Abstract
The role of hemodynamics in cardiovascular development is not well understood. Indeed, it would be remarkable if it were, given the dauntingly complex array of intricately synchronized genetic, molecular, mechanical, and environmental factors at play. However, with congenital heart defects affecting around 1 in 100 human births, and numerous studies pointing to hemodynamics as a factor in cardiovascular morphogenesis, this is not an area in which we can afford to remain in the dark. This review seeks to present the case for the importance of research into the biomechanics of the developing cardiovascular system. This is accomplished by i) illustrating the basics of some of the highly complex processes involved in heart development, and discussing the known influence of hemodynamics on those processes; ii) demonstrating how altered hemodynamic environments have the potential to bring about morphological anomalies, citing studies in multiple animal models with a variety of perturbation methods; iii) providing examples of widely used technological innovations which allow for accurate measurement of hemodynamic parameters in embryos; iv) detailing the results of studies in avian embryos which point to exciting correlations between various hemodynamic manipulations in early development and phenotypic defect incidence in mature hearts; and finally, v) stressing the relevance of uncovering specific biomechanical pathways involved in cardiovascular formation and remodeling under adverse conditions to the potential treatment of human patients. The time is ripe to unravel the contributions of hemodynamics to cardiac development, and to recognize their frequently neglected role in the occurrence of heart malformation phenotypes.
Keywords: heart formation, congenital heart disease, hemodynamics, mechanotransduction
1. Introduction
Cardiac formation and the dynamics of blood flow, or hemodynamics, are intrinsically linked. The heart’s main function is to pump blood to the lungs for oxygenation and to the systemic circulation for oxygen and nutrient exchange. Looking at the heart from this ‘mechanical’ perspective, cardiac development must end with a fully functional pump. Heart development has to be exquisitely sensitive to the mechanics of pumping and blood circulation to ensure optimal cardiac performance; to maximize the chances of the embryo surviving under different environments, it has to be adaptable to changes. This is accomplished in part by signaling pathways that sense and respond to mechanical stimuli from blood flow during heart formation and, in so doing, modulate developmental processes.
Extensive research has been directed at understanding the mechanisms that drive the various stages of cardiac development. As a result, a wealth of information is now available on regulatory pathways and processes that act during heart development. Furthermore, the consequences of genetic anomalies and disruptions in normal processes via teratogen exposure are being actively investigated. Efforts have also been directed at understanding both how blood flow dynamics regulates cardiovascular development and how perturbing normal blood flow conditions impacts cardiac formation. Ultimately, the goal of these investigations is to fully comprehend the processes governing cardiac formation and how human babies develop congenital heart defects. Because blood flow is an integral part of heart development, it influences heart formation under normal and anomalous conditions. The effects of blood flow on cardiovascular development therefore cannot be neglected.
The ways in which hemodynamic conditions modulate cardiac development remain unclear. Several pathways and molecular mechanisms affected by hemodynamics are beginning to be examined and understood, but much research is still needed. The inherent complexity of the response of cardiac tissue to blood flow, with its diverse, interconnected and potentially redundant array of mechanosensors and mechanotransducers, can initially be overwhelming. Looking at heart development from a more macroscopic perspective, however, it is certainly possible to observe outcomes and infer general ‘rules’ (whether biomechanical, biophysical and/or biochemical) by which the heart responds to disruptions. For example, by determining cardiac anomalies after different exposures, it is possible to formulate a general theory about when malformation phenotypes appear and in response to what. In turn, this knowledge can point to developmental pathways of interest and inform future investigations.
Overall, about 1% of newborns have some form of congenital heart disease (Mozaffarian et al., 2015), a malformation of the heart that is present at birth. The most severe cases undergo cardiac surgery as soon as hours after birth. Nowadays, and thanks to many advances, more children with congenital heart disease are surviving into adulthood, but better strategies for treatment and follow up are needed. This can only be achieved by continuing to enhance our understanding of heart formation and the role of different factors on cardiac development and cardiovascular adaptation before and after birth.
In this review, we will first briefly summarize heart development, highlighting the role of hemodynamics. Next, we will discuss the different methods used to perturb blood flow in animal models with the goal of understanding the role of hemodynamics on cardiac formation, and review the common techniques used to measure and quantify cardiac blood flow parameters during embryonic development. Finally, and as an example, we will analyze the immediate and delayed hemodynamic and morphological effects of perturbing blood flow in an avian model during a specific developmental window.
2. Heart Development
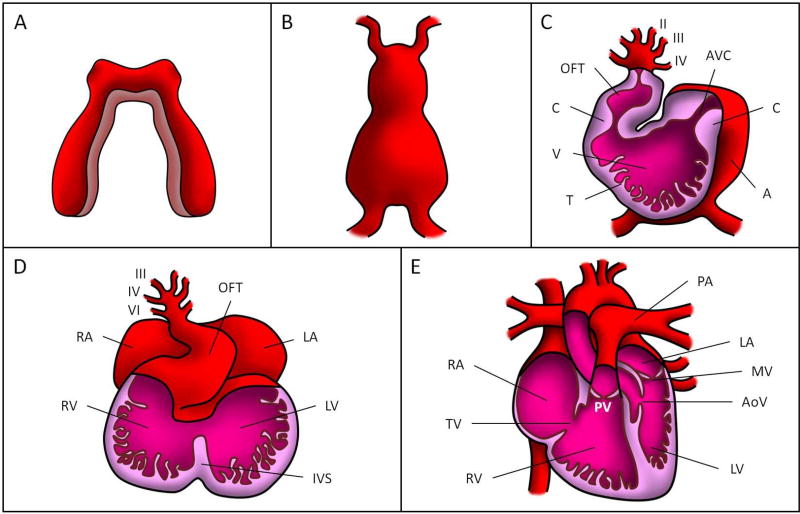
Cardiac development starts during the early embryonic stages and consists of a complex progression of events that ultimately mold the mature heart (see Figure 1 and Table 1). In the pre-somite embryo, the splanchnic mesoderm differentiates into a layer of myocardial progenitors with a plexus of elongated endothelial precursors beneath them (DeRuiter et al., 1992). Left and right populations of the differentiated mesoderm migrate and align themselves into bilaterally-paired fields, the cardiogenic cords (Figure 1A). The endothelial precursors form paired endocardial tubes that later fuse into a single tube (Kirby, 2007). The primitive heart tube is complete when the myocardial mantles merge to fully envelop the newly-fused endocardial tube and the myocardium secretes cardiac jelly, composed of extracellular matrix (ECM), into the space between the outer myocardial tube and the inner endocardial tube (Figure 1B) (Martinsen and Lohr, 2015). Therefore, the early tubular heart wall consists of three layers: i) an active myocardium layer that contracts cyclically; ii) an endocardium layer, a monolayer of endocardial cells in contact with blood flow; and iii) a thin cardiac jelly layer in between.
Figure 1. Cardiac formation stages.
(A) Cardiogenic cords, (B) linear tubular heart, (C) looped tubular heart, (D) cardiac septation, and (E) fully formed four-chambered heart. OFT: outflow tract, AVC: atrioventricular canal, C: endocardial cushion, V: primitive ventricle, T: trabeculae, A: primitive atrium, RA: right atrium, LA: left atrium, RV: right ventricle, LV: left ventricle, IVS: interventricular septum, PV: pulmonary valve, PA: pulmonary artery, MV: mitral valve, AoV: aortic valve, TV: tricuspid valve. The roman numbers in (C) and (D) correspond to the numbers assigned to the pharyngeal arch arteries (6 in total over developmental stages).
Table 1.
Timings of major events in heart development.
| Human(days) | Mouse(E) | Chick (HH (days)) | Zebrafish (hpf) | Major events in heart development |
|---|---|---|---|---|
| 15 | 7.5 | 7 (1) | 17.5 | Cardiac crescent |
| 21–22 | 8.5 | 8–9 (1.3) | 18–19.5 | Fusion of paired heart tubes |
| 22 | 8.5 | 10 (1.5) | 22 | First myocardial contractions |
| 22 | 8.5 | 9–10 (1.5) | 30 | Cardiac looping initiates |
| 24 | 8.5 | 10 (1.5) | 24 | First blood flow through the heart |
| 26 | 9.5 | 16–17 (2.5) | 48 | Ventricular trabeculation starts |
| 28 | 9.5 | 12–13 (2) | 60 | First definable endocardial cushions |
| 56 | 12.5 | 21–23 (3.5–4) | 105 | Appearance of primordial atrioventricular valves |
| 56 | 12.5 | 28–29 (5.5–6) | Appearance of primordial semilunar valves | |
| 48–56 | 10.5–13.5 | 16–46 (2–21) | Atrial septation | |
| 52–56 | 11.5–13.5 | 25–34 (4.5–8) | Outflow tract septation | |
| 52–64 | 11.5–13.5 | 19–34 (3–8) | Ventricular septation |
Comparison among human, mouse, chick and zebrafish; the latter three are frequently used as models of heart development. Human staging is given in days, mouse staging in embryonic day (E), chick staging in Hamburger-Hamilton staging (HH)(Hamburger and Hamilton, 1992) and approximate days of incubation, and zebrafish staging in hours post fertilization (hpf).
Note: The zebrafish heart has a single atrium and ventricle thus some aspects of heart morphogenesis differ from those of human, mouse and chicken. Adapted from (Lindsey et al., 2014)with additional data from (Stainier et al., 1993; Hu et al., 2000; Beis et al., 2005; Martinsen, 2005; Martinsen and Lohr, 2005; Martin and Bartman, 2009; Krishnan et al., 2014; Captur et al., 2016).
Soon after formation, the primitive heart tube starts to beat and cardiac looping begins. Through accretion of cells at each end, the heart tube elongates, causing rightward bending and torsion (Figure 1C). The straight heart tube is transformed into an s-shape heart tube, with the inflow and outflow poles in close proximity. Ventricular and atrial chambers then form by cell proliferation and migration (Moorman & Christoffels, 2003). These primitive chambers are connected by the atrio-ventricular canal (AVC), with the ventricle further connected to the circulation by the outflow tract (OFT). In the AVC and the OFT, the myocardium secretes additional ECM into the cardiac jelly to form paired localized swellings, the endocardial cushions, which bulge into the lumen. These cardiac cushions are the precursors of heart valves and are crucial to the development of portions of the atrial and ventricular septa (Kirby, 2007; Miquerol and Kelly, 2013). Even at this early stage, the cardiac cushions act as primitive valves, maintaining directional flow in the heart tube (Kirby, 2007; Combs and Yutzey, 2009). Before the cushions morph into valves, they undergo extensive remodeling, driven in part by a process known as endothelial-to-mesenchymal transition (EMT). Signaling between the myocardium and endocardium in the AVC and OFT initiates EMT in their cushion endocardial cells, causing them to acquire a mesenchymal phenotype and invade the cardiac jelly. Around the same time, ridges of endocardial cell-covered myocardium, called trabeculations, begin to form in the primitive ventricle (Kirby, 2007).
After cardiac looping is complete, the processes of chamber specification and septation partition the heart into four distinct chambers (Figure 1D). Left and right atrial and ventricular chambers are established as the AVC cushions grow outwardly and fuse together: The atrial chambers are separated by the growth of a muscular atrio-ventricular septum which descends from the atrial roof; meanwhile, the formation of the interventricular septum initiates from the deepest curve of the heart tube and grows superiorly before fusing with the AVC cushions (Kirby, 2007; Lin et al., 2012), establishing the left and right ventricles. The interventricular septum ultimately fuses with the OFT cushions to create ventricular outlets (Lin et al., 2012).
