Abstract
Background
Chagas disease is caused by Trypanosoma cruzi, a parasite endemic to Latin America. Most infections occur in children by vector or congenital transmission. Trypanosoma cruzi establishes a complexity of specific molecular parasite-host cell interactions to invade the host. However, most studies have been mainly focused on the interaction between the parasite and different cell types, but not on the infection and invasion on a tissue level. During congenital transmission, T. cruzi must cross the placental barrier, composed of epithelial and connective tissues, in order to infect the developing fetus. Here we aimed to study the global changes of transcriptome in the placental tissue after a T. cruzi challenge.
Results
Strong changes in gene expression profiling were found in the different experimental conditions, involving the reprogramming of gene expression in genes involved in the innate immune response.
Conclusions
Trypanosoma cruzi induces strong changes in genes involved in a wide range of pathways, especially those involved in immune response against infections.
Electronic supplementary material
The online version of this article (10.1186/s13071-018-2988-0) contains supplementary material, which is available to authorized users.
Keywords: Trypanosoma cruzi, Placenta, Global gene expression, Microarray
Background
Chagas disease is a zoonotic disease caused by Trypanosoma cruzi, a parasite endemic to Latin America. Most infections occur in children by vector or congenital transmission. The prevalence of Chagas disease in pregnant women in Latin America ranges between 5–40% depending on the geographical area and the rate of congenital transmission is estimated to be 1–12% [1]. In addition, due to population mobility, Chagas disease has been increasingly detected in other non-endemic countries and continents (where the vector does not exist) such as the USA, Canada, Australia, Europe and Asia [2, 3]. Congenital transmission, in spite of its low transmission rates, is partially responsible for the progressive globalization of the disease [2, 4, 5]. Importantly, congenital infection is responsible for an estimated 22% of new infections in 2010, making this form of transmission epidemiologically relevant [6].
The parasite presents a complex life-cycle that occurs in both vertebrate and invertebrate hosts, where three major developmental stages are observed: epimastigotes, trypomastigotes and amastigotes. Trypomastigotes constitute the extracellular infective form in mammals where they are able to infect a wide range of nucleated mammalian cells [7]. Interestingly, T. cruzi has co-evolved with mammals to establish a complexity of specific molecular parasite-host cell interactions to invade host cells and tissues, to evade the host immune system and to undergo intracellular replication [8]. Key steps in parasite infection include its host cell penetration and replication of the protozoa in the cytoplasm of infected cells. The application of oligonucleotide and cDNA microarray technologies in the study of host-parasite interactions have permitted rapid and unbiased examination of changes in expression of a large number of genes at the level of transcription [9, 10]. However, these studies have been mainly focused on the interaction between the parasite and different cell types, but not on the infection and invasion on a tissue level.
During congenital transmission, T. cruzi must cross the placental barrier in order to infect the developing fetus [3, 11]. This anatomical barrier is formed by the trophoblast, a two-layer epithelium which is in direct contact with maternal blood, the fetal connective tissue (villous stroma), the endothelium of fetal vessels and the basal laminae that support the epithelia [3, 12]. Interestingly, the congenital transmission rate for T. cruzi is low [4, 13] and it has been proposed that the placenta might play an important role avoiding parasite infection [3].
The study of gene expression profiles during infection constitutes a very powerful tool to analyze global responses of several kinds of cells and tissues, allowing the identification of new genes and/or pathways implicated in the establishment of the infection and pathogenesis as well as possible local tissue responses [3, 10]. Therefore, here we aimed to study the global changes of transcriptome in the placental tissue after T. cruzi challenge.
Methods
Parasite harvesting
Trypomastigotes from T. cruzi (Y Strain, T. cruzi II) were obtained from previously infected Vero cells (ATCC® CCL-81) grown in RPMI medium supplemented with 5% fetal bovine serum (FBS) and antibiotics (penicillin-streptomycin) at 37 °C in a humid atmosphere at 5% CO2. Parasites invaded the cells and replicated intracellularly as amastigotes, after 48–72 h; amastigotes transformed back to trypomastigotes and lysed host cells. The infective trypomastigotes were separated from cellular debris by low speed centrifugation (500× g). From the supernatant, the parasites were isolated by centrifugation at 3500× g, suspended in RPMI media (without FBS, 1% antibiotics; RPMI 1640, Biological Industries Ltd., Kibbutz Beit Haemek, Israel) and quantified in a Neubauer Chamber [14, 15].
HPE infection
HPE were obtained from healthy mothers with uncomplicated pregnancies by cesarean delivery. Placentas were processed in a class II laminar flow hood immediately after delivery. The maternal and fetal surfaces were discarded and villous tissue was obtained from the central part of the cotyledons. The dissected explants were washed with sterile PBS in order to get rid of the blood and co-cultivated with T. cruzi trypomastigotes in serum free RPMI media. HPE were challenged with 105 or 106 parasites/ml, since these concentrations have been proposed to correlate with low or high parasitaemia, respectively [16]. For validation experiments, LPS (10 ng/ml) was used as positive control. After 2 or 24 h of infection (in order to study early and late placental responses [16–18], explants were collected in RNA later solution (Thermo Fisher Scientific, Waltham, Massachusetts, USA), stored at 4 °C for 24 h and at -80 °C for posterior RNA isolation [19].
RNA purification and microarray experiment
Total RNA was isolated with a Purelink RNA isolation kit (Thermo Fisher Scientific) according to the manufacturer’s instructions. RNA integrity was analyzed with a Bioanalyzer 2100 (Agilent Technologies, Santa Clara, California, USA) obtaining RNA integrity numbers (RIN) above 8 for all samples (on a scale based on an rRNA 28S/18S ratio where a RIN of 1 corresponds to a totally degraded RNA and 10 to a totally non-degraded RNA). RNA concentration was quantified by spectrophotometry (Nanodrop, Thermo Fisher Scientific). One hundred nanograms of total RNA was reverse-transcribed into cDNA, then transcribed to cRNA and Cy3-labeled with a Low Input Quick Amp-One Color Labeling Kit (Agilent Technologies). The labeled cRNA was purified with an illustra RNAspin Mini Isolation Kit (GE Healthcare, Little Chalfont, UK) and the total yield was measured with a Qubit RNA HS Kit (Thermo Fisher Scientific). Hybridization, washing, assembling of the chips, and scanning were performed according to the manufacturers’ instructions. Briefly, labeled samples were hybridized with SurePrint G3 Human GE 8x60K chips for 17 h at 60 °C in an Agilent hybridization oven at 10× rpm. Posterior washing, stabilization and drying procedures were performed according to Agilent’s Low Input Quick Amp Labeling Kit instructions [10].
