Abstract
Background
The major factors contributing for nerve damage and permanent disabilities in leprosy are type 1 or reversal reactions (RR) and type 2 or erythema nodosum leprosum (ENL). Gene profiling of leprosy reactions have shown that different pathways are activated during the course of reactions, which is consistent with the exacerbated immune response exhibited by these patients.
Methods
We used qPCR to screen a panel of 90 genes related to the immune response in leprosy in RNA-derived peripheral leukocytes of patients with (N = 94) and without leprosy reactions (N = 57) in order to define expression signatures correlated to RR or ENL.
Results
Our results show that there is a marked signature for RR in the blood, comprising genes mostly related to the innate immune responses, including type I IFN components, autophagy, parkins and Toll like receptors. On the other hand, only Parkin was differentially expressed in the ENL group.
Conclusions
The data put together corroborates previous work that brings evidence that an acute uncontrolled exacerbated immune response designed to contain the spread of M. leprae antigens might be cause of RR pathogenesis. Identifying a blood profile useful to predict leprosy reactions prior to its development might help to reduce the morbidity associated to this disabling disease.
Keywords: Leprosy reactions; gene expression; profile, Parkin, Pro-inflammatory, Type-I IFN, OASL
Background
Leprosy is a chronic infectious disease caused by Mycobacterium leprae. The bacilli invades Schwann cells and macrophages of the skin leading the tissue injury, which is the major reason for its pathogenesis [1–3]. Leprosy presents a wide variety of clinical presentations, including the indeterminate (I), tuberculoid (TT), borderline (BT, BB, BL) and lepromatous (LL) forms. In addition, about 20–50% of leprosy patients, depending on the population studied, can be affected by acute inflammatory episodes known as leprosy reactions, as so called type 1 (Reversal Reaction) or type 2 (Erythema Nodosum Leprosum- ENL) [4, 5]. Either RR or ENL are observed in all borderline forms prior, during or after completion of multidrug therapy. RR involves the active participation of T lymphocytes and abrupt episodes of intense local delayed-type hypersensitivity to M. leprae in skin and/or nerves. On the other hand, ENL is typical of the BL and LL forms and is correlated to a systemic reaction involving a cytokine storm and also deposition of immune complexes in skin and organs [6, 7]. Regardless its type, leprosy reactions are an important contributing factor of nerve damage among patients with leprosy. The identification of host-derived biomarkers correlated to leprosy reactions might point out new tests to predict increased risk of developing the occurrence of reactional episodes thus helping to prevent its irreversible sequels. There are only a few transcriptomic studies searching for genes related to the development of leprosy reactions. Among these, a role for pro and anti-inflammatory regulators, IFN-induced genes, complement components, among others have been described [5, 6, 8].
Methods
In this study we analyzed the expression of a panel of relevant immune response genes in RNA-derived from peripheral blood leukocytes of leprosy patients with and without reactions in order to identify gene expression signatures associated with either RR or ENL. One hundred and fifty one cDNA samples of patients divided into three groups were used: 57 patients with no evidence of reactions (hereinafter referred to as No Reaction Group - NR), 50 patients with RR and 44 patients with ENL. Subjects were diagnosed according to the Brazilian’s Ministry of Health guidelines in the leprosy outpatient clinics from Hospital Universitário