Concurrently with ventricular septation, the OFT is divided into the ascending aorta and pulmonary trunk by the formation of the aorticopulmonary septum. This septum originates between the IV and VI pharyngeal arch arteries and converges with the fused OFT cushions (Anderson et al., 2008). Valve morphogenesis occurs as the mesenchymalized cushions elongate and remodel into primitive valves that will ultimately transform into thin valve leaflets (Lin et al., 2012). The mitral and tricuspid AV valves arise from the AVC cushions, while the aortic and pulmonic semilunar valves originate from the OFT cushions (Figure 1E).
2.1 Endocardial-to-mesenchymal Transition and Valve Formation
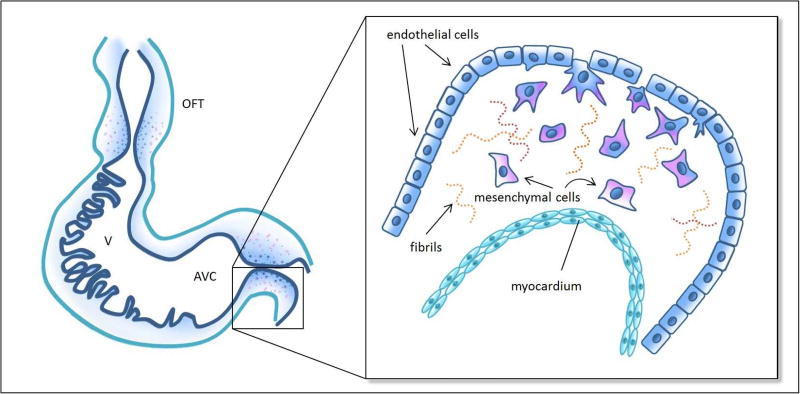
Shortly after the formation of endocardial cushions, EMT induces cushion remodeling that is necessary for subsequent valve formation. EMT is initiated when signaling from the adjacent myocardium, through transforming growth factor beta (TGFβ) and bone morphogenetic protein (BMP) pathways, causes activation of endocardial cells (Runyan et al., 1990; Garside et al., 2013). Full activation is characterized by the loss of cell-cell adhesion molecules, specifically platelet endothelial cell adhesion molecule (PECAM) and vascular endothelial cadherin (VE-Cadherin), and subsequent delamination of endocardial cells from the endocardium (Markwald et al., 1976; Baldwin et al., 1994). Activated cells acquire an invasive phenotype: they will elongate, develop filopodia, and migrate into the cardiac jelly (Kinsella and Fitzharris, 1980) (Figure 2). Select endocardial cells that do not undergo EMT proliferate to maintain the local integrity of the endocardium (Kirby, 2007); the newly transformed mesenchymal cells invading the cushions proliferate, densely populating the cardiac jelly (Kirby, 2007). Not surprisingly, EMT depends on ECM components, growth factors, and various transcriptional factors that mediate both cellular changes within the endocardial cells and remodeling in the cardiac jelly (Garside et al., 2013). Despite extensive research, the signaling pathways that initiate and regulate EMT in the cardiac cushions are not fully understood, and researchers continue to discover novel EMT regulatory mechanisms.
Figure 2. Schematics of endothelial-to-mesenchymal transition (EMT).
Left: schematic representation of the looped tubular heart depicting cushions in the outflow tract (OFT) and atrioventricular canal (AVC), as well as trabecular structures in the primitive ventricle (V). Inset: details of EMT in the AVC cushions, wherein a subset of activated endothelial cells (navy) delaminate from the endocardium, elongate, develop filopodia, migrate into the cardiac jelly, which is composed of extracellular matrix including some fibril proteins, and acquire a mesenchymal phenotype (pink). See Table 1 for relevant developmental periods.
While similar developmental mechanisms mediate maturation of the OFT and AVC cushions, there are notable differences. EMT is initiated earlier in the AVC cushions than in the OFT cushions (Camenisch et al., 2002), and cellularization of the AVC cushions occurs exclusively by EMT (Kirby, 2007). In contrast, secondary heart field cells also contribute to cushion cellularization in the proximal OFT cushions, and a small population of migratory neural crest cells invade the distal OFT cushions and contribute to their mesenchymalization as well (Webb et al., 2003; Kirby, 2007; Hinton and Yutzey, 2011). In addition, while many of the accepted molecular hierarchies driving EMT have been defined using AVC cushion explants from mouse or chicken embryos (Combs and Yutzey, 2009), in vivo studies of OFT cushions have confirmed that there are subtle differences in the molecular mechanisms driving the mesenchymalization of the AVC and OFT endocardial cushions. For example, vascular endothelial growth factor (VEGF) has distinctive roles in OFT versus AVC cushion development. VEGF is required for complete transformation of endothelial cells into mesenchymal cells during EMT in the OFT cushions; however, in the AVC cushions this is accomplished through alternate signaling, and instead VEGF is required for proper valve elongation (Stankunas et al., 2010; Bai et al., 2013). The observed differences are most likely attributable to the fact that the OFT and AVC cushions develop into mature valves with disparate forms and functions (Stankunas et al., 2010; Kruithof et al., 2012).
Anomalous cushion development has been implicated in the pathogenesis of congenital heart defects; thus endocardial cushion EMT has become the subject of intense investigation (Gong et al., 2017). There are several excellent reviews that cover the signaling pathways and molecular regulation of EMT in the endocardial cushions (Camenisch et al., 2010; Grego-Bessa et al., 2010; Garside et al., 2013). Numerous studies have also indicated that mechanotransduction pathways, especially in response to blood flow dynamics, are key regulators of EMT and cushion development, e.g. (Slough et al., 2008; Tan et al., 2013; Biechler et al., 2014; Goetz et al., 2014; Heckel et al., 2015; Menon et al., 2015).
2.2 Effect of Hemodynamics on Cardiac Formation
Blood flow is initiated with the beating of the heart tube early during embryonic development (Table 1). Therefore, cardiac looping, primitive chamber formation and alignment, cardiac septation, valve formation, and later cardiac maturation all occur under blood flow conditions. Concurrent with the earliest stages of cardiac looping, there is a dramatic increase in heart rate, blood pressure, and blood volume (Andrés-Delgado and Mercader, 2016; Haack and Abdelilah-Seyfried, 2016).
These hemodynamic changes in the heart are sensed by several different mechanosensors on cardiac cells (including endothelial, mesenchymal and myocardial cells). Examples of mechanosensors include cilia in endothelial cells, mechanosensitive ion channels, focal adhesions, cell membrane receptor kinases, and membrane lipids. These mechanosensors relay information about flow through mechanotransduction pathways that elicit regulatory responses via downstream signaling pathways like TGFβ/BMP, VEGF, and Notch (Andrés-Delgado and Mercader, 2016; Haack and Abdelilah-Seyfried, 2016). In order to understand these mechanosensitive pathways, we must consider the physical forces and stimuli acting on cardiac cells.
During the cardiac cycle, the heart walls are subjected to multiple mechanical stresses of different origins. These stresses are generated by the active contraction and passive expansion of the myocardium during the cardiac cycle; and the interaction of cardiac walls with blood. Importantly, blood flow exerts shear stress on the endocardium, a frictional force per unit area that is tangential to the surface and that arises due to the motion of blood. While this shear stress contributes to the cumulative stress experienced by cardiovascular walls, it is frequently considered separately because endocardial and endothelial cells, in direct contact with blood flow, are exquisitely sensitive to shear stress. In what follows, to avoid confusion, we will refer to stresses in the heart wall (encompassing all stresses), or to shear stress on the endocardium specifically.
The shear stress exerted by blood flow provides endocardial cells with a critical input necessary to direct their responses to dynamic flows through changes in proliferation, differentiation, cell shape, and permeability(Haack & Abdelilah-Seyfried, 2016; Hahn & Schwartz, 2009). These shear stresses vary temporally and spatially due to the progression of cardiac looping and the highly dynamic nature of blood flow during early heart morphogenesis (Haack & Abdelilah-Seyfried, 2016). An array of mechanosensors on the surface of endocardial cells, including primary cilia and ion channels Trpv4/Trpp2, transmit information about the hemodynamic environment in order to coordinate cellular responses during development (Andrés-Delgado & Mercader, 2016). In particular, primary cilia may play a critical role in sensing low velocity flows and can mediate proliferation and differentiation of neighboring cardiomyocytes through Notch1 signaling(Haack & Abdelilah-Seyfried, 2016; Samsa et al., 2015). Also, prior to valve formation, blood flow is oscillatory indicating that endocardial cells will be exposed to rapidly reversing shear stresses during the cardiac cycle(Haack & Abdelilah-Seyfried, 2016; Heckel et al., 2015). Studies in zebrafish embryos suggest that the mechanosensitive ion channels Trpv4/Trpp2 are activated by these oscillatory flows and mediate subsequent valvulogenesis by promoting endocardial cushion development until unidirectional flow is established (Haack & Abdelilah-Seyfried, 2016; Heckel et al., 2015; Koefoed, Veland, Pedersen, Larsen, & Christensen, 2014).
Endocardial cells possess the unique ability to directly sense shear stress from blood flow, but like all cardiac cells in the heart wall, they too experience stress from the cyclic active contraction and expansion of the myocardium. These stresses result in cell deformation (or stretch), which can also be sensed by cells and mediate responses to blood flow. In endocardial cells, cell-cell adhesion complexes, like VE-cadherin and platelet endothelial cell adhesion molecule 1(PEcam1), and integrins at the basal membrane can transmit tension and induce changes in the neighboring myocardium via nitric oxide and endothelin signaling(Andrés-Delgado & Mercader, 2016).
Similar to endocardial cells, myocardial cells, also known as cardiomyocytes, can sense mechanical forces via complexes at their cell borders. However, cardiomyocytes directly experience cyclic stress due to their intrinsic contractile behavior and as part of their interaction with the flow of blood (Jacot et al., 2010). Mechanosensors attached to the actomyosin cytoskeleton enable transmission of intercellular, intracellular, and extracellular mechanical stimuli. Integrins act as mechanosensors at the cell-ECM interface through costameric and focal adhesions to the ECM whereas neighboring cardiomyocytes transmit mechanical force via N-cadherin adhesion complexes found on their intercalated disks at cell-cell junctions(Chopra, Tabdanov, Patel, Janmey, & Kresh, 2011; Hersch et al., 2013; Li et al., 2015). Through these mechanosensors, cardiomyocytes are able to respond to cardiac tissue motion due to blood flow and adapt their form and function to adjust cardiac output (Chopra et al., 2011; Li et al., 2015). For example, during heart morphogenesis, cardiomyocytes respond to stress by altering their morphology (elongating in the direction of maximal stretch) as well as proliferation rates (Lindsey et al., 2014; Andrés-Delgado and Mercader, 2016). Furthermore, many cellular and molecular events driving heart morphogenesis seem dependent on normal cardiomyocyte contractility (Koushik et al., 2001; Berdougo et al., 2003; Huang et al., 2003; Bartman et al., 2004; Granados-Riveron and Brook, 2012). Thus, there exists a complex interplay of mechanical stimuli and differential cellular responses which allows the myocardium and the developing heart, as a whole, to adapt to changing hemodynamic conditions.