Data analysis
Chips were scanned with an Agilent microarray scanner G2565BA; the software Agilent Feature Extraction (version 9.5.1), was used for quality control, data filtering and data normalization. Extracted data from the SurePrintG3 8x60K chips were analyzed using GeneSpring GX 13.0 software. Genes showing a 2-fold change in their expression (or more) with P ≤ 0.05 were considered differentially expressed using ANOVA and Benjamini-Hochberg false discovery rate correction for multiple testing. Analysis of interaction networks between upregulated genes in each experimental group was performed with Cytoscape network visualization and integration software and GeneMania open-source gene function prediction service plug-in (http://www.genemania.org/) and visualized by the corresponding Cytoscape software version 3.0.2 plug-ins [20] The weighting of the network attributes was set to Gene Ontology (GO)-based weighting for biological processes. Gene set enrichment analysis (GSEA) was performed with GSEA 3.0 software (Broad Institute, Cambridge, Massachussets, USA) [21]. Each gene set permutation was performed 1000 times, analyses were based on the GO pathways database (http://geneontology.org/) [22] and a normalized enrichment score (NES) was obtained for each gene set. An enrichment map from data obtained with GSEA was generated with the Enrichment Map plug-in for Cytoscape 3.0 [23]. NES and false discovery rate (FDR) q-value were considered as parameters for the analysis. The NES value allows the comparison of analysis results across gene sets because it considers differences in gene sets sizes corrected by the size of the expression dataset; the FDR represents the estimated probability that a gene set with a given NES represents a false positive finding [22].
RT-qPCR
One hundred picograms of total RNA was retro-transcribed to cDNA using an M-MLV Reverse Transcriptase system with Oligo(dT) primers (Thermo Fisher Scientific). For real-time reactions, 10 μl of Sensifast qPCR Master Mix (Bioline, London, UK) was mixed with 100 mM forward and reverse primers and 2 μl of cDNA. Samples were analyzed in an ABI 7300 real-time PCR system (Applied Biosystems) with an initial step of 3 minutes at 95°C for polymerase activation, followed by 40 cycles at 95°C for 5 seconds for denaturation, and 60°C for 15 seconds for annealing/extension.333. Results were analyzed against human GAPDH as a housekeeping gene and expressed using the ΔΔCT method (Pfaffl, 2001). The sequences of primers can be found in Table 1. The results were expressed as the mean ± SD. The significance of differences was evaluated using ANOVA followed by Dunnett’s post-hoc test as indicated.
Table 1.
Sequences of primers used in RT-qPCR
| Gene | Forward primer | Reverse primer |
|---|---|---|
| TLR2 | TCGGAGTTCTCCCAGTGTTTG | GCAGTGAAAGAGCAATGGGC |
| TLR4 | GGTCAGACGGTGATAGCGAG | TTTAGGGCCAAGTCTCCACG |
| TLR7 | TCCATGCCATCAAGAAAGTTGA | GTCTGTGCAGTCCACGATCA |
| TLR9 | CAGCATGGGTTTCTGCCG | GGGCAGTTCCACTTGAGGTT |
| NOD1 | CCTGGTGGCCAAGTGATTGTA | CCAAGCCTGCGATTCCCATA |
| NOD2 | ATCCGGAGCCTGTACGAGAT | CGCGCAAATACAGAGCCTTG |
| IL-1β | CTTCGAGGCACAAGGCACAA | CTGGAAGGAGCACTTCATCTGT |
| IL6 | ACCCCCAATAAATATAGGACTGGA | CGAAGGCGCTTGTGGAGAA |
| IL-12α | GCTCCAGAAGGCCAGACAAA | GCCAGAGCCTAAGACCTCAC |
| IFN-ɣ | TGGAAAGAGGAGAGTGACAGA | CTGTTTTAGCTGCTGGCGAC |
| IL-10 | CGAGATGCCTTCAGCAGAGT | GGCAACCCAGGTAACCCTTA |
| TGFβ | TACCTGAACCCGTGTTGCTC | CCGGTAGTGAACCCGTTGAT |
| IL-17 | TGGAATCTCCACCGCAATGA | GCTGGATGGGGACAGAGTTC |
| GAPDH | AACAGCGACACCCACTCCTC | GGAGGGGAGATTCAGTGTGGT |
Results
Trypanosoma cruzi changes the gene expression profile in HPE
The effect of the parasite on placental tissue was assayed in HPE after challenges with a low (105 parasites/ml) or a high (106 parasites/ml) concentration of trypomastigotes for 2 or 24 h.
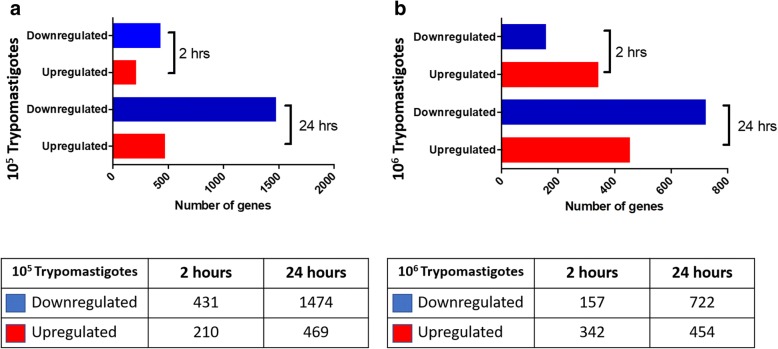
Total RNA extracted from infected and non-infected control HPE, was labeled and hybridized to a Human GE 60K Microarray, which allows the evaluation of the gene expression profile of 26,083 different human genes. Genes showing at least a 2-fold change in their expression and a 95% probability of being differentially expressed (P ≤ 0.05) were significantly regulated during parasite challenge. Figure 1 shows the total number of significant differentially expressed genes between infected and non-infected control HPE. A low parasite concentration induces the downregulation of 431 and 1474 genes as well as the upregulation of 210 and 469 genes after 2 and 24 h of parasite challenge, respectively (Fig. 1a). After a high parasite concentration challenge, 157 and 722 genes were downregulated, and 342 and 454 were upregulated after 2 and 24 h, respectively (Fig. 1b). Major changes occurred after 24 h of parasite challenge with the lowest parasite concentration. A selection of the most upregulated and downregulated genes (fold change range between 34.70 and 71.43) is shown in Table 2. Among the most upregulated genes are those involved in immune response such as CXCL9, TLR-7, TLR-8, CD46, C1qTNF3, HLA-DQB1 and CCL20; genes involved in extracellular matrix (ECM) remodeling (ADAM12, ADAMTSL3, MMP10) and related to pregnancy. The list of most downregulated genes also includes genes related to immunity such as LBP, CD14, DCD and IL-6.
Fig. 1.
Differential gene expression in Trypanosoma cruzi infected HPCVE at 2 and 24 h compared to not-infected control samples. HPCVE were challenged with 105 (a) or 106 (b) trypomastigotes/ml. Blue bars indicate downregulated genes and red bars indicate upregulated genes. Inset table shows the number of differentially expressed genes for each condition. (≥ 2-fold, P ≤ 0.05)
Table 2.