Professor Edgard Santos and Hospital Couto Maia in the city of Salvador-Bahia, Brazil. Patients were classified according to a Ridley–Jopling classification and by the WHO field classification [9, 10], as previously reported for studies of patients recruited from this hospital in Salvador [11]. Detailed complementary data about the participants are described in Table 1. Written informed consent was obtained from all patients after approval of the study by the Ethics Committee from the Federal University of Bahia (number 891.963). Peripheral leukocytes from patients free of immunosuppressants such as thalidomide or prednisone were homogenized in TRIzol (Thermo Fisher Scientific). RNA was extracted using the PureLink ™ RNA Mini Kit (Thermo Fisher Scientific) and the total RNA concentration was determined in optical density spectrophotometer (260 and 280 nm). The cDNA conversion was performed using the High Capacity cDNA Reversion Transcription Kit (Applied Biosystems) following the manufacturer’s instructions. The expression of 90 target genes and 4 normalizing genes was performed by medium- throughput quantitative q-PCR using the microfluidic system Biomark (Fluidigm, CA). The analysis was performed from the real-time fluorescence accumulation data of each sample (ΔRn), using the logistic function adjustment of four parameters to represent each amplification curve by the library of qpcR (R Development Core Team, 2009) version 2.922. Results: After filtering by QC, 35 genes were excluded and 55 analyzed. We first compared the paucibacillary (PB) versus multibacillary (MB) leprosy within the unreactional (NR) group checking for differences regarding these two disease poles. This analysis did not show any significant differences (p < 0.05, data not shown). Nevertheless, there was a differential pattern of gene expression between the NR and RR group as shown in Table 2. A set of genes belonging to different pathways that includes the parkin pathway, pattern recognition receptors (PRRs), type I IFNs precursors, inflammatory cytokines and chemokines and eicosanoid metabolism were significantly more expressed in RR patients as compared to the NR group (Fig. 1). This peculiar inflammatory signature for type 1 reaction has been described in previous works [5, 8] that also underpinned a mixed immune activation that seems to lead to the RR pathogenesis. On the other hand, only PARK2 was significantly more expressed in leucocytes of ENL compared to unreactional patients (logFC =2.13 e p = 0.04), as well as TLR7 between RR and ENL subjects (logFC = − 2.72 e p = 0.02).
Table 1.
Demographic and clinical characteristics of leprosy patients
| A - Characteristics of the samplea | ||||
| N individuals | Age, years ± SD |
Gender M:F | ||
| Cases / Reaction | 94 | 44.05 (13.31) | 58:36 | |
| Controls / No Reaction | 57 | 45.21 (14.98) | 28:29 | |
| B - Clinical characteristics of the cohorta | ||||
| Clinical phenotype | n | (%) | ||
| Tuberculoid (TT) | 20 | (13) | ||
| Borderline tuberculoid (BT) | 30 | (20) | ||
| Borderline (BB) | 18 | (12) | ||
| Borderline lepromatous (BL) | 17 | (11) | ||
| Lepromatous (LL) | 49 | (32) | ||
| Indeterminate leprosy (I) | 11 | (7) | ||
| Other forms (Neural) | 6 | (4) | ||
| Total# | 151 | (100) | ||
| C - Patients with reaction episodea | ||||
| RR | 50 | (53) | ||
| ENL | 44 | (47) | ||
| Total# | 94 | (62) | ||
| D - Patients without reaction episodea | ||||
| PB | 47 | (82) | ||
| MB | 10 | (18) | ||
| Total# | 57 | (38) | ||
aResults are shown as N(%). Abbreviations: SD Standart deviation, M male, F female, PB paucibacillary, MB multibacillary, RR reversal reaction, ENL erythema nodosum leprosum. Patients were also classified under leprosy clinical spectrum according to Ridley & Jopling [9]
Table 2.
