Abstract
Background
This study aimed to investigate the correlation of brain perfusion with white matter hyperintensity (WMH), brain atrophy, and cognition in patients with moderate to severe posterior cerebral artery stenosis (PCAS).
Material/Methods
65 patients with memory decline as the main complaint and no history of brain infarction were recruited from the Department of Neurology of Tongji Hospital. Patients with moderate to severe PCAS were included in case group, and subjects with normal intracranial blood vessels served as controls. The demographics and vascular risk factors were recorded. Montreal Cognitive Assessment (MoCA) was used to evaluate the cognition. CT perfusion imaging was performed, and WASID was employed for the assessment of intracranial artery stenosis. The region of interest (ROI) was analyzed based on the whole brain perfusion. Cranial MRI was performed, and Scheltens scoring system was used for the assessment of WMH on FLAIR. T1 weighed images were obtained, and global cortical atrophy (GCA) scale was employed for the assessment of brain atrophy. The detections of brain perfusion, WMH and brain atrophy were done at centrum ovale, parietal lateral ventricle and basal ganglia layers.
Results
In PCAS patients we found low perfusion in the antecornu and postcornu blood supply areas at the lateral ventricle, the blood supply area of the anterior cerebral artery, the blood supply area of the posterior cerebral artery, and the blood supply area at the hippocampus as compared with control subjects (p<0.05). As compared with control subjects, the incidence of WMH in the blood supply areas at the deep brain and lateral ventricle was significantly higher in PCAS patients (p<0.05). When compared with controls, the incidence of brain atrophy increased significantly in PCAS patients (p<0.01). Correlation analysis showed the brain perfusion at the blood supply area of the posterior cerebral artery was positively correlated to the total MoCA score and negatively correlated to the severity of WMH at the blood supply area of the posterior cerebral artery (p<0.05). Further analysis showed the brain perfusion at the blood supply area of the posterior cerebral artery was negatively associated with cortex supplied by the posterior cerebral artery, posterior cingulate, and hippocampus (p<0.01).
Conclusions
PCAS patients have a higher incidence of brain atrophy, and the perfusion at the area supplied by the posterior cerebral artery is correlated to the severity of brain atrophy and of WMH, as well as to cognition decline.
MeSH Keywords: Cognition, Leukoencephalopathies, Perfusion Imaging, Posterior Cerebral Artery
Background
Computerized tomography angiography (CTA) and magnetic resonance angiography have become widely used in clinical practice with the development of imaging technology; therefore, the detection rate and incidence rate of asymptomatic intracranial artery stenosis have been steadily increasing [1]. In recent years there has been increasing clinical attention to focused on cognition impairment secondary to vascular stenosis. The blood supply to the hippocampus, occipital lobe, and lower surface of the temporal lobe is mainly dependent on the posterior cerebral artery (PCA), and these structures are also closely related to memory and cognition [2]. However, the relationship between PCA stenosis (PCAS) and cognition remains unclear, and most studies have focused on mechanisms underlying carotid artery stenosis-related cognition impairment. Some clinicians have investigated the influence of hemodynamics after carotid artery stenosis on cognition, but there is still controversy on this issue [3,4]. Kurata et al. found that hemodynamics after internal carotid artery stenosis was closely related to white matter hyperintensity (WMH) [5]. Patankar et al. speculated that major artery stenosis could promote the development of WMH, but the specific mechanism is unclear [6]. A recent study revealed that carotid artery stenosis could increase risk of low brain perfusion [7], which facilitates development of brain atrophy and cognition decline [8]. However, there is no direct evidence supporting a relationship between intracranial artery stenosis and brain atrophy. A variety of studies have been conducted to investigate the mechanism underlying carotid artery stenosis related cognition impairment, but little is known about the relationship between PCAS and cognition impairment. The present study aimed to investigate the relationship of PCAS with brain perfusion, WMH, brain atrophy, and cognition.
Material and Methods
Subjects
The study was approved by the Ethics Committee of Tongji Hospital, and all subjects or their legal representatives gave their written informed consent. Patients with memory decline as the main complaint and without any history of brain infarction were recruited from Tongji Hospital, between Nov. 2015 and Dec. 2016. Using our inclusion and exclusion criteria, we included 65 patients. PCAS was found in 34 patients (left: n=9; right: n=15; bilateral: n=10), in whom there were 12 males and 22 females, with a mean age of 74.71±8.68 years old. In the remaining 31 patients, there were 11 males and 20 females, with a mean age of 64.52±7.68 years old.
Inclusion criteria
PCAS group: PCAS was diagnosed based on CTA (≥50% stenosis). Control group: Intracranial artery stenosis was not identified by CTA.
Exclusion criteria
Patients with intracranial artery stenosis except for the posterior cerebral artery were excluded. Brain infarction or hemorrhage was excluded by cranial computerized tomography (CT) or magnetic resonance imaging (MRI). Patients with a history of severe brain trauma or atrial fibrillation were excluded. Patients with heart, liver, kidney, or lung dysfunction were excluded. Patients with malignancies or severe infection were excluded. Patients unable to cooperate with examinations due to visual, hearing, or verbal dysfunction were excluded.