In order for cardiac cells to respond to stress/stretch, their mechanosensors must transduce this mechanical stimuli into a biochemical response. Mechanotransduction pathways in cardiac cells are incredibly intricate and are further complicated by the many levels of cross talk between pathways. A detailed discussion of mechanotransduction pathways is out of the scope of this review. However, in order to provide a sense of how mechanotransduction pathways relate to some of the aforementioned mechanosensors, we would like to briefly discuss the zinc-finger transcriptional regulator Krüppel-like factor 2 (KLF2) which has emerged as a key mechanotransducer of shear stress in the developing heart., In embryonic chicken hearts, for instance, expression of Klf2 in the endocardium correlates with wall shear stress: regions of high shear stress on the endocardium, such as endocardial cushions, present elevated expression of Klf2 (Groenendijk et al., 2007). In general, KLF2 expression is thought to guide heart morphogenesis through activation or suppression of critical developmental pathways involving Notch, TGFβ, VEGF, and nitric oxide (NO) (Dietrich et al., 2014; Andrés-Delgado and Mercader, 2016). As described previously, primary cilia in endothelial cells are membrane-bound organelles that act as flow sensors. Studies in zebrafish endothelium have revealed that the degree of cilia bending, which is related to the shear stress exerted by flow on the endothelium, also correlates with intracellular Ca2+ signaling and subsequent activation of klf2a (the zebrafish ortholog to Klf2) (Goetz et al., 2014). Further, zebrafish models have implicated klf2a as an important mediator of endocardial cell shape and size during development, implying that it is also essential for normal valvulogenesis and primitive chamber formation (Dietrich et al., 2014; Heckel et al., 2015; Andrés-Delgado and Mercader, 2016). Interestingly, ciliated endocardial cells are most abundant in regions of low flow (and low wall shear stress) and absent from the endocardial cushion area, a region with the highest shear stress and associated with high expression of Klf2 in chicken embryos (Egorova et al., 2011). Experiments with chick endocardial cells suggest that absence of cilia at the endocardial cushions is crucial for activation of the Tgfβ/Alk5 signaling pathway (Egorova et al., 2011; Andrés-Delgado and Mercader, 2016) required for EMT, and thus cilia absence may be required for normal progression of EMT in endocardial cushions (Koefoed et al., 2014). While there is still much to be learned about how the developing heart senses and responds to hemodynamic conditions, the unique mechanical cues sensed by endocardial, myocardial and mesenchymal cells during early development are known to actively mediate heart and valve formation.
3. Altering Hemodynamic Conditions in Animal Models: Lessons Learned
In vivo interventions in animal models are a powerful tool for studying how the development of the heart is affected by blood flow. There are multiple ways to alter hemodynamic conditions, with procedures generally categorized as surgical manipulations, environmental or drug exposure, and genetic manipulations. These different procedures alter the embryonic hemodynamic environment by changing the blood viscosity, modifying pressure gradients, and/or modifying cardiac cycle timings (Vermot et al., 2009). During early developmental stages, when the heart is forming (looping and septation stages), the tiny (< 1mm) dimensions of the heart and relatively small volume flow rate render small Reynolds and Womersley number flows (Hove et al., 2003; Forouhar et al., 2006; Vermot et al., 2009). The flow of blood is therefore viscous-dominated.. However, it is important to note that cardiac function and blood flow are intricately linked to each other in a closed-looped system. As such, changing blood pressure affects blood flow, and thus shear stress on the endocardium; changing viscosity also affects blood pressure and blood flow velocity. This implies that after interventions shear stresses on endocardial cells as well as stresses in the heart wall will both be modified. Thus, while different mechanisms can be investigated, when experimenting in vivo, it is impossible to completely uncouple the effects of shear stress on the endocardium from those of blood pressure and cardiac motion that affect stresses in the heart wall.
Because the early processes of heart formation are highly conserved amongst vertebrates, a variety of species are used to study the effects of altered hemodynamics on cardiac development, including chicken (Gallus gallus), zebrafish (Danio rerio), mouse (Mus musculus), and sheep (Ovis aries). The avian, mouse, and sheep heart, like the human heart, consists of four chambers (two ventricles and two atria). These models are useful to assess four-chamber cardiac function and to reproduce conditions found in human babies. Zebrafish have a two-chambered heart consisting of a single atrium and ventricle that push blood into capillary beds in the gills before circulating throughout the body. Hence, their use as a model is more restricted, but is still extremely valuable for understanding early developmental processes such as valve formation. Avian and zebrafish embryos are generated quickly, are economical to procure and maintain, and are readily accessible for imaging and manipulation. Zebrafish tissue is optically clear, as is avian tissue at early stages (looping stages and before), making them good candidates for video or light microscopy studies. Zebrafish have the unique ability to survive without a heartbeat (i.e. circulatory flow) throughout early stages of development, which enables researchers to manipulate hemodynamic forces to a greater extent and further isolate their effects during cardiac development (Kopp et al., 2005). Countless mutant lines are available for mice and zebrafish, while genetic manipulation is hardly seen in avian models or sheep. Mice and sheep can be used to study maternal-fetal hemodynamic interactions, especially during late gestational stages (equivalent to third trimester pregnancy in humans). Extensive study of fetal hemodynamic parameters in mammals often involves difficult invasive surgery and instrumentation, which can be prohibitive in mice, and carries a high financial cost in sheep. Each model, therefore, has advantages that can be exploited and disadvantages to overcome.
In summary, sheep models have been used to monitor the effects of blood flow conditions during fetal, third-trimester human equivalent, stages. Meanwhile, early embryonic studies have been performed in mouse, chicken and zebrafish models; chicken and zebrafish are preferred due to their optical access and the relative ease of monitoring their blood flow. The chicken embryo is widely used for surgical interventions to alter blood flow; mice and zebrafish mutants are widely available for genetic studies. While the methods, stages, and specific parameters assessed vary widely depending on the chosen animal model, the different procedures to alter blood flow dynamics have been used to study complementary aspects of the response of the developing heart to hemodynamic conditions.
3.1 Surgical Manipulations
In order to study the direct effects of altered hemodynamics without the confounding effects of drugs or genetic anomalies, many studies surgically modify blood flow to cause localized and systemic changes in pressure and flow distributions. Because of their ease of access in ovo, avian embryos are primarily used in such studies, although certainly not to the exclusion of other species.
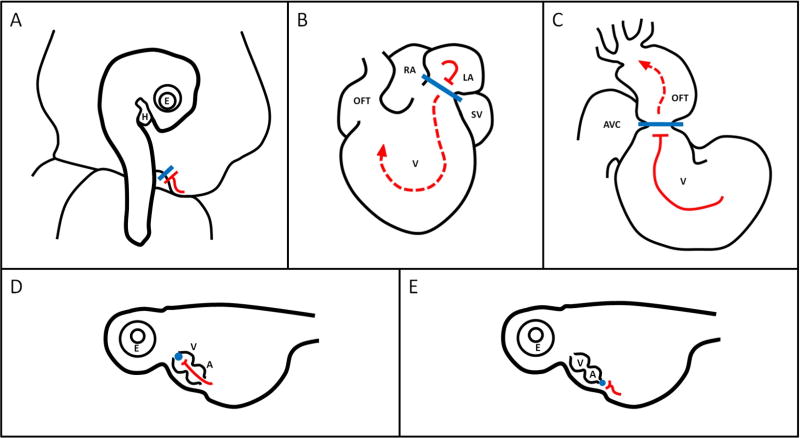
Most surgical interventions to alter blood flow simply block or diminish flow going into or out of the heart (Figure 3). Reducing blood flow into the heart frequently decreases cardiac blood pressure and stroke volume, and therefore decreases cardiac hemodynamic load. Occluding flow out of the heart results in increased cardiac blood pressure (with or without a decrease in stroke volume), and thus increases cardiac hemodynamic load. In avian models, surgical sutures or clips are used to reduce or halt blood flow through vessels or the heart. Common manipulations to decrease load during tubular heart stages are left atrial ligation (LAL) and left or right vitelline vein ligation (VVL) (Figure 3A and 3B, respectively); to increase hemodynamic load, conotruncal or outflow tract banding (OTB) is performed (Figure 3C) (Midgett and Rugonyi, 2014). Similar surgeries, albeit during fetal stages (fully formed heart), have been performed in lambs wherein the left atrium is occluded with a balloon catheter to block left ventricular inflow, or the ascending aorta is banded to partially occlude left ventricular outflow (Fishman et al., 1978). In zebrafish, both heart outflow and inflow have been occluded by inserting a microbead (Figure 3D and 3E) (Hove et al., 2003). Cardiac defect phenotypes observed after interventions are listed in Table 2. Note that the wide range of defects found in chicken embryos is in part due to different studies targeting slightly different cardiac developmental windows, with perhaps slight differences in interventions (for example, OTB phenotypes depend on band tightness and also the exact location of the band or surgical suture employed). In all cases, both shear stress on the endocardium and stresses (and deformations) in the cardiac wall have an important role in the tissue remodeling that follows intervention, and perturbation in blood flow consequently leads to structural cardiac defects. The range of cardiac defects found, moreover, is indicative of the complexity and interlinkage of the mechanisms at play.
Figure 3. Surgical Manipulations.
Illustrations of (A) VVL in an HH17 avian embryo (B) LAL in an HH21 avian heart, (C) OTB in an HH18 avian heart, (D) microbead outflow occlusion and (E) microbead inflow occlusion in a 57 hpf zebrafish heart. See Table 1 for staging details. Illustrations adapted from Midgett 2014 and Hove et al 2003, with reference to videos from (Al Naieb et al., 2013). Blue shapes indicate sutures/clips/beads, red lines indicate blood flow. A lone bar-headed line indicates complete occlusion (A,D, and E) and a bar-headed line paired with a dashed arrow-headed line (B, C) indicates partial occlusion and perturbed flow. H: heart, E: eye, AVC: atrioventricular canal, OFT: outflow tract, V: ventricle, RA: right atrium, LA: left atrium, SV: sinus venosis, A: atrium.
Table 2.
Common surgical manipulations to alter hemodynamics and their effects on heart development.
| Decreased hemodynamic load | Increased hemodynamic load | |
|---|---|---|
|
| ||
| Avian (Midgett 2014) |
VVL:
|
OTB:
|
LAL:
| ||
|
| ||
| Zebrafish (Hove et al 2003) |
Blocked inflow:
|
Blocked outflow:
|
|
| ||
| Lamb (Fishman et al 1978) |
LV inflow obstruction:
|
LV outflow obstruction:
|
VSD: ventricular septal defect, PAA: pharyngeal arch artery malformation, AVV: atrioventricular valve malformation, SLV: semilunar valve malformation, DORV: double outlet right ventricle, LHH: left heart hypoplasia, LVO: left ventricular output; LV: left ventricle, RV: right ventricle.
3.2 Teratogen or Pharmacological Exposure
When exposing embryos to potentially toxic environmental factors that alter blood flow, it can be difficult to determine whether the observed effects are due to toxicity, unintended consequences of the intervention, or the actual change in blood flow. There are, however, a number of studies that subject embryos to substances specifically targeted at disrupting hemodynamics. Since access to the cardiovascular system to surgically perturb blood flow is difficult in zebrafish due to a proteinaceous membrane (chorion) enveloping the embryo (Kohli and Elezzabi, 2008), pharmacological interventions are particularly common in zebrafish embryos, although they exist in other animal models as well. For example, in zebrafish, treatment with the calcium channel blocker and antihypertensive drug verapamil caused a 50% decrease in ejection fraction, abnormally non-zero end-systolic area, and decreased rates of diastolic filling and systolic pumping (Deniz et al., 2012). Anesthetics, like tricaine, can also be used to reduce blood flow in zebrafish. Further, a compound like 2,3-Butanedione monoxime, an inhibitor of cardiac muscle contractions, affords researchers the opportunity to study endocardial cushion formation and trabeculation in the absence of blood flow and myocardial function (Bartman et al., 2004; Denvir et al., 2008). Avian embryos exposed to trichloroethylene, an industrial solvent and short-term surgical anesthetic, showed reduced dorsal aortic blood flow, increased passive-to-active atrioventricular blood flow ratio, and increased mortality (Drake et al., 2006). Ethanol, a well-known teratogen still being explored for its specific mechanisms of damage, caused abnormal Doppler waveforms and increased levels of retrograde flow in conjunction with underdeveloped atrioventricular cushions in avian embryos (Karunamuni et al., 2014). Mouse embryos subjected to maternal lithium had both semilunar and atrioventricular valve regurgitation, displayed a variety of altered hemodynamic parameters, and had decreased morphometric measurements as compared to controls (Han et al., 2009). In all these cases, although hemodynamic parameters are altered, other off-target pharmacological effects cannot be ignored as they may lead investigators to make inaccurate conclusions. However, with full characterization of compounds and careful experimental design (e.g. use of complementary alternative models), pharmacological agents can be valuable experimental tools for studying the effects of altering embryonic hemodynamics during heart formation.
