Abstract
Adipocyte-derived extracellular vesicles (EVs) may serve as novel endocrine mediators of adipose tissue and impact upon vascular health. However, it is unclear whether adipocyte-derived EVs are present in the human circulation. Therefore, the purpose of this study was to seek evidence for the presence of adipocyte-derived EVs in circulating plasma. Size-exclusion chromatography of platelet-free plasma identified fractions 5 to 10 as containing EVs by a peak in particle concentration, which corresponded with the presence of EV and adipocyte proteins. Pooling fractions 5 to 10 and subjecting to ultracentrifugation yielded a plasma EV sample, as verified by transmission electron microscopy (TEM) showing EV structures and Western blotting for EV (e.g., CD9 and Alix) and adipocyte markers. Magnetic beads and a solid-phase assay were used to deplete the EV sample of the four major families of circulating EVs: platelet-derived, leukocyte-derived, endothelial-derived, and erythrocyte-derived EVs. Postdepletion samples from both techniques contained EV structures as visualized by TEM, as well as CD9, Alix, and classic adipocyte proteins. Postdepletion samples also contained a range of other adipocyte proteins from an adipokine array. Adipocyte proteins and adipokines are expressed in optimally processed plasma EV samples, suggesting that adipocyte-derived EVs are secreted into the human circulation.
Optimally isolated, human plasma-derived extracellular vesicles were found to contain multiple adipocyte markers, even after the depletion of major circulating extracellular vesicle populations.
The endocrine functions of adipose tissue have largely been attributed to adipokines, an array of soluble bioactive molecules secreted from adipocytes such as adiponectin and fatty acid binding protein 4 (FABP4) (1). Dysregulation of adipokine secretion is associated with obesity-related cardiovascular disease, insulin resistance, and type 2 diabetes (2). Adiponectin, peroxisome proliferator–activated receptor γ (PPARγ), FABP4, and perilipin have been detected within adipocyte-derived extracellular vesicles (EVs) in vitro (3–12), indicating an additional method for endocrine signaling from adipose tissue. Dysfunctional adipocytes in obese adipose tissue may release an altered complement of EVs, which, in addition to dysregulated adipokine secretion, help to promote the cardiovascular complications associated with obesity. Therefore, there is a need for comprehensive evidence for the existence of adipocyte-derived EVs in vivo to explore their potential as novel circulating biomarkers of adipocytes in vivo.
EVs are heterogeneous submicron vesicles released from almost all cells in response to cellular stress, activation, or apoptosis. EVs may originate from cytoplasmic multivesicular bodies that fuse with the plasma membrane to release vesicles typically <120 nm in diameter, often referred to as exosomes. EVs also include microvesicles, which are ∼100 to ∼1000 nm in size and bud directly from the plasma membrane into the extracellular space. Both subclasses of EVs have a biomolecular composition similar to that of the original cell, including specific lipids, proteins, and nucleic acids. Recent advances in methodology have enabled standardization of nomenclature and characterization of EV populations (13).
Most studies examining the release of EVs from adipocytes have been conducted in vitro using 3T3-L1 cells (3, 6–9, 11, 12), a murine adipocyte cell line frequently used to model adipocyte functions. Others have also isolated EVs from human adipocytes and adipose tissue extracts (4, 5, 10). These studies have demonstrated the functional relevance of adipocyte-derived EVs in the paracrine regulation of adipocyte metabolism (14), monocyte to macrophage differentiation (4), and regulation of hepatic insulin signaling (5). Effects on vascular homeostasis have also been shown, including induction of neovascularization and angiogenesis (15, 16), suggesting that adipocyte-derived EVs may influence vascular health within and at sites remote to adipose tissue. However, evidence for the presence of adipocyte-derived EVs in the human circulation has not yet been confirmed, because EVs in blood are thought to derive primarily from platelets [with leukocyte-derived, endothelial-derived, and erythrocyte-derived EVs contributing smaller populations (17–19)], and adipocytes lack a unique marker to readily distinguish them from other cells. Preliminary evidence from flow cytometric analyses showed that EVs contain the adipocyte markers FABP4 and adiponectin in human and mouse plasma (18, 20). However, the use of direct flow cytometry for EV measurements is suboptimal, as the lower limit of detection for many conventional flow cytometers is ∼300 nm (21), resulting in an incomplete assessment of the EV population. Separate studies have also shown that adiponectin, FABP4, perilipin, and PPARγ were associated with plasma EVs (4, 11, 22), though in most cases, plasma samples were not processed in accordance with guidelines set by the International Society for Extracellular Vesicles (ISEV) (13). This may lead to false-positive results from contamination of soluble adipokines present in the larger plasma protein pool.
In light of these uncertainties, we used a combination of adipocyte markers and sample processing according to ISEV recommendations to seek evidence for the presence of adipocyte-derived EVs in healthy human plasma.
Materials and Methods
Plasma EV isolation
Ethical approval for this study was granted by the Cardiff Metropolitan University School Research Ethics Committee, and informed consent was obtained from each volunteer. Blood was drawn from seven healthy volunteers (three males and four females) using a 19-gauge needle into 3.2% (w/v) sodium citrate vacutainers and immediately centrifuged (2500g, 15 minutes, 21°C) to isolate platelet-poor plasma. The first 3 mL of blood was discarded in line with recommended guidelines for collection of EVs from blood (23, 24). Platelet-poor plasma was then pooled and centrifuged as above to isolate platelet-free plasma. Platelet-free plasma (1 mL) was then loaded onto Exo-spin™ midi size-exclusion columns (Cell Guidance Systems, Cambridge, United Kingdom), and 30 × 500-µL fractions were collected. Fractions 5 to 10 were then pooled and ultracentrifuged (100,000g, 1 hour, 4°C) to pellet EVs (hereafter referred to as “pooled EVs”).
