Abstract
Background
CD81, a member of the tetraspanin family, is overexpressed in several tumor types, but its role in breast cancer remains unknown. The present study aimed to investigate the effects of increased CD81 expression on cell migration and proliferation in breast cancer cell lines, MDA-MB-231 and MDA-MB-435S in vitro, and the effects of increased CD81 expression in breast cancer tissue microarrays on patient prognosis.
Material/Methods
The expression of CD81 was evaluated using immunohistochemistry in a human breast cancer tissue microarray containing 140 tumor tissues and a microarray containing 77 normal breast tissues. The effects of increased CD81 expression on cell proliferation and migration in breast cancer cell lines, MDA-MB-231 and MDA-MB-435S, were evaluated by proliferation, transwell migration, and cell counting kit-8 (CCK-8) assays. CD81-expressing plasmid transfection upregulated CD81 expression, and short hairpin RNA (shRNA) lentivirus silenced CD81 expression in vitro.
Results
CD81 expression was significantly increased in breast cancer tissues compared with normal breast tissues (P<0.05). Increased expression of CD81 was significantly associated with lymph node metastasis (P<0.05), clinical stage (P<0.05) and with reduced overall survival (OS) in patients with breast cancer (P<0.05). Increased CD81 expression in MDA-MB-231 and MDA-MB-435S cells promoted cell proliferation and migration, which were inhibited by CD81 silencing.
Conclusions
The findings of the study showed that CD81 might be a potential prognostic biomarker associated with poor patient prognosis in breast cancer. These findings should be investigated further with large-scale controlled prospective studies in patients with breast cancer.
MeSH Keywords: Antigens, CD81; Breast Neoplasms; Cell Proliferation; Prognosis; Transcellular Cell Migration
Background
Worldwide, breast cancer remains the most prevalent cancer in women and the second most common cause of cancer-related death among women, particularly in developing countries [1]. Despite new targeted therapies and increased understanding of the molecular heterogeneity of breast cancer, which resulted in some improvement in the survival rates of patients with breast cancer, drug resistance and more rapid tumor progression in some patients are issues that remain to be resolved [1,2]. Further molecular studies are required to identify prognostic biomarkers and to develop further targeted therapeutic strategies for patients with breast cancer.
The tetraspanin family is a large and highly-conserved group of membrane proteins that are ubiquitously expressed in eukaryotic cells [3]. Tetraspanin-enriched microdomains can form in the cell membrane with their partner proteins, including integrins and immunoglobulins, to regulate cell adhesion, migration, and invasion and through these mechanisms, tetraspanins also play a role in tumor progression [4,5]. Expression of the tetraspanin, KAI1/CD82, has been shown to inhibit tumor metastasis in several types of cancer [6–8]. However, the CD151 gene and the TSPAN8 gene have been shown to be potential oncogenes in breast cancer, melanoma, and lung cancer [9,10].
CD81, is also known as the target of the antiproliferative 1 (TAPA1), is also a member of the tetraspanin family [11]. The normal physiological role of CD81 involves immune function. In B-cells, CD81 associates with CD19 transports it to the cell membrane [12]. Also, CD81 plays an important role in the activation of B-cells by facilitating connections to the molecules required for B-cell receptor signaling [13]. In T-cells, CD81 is a co-stimulatory molecule of CD3 and regulates the development of T-cells in the thymus [13,14].
Recently, there have been some studies that have shown CD81 to be involved in the progression of human malignancy, including the promotion of tumor growth and metastasis in melanoma [15]. However, CD81 expression has also been shown to be negatively correlated with the development of metastases in patients with hepatocellular carcinoma (HCC) [16]. The role of CD81 expression in human malignancy is controversial, and CD81 expression in breast cancer remains to be studied.
Therefore, the aims of the present were to investigate the effects of increased CD81 expression on cell migration and proliferation in breast cancer cell lines, MDA-MB-231 and MDA-MB-435S in vitro, and the effects of increased CD81 expression in breast cancer on patient prognosis by evaluating CD81 expression in breast cancer tissue microarrays.
