Abstract
Background:
With the exception of the presence of the FIP1L1-PDGFRA fusion gene, little is known about predictors of imatinib response in clinically-defined hypereosinophilic syndrome (HES).
Methods:
Subjects with FIP1L1-PDGFRA-myeloid neoplasm (FP; n =12), PDGFRA-negative HES with ≥4 criteria suggestive of a myeloid neoplasm (MHES; n =10), or steroid-refractory PDGFRA-negative HES with <4 myeloid criteria (SR; n = 5) were enrolled in a prospective study of imatinib therapy (NCT00044304: registered at clinicaltrials.gov). The primary outcome was an eosinophil count <1.5 × 109/L at one month and improvement of clinical symptoms. Clinical, molecular, and bone marrow responses to imatinib were assessed. A retrospective cohort of 18 subjects with clinically-defined HES who received imatinib (300–400 mg daily ≥ 1 month) were classified according to the criteria used in the prospective study.
Results:
Overall, imatinib response rates were 100% in the FP group (n = 16), 54% in the MHES group (n = 13) and 0% in the SR group (n = 16). The presence of ≥ 4 myeloid features was the sole predictor of response. After ≥ 18 months in complete remission, imatinib was tapered and discontinued in 8 FP and 1 MHES subjects. Seven subjects (6 FP, 1 MHES) remain in remission off therapy for a median of 29 months (range 14–36).
Conclusions:
Clinical features of MHES predict imatinib response in PDGFRA-negative HES.
Keywords: eosinophilia, hypereosinophilic syndrome, imatinib, myeloid neoplasm, PDGFRA-negative
Imatinib, a tyrosine kinase inhibitor that selectively inhibits bcr-abl, c-kit, and the platelet-derived growth factor receptor (PDGFR) tyrosine kinases, was FDA-approved for use in Ph+ CML in 2001 and for hypereosinophilic syndromes, including PDGFRα (PDGFRA)-positive myeloid neoplasms (MN), in 2006. Although imatinib responsiveness is nearly universal in patients with PDGFRA-associated MN (1–8), reports in PDGFRA-negative HES have been mixed, with response rates ranging from 9 to 60% depending on the series (1, 6–9). Whereas varied dosing regimens and heterogeneous patient populations likely account for some of the variability in initial response to imatinib in PDGFRA-negative HES, predictors of response have not been examined systematically in any of the studies published to date.
To address this issue, a prospective study of imatinib in subjects with PDGFRA-associated MN and HES with features suggestive of a myeloid neoplasm was expanded to include subjects with steroid-resistant HES without these features. Baseline clinical and laboratory characteristics were compared between imatinib responders and nonresponders in the prospective study and in a retrospective analysis of subjects enrolled on a separate natural history protocol. Long-term outcomes in PDGFRA-associated and PDGFRA-negative imatinib responders in the prospective cohort were also assessed.
Patients and methods
Study participants were evaluated on Institutional Review Board-approved research protocols to study eosinophilia (NCT00001406) or the safety and efficacy of imatinib treatment for HES (NCT00044304) after providing informed consent. HES was defined as absolute eosinophil count (AEC) >1.5 × 109/1 on two occasions, no secondary etiology for the eosinophilia, and evidence of end organ damage (histologic evidence of tissue infiltration by eosinophils and/or objective evidence of organ involvement temporally associated with eosinophilia not attributable to another cause).
Prospective study
Between August 24, 2006, and September 30, 2013, subjects with a clinical diagnosis of HES were recruited for a prospective study of imatinib mesylate if they were negative for BCR-ABL and met one of three criteria: (1) documentation of the FIP1L1-PDGFRA fusion gene in peripheral blood by RT-PCR (FP), (2) FIP1L1-PDGFRA-negative but ≥4 of the following laboratory criteria suggestive of a FIP1L1-PDGFRA-associated myeloid neoplasm (MHES): dysplastic eosinophils on peripheral smear (abnormal nuclear lobation (hypo and hyper), uneven granulation, hypogranulation, agranulation), serum B12 level ≥1000 pg/ml, serum tryptase level ≥12 ng/mL, anemia and/or thrombocytopenia, bone marrow cellularity >80% with left shift in maturation, dysplastic (spindle-shaped) mast cells on bone marrow biopsy, evidence of reticulin fibrosis ≥2+ on bone marrow biopsy, dysplastic megakaryocytes on bone marrow biopsy, or (3) FIP1L1-PDGFRA-negative with <4 of the above criteria but steroid-refractory (SR). Steroid-refractory was defined for the purposes of the study as persistent eosinophilia and symptoms despite prednisone therapy (≥10 mg daily). Briefly, eligible subjects were treated with imatinib 300–400 mg daily. The primary efficacy outcome was AEC <1.5 × 109/1 after 1 month of therapy and clinical improvement. Clinical, bone marrow and molecular remission (FP subjects) were assessed after 4–8 weeks, and longitudinal clinical, hemato-logic, and molecular assessments were performed in imatinib responders every 3 months thereafter with tapering occurring according to a standardized schedule (Online methods).