Upregulated and downregulated genes with fold change (FC) ≥ 20 at 2 and 24 h post-infection
| Gene ID | Description | FC | Gene ID | Description | FC |
|---|---|---|---|---|---|
| 2 h | |||||
| Upregulated 105 trypomastigotes | Downregulated 105 trypomastigotes | ||||
| LDOC1 | Leucine zipper, downregulated in cancer 1 | 71.43 | SLC9B1 | Solute carrier family 9, subfamily B (NHA1, cation proton antiporter 1), member 1 | 65.64 |
| ADAM12 | ADAM metallopeptidase domain 12 | 54.99 | LBP | Lipopolysaccharide binding protein | 60.23 |
| PNMT | Phenylethanolamine N-methyltransferase | 51.74 | MEDAG | Mesenteric estrogen-dependent adipogenesis | 52.12 |
| ADORA3 | Adenosine A3 receptor | 48.44 | SYT1 | Synaptotagmin I | 49.30 |
| GH2 | Growth hormone 2 | 44.96 | RGS7BP | Regulator of G-protein signaling 7 binding protein | 49.08 |
| PCDHB13 | Protocadherin beta 13 | 44.71 | MT1H | Metallothionein 1H | 47.18 |
| CXCL9 | Chemokine (C-X-C motif) ligand 9 | 26.61 | CAPN8 | Calpain 8 | 46.86 |
| TLR7 | Toll-like receptor 7 | 23.52 | DCD | Dermcidin | 42.36 |
| TLR8 | Toll-like receptor 8 | 21.74 | HTR5A | 5-hydroxytryptamine (serotonin) receptor 5A, G protein-coupled | 42.25 |
| CD46 | CD46 molecule, complement regulatory protein | 21.48 | KALRN | Kalirin, RhoGEF kinase | 42.01 |
| C1QTNF3 | C1q and tumor necrosis factor related protein 3 | 21.41 | CDH18 | Cadherin 18, type 2 | 35.52 |
| Upregulated 106 trypomastigotes | Downregulated 106 trypomastigotes | ||||
| LOC101060810 | Zinc finger protein 98-like | 68.80 | SNRPG | Small nuclear ribonucleoprotein polypeptide G | 73.40 |
| LMOD1 | Leiomodin 1 (smooth muscle) | 62.19 | TUBA1C | Tubulin, alpha 1c | 71.97 |
| SPIN4 | Spindlin family, member 4 | 53.45 | TSC22D1 | TSC22 domain family, member 1 | 71.15 |
| LINC00551 | Long intergenic non-protein coding RNA 551 | 52.98 | CD14 | CD14 molecule | 70.98 |
| PSG3 | Pregnancy specific beta-1-glycoprotein 3 | 47.68 | MBNL2 | Muscleblind-like splicing regulator 2 | 64.74 |
| PSG8 | Pregnancy specific beta-1-glycoprotein 8 | 45.17 | FUT3 | Fucosyltransferase 3 (galactoside 3(4)-L-fucosyltransferase. Lewis blood group) | 63.73 |
| PSPHP1 | Phosphoserine phosphatase pseudogene 1 | 44.87 | GCGR | Glucagon receptor | 62.52 |
| PSG1 | Pregnancy specific beta-1-glycoprotein 1 | 42.74 | IL6 | Interleukin 6 (interferon, beta 2) | 62.25 |
| PCDHB13 | Protocadherin beta 13 | 41.77 | NOG | Noggin | 60.99 |
| HULC | Hepatocellular carcinoma upregulated long non-coding RNA | 40.94 | SBSN | Suprabasin | 60.01 |
| ADAMTSL3 | ADAMTS-like 3 | 34.70 | FAM89A | Family with sequence similarity 89, member A | 57.57 |
| 24 h | |||||
| Upregulated 105 trypomatigotes | Downregulated 105 trypomastigotes | ||||
| GCGR | Glucagon receptor | 26.78 | GEMIN2 | Gem (nuclear organelle) associated protein 2 | 66.26 |
| CHRDL2 | Chordin-like 2 | 26.46 | GUCA1A | Guanylate cyclase activator 1A (retina) | 50.37 |
| TAC3 | Tachykinin 3 | 23.82 | LOC340515 | Uncharacterized LOC340515 | 47.71 |
| STC1 | Stanniocalcin 1 | 21.80 | LINC00200 | Long intergenic non-protein coding RNA 200 | 42.88 |
| SLC43A3 | Solute carrier family 43, member 3 | 21.60 | FAM182B | Family with sequence similarity 182, member B | 41.35 |
| PENK | Proenkephalin | 21.06 | SEC14L4 | SEC14-like 4 (S. cerevisiae) | 38.81 |
| SLC44A4 | Solute carrier family 44, member 4 | 21.02 | CLEC6A | C-type lectin domain family 6, member A | 38.42 |
| HLA-DQB1 | Major histocompatibility complex, class II, DQ beta 1 | 20.99 | SNORA65 | Small nucleolar RNA, H/ACA box 65 | 36.94 |
| ANTXR1 | Anthrax toxin receptor 1 | 20.21 | LOC541473 | FK506 binding protein 6, 36kDa pseudogene | 36.81 |
| SBSN | Suprabasin | 20.18 | AKR7L | Aldo-keto reductase family 7-like | 36.51 |
| Upregulated 106 trypomastigotes | Downregulated 106 trypomastigotes | ||||
| MMP10 | Matrix metallopeptidase 10 (stromelysin 2) | 105.67 | DNMT3L | DNA (cytosine-5-)-methyltransferase 3-like | 63.39 |
| CSH2 | Chorionic somatomammotropin hormone 2 | 68.94 | KLRG2 | Killer cell lectin-like receptor subfamily G, member 2 | 46.45 |
| GH2 | Growth hormone 2 | 68.66 | MS4A6A | Membrane-spanning 4-domains. subfamily A, member 6A | 40.49 |
| CCL20 | Chemokine (C-C motif) ligand 20 | 58.87 | TMEM45B | Transmembrane protein 45B | 40.27 |