3.3 Genetic Manipulations
Genetic manipulations are not commonly used to specifically investigate the relationship between altered hemodynamic conditions and heart development; however, a handful of transgenic and morphant models with altered flow have been instrumental in defining the role of hemodynamics on cardiac formation. While surgical interventions physically impede or restrict blood flow, genetic manipulations achieve perturbed blood flow by targeting genes that regulate hematopoiesis (and therefore blood viscosity), cardiac contractility, or pace making. Another interesting approach is to target genes that sense blood flow in order to alter the normal response to hemodynamics. As with teratogenic or pharmacological interventions, however, it can be difficult to tease apart the effects of genetically altered flow from those of unintended genetic interactions and/or compensatory pathways.
Mouse and zebrafish models have been used extensively for genetic manipulations, and transgenic lines are widely available for both species (Koushik et al., 2001; Huang et al., 2003; Hwa et al., 2017). Transgenic lines that express fluorescent probes driven by a chosen promoter have been widely used both for visualization and lineage studies, and are often bred into other mutant lines to augment analysis (Auman et al., 2007; Zhang et al., 2014; Samsa et al., 2015; Lee et al., 2016; Battista et al., 2017). Zebrafish embryos are especially good candidates for genetic manipulation in developmental studies due to their small size, external fertilization, rapid ontogeny, optical clarity, and accessibility. Transgenic mouse models have played an important role in shaping our understanding of heart development; however, in comparison to zebrafish, generation time is longer and associated costs are higher. Moreover, as a mammalian model, access to the mouse embryo for in vivo imaging and manipulation is hampered. Nevertheless, since some processes in zebrafish heart development are divergent from those of mammals (e.g., the zebrafish heart has only two chambers, and the placenta is absent), mouse models are irreplaceable in some studies.
Genetic manipulations to alter flow mainly include approaches aimed at altering hematopoiesis and cardiac contraction. For example, by silencing gata1 and gata2 genes, which control early hematopoiesis in zebrafish, morphants with significantly reduced blood viscosity were obtained. Gata1 morphants had no circulating red blood cells and a 90% reduction in blood viscosity; gata2 morphants had 72% fewer red blood cells and 70% lower blood viscosity compared to control embryos (Vermot et al., 2009). These morphants were used to establish the role of oscillating flows and shear stress on the endocardium in valvulogenesis (Vermot et al., 2009) and ventricular trabeculation (Lee et al., 2016).
Another commonly studied example is the silent heart (sih−/−) zebrafish, which has a mutation in the gene encoding cardiac troponin T (Tnnt2), a protein that is essential for myocardial sarcomere assembly and contractility. Silent heart mutants lack a heartbeat and therefore blood flow (Bartman et al., 2004). Similarly, by knocking out the Ncx1 gene encoding the sodium-calcium exchanger protein 1 in mice, mutant embryos without a heartbeat or blood flow were obtained. In both the sih−/− zebrafish and the Ncx1 knockout, embryos lacked endocardial cushion development, while looping and chamber specification remained unaffected (Koushik et al., 2001; Sehnert et al., 2002; Bartman et al., 2004). Studies with sih−/− zebrafish and Ncx1 knockout mice support the idea that endocardial cushion development is exquisitely sensitive to changes in myocardial function (Koushik et al., 2001; Bartman et al., 2004; Bartman and Hove, 2005; Hwa et al., 2017).
As an alternative to direct perturbation of embryonic blood flow, other studies have used genetic approaches to target mechanosensory pathways to impair the developing cardiovasculature’s ability to sense changes in hemodynamics. Knockdown of a gene encoding a protein subunit required for assembly of primary cilia, the intraflagellar transport protein 88 (Ift88), resulted in mouse embryos with non-ciliated endothelial cells (Clement et al., 2009). Phenotypic analysis revealed malformations in the OFT and ventricles of Ift88−/− embryos. These findings support the idea that primary cilia act as wall shear stress mechanosensors for endothelial and endocardial cells, allowing them to regulate OFT maturation and trabecular development in the ventricles in response to flow (Clement et al., 2009; Andrés-Delgado and Mercader, 2016). The observed structural anomalies of Ift88−/− embryos, however, undoubtedly alter blood flow patterns, making it difficult to isolate the primary and secondary effects of cilia loss (Slough et al., 2008; Clement et al., 2009). As is the case with any genetic model, careful consideration must be given to the unintended effects of targeted knockouts.
3.4 Altered Hemodynamics: an Underlying Cause of Cardiac Malformation
Numerous studies have demonstrated that hemodynamics cannot be neglected as a variable affecting cardiac development. Blood flow is intricately linked to any manipulation that alters heart development, including genetic modifications, which frequently result in perturbations to blood flow. Perhaps not surprisingly, perturbed blood flow conditions lead to cardiac malformation phenotypes that independently arise from known gene mutations and teratogen exposures (Rugonyi, 2016; Midgett et al., 2017b). Whether some of these mutations and exposures also lead to altered blood flows that could ultimately underlie the cardiac malformation is not known, but it is certainly an interesting topic of investigation. It is worth mentioning, however, that since different processes that shape heart development occur during specific developmental periods, the temporal window of exposure to altered blood flow conditions (as well as teratogens and genetic modifications) plays an important role in the range of cardiovascular defects that could result from the exposure. When taken together, the wealth of studies done over the last half century (and more) point to a highly complex interplay of blood flow patterns and tissue remodeling in which physical and biological variables play intricately interwoven and crucial roles in the development of the cardiovascular system.
4. Characterizing Early Changes in Hemodynamic Conditions
A growing number of methods exist for characterizing the hemodynamic conditions under which the early embryonic heart develops. The challenges presented by the small size of the embryonic heart, the frequency at which it beats, and its optical access are considerable, and technology has been continually evolving to provide more detailed and accurate information about flow dynamics within the embryonic cardiovascular system. Each measurement technique has its own advantages and limitations and must be carefully chosen to suit the experimental application, taking into consideration the species, stage of development, and parameters of interest. Moreover, methods are frequently used in conjunction with each other to present a more complete picture of the hemodynamic environment. Common technologies for direct measurement of embryonic hemodynamic parameters and cardiac motion currently include servo-null micropressure systems, ultrasound, video microscopy, confocal microscopy, light sheet microscopy, and optical coherence tomography (OCT). In addition, particle image velocimetry (PIV) is a common technique that uses data from diverse imaging modalities to compute velocity profiles. Moreover, computational fluid dynamics (CFD) modeling is used to precisely compute shear stresses on the endocardium and the details of blood flow velocity distributions in two or three dimensional models of the heart or region of interest within the heart or vasculature.
4.1 Servo-null Micropressure Systems
The instrument most often employed to measure embryonic blood pressure is the servo-null micropressure system, which is specifically designed to measure small blood pressure values in tiny regions with good dynamic performance. The method is widely applied and has been used in chick, zebrafish, mice, rat, and frog embryos (Nakazawa et al., 1985; Stewart et al., 1986; Nakazawa et al., 1988; Hu and Clark, 1989; Hou and Burggren, 1995; Chabert and Taber, 2002; Ishiwata et al., 2003; Kopp et al., 2005; Lucitti et al., 2005). In a servo-null system, blood pressure is measured at the tip of a fluid-filled micropipette inserted into the lumen of the heart or vascular vessel. The saline fluid inside the micropipette (which produces an electron concentration gradient) is displaced when the lumen pressure changes. By measuring the internal pressure needed to force the micropipette fluid back to a user-set neutral point, blood pressure can be measured accurately and with good temporal resolution (https://www.wpiinc.com/products/top-products/sys-900a-micropressure-system/). In contrast, traditional solid-state catheter pressure systems are generally too large for use in developing embryos (Le et al., 2012), and conventional fluid-filled transducers have poor temporal resolution for the high frequency at which the embryonic heart beats (Ishii et al., 2001; Le et al., 2012). The drawbacks of the servo-null system include its requirement for custom-pulled nonconductive glass or polyethylene micropipettes (Heineman and Grayson, 1985; Le et al., 2012), and that the system’s zero balance is sensitive to temperature (Nakazawa et al., 1986). However, the micropipettes’ small size (0.5–10µm (Wiederhielm et al., 1964; Heineman and Grayson, 1985; Stewart et al., 1986; Nakazawa et al., 1988; Hu and Clark, 1989; Chabert and Taber, 2002), depending on the application) minimally disturbs the blood flow environment while an accurate pressure curve is recorded. Servo-null blood pressure measurements are often taken in the ventricle(s)(Hou and Burggren, 1995; Chabert and Taber, 2002; Shi et al., 2013), vitelline arteries (Nakazawa et al., 1985; Nakazawa et al., 1986; Stewart et al., 1986; Hu and Clark, 1989; Broekhuizen et al., 1999), and the dorsal aorta (Lucitti et al., 2005; Shi et al., 2013), and have been taken in the truncus arteriosus and conus arteriosus as well (Hou and Burggren, 1995). This method allows researchers to monitor blood pressure in the embryonic cardiovascular system in vivo.
4.2 Ultrasound
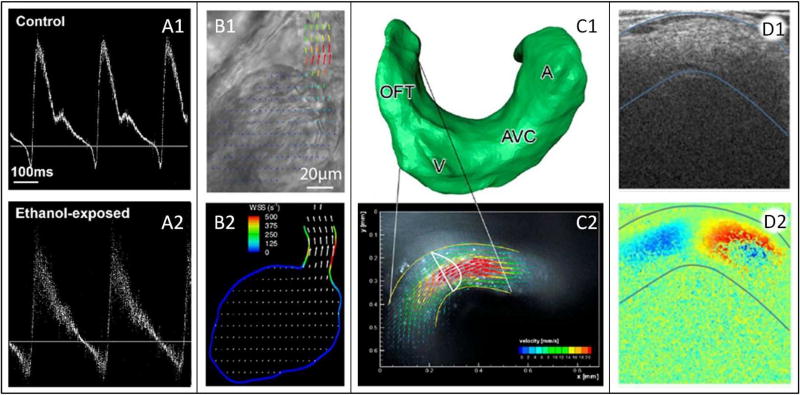
Ultrasound is possibly the most ubiquitous method for assessing hemodynamic parameters in developing embryos. It is popular for use in humans (Chang et al., 2000; Liu et al., 2016) and in mice, and while the size of the probe can be cumbersome for chick studies; it has been used both in ovo and ex ovo (Figure 4A) (Stewart et al., 1986; Hu and Clark, 1989; Lucitti et al., 2005; McQuinn et al., 2007; Oosterbaan et al., 2009). In conjunction with ultrasound structural images, pulsed-wave Doppler ultrasound enables depth-specific blood velocity measurement (Atkinson and Wells, 1977) and is the basis for color Doppler technology, which provides images of color-mapped flow fields. However, because velocity is measured in the direction of the Doppler beam, the angle of the vessel centerline and the angle of the beam must be known in order to calculate absolute velocity magnitude (Kowalski et al., 2014). While this is a limiting factor, the non-invasive nature, good temporal resolution and availability of ultrasound systems have made ultrasound the standard method for decades (Hove, 2006; Kowalski et al., 2014). High-frequency ultrasound, also known as ultrasound biomicroscopy (UBM), has been further developed to examine morphological changes in the smaller embryonic cardiovascular systems. UBM transducers have frequencies between 30 and 55 MHz, considerably higher than the 2–15 MHz used in human clinical applications (McQuinn et al., 2007; Hahurij et al., 2014; Liu et al., 2016). These high frequencies allow for up to 30 µm axial and 75 µm lateral spatial resolution (Phoon and Turnbull, 2003; McQuinn et al., 2007). Such detail is sufficient to capture accurate structural and flow velocity images of mouse and chick embryonic morphology and heart dynamics, as early as late tubular stages. UBM in combination with Doppler ultrasonography is a powerful tool for studying the mechanics of the developing heart.