Retrospective study
A retrospective chart review of 302 eosinophilic subjects seen at the NIH between November 3, 1999, and September 30, 2013, identified 39 patients with HES who were not enrolled on the prospective study, but had received imatinib ≥300 mg daily for at least one month. The subjects were classified into FP, SR, and MHES groups, and imatinib response was assessed using the same criteria as in the prospective analysis.
Assessment of bone marrow pathology
Bone marrow biopsies were reviewed by a hematopathologist and a hematologist in the Clinical Pathology Department of the NIH Clinical Center.
Cytogenetic and molecular analysis
Cytogenetic studies were performed prior to imatinib therapy in 19 of the 42 patients in the NCI laboratory using standard methods (10). (see Online methods for description). The remaining 23 studies were performed in various commercial laboratories and the results reviewed at NIH.
Statistical analysis
Statistical analyses of demographic and clinical predictors of response were performed using the Mann-Whitney U-test and the Fisher’s exact test for assessment of response at one month. Model fitting was performed with logistic regression using a likelihood ratio test. Missing covariates are assumed missing completely at random. Holm’s correction was used for multiple comparisons in the univariate model. Multiple correction for bivariate models used a resampling based multiple correction (11). The central Fisher’s exact inferences used the exact 2×2 R package (12). All analyses were performed using Prism V6.0 (GraphPad Software, LaJolla, CA, USA) or R version 3.1.2.
Results
Study population
Of the 27 subjects enrolled on the prospective imatinib study, 12 were FIP1L1-PDGFRA-associated (FP), ten had ≥4 features suggestive of a myeloid neoplasm but were FIP1L1-PDGFRA-negative (MHES), and five were corticosteroid-refractory (SR). A detailed description of the results for the first seven FP subjects has been published previously (5, 13). Eighteen subjects (four FP, three MHES, and 11 SR) met criteria for the retrospective study. Five subjects were excluded because the data available was insufficient for classification, and 16 subjects were excluded because they did not meet criteria for MHES and demonstrated a clinical and hematologic response to <20 mg prednisone (steroid-responsive). Baseline characteristics of the subjects are given in Table 1 and were similar between the retrospective and prospective groups (Table S1) with the exception of splenomegaly (11/25 vs 1/17, P = 0.01), median serum tryptase (14 vs 4.47; P = 0.018), and median B12 levels (1579 vs 781; P = 0.043), which were all significantly increased in the prospective cohort, consistent with the increased number of FP subjects in this group. No subjects in any of the groups were positive for BCR-ABL.
Table 1.
Comparison of demographic and clinical characteristics between clinical subgroups in the combined prospective and retrospective cohorts
| Group* | FP (n = 16) | MHES (n = 13) | SR (n = 16) |
|---|---|---|---|
| Demographics | |||
| Median age, years (range) | 39 (17–61) | 43 (3–84) | 43 (11–65) |
| Sex, M/F (%) | 15/1 (94/6) | 6/7 (41/59) | 6/11 (54/46) |
| Race | |||
| African American | 3 | 4 | 4 |
| Asian | 1 | 1 | 1 |
| White | 11 | 7 | 10 |
| Hispanic | 1 | 1 | 1 |
| Clinical manifestations | |||
| Cardiac | 4 | 4 | 3 |
| Splenomegaly | 7/14 | 4/13 | 1/15 |
| Laboratory parameters | |||
| Median AEC x 109/l* (n; range) | 7.707 (16; 4.342–27.555) | 6.42 (13; 1.57–150.48) | 4.747 (16; 1.29–70.29) |
| Median peak AEC × 109/l (n; range) | 12.117 (16; 4.830–120.0) | 13.226 (13; 3.76–151.0) | 8.95 (16; 2.856–82.0) |
| Serum tryptase, ng/mL (n; range) | 19.6 (12; 3.5–47.5) | 6.22 (12; 0.99–27) | 4.63 (16; 0.99–19.1) |
| Serum B12, pg/mL (n; range) | 2966 (14; 313–27529) | 1.28 (12; 372–5055) | 711 (16; 368–1316) |
| Serum IgE, pg/mL (n; range) | 12 (11; 1.5–5027) | 78 (11; 8–1854) | 223 (16; 3.9–42874) |
| Serum LDH, U/l (n; range) | 279 (11; 123–663) | 290 (11; 100–587) | 235 (15; 126–600) |
| Clonal TCR rearrangement | 2/16 | 1/13 | 6/16 |
| Aberrant T lymphocyte population by flow | 0/16 | 1/13** | 4/16*** |
| Cytogenetic abnormality | 1/14 | 3/13 | 1/14 |
FP—FIP1L1-PDGFRA-associated myeloid neoplasm, MHES—FIP1L1-PDGFRA-negative, myeloid HES, SR—FIP1L1-PDGFRA-negative, nonmyeloid, corticosteroid nonresponder. Abbreviations: AEC, absolute eosinophil count; TCR, T-cell receptor; LDH, lactate dehydrogenase; IgE, immunoglobulin E.