| ENO2 | Enolase 2 (gamma, neuronal) | 53.70 | IGFBP1 | Insulin-like growth factor binding protein 1 | 39.99 |
| SOD2 | Superoxide dismutase 2, mitochondrial | 53.42 | PSPHP1 | Phosphoserine phosphatase pseudogene 1 | 39.83 |
| PCDHB13 | Protocadherin beta 13 | 53.33 | TPTE2P3 | Transmembrane phosphoinositide 3-phosphatase and Tensin homolog 2 pseudogene 3 | 39.87 |
| SELE | Selectin E | 52.93 | MNDA | myeloid cell nuclear differentiation antigen | 38.99 |
| ABO | ABO blood group (transferase A, alpha 1-3-N-acetylgalactosaminyltransferase; transferase B, alpha 1-3-galactosyltransferase) | 51.78 | FGF14-AS2 | FGF14 antisense RNA 2 | 38.98 |
| STC1 | Stanniocalcin 1 | 51.40 | PTX3 | Pentraxin 3, long | 38.59 |
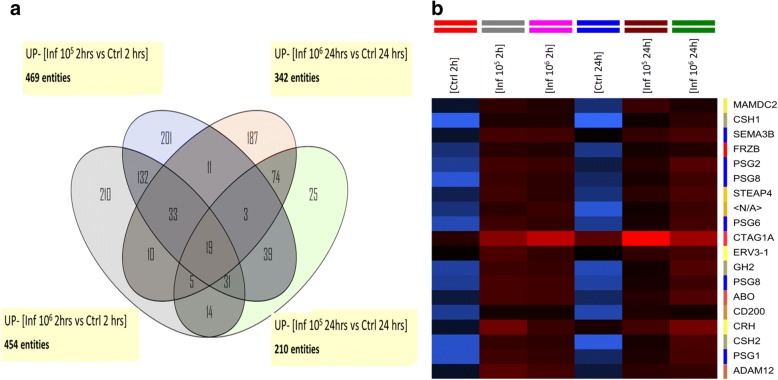
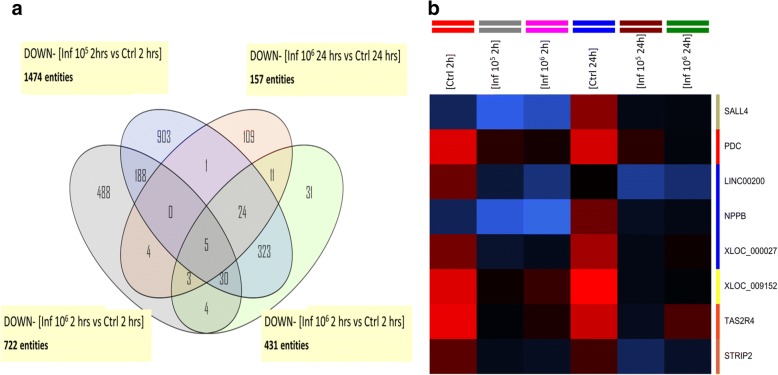
The Venn diagrams in Fig. 2a show that 19 genes are upregulated in the four different experimental conditions compared to control non-infected samples, which are also shown in the corresponding heatmap (Fig. 2b) listed in Table 3. Contrarily, only 5 genes are downregulated in the same conditions (Fig. 3a, b), which are listed in Table 3. Most of the upregulated genes are related to pregnancy processes.
Fig. 2.
Venn diagrams comparing common differentially upregulated genes. HPCVE were incubated for 2 and 24 h with 105 or 106 T. cruzi trypomastigotes/ml. All samples were compared to the respective uninfected control. The diagram in a shows the upregulated genes at both parasite concentrations and incubation times, b corresponds to the heatmap of the differentially expressed genes in the central intersection
Table 3.
Upregulated and downregulated genes in the four different experimental conditions compared to control non-infected samples
| Gene symbol | Description |
|---|---|
| Upregulated genes | |
| MAMDC2 | Homo sapiens MAM domain containing 2 (MAMDC2), mRNA [NM_153267] |
| CSH1 | Homo sapiens chorionic somatomammotropin hormone 1 (placental lactogen) (CSH1), mRNA [NM_001317] |
| SEMA3B | Homo sapiens sema domain, immunoglobulin domain (Ig), short basic domain, secreted, (semaphorin) 3B (SEMA3B), transcript variant 1, mRNA [NM_004636] |
| FRZB | Homo sapiens frizzled-related protein (FRZB), mRNA [NM_001463] |
| PSG2 | Homo sapiens pregnancy specific beta-1-glycoprotein 2 (PSG2), mRNA [NM_031246] |
| PSG8 | Homo sapiens pregnancy specific beta-1-glycoprotein 8 (PSG8), transcript variant 1, mRNA [NM_182707] |
| STEAP4 | Homo sapiens STEAP family member 4 (STEAP4), transcript variant 2, mRNA [NM_001205315] |
| Uncharacterized protein [Source: UniProtKB/TrEMBL; Acc: B8ZZY5] [ENST00000409490] | |
| PSG6 | Homo sapiens pregnancy specific beta-1-glycoprotein 6 (PSG6), transcript variant 1, mRNA [NM_002782] |
| CTAG1A | Homo sapiens cancer/testis antigen 1A (CTAG1A), mRNA [NM_139250] |
| ERV3-1 | Homo sapiens endogenous retrovirus group 3, member 1 (ERV3-1), mRNA [NM_001007253] |
| GH2 | Homo sapiens growth hormone 2 (GH2), transcript variant 3, mRNA [NM_022558] |
| PSG8 | Homo sapiens pregnancy specific beta-1-glycoprotein 8 (PSG8), transcript variant 1, mRNA [NM_182707] |
| ABO | ABO blood group (transferase A, alpha 1-3-N-acetylgalactosaminyltransferase; transferase B, alpha 1-3-galactosyltransferase) [Source: HGNC Symbol; Acc:79] [ENST00000319878] |
| CD200 | Homo sapiens CD200 molecule (CD200), transcript variant 2, mRNA [NM_001004196] |
| CRH | Homo sapiens corticotropin releasing hormone (CRH), mRNA [NM_000756] |
| CSH2 | Homo sapiens chorionic somatomammotropin hormone 2 (CSH2), transcript variant 2, mRNA [NM_022644] |
| PSG1 | Homo sapiens pregnancy specific beta-1-glycoprotein 1 (PSG1), transcript variant 1, mRNA [NM_006905] |
| ADAM12 | Homo sapiens ADAM metallopeptidase domain 12 (ADAM12), transcript variant 2, mRNA [NM_021641] |
| Downregulated genes | |
| SALL4 | Homo sapiens spalt-like transcription factor 4 (SALL4), mRNA [NM_020436] |
| LINC00200 | Homo sapiens long intergenic non-protein coding RNA 200 (LINC00200), long non-coding RNA [NR_015376] |
| NPPB | Homo sapiens natriuretic peptide B (NPPB), mRNA [NM_002521] |
| TAS2R4 | Homo sapiens taste receptor, type 2, member 4 (TAS2R4), mRNA [NM_016944] |
| STRIP2 | Homo sapiens striatin interacting protein 2 (STRIP2), transcript variant 1, mRNA [NM_020704] |
| FRZB | Homo sapiens frizzled-related protein (FRZB), mRNA [NM_001463] |
Fig. 3.