CT scanning
The whole-brain perfusion imaging was done by CT (Toshiba Aquilion ONE 320). Coverage of the brain was 140 mm with volume scanning. Parameters were as follows: slice thickness, 0.5 mm; field of view (FOV), 220 mm; matrix, 512×512; voltage, 100 kV; current, 150 mA. A 50 mL bolus of iodinated contrast agent (iohexol: 350 mg/ml) was injected at 5 ml/s into the antecubital vein, and the first scanning was done 7 s later. Thereafter, intermittent scanning was done with 2 s interval after 12 s. In the arterial phase, the image was visualized after 12 s and peaked at 18–28 s. In the venous phase, intermittent scanning was done every 5 s, from 40 s to 60 s. The single rotation time was 0.5 s and total scanning time was 60 s. Total volume data were obtained.
MRI scanning
Superconducting magnetic resonance (Siemens, Germany) and standard orthogonal coil were used for MRI. In brief, patients were placed in a supine position with the head fixed on a head pad. A standard head coil was used. Then, plain scanning was performed first under the following parameters: for T1-weighted scanning: TR, 1530 s; TE, 9 ms; slice thickness, 5 mm; internal, 1 mm; FOV, 230×230 mm, and for T2-weighted scanning: TR, 4210 ms, TE, 96 ms; slice thickness, 5 mm; interval, 1 mm, FOV, 230×230 mm; FLAIR scanning, TR, 5000 ms; TE, 94 ms; TI, 1800 ms; slice thickness, 5 mm; interval, 1 mm; FOV, 230×230 mm.
Construction of cerebral blood flow map and production of CTA data
The Perfusion Mismatch Analyzer (PMA; Advanced Medical Science Center, Iwate Medical University, Iwate, Japan) was used to calculate the cerebral blood flow (CBF). The software automatically selects ten arterial input functions (AIFs) in one slice, which was set at the level of the Circle of Willis. The venous output function (VOF) was automatically chosen in the intracranial veins above the skull base. The perfusion map was calculated with convolution method with matrix of 512×512, slice thickness of 0.1 mm and other default values in PMA. For quantification, the perfusion map after PMA reconstruction was input into Mango (http://rii.uthscsa.edu/mango/), and the region of interest (ROI) was delineated. The volume data were input into a 3-D CTA imaging system (Toshiba) for subtraction angiography. The 3-D images were obtained from CTA.
Assessment of intracranial artery stenosis
The Warfarin-Aspirin Symptomatic Intracranial Disease (WASID) method [9] was used to assess the intracranial artery stenosis as% stenosis=1–(diameter of stenotic artery/diameter of distal normal artery)×100. The bilateral internal carotid arteries, anterior cerebral artery, vertebral artery, basilar artery, and PCA were assessed for stenosis (Figure 1). Image analysis was performed on a consensus basis with all images examined as distinct samples by an experienced neurological physician who was unaware of the radiology reports and clinical history, followed by final verification by a similarly blinded and experienced neuroradiologist. The distribution of stenosis was further analyzed.
Figure 1.
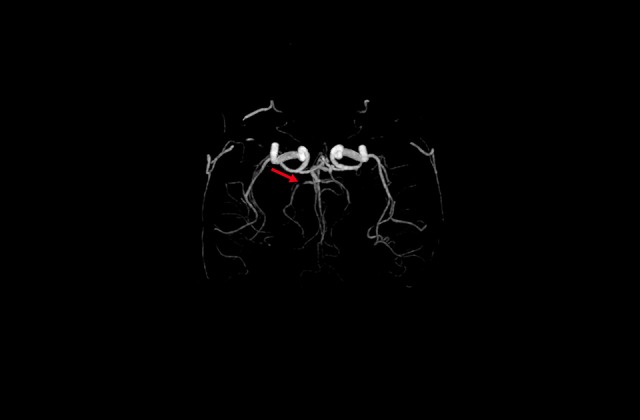
As shown by the red arrow in the figure, only the right posterior cerebral artery develops severe stenosis, while other intracranial arteries are normal.
Assessment of white-matter lesion
Three representative layers (centrum ovale, parietal lateral ventricle, and basal ganglia) were selected for the assessment of WMH with the modified Scheltens scoring system [10]. The periventricular areas (antecornu, postcornu and para-lateral ventricle) were assessed for hyperintensity: 0, no change; 1, lesion smaller than 5 mm; 2, lesion of 6–10 mm. The deep white matter was assessed at the areas supplied by the anterior cerebral artery and middle cerebral artery, the subcortical area supplied by the middle cerebral artery, and the area supplied by PCA: 0, no abnormality; 1, lesion no larger than 3 mm and no more than 5; 2, lesion no larger than 3 mm and no more than 6 mm; 3, lesion of 4–10 mm and no more than 5 mm; 4, lesion of 4–10 mm and no less than 6; 5, lesion no smaller than 11 and no less than 1 mm; 6, merged lesion. The WMH score at different ROIs was obtained. The ROI of the affected side was used for unilateral artery stenosis; the mean WMH score of bilateral ROIs was obtained for patients with bilateral artery stenosis and those in the control group. One was used as the cut-off value in the assessment of WMH, and the incidence of WMH at different ROIs was compared between 2 groups.
