), and were housed under constant temperature- and humidity-controlled conditions within a 12 h light/dark cycle (light period 0700h-1900h), with water and food available ad libitum. Litters were culled to 10 pups on postnatal day (P)1, maintaining as equal ratios as possible of males and females. Ten litters were designated as maternal separation (MS), wherein each pup was separated in an isolated container for 4 hours/day (1000h-1400h) from P2-P20 in a thermo-regulated environment at 37°C. Apart from weighing and normal husbandry procedures, twenty control (Con) litters were undisturbed until weaning (P21) when all animals were rehoused with a cage-mate matched for sex and group (free-fed or food-restricted). Only one male and one female per litter per group were used, to avoid any litter effects. Therefore, each of ten MS litters and twenty Con litters supplied two males and two females.
Beginning on P36, half of MS animals and half of Con animals (9-12/sex/group) were placed on a food-restricted diet that aimed to reduce body weight to ~80% free-fed weight. The paradigm was developed based on earlier experiments in our laboratory wherein we observed that free-fed males and females between P35-P55 consume between 15-25g or 12-23g, respectively, increasing daily intake by approximately one gram every two days [unpublished observations, but similarly reported in (Amancio-Belmont, Romano-Lopez, Ruiz-Contreras, Mendez-Diaz, & Prospero-Garcia, 2017) and (Robison et al., 2017)]. We have observed that limiting food to 85-90% of free-fed intake reduced subject weight to ~80% of free-fed weight. Therefore, all food was removed from the food hopper on P36 and rats were fed 10g – 20 g/day (females) or 13g – 23 g/day (males) of their Prolab® diet (RMA-3000, 5P00, TestDiet®, St. Louis, MO), beginning with 10 g/day (females) or 13 g/day (males) on P36 and increasing by 2g every four days. Food was supplied daily at approximately 1600h. Free-fed animals remained on an ad libitum feeding regimen. On P55, all rats were weighed and sacrificed for analysis of PFC Iba-1 expression and microglial morphology.
IBA-1 immunohistochemistry
For immunohistochemical analyses, animals were euthanized and intracardially perfused with ice-cold 4% paraformaldehyde (in 0.1 M phosphate buffer [PB], pH 7.4). Tissue was processed using standard immunohistochemical methods. Briefly, brains were cryoprotected in 30% sucrose solution in 0.1 M PB, and then sectioned on a freezing microtome at a thickness of 40μm. Sections were washed with phosphate-buffered saline (PBS) and blocked in PBS with 10% normal donkey serum (NGS) and 0.1% Triton X. Sections were subsequently incubated with a monoclonal antibody raised in rabbit against Iba-1 (1:1000, Wako Chemicals, Richmond, VA, USA) overnight at 4°C. On day 2, sections were incubated in biotinylated anti-rabbit secondary serum conjugated with Alexa Fluor 488 (1:500, ThermoFisher Scientific, Waltham, MA). Following this, sections were mounted on gelatin-coated slides, and coverslipped with Fluoromount (ThermoFisher Scientific, Waltham, MA).
Image capture and analysis
Images of PFC Iba-1 immunofluorescence were taken at 20x magnification on a Zeiss AxioImager M2 microscope system. Exposure times were set such that background from primary delete control would be zero, and kept consistent across samples. Quantification of microglial surface area in tissue sections was performed using ImageJ (1.48v, http://imagej.nih.gov.ij), a publically available image analysis package. For each animal, ten regions of interest were taken within the prelimbic region of the PFC, over five coronal sections (two 430μm × 330μm regions of interest/section; see Figure 1). The adjusted threshold function was used to filter out non-specific signals. Three stringency levels were generated (“low”, “medium”, “high”), representing the light intensity of Iba-1 immunofluorescence. Thereafter, the percent surface area of Iba-1 positive regions over all regions of interest were measured and confirmed for robustness across the set thresholds.
Figure 1.