Venn diagrams comparing common differentially downregulated genes. HPCVE were incubated for 2 and 24 h with 105 or 106 T. cruzi trypomastigotes/ml. All samples were compared to the respective uninfected control. The diagram in a shows the downregulated genes at both parasite concentrations and incubation times, b corresponds to the heatmap of the differentially expressed genes in the central intersection
Trypanosoma cruzi alters a wide range of biological processes in HPE
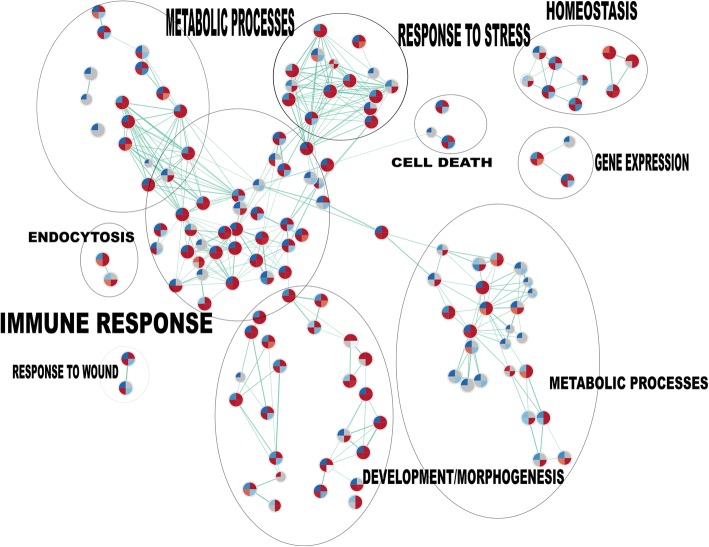
GO and pathway analysis were performed using GeneSpringGX 13.0 software (Agilent Technologies), comparing the different experimental conditions described above. Our results indicate that a wide range of biological processes are altered at the different conditions in presence of the parasite (Table 4). The different biological processes detected include immune response, pregnancy related processes and signaling. In order to understand the relationships between those biological processes, we performed a GSEA analysis of gene sets at different times and parasite load challenges. We analyzed biological processes pathways based on gene ontology results (Fig. 4). The biggest cluster is composed of pathways related with immune response, followed by development morphogenesis cluster, regulation of metabolic processes and signal transduction genes, metabolic processes, homeostasis, response to stimulus, cell death and endocytosis (Fig. 4).
Table 4.
Biological processes predicted to be modulated during T. cruzi infection
| GO Accession | GO term | No. of genes/condition | |||
|---|---|---|---|---|---|
| 105 2 h | 106 2 h | 105 24 h | 106 24 h | ||
| Upregulated biological processes | |||||
| GO:0022414 | Reproductive process | 45 | 42 | 45 | 114 |
| GO:0032501|GO:0050874 | Multicellular organismal process | 191 | 189 | 326 | 550 |
| GO:0050896|GO:0051869 | Response to stimulus | 208 | 196 | 728 | 623 |
| GO:0051704|GO:0051706 | Multi-organism process | 56 | 58 | 204 | 197 |
| GO:0065007 | Biological regulation | 269 | 255 | 977 | 836 |
| GO:0002376 | Immune system process | 39 | 36 | 236 | 215 |
| GO:0009987|GO:0008151|GO:0050875 | Cellular process | 346 | 334 | 1161 | 987 |
| GO:0022610 | Biological adhesion | 35 | 23 | 134 | 99 |
| GO:0023052|GO:0023046 | Signaling | 141 | 132 | 543 | 464 |
| GO:0044699 | Single-organism process | 342 | 0 | 1125 | 972 |
| GO:0051179 | Localization | 125 | 0 | 415 | 372 |
| GO:0071840|GO:0071841 | Cellular component organization or biogenesis | 105 | 0 | 427 | 307 |
| GO:0008152 | Metabolic process | 238 | 0 | 0 | 629 |
| GO:0032502 | Developmental process | 144 | 0 | 0 | 434 |
| Downregulated biological processes | |||||
| GO:0009987|GO:0008151|GO:0050875 | Cellular processes | 902 | 681 | 937 | 648 |
| GO:0023052|GO:0023046 | Signaling | 388 | 0 | 376 | 0 |
| GO:0032501|GO:0050874 | Multicellular organismal process | 516 | 0 | 490 | 0 |
| GO:0044699 | Single-organism processes | 903 | 620 | 880 | 615 |
| GO:0050896|GO:0051869 | Response to stimulus | 547 | 371 | 507 | 328 |
| GO:0065007 | Biological regulation | 697 | 505 | 734 | 487 |
| GO:0071840|GO:0071841 | Cellular component organization or biogenesis | 0 | 240 | 0 | 248 |
| GO:0022414 | Reproductive processes | 0 | 0 | 106 | 0 |
| GO:0051179 | Localization | 0 | 0 | 275 | 0 |
| GO:0051704|GO:0051706 | Multi-organism processes | 0 | 0 | 123 | 0 |
Fig. 4.
Enrichment map of gene sets of biological processes pathways. HPCVE were incubated for 2 and 24 h with 105 or 106 T. cruzi trypomastigotes/ml. Genes with fold changes ≥ 2 (P ≤ 0.05) were considered for the analysis. Gene set analysis (GSEA) was performed based in pathways from GO biological processes. Nodes correspond to gene sets and connecting lines to overlapping member genes between nodes. Divided circles represent predicted pathways, each segment of the circle represents a different experimental group according to attached legend and colors depict upregulated (red) or downregulated (blue) genes. Clusters grouped by biological function were manually labeled
Several gene sets grouped within the main cluster (immune response), are enriched at 2 or 24 h post-infection after parasite challenges of 105 trypomastigotes/ml but not of 106 parasites. Thus, immune system process pathways are positively regulated against 105 parasites at 2 h (NES: 1.57; FDR q-value = 0.0013) and 24 h (NES: 2.75; FDR q-value = 0.0018), downregulated with 106 trypomastigotes/ml at 2 h (NES: -2.1; FDR q-value = 0.0087) but not at 24 h (NES: 1.44; FDR q-value = 0.18). Regulation of immune response is positively regulated with 105 parasites at 2 h (NES: 1,50; FDR q-value = 0.022) and 24 h (NES: 1.98; FDR q-value = 0.01) but negatively with 106 parasites at 2 h (NES: -1,77; FDR q-value = 0.067) and without significant changes at 24 h (NES: -0,83; FDR q-value not significant).
Gene Ontology process regulation of cytokine production is upregulated 2 h post-infection (NES: 1.36; FDR q-value = 0.023) but not in the other experimental groups. The inflammatory response pathway is upregulated 24 h post-infection against 105 trypomastigotes (NES: 1.65; FDR q-value = 0.049) but downregulated with 106 parasites 2 h post-infection (NES: 1.99; FDR q-value = 0.023). Amongst the changes of pathways related to other processes, it can be highlighted the upregulation of cell proliferation pathway 24 h post-infection (NES: 2.18; FDR q-value = 0.003) and cellular response to hormone stimulus (2 h 105 Trypos NES: 2.53; FDR q-value = 0.005).
To understand the nature of the interaction between upregulated or downregulated genes, we performed a gene interaction analysis using the GeneMANIA plug-in for Cytoscape 3.0 software [24, 25]. For each experimental condition, we analyzed co-expression, co-localization, physical interactions, genetic interactions shared protein domains and pathways amongst all the differentially expressed genes (Fc ≥ 2) in both upregulated or downregulated gene lists. The relative weight of each process between all interacting genes is depicted in Table 5. In all experimental conditions, co-expression (a category where two genes have similar expression levels) is the predominating interaction, co-localization (genes expressed in the same tissue) the second in the upregulated conditions; however, in downregulated groups physical interactions (when two gene products are found to interact in protein-protein interaction studies) is the second most common interaction. Shared protein domains, genetic and pathways interaction represent marginal interactions in all groups. A circular layout of the interaction network for each is shown in Additional file 1: Figure S1.