Material and Methods
Study approval
The study protocol was approved by the Independent Ethics Committee of The Fifth Peoples’ Hospital of Shanghai, Fudan University (#2012-041).
Tissue microarrays
A human breast cancer tissue microarray containing 140 tumor tissues (HBreD140Su01) and a human breast tissue microarray consisting of 77 adjacent normal breast tissues (HBreD077Su01) from patients with breast cancer were purchased from Outdo Biotech Co., Ltd., (Shanghai, China). The tumor tissue samples were obtained from patients who also provided detailed clinicopathological information (for 139/140 samples).
Immunohistochemical staining for CD81 in breast tissue microarrays
The tissue sections underwent immunohistochemical staining using a primary antibody to CD81 (Proteintech, Wuhan, China) (Cat No.18250-1-AP) at a dilution of 1: 500.
Evaluation of immunohistochemical staining for CD81 in breast tissue microarrays
CD81 staining results were stratified into negative and positive groups according to the proportion of CD81-positive cells and staining intensity. The scores of CD81-positive cells were assigned as follows: 0, ≤10% positive cells; 1, 11–25% positive cells; 2, 26–50%, positive cells; 3, >50% positive cells. The staining intensity was scored as follows: 0, no staining; 1, light brown; 2, brown; 3, dark brown. Total scores were obtained by multiplying the percentage and intensity scores. A total score ≤3 was considered to be negative, and a total score >3 was considered to be positive.
Cell culture of MDA-MB-231 and MDA-MB-435S breast cancer cells
Human breast cancer cell lines MDA-MB-231 and MDA-MB-435S were obtained from the Shanghai Cell Bank of the Chinese Academy of Science, Shanghai, China. Cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Corning, U.S.A.) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. All cells were cultured in a humidified incubator (Thermo Fisher Scientific, Waltham, MA, U.S.A.) with 5% CO2 at 37°C.
CD81 overexpression and silencing in MDA-MB-231 and MDA-MB-435S breast cancer cells in vitro
To generate CD81-expressing recombinant plasmid, the full-length cDNA of CD81 (GeneBank accession no. NM_004356.3) was amplified by polymerase chain reaction (PCR), and the PCR products were inserted into a pcDNA3.1 expression vector. DNA sequencing was used to verify the sequences of the recombinant plasmid.
To inhibit CD81 expression in breast cancer cells, CD81 short hairpin RNA (shRNA) (shCD81) lentivirus was packaged and obtained from GenePharma (Shanghai, China). The following target sequence was used: sense 5-UGAUGUUCGUUGBCUUCCUUU-3. The stably infected cells were screened using puromycin. For transfection, Lipofectamine 3000 (Invitrogen, Waltham, MA, U.S.A.) was used according to the manufacturer’s protocol.
Cell counting kit-8 (CCK-8) cell proliferation assay of MDA-MB-231 and MDA-MB-435S breast cancer cells in vitro
A cell proliferation assay was performed using cell counting kit-8 (CCK-8) assay (Dojindo, Japan) according to the manufacturer’s protocol. Then, 24 h after transfection, breast cancer cells were seeded into a 96-well plate at a density of 3×103 cells per well and cultured with 100 μl of 10% FBS in culture medium, and 10 μl of CCK-8 reagent was added into each well and incubated for 1 h or at the scheduled time points. An automated microplate reader read the absorbance at 450 nm. Each experiment was performed in triplicate.
Colony formation assays for MDA-MB-231 and MDA-MB-435S breast cancer cells in vitro
The colony formation assay involved seeding of the MDA-MB-231 and MDA-MB-435S cells at a density of 1,000 cells per well into a 6-well plate and maintained in 2 ml DMEM medium containing 10% FBS. After three weeks, the colonies that formed were fixed using 4% paraformaldehyde and stained with 0.05% crystal violet for 10–30 min and the stained colonies were counted and photographed. Each experiment was performed in triplicate.