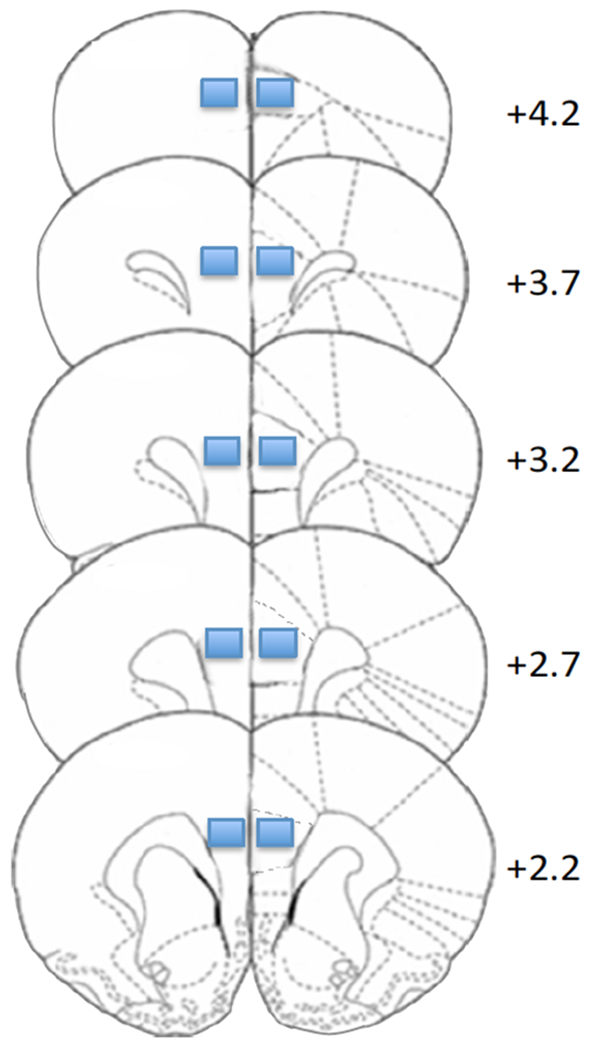
Illustration of regions of interest (rectangles) analyzed for prelimbic PFC Iba-1. Numerals indicate mm from bregma (Paxinos and Watson, 1998)
A second analysis of the same regions was performed to determine morphological differences in microglia, as previously described (Vollmer et al., 2016). Briefly, acquired images were analyzed using ImageJ software to quantify soma area and microglial process complexity and length (ramification). The area of each soma that was in full-focus was measured using the “Freehand line” followed by “Analyze and Measure,” and an average was taken of all cells in each image. Images were then processed using ImageJ “Despeckle” to remove single pixel background staining, then converted to binary images and skeletonized. The “Analyze Skeleton” ImageJ plugin (Arganda-Carreras, Fernandez-Gonzalez, Munoz-Barrutia, & Ortiz-De-Solorzano, 2010) was then used to quantify the length and number of endpoints for each branch. These measures were then summed and normalized to the number of cells in each image.
Statistics
Differences in weight on the day of sacrifice and % change in weight from P36 to P55 were measured between groups using a 3-way (Rearing Group × Feeding Group × Sex) analysis of variance (ANOVA), with Bonferroni correction for multiple comparisons. Differences in percent surface area of PFC Iba-1 expression were first compared across thresholding parameters using a mixed (Rearing Group × [Threshold]) ANOVA in males and females separately. When no interaction with threshold was found, values across thresholds were averaged and percent surface area was compared using 3-way (Rearing Group × Feeding Group × Sex) ANOVA with Tukey correction for multiple comparisons. Branch length and number of end-points were compared using 3-way (Rearing Group × Feeding Group × Sex) ANOVA with Tukey correction for multiple comparisons. Finally, body weight change and Iba-1 expression levels in food-restricted rats were analyzed for correlation using Pearson correlational analysis.
Results
Data for body weight was lost for 15 total animals (2-3/group) due to a record-keeping error. As expected, food restricted rats weighed significantly less than free-fed rats (Main Effect of Feeding: F1,56=114.1; p<0.001; Figure 2a). This difference was not affected by previous MS however a Feeding × Sex interaction (F1,56=11.44; p=0.001) revealed that food restriction yielded a greater weight difference in males compared to females. Notably, our food-restriction paradigm yielded a greater reduction in body weight than originally expected in males (72% of free-fed weight in Con males, and 77% of free-fed weight in MS-exposed males). Additionally, the average weight-gain of food-restricted adolescent males and females between P36-P55 was not affected by MS or sex but was significantly less than the weight gained by free-fed animals (Main Effect of Feeding: F1,56=21.77; p<0.001 Figure 2b)
Figure 2.