Acquisition and measurement of blood flow perfusion at different ROIs
The CBF map was input into Mango (http://rii.uthscsa.edu/mango/), and ROI was determined at the centrum ovale, parietal lateral ventricle, and basal ganglia layers. The CBF of different ROIs was calculated. At these layers, the CBF was calculated at bilateral areas supplied by the anterior cerebral artery, the area supplied by middle cerebral artery, the subcortical area supplied by the middle cerebral artery, the area besides the ventricle, and the area supplied by PCA and the hippocampus. The sinus, large vessels, and ventricles were avoided during the measurement of CBF. The CBF of ROI at different layers was determined. The CBF of ROI of the affected side was used for unilateral artery stenosis; the mean CBF of bilateral ROIs was obtained for patients with bilateral artery stenosis and those in control group. The mean CBF of ROI was compared between 2 groups.
Assessment of brain atrophy
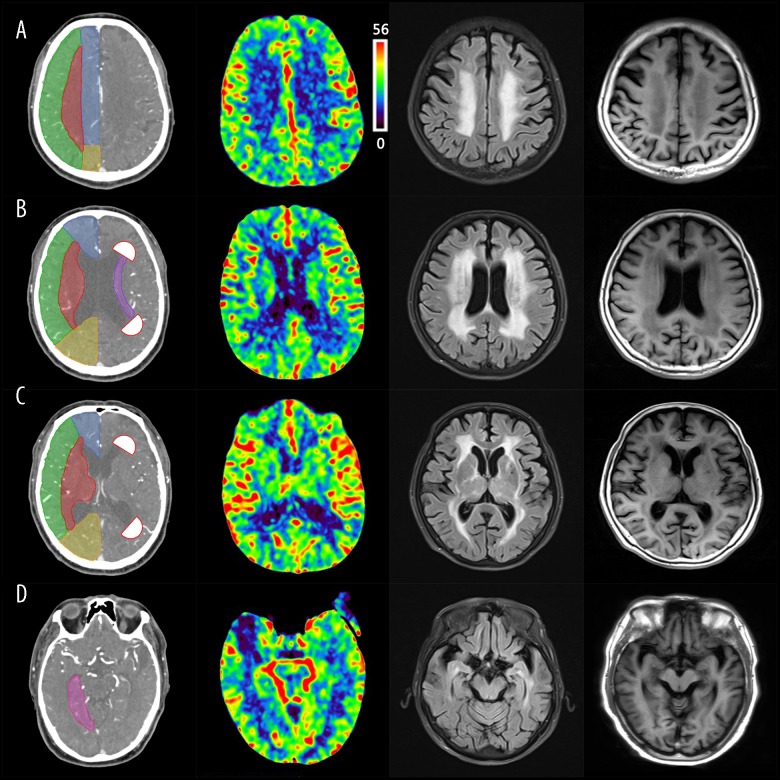
The brain atrophy was assessed at the centrum ovale, parietal lateral ventricle, and basal ganglia layers using the modified Global Cortical Atrophy Scale [11]. The cortex supplied by the anterior cerebral artery, the cortex supplied by the middle cerebral artery, and the cortex supplied by the PCA, posterior cingulate, and hippocampus were assessed for atrophy. The Global Cortical Atrophy Scale was assessed with a 4-point scoring system: 0, no sulcal opening and lobe atrophy; 1, the visualization of sulcal opening peripherally; 2, evident sulcal opening and deepening; 3, evident sulcal opening and gyral thinning. Hippocampal atrophy was assessed with the medial temporal lobe atrophy (MTA) scale, which is a 5-point scale, ranging from 0 to 4: 0, merging of hippocampus and temporal lobe; 1, structural deficiency or unclear structure between hippocampus and temporal lobe; 2, widened structural deficiency or unclear structure; 3, absence of connection between head and body besides findings in grade 2; 4, brain atrophy more severe than in grade 2 without evident hippocampus. We used the atrophy score at different ROIs and hippocampus, with 1 as the cut-off value for the assessment of brain atrophy. The incidence of brain atrophy was compared between 2 groups (Figure 2).
Figure 2.
Outlines of region of interest (centrum ovale, parietal lateral ventricle and basal ganglia layers) are described as A, B, C, respectively. ACA (territory of anterior cerebral artery) is indicated by blue; MCA (territory of middle cerebral artery) is indicated by green; PCA (territory of posterior cerebral artery) is indicated by yellow; Sub_MCA (territory of Sub cortex of Middle cerebral artery) is indicated by red. In panel B, antecornu and postcornu are indicated by white, semi-circular areas, which are presented as 1 cm in diameter; periventricular regions territory was indicated by purple; In panel D, hippocampus is indicated by pink. From left to right, consisting of relative cerebral blood flow, FLAIR MR, T1-weighted MR. Images in Figure 1 were obtained from the Department of Radiology of Tongji Hospital.
Assessment of cognition
The Montreal Cognitive Assessment (MoCA), Chinese version, was used for the assessment of cognition. The MoCA scores range from 0 to 30 and are divided into 7 subscores. The score was increased by 1 when the educational level was ≤12 years, and the accumulative total score was no higher than 30. The total MoCA score was ≥24 for subjects with educational level of primary school or higher and ≥20 for those with education level lower than primary school. Subjects filled in the form in a quiet and calm environment. The total MoCA score and score of each domain were obtained.