Cell migration assays for MDA-MB-231 and MDA-MB-435S breast cancer cells in vitro
The cell migration assays were performed using transwell chambers (Corning, U.S.A.) with a pore size of 8 μm. Approximately 3×104 of the MDA-MB-231 and MDA-MB-435S cells, maintained in 400 μl medium without FBS were seeded into the upper chamber, while the lower chamber was filled with 800 μl medium with 10% FBS. After cell culture for 24 h at 37°C, the migrating cells were fixed and stained with 0.05% crystal violet for 20 min, after which the migrated cells were counted and photographed in five random fields using light microscopy.
Western blot
Proteins extracted from cells were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA, U.S.A.), which were then blocked using 5% dried skimmed milk powder. Blots were incubated with primary antibodies at 4°C overnight and then incubated with the secondary antibody for 1 h at room temperature. Specific proteins were visualized using the Odyssey Infrared Imaging System (Li-COR, Lincoln, NE, U.S.A.) according to the manufacturer’s instructions. The primary antibodies and their dilutions used included anti-CD81 (1: 500) (Proteintech, Wuhan, China) (Cat. No. 18250-1-AP), anti-GAPDH (1: 100000) (Proteintech, Wuhan, China) (Cat. No. 60004-1-Ig).
Statistical analysis
The chi-squared (χ2) test was used for comparative correlations between CD81 expression and clinicopathological parameters. Valued data were represented as the mean ± standard deviation (SD) of three independent experiments. Kaplan–Meier analysis was used to estimate the overall survival (OS) rates for breast cancer patients from different groups. Student’s t-test was performed for comparison between two groups. P<0.05 was considered statistically significant.
Results
Immunohistochemistry showed increased expression of CD81 in breast cancer tissues
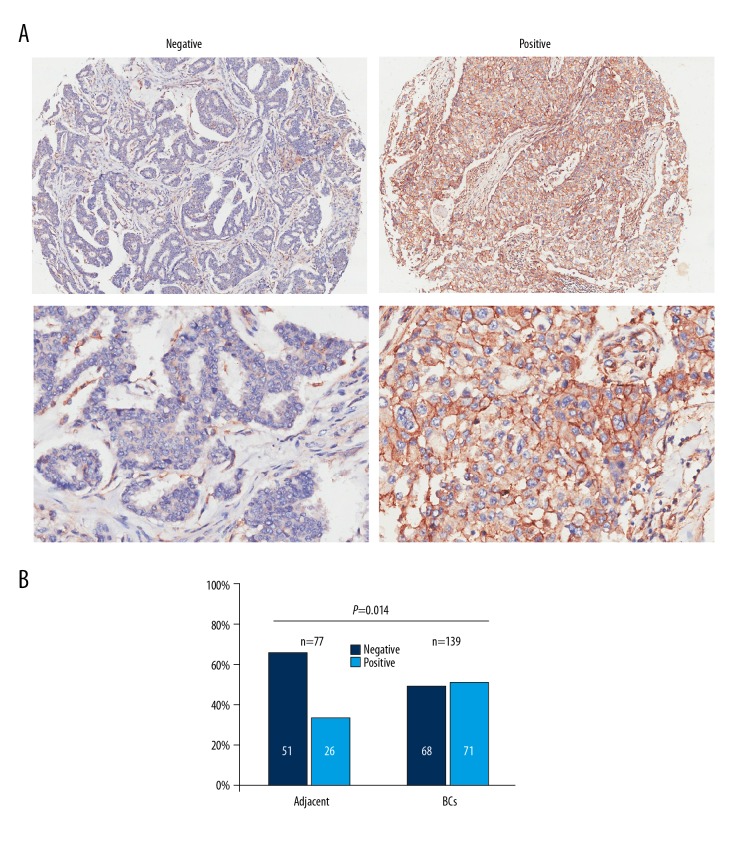
Representative photomicrograph images of CD81-negative immunostained breast tissues and CD81-positive immunostained breast tissues are shown in Figure 1A. The light microscopy semi-quantitative method for CD81 expression was used in two tissue microarrays consisting of 140 breast cancer tumor tissues and 70 adjacent normal breast tissues. The proportion of CD81-positive tissues (71/140, 51%) was significantly increased in breast cancer tissue samples compared with normal breast tissue samples (28/77, 36%) (P <0.05) (Figure 1B), indicating that CD81 was significantly overexpressed in breast cancer tissues.