Normalized gene expression values of whole blood leukocytes samples of leprosy patients with reactions (n = 94) and leprosy patients without reactions (n = 57)
| Reaction vs No Reaction | |||
|---|---|---|---|
| Gene | Description | log fold change | p.value* |
| CCL2 | C-C Motif Chemokine Ligand 2 | 4.04 | 0.0016 |
| PARK | parkinson protein 2, E3 ubiquitin protein ligase | 3.27 | 0.0036 |
| ALOX5 | Arachidonate 5-Lipoxygenase | 2.99 | 0.0108 |
| TLR7 | Toll Like Receptor 7 | 2.98 | 0.011 |
| LRRK2 | Leucine Rich Repeat Kinase 2 | 3.09 | 0.0161 |
| IFNB | interferon beta 1 | 2.36 | 0.0228 |
| TLR10 | Toll Like Receptor 10 | 3.07 | 0.0236 |
| IL18 | Interleukin 18 | 2.34 | 0.0326 |
| TLR3 | Toll Like Receptor 3 | 2.74 | 0.0338 |
| CLEC5A | C-Type Lectin Domain Containing 5A | 2.81 | 0.0446 |
| RR vs No Reaction | |||
| CCL2 | C-C Motif Chemokine Ligand 2 | 4.31 | 0.0002 |
| TLR7 | Toll Like Receptor 7 | 3.81 | 0.0012 |
| PARK | parkinson protein 2, E3 ubiquitin protein ligase | 3.16 | 0.002 |
| ALOX5 | Arachidonate 5-Lipoxygenase | 3.32 | 0.0044 |
| LRRK2 | Leucine Rich Repeat Kinase 2 | 3.28 | 0.0056 |
| IFNB | interferon beta 1 | 2.64 | 0.0104 |
| TLR10 | Toll Like Receptor 10 | 3.08 | 0.0122 |
| TLR3 | Toll Like Receptor 3 | 3.04 | 0.0122 |
| IL18 | Interleukin 18 | 2.65 | 0.014 |
| OAS1 | 2′-5′-oligoadenylate synthetase 1 | 2.51 | 0.0376 |
| IL15 | Interleukin 15 | 2.42 | 0.0446 |
| ENL vs No Reaction | |||
| PARK | parkinson protein 2, E3 ubiquitin protein ligase | 2.13 | 0.0436 |
| ENL vs RR | |||
| TLR7 | Toll Like Receptor 7 | −2.72 | 0.0214 |
*The genes were defined as differentially expressed by the criterion of p-value adjusted for multiple comparisons. Bayesian statistical analysis used a log fold change cutoff of > 1 and adjusted p value of < 0.05. - Abbreviations: RR reversal reaction, ENL erythema nodosum leprosum
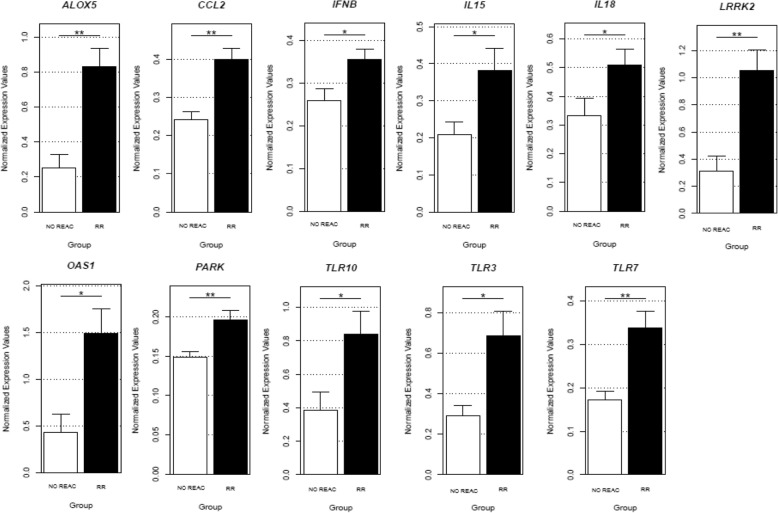
Fig. 1.
Gene expression of whole blood leukocytes in patients with RR as compared to patients without leprosy reactions. A group of genes differentially expressed in RR samples was identified, consisting of 1 chemokine gene (CCL2 logFC = 4.04), 2 PARK genes (PARK logFC =3.27, LRRK2 logFC =3.09), 1 eicosanoid metabolism gene (ALOX5 logFC =2.99), 4 pattern recognition receptor genes (Toll-like receptors -TLRs: TLR7 logFC =2.98, TLR10 logFC =3.07, TLR3 logFC =2.74, and C-lectin receptors-CLRs: CLEC5A logFC =2.81), 2 cytokine genes (IL18 logFC =2.34, IL15 logFC = 2.42) and 2 type I IFN genes (IFNB logFC =2.36, OAS1 logFC = 2.51)
Discussion
Our RR signature corroborates data showing that M. leprae components and host cell destruction continue to stimulate the immune response in a sudden and acute manner during RR. Most pathogen-associated molecular patterns (PAMPs) and damage-associated molecular pattern molecules (DAMPs) bind specific PRRs such as Toll-like receptors and NOD-like receptors to orchestrate both, autophagy and IFN signaling [12, 13]. We hypothesized that the continued binding of PAMPs and DAMPs to TLRs caused by the pathogen components after killing destruction provides the necessary trigger for maintenance of the inflammatory process. The stimulation of innate mechanisms that comprise genes with autophagic activities such as PARK and LRRK2, in addition to the type I IFNs in the beginning of the process seems to be activated in order to clear killed mycobacteria, but it is unbalanced and exacerbated. Regarding the IFNs, the genes IFNB and OAS1 (2–5 ‘oligoadenylate synthetase-1 gene) had a greater expression in RR samples. OASL was also shown to be upregulated in M. leprae–infected human macrophage cell lineages, primary monocytes, and skin lesion from patients with a disseminated form of leprosy; whereas OASL knock down was associated with decreased viability of M. leprae and upregulation of autophagy