Figure 4. Characterizing Hemodynamics.
(A) Ultrasound trace for 1) untreated and 2) ethanol-injected HH19 avian embryos. Reproduced from (Peterson et al., 2017). (B1) video microscopy frame of a 3 dpf zebrafish heart, overlaid with 2D velocity vector field calculated using PIV; (B2) 2D WSS distribution calculated using the velocities from (B1). Reproduced from (Jamison et al., 2013). (C1) surface reconstructed from a confocal microscopy z-stack of an HH14 avian embryo; (C2) confocal microscopy image, overlaid with 3D velocity vector field calculated using PIV and validated with CFD performed using the mesh from (C1). Reproduced from (Hierck et al., 2008). (D1) OCT structural image of an HH18 avian outflow tract; (D2) concurrently collected Doppler OCT image (both overlaid with blue curves delineating the myocardium). Adapted from (Midgett et al., 2014). WSS: wall shear stress, OFT: outflow tract, V: ventricle, AVC: atrioventricular canal, A: atrium, dpf: days post fertilization.
4.3 Video Microscopy
For optically accessible hearts, such as those in early chick (<5 days of incubation) (McQuinn et al., 2007), frog (Deniz et al., 2012), and zebrafish embryos, simple video microscopy can provide a wealth of information on the hemodynamic conditions within the heart. A camera mounted on an inverted microscope records the beating heart, and the resulting images are used to measure heart rate, cardiac rhythm, vessel diameters, blood velocity, heart tube shape, and/or changes in heart wall motion (Suga et al., 1973; Hou and Burggren, 1995; Kopp et al., 2005; Lucitti et al., 2005; Jamison et al., 2013; Liu et al., 2016). From these measurements and possibly in combination with pressure data, parameters such as shear stress on the endocardium, stroke volume, cardiac output, ejection fraction, systolic and diastolic area as well as inflow/outflow rates, total arterial compliance, and total vascular resistance may be calculated (Kopp et al., 2005; Lucitti et al., 2005; Deniz et al., 2012; Jamison et al., 2013; Stovall et al., 2016). Video microscopy is limited in that it can provide very little information in the direction perpendicular to the imaging plane. However, depending on the capabilities of the camera and optical microscope, spatial and temporal resolution can be excellent, making video microscopy an ideal and readily available method for experimental use in cardiac developmental studies (Figure 4B).
4.4 Confocal and Light Sheet Microscopy
Confocal and light sheet microscopy are used on optically clear (living or fixed) tissue samples that have endogenous or labeled fluorescence. Due to the combined need for easy access and optically clear tissue, these techniques have been used in vivo mainly with chicken and zebra fish embryos. In confocal microscopy, a laser beam focused to a point through a pinhole (Liebling et al., 2005; Huss et al., 2015) excites the fluorophores in a specific small region of the sample, and the fluorescent light intensity is collected and recorded to form an image. In light sheet microscopy, a plane of tissue is excited by illuminating the sample through a slit aperture. The emitted fluorescence perpendicular to the plane is then captured and recorded, forming an image (Lee et al., 2016). Fluorescence from cardiovascular tissue yields structural information (Hove et al., 2003; Hove, 2006; Hierck et al., 2008), and, when combined with either time lapse microscopy or a fast acquisition system, can provide data on morphogenesis and tissue motion (Liebling et al., 2005; Huss et al., 2015; Lee et al., 2016). Fluorescence from labeled blood cells, on the other hand, renders data on flow dynamics (Hove et al., 2003; Hove, 2006; Lucitti et al., 2007; Larina et al., 2009; Boselli and Vermot, 2016). Images are typically collected in plane, but 3D (Hierck et al., 2008) and 4D (3D through time) (Liebling et al., 2005; Lee et al., 2016) reconstructions can be assembled by collecting image sequences in z-stacks (Figure 4C). Confocal and light sheet microscopy, therefore, have the capability to provide all the information of video microscopy with the crucial addition of the third (perpendicular to the imaging plane) spatial dimension.
4.5 Optical Coherence Tomography
OCT is often described as the optical analog to ultrasound. Light interferometry is used to measure the echo time delay of a light beam directed at a sample, which captures the native contrast between tissue layers (Figure 4 D1), enabling tomographic images. Additionally, the velocity of light-scattering particles (i.e. red blood cells) can be calculated from their Doppler phase shift between adjacent scan lines (Larina et al., 2011; Midgett et al., 2015) (Figure 4 D2). A main advantage of OCT is that it can simultaneously acquire structural and Doppler images of the heart or a portion of the vasculature, allowing evaluation of both cardiac motion and blood flow dynamics. Penetration depth, resolution, and acquisition speed all depend on the specifics of the system. Penetration depth in embryonic tissue is at most a couple of millimeters, but OCT is capable of axial resolution as fine as 2µm (Jenkins et al., 2007; Larina et al., 2011). The most advanced OCT systems can gather 2D image sequences at well over a hundred frames per second (Jenkins et al., 2007; Shi et al., 2013), enabling accurate structural and Doppler velocity acquisition over the embryonic cardiac cycle. OCT’s ability to capture detailed structure and flow information in real time has made it an increasingly popular tool for studying embryonic hemodynamics, and it has been applied to studies in avian, frog, zebrafish, mouse, and rat embryos (Larina et al., 2011).
4.6 Particle Image Velocimetry
PIV is used to calculate flow velocity profiles and flow fields from in vivo images. Through a specialized algorithm, PIV tracks the motion of blood cells or other particles (typically added to the flow) imaged with modalities such as video microscopy (Jamison et al., 2013), confocal microscopy, light sheet microscopy or other methods (Poelma et al., 2010). By tracking motion, in-plane particle velocities can be computed and velocity profiles depicted on a 2D region of interest (Vennemann et al., 2006; Poelma et al., 2010) (Figure 4B). While optical access is typically required, PIV provides information on blood flow velocity distributions in 2D. PIV is useful in cases where ultrasound is infeasible, such as areas of very slow flow or in very small, early embryos, and when the velocity profile is needed to estimate shear stress on the endocardium or secondary flows.
4.7 Computational Fluid Dynamics
Computational methods typically take as input experimental data and use theoretical models to calculate parameters that cannot be measured directly. CFD can calculate detailed 3D and 4D blood flow fields (3D velocity vectors over time) and distributions of shear stress on the endocardium using cardiovascular geometries extracted from micro computed tomography (microCT) (Butcher et al., 2007; Kowalski et al., 2013), OCT (Goenezen et al., 2015; Midgett et al., 2015), confocal microscopy (Hierck et al., 2008; Menon et al., 2015; Boselli and Vermot, 2016), light sheet microscopy (Lee et al., 2013; Lee et al., 2016) or other imaging modalities (Figure 4C). This is in contrast to Doppler techniques, which can only measure one component of the three-dimensional velocity vector, and PIV techniques, which measure two dimensions. Furthermore, CFD can be applied in vessel structures that are not optically accessible for PIV (Kowalski et al., 2014) and/or too small for accurate Doppler ultrasound acquisition. Reconstruction of cardiovascular geometries and their motion over the cardiac cycle for CFD simulations is achieved through a combination of image acquisition strategies and image registration algorithms. Care must be taken with assumptions about material properties, and validation strategies are required to ensure that calculated velocities are accurate, but the ease with which detailed flow distributions within the lumen volume (including recirculating regions and secondary flows) and shear stresses on endocardial and endothelial layers can be calculated makes CFD an invaluable methodological asset.
In practice, a combination of measurements and flow modeling techniques are used to quantify hemodynamics during heart development. These quantifications will eventually lead to a better understanding of the forces and hemodynamic stimuli exerted by blood flow during development and how these stimuli influence cardiovascular formation.
5. Altering Hemodynamic Conditions in Chicken Embryos during EMT progression in the OFT
The focus of this section is on reviewing the effects of altering hemodynamic conditions in chicken embryos during a particular developmental window. The targeted developmental window is important, as it defines the developmental processes that might be affected by perturbations in blood flow. The looping stages of cardiac development have been the target of several interventions to alter blood flow, e.g., (Clark et al., 1989; Hogers et al., 1997; Keller, 1998; Sedmera et al., 1999; Midgett and Rugonyi, 2014; Menon et al., 2015; Rennie et al., 2017), because the looping tubular heart is very sensitive to flow conditions. In chicken embryos, altering blood flow between HH18 and HH24 (about 3 to 4 days of incubation) potentially affects the development of ventricular trabeculation, OFT EMT, migration and proliferation of secondary heart field and mesenchymal cells, and in general the maturation of myocardial cells and the cardiac conduction system. We will focus here on previous studies by our group which examined flow perturbations in this developmental window (HH18 to HH24; see Figure 5).
Figure 5. Schematics of experimental design.
Blood flow was perturbed at HH18 through VVL or OTB, and resulting blood flow dynamics were measured 2 hours after intervention. Embryos were then reincubated to HH24 (~24 hours after intervention) and either collected for further analysis or had their hemodynamics restored (for OTB embryos). Embryos that were not collected were then re-incubated and analyzed for structural cardiac malformations at HH38, when the heart was fully formed.