Nanoparticle-tracking analysis
Quantification of EV populations was performed using nanoparticle-tracking analysis (NTA) with a NanoSight LM10 instrument configured with a 488-nm laser and an sCMOS camera (Malvern Instruments Ltd, Malvern, United Kingdom). A Harvard Apparatus syringe pump was used for EV measurements at a constant flow rate of 20 arbitrary units. Camera shutter speed and gain were maintained at 607 and 15, respectively. Sample videos were recorded for 60 seconds in repetitions of 5 using a capture screen gain of 8 to 11 and a camera level of 8 to 10. Samples were processed using a screen gain of 20 and a detection threshold of 4 to 6. Software version 3.1 (build 3.1.54) was used for capture and analysis. All experiments were performed in a temperature-controlled room at 22°C. Results are presented as particles per milliliter.
Western blotting
The protein concentration of individual column fractions (1 to 30) was determined using a NanoDrop 1000 Spectrophotometer (Thermo Fisher Scientific, Waltham, MA). Samples (8 µg) of fractions 2 to 28, pooled EVs, and postdepletion samples were prepared to 30 µL (neat or diluted with 1× PBS), boiled for 8 minutes on a heat block, centrifuged (12,000g, 5 minutes, 4°C), and kept on ice before loading onto 4% to 12% Bis-Tris gels (Thermo Fisher Scientific). InstantBlue™ Protein Stain (Expedeon Ltd, Cambridge, United Kingdom) was used as a loading control. Amersham Hybond P 0.45-µm polyvinylidene difluoride membranes (GE Healthcare, Amersham, United Kingdom) were probed with the following antibodies [diluted 1:500 in either 5% (w/v) skimmed milk or 5% (w/v) BSA both in Tris-buffered saline with 0.05% (v/v) Tween 20]: mouse monoclonal anti-Alix (25), rabbit polyclonal anti-CD63 (26) (both from Santa Cruz Biotechnology, Santa Cruz, CA); mouse monoclonal anti-CD81 (27) (Bio-Rad, Hertfordshire, United Kingdom); rabbit monoclonal anti-adiponectin (28) (Abcam, Cambridge, United Kingdom); rabbit monoclonal anti-CD9 (29); rabbit monoclonal anti-FABP4 (30); rabbit monoclonal anti-perilipin (31); and rabbit monoclonal anti-PPARγ (32) (Cell Signaling Technology, Danvers, MA). Proteins were analyzed using reducing conditions with the exception of the tetraspanins (CD9, CD63, and CD81), which were analyzed using nonreducing conditions. Signals were detected using either goat anti-mouse IgG–horseradish peroxidase (33) or donkey anti-rabbit IgG–horseradish peroxidase (34) [diluted 1:1000 in 5% (w/v) skimmed milk in Tris-buffered saline with 0.05% (v/v) Tween 20] followed by Amersham ECL Western Blotting Detection Reagents (GE Healthcare).
Transmission electron microscopy
Pooled EVs were resuspended in 1× 0.22-µm filtered PBS, then fixed with an equal volume of 4% (v/v) paraformaldehyde, and kept at 4°C until processing for transmission electron microscopy (TEM) the next day. Briefly, EVs (10 µL) were adsorbed onto glow-discharged carbon formvar 200 mesh copper grids for 2 minutes. Grids were then blotted using filter paper, stained for 10 seconds with 2% (w/v) uranyl acetate before surplus stain was removed, and grids were air-dried. Grids were imaged using an FEI Tecnai 12 TEM at 120 kV fitted with a Gatan OneView CMOS camera (Gatan, Inc., Abingdon, United Kingdom).
Sequential depletion of EV populations using magnetic beads
Pooled EVs were diluted to a concentration of 1 × 1011 particles per milliliter using 1× 0.22-µm filtered PBS in replicates of three. EVs were then incubated for 2 hours at room temperature with 3 µg/mL rabbit monoclonal anti-CD41 antibody (35) (Abcam). Fifty microliters (per sample) of prewashed Dynabeads™ M-280 sheep anti-rabbit IgG magnetic beads (Life Technologies, Paisley, United Kingdom) were added to EVs/anti-CD41 and incubated with mixing for 30 minutes at room temperature. Samples were then introduced into the magnet (DynaMag™-2; Life Technologies) to deplete CD41+ EVs: this was quantified using NTA. The process was repeated sequentially with 3 µg/mL rabbit monoclonal anti-CD11b (36), rabbit polyclonal anti-CD144 (37), and rabbit monoclonal anti-CD235a (38) antibodies (all from Abcam) to deplete CD11b+, CD144+, and CD235a+ EVs. Final supernatants were quantified using NTA and analyzed by Western blot with predepletion samples for the presence of adipocyte and EV markers.