Table 5.
Relative weight of gene interactions in T. cruzi-infected HPE
| Network group | 2 h/105 Up | 2 h/105 Down | 2 h/106 Up | 2 h/106 Down | 24 h/105 Up | 24 h/105 Down | 24 h/106 Up | 24 h/106 Down |
|---|---|---|---|---|---|---|---|---|
| Co-expression | 72 | 61 | 64 | 42 | 75 | 66 | 75 | 75 |
| Co-localization | 13 | 7 | 18 | 9 | 11 | 6 | 14 | 3 |
| Physical interactions | 7 | 14 | 11 | 31 | 6 | 17 | 6 | 9 |
| Genetic interactions | 4 | 0 | 2 | 4 | 3 | 0 | 0 | 1 |
| Shared protein domains | 0 | 1 | 1 | 3 | 0 | 1 | 1 | 0 |
| Pathway | 0 | 1 | 1 | 3 | 0 | 0 | 1 | 7 |
| Others | 4 | 16 | 3 | 8 | 5 | 10 | 3 | 5 |
Abbreviations: Up, upregulated; Down, downregulated; H, hours of HPE incubation with the parasite; 105, challenge with 105 parasites/ml; 106, challenge with 106 parasites/ml
Trypanosoma cruzi induces differential expression of pathogen pattern recognition receptors in HPE
Based on the results of the microarray analysis showing an activation of local immune processes, we decided to validate by RT-qPCR PRR activation and cytokine production. Explants were co-incubated with 105 T. cruzi trypomastigotes for 2 h and the expression of Toll-like receptors (TLRs) and NOD-like receptors (NLRs) was assayed. Trypanosoma cruzi trypomastigotes induce statistically significant increases of TLR-2 (96.13 ± 61.6%; F(2, 9) = 5.409; P ≤ 0.01, Fig. 5a), TLR-4 (47.56 ± 27.99%; F(2, 45) = 2.173; P ≤ 0.0001, Fig. 5b), TLR-7 (57.29 ± 24.59%; F(2, 2) = 6.048; P ≤ 0.05, Fig. 5c) and TLR-9 (61.56 ± 5.11%; F(2, 6) = 1.630; P ≤ 0.01, Fig. 5d) expression compared to non-infected HPE. However, T. cruzi does not increase significantly NOD-1 and NOD-2 receptors (Fig. 5d, e).
Fig. 5.
Trypanosoma cruzi induce differential expression of pathogen pattern recognition receptors in HPE. Explants were co-incubated with 105 T. cruzi trypomastigotes/ml for 2 h and the expression of TLR-2 (a), TLR-4 (b), TLR-7 (c), TLR-9 (d), NOD-1 (e) and NOD-2 (f) were assayed. Samples were processed for RT-qPCR. The sequences of primers can be found in Table 1. The significance of differences was evaluated using Student’s t-tests for paired data. *P ≤ 0.05, **P ≤ 0.01, ****P ≤ 0.0001
Trypanosoma cruzi increases pro-inflammatory and immune-modulating cytokines in HPE
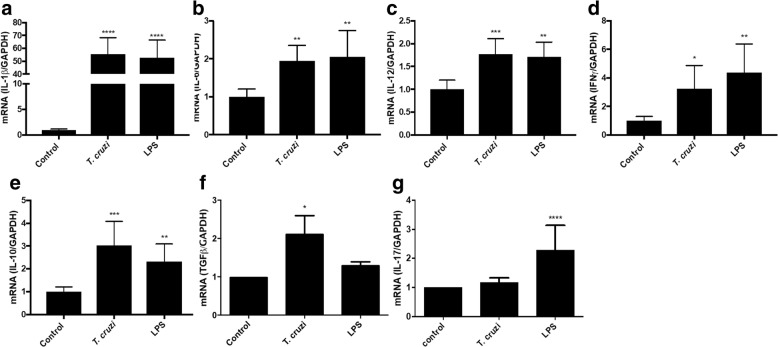
HPE were co-incubated with 105 T. cruzi trypomastigotes for 2 h as well as in the presence and absence of LPS as positive controls. T. cruzi induces significant increases of the pro-inflammatory cytokines IL-1β (5542.55 ± 1090.11%; F(2, 19) = 64.91; P ≤ 0.0001, Fig. 6a), IL-6 (94.70 ± 40.38%; F(2, 15) = 8.849; P ≤ 0.01, Fig. 6b), IL-12α (77.08 ± 33.01%; F(2, 19) = 64.91; F(2, 15) = 16.13; P ≤ 0.01, Fig. 6c), IFNγ (329.29 ± 162.22%; F(2, 17) = 7.729; P ≤ 0.05, Fig. 6d) and of the immune-modulating cytokines IL-10 (303.34 ± 104.28%; F(2, 20) = 14.87; P ≤ 0.001, Fig. 6e) and TGF β (329.29 ± 162.22%; F(2, 11) = 1.684; P ≤ 0.05, Fig. 6f) but not of IL-17 (Fig. 6g).
Fig. 6.
Trypanosoma cruzi increases pro-inflammatory and immune-modulating cytokines in HPE. HPE were co-incubated with 105 T. cruzi trypomastigotes/ml for 2 h as well as in the presence and absence of LPS as positive control. Expression of IL-1β (a), IL-6 (b), IL-12α (c), IFNγ (d), IL-10 (e), TGF β (f) and IL-17 (g) was assayed. Samples were processed for RT-qPCR. The sequences of primers can be found in Table 1. The significance of differences was evaluated using Student’s t-tests for paired data. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001
Discussion
The interaction between the host and pathogens, including T. cruzi, is the most important factor in determining whether an infection is successful. Host-parasite interaction includes invasion of the host through primary barriers (such as the placental barrier), evasion of host defenses, pathogen replication in the host, and immunological capacity of the host to control or eliminate the pathogen [26]. Importantly, infected organisms are capable of sensing the intrusion by pathogens and react by triggering host defenses [17, 26]. On the other hand, the parasite is equipped with multiple tools to establish a long-term relationship with the infected host. Tissue infection in particular is relevant during disease progression. The presence of the parasite provokes tissue damage as well as immune and reparatory responses, which can lead to fibrosis and tissue dysfunction as observed in chagasic cardiomyopathy [27]. Considering the temporary existence of the placenta, the effect on parasite infection on this particular tissue is relevant to understand the physiopathology of congenital transmission in order to obtain tools for diagnosis, prognosis and treatment of the disease.