Large granular lymphocytes.
CD3dimCD4+.
Initial response to imatinib
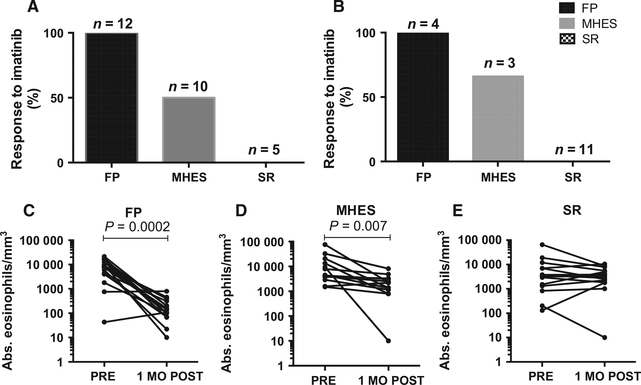
Among the MHES subjects, ≥50% of the subjects in each cohort (5/10 in the prospective cohort and 2/3 in the retro-spective cohort) met the primary study outcome of AEC<1.5 × 109/1 after 1 month of therapy (Fig. 1A and B), although the eosinophil response was typically slower than in the FP cohort (median 5 weeks (MHES) vs 1 week (FP); P = 0.005). Serum tryptase and B12 levels also decreased significantly in the FP and MHES subjects in the prospective cohort in response to imatinib (Fig. S1).
Figure 1.
Imatinib response rates differ between corticosteroid nonresponder (SR), PDGFRA-associated (FP), and myeloid (MHES) groups. Response rates are shown separately for the prospective (A) and retrospective (B) cohorts. Peripheral eosinophil counts pre- and 1 month post-imatinib are shown for the prospective and retrospective cohorts combined (C-E). FP—FIP1L1-PDGFRA-associated myeloid neoplasm, MO—month, MHES—PDGFRA-negative, myeloid HES, SR —PDGFRA-negative, nonmyeloid, corticosteroid nonresponder.
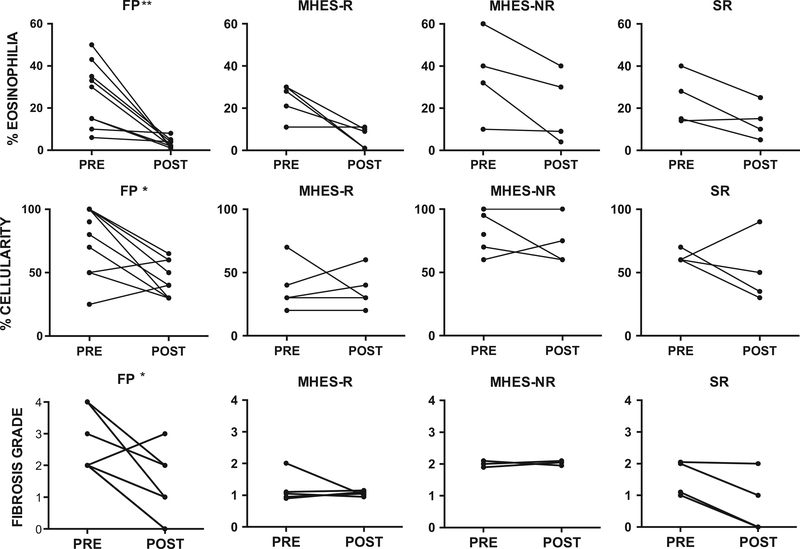
Nine subjects in the prospective study, five of whom met the primary endpoint, had bone marrow biopsies performed before and at 4–8 weeks postinitiation of treatment. There was no significant change in bone marrow cellularity in MHES subjects regardless of imatinib response, although responders had significantly lower bone marrow cellularity prior to therapy than nonresponders (median 30% vs 80%, P = 0.02), which may have obscured any treatment effect. Bone marrow eosinophilia decreased in all seven subjects who had bone marrow eosinophilia >20% prior to imatinib treatment, but remained >20% in two of three subjects who did not meet the primary endpoint as compared to none of four who did (Fig. 2). Mast cell increases (2–6%) were present in eight of nine subjects prior to treatment, with >25% spindle-shaped morphology noted in five subjects and aggregates in one subject. At follow-up, normalization of mast cells had occurred in all but three subjects. Reticulin fibrosis was 1–2+ in all MHES subjects prior to therapy and remained unchanged.