Solid-phase–based depletion of EV populations
High-binding ELISA plates (Greiner Bio-One, Gloucestershire, United Kingdom) were coated in triplicate with rabbit monoclonal anti-CD41, anti-CD11b, anti-CD144, or anti-CD235a antibodies (Abcam) diluted to 3 µg/mL in PBS overnight at 4°C. Pooled EVs were diluted to a concentration of 1 × 1011 particles per milliliter as above and incubated for 2 hours at room temperature in wells containing anti-CD41 antibody to deplete CD41+ EVs. Supernatants were then transferred to wells containing anti-CD11b antibody for 2 hours at room temperature to deplete CD11b+ EVs. This process was then repeated sequentially with wells containing anti-CD144 antibody and anti-CD235a antibody to deplete CD144+ and CD235a+ EVs. Final supernatants were analyzed as above.
Time-resolved fluorescence
The efficiency of depletion of major circulating EV populations was assessed using time-resolved fluorescence (TRF) as previously described (39, 40). Briefly, EVs were normalized to a concentration of 1 × 1011 particles per milliliter in predepletion, post-CD41, post-CD11b, post-CD144, and post-CD235a samples from magnetic-bead and solid-phase–based depletion. EVs were then immobilized on high-binding ELISA plates (Greiner Bio-One) overnight at 4°C. EVs were blocked for 2 hours at room temperature using 1% (w/v) BSA before adding 3 µg/mL primary antibodies of interest (anti-CD41, anti-CD11b, anti-CD144, and anti-CD235a, as detailed above) in 0.1% (w/v) BSA overnight at 4°C. Primary antibodies were detected using a biotin-labeled goat anti-rabbit IgG secondary antibody (41) (diluted 1:2500 in 0.1% BSA; PerkinElmer, Buckinghamshire, United Kingdom) for 1 hour at room temperature, followed by a streptavidin-europium conjugate (diluted 1:1000 in red assay buffer, both from PerkinElmer) for 45 minutes at room temperature. TRF was measured on a BMG CLARIOstar® plate reader (BMG Labtech, Bucks, United Kingdom).
Detection of an array of adipokines in plasma EV samples
A commercially available Proteome Profiler Human Adipokine Array Kit (R&D Systems, Bio-Techne, Abingdon, United Kingdom) was used to analyze 58 adipocyte-related molecules in predepletion, post–magnetic-bead depletion, and post–solid-phase depletion EV samples. Samples were diluted to load an absolute concentration of 2 × 1010 particles. The remainder of the experiment was performed according to the manufacturer’s protocol. Dot assays were detected using Amersham ECL Hyperfilm following 15-minute and 60-minute exposures. Blots were scanned and pixel densities analyzed using HLImage++ (Western Vision Software, Salt Lake City, UT). A full list of analytes included in the kit is shown in Supplemental Table 1.
Statistical analysis
Data are presented as mean ± SEM. A one-way ANOVA with Tukey multiple-comparison test was used to analyze the difference between means. A P value of <0.05 was considered significant. Data were analyzed using GraphPad Prism (version 5; GraphPad Software Inc., La Jolla, CA).
Results
Preparation of plasma-derived EVs using size-exclusion chromatography
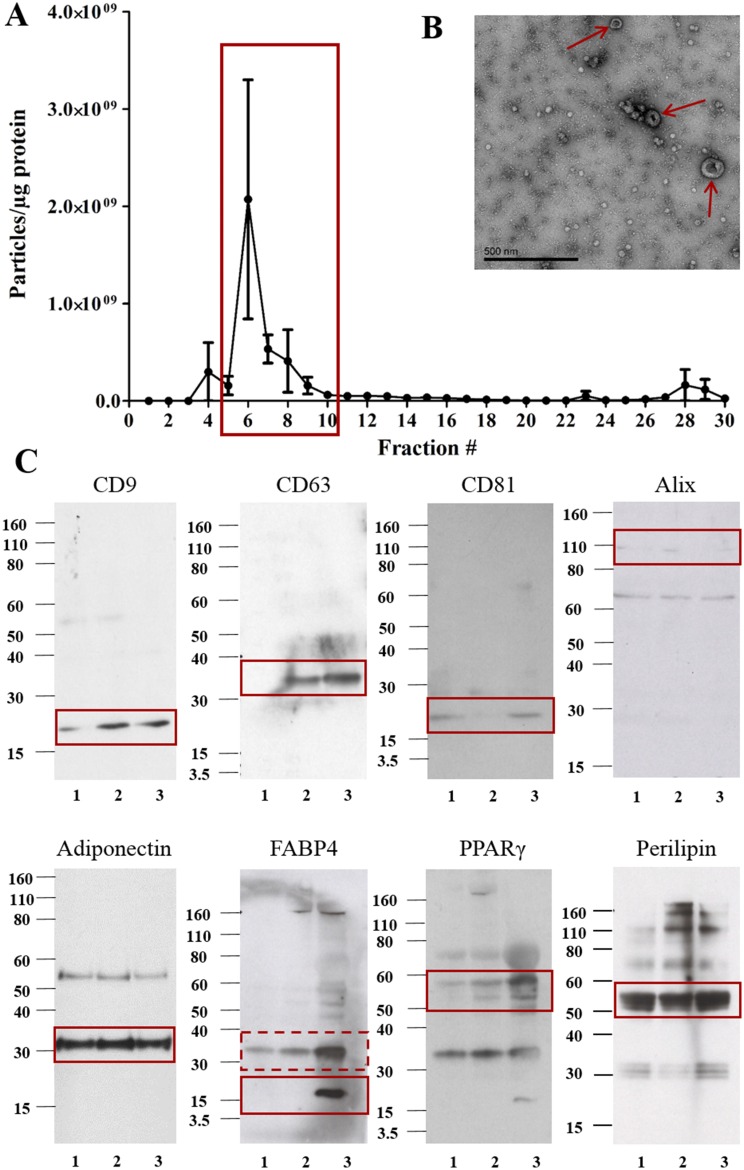
Analysis of individual column fractions using NTA showed a small peak in the concentration of particles per milliliter between fractions 5 to 10, followed by a large peak in particles and protein from fractions 12 to 26. Western blot analysis of fractions 2 to 28 showed the presence of both EV and adipocyte markers in fractions 6 to 10 but only adipocyte markers in fractions 11 to 28 (Supplemental Fig. 1). Plotting the ratio of particle concentration to protein concentration as described previously (42) showed fractions 5 to 10 to contain the highest number of particles to protein (Fig. 1A). Therefore, these fractions were pooled and ultracentrifuged to pellet plasma-derived EVs. TEM of pelleted EVs indicated the presence of vesicle structures, and Western blot analysis showed the presence of classical EV and adipocyte markers in the pooled EVs of three different individuals (Fig. 1B and 1C). Pooled EVs were shown to be deficient in the endoplasmic reticulum marker Grp-94 (Supplemental Fig. 2A), in accordance with ISEV guidelines for expected proteins in EV isolates (13). The supernatant of pelleted EVs following ultracentrifugation was deficient in CD9 (Supplemental Fig. 2B), indicating EVs were successfully pelleted by ultracentrifugation.