Figure 1.
CD81 expression was upregulated in breast cancer tissues in tissue microarrays. (A) Representative photomicrographs show CD81 expression using immunohistochemistry in breast cancer tissues and adjacent normal breast tissues. Magnification ×40 (upper) and ×200 (lower). (B) The difference in CD81 expression levels between breast cancer tissues and adjacent normal breast tissues. * P<0.05.
Increased expression of CD81 was correlated with clinicopathological parameters and was associated with reduced overall survival (OS) in patients with breast cancer
The relationship between CD81 expression and the progression of breast cancer was analyzed statistically, and the results are summarized in Table 1. CD81 expression levels in breast cancer tissues were significantly associated with the presence of lymph node metastasis (P<0.05), although the degree of statistical significance was weak. CD81 expression intensity in breast cancer tissue was significantly correlated with TNM stage of patients with breast cancer (P<0.05). The Kaplan-Meier survival analysis curve analysis used to assess the correlation between CD81 expression and prognosis of patients with breast cancer showed that CD81-positive patients had a worse prognosis than CD81-negative patients (P<0.05). Figure 2 shows the Kaplan-Meier curve for the OS rate between the two patient groups. These results support that high CD81 expression in breast cancer tissues is correlated with clinicopathological parameters and predicts poor prognosis in patients with breast cancer.
Table 1.
Correlation between CD81 expression with clinicopathological parameters of breast cancer patients (n=139) from breast cancer tissue microarrays.
| Parameters | CD81 expression | P value | ||
|---|---|---|---|---|
| Negative | Positive | Total | ||
| Age (years) | 0.366642 | |||
| <60 | 48 (51.6%) | 45 (48.4%) | 93 | |
| ≥60 | 20 (43.5%) | 26 (56.5%) | 46 | |
| Tumor infiltration | / | |||
| T1–T2 | 67 (48.9%) | 70 (51.1%) | 137 | |
| T3–T4 | 1 (50.0%) | 1 (50.0%) | 2 | |
| Lymph node metastasis | 0.04862 | |||
| LN+ | 26 (40.0%) | 39 (60.0%) | 65 | |
| LN− | 42 (56.8%) | 32 (43.2%) | 74 | |
| Grade | 0.567629 | |||
| 1–2 | 49 (50.5%) | 48 (49.5%) | 97 | |
| 3–4 | 19 (45.2%) | 23 (54.8%) | 42 | |
| Stage | 0.019019 | |||
| I–II | 52 (55.9%) | 41 (44.1%) | 93 | |
| III–IV | 16 (34.8%) | 30 (65.2%) | 46 | |
| Recrudescence | 0.080568 | |||
| Yes | 14 (36.8%) | 24 (63.2%) | 38 | |
| No | 54 (53.5%) | 47 (46.5%) | 101 | |
The 7th American Joint Committee on Cancer (AJCC) TNM system was used for the classification and staging of breast cancer.
Figure 2.
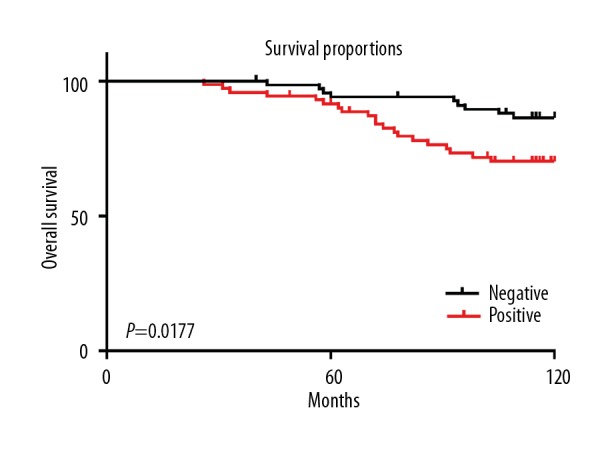
Correlation between CD81 expression levels in breast cancer tissues and overall survival (OS) rates of patients with breast cancer. According to the expression of CD81 in breast cancer tissues, the patients were classified into two groups: negative (68 patients) and positive (71 patients). The grouping method is described in the Methods section of the manuscript.