Figure 2.
Bone marrow cellularity, eosinophilia, and fibrosis were significantly improved in FP subjects but not in MHES or SR subjects in the prospective cohort. Bone marrow percent cellularity, percent eosinophilia, and fibrosis grade before and one month after initiation of imatinib therapy are shown for subjects with PDGFRA-associated disease (FP) (n = 11), PDGFRA-negative MHES who were imatinib-responsive (MHES-R) (n = 5) or imatinib-unresponsive (MHES-NR) (n = 3), and PDGFRA-negative, corticosteroid-resistant HES without myeloid features (SR) (n = 4). *P < 0.05 or **P < 0.01 as compared to pre, Wilcoxon matched pairs signed rank test.
None of the SR subjects in either cohort met the primary endpoint of AEC less than 1.5 × 109/1 and clinical improvement with imatinib (Table 2 and Fig. 2). Consequently, repeat bone marrow examinations were performed in only four subjects in the prospective cohort during imatinib treatment. Imatinib was discontinued in nonresponders after 4 weeks in four subjects in the prospective cohort. One subject was on high-dose corticosteroids at entry onto the protocol and at the time of the 1 month evaluation with continued disease manifestations despite a low AEC. Subsequent tapering of background prednisone resulted in rebound eosinophilia (AEC 3.4 × 109/1) and imatinib was stopped. One subject in the SR group had significant toxicity from imatinib with no clinical improvement and rising AEC (1.1 × 109/1) and imatinib was stopped due to toxicity. Subjects in the retrospective cohort for whom the data was available (10/11) stopped imatinib after 4–21 weeks (median 8 weeks).
Table 2.
Bone marrow response to imatinib
| Group* | N | Median % cellularity (range) |
Median % eosinophils (range) |
Median % mast cells (range) |
|||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | ||
| FP | 9 | 90 (25–100) | 40 (30–65)** | 30 (6–50) | 2 ( 1 –8)† | 4 (2–5) | 0 (0–3)** |
| MHES-R | 5 | 35 (20–70) | 30 (20–60) | 28 (11–30) | 9 (1–111) | 5 (2–6) | 1 (0–2) |
| MHES-NR | 4 | 83 (60–100) | 68 (60–100) | 36 (10–60) | 20 (4–40) | 4 (0–5) | 2 (1–7.5) |
| SR | 4 | 60 (60–70) | 43 (30–90) | 22 (14–40) | 13 (5–25) | 1 (1–2) | 0 (0–1) |
R—imatinib responder; NR—imatinib nonresponder; FP—FIP1L1-PDGFRA-associated myeloid neoplasm; MHES—FIP1L1-PDGFRA-negative, myeloid HES; SR—FIP1L1-PDGFRA-negative, nonmyeloid corticosteroid nonresponder.
P ≤ 0.02, Wilcoxon matched pairs signed rank test comparing pre and post values.
P < 0.001, Wilcoxon matched pairs signed rank test comparing pre and post values.
As expected, all 16 FP subjects demonstrated a rapid and dramatic response to imatinib with normalization of the AEC to <500 × 109/1 after 1 month of therapy (Fig. 1A and B) and improvement in symptoms. Complete molecular remission was observed in 11 of 12 prospective subjects with a median time to remission of 5 months (range 1–21 months). One subject died prior to repeat molecular testing. In the nine subjects in the prospective cohort for whom bone marrow data were available prior to and after 1–5 months of imatinib therapy, dramatic improvement in bone marrow pathology (cellularity, eosinophilia, decreased mast cells, and reticulin fibrosis) was also observed (Table 2 and Fig. 2). Median eosinophilia decreased from 30% to 2% (P < 0.001). Increased mast cells (2–5%) were present in all nine subjects prior to treatment, with >25% spindle-shaped morphology noted in seven subjects and aggregates in three subjects. At follow-up, only one subject had >1% mast cells (P = 0.02) and no subject had abnormal mast cell morphology or aggregates. Reticulin fibrosis was increased (2–4+) in all nine subjects prior to initiation of imatinib and decreased by at least one grade in response to imatinib in all but one subject (P < 0.02).