Figure 1.
Detailed analysis of pooled plasma EVs. (A) Fractions 5 to 10 showed the highest ratio of particles to protein and (B) the presence of EV structures by TEM (red arrows indicate EV structures) following ultracentrifugation. (C) Pooled EVs from three different individuals (labeled 1, 2, and 3) were analyzed by Western blot for EV markers (CD9, CD63, CD81, and Alix) and adipocyte markers (adiponectin, FABP4, PPARγ, and perilipin) (n = 3). Solid red boxes indicate the predicted molecular weight for each antigen; the dotted red box may indicate an FABP4 dimer ∼32 kDa.
Adipocyte markers remain following sequential depletion of major EV families
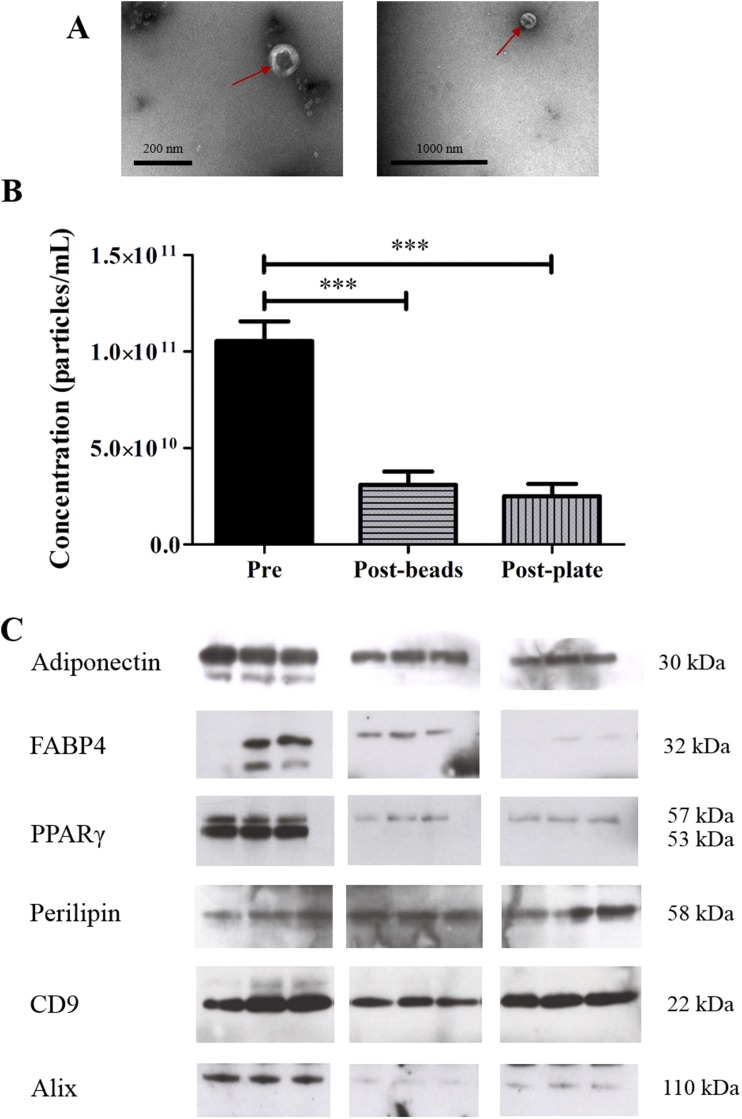
Magnetic beads and a solid-phase–based method were used to sequentially deplete EVs bearing markers of the four major EV populations in the circulating plasma of three different individuals. TEM analysis revealed EV structures to be present in both post–magnetic-bead and post–solid-phase depletion samples (Fig. 2A). EV concentration was reduced by ∼75% in both post–magnetic-bead and post–solid-phase depletion samples: 1.01 × 1011 ± 1.00 × 1010 particles per milliliter to 3.10 × 1010 ± 6.90 × 109 particles per milliliter and 2.50 × 1010 ± 6.50 × 109 particles per milliliter, respectively (P < 0.001; n = 5; Fig. 2B). The detection of markers of the main EV populations in plasma (platelet, CD41; monocytes, CD11b; endothelial cells, CD144; and erythrocytes, CD235a) were reduced in postdepletion samples following magnetic bead and solid-phase–based methods (Supplemental Fig. 3). Adiponectin, FABP4, PPARγ, perilipin, CD9, and Alix were reduced but still detectable in post–magnetic-bead and post–solid-phase depletion samples (Fig. 2C). Interestingly, only the adipocyte-specific PPARγ2 isoform remained in postdepletion samples.