Previous studies about transcriptomics related to T. cruzi and Chagas disease have been focused on a single type cell response [10, 28] or on tissues or organs in animal models [24, 29] but not on human tissues. Here, we describe for the first time, the transcriptomics of an ex vivo human placental tissue model in response to challenges with the parasite.
As expected, T. cruzi modifies an ample range of biological processes during tissue invasion and infection. As described before, the parasite dramatically changes the gene expression in single cells [10]. However, in tissue and organ samples a more complex change in gene expression can be expected since they are composed of different cell types or tissues. For instance, in HPE epithelial cells derived from the trophoblast and fetal capillaries as well as fibroblasts and macrophages in the fetal connective tissue can be found, between others [3]. In addition, ECM components, that are synthetized by the resident cells are also present in tissue and organ samples [18, 19]. Similar results have been obtained in animal models, where important changes in murine myocardium metabolic pathways [29] and in murine placental response [24] are described. The increase of gene expression of proteases involved in ECM-remodeling (Table 2) is in concordance with our previous results showing that the parasite increased expression and activity of matrix metalloproteases (MMP-2 and MMP-9) in human placenta [19]. The profound changes in genes involved in signaling agrees with numerous previous studies that showed that T. cruzi activates or inhibits several signal transduction pathways [14, 28, 30].
The effect of T. cruzi on the immune system responses are particularly relevant since they are our main defense against the pathogen. The parasite dramatically changes host genes involved in the immune response. The expression of an important number of genes of innate immunity is increased. Thus, genes related to complement regulation and function such as CD46 and C1q are upregulated. We have previously shown, that during ex vivo infection of HPE, T. cruzi calreticulin (TcCRT) acts as a virulence factor since it binds maternal classical complement component C1q and increases parasite infectivity [31]. On the other hand, TLRs are also increased, particularly TLR-7 and TLR-8 which are increased over 20-fold. The validation experiments show that both mentioned TLRs as well as TLR-2, TLR-4 and TLR-9 are significantly increased (Fig. 5). However, TLR-2, the TLR whose inhibition increases parasite infection in HPE as well as parasite-induced tissue damage [17] showed no significant increase in the microarray analysis (Additional file 1: Figure S1).
A similar contradictory result was obtained with IL-6; a high parasite concentration decreases the expression of this cytokine more than 60-fold (Table 2). However, a low parasite concentration does not change IL-6 expression (Additional file 1: Figure S1). However, our RT-qPCR data show a significant increase of IL-6 (Fig. 5) that is in concordance with the increase of IL-6 protein in the culture media of HPE after parasite challenge in the same condition [17], suggesting regulation at post-transcriptional levels.
Another important group of genes that change their expression are those related to pregnancy. Most of the 19 upregulated genes in the four different experimental conditions compared to control samples are related to pregnancy processes (Table 3) and to the maintenance and development of the fetus such as pregnancy specific beta-1-glycoproteins, GH2 (growth hormone 2), CSH1 and CSH2 (chorionic somatomammotropin hormone 1 and 2). Given that the placenta is the sole interface between mother and fetus and that this organ not only protects the fetus from infection but also regulates important metabolic and other physiological processes [32], it appears easily explainable that different pregnancy related processes are affected.
Conclusions
Trypanosoma cruzi induces strong changes in genes involved in a wide range of pathways, especially those involved in immune response against infections.
Additional file
Figure S1. Interaction networks in differentially expressed genes from each experimental group. HPCVE were incubated during 2 and 24 h with 105 or 106 T. cruzi trypomastigotes. Interaction networks from differentially expressed genes (FC ≥ 2) compared with uninfected control with GeneMANIA function prediction service plug-in in Cytoscape software. Co-expression, co-localization, physical interactions, genetic interactions shared protein domains and pathways are shown and each color represents specific interactions according to legend. (PDF 707 kb)
Acknowledgements
Not applicable.
Funding
This work was supported by ERANET-LAC grant ELAC2014/HID-0328 (to UK and AGS), REDES130118 (to UK and CR), UREDES URC-024/16 (to UK) and FONDECYT 3180452 (to ChC).
Availability of data and materials
The datasets generated during and analyzed during the current study are available in the GEO repository under the accession number GSE113155.
Authors’ contributions
UK, CR and ChC conceived and designed the experiments. ChC, GL, IC and NJ performed the experiments. ChC, GL, IC, CR, AS and UK analyzed the data. ChC, AJ and UK wrote the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was conducted in accordance with Ethic Committee of the Faculty of Medicine, University of Chile (No. 041-2011). Each patient signed an informed consent for experimental use.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Christian Castillo, Email: christiancastillor@gmail.com.
Ileana Carrillo, Email: icarrillow@gmail.com.
Gabriela Libisch, Email: glibisch@pasteur.edu.uy.
Natalia Juiz, Email: nataliaajuiz@gmail.com.
Alejandro Schijman, Email: aleschijman@gmail.com.
Carlos Robello, Email: robello@pasteur.edu.uy.
Ulrike Kemmerling, Email: ukemmerling@uchile.cl.