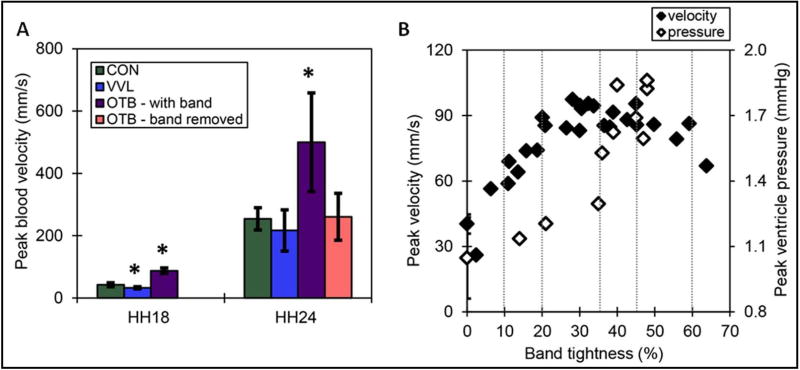
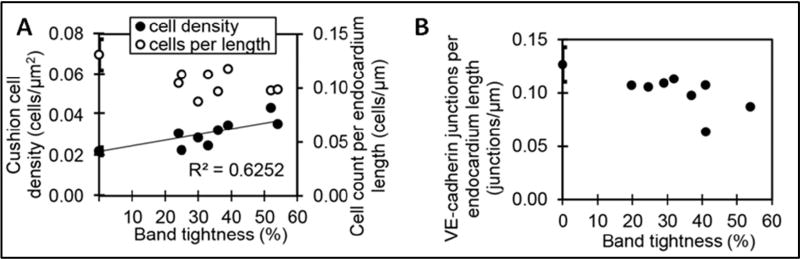
Surgical interventions to alter hemodynamics (OTB and VVL, Figures 3C and 3A, respectively) were performed at HH18 (~day 3 of incubation, Figure 5). In VVL embryos, blood flow was totally obstructed in all samples and angiogenesis bypassed the ligation about 5 hours after intervention, which resulted in a transient but consistent reduction in flow of about 50% (Figure 6A) (Midgett et al., 2017b). In contrast, flow disruptions from OTB persisted until the band was removed 24 hours later (Figure 6A), and increases in blood flow velocity at the band site depended on the degree of constriction of the outflow tract, i.e. the band tightness (see Figure 6B) (Shi et al., 2013; Midgett et al., 2014). Furthermore, the band tightness had a direct effect on the degree of remodeling within the banded OFT cushions: Relative to controls at the same stage, cardiac tissues collected 24 hours after OTB showed increases in cushion cell density and decreases in endocardial cell and VE-cadherin surface density proportionate to their band tightness (see Figure 7) (Midgett et al., 2017a). Since anomalous endocardial cushion formation and remodeling are associated with heart malformations, these studies imply that varying the degree of hemodynamic perturbation could lead to specific cardiac anomalies in a ‘dose-response’ way.
Figure 6. Measured hemodynamics after interventions.
(A) Maximum blood velocity after interventions. Interventions performed at HH18; band removed at HH24. Constriction of banded embryos ranged between 21 and 52% tightness. Standard deviation is displayed as error bars. Asterisk: statistically significant differences between experimental and control embryos (n=8, p<0.05). (B) Altered hemodynamics after outflow tract banding. Hemodynamic response to outflow tract band tightness, produced from previously published data (Shi et al., 2013; Midgett et al., 2014). Vertical lines outline ranges of constriction used for further analysis, and standard deviation of controls are displayed as error bars. CON: surgical sham control, VVL: vitelline vein ligated, OTB: outflow tract banded. Reproduced from (Midgett et al., 2017b).
Figure 7. EMT vs band tightness in chicken embryos at HH24 with banding performed at HH18.
(A) Cushion cell density and cell count per endocardium length quantitated from DAPI stain imaged with confocal microscopy. (B) Endocardial cell junctions per endocardium length quantitated from confocal immunofluorescent images stained for VE-cadherin. Reproduced from (Midgett et al., 2017a).
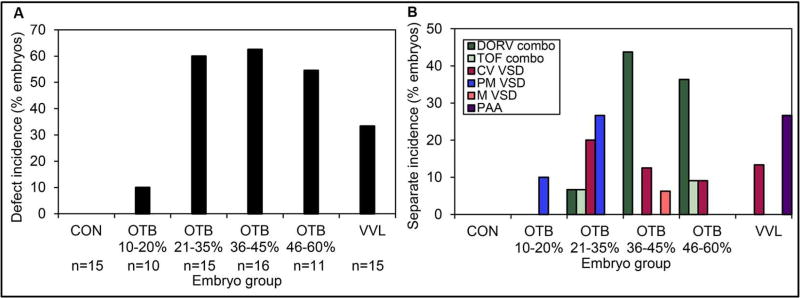
In order to investigate whether type and degree of hemodynamic perturbation would indeed lead to distinct malformation phenotypes, embryos were incubated until HH38, when the heart is fully formed (Figure 5). By carefully analyzing in vivo cardiac ultrasound data and microCT images of the HH38 hearts, we were able to draw two main conclusions about the effects of early transient flow perturbation on heart formation. First, we found that the number of hearts with overt structural malformations was significantly higher for surgically intervened embryos than for controls. Control embryos had no defects, VVL showed about 35% incidence of defects, and for the more constricted banded cases (20–60% band tightness), defect incidence in surviving embryos was as high as 60% (Figure 8A). Second, we found that phenotypic incidence seemed to depend on the hemodynamic perturbation (Midgett et al., 2017b) (Figure 8B). Among malformed hearts, by far the most common malformation was a ventricular septal defect (VSD), which is also the most common cardiac malformation in humans. Perimembranous VSD, which is a discontinuity in the upper section of the interventricular septum, occurred in the range of 20–40% band tightness. Conoventricular VSD, wherein the septum is discontinuous just below the semilunar valves, occurred for > 30% band tightness and in VVL embryos. There was a single incidence of a muscular VSD, characterized by discontinuity in the lower, muscular section of the septum, in the 30–40% band tightness range. We also found cases of Tetralogy of Fallot (TOF), characterized by VSD in combination with pulmonary stenosis, right ventricular hypertrophy, and oversized, overriding aorta; and cases of double outlet right ventricle (DORV), which is characterized by VSD in combination with an anomalous alignment of the great arteries with the right ventricle. Both TOF and DORV are rare malformations in human babies, but are also among the most serious conditions; they occurred for >30% band tightness, with DORV appearing more frequently than TOF. The incidence of TOF and DORV for the more constrained cases (> 30% band tightness), which underwent the most severe changes in blood pressure and shear stress on the OFT endocardium (Figure 6B), perhaps reflects on the low incidence of these defects among human babies. We also found cases of pharyngeal arch artery (PAA) malformation. Interestingly, PAA alone (not in combination with DORV or TOF) was only found in VVL embryos. In sum, while a given early hemodynamic perturbation does not exclusively correspond to a specific cardiac defect, malformation phenotypes occur within specific and sometimes distinct ranges of hemodynamic perturbation.
Figure 8. Cardiac defects depend on the level of hemodynamic perturbation.
(A) Overall defect incidence among surviving embryos. Embryos were grouped by band tightness, VVL intervention, and controls were also included. (B) Separate defect type incidence among surviving embryos, by embryo group. CON: normal control, OTB: outflow tract banded embryos, VVL: vitelline vein ligated embryos, VSD: ventricular septal defect, CV VSD: conoventricular VSD, PM VSD: perimembranous VSD, M VSD: muscular VSD, DORV: double outlet right ventricle, TOF: Tetralogy of Fallot. Reproduced from (Midgett et al., 2017b).
6. Conclusions
Human babies with congenital heart disease present with many different malformation phenotypes. While the most common form of cardiac malformation is VSD, certainly other cardiac defects such as TOF, DORV, and valve malformations, among others, occur. Interestingly, these defects can be reproduced by genetic manipulations, teratogen exposure, and particular perturbations in blood flow. The several different etiologies that lead to the same heart malformation argue in favor of a complex interplay among diverse factors (genetic, biochemical, biomechanical, environmental) leading to cardiac malformations. Hemodynamics plays a historically underappreciated but essential role in regulating cardiac morphology and cardiac tissue responses. New research into how exactly hemodynamics regulate cardiac development and the effects of disturbing those regulatory mechanisms is emerging and has the potential to revolutionize the way we understand malformation phenotypes and the causes of congenital heart disease.
In the meantime, it is interesting to consider how seemingly different insults to the developing cardiovascular system can all lead to the same heart defect(s), and vice versa. In analyzing and treating human cases with the same diagnosis, i.e. the same cardiac malformation phenotype, the first and perhaps more important issue may be to question how similar the cases really are. In particular, was convergence to the same phenotype achieved through entirely different biological paths, or through paths that converged before the malformation fully developed? The answer to this question may have important implications for how we treat congenital heart disease. If different etiologies for the same malformation lead to different heart tissue compositions or cellular responses (meaning, the same phenotype was achieved through different biological paths), then each case might need to be considered independently, through personalized approaches. On the other hand, if similar malformation phenotypes are also accompanied (perhaps in a subset of cases) by similar cardiac composition and cell responses (phenotype convergence was through convergent paths), then we can envision treatment strategies – beyond surgical repair – tailored to the phenotype (e.g. anti-fibrotic treatment). In the latter case, an interesting question also emerges: how is it that different insults result in exactly the same defects? What is it that ultimately drives convergence to the malformation phenotype? In this context, it is also interesting to point that the same ‘insult’ such as a change in hemodynamic conditions can lead to different malformation phenotypes depending on the level (‘dose’) of the perturbation. This finding perhaps argues for some level of predictability in the response to flow. It also prompts a fundamental question, can flow be manipulated early on to avoid or diminish the severity of heart malformations? In answering all these questions, we might find ways of treating cardiac malformations before they completely develop, and ultimately devise ways of rescuing babies from congenital heart disease.
Acknowledgments
This work was partially supported by grants from the National Institutes of Health (NIH) R01 HL094570 and American Heart Association (AHA) 16GRNT29840002. The content is solely the responsibility of the authors and does not necessarily represent the official views of grant giving bodies.