Figure 2.
Adipocyte and EV markers were maintained post–magnetic-bead and post–solid-phase depletion. (A) EV structures were visible by TEM in post–magnetic-bead depletion (left; scale bar, 200 nm) and post–solid-phase depletion (right; scale bar, 1000 nm). (B) EV concentration was reduced following sequential depletion of major EV families using magnetic beads or a solid-phase method. ***P = 0.005 (n = 5). (C) Adiponectin, FABP4, PPARγ2, perilipin, CD9, and Alix were still present in postdepletion samples of three different individuals.
Major adipokines are expressed in predepletion and postdepletion samples
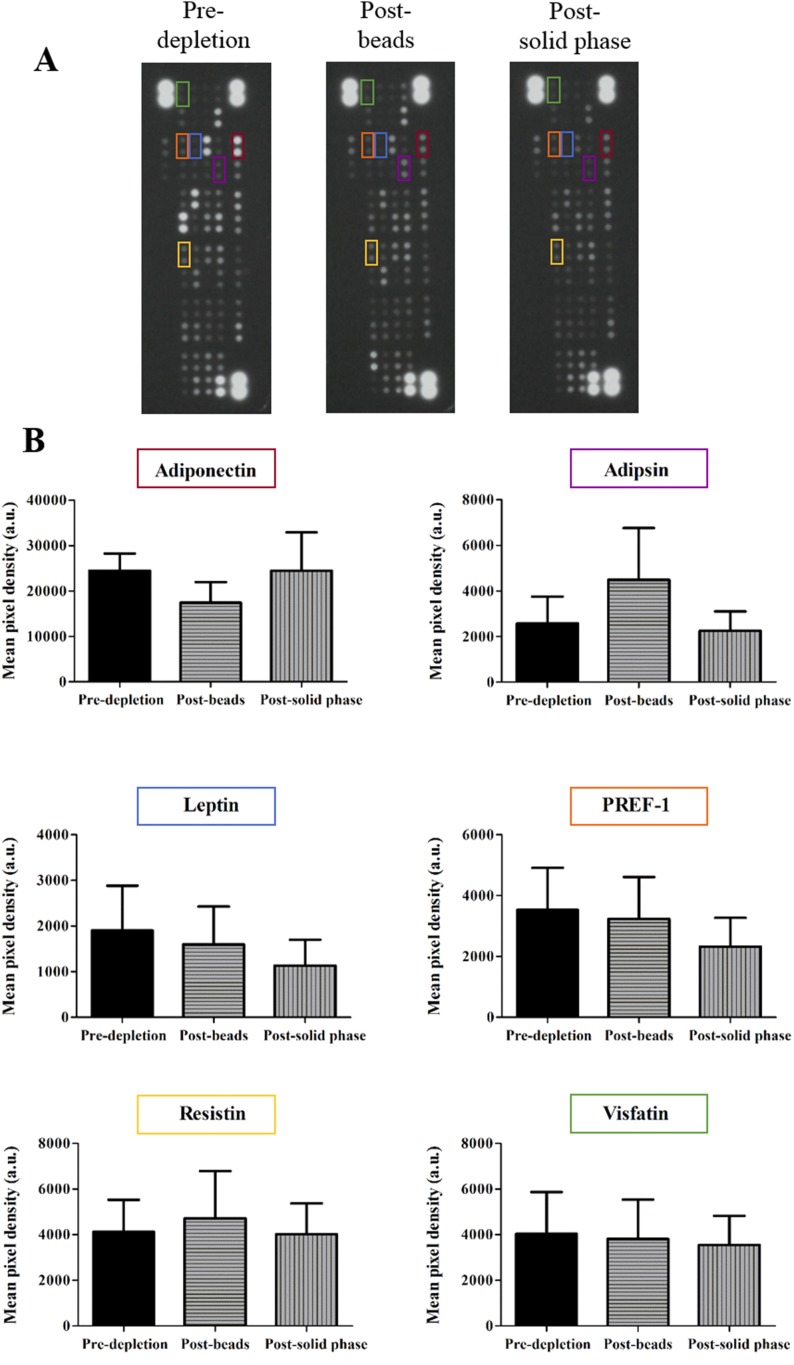
An adipokine array kit was used to probe for 58 adipokines (Supplemental Table 1) in predepletion and post–magnetic-bead, and post–solid-phase depletion plasma EV samples (Fig. 3A). Major adipokines, including adiponectin, adipsin, leptin, preadipocyte factor-1, resistin, and visfatin, were detected in all samples (Fig. 3B). No significant differences were observed between samples.
Figure 3.