Body weight in free-fed and food-restricted adolescents. (a) Food restriction resulted in lower body weight at P55 in all animals regardless of MS exposure, with a greater reduction in males than in females. (b) Food-restricted subjects gained less weight during the restriction period, compared to free-fed subjects, regardless of rearing or sex. Means ± SEM are shown. MS: maternal separation history; Con: control-reared.
No 3-way interaction was found for effects of Rearing × Feeding × [Threshold] on Iba-1 density (p=0.535 in males and p=0.957 in females; Figure 3). Averages were then taken across all three thresholded values and compared between groups. Effects of MS exposure, adolescent food restriction, and sex are illustrated in Figure 4a/b. While no Sex × Rearing × Feeding interaction was found in a 3-way ANOVA (p=0.396), a Main Effect of Feeding was found (F1,69=7.022; p=0.01), as well as a significant Sex × Rearing interaction (F1,69=8.07; p=0.006). After a Sex × Rearing interaction was found, post-hoc 2-way (Rearing × Feeding) ANOVAs were run separately in males and females. In males, a Rearing × Feeding interaction (F1,33=4.54; p=0.041) revealed that the effect of feeding was only observed in MS-exposed (Adjusted p=0.015) but not Con (Adjusted p=0.56) males. Food restriction increased Iba-1 in all females, regardless of exposure to MS (Main Effect of Feeding: F1,37=4.635; p=0.038).
Figure 3.
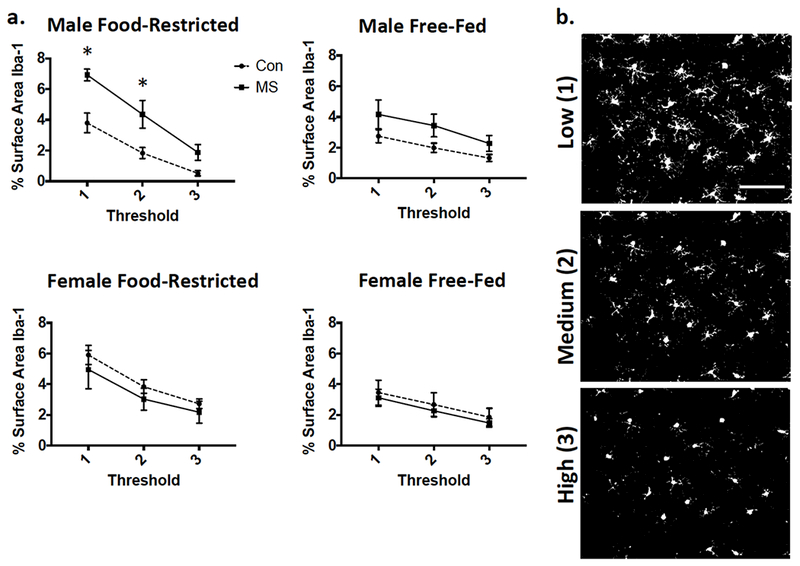
Iba-1 immunofluorescence as expressed by % surface area at each of three thresholded analyses. (a) Graphical representation of means ± SEM (n=9-12). Con: control-reared; MS: maternally separated. (b) Examples of analyzed images after threhsolding at each level. Images taken from a control-reared, food-restricted male. Scale bar: 100μm
Figure 4.
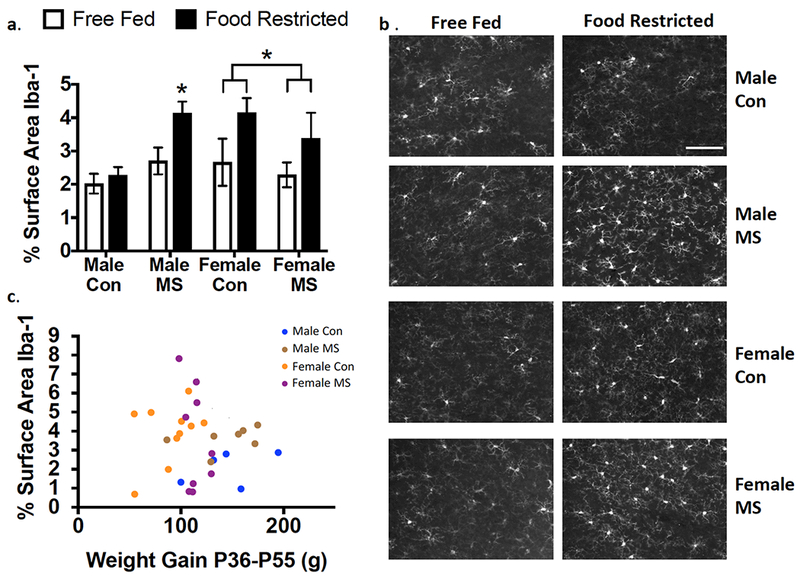
Percent Iba-1 immunofluorescence in the PFC of adolescents is altered by food restriction in a sex and rearing-dependent manner. (a) Quantification of percent Iba-1 immunofluorescence as a factor of sex, rearing, and food restriction. Means ± SEM are shown (n=9-12). *p<0.05. Brackets illustrate a main effect of feeding in females, wherein food-restricted females expressed higher levels of Iba-1 regardless of rearing group. (b) Representative photomicrographs showing Iba-1 immunofluorescence in each group. Scale bar: 100μm. (c) Weight gain between P36-P55 did not correlate with Iba-1 immunofluorescence in food-restricted rats. Con: control-reared; MS: maternally separated. Pearson r = 0.10; p=0.59.