Statistical analysis
Statistical analysis was performed with SPSS version 20.0. Data are expressed as mean ± standard deviation or percentages, and the demographics were described. The CBF was compared with one-way analysis of variance. The chi-square test was employed for the comparison of percentages. Spearman correlation analysis was used for assessment of the relationship of brain perfusion with WMH, brain atrophy, and cognition. A value of two-sided p≤0.05 was considered statistically significant.
Results
General characteristics
There were no significant differences in gender, educational level, smoking status, drinking status, family history, hyperlipidemia, and hyperhomocysteinemia between the 2 groups (p>0.05), but there was a highly significant difference in age between the 2 groups, and significant differences were noted for hypertension and diabetes mellitus (p<0.05) (Table 1).
Table 1.
Baseline characteristics of the study population.
| Control group (n=31) | Stenosis group (n=34) | P value | |
|---|---|---|---|
| Age (mean ±SD) | 64.52±7.68 | 74.63±8.57 | <0.01** |
| Sex (Male %) | 11 (35.5) | 12 (35.3) | 0.92 |
| Education years | 10.26±3.17 | 8.91±4.44 | 0.17 |
| Smoking (%) | 5 (16.1) | 6 (17.6) | 0.87 |
| Alcohol consumption (%) | 1 (3.2) | 2 (5.9) | 1.00 |
| Hypertension (%) | 12 (38.7) | 22 (64.7) | 0.036* |
| Diabetes (%) | 3 (9.7) | 10 (29.4) | 0.047* |
| Family history of dementia (%) | 1 (3.2) | 2 (5.9) | 1.00 |
| Hyperlipidemia (%) | 13 (46.4) | 17 (70.8) | 0.076 |
| Homocysteine (%) | 0 (0) | 3 (17.6) | 0.27 |
| Whole brain cerebral blood flow (CBF) | 32.47±7.82 | 27.79±8.44 | 0.024* |
| White matter hyperintensity score | 5.5 (2.5, 9.5) | 19.5 (11.75, 26.25) | <0.01** |
| Cerebral atrophy score | 2.08 (1, 6.25) | 7.5 (3.29, 11.54) | 0.001** |
| Incidence of cognitive impairment (%) | 54.80 | 79.40 | 0.034* |
p<0.05;
p<0.01.
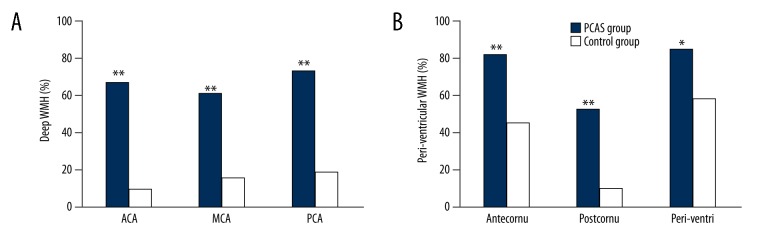
Incidence of WMH at different sites
In the PCAS group, the deep brain areas had a higher incidence of WMH as compared with the control group (p<0.01). In the PCAS group, the incidence of WMH in the periventricular area (antecornu, postcornu, and para-lateral ventricle) was significantly higher than in the control group (p<0.05) (Figure 3).
Figure 3.
Incidence of white matter hyperintensity in different brain areas between the 2 groups. (A) Shows deep white matter hyperintensity in different territories in anterior cerebral artery, middle cerebral artery, and posterior cerebral artery. (B) Illustrates white matter hyperintensity in periventricular regions. ACA – territory of anterior cerebral artery; MCA – territory of middle cerebral artery; PCA – territory of posterior cerebral artery; Sub_MCA – territory of subcortex of Middle cerebral artery; antecornu – territory of antecornu; postcornu – territory of postcornu; Periventri – territory of periventricular regions. * p<0.05, ** p<0.01.
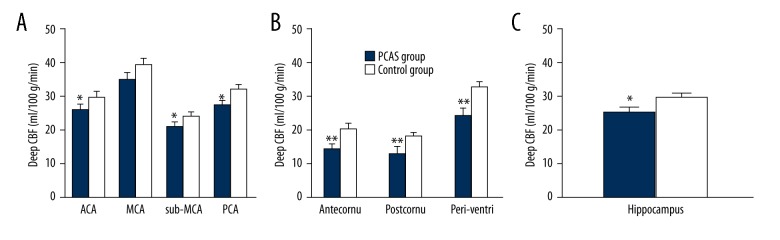
CBF at different sites
In the PCAS group, the CBF at the area supplied by the anterior cerebral artery, the area supplied by the PCA, and the subcortical area supplied by the middle cerebral artery was significantly lower than in the control group (p<0.05). A significant difference was also observed in the hippocampal CBF between the 2 groups (p<0.05), but there was no significant difference in the CBF of the area supplied by the middle cerebral artery (p>0.05). In the PCAS group, the CBF of the area besides the ventricle (antecornu, postcornu, and para-lateral ventricle) was significantly lower than in the control group (p<0.05) (Figure 4).