Abbreviations
Cardiac defect phenotypes
- VSD
ventricular septal defect
- TOF
Tetralogy of Fallot
- DORV
double outlet right ventricle
- AVV
atrioventricular valve malformation
- PAA
pharyngeal arch artery malformation
Techniques to measure and quantify cardiac flow and form
- OCT
optical coherence tomography
- PIV
particle image velocimetry
- UBM
ultrasound biomicroscopy
- microCT
micro computed tomography
- CFD
computational fluid dynamics
Interventions to alter flow in chicken embryos
- OTB
outflow tract (conotruncal) banding
- VVL
vitelline vein ligation
- LAL
left atrial ligation
Miscellaneous
- OFT
outflow tract (conotruncus)
- AVC
atrio-ventricular canal
- HH
Hamburger-Hamilton stage
- EMT
endothelial-to-mesenchymal transition
- ECM
extracellular matrix
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [Accessed November 20, 2017];Measure hydrostatic pressure in small vessels and oocytes. [Online]. https://www.wpiinc.com/products/top-products/sys-900a-micropressure-system/
- Al Naieb S, Happel CM, Yelbuz TM. A detailed atlas of chick heart development in vivo. Annals of Anatomy - Anatomischer Anzeiger. 2013;195(4):324–341. doi: 10.1016/j.aanat.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Anderson RH, Brown N, Webb S, Henderson D. Lessons learnt with regard to aortopulmonary window. Cardiology in the Young. 2008;18(5):451–457. doi: 10.1017/S1047951108002709. [DOI] [PubMed] [Google Scholar]
- Andrés-Delgado L, Mercader N. Interplay between cardiac function and heart development. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2016;1863(7, Part B):1707–1716. doi: 10.1016/j.bbamcr.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson P, Wells PNT. Pulse-Doppler Ultrasound and Its Clinical Application. The Yale Journal of Biology and Medicine. 1977;50(4):367–373. [PMC free article] [PubMed] [Google Scholar]
- Auman HJ, Coleman H, Riley HE, Olale F, Tsai H-J, Yelon D. Functional Modulation of Cardiac Form through Regionally Confined Cell Shape Changes. PLOS Biology. 2007;5(3):e53. doi: 10.1371/journal.pbio.0050053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y, Wang J, Morikawa Y, Bonilla-Claudio M, Klysik E, Martin JF. Bmp signaling represses Vegfa to promote outflow tract cushion development. Development (Cambridge, England) 2013;140(16):3395–3402. doi: 10.1242/dev.097360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin HS, Shen HM, Yan HC, DeLisser HM, Chung A, Mickanin C, et al. Platelet endothelial cell adhesion molecule-1 (PECAM-1/CD31): alternatively spliced, functionally distinct isoforms expressed during mammalian cardiovascular development. Development. 1994;120(9):2539–2553. doi: 10.1242/dev.120.9.2539. [DOI] [PubMed] [Google Scholar]
- Bartman T, Hove J. Mechanics and function in heart morphogenesis. Developmental Dynamics. 2005;233(2):373–381. doi: 10.1002/dvdy.20367. [DOI] [PubMed] [Google Scholar]
- Bartman T, Walsh EC, Wen K-K, McKane M, Ren J, Alexander J, et al. Early Myocardial Function Affects Endocardial Cushion Development in Zebrafish. PLOS Biology. 2004;2(5):e129. doi: 10.1371/journal.pbio.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battista NA, Lane AN, Liu J, Miller LA. Fluid dynamics in heart development: effects of hematocrit and trabeculation. Mathematical Medicine and Biology: A Journal of the IMA. 2017:dqx018–dqx018. doi: 10.1093/imammb/dqx018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beis D, Bartman T, Jin S-W, Scott IC, D'Amico LA, Ober EA, et al. Genetic and cellular analyses of zebrafish atrioventricular cushion and valve development. Development. 2005;132(18):4193–4204. doi: 10.1242/dev.01970. [DOI] [PubMed] [Google Scholar]
- Berdougo E, Coleman H, Lee DH, Stainier DYR, Yelon D. Mutation of weak atrium/atrial myosin heavy chain disrupts atrial function and influences ventricular morphogenesis in zebrafish. Development. 2003;130(24):6121–6129. doi: 10.1242/dev.00838. [DOI] [PubMed] [Google Scholar]
- Biechler SV, Junor L, Evans AN, Eberth JF, Price RL, Potts JD, et al. The impact of flow-induced forces on the morphogenesis of the outflow tract. Frontiers in Physiology. 2014;5:225. doi: 10.3389/fphys.2014.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boselli F, Vermot J. Live imaging and modeling for shear stress quantification in the embryonic zebrafish heart. Methods. 2016;94(Supplement C):129–134. doi: 10.1016/j.ymeth.2015.09.017. [DOI] [PubMed] [Google Scholar]
- Broekhuizen MLA, Hogers B, DeRuiter MC, Poelmann RE, Groot ACGD, Wladimiroff JW. Altered hemodynamics in chick embryos after extraembryonic venous obstruction. Ultrasound in Obstetrics & Gynecology. 1999;13(6):437–445. doi: 10.1046/j.1469-0705.1999.13060437.x. doi: [DOI] [PubMed] [Google Scholar]
- Butcher JT, Sedmera D, Guldberg RE, Markwald RR. Quantitative volumetric analysis of cardiac morphogenesis assessed through micro-computed tomography. Developmental Dynamics. 2007;236:802–809. doi: 10.1002/dvdy.20962. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Molin DGM, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, et al. Temporal and Distinct TGFβ Ligand Requirements during Mouse and Avian Endocardial Cushion Morphogenesis. Developmental Biology. 2002;248(1):170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Runyan RB, Markwald RR. Heart Development and Regeneration. Boston: Academic Press; 2010. Chapter 6.1 - Molecular Regulation of Cushion Morphogenesis; pp. 363–387. [Google Scholar]
- Captur G, Wilson R, Bennett MF, Luxán G, Nasis A, de la Pompa JL, et al. Morphogenesis of myocardial trabeculae in the mouse embryo. Journal of Anatomy. 2016;229(2):314–325. doi: 10.1111/joa.12465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabert S, Taber LA. Intramyocardial pressure measurements in the stage 18 embryonic chick heart. American Journal of Physiology-Heart and Circulatory Physiology. 2002;282(4):H1248–H1254. doi: 10.1152/ajpheart.00364.2001. [DOI] [PubMed] [Google Scholar]
- Chang C-H, Chang F-M, Yu C-H, Liang R-I, Ko H-C, Chen H-Y. Systemic assessment of fetal hemodynamics by Doppler ultrasound. Ultrasound in Medicine & Biology. 2000;26(5):777–785. doi: 10.1016/S0301-5629(00)00207-6. [DOI] [PubMed] [Google Scholar]
- Chopra A, Tabdanov E, Patel H, Janmey PA, Kresh JY. Cardiac myocyte remodeling mediated by N-cadherin-dependent mechanosensing. American Journal of Physiology - Heart and Circulatory Physiology. 2011;300(4):H1252–H1266. doi: 10.1152/ajpheart.00515.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffels VM, Habets PEMH, Franco D, Campione M, de Jong F, Lamers WH, et al. Chamber Formation and Morphogenesis in the Developing Mammalian Heart. Developmental Biology. 2000;223(2):266–278. doi: 10.1006/dbio.2000.9753. [DOI] [PubMed] [Google Scholar]
- Clark EB, Hu N, Frommelt P, Vandekieft GK, Dummett JL, Tomanek RJ. Effect of increased pressure on ventricular growth in stage 21 chick embryos. American Journal of Physiology - Heart and Circulatory Physiology. 1989;257(1):H55–H61. doi: 10.1152/ajpheart.1989.257.1.H55. [DOI] [PubMed] [Google Scholar]
- Clement CA, Kristensen SG, Møllgård K, Pazour GJ, Yoder BK, Larsen LA, et al. The primary cilium coordinates early cardiogenesis and hedgehog signaling in cardiomyocyte differentiation. Journal of Cell Science. 2009;122(17):3070–3082. doi: 10.1242/jcs.049676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs MD, Yutzey KE. Heart Valve Development: Regulatory Networks in Development and Disease. Circulation Research. 2009;105(5):408–421. doi: 10.1161/circresaha.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniz E, Jonas S, Khokha M, Choma MA. Endogenous contrast blood flow imaging in embryonic hearts using hemoglobin contrast subtraction angiography. Optics letters. 2012;37(14):2979–2981. doi: 10.1364/OL.37.002979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denvir MA, Tucker CS, Mullins JJ. Systolic and diastolic ventricular function in zebrafish embryos: Influence of norepenephrine, MS-222 and temperature. BMC Biotechnology. 2008;8(1):21. doi: 10.1186/1472-6750-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRuiter MC, Poelmann RE, VanderPlas-de Vries I, Mentink MM, Gittenberger-de Groot AC. The development of the myocardium and endocardium in mouse embryos. Fusion of two heart tubes? Anatomy and Embryology. 1992;185(5):461–473. doi: 10.1007/BF00174084. [DOI] [PubMed] [Google Scholar]
- Dietrich A-C, Lombardo Verónica A, Veerkamp J, Priller F, Abdelilah-Seyfried S. Blood Flow and Bmp Signaling Control Endocardial Chamber Morphogenesis. Developmental Cell. 2014;30(4):367–377. doi: 10.1016/j.devcel.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Drake VJ, Koprowski SL, Lough J, Hu N, Smith SM. Trichloroethylene Exposure during Cardiac Valvuloseptal Morphogenesis Alters Cushion Formation and Cardiac Hemodynamics in the Avian Embryo. Environmental Health Perspectives. 2006;114(6):842–847. doi: 10.1289/ehp.8781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer LA, Kirby ML. Sonic hedgehog maintains proliferation in secondary heart field progenitors and is required for normal arterial pole formation. Developmental biology. 2009;330(2):305–317. doi: 10.1016/j.ydbio.2009.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egorova AD, Khedoe PPSJ, Goumans M-JTH, Yoder BK, Nauli SM, Dijke Pt, et al. Lack of primary cilia primes shear-induced Endothelial-to-Mesenchymal Transition. Circulation research. 2011;108(9):1093–1101. doi: 10.1161/CIRCRESAHA.110.231860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finsterer J, Stollberger C, Towbin JA. Left ventricular noncompaction cardiomyopathy: cardiac, neuromuscular, and genetic factors. Nat Rev Cardiol. 2017;14(4):224–237. doi: 10.1038/nrcardio.2016.207. [DOI] [PubMed] [Google Scholar]
- Fishman NH, Hof RB, Rudolph AM, Heymann MA. Models of congenital heart disease in fetal lambs. Circulation. 1978;58(2):354–364. doi: 10.1161/01.cir.58.2.354. [DOI] [PubMed] [Google Scholar]
- Forouhar AS, Liebling M, Hickerson A, Nasiraei-Moghaddam A, Tsai H-J, Hove JR, et al. The Embryonic Vertebrate Heart Tube Is a Dynamic Suction Pump. Science. 2006;312(5774):751–753. doi: 10.1126/science.1123775. [DOI] [PubMed] [Google Scholar]
- Garside VC, Chang AC, Karsan A, Hoodless PA. Co-ordinating Notch, BMP, and TGFβ Signalling During Heart Valve Development. Cellular and molecular life sciences : CMLS. 2013;70(16):2899–2917. doi: 10.1007/s00018-012-1197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenezen S, Chivukula VK, Midgett M, Phan L, Rugonyi S. 4D Subject-Specific Inverse Modeling of the Chick Embryonic Heart Outflow Tract Hemodynamics. Biomechanics and Modeling in Mechanobiology. 2015 doi: 10.1007/s10237-015-0720-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz Jacky G, Steed E, Ferreira Rita R, Roth S, Ramspacher C, Boselli F, et al. Endothelial Cilia Mediate Low Flow Sensing during Zebrafish Vascular Development. Cell Reports. 2014;6(5):799–808. doi: 10.1016/j.celrep.2014.01.032. [DOI] [PubMed] [Google Scholar]
- Gong H, Lyu X, Wang Q, Hu M, Zhang X. Endothelial to mesenchymal transition in the cardiovascular system. Life Sciences. 2017;184(Supplement C):95–102. doi: 10.1016/j.lfs.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Granados-Riveron JT, Brook JD. The Impact of Mechanical Forces in Heart Morphogenesis. Circulation: Cardiovascular Genetics. 2012;5(1):132–142. doi: 10.1161/circgenetics.111.961086. [DOI] [PubMed] [Google Scholar]
- Grego-Bessa J, Pérez-Pomares JM, Luis de la Pompa J. Heart Development and Regeneration. Boston: Academic Press; 2010. Chapter 6.2 - Signaling Pathways in Valve Formation: The Origin of Congenital Defects; pp. 389–413. [Google Scholar]
- Groenendijk BC, Van der Heiden K, Hierck BP, Poelmann RE. The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiology. 2007;22:380–389. doi: 10.1152/physiol.00023.2007. [DOI] [PubMed] [Google Scholar]
- Haack T, Abdelilah-Seyfried S. The force within: endocardial development, mechanotransduction and signalling during cardiac morphogenesis. Development. 2016;143(3):373–386. doi: 10.1242/dev.131425. [DOI] [PubMed] [Google Scholar]