Since Feeding affected weight, we analyzed whether Iba-1 levels correlated with weight-change in food-restricted rats. As shown in Figure 4c, no correlation between weight-change and % surface area Iba-1 was found (r= 0.10; p=0.59).
Soma area was not affected by rearing, feeding, or sex (Table 1). When branch length (ramification) was analyzed, a 3-way Rearing × Feeding × Sex interaction (F1,64=26.26; p<0.0001) revealed that Male MS (Adjusted p=0.04) and Female Con (Adjusted p=0.003) animals displayed increased microglial branch length following food restriction (Figure 5b). While no 3-way interaction was found for number of end-points (p=0.557), a Rearing × Feeding interaction (F1,64=15.86; p=0.0002) was found. A post-hoc 2-way (Rearing × Feeding) ANOVA then revealed that only Female MS animals exposed to food restriction displayed significantly fewer end-points than free-fed Female MS animals (Adjusted p=0.03; Figure 5c). Therefore, while Male MS, Female Con, and Female MS animals all displayed higher Iba-1 expression following food restriction, the corresponding morphology changes were notably different in Female MS animals.
Table 1:
Means and SEM of microglia soma area. Soma size was unchanged by sex and experience.
| Group | Average Soma Area (μm2) |
|---|---|
| Male Con Free-Fed | 68.0 ± 4.49 |
| Male Con Restricted | 67.3 ± 1.95 |
| Male MS Free-Fed | 74.4 ± 3.23 |
| Male MS Restricted | 67.9 ± 2.21 |
| Female Con Free-Fed | 65.8 ± 5.79 |
| Female Con Restricted | 59.7 ± 4.27 |
| Female MS Free-Fed | 68.5 ± 3.86 |
| Female MS Restricted | 67.3 ± 5.91 |
Figure 5:
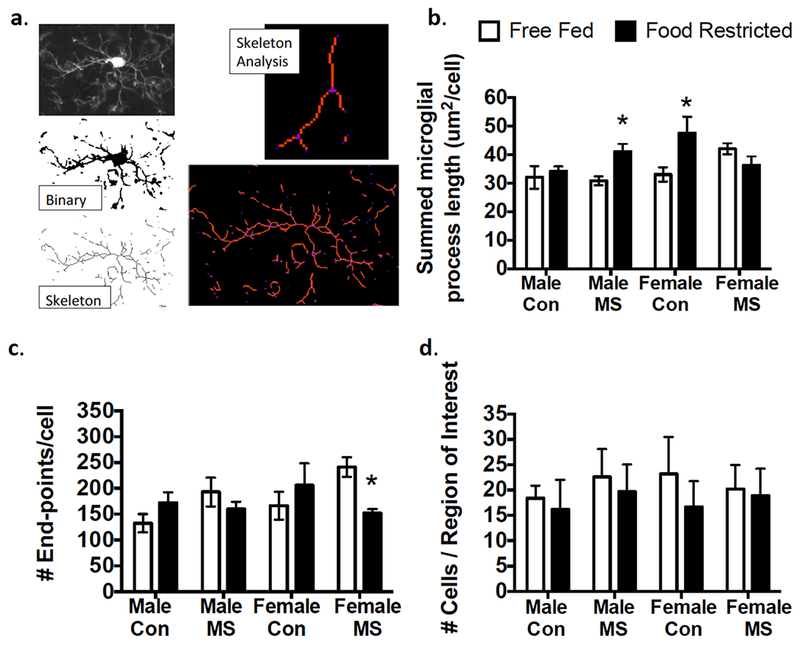
Effects of MS and food restriction on PFC microglial morphology. (a) Illustration of skeleton analyses, with a representative microglia as a binary image, a skeletonized image, and a tagged skeleton. The total process length (orange) and number of microglia process endpoints (blue) were summarized for statistical comparisons from Analyze Skeleton plugin by ImageJ. (b) Process length/cell was higher in food-restricted males exposed to maternal separation (MS) and control (Con) females. (c) Food-restricted females exposed to maternal separation (MS) displayed fewer end-points per cell than those that were free-fed. (d) No group differences were observed in microglial cell number. Means ± SEM are shown (n=9-12) *p<0.05 difference from Free-Fed.