Major adipokines were present in post–magnetic-bead and post–solid-phase depletion samples. (A) Inverted raw data of dot blots predepletion, post–magnetic-bead depletion, and post–solid-phase depletion. (B) Major adipokines are highlighted with corresponding pixel densities. Representative dot blots of n = 3.
Discussion
This study presents a variety of evidence for the presence of adipocyte-derived EVs in the circulating plasma of healthy individuals. A panel of adipocyte markers and adipokines were detected in plasma EV samples after careful sample processing and depletion of EVs from major circulating sources. Adipocyte-derived EVs have proven to be important, novel endocrine mediators of adipocytes in vitro; thus, their detection in the human circulation is an important step toward understanding their roles as mediators of adipocyte function, including potential effects on vascular health.
Due to the complexity of plasma as a biofluid, platelet-depleted plasma was loaded onto size-exclusion chromatography (SEC) columns. SEC has previously been shown to separate EVs quickly and effectively from the majority of nonvesicular protein in plasma (39, 43). In this study, EVs were identified in fractions 5 to 10 from the high particle-to-protein ratio and the presence of EV markers, CD9, CD81, and Alix in these fractions (Fig. 1 and Supplemental Fig. 1). Later fractions had a low particle-to-protein ratio and EV markers were not identified in these fractions. Additionally, the adipocyte markers adiponectin, FABP4, perilipin, and PPARγ were detected in fractions 5 to 10 but were also present in later fractions. Detection of these markers is in keeping with previous studies that have identified adipocyte markers within EVs from human plasma (4, 11, 18, 22). However, our data indicate that markers previously used to identify adipocyte-derived EVs in unpurified plasma samples are largely soluble and likely not associated with EVs as illustrated in Supplemental Fig. 1, in which we show adiponectin, FABP4, perilipin, and PPARγ are all detected as soluble protein in SEC fractions not containing EVs, despite loading up to 55 times less volume. This finding has important implications for the measurement of adipocyte EV markers in human plasma and highlights the importance of techniques such as SEC prior to analysis of adipocyte markers to avoid erroneous overestimations from soluble material. Pooling and subsequent ultracentrifugation of these fractions confirmed the presence of EV structures by TEM and both EV and adipocyte proteins by Western blotting (Fig. 1 and Supplemental Fig. 2A and 2B). We also observed the presence of adipokines in the supernatant of pelleted EVs, highlighting the importance of the ultracentrifugation step after SEC. This is in keeping with previous studies, which have shown that SEC is effective in removing ∼95% of nonvesicular protein in a single step, but the EV-free supernatant is likely to contain residual, non-EV–associated plasma proteins, including adipokines (39, 43).
The majority of plasma-derived EVs originate from cells that are in direct contact with blood, such as platelets, leukocytes, vascular endothelial cells, and erythrocytes (44). The location of adipocytes within adipose tissue may hinder the majority of adipocyte-derived EVs reaching the systemic circulation. Consequently, adipocyte-derived EVs are likely to form only a minor proportion of plasma-derived EVs. Furthermore, markers that uniquely identify adipocytes, such as adiponectin, are readily secreted. High-speed centrifugation used for EV isolation may copellet these soluble markers with EVs, lending a false adipocyte character. We therefore applied two separate techniques to deplete the major circulating populations of plasma-derived EVs to establish whether adipocyte markers were reduced by depletion of nonadipocyte EVs and whether an adipocyte protein signature was retained postdepletion. EV structures were visible by TEM following sequential depletion of major plasma EV populations (Fig. 2A), though the overall concentration of EVs detected by NTA was reduced by ∼75% (Fig. 2B). Both techniques were shown to reduce the expression of each marker used for depletion, with the magnetic bead–based approach depleting these markers beyond detection by TRF (Supplemental Fig. 3). This suggests both techniques are effective in reducing the populations of major circulating EVs in plasma. Expression of adiponectin, FABP4, and PPARγ was reduced postdepletion (Fig. 2C), suggesting a proportion of these markers are in some way associated with EVs from nonadipocyte populations. Although their expression is predominantly associated with adipocytes, both FABP4 and PPARγ have previously been shown to be produced by other cells including macrophages (45, 46), perhaps explaining the partial loss in signal postdepletion. FABP4 was not detected in all samples (possibly due to individual variations in donors) and was often detected at a higher molecular weight than expected. FABP4 has previously been reported to form homodimers, particularly upon ligand activation (47), though the absence of expression in some samples reaffirms the need to use multiple markers when analyzing adipocyte-derived EVs. Although both isoforms of PPARγ were detected in predepletion samples, only PPARγ2 remained in postdepletion samples. PPARγ2 is an adipocyte-specific nuclear transcription factor (48), and its presence in combination with adiponectin, FABP4, and perilipin in postdepletion samples is highly indicative of adipocyte origin. Furthermore, a number of major adipokines were detected in postdepletion samples using an adipokine array kit, including the angiogenic factors leptin and resistin, and adipokines adipsin, preadipocyte factor-1, and visfatin (Fig. 3). This further evidences the presence of adipocyte markers in EV samples that have been depleted of major circulating plasma EV populations. The EV markers Alix and CD9 were reduced but still present in postdepletion samples (Fig. 2C). This tallies with a reduced concentration of EVs but also indicates that EVs are still present in postdepletion samples, supporting the TEM data. Taken together, our data show that after depleting EVs from major sources in plasma using either magnetic beads or solid-phase depletion, adipocyte and EV markers are still detectable, supporting the presence of adipocyte-derived EVs. It is important to note that we observed differences in expression patterns of both EV and adipocyte markers between individuals; however, this is most likely due to natural biological variation within our small group of donors.