- Hahn C, Schwartz MA. Mechanotransduction in vascular physiology and atherogenesis. Nature reviews. Molecular cell biology. 2009;10(1):53–62. doi: 10.1038/nrm2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahurij ND, Calkoen EE, Jongbloed MRM, Roest AAW, Gittenberger-de Groot AC, Poelmann RE, et al. Echocardiographic Assessment of Embryonic and Fetal Mouse Heart Development: A Focus on Haemodynamics and Morphology. The Scientific World Journal. 2014;2014:11. doi: 10.1155/2014/531324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. Developmental Dynamics. 1992;195(4):231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Han M, Serrano MC, Lastra-Vicente R, Brinez P, Acharya G, Huhta JC, et al. Folate rescues lithium-, homocysteine- and Wnt3A-induced vertebrate cardiac anomalies. Disease Models & Mechanisms. 2009;2(9–10):467–478. doi: 10.1242/dmm.001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckel E, Boselli F, Roth S, Krudewig A, Belting H-G, Charvin G, et al. Oscillatory Flow Modulates Mechanosensitive <em>klf2a</em> Expression through <em>trpv4</em> and <em> trpp2</em> during Heart Valve Development. Current Biology. 2015;25(10):1354–1361. doi: 10.1016/j.cub.2015.03.038. [DOI] [PubMed] [Google Scholar]
- Heineman FW, Grayson J. Transmural distribution of intramyocardial pressure measured by micropipette technique. American Journal of Physiology-Heart and Circulatory Physiology. 1985;249(6):H1216–H1223. doi: 10.1152/ajpheart.1985.249.6.H1216. [DOI] [PubMed] [Google Scholar]
- Hersch N, Wolters B, Dreissen G, Springer R, Kirchgeßner N, Merkel R, et al. The constant beat: cardiomyocytes adapt their forces by equal contraction upon environmental stiffening. Biology Open. 2013;2(3):351–361. doi: 10.1242/bio.20133830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hierck BP, Van der Heiden K, Poelma C, Westerweel J, Poelmann RE. Fluid shear stress and inner curvature remodeling of the embryonic heart. Choosing the right lane. Scientific World Journal. 2008;8:212–222. doi: 10.1100/tsw.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB, Yutzey KE. Heart Valve Structure and Function in Development and Disease. Annual review of physiology. 2011;73:29–46. doi: 10.1146/annurev-physiol-012110-142145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogers B, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Unilateral Vitelline Vein Ligation Alters Intracardiac Blood Flow Patterns and Morphogenesis in the Chick Embryo. Circulation Research. 1997;80(4):473–481. doi: 10.1161/01.res.80.4.473. [DOI] [PubMed] [Google Scholar]
- Hou PC, Burggren WW. Blood pressures and heart rate during larval development in the anuran amphibian Xenopus laevis. American Journal of Physiology. 1995;269(5 Pt 2):R1120–1125. doi: 10.1152/ajpregu.1995.269.5.R1120. [DOI] [PubMed] [Google Scholar]
- Hove JR. Quantifying Cardiovascular Flow Dynamics During Early Development. Pediatric Research. 2006;60:6. doi: 10.1203/01.pdr.0000219584.22454.92. [DOI] [PubMed] [Google Scholar]
- Hove JR, Koster RW, Forouhar AS, Acevedo-Bolton G, Fraser SE, Gharib M. Intracardiac fluid forces are an essential epigenetic factor for embryonic cardiogenesis. Nature. 2003;421:172–177. doi: 10.1038/nature01282. [DOI] [PubMed] [Google Scholar]
- Hu N, Clark EB. Hemodynamics of the stage 12 to stage 29 chick embryo. Circ Res. 1989;65(6):1665–1670. doi: 10.1161/01.res.65.6.1665. [DOI] [PubMed] [Google Scholar]
- Hu N, Sedmera D, Yost HJ, Clark EB. Structure and function of the developing zebrafish heart. The Anatomical Record. 2000;260(2):148–157. doi: 10.1002/1097-0185(20001001)260:2<148::AID-AR50>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Huang C, Sheikh F, Hollander M, Cai C, Becker D, Chu P-H. Embryonic atrial function is essential for mouse embryogenesis, cardiac morphogenesis and angiogenesis. Development. 2003;130(24):6111–6119. doi: 10.1242/dev.00831. [DOI] [PubMed] [Google Scholar]
- Huss D, Benazeraf B, Wallingford A, Filla M, Yang J, Fraser SE, et al. A transgenic quail model that enables dynamic imaging of amniote embryogenesis. Development. 2015;142(16):2850–2859. doi: 10.1242/dev.121392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwa JJ, Beckouche N, Huang L, Kram Y, Lindskog H, Wang RA. Abnormal arterialvenous fusions and fate specification in mouse embryos lacking blood flow. Scientific Reports. 2017;7:11965. doi: 10.1038/s41598-017-12353-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Kuwaki T, Masuda Y, Fukuda Y. Postnatal development of blood pressure and baroreflex in mice. Autonomic Neuroscience. 2001;94(1):34–41. doi: 10.1016/S1566-0702(01)00339-3. [DOI] [PubMed] [Google Scholar]
- Ishiwata T, Nakazawa M, Pu WT, Tevosian SG, Izumo S. Developmental Changes in Ventricular Diastolic Function Correlate With Changes in Ventricular Myoarchitecture in Normal Mouse Embryos. Circulation Research. 2003;93(9):857–865. doi: 10.1161/01.res.0000100389.57520.1a. [DOI] [PubMed] [Google Scholar]
- Jacot JG, Martin JC, Hunt DL. Mechanobiology of cardiomyocyte development. Journal of biomechanics. 2010;43(1):93–98. doi: 10.1016/j.jbiomech.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamison RA, Samarage CR, Bryson-Richardson RJ, Fouras A. In Vivo Wall Shear Measurements within the Developing Zebrafish Heart. PLOS ONE. 2013;8(10):e75722. doi: 10.1371/journal.pone.0075722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins MW, Adler DC, Gargesha M, Huber R, Rothenberg F, Belding J, et al. Ultrahigh-speed optical coherence tomography imaging and visualization of the embryonic avian heart using a buffered fourier domain mode locked laser. Optics Express. 2007;15:6251–6267. doi: 10.1364/oe.15.006251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunamuni G, Gu S, Doughman YQ, Peterson LM, Mai K, McHale Q, et al. Ethanol exposure alters early cardiac function in the looping heart: a mechanism for congenital heart defects? American Journal of Physiology - Heart and Circulatory Physiology. 2014;306(3):H414–H421. doi: 10.1152/ajpheart.00600.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller BB. Embryonic cardiovascular function, coupling and maturation: a species view. In: Burggren WW, Keller BB, editors. Development of Cardiovascular Systems. Cambridge, MA: University Press; 1998. [Google Scholar]
- Kinsella M, Fitzharris T. Origin of cushion tissue in the developing chick heart: cinematographic recordings of in situ formation. Science. 1980;207(4437):1359–1360. doi: 10.1126/science.7355294. [DOI] [PubMed] [Google Scholar]
- Kirby ML. Cardiac Development. Oxford, UK: Oxford University Press; 2007. [Google Scholar]
- Koefoed K, Veland IR, Pedersen LB, Larsen LA, Christensen ST. Cilia and coordination of signaling networks during heart development. Organogenesis. 2014;10(1):108–125. doi: 10.4161/org.27483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli V, Elezzabi AY. Laser surgery of zebrafish (Danio rerio) embryos using femtosecond laser pulses: Optimal parameters for exogenous material delivery, and the laser's effect on short- and long-term development. BMC Biotechnology. 2008;8(1):7. doi: 10.1186/1472-6750-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopp R, Schwerte T, Pelster B. Cardiac performance in the zebrafish <em>breakdance</em> mutant. Journal of Experimental Biology. 2005;208(11):2123–2134. doi: 10.1242/jeb.01620. [DOI] [PubMed] [Google Scholar]
- Koushik SV, Wang J, Rogers R, Moskophidis D, Lambert NA, Creazzo TL, et al. Targeted inactivation of the sodium-calcium exchanger (Ncx1) results in the lack of a heartbeat and abnormal myofibrillar organization. The FASEB Journal. 2001 doi: 10.1096/fj.00-0696fje. [DOI] [PubMed] [Google Scholar]
- Kowalski WJ, Dur O, Wang Y, Patrick MJ, Tinney JP, Keller BB, et al. Critical Transitions in Early Embryonic Aortic Arch Patterning and Hemodynamics. PLOS ONE. 2013;8(3):e60271. doi: 10.1371/journal.pone.0060271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalski WJ, Pekkan K, Tinney JP, Keller BB. Investigating developmental cardiovascular biomechanics and the origins of congenital heart defects. Frontiers in Physiology. 2014;5(408) doi: 10.3389/fphys.2014.00408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan A, Samtani R, Dhanantwari P, Lee E, Yamada S, Shiota K, et al. A Detailed Comparison of Mouse and Human Cardiac Development. Pediatric research. 2014;76(6):500–507. doi: 10.1038/pr.2014.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruithof BPT, Duim SN, Moerkamp AT, Goumans M-J. TGFβ and BMP signaling in cardiac cushion formation: Lessons from mice and chicken. Differentiation. 2012;84(1):89–102. doi: 10.1016/j.diff.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Larina IV, Ivers S, Syed S, Dickinson ME, Larin KV. Hemodynamic measurements from individual blood cells in early mammalian embryos with Doppler swept source OCT. Optics letters. 2009;34(7):986–988. doi: 10.1364/ol.34.000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larina IV, Larin KV, Justice MJ, Dickinson ME. Optical Coherence Tomography for live imaging of mammalian development. Current opinion in genetics & development. 2011;21(5):579–584. doi: 10.1016/j.gde.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le VP, Kovacs A, Wagenseil JE. Measuring Left Ventricular Pressure in Late Embryonic and Neonatal Mice. 2012;(60):e3756. doi: 10.3791/3756. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Fei P, Packard RRS, Kang H, Xu H, Baek KI, et al. 4-Dimensional light-sheet microscopy to elucidate shear stress modulation of cardiac trabeculation. The Journal of Clinical Investigation. 2016;126(5):1679–1690. doi: 10.1172/JCI83496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Moghadam ME, Kung E, Cao H, Beebe T, Miller Y, et al. Moving Domain Computational Fluid Dynamics to Interface with an Embryonic Model of Cardiac Morphogenesis. PLOS ONE. 2013;8(8):e72924. doi: 10.1371/journal.pone.0072924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Gao E, Vite A, Yi R, Gomez L, Goossens S, et al. Alpha-Catenins Control Cardiomyocyte Proliferation by Regulating Yap Activity. Circulation research. 2015;116(1):70–79. doi: 10.1161/CIRCRESAHA.116.304472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebling M, Forouhar AS, Gharib M, Fraser SE, Dickinson ME. Four-dimensional cardiac imaging in living embryos via postacquisition synchronization of nongated slice sequences. SPIE; p. 10. (Year) [DOI] [PubMed] [Google Scholar]
- Lin C-J, Lin C-Y, Chen C-H, Zhou B, Chang C-P. Partitioning the heart: mechanisms of cardiac septation and valve development. Development (Cambridge, England) 2012;139(18):3277–3299. doi: 10.1242/dev.063495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey SE, Butcher JT, Yalcin HC. Mechanical Regulation of Cardiac Development. Frontiers in Physiology. 2014;5 doi: 10.3389/fphys.2014.00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Liu Y, Lai Y-P, Gu X-N, Liu D-M, Yang M. Fetal Hemodynamics and Fetal Growth Indices by Ultrasound in Late Pregnancy and Birth Weight in Gestational Diabetes Mellitus. Chinese Medical Journal. 2016;129(17):2109–2114. doi: 10.4103/0366-6999.189057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti JL, Jones EAV, Huang C, Chen J, Fraser SE, Dickinson ME. Vascular remodeling of the mouse yolk sac requires hemodynamic force. Development. 2007;134(18):3317–3326. doi: 10.1242/dev.02883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucitti JL, Tobita K, Keller BB. Arterial hemodynamics and mechanical properties after circulatory intervention in the chick embryo. Journal of Experimental Biology. 2005;208:1877–1885. doi: 10.1242/jeb.01574. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. American Journal of Anatomy. 1976;148:85–120. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- Martin RT, Bartman T. Analysis of heart valve development in larval zebrafish. Developmental Dynamics. 2009;238(7):1796–1802. doi: 10.1002/dvdy.21976. doi: [DOI] [PubMed] [Google Scholar]
- Martinsen BJ. Reference