Increased expression of CD81 promoted cell proliferation and migration in MDA-MB-231 and MDA-MB-435S breast cancer cells in vitro
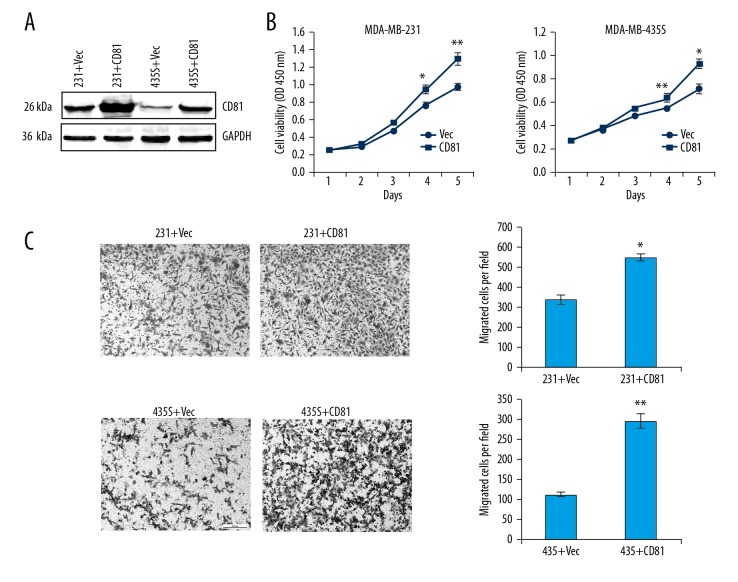
Based on the clinical data, functional cell analysis was used to observe the effects of CD81 expression on cell proliferation and migration of breast cancer cell lines. The CD81-expressing plasmid was transfected into MDA-MB-231 and MDA-MB-435S cells to upregulate CD81 expression. Western blot analysis confirmed the efficiency of the plasmid transfection (Figure 3A, Supplementary Figure 1). The cell counting kit-8 (CCK-8) cell proliferation assay was used to determine the effects of overexpression of CD81 on cell proliferation of breast cancer cells in vitro. As shown in Figure 3B, the cell growth rate was significantly increased in cells with overexpression of CD81 compared with the control groups. Cell migration assays showed that the effect of overexpression of CD81 significantly promoted cell migration in the two breast cancer cell lines in vitro (P<0.01) (Figure 3C). The results of the functional analysis showed that CD81 expression regulated cell proliferation and migration in breast cancer cells in vitro.
Figure 3.
Overexpression of CD81 promoted cell proliferation and migration of MDA-MB-231 and MDA-MB-435S breast cancer cells in vitro. (A) Western blot analysis confirmed the efficacy of the CD81-expressing plasmid. (B) Growth curves of MDA-MB-231-vec/MDA-MB-231-CD81 and MDA-MB-435S-vec/MDA-MB-435S-CD81 cells are shown. * P<0.05; ** P<0.01. (C) Overexpression of CD81 significantly promoted cell migration of MDA-MB-231 and MDA-MB-435S cells. * P<0.05; ** P<0.01.