Figure 4.
Cerebral blood flow comparison at different sites between the 2 groups. (A) Shows CBF of deep regions territories. (B) Shows CBF of periventricular regions. (C) Shows CBF of Hippocampus. ACA – territory of Anterior cerebral artery; MCA – territory of middle cerebral artery; PCA – territory of posterior cerebral artery; Sub_MCA – territory of Subcortex of middle cerebral artery; antecornu – territory of antecornu; postcornu – territory of postcornu; periventri – territory of periventricular regions, CBF – cerebral blood flow. * p<0.05, ** p<0.01.
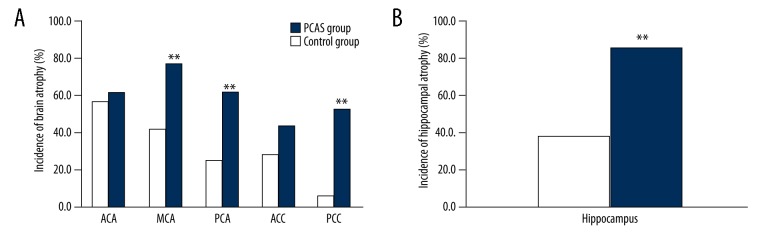
Incidence of brain atrophy
In the PCAS group, the incidences of brain atrophy at the area supplied by the middle cerebral artery and the area supplied by the PCA, posterior cingulate, and hippocampus were significantly higher than in the control group (p<0.01), but there were no significant differences in the incidences of brain atrophy in the area supplied by anterior cerebral artery and the anterior cingulate between the 2 groups (p>0.05) (Figure 5).
Figure 5.
Incidence of brain atrophy in different brain structures. (A) Demonstrates incidence of brain atrophy in different brain structures. (B) Demonstrates incidence of brain atrophy of hippocampus. ACA – territory of anterior cerebral artery; MCA – territory of middle cerebral artery; PCA – territory of posterior cerebral artery; ACC – territory of anterior cingulate cortex; PCC – territory of posterior cingulate cortex; * p<0.05, ** p<0.01.
Correlation of CBF with cognition and WMH severity
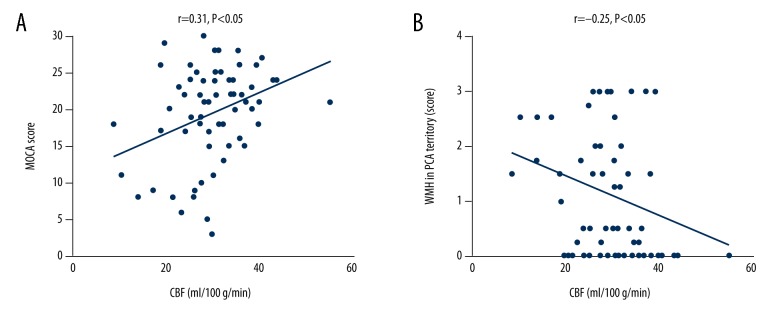
The CBF of the area supplied by the PCA was positively related to total MoCA score (r=0.31, p<0.05) and negatively related to the severity of WMH at the area supplied by the PCA (r=−0.25, p<0.05) (Figure 6).
Figure 6.
Correlation between CBF and MoCA score, White matter hyperintensities. (A) Illustrated correlation between CBF of PCA territory with total MoCA score. (B) Illustrates correlation between CBF of PCA territory with WMH in PCA territory. CBF – cerebral blood flow; MoCA – Montreal Cognitive Assessment; PCA – territory of posterior cerebral artery.
Correlation between CBF and brain atrophy
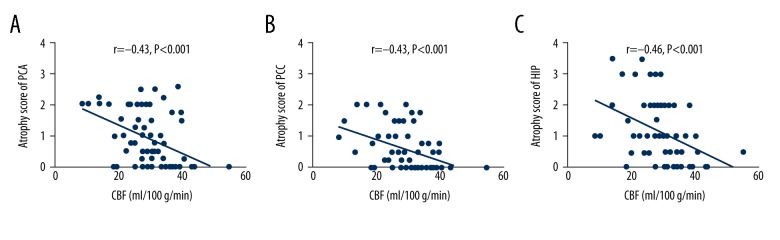
The CBF of the area supplied by the PCA was negatively associated with the atrophy severity of the cortex supplied by the PCA (r=−0.41, p<0.01), posterior cingulate (r=−0.41, p<0.01), and hippocampus (r=−0.42, p<0.01) (Figure 7).
Figure 7.
Correlation between CBF in PCA territory with brain atrophy in posterior cerebral regions. (A) Shows correlation between CBF of PCA territory with atrophy score of PCA; (B) Shows correlation between CBF of PCA territory with atrophy score of PCC; (C) shows correlation between CBF of PCA territory with atrophy score of hippocampus. PCA – territory of posterior cerebral artery cortex; PCC – territory of posterior cingulate cortex; HIP – hippocampus; CBF – cerebral blood flow.