levels [14]. Additionally, the chemokine CCL2 was the most expressed gene in our RR group. Recent reports have linked the STING signaling, type I IFN and CCL2 activation [14, 15]. During mycobacterial infection, this chemokine can be produced in a STING-dependent manner and it is actively involved in the recruitment of monocytes to the infection site [15] and also related to mycobacterial survival within macrophages [14]. Other works have pointed the participation of CCL2 in the pathogenesis of several inflammatory disorders such as atherosclerosis and autoimmune diseases [16–18]. Here, we could speculate that a prominent triggering of STING signaling and high expression of type I IFN and CCL2 may contribute to the attraction of immune cells and enhancement of inflammatory response during leprosy reaction.
Type 1 reaction or RR is caused by an amplified immune response possibly triggered by fragmented bacillary antigens available in the cell medium [19]. The main issue however, is that a dysregulated process of gene activation, aiming to contain the progress of M. leprae and eliminate the infection, will lead to the nerve and tissue damage. Indeed, persons with history of RR can keep an altered response to M. leprae antigens that differs from patients with unreactional leprosy for years after resolution of RR [8]. Additionally, our results show that the expression of TLR3, TLR7 and TLR10 were significantly increased in the reactions per se as well as in RR with TLR7 and TLR10 corroborating with data that fragments of bacterial destruction may be giving continuity to the characteristic inflammatory process of both reactional episodes in leprosy. On the other hand, ENL is characterized by a systemic inflammatory reaction. In this case, it might be possible that other set of genes related to the humoral immune response would be more active in these leucocytes. We need to expand our panel in order to identify which profile explains ENL.
Conclusion
Overall our data strength previous data and reinforces a signature for RR that could help to guide future studies for developing tools to predict this condition among leprosy patients. Personalizing the treatment of individuals susceptible to the development of reactions will help increase the effectiveness of treatment and reduce morbidity and disability in leprosy.
Acknowledgements
We thank the staff of outpatients leprosy clinics from Hospital Universitário Professor Edgard Santos and Hospital Couto Maia for the support in the subjects recruitment and sample collection.
Funding
National Institute of Science and Technology in Tropical Diseases, Brazil (N° 573839/2008–5), CNPq, Brazil (N°404277/2012–8 and N° 309397/2013–8) and FAPERJ (N° E9/2016). We also thank CAPES by granting scholarships.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
PM diagnosed, treated and included the patients in the study. JLR and NLS collected the samples and extracted the RNA from the patients. JLR and TGT performed the gene expression experiments. MRA and TGT analyzed the data. LCC supervised the samples processing and wrote the manuscript. MOM supervised the data and manuscript preparation. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study has been approved by the Ethical Committee of the Hospital Universitário Prof. Edgar Santos, Federal University of Bahia, number 891.963. All patients read and signed an informed consent form.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jamile Leão Rêgo, Email: jamileao@hotmail.com.
Nadja de Lima Santana, Email: nlimas@hotmail.com.
Paulo Roberto Lima Machado, Email: 19pmachado@gmail.com.
Marcelo Ribeiro-Alves, Email: mribalves@gmail.com.
Thiago Gomes de Toledo-Pinto, Email: thiago_gtp@hotmail.com.
Léa Cristina Castellucci, Email: leacastel@hotmail.com.
Milton Ozório Moraes, Email: mmoraes@fiocruz.br.