Silencing of CD81 expression decreased proliferation and migration of MDA-MB-231 and MDA-MB-435S breast cancer cells in vitro
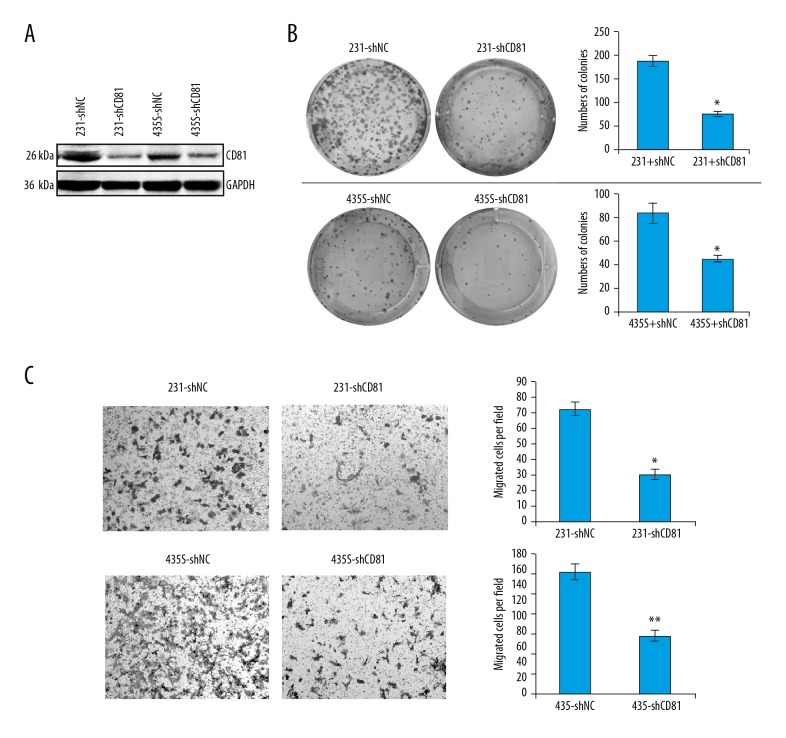
Knockdown of CG81 expression in MDA-MB-231 and MDA-MB-435S cells was performed using short hairpin RNA (shRNA) lentivirus in vitro (shCD81). Western blot results showed that CD81 expression was successfully inhibited in MDA-MB-231-shCD81 and MDA-MB-435S-shCD81 cells compared with their control groups (Figure 4A, Supplementary Figure 1). In the colony formation assays, knockdown of CD81 significantly suppressed the number and volume of the colonies formed from breast cancer cells, suggesting that silencing CD81 could attenuate the proliferation capacity of breast cancer cells (Figure 4B). The results of cell migration assays also showed that knockdown of CD81 expression in breast cancer cells led to reduced cell migration (Figure 4C). These results supported that CD81 expression could enhance breast cancer cell growth and migration in vitro.
Figure 4.
Silencing of CD81 inhibited proliferation and migration of MDA-MB-231 and MDA-MB-435S breast cancer cells in vitro. (A) The results of Western blot analysis show that CD81 expression was successfully inhibited in MDA-MB-231-shCD81 and MDA-MB-435S-shCD81 cells comparing with the control groups. (B) Colony formation assays determined the effects of CD81 on the colony formation ability of MDA-MB-231 and MDA-MB-435S cells. * P<0.05. (C) Silencing of CD81 significantly inhibited cell migration of MDA-MB-231 and MDA-MB-435S cells. * P<0.05; ** P<0.01.
Discussion
The findings of the present study showed that CD81 expression was upregulated in breast cancer tissues compared with normal breast tissues and that high expression levels of CD81 were correlated with poor prognosis or reduced overall survival (OS) in patients with breast cancer. Cell functional analysis using MDA-MB-231 and MDA-MB-435S human breast cancer cell showed that CD81 expression promoted the proliferation and migration of breast cancer cells in vitro. These findings indicate that CD81 might be a potential prognostic biomarker or oncogene product in human breast cancer.
As a member of the tetraspanin family, CD81 has previously been shown to be involved in the immune system and infection. In the immune system, CD81 regulates the activation of B-cells and T-cells, and immune receptor signaling [13]. During infection, CD81 is reported to be involved as a gateway molecule in hepatocytes for hepatitis C virus infection [17], and may have a similar role in Plasmodium infection of red blood cells in malaria [18]. Although the diverse functions of CD81 have been reported in varied biological processes [18,19], its role in human malignancy remains poorly understood. Considering the high morbidity and mortality of breast cancer [20], there is a need to understand the molecular mechanisms of breast cancer development, to overcome the problems of drug resistance and the early development of metastases in breast cancer.