Predictors of response to imatinib in PDGFRA-negative HES
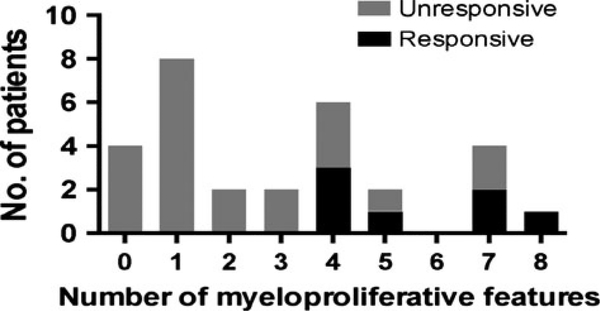
Evaluation of all PDGFRA-negative subjects in both the retrospective and prospective cohorts (n = 29) revealed seven covariates of 19 tested that significantly predicted imatinib response. Only two covariates (number of criteria suggestive of a myeloid neoplasm and dysplastic eosinophils) were significant after multiple testing (covariates tested are listed in Supplemental Table 2). When PDGFRA-negative subjects were dichotomized by ≥ or <4 myeloid criteria and the presence or absence of dysplastic eosinophils, those subjects with ≥4 myeloid criteria were more likely to respond (two-sided P = 0.002 (≥4 myeloid criteria), P = 0.004 (dysplastic eosinophils); central Fisher’s exact test). Because all the PDGFRA-negative responders had ≥4 myeloid criteria and dysplastic eosinophils, the sample odds ratio was infinite, but the lower one-sided 97.5% confidence limits show that the lower limit of the odds ratios are 2.7 for ≥4 myeloid criteria and 2.3 for dysplastic eosinophils. Subjects in the SR cohort had <4 myeloid criteria by definition, and none responded to imatinib. Increasing the number of myeloid criteria above four did not increase the likelihood of response, which remained stable at 50% (Fig. 3). The predictive value of either myeloid criteria or the presence of dysplastic eosinophils could not be significantly improved by correcting for additional variables.
Figure 3.
Clinical features suggestive of a myeloid neoplasm are associated with imatinib responsiveness in PDGFRA-negative patients. The response to imatinib (responders—black bars, nonresponder—gray bars) is shown as a function of the number of features suggestive of a myeloid neoplasm in the PDGFRA-negative subjects in the combined prospective and retrospective cohorts.
Cytogenetic abnormalities were detected in bone marrow aspirates of only five of the 41 subjects tested (Table 1). One FP patient had a t(6;8)(q21;p12) involving the FGFR1 gene on 8p in 13 of 20 metaphase cells analyzed, and one SR patient had a del(5)(q32q33) in four of 19 cells analyzed. The remaining three abnormalities were detected in MHES patients including one female patient with loss of one X as the sole abnormality in four of 20 metaphases, one 3-year-old child with a t(5;13)(q33;q12) in three of six cells analyzed, and a 29-year-old man who had normal marrow cytogenetics at presentation but returned 1 month after initiation of imatinib therapy with pre-B cell ALL with three closely related, pseudodiploid abnormal cell lines including the known, recurring t(5;14)(q31;q32) in all of the cells. PDGFRB fusions were not detected by FISH in any of the five subjects tested, including one subject with bone marrow features consistent with CMML. Although two of the patients with abnormal cytogenetics had breaks in 5q33 where PDGFRB is located, neither demonstrated a complete response to imatinib. JAK2 mutations (V617F in three subjects and an exon 13 mutation in one subject) were identified in four of ten subjects, all of whom were in the MHES group and presented with classic manifestations of HES.
Longitudinal follow-up of imatinib-responsive subjects in the prospective cohort
Of the five complete responders in the MHES cohort, one subject remains in complete clinical and hematologic remission on continuous imatinib therapy (current dose, 100 mg; duration, 27 months). Imatinib was tapered and discontinued after 75 months in one subject, who remains in clinical and hematologic remission off imatinib for 34 months. Drug was discontinued in a second subject 36 months ago (after 46 months of continuous therapy) in anticipation of renal transplant. She remains stable on immunosuppressive therapy off imatinib. The final two subjects who demonstrated complete response at 1 month developed recurrent symptoms and eosinophilia despite continuous imatinib therapy. The JAK2 V617F mutation-positive subject who relapsed at 10 months was treated with nilotinib (400 mg bid) with only a transient, partial response. Nilotinib was discontinued after 5 weeks, and a partial response was achieved with interferon-α and hydroxyurea. The second subject relapsed after 8 months of imatinib therapy and ultimately died of progressive disease.