References
- 1.Agrawal A, et al. Neurological manifestations of Hansen's disease and their management. Clin Neurol Neurosurg. 2005;107(6):445–454. doi: 10.1016/j.clineuro.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Eichelmann K., G.G.S., Salas-Alanis JC, Ocampo-Candiani J., Leprosy An update: definition, pathogenesis, classification, diagnosis, and treatment. Actas Dermo-Sifiliográficas, 2013. 104(7): p. 554–563. [DOI] [PubMed]
- 3.Lastória JC, de Abreu MAMM. Leprosy: review of the epidemiological, clinical, and etiopathogenic aspects - part 1. An Bras Dermatol. 2014;89(2):205–218. doi: 10.1590/abd1806-4841.20142450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rêgo JL, et al. The role of ERBB2 gene polymorphisms in leprosy susceptibility. Braz J Infect Dis. 2015;19:206–208. doi: 10.1016/j.bjid.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Geluk A, et al. Longitudinal immune responses and gene expression profiles in type 1 leprosy reactions. J Clin Immunol. 2014;34(2):245–255. doi: 10.1007/s10875-013-9979-x. [DOI] [PubMed] [Google Scholar]
- 6.Dupnik KM, et al. Transcriptional changes that characterize the immune reactions of leprosy. J Infect Dis. 2015;211(10):1658–1676. doi: 10.1093/infdis/jiu612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moraes MO, et al. Anti-inflammatory drugs block cytokine mRNA accumulation in the skin and improve the clinical condition of reactional leprosy patients. J Investig Dermatol. 2000;115(6):935–941. doi: 10.1046/j.1523-1747.2000.00158.x. [DOI] [PubMed] [Google Scholar]
- 8.Orlova M, et al. Gene set signature of reversal reaction type I in leprosy patients. PLoS Genet. 2013;9(7):e1003624. doi: 10.1371/journal.pgen.1003624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ridley D, Jopling W. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis. 1966;34(3):255–273. [PubMed] [Google Scholar]
- 10.WHO, Action Programme for the Elimination of Leprosy . Efficacy of single dose multi drug therapy for the treatment of single-lesion paucibacillary leprosy. Geneva: World Health Organization; 1997. pp. 121–129. [Google Scholar]
- 11.Machado PRL, et al. Viral co-infection and leprosy outcomes: a cohort study. PLoS Negl Trop Dis. 2015;9(8):e0003865. doi: 10.1371/journal.pntd.0003865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang F, et al. Genomewide association study of leprosy. N Engl J Med. 2009;361(27):2609–2618. doi: 10.1056/NEJMoa0903753. [DOI] [PubMed] [Google Scholar]
- 13.Saxena M, Yeretssian G. NOD-like receptors: master regulators of inflammation and cancer. Front Immunol. 2014;5:327. doi: 10.3389/fimmu.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Toledo-Pinto TG, et al. STING-dependent 2′-5′ Oligoadenylate Synthetase–like production is required for intracellular Mycobacterium leprae survival. J Infect Dis. 2016;214(2):311–320. doi: 10.1093/infdis/jiw144. [DOI] [PubMed] [Google Scholar]
- 15.Cambier CJ, et al. Phenolic glycolipid facilitates mycobacterial escape from Microbicidal tissue-resident macrophages. Immunity. 2017;47(3):552–565.e4. doi: 10.1016/j.immuni.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tracy OC, Lubor B, Mathias H. CCL2-CCR2 signaling in disease pathogenesis. Endocrine, Metabolic & Immune Disorders - Drug Targets. 2015;15(2):105–118. doi: 10.2174/1871530315666150316120920. [DOI] [PubMed] [Google Scholar]
- 17.Gosling J, et al. MCP-1 deficiency reduces susceptibility to atherosclerosis in mice that overexpress human apolipoprotein B. J Clin Invest. 1999;103(6):773–778. doi: 10.1172/JCI5624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Behfar S, et al. A brief look at the role of monocyte chemoattractant protein-1 (CCL2) in the pathophysiology of psoriasis. Cytokine. 2017 [DOI] [PubMed]
- 19.Mendonça VA, et al. Immunology of leprosy. An Bras Dermatol. 2008;83(4):343–350. doi: 10.1590/S0365-05962008000400010. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.