Discussion
Here we report effects of food restriction and neonatal MS on microglial activation in the PFC during adolescence (P55). Food restriction increased Iba-1 expression in the PFC, with different alterations of microglial morphology depending on sex and MS history. However, our food-restriction paradigm unexpectedly reduced weight to a greater extent in males than in females, confounding the interpretation of whether these interactive effects are sex-dependent. Additionally, MS during lactation exposes pups to decreased nutrient supply, especially since maternal care is greater in the light cycle than during the dark (Hall and Rosenblatt, 1978). Thus, MS exposure has been shown to yield altered energy metabolism and feeding behaviors later in life (da Silva et al., 2014). For example, adult rats exposed to MS display a blunted hypothalamic-pituitary-thyroid axis response to 48h of fasting (Jaimes-Hoy et al., 2016), therefore food restriction may induce differential physiological changes in MS and Con rats. Reduced access to nutrients during MS may also induce hypersensitivity to food-related stressors, as starvation increases corticosterone to a greater extent in MS-exposed males, but not females, compared to control-reared subjects (Jaimes-Hoy, et al., 2016). This sex-specific effect supports the hypothesis that males and females are differently impacted by a subsequent stressor following MS, however the current findings are limited in their conclusiveness regarding sex differences given the different effect of our restriction paradigm on males and females.
The mammalian brain undergoes dramatic functional and developmental changes during adolescence, particularly in the PFC (Brenhouse and Schwarz, 2016). Our results show that adolescents are sensitive to food restriction, which is unsurprising since much of immune regulation, priming, and programming is being fine-tuned during this time frame. While it remains to be seen as to whether such environmental conditions uniquely affect adolescence, and not other ages, studies show that food restriction reduces microglial activation during aging (Morgan et al., 1997). Such evidence suggests that food deprivation may actually have a beneficial effect in aged animals. Together, these data highlight that food restriction may have additional effects aside from increased motivation that is currently utilized in behavioral studies (Carr, 2007). Notably, the evidence presented here is limited to microglial alterations in the PFC. Going forward, it is imperative to explore the effects of food restriction in additional brain regions in order to further elucidate the unintended effects of this motivational paradigm.
The requirement that animals be food restricted for certain behavioral tasks may also be noteworthy when using female animals because it could possibly interfere with their estrous cycle. While there are a number of studies on the disruptive effects of severe food restriction on estrous, little is known regarding the impact of mild food deprivation, which is the normal manipulation for most behavioral tests. Sprague-Dawley rats with reliable estrous cycles display only slightly disrupted cycling after seven days of mild food restriction (Tropp and Markus, 2001). The current study was not sufficiently powered to account for differences in estrous phase, therefore the mediation of hormonal status at the time of sacrifice on microglia cannot be ruled out.
Another important limitation of this study is our use of timed pregnant dams purchased and delivered to the colony at gestational-day 15. While both Con and MS animals were offspring from dams that had undergone the stress of shipment, it is possible that an interaction between prenatal stress and postnatal stress yielded the effects reported here. It is also important to note that our study only measured Iba-1, which is one of many markers of microglial activity and proliferation. Recent years have seen the identification of various microglia-specific marker proteins, including, but not limited to, CD11b, Cx3Cr1 and P2Y12. Measurement of Iba-1 alone permits only a limited understanding of the activation patterns of microglia under normal and detrimental conditions.