Of the 12 FP subjects in the prospective cohort, one subject is in complete remission on continuous imatinib therapy for 41 months. Two subjects died of complications of endomyocardial fibrosis despite complete hematologic and molecular remission, one after 27 days and the second after 8.5 years of continuous imatinib therapy. The remaining nine subjects underwent at least one interruption of therapy. As previously reported (13), imatinib was discontinued in four subjects between 2004 and 2007 after a median of 28 months in molecular remission (range 19–32). Molecular relapse occurred in all four subjects within 5 months of drug discontinuation. One additional subject in the dose descalation trial relapsed while on 100 mg of imatinib.
A second imatinib interruption was initiated in 2010 after reports demonstrating durable remission after stopping imatinib in patients with CML (14). Four of the five subjects who had relapsed previously with dose tapering (n = 1) or discontinuation (n = 3) (13) underwent imatinib interruption after a median of 92 months of continuous imatinib therapy (range 89–97 months). All remain in hematologic and molecular remission 18–34 months after imatinib discontinuation. Imatinib therapy was interrupted in four additional subjects. Two subjects, who had received imatinib for 77 and 140 months, remain in molecular and clinical remission for 34 and 13 months, respectively. One subject, who had been on imatinib for 19 months at the time of discontinuation, relapsed after two months off therapy. An additional subject discontinued imatinib after 18 months of therapy when he was temporarily lost to follow up. Molecular testing performed 6 months later was positive for FIP1L1-PDGFRA and imatinib therapy was restarted.
Discussion
A myeloproliferative variant of HES was described over twenty years ago (15). Since that time, the discovery of the imatinib-responsive FIP1L1-PDGFRA fusion gene in 10–20% of patients with a provisional diagnosis of HES has simplified the treatment algorithm for these individuals (16).
The remaining patients are a heterogeneous group, comprised of patients with primary myeloid neoplasms, lymphocyte-driven eosinophilic disorders, and idiopathic HES (17). Although reported response rates to imatinib have been highly variable in these PDGFRA-negative patients, predictors of response have not been explored to date. Given the high cost of tyrosine kinase inhibitors, and the potential for disease progression if an ineffective therapy is selected, this remains an important question.
In the present study, clinical and laboratory parameters were assessed in relation to treatment response in subjects with PDGFRA-negative HES enrolled in a prospective trial of imatinib therapy. The same parameters were also assessed in a retrospective cohort of subjects with PDGFRA-negative HES who received imatinib for >1 month as part of routine care and for whom adequate data were available. In both the prospective and retrospective cohorts, a strong predictor of imatinib response was the presence of a myeloid (MHES) phenotype (i.e., the presence of ≥4 features suggestive of FP, as defined in the prospective trial). In fact, none of the 16 subjects without these features responded to imatinib.
The most plausible explanation for the association between the presence of the MHES phenotype and imatinib responsiveness is that these criteria are sufficient to distinguish between subjects with primary (myeloid) HES and secondary (lymphocyte-driven) or idiopathic HES and that subjects with primary myeloid HES are more likely to have imatinib-sensitive mutations. In fact, the defining criteria for the MHES group in the present study were based on the characteristics of a subgroup of patients with imatinib-responsive HES who participated in a pilot study conducted prior to the discovery of the FIP1L1-PDGFRA fusion gene (18). Known imatinib-sensitive mutations other than FIP1L1-PDGFRA are uncommon causes of HES (19, 20). Although some of these rearrangements, including BCR/ABL and PDGFRB, can be identified by standard cytogenetic and/or FISH analysis, others, such as point mutations in PDGFRA (21), require sequence analysis. Surrogate markers of imatinib response are clearly needed.
Another important issue in the treatment of HES is duration of therapy. Despite persistence of leukemia as assessed by a highly sensitive patient-specific nested PCR assay (22), several studies have demonstrated stable complete molecular response after imatinib interruption in approximately 40% of patients with CML and undetectable minimal residual disease (UMRD) for a minimum of two years (23). Factors reported to be associated with a lower risk of relapse include prior interferon-alpha therapy, shorter time to UMRD, lower Sokal score, and longer duration of imatinib treatment (19). These data suggest that imatinib discontinuation may be safe and effective in FP patients in molecular remission for >24 months.
In the present trial, imatinib therapy was interrupted a total of 14 times in nine FP and two MHES subjects. Six FP subjects (including three who had failed a prior interruption of therapy) are currently in hematologic and molecular remission for > 1 year (range 13–34 months). The only apparent difference between the interruptions leading to UMRD > 1 year and those resulting in molecular relapse is the duration of continuous imatinib therapy prior to interruption (median of 92 months vs 22 months, P < 0.01, Mann-Whitney U-test). Although the duration of therapy was also prolonged (>4 years) in a recent report of sustained remission in two FP patients after imatinib discontinuation (24), the effect of duration of remission on relapse rate was not confirmed in a larger series, in which imatinib was dis-continued in 11 FP patients, of whom four remained in molecular remission off imatinib for 9–88 months (25).