To our knowledge, this study has been the first to show that the tetraspanin, CD81, might be a potential prognostic biomarker that could be incorporated into the tissue diagnosis panel of immunomarkers, and there is also the possibility that CD81 may be a future therapeutic target in patients with breast cancer. Future large-scale controlled prospective studies are required to evaluate these possibilities.
In the present study, in breast cancer tissues, CD81 expression was significantly upregulated when compared with adjacent normal breast tissues, and its expression levels were significantly associated with lymph node metastasis and TNM stage of patients with breast cancer. However, the association between CD81 expression and the presence of lymph node metastasis is not very strong (P=0.048). Therefore, studies that include large sample sizes are needed to verify this association. Although this was a preliminary study, the findings suggest that CD81 might be involved in the development of breast cancer, but this association also requires verification with further studies. The results of this study also confirmed that CD81 expression by human breast cancer cells promoted cell proliferation and migration, which are the properties of malignant tumors that enable invasion into vessels and tumor metastasis. These results support the possibility that expression of CD81 might have a role in the progression of breast cancer, but this possibility also requires further investigation.
From the findings of the present study, it is clear that CD81 has a potential prognostic and functional role in human breast cancer and that further studies are needed to elucidate the underlying mechanisms for the effects of CD81 in this type of malignancy. It is possible that CD81 has a role in regulating the activation of the immune system in patients with breast cancer, as recent studies have shown that exosomes derived from tumor cells contribute to the genesis and development of several types of cancer [21–23]. Also, CD81 is an important exosome membrane protein, and it is also possible that the role of CD81 in breast cancer may be related to exosome function [24]. The potential for multiple roles for CD81 in different types of malignant tumor may explain the varied reports in the published literature and might indicate that CD81 has functions that are specific to tumor types and are context-dependent, which mean that its role in other types of malignancy may not be the same as for breast cancer. However, these hypotheses require further studies.
Conclusions
The findings of the present study showed that CD81, a member of the tetraspanin family, was significantly overexpressed in breast cancer tissues compared with adjacent normal breast tissues and that increased expression levels of CD81 in tumor tissues from patients with breast cancer were significantly associated with reduced overall survival (OS). Further in vitro studies, using two human breast cancer cell lines, showed that increased expression of CD81 promoted tumor cell proliferation and migration. The findings of the study showed that CD81 might be a potential prognostic biomarker associated with poor patient prognosis in breast cancer. These findings should be investigated further with large-scale controlled prospective studies in patients with breast cancer.
Supplementary Figure
CD81 expression levels on Western blot in MDA-MB-231 and MDA-MB-435S breast cancer cells in vitro after overexpression or knockdown. (A) CD81 expression in MDA-MB-231 cells. (B) CD81 expression in MDA-MB-435S cells.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Levy S, Shoham T. The tetraspanin web modulates immune-signalling complexes. Nat Rev Immunol. 2005;5:136–48. doi: 10.1038/nri1548. [DOI] [PubMed] [Google Scholar]
- 4.Hemler ME. Tetraspanin functions and associated microdomains. Nat Rev Mol Cell Biol. 2005;6:801–11. doi: 10.1038/nrm1736. [DOI] [PubMed] [Google Scholar]