In conclusion, this provides evidence for the presence of adipocyte-derived EVs in circulating plasma using multiple adipocyte and EV markers and conducted in accordance with international recommendations. Our data also emphasize the need for careful EV preparation when analyzing adipocyte markers to avoid contribution of signal from soluble adipocyte material. Although adipocyte-derived EVs may only constitute a relatively small fraction of the total EV population in circulating plasma, this may not necessarily reflect a minor effect on vascular health because the content of the EVs is likely to dictate their function, particularly in EVs derived from dysfunctional adipocytes. Our data thus provide a platform for future investigations into circulating adipocyte-derived EVs as potential biomarkers of adipocytes in health and obesity-driven cardiovascular disease.
Supplementary Material
Acknowledgments
The authors thank Dr. Justyna Witczak and Margaret Munnery for the phlebotomy required for this work, the healthy individuals who volunteered for the study, and the Sir William Dunn School of Pathology Electron Microscopy facility for the TEM analyses.
Financial Support: This work was supported by the Ewen Maclean scholarship fund (Cardiff University), the British Heart Foundation, and Cardiff Metropolitan University.
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- EV
extracellular vesicle
- FABP4
fatty acid binding protein 4
- ISEV
International Society for Extracellular Vesicles
- NTA
nanoparticle-tracking analysis
- PPARγ
peroxisome proliferator–activated receptor γ
- SEC
size-exclusion chromatography
- TEM
transmission electron microscopy
- TRF
time-resolved fluorescence
References
- 1. Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br J Nutr. 2004;92(3):347–355. [DOI] [PubMed] [Google Scholar]
- 2. Wang B, Wood IS, Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455(3):479–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kralisch S, Ebert T, Lossner U, Jessnitzer B, Stumvoll M, Fasshauer M. Adipocyte fatty acid-binding protein is released from adipocytes by a non-conventional mechanism. Int J Obes. 2014;38(9):1251–1254. [DOI] [PubMed] [Google Scholar]
- 4. Kranendonk MEG, Visseren FLJ, van Balkom BWM, Nolte-’t Hoen ENM, van Herwaarden JA, de Jager W, Schipper HS, Brenkman AB, Verhaar MC, Wauben MHM, Kalkhoven E. Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring). 2014;22(5):1296–1308. [DOI] [PubMed] [Google Scholar]
- 5. Kranendonk MEG, Visseren FLJ, van Herwaarden JA, Nolte-’t Hoen ENM, de Jager W, Wauben MHM, Kalkhoven E. Effect of extracellular vesicles of human adipose tissue on insulin signaling in liver and muscle cells. Obesity (Silver Spring). 2014;22(10):2216–2223. [DOI] [PubMed] [Google Scholar]
- 6. Connolly KD, Guschina IA, Yeung V, Clayton A, Draman MS, Von Ruhland C, Ludgate M, James PE, Rees DA. Characterisation of adipocyte-derived extracellular vesicles released pre- and post-adipogenesis. J Extracell Vesicles. 2015;4(1):29159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. DeClercq V, d’Eon B, McLeod RS. Fatty acids increase adiponectin secretion through both classical and exosome pathways. Biochim Biophys Acta. 2015;1851(9):1123–1133. [DOI] [PubMed] [Google Scholar]
- 8. Eguchi A, Mulya A, Lazic M, Radhakrishnan D, Berk MP, Povero D, Gornicka A, Feldstein AE. Microparticles release by adipocytes act as “find-me” signals to promote macrophage migration. PLoS One. 2015;10(4):e0123110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ertunc ME, Sikkeland J, Fenaroli F, Griffiths G, Daniels MP, Cao H, Saatcioglu F, Hotamisligil GS. Secretion of fatty acid binding protein aP2 from adipocytes through a nonclassical pathway in response to adipocyte lipase activity. J Lipid Res. 2015;56(2):423–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ferrante SC, Nadler EP, Pillai DK, Hubal MJ, Wang Z, Wang JM, Gordish-Dressman H, Koeck E, Sevilla S, Wiles AA, Freishtat RJ. Adipocyte-derived exosomal miRNAs: a novel mechanism for obesity-related disease. Pediatr Res. 2015;77(3):447–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eguchi A, Lazic M, Armando AM, Phillips SA, Katebian R, Maraka S, Quehenberger O, Sears DD, Feldstein AE. Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J Mol Med (Berl). 2016;94(11):1241–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Durcin M, Fleury A, Taillebois E, Hilairet G, Krupova Z, Henry C, Truchet S, Trötzmüller M, Köfeler H, Mabilleau G, Hue O, Andriantsitohaina R, Martin P, Le Lay S. Characterisation of adipocyte-derived extracellular vesicle subtypes identifies distinct protein and lipid signatures for large and small extracellular vesicles. J Extracell Vesicles. 2017;6(1):1305677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lötvall J, Hill AF, Hochberg F, Buzás EI, Di Vizio D, Gardiner C, Gho YS, Kurochkin IV, Mathivanan S, Quesenberry P, Sahoo S, Tahara H, Wauben MH, Witwer KW, Théry C. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. 2014;3(1):26913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Müller G, Jung C, Straub J, Wied S, Kramer W. Induced release of membrane vesicles from rat adipocytes containing glycosylphosphatidylinositol-anchored microdomain and lipid droplet signalling proteins. Cell Signal. 2009;21(2):324–338. [DOI] [PubMed] [Google Scholar]