Microglial morphology effects reported here highlight the multifaceted nature of activation, since food restriction stress led to hyper-ramification in males that had been exposed to MS, as well as in control females. While process shortening and increased soma size has traditionally been associated with microglial activation, increasing evidence points to the fact that there are many types of microglial “activation”. In fact, hyper-ramification of microglia in the PFC and hippocampus has been observed in other models of stress, such as repeated swimming in a chronic behavioral despair model (Hellwig et al., 2016) and chronic restraint stress (Hinwood et al., 2013). In contrast to the traditional pro-inflammatory form of microglial activation, little is known about hyper-ramification of microglia or about the function of hyper-ramified microglia (Hinwood et al., 2013). Surprisingly, increased Iba-1 expression in all females as well as MS males did not signify an identical effect of food restriction on all three groups of animals. Females with a history of MS responded to food restriction with fewer microglial endpoints, rather than hyper-ramification. Fewer end-points suggest a decreased complexity of microglial processes, and while branch thickness was not included in our current analysis, thicker branches may have contributed to the increased Iba-1-positive surface area observed in MS-exposed females. Taken together, further studies will be necessary to examine more specific ways in which microglia are affected by food deprivation.
In summary, these findings indicate that food restriction may have immune effects in adolescents in the form of altered microglia activation. Further, the effects of food restriction in a repeated-stress paradigm may be sex-specific. These data in adolescents are dissimilar from past research demonstrating that food-restricted older animals experience reduced microglia activation, suggesting an age-related distinction in immune response resulting from food restriction. Taken together, our findings provide evidence that food restriction alters microglial activation in adolescence in an experience-dependent manner, and introduces the possibility that utilizing food restriction paradigms in behavioral protocols may result in unintended consequences.
Lay Summary.
This article describes the impact of early life experiences on later responses of microglia—the brain’s resident immune cells—to stress. We report that the stress of food restriction yields higher levels of microglial activation in animals that had also been exposed to early life stress, compared to those that were raised in control environments.
Footnotes
Declaration of Interest
The authors have no conflicts of interest to disclose.
References
- Alexander GE, & Goldman PS (1978). Functional development of the dorsolateral prefrontal cortex: an analysis utlizing reversible cryogenic depression. Brain Res, 143(2), pp. 233–249. [DOI] [PubMed] [Google Scholar]
- Amancio-Belmont O, Romano-Lopez A, Ruiz-Contreras AE, Mendez-Diaz M, & Prospero-Garcia O (2017). From adolescent to elder rats: Motivation for palatable food and cannabinoids receptors. Dev Neurobiol, 77(8), pp. 917–927. doi:10.1002/dneu.22472 Retrieved from 10.1002/dneu.22472https://www.ncbi.nlm.nih.gov/pubmed/27935269 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27935269 [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH (2008). Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci, 31(4), pp. 183–191. [DOI] [PubMed] [Google Scholar]
- Anderson RI, Bush PC, & Spear LP (2013). Environmental manipulations alter age differences in attribution of incentive salience to reward-paired cues. Behav Brain Res, 257, pp. 83–89. doi: 10.1016/j.bbr.2013.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arganda-Carreras I, Fernandez-Gonzalez R, Munoz-Barrutia A, & Ortiz-De-Solorzano C (2010). 3D reconstruction of histological sections: Application to mammary gland tissue. Microsc Res Tech, 73(11), pp. 1019–1029. doi:10.1002/jemt.20829 Retrieved from 10.1002/jemt.20829https://www.ncbi.nlm.nih.gov/pubmed/20232465 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20232465 [DOI] [PubMed] [Google Scholar]
- Bayer TA, Falkai P, & Maier W (1999). Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis”. J Psychiatr Res, 33(6), pp. 543–548. [DOI] [PubMed] [Google Scholar]