Hypereosinophilic syndrome is a rare disease, and as such, the sample size of this study was small and the data are insufficient to exclude the possibility that a rare patient with HES without myeloid features will respond to imatinib. Nevertheless, our data suggest that an imatinib trial should be considered early in patients presenting clinical features consistent with MHES and that conversely, given the low likelihood of imatinib response and the potential for complications of eosinophilia during ineffective therapy trials, agents other than imatinib should be used as second-line therapy for steroid-refractory patients with HES that do not meet criteria for MHES. Finally, our data confirm recently published reports of durable molecular remission in some FP patients after imatinib discontinuation and suggest that durable clinical and hematologic remission can also be achieved in PDGFRA-negative patients with MHES.
Supplementary Material
Acknowledgments
The authors would like to thank the clinical providers who provided support and care for the patients including Cheryl Talar-Williams, Gyan Joshi for statistical support, and the subjects who donated time, blood, and tissue samples.
Funding
This research was supported by the Division of Intramural Research, NIAID, National Institutes of Health, Division of Intramural Research, NHLBI, National Institutes of Health; Division of Intramural Research, NCI, National Institutes of Health. This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institute of Health, under contract No. HHSN2612 00800001E. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was supported in part by the National Institute of Allergy and Infectious Diseases. Clinicaltrials.gov identifiers: NCT00001406 and NCT00044304.
Footnotes
Conflicts of interest
No conflicts of interest are reported for any of the authors.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Comparison of subject characteristics between the prospective and retrospective cohorts.
Table S2. Predictors used in univariate model.
Figure S1. Pre- and post imatinib laboratory values in the prospective cohort by clinical group.
References
- 1.Baccarani M, Cilloni D, Rondoni M, Ottaviani E, Messa F, Merante S et al. The efficacy of imatinib mesylate in patients with FIP1L1-PDGFRalpha-positive hypere-osinophilic syndrome. Results of a multicenter prospective study. Haematologica 2007;92:1173–1179. [DOI] [PubMed] [Google Scholar]
- 2.Cools J, DeAngelo DJ, Gotlib J, Stover EH, Legare RD, Cortes J et al. A tyrosine kinase created by fusion of the PDGFRA and FIP1L1 genes as a therapeutic target of imatinib in idiopathic hypereosinophilic syndrome. N Engl J Med 2003;348:1201–1214. [DOI] [PubMed] [Google Scholar]
- 3.Helbig G, Hus M, Halasz M, Dudzinski M, Wieclawek A, Stachowicz M et al. Imatinib mesylate may induce long-term clinical response in FIP1L1-PDGFRalpha-negative hypereosinophilic syndrome. Med Oncol 2012;29:1073–1076. [DOI] [PubMed] [Google Scholar]
- 4.Helbig G, Moskwa A, Hus M, Piszcz J, Swiderska A, Urbanowicz A et al. Durable remission after treatment with very low doses of imatinib for FIP1L1-PDGFRalpha-positive chronic eosinophilic leukaemia. Cancer Chemother Pharmacol 2011;67:967–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klion AD, Robyn J, Akin C, Noel P, Brown M, Law M et al. Molecular remission and reversal of myelofibrosis in response to imatinib mesylate treatment in patients with the myeloproliferative variant of hypere-osinophilic syndrome. Blood 2004; 103:473–478. [DOI] [PubMed] [Google Scholar]
- 6.Metzgeroth G, Walz C, Erben P, Popp H, Schmitt-Graeff A, Haferlach C et al. Safety and efficacy of imatinib in chronic eosinophilic leukaemia and hypereosinophilic syndrome: a phase-II study. Br J Haematol 2008;143:707–715. [DOI] [PubMed] [Google Scholar]
- 7.Ogbogu PU, Bochner BS, Butterfield JH, Gleich GJ, Huss-Marp J, Kahn JE et al. Hypereosinophilic syndrome: a multicenter, retrospective analysis of clinical characteristics and response to therapy. J Allergy Clin Immunol 2009;124:1319–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pardanani A, Brockman SR, Paternoster SF, Flynn HC, Ketterling RP, Lasho TL et al. FIP1L1-PDGFRA fusion: prevalence and clinicopathologic correlates in 89 consecutive patients with moderate to severe eosinophilia. Blood 2004;104:3038–3045. [DOI] [PubMed] [Google Scholar]