Discussion
Correlation of PCAS with low brain perfusion and cognition
Studies have shown that carotid artery stenosis is often accompanied by cognition decline and may be a risk factor of cognition decline [12]. Age is an independent risk factor for the development of intracranial atherosclerosis. A study conducted in the South Korean population observed that for every 10 years of age, the odds of intracranial disease increased by 1.2 [13] and a highly significant difference were noted in the age in our study. In addition, our results also demonstrated the significant differences in hypertension and diabetes mellitus between the 2 groups in accordance with a previous report [14]. Due to the limitations of cross-sectional studies, cohort studies need to be done to confirm these findings. This study showed that the incidence of cognition impairment was as high as 79.4% in patients with PCAS, which was significantly higher than in the control group. This indicated that vascular stenosis was closely related to cognition, which was consistent with a previous report [15]. Chronic low perfusion is widely accepted as a potential mechanism underlying the change in cognition of patients with internal carotid artery stenosis [5]: the long-term low brain perfusion reduces the blood flow in the brain, chronic cerebral ischemia may cause WMH, and white matter ischemia may cause damage to cognition and memory [16,17]. This study also revealed that the areas besides the ventricle (antecornu, postcornu, and para-lateral ventricle) and the deep brain areas (supplied by the anterior cerebral artery, middle cerebral artery, and PCA) were more likely to develop low perfusion. We speculate that this might be related to the vascular distribution in the brain: The deep and superficial perforating arteries meet in a junctional zone, where watershed ischemia or infarct can occur [18], periventricular regions areas of the brain that are fed by distal pial end arterioles that form an arterial border zone, and thus is susceptible to the damage induced by systemic or focal reduction in blood flow [19].
Correlation of low brain perfusion with WMH and cognition impairment
Some studies have shown that WMH is closely related to small vessel diseases of the brain [20]. Recent studies indicate major artery stenosis may promote the development of WMH [5]. In this study, results indicated that the antecornu, postcornu, para-lateral ventricle, areas supplied by the anterior and middle cerebral arteries, subcortical area supplied by middle cerebral artery, and area supplied by PCA in PCAS patients were more likely to develop WMH. There is no consensus on the pathogenesis of WMH, and chronic low brain perfusion theory [21], brain blood barrier impairment theory [22], inflammatory injury theory [23], and age related white matter lesion theory [24] have been proposed for the pathogenesis of WMH. The above findings suggest that the antecornu, postcornu, para-lateral ventricle, areas supplied by the anterior and middle cerebral arteries, subcortical area supplied by middle cerebral artery, and area supplied by PCA had reduced perfusion are closely related to low brain perfusion. In addition, this study indicated the change in CBF of the area supplied by the PCA was closely related to the severity of WMH at the area supplied by the PCA and cognition impairment. This suggests that cognition impairment is associated with low brain perfusion and the subsequent development of WMH. The Papez circuit begins with the hippocampus and projects back to the hippocampus via the fornix, mamillary bodies, and posterior cingulate cortex. It has been confirmed that the Papez circuit is closely related to memory [25]. Thus, we speculate that the PCAS-related cognition impairment might be related to injury to the Papez circuit. This was a cross-sectional study, and causal relationships should be further validated in more longitudinal studies.
Correlation of low brain perfusion with brain atrophy and cognition impairment
Studies had indicated that carotid artery stenosis may cause extensive cortical and subcortical atrophy [26]. Kitanit et al. [27] conducted a longitudinal study on ischemic vascular diseases, and found that general brain atrophy was positively related to the reduced brain blood flow. This study showed that the incidence of atrophy at the areas supplied by the middle cerebral artery and PCA, posterior cingulate, and hippocampus in PCAS patients was significantly higher than in the control group. Further correlation analysis showed the CBF at the area supplied by the PCA was closely related to the severity of atrophy at these areas. On the basis of vascular distribution, blood supply area of posterior cerebral artery, posterior cingulated and hippocampus were mainly dependent on the PCA. Thus, the occurrence of brain atrophy was closely related to low brain perfusion: (1) The reduced brain perfusion might cause ischemia/hypoxia in bilateral cortexes, which triggered microcirculation disorder and deteriorates neurodegeneration [28]; (2) White matter degeneration may cause cortical atrophy: the damage to the connection between white matter fibers may cause secondary cortical injury [29]; (3) Hypertension may promote the development of brain atrophy and long-term hypertension may thicken the intima of arterioles in the brain, which reduces brain blood flow and brain perfusion, resulting in diffuse demyelination [30]. In addition, there is evidence showing that the occurrence of cognition impairment might be a consequence of accumulative WMH and brain atrophy [31]. This suggests that the cognition impairment results from the action of multiple risk factors, and more longitudinal studies were needed to elucidate this issue.
This study investigated the brain perfusion, WMH, and brain atrophy in PCAS patients on the basis of the correlation between PCAS and cognition impairment. Correlation analysis showed that change in brain perfusion at the area supplied by the PCA was significantly related to the severity of WMH and brain atrophy at different sites and cognition decline. These findings indicate that intracranial artery stenosis plays important roles in the occurrence and development of cognition impairment. Thus, we speculate that PCAS causes reduction in focal brain perfusion, which leads to damage to white matter, and long-term ischemia/hypoxia increases the risk for brain atrophy, resulting in the occurrence of cognition impairment.