- 15. Han YD, Bai Y, Yan XL, Ren J, Zeng Q, Li XD, Pei XT, Han Y. Co-transplantation of exosomes derived from hypoxia-preconditioned adipose mesenchymal stem cells promotes neovascularization and graft survival in fat grafting. Biochem Biophys Res Commun. 2018;497(1):305–312. [DOI] [PubMed] [Google Scholar]
- 16. Kang T, Jones TM, Naddell C, Bacanamwo M, Calvert JW, Thompson WE, Bond VC, Chen YE, Liu D. Adipose-derived stem cells induce angiogenesis via microvesicle transport of miRNA-31. Stem Cells Transl Med. 2016;5(4):440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Berckmans RJ, Nieuwland R, Böing AN, Romijn FP, Hack CE, Sturk A. Cell-derived microparticles circulate in healthy humans and support low grade thrombin generation. Thromb Haemost. 2001;85(4):639–646. [PubMed] [Google Scholar]
- 18. Gustafson CM, Shepherd AJ, Miller VM, Jayachandran M. Age- and sex-specific differences in blood-borne microvesicles from apparently healthy humans. Biol Sex Differ. 2015;6(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Arraud N, Linares R, Tan S, Gounou C, Pasquet J-M, Mornet S, Brisson AR. Extracellular vesicles from blood plasma: determination of their morphology, size, phenotype and concentration. J Thromb Haemost. 2014;12(5):614–627. [DOI] [PubMed] [Google Scholar]
- 20. Phoonsawat W, Aoki-Yoshida A, Tsuruta T, Sonoyama K. Adiponectin is partially associated with exosomes in mouse serum. Biochem Biophys Res Commun. 2014;448(3):261–266. [DOI] [PubMed] [Google Scholar]
- 21. van der Pol E, Hoekstra AG, Sturk A, Otto C, van Leeuwen TG, Nieuwland R. Optical and non-optical methods for detection and characterization of microparticles and exosomes. J Thromb Haemost. 2010;8(12):2596–2607. [DOI] [PubMed] [Google Scholar]
- 22. Looze C, Yui D, Leung L, Ingham M, Kaler M, Yao X, Wu WW, Shen R-F, Daniels MP, Levine SJ. Proteomic profiling of human plasma exosomes identifies PPARgamma as an exosome-associated protein. Biochem Biophys Res Commun. 2009;378(3):433–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shah MD, Bergeron AL, Dong J-F, López JA. Flow cytometric measurement of microparticles: pitfalls and protocol modifications. Platelets. 2008;19(5):365–372. [DOI] [PubMed] [Google Scholar]
- 24. Witwer KW, Buzás EI, Bemis LT, Bora A, Lässer C, Lötvall J, Nolte-’t Hoen EN, Piper MG, Sivaraman S, Skog J, Théry C, Wauben MH, Hochberg F. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. RRID:AB_10608844.
- 26.RRID:AB_648179.
- 27.RRID:AB_323286.
- 28.RRID:AB_1523093.
- 29.RRID:AB_2732848.
- 30.RRID:AB_2278527.
- 31.RRID:AB_10829911.
- 32.RRID:AB_10694772.
- 33.RRID:AB_650499.
- 34.RRID:AB_2722659.
- 35.RRID:AB_2732852.
- 36.RRID:AB_868788.
- 37.RRID:AB_870662.
- 38.RRID:AB_2732853.
- 39. Welton JL, Webber JP, Botos L-A, Jones M, Clayton A. Ready-made chromatography columns for extracellular vesicle isolation from plasma. J Extracell Vesicles. 2015;4(1):27269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burnley-Hall N, Abdul F, Androshchuk V, Morris K, Ossei-Gerning N, Anderson R, Rees DA, James PE. Dietary nitrate supplementation reduces circulating platelet-derived extracellular vesicles in coronary artery disease patients on clopidogrel therapy: a randomised, double-blind, placebo-controlled study. Thromb Haemost. 2018;118(1):112–122. [DOI] [PubMed] [Google Scholar]
- 41.RRID:AB_2732854.
- 42. Webber J, Clayton A. How pure are your vesicles? J Extracell Vesicles. 2013;2(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Böing AN, van der Pol E, Grootemaat AE, Coumans FAW, Sturk A, Nieuwland R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J Extracell Vesicles. 2014;3(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Christersson C, Johnell M, Siegbahn A. Evaluation of microparticles in whole blood by multicolour flow cytometry assay. Scand J Clin Lab Invest. 2013;73(3):229–239. [DOI] [PubMed] [Google Scholar]
- 45. Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, Linton MF. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7(6):699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosen ED, Sarraf P, Troy AE, Bradwin G, Moore K, Milstone DS, Spiegelman BM, Mortensen RM. PPAR gamma is required for the differentiation of adipose tissue in vivo and in vitro. Mol Cell. 1999;4(4):611–617. [DOI] [PubMed] [Google Scholar]
- 47. Gillilan RE, Ayers SD, Noy N. Structural basis for activation of fatty acid-binding protein 4. J Mol Biol. 2007;372(5):1246–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene. Mol Cell Biol. 1995;15(1):351–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.