- 9.Jain N, Cortes J, Quintas-Cardama A, Manshouri T, Luthra R, Garcia-Manero G et al. Imatinib has limited therapeutic activity for hypereosinophilic syndrome patients with unknown or negative PDGFRalpha mutation status. Leuk Res 2009;33:837–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Standing Committee on Human Cytogenetic Nomenclature ISCN 2013: an International System for Human Cytogenetic Nomenclature (2013). In: Shaffer LG, McGowan-Jordan J, Schmid M (editors). Basel: Karger, 2013. [Google Scholar]
- 11.Westfall PH, Young SS. Resampling-Based Multiple Testing: Examples and Methods for P-Value Adjustment. New York: Wiley, 1993. [Google Scholar]
- 12.Fay MP. Confidence intervals that match Fisher’s exact or Blaker’s exact tests. Bio-statistics 2010;11:373–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klion AD, Robyn J, Maric I, Fu W, Schmid L, Lemery S et al. Relapse following discontinuation of imatinib mesylate therapy for FIP1L1/PDGFRA-positive chronic eosinophilic leukemia: implications for optimal dosing. Blood 2007;110:3552–3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rousselot P, Huguet F, Rea D, Legros L, Cayuela JM, Maarek O et al. Imatinib mesylate discontinuation in patients with chronic myelogenous leukemia in complete molecular remission for more than 2 years. Blood 2007;109:58–60. [DOI] [PubMed] [Google Scholar]
- 15.Weller PF, Bubley GJ. The idiopathic hyper-eosinophilic syndrome. Blood 1994;83:2759–2779. [PubMed] [Google Scholar]
- 16.Gotlib J, Cools J. Five years since the discovery of FIP1L1-PDGFRA: what we have learned about the fusion and other molecularly defined eosinophilias. Leukemia 2008;22:1999–2010. [DOI] [PubMed] [Google Scholar]
- 17.Valent P, Klion AD, Horny HP, Roufosse F, Gotlib J, Weller PF et al. Contemporary consensus proposal on criteria and classification of eosinophilic disorders and related syndromes. J Allergy Clin Immunol 2012;130:607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klion AD, Noel P, Akin C, Law MA, Gilliland DG, Cools J et al. Elevated serum tryptase levels identify a subset of patients with a myeloproliferative variant of idiopathic hypereosinophilic syndrome associated with tissue fibrosis, poor prognosis, and imatinib responsiveness. Blood 2003;101:4660–4666. [DOI] [PubMed] [Google Scholar]
- 19.Apperley JF, Gardembas M, Melo JV, Russell-Jones R, Bain BJ, Baxter EJ et al. Response to imatinib mesylate in patients with chronic myeloproliferative diseases with rearrangements of the platelet-derived growth factor receptor beta. N Engl J Med 2002;347:481–487. [DOI] [PubMed] [Google Scholar]
- 20.Golub TR, Barker GF, Lovett M, Gilliland DG. Fusion of PDGF receptor beta to a novel ets-like gene, tel, in chronic myelomonocytic leukemia with t(5;12) chromosomal translocation. Cell 1994;77:307–316. [DOI] [PubMed] [Google Scholar]
- 21.Erben P, Gosenca D, Muller MC, Reinhard J, Score J, Del Valle F et al. Screening for diverse PDGFRA or PDGFRB fusion genes is facilitated by generic quantitative reverse transcriptase polymerase chain reaction analysis. Haematologica 2010;95:738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Bartley PA et al. Patients with chronic myeloid leukemia who maintain a complete molecular response after stopping imatinib treatment have evidence of persistent leukemia by DNA PCR. Leukemia 2010;24:1719–1724. [DOI] [PubMed] [Google Scholar]
- 23.Ross DM, Branford S, Seymour JF, Schwarer AP, Arthur C, Yeung DT et al. Safety and efficacy of imatinib cessation for CML patients with stable undetectable minimal residual disease: results from the TWISTER study. Blood 2013;122:515–522. [DOI] [PubMed] [Google Scholar]
- 24.Helbig G, Kyrcz-Krzemien S. Cessation of imatinib mesylate may lead to sustained hematologic and molecular remission in FIP1L1-PDGFRA-mutated hypere-osinophilic syndrome. Am J Hematol 2014;89:115. [DOI] [PubMed] [Google Scholar]
- 25.Legrand F, Renneville A, Macintyre E, Mastrilli S, Ackermann F, Cayuela JM et al. , on behalf of the French Eosinophil Network. The spectrum of FIP1L1-PDGFRA-associated chronic eosinophilic leukemia: new insights based on a survey of 44 cases. Medicine (Baltimore) 2013;92:e1–e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.