To the best of our knowledge, this study is the first to investigate the relationship of PCAS with brain perfusion, WMH, brain atrophy, and cognition. Different from previous studies analysis in this study was done at different layers, and the roles of vascular risk factors, imaging findings, and cognition assessment were independently investigated, which is helpful for the identification of lesions at specific layers and sites as well as the severity of lesions. Findings of this study will benefit future clinical diagnosis and treatment of cognition impairment. However, there were still limitations in this study: (1) This was a cross-sectional study with small sample size, and results were preliminary, and more longitudinal studies with large sample size are needed to confirm these findings; (2) White matter lesions and brain atrophy were assessed with a visual assessment scale, which has several limitations (such as being insensitive to small lesions); (3) In the PCAS group, patients with ≥50% stenosis were included, and there were no patients with mild stenosis (<30% stenosis) and More studies are needed to investigate the imaging findings and clinical behaviors of patients with different severity of PCAS; (4) Collateral circulation was not evaluated in this study, and we could not determine the influence of collateral circulation opening on the brain perfusion. Thus, future studies with larger sample sizes are needed to further investigate the influence of collateral circulation, which will make these findings more convincing.
Conclusions
Old age, hypertension, and diabetes mellitus may be risk factors of PCAS. PCAS patients are more likely to develop cognition impairment. PCAS patients have focal reduction in brain perfusion, and cognition decline is closely related to the reduced perfusion at the area supplied by the PCA. PCAS patients have a higher incidence of WMH, and the change in brain perfusion at the area supplied by the PCA is closely associated with the severity of WMH at the area supplied by the PCA. The incidence of brain atrophy in PCAS patients increases significantly, and the change in brain perfusion at the area supplied by the PCA is closely associated with the severity of brain atrophy.
Footnotes
Source of support: This work was supported in part by the National Science Foundation of China (No. 81671307) and the Pudong New Area Commission of Health and Family Planning PW-2015A-27 and Priority of Shanghai key discipline of medicine(2017ZZ02020)
References
- 1.Huang HW, Guo MH, Lin R, et al. Prevalence and risk factors of middle cerebral artery stenosis in asymptomatic residents in Rongqi County, Guangdong. Cerebrovasc Dis. 2007;24(1):111–15. doi: 10.1159/000103125. [DOI] [PubMed] [Google Scholar]
- 2.Kocer A. Cognitive problems related to vertebrobasilar circulation. Turk J Med Sci. 2015;45(5):993–97. doi: 10.3906/sag-1403-100. [DOI] [PubMed] [Google Scholar]
- 3.Landgraff NC, Whitney SL, Rubinstein EN, Yonas H. Cognitive and physical performance in patients with asymptomatic carotid artery disease. J Neurol. 2010;257(6):982–91. doi: 10.1007/s00415-009-5449-z. [DOI] [PubMed] [Google Scholar]
- 4.Madl C, Grimm G, Kramer L, et al. Cognitive brain function in non-demented patients with low-grade and high-grade carotid artery stenosis. Eur J Clin Invest. 1994;24(8):559–64. doi: 10.1111/j.1365-2362.1994.tb01107.x. [DOI] [PubMed] [Google Scholar]
- 5.Kurata M, Okura T, Watanabe S, Higaki J, et al. Association between carotid hemodynamics and asymptomatic white and gray matter lesions in patients with essential hypertension. Hypertens Res. 2005;28(10):797–803. doi: 10.1291/hypres.28.797. [DOI] [PubMed] [Google Scholar]
- 6.Patankar T, Widjaja E, Chant H, et al. Relationship of deep white matter hyperintensities and cerebral blood flow in severe carotid artery stenosis. Eur J Neurol. 2006;13(1):10–16. doi: 10.1111/j.1468-1331.2006.01115.x. [DOI] [PubMed] [Google Scholar]
- 7.Bokkers RP, van der Worp HB, Mali WP, Hendrikse J. Noninvasive MR imaging of cerebral perfusion in patients with a carotid artery stenosis. Neurology. 2009;73(11):869–75. doi: 10.1212/WNL.0b013e3181b7840c. [DOI] [PubMed] [Google Scholar]
- 8.Poels MM, Ikram MA, Vernooij MW, et al. Total cerebral blood flow in relation to cognitive function: the Rotterdam Scan Study. J Cereb Blood Flow Metab. 2008;28(10):1652–55. doi: 10.1038/jcbfm.2008.62. [DOI] [PubMed] [Google Scholar]
- 9.Samuels OB, Joseph GJ, Lynn MJ, et al. A standardized method for measuring intracranial arterial stenosis. Am J Neuroradiol. 2000;21(4):643–46. [PMC free article] [PubMed] [Google Scholar]
- 10.Scheltens P, Barkhof F, Leys D, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. 1993;114(1):7–12. doi: 10.1016/0022-510x(93)90041-v. [DOI] [PubMed] [Google Scholar]