- Brenhouse HC, & Schwarz JM (2016). Immunoadolescence: Neuroimmune development and adolescent behavior. Neurosci Biobehav Rev, 70, pp. 288–299. doi:10.1016/j.neubiorev.2016.05.035 Retrieved from 10.1016/j.neubiorev.2016.05.035https://www.ncbi.nlm.nih.gov/pubmed/27260127 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27260127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, & Howes OD (2016). Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology (Berl), 233(9), pp. 1637–1650. doi: 10.1007/s00213-016-4218-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD (2007). Chronic food restriction: enhancing effects on drug reward and striatal cell signaling. Physiol Behav, 91(5), pp. 459–472. doi:10.1016/j.physbeh.2006.09.021 Retrieved from 10.1016/j.physbeh.2006.09.021https://www.ncbi.nlm.nih.gov/pubmed/17081571 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17081571 [DOI] [PubMed] [Google Scholar]
- Chocyk A, Dudys D, Przyborowska A, Mackowiak M, & Wedzony K (2010). Impact of maternal separation on neural cell adhesion molecules expression in dopaminergic brain regions of juvenile, adolescent and adult rats. Pharmacol Rep, 62(6), pp. 1218–1224. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=21273681 [DOI] [PubMed] [Google Scholar]
- da Silva MC, de Souza JA, Dos Santos LO, Pinheiro IL, Borba TK, da Silva AA, … de Souza SL (2014). Effects of maternal separation on the dietary preference and behavioral satiety sequence in rats. J Dev Orig Health Dis, 5(3), pp. 219–228. doi: 10.1017/S204017441400018X Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24901662 [DOI] [PubMed] [Google Scholar]
- Davey C, Yucel M, & Allen N (2008). The emergence of depression in adolescence: Development of the prefrontal cortex and the representation of reward. Neurosc Biobeh Rev, 32, pp. 1–19. [DOI] [PubMed] [Google Scholar]
- Deroche V, Marinelli M, Maccari S, Le Moal M, Simon H, & Piazza PV (1995). Stress-induced sensitization and glucocorticoids. I. Sensitization of dopamine-dependent locomotor effects of amphetamine and morphine depends on stress-induced corticosterone secretion. J Neurosci, 15(11), pp. 7181–7188. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7472472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deslauriers J, Racine W, Sarret P, & Grignon S (2014). Preventive effect of alpha-lipoic acid on prepulse inhibition deficits in a juvenile two-hit model of schizophrenia. Neuroscience, 272, pp. 261–270. doi: 10.1016/j.neuroscience.2014.04.061 [DOI] [PubMed] [Google Scholar]
- Diz-Chaves Y, Astiz M, Bellini MJ, & Garcia-Segura LM (2013). Prenatal stress increases the expression of proinflammatory cytokines and exacerbates the inflammatory response to LPS in the hippocampal formation of adult male mice. Brain Behav Immun, 28, pp. 196–206. doi:10.1016/j.bbi.2012.11.013 Retrieved from 10.1016/j.bbi.2012.11.013https://www.ncbi.nlm.nih.gov/pubmed/23207108 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23207108 [DOI] [PubMed] [Google Scholar]
- Ganguly P, & Brenhouse HC (2015). Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Dev Cogn Neurosci, 11, pp. 18–30. doi:10.1016/j.dcn.2014.07.001 Retrieved from 10.1016/j.dcn.2014.07.001http://www.ncbi.nlm.nih.gov/pubmed/25081071 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25081071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovanoli S, Engler H, Engler A, Richetto J, Voget M, Willi R, … Meyer U (2013). Stress in puberty unmasks latent neuropathological consequences of prenatal immune activation in mice. Science, 339(6123), pp. 1095–1099. doi:10.1126/science.1228261 Retrieved from 10.1126/science.1228261https://www.ncbi.nlm.nih.gov/pubmed/23449593 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23449593 [DOI] [PubMed] [Google Scholar]
- Hall WG, & Rosenblatt JS (1978). Development of nutritional control of food intake in suckling rat pups. Behav Biol, 24(4), pp. 413–427. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/747582 [DOI] [PubMed] [Google Scholar]
- Hellwig S, Brioschi S, Dieni S, Frings L, Masuch A, Blank T, & Biber K (2016). Altered microglia morphology and higher resilience to stress-induced depression-like behavior in CX3CR1-deficient mice. Brain Behav Immun, 55, pp. 126–137. doi: 10.1016/j.bbi.2015.11.008 [DOI] [PubMed] [Google Scholar]