References
- 1.Moscatelli G, Moroni S, Garcia-Bournissen F, Ballering G, Bisio M, Freilij H, et al. Prevention of congenital Chagas through treatment of girls and women of childbearing age. Mem Inst Oswaldo Cruz. 2015;110:507–509. doi: 10.1590/0074-02760140347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schmunis G. Epidemiology of Chagas disease in non-endemic countries: the role of international migration. Mem Inst Oswaldo Cruz. 2007;102:75–85. doi: 10.1590/S0074-02762007005000093. [DOI] [PubMed] [Google Scholar]
- 3.Liempi A, Castillo C, Carrillo I, Munoz L, Droguett D, Galanti N, et al. A local innate immune response against Trypanosoma cruzi in the human placenta: the epithelial turnover of the trophoblast. Microb Pathog. 2016;99:123–129. doi: 10.1016/j.micpath.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 4.Perez-Molina JA, Perez AM, Norman FF, Monge-Maillo B, Lopez-Velez R. Old and new challenges in Chagas disease. Lancet Infect Dis. 2015;15:1347–1356. doi: 10.1016/S1473-3099(15)00243-1. [DOI] [PubMed] [Google Scholar]
- 5.Castillo C, Munoz L, Carrillo I, Liempi A, Medina L, Galanti N, et al. Toll-like receptor-2 mediates local innate immune response against Trypanosoma cruzi in ex vivo infected human placental chorionic villi explants. Placenta. 2017;60:40–46. doi: 10.1016/j.placenta.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 6.Kaplinski M, Jois M, Galdos-Cardenas G, Rendell VR, Shah V, Do RQ, et al. Sustained domestic vector exposure is associated with increased Chagas cardiomyopathy risk but decreased parasitemia and congenital transmission risk among young women in Bolivia. Clin Infect Dis. 2015;61:918–926. doi: 10.1093/cid/civ446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrias ES, de Carvalho TM, De Souza W. Trypanosoma cruzi: entry into mammalian host cells and parasitophorous vacuole formation. Front Immunol. 2013;4:186. doi: 10.3389/fimmu.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker DM, Oghumu S, Gupta G, McGwire BS, Drew ME, Satoskar AR. Mechanisms of cellular invasion by intracellular parasites. Cell Mol Life Sci. 2014;71:1245–1263. doi: 10.1007/s00018-013-1491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shigihara T, Hashimoto M, Shindo N, Aoki T. Transcriptome profile of Trypanosoma cruzi-infected cells: simultaneous up- and down-regulation of proliferation inhibitors and promoters. Parasitol Res. 2008;102:715–722. doi: 10.1007/s00436-007-0819-x. [DOI] [PubMed] [Google Scholar]
- 10.Chiribao ML, Libisch G, Parodi-Talice A, Robello C. Early Trypanosoma cruzi infection reprograms human epithelial cells. Biomed Res Int. 2014;2014:439501. doi: 10.1155/2014/439501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carlier Y, Truyens C, Deloron P, Peyron F. Congenital parasitic infections: a review. Acta Trop. 2012;121:55–70. doi: 10.1016/j.actatropica.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Arora N, Sadovsky Y, Dermody TS, Coyne CB. Microbial vertical transmission during human pregnancy. Cell Host Microbe. 2017;21:561–567. doi: 10.1016/j.chom.2017.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rendell VR, Gilman RH, Valencia E, Galdos-Cardenas G, Verastegui M, Sanchez L, et al. Trypanosoma cruzi-infected pregnant women without vector exposure have higher parasitemia levels: implications for congenital transmission risk. PLoS One. 2015;10:e0119527. doi: 10.1371/journal.pone.0119527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castillo C, Villarroel A, Duaso J, Galanti N, Cabrera G, Maya JD, et al. Phospholipase C gamma and ERK1/2 mitogen activated kinase pathways are differentially modulated by Trypanosoma cruzi during tissue invasion in human placenta. Exp Parasitol. 2013;133:12–17. doi: 10.1016/j.exppara.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Liempi A, Castillo C, Duaso J, Droguett D, Sandoval A, Barahona K, et al. Trypanosoma cruzi induces trophoblast differentiation: a potential local antiparasitic mechanism of the human placenta? Placenta. 2014;35:1035–1042. doi: 10.1016/j.placenta.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Fretes RE, Kemmerling U. Mechanism of Trypanosoma cruzi placenta invasion and infection: the use of human chorionic villi explants. J Trop Med. 2012;2012:614820. doi: 10.1155/2012/614820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Castillo C, Munoz L, Carrillo I, Liempi A, Gallardo C, Galanti N, et al. Ex vivo infection of human placental chorionic villi explants with Trypanosoma cruzi and Toxoplasma gondii induces different Toll-like receptor expression and cytokine/chemokine profiles. Am J Reprod Immunol. 2017. 10.1111/aji.12660. [DOI] [PubMed]
- 18.Duaso J, Rojo G, Cabrera G, Galanti N, Bosco C, Maya JD, et al. Trypanosoma cruzi induces tissue disorganization and destruction of chorionic villi in an ex vivo infection model of human placenta. Placenta. 2010;31:705–711. doi: 10.1016/j.placenta.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 19.Castillo C, Lopez-Munoz R, Duaso J, Galanti N, Jaña F, Ferreira J, et al. Role of matrix metalloproteinases 2 and 9 in ex vivo Trypanosoma cruzi infection of human placental chorionic villi. Placenta. 2012;33:991–997. doi: 10.1016/j.placenta.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juiz NA, Torrejon I, Burgos M, Fernanda Torres AM, Duffy T, Cayo NM, et al. Alterations in placental gene expression of pregnant women with chronic Chagas disease. Am J Pathol. 2018;188:1345–1353. doi: 10.1016/j.ajpath.2018.02.011. [DOI] [PubMed] [Google Scholar]
- 23.Merico D, Isserlin R, Stueker O, Emili A, Bader GD. Enrichment map: a network-based method for gene-set enrichment visualization and interpretation. PLoS One. 2010;5:e13984. doi: 10.1371/journal.pone.0013984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Juiz NA, Solana ME, Acevedo GR, Benatar AF, Ramirez JC, da Costa PA, et al. Different genotypes of Trypanosoma cruzi produce distinctive placental environment genetic response in chronic experimental infection. PLoS Negl Trop Dis. 2017;11:e0005436. doi: 10.1371/journal.pntd.0005436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Montojo J, Zuberi K, Rodriguez H, Bader GD, Morris Q. GeneMANIA: Fast gene network construction and function prediction for Cytoscape. F1000Res. 2014;3:153. doi: 10.12688/f1000research.4572.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sen R, Nayak L, De RK. A review on host-pathogen interactions: classification and prediction. Eur J Clin Microbiol Infect Dis. 2016;35:1581–1599. doi: 10.1007/s10096-016-2716-7. [DOI] [PubMed] [Google Scholar]
- 27.Nagajyothi F, Machado FS, Burleigh BA, Jelicks LA, Scherer PE, Mukherjee S, et al. Mechanisms of Trypanosoma cruzi persistence in Chagas disease. Cell Microbiol. 2012;14:634–643. doi: 10.1111/j.1462-5822.2012.01764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y, Shah-Simpson S, Okrah K, Belew AT, Choi J, Caradonna KL, et al. Transcriptome remodeling in Trypanosoma cruzi and human cells during intracellular infection. PLoS Pathog. 2016;12:e1005511. doi: 10.1371/journal.ppat.1005511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Girones N, Carbajosa S, Guerrero NA, Poveda C, Chillon-Marinas C, Fresno M. Global metabolomic profiling of acute myocarditis caused by Trypanosoma cruzi infection. PLoS Negl Trop Dis. 2014;8:e3337. doi: 10.1371/journal.pntd.0003337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maeda FY, Cortez C, Yoshida N. Cell signaling during Trypanosoma cruzi invasion. Front Immunol. 2012;3:361. doi: 10.3389/fimmu.2012.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Castillo C, Ramirez G, Valck C, Aguilar L, Maldonado I, Rosas C, et al. The interaction of classical complement component C1 with parasite and host calreticulin mediates Trypanosoma cruzi infection of human placenta. PLoS Negl Trop Dis. 2013;7:e2376. doi: 10.1371/journal.pntd.0002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayhew TM. Turnover of human villous trophoblast in normal pregnancy: what do we know and what do we need to know? Placenta. 2014;35:229–240. doi: 10.1016/j.placenta.2014.01.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Interaction networks in differentially expressed genes from each experimental group. HPCVE were incubated during 2 and 24 h with 105 or 106 T. cruzi trypomastigotes. Interaction networks from differentially expressed genes (FC ≥ 2) compared with uninfected control with GeneMANIA function prediction service plug-in in Cytoscape software. Co-expression, co-localization, physical interactions, genetic interactions shared protein domains and pathways are shown and each color represents specific interactions according to legend. (PDF 707 kb)
Data Availability Statement
The datasets generated during and analyzed during the current study are available in the GEO repository under the accession number GSE113155.