- 11.Del Brutto OH, Mera RM, Zambrano M, et al. Global cortical atrophy (GCA) associates with worse performance in the Montreal Cognitive Assessment (MoCA). A population-based study in community-dwelling elders living in rural Ecuador. Arch Gerontol Geriatr. 2015;60(1):206–9. doi: 10.1016/j.archger.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Y, Wang YJ, Yan JC, et al. Effects of carotid artery stenting on cognitive function in patients with mild cognitive impairment and carotid stenosis. Exp Ther Med. 2013;5(4):1019–24. doi: 10.3892/etm.2013.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bae HJ, Lee J, Park JM, et al. Risk factors of intracranial cerebral atherosclerosis among asymptomatics. Cerebrovasc Dis. 2007;24(4):355–60. doi: 10.1159/000106982. [DOI] [PubMed] [Google Scholar]
- 14.Du YL, Chen SX, Hu YR, et al. [Prevalence and risk factors of asymptomatic intracranial vascular stenosis in patients with essential hypertension]. Zhonghua Xin Xue Guan Bing Za Zhi. 2007;35(10):893–96. [in Chinese] [PubMed] [Google Scholar]
- 15.Silvestrini M, Paolino I, Vernieri F, et al. Cerebral hemodynamics and cognitive performance in patients with asymptomatic carotid stenosis. Neurology. 2009;72(12):1062–68. doi: 10.1212/01.wnl.0000345015.35520.52. [DOI] [PubMed] [Google Scholar]
- 16.Lee YH, Yeh SJ. Correlation of common carotid artery intima media thickness, intracranial arterial stenosis and post-stroke cognitive impairment. Acta Neurol Taiwan. 2007;16(4):207–13. [PubMed] [Google Scholar]
- 17.Shi D, Li-yuan H, Ai-hong Z, et al. The effect of cerebral vascular stenosis or occlusion on cognitive function. Chin J Contemp Neurol Neurosurg. 2008;(06):514–19. [Google Scholar]
- 18.Gandolfo C, Del Sette M, Finocchi C, et al. Internal borderzone infarction in patients with ischemic stroke. Cerebrovasc Dis. 1998;8(5):255–58. doi: 10.1159/000015862. [DOI] [PubMed] [Google Scholar]
- 19.Wallin A, Sjögren M, Edman A, et al. Symptoms, vascular risk factors and blood-brain barrier function in relation to CT white-matter changes in dementia. Eur Neurol. 2000;44(4):229–35. doi: 10.1159/000008242. [DOI] [PubMed] [Google Scholar]
- 20.Erkinjuntti T. Subcortical vascular dementia. Cerebrovasc Dis. 2002;13(Suppl 2):58–60. doi: 10.1159/000049152. [DOI] [PubMed] [Google Scholar]
- 21.Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37(6):1391–98. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 22.Kimura M, Tanaka A, Yoshinaga S. Significance of periventricular hemodynamics in normal pressure hydrocephalus. Neurosurgery. 1992;30(5):701–4. discussion 704–5. [PubMed] [Google Scholar]
- 23.Black S, Gao G, Bilbao J. Understanding white matter disease: Imaging-pathological correlations in vascular cognitive impairment. Stroke. 2009;40(3 Suppl):S48–52. doi: 10.1161/STROKEAHA.108.537704. [DOI] [PubMed] [Google Scholar]
- 24.Sala S, Agosta F, Pagani E, et al. Microstructural changes and atrophy in brain white matter tracts with aging. Neurobiol Aging. 2012;33(3):488–98.e2. doi: 10.1016/j.neurobiolaging.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 25.Aggleton JP, Pralus A, Nelson AJ, Hornberger M. Thalamic pathology and memory loss in early Alzheimer’s disease: moving the focus from the medial temporal lobe to Papez circuit. Brain. 2016;139(7):1877–90. doi: 10.1093/brain/aww083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muller M, van der Graaf Y, Algra A, et al. Carotid atherosclerosis and progression of brain atrophy: the SMART-MR study. Ann Neurol. 2011;70(2):237–44. doi: 10.1002/ana.22392. [DOI] [PubMed] [Google Scholar]
- 27.Kitani M, Kobayashi S, Yamaguchi S, Okada K. Cerebral atrophy precedes the change in cerebral blood flow in patients with ischemic cerebrovascular disease: a short-term follow-up study. Gerontology. 1992;38(1–2):1–8. doi: 10.1159/000213300. [DOI] [PubMed] [Google Scholar]
- 28.Vernieri F, Pasqualetti P, Matteis M, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke. 2001;32(7):1552–58. doi: 10.1161/01.str.32.7.1552. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt R, Ropele S, Enzinger C, et al. White matter lesion progression, brain atrophy, and cognitive decline: The Austrian stroke prevention study. Ann Neurol. 2005;58(4):610–16. doi: 10.1002/ana.20630. [DOI] [PubMed] [Google Scholar]
- 30.Yang L, Ning XJ, Cheng Y. Factors contributing to the mild cognitive impairment in healthy elderly persons with general cerebral atrophy. Chin J Geria Heart Brain Vess Dis. 2002;(04):239–42. [Google Scholar]
- 31.Godin O, Tzourio C, Rouaud O, et al. Joint effect of white matter lesions and hippocampal volumes on severity of cognitive decline: the 3C-Dijon MRI study. J Alzheimers Dis. 2010;20(2):453–63. doi: 10.3233/JAD-2010-1389. [DOI] [PubMed] [Google Scholar]