- Helmeke C, Ovtscharoff WJ, Poeggel G, & Braun K (2008). Imbalance of immunohistochemically characterized interneuron populationsn in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience, 152(1), pp. 18–28. [DOI] [PubMed] [Google Scholar]
- Hinwood M, Tynan RJ, Charnley JL, Beynon SB, Day TA, & Walker FR (2013). Chronic stress induced remodeling of the prefrontal cortex: structural re-organization of microglia and the inhibitory effect of minocycline. Cereb Cortex, 23(8), pp. 1784–1797. doi:10.1093/cercor/bhs151 Retrieved from 10.1093/cercor/bhs151https://www.ncbi.nlm.nih.gov/pubmed/22710611 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22710611 [DOI] [PubMed] [Google Scholar]
- Jaimes-Hoy L, Gutierrez-Mariscal M, Vargas Y, Perez-Maldonado A, Romero F, Sanchez-Jaramillo E, Joseph-Bravo P (2016). Neonatal Maternal Separation Alters, in a Sex-Specific Manner, the Expression of TRH, of TRH-Degrading Ectoenzyme in the Rat Hypothalamus, and the Response of the Thyroid Axis to Starvation. Endocrinology, 157(8), pp. 3253–3265. doi:10.1210/en.2016-1239 Retrieved from 10.1210/en.2016-1239https://www.ncbi.nlm.nih.gov/pubmed/27323240 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27323240 [DOI] [PubMed] [Google Scholar]
- Kozlowski C, & Weimer RM (2012). An automated method to quantify microglia morphology and application to monitor activation state longitudinally in vivo. PLoS One, 7(2), p e31814. doi:10.1371/journal.pone.0031814 Retrieved from 10.1371/journal.pone.0031814https://www.ncbi.nlm.nih.gov/pubmed/22457705 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22457705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TE, Rozovsky I, Goldsmith SK, Stone DJ, Yoshida T, & Finch CE (1997). Increased transcription of the astrocyte gene GFAP during middle-age is attenuated by food restriction: implications for the role of oxidative stress. Free Radic Biol Med, 23(3), pp. 524–528. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9214592 [DOI] [PubMed] [Google Scholar]
- Paxinos G, & Watson C (1998). The Rat Brain in Stereotaxic Coordinates, Fourth Edition: Academic Press. [Google Scholar]
- Robison LS, Michaelos M, Gandhi J, Fricke D, Miao E, Lam CY, … Thanos PK (2017). Sex Differences in the Physiological and Behavioral Effects of Chronic Oral Methylphenidate Treatment in Rats. Front Behav Neurosci, 11, p 53. doi:10.3389/fnbeh.2017.00053 Retrieved from 10.3389/fnbeh.2017.00053https://www.ncbi.nlm.nih.gov/pubmed/28400722 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28400722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson CW, Marsden CA, & Mason R (2008). Early life stress causes FG-7142-induced corticolimbic dysfunction in adulthood. Brain Res, 1193, pp. 43–50. [DOI] [PubMed] [Google Scholar]
- Takatsuru Y, Nabekura J, Ishikawa T, Kohsaka S, & Koibuchi N (2015). Early-life stress increases the motility of microglia in adulthood. J Physiol Sci, 65(2), pp. 187–194. doi:10.1007/s12576-015-0361-z Retrieved from 10.1007/s12576-015-0361-zhttps://www.ncbi.nlm.nih.gov/pubmed/25702174 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25702174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Tomoda A, & Andersen SL (2006). Neurobiological consequences of early stress and childhood maltreatment: are results from human and animal studies comparable? Ann N Y Acad Sci, 1071, pp. 313–323. Retrieved from http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=16891580 [DOI] [PubMed] [Google Scholar]
- Tropp J, & Markus EJ (2001). Effects of mild food deprivation on the estrous cycle of rats. Physiol Behav, 73(4), pp. 553–559. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11495659 [DOI] [PubMed] [Google Scholar]
- Viviani B, Boraso M, Valero M, Gardoni F, Marco EM, Llorente R, … Viveros MP (2013). Early maternal deprivation immunologically primes hippocampal synapses by redistributing interleukin-1 receptor type I in a sex dependent manner. Brain Behav Immundoi: 10.1016/j.bbi.2013.09.008 [DOI] [PubMed] [Google Scholar]
- Vollmer LL, Ghosal S, McGuire JL, Ahlbrand RL, Li KY, Santin JM, … Sah R (2016). Microglial Acid Sensing Regulates Carbon Dioxide-Evoked Fear. Biol Psychiatry, 80(7), pp. 541–551. doi:10.1016/j.biopsych.2016.04.022 Retrieved from 10.1016/j.biopsych.2016.04.022https://www.ncbi.nlm.nih.gov/pubmed/27422366 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27422366 [DOI] [PMC free article] [PubMed] [Google Scholar]