- 5.Bassani S, Cingolani LA. Tetraspanins: Interactions and interplay with integrins. Int J Biochem Cell Biol. 2012;44:703–8. doi: 10.1016/j.biocel.2012.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Dong JT, Lamb PW, Rinker-Schaeffer CW, et al. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268:884–86. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- 7.Tang Y, Cheng Y, Martinka M, et al. Prognostic significance of KAI1/CD82 in human melanoma and its role in cell migration and invasion through the regulation of ING4. Carcinogenesis. 2014;35:86–95. doi: 10.1093/carcin/bgt346. [DOI] [PubMed] [Google Scholar]
- 8.Adachi M, Taki T, Ieki Y, et al. Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res. 1996;56:1751–55. [PubMed] [Google Scholar]
- 9.Testa JE, Brooks PC, Lin JM, Quigley JP. Eukaryotic expression cloning with an antimetastatic monoclonal antibody identifies a tetraspanin (PETA-3/CD151) as an effector of human tumor cell migration and metastasis. Cancer Res. 1999;59:3812–20. [PubMed] [Google Scholar]
- 10.Zoller M. Tetraspanins: push and pull in suppressing and promoting metastasis. Nat Rev Cancer. 2009;9:40–55. doi: 10.1038/nrc2543. [DOI] [PubMed] [Google Scholar]
- 11.Oren R, Takahashi S, Doss C, et al. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990;10:4007–15. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shoham T, Rajapaksa R, Kuo CC, et al. Building of the tetraspanin web: Distinct structural domains of CD81 function in different cellular compartments. Mol Cell Biol. 2006;26:1373–85. doi: 10.1128/MCB.26.4.1373-1385.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levy S. Function of the tetraspanin molecule CD81 in B and T cells. Immunol Res. 2014;58:179–85. doi: 10.1007/s12026-014-8490-7. [DOI] [PubMed] [Google Scholar]
- 14.Mittelbrunn M, Yanez-Mo M, Sancho D, et al. Cutting edge: Dynamic redistribution of tetraspanin CD81 at the central zone of the immune synapse in both T lymphocytes and APC. J Immunol. 2002;169:6691–95. doi: 10.4049/jimmunol.169.12.6691. [DOI] [PubMed] [Google Scholar]
- 15.Hong IK, Byun HJ, Lee J, et al. The tetraspanin CD81 protein increases melanoma cell motility by upregulating metalloproteinase MT1-MMP expression through the pro-oncogenic Akt-dependent Sp1 activation signaling pathways. J Biol Chem. 2014;289:15691–704. doi: 10.1074/jbc.M113.534206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Inoue G, Horiike N, Onji M. The CD81 expression in liver in hepatocellular carcinoma. Int J Mol Med. 2001;7:67–71. doi: 10.3892/ijmm.7.1.67. [DOI] [PubMed] [Google Scholar]
- 17.Pileri P, Uematsu Y, Campagnoli S, et al. Binding of hepatitis C virus to CD81. Science. 1998;282:938–41. doi: 10.1126/science.282.5390.938. [DOI] [PubMed] [Google Scholar]
- 18.Silvie O, Rubinstein E, Franetich JF, et al. Hepatocyte CD81 is required for Plasmodium falciparum and Plasmodium yoelii sporozoite infectivity. Nat Med. 2003;9:93–96. doi: 10.1038/nm808. [DOI] [PubMed] [Google Scholar]
- 19.Rubinstein E, Ziyyat A, Prenant M, et al. Reduced fertility of female mice lacking CD81. Dev Biol. 2006;290:351–58. doi: 10.1016/j.ydbio.2005.11.031. [DOI] [PubMed] [Google Scholar]
- 20.DeSantis C, Ma J, Bryan L, Jemal A. Breast cancer statistics, 2013. Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 21.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 22.Baginska J, Viry E, Paggetti J, et al. The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front Immunol. 2013;4:490. doi: 10.3389/fimmu.2013.00490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lowry MC, Gallagher WM, O’Driscoll L. The role of exosomes in breast cancer. Clin Chem. 2015;61:1457–65. doi: 10.1373/clinchem.2015.240028. [DOI] [PubMed] [Google Scholar]
- 24.Mathivanan S, Ji H, Simpson RJ. Exosomes: Extracellular organelles important in intercellular communication. J Proteomics. 2010;73:1907–20. doi: 10.1016/j.jprot.2010.06.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CD81 expression levels on Western blot in MDA-MB-231 and MDA-MB-435S breast cancer cells in vitro after overexpression or knockdown. (A) CD81 expression in MDA-MB-231 cells. (B) CD81 expression in MDA-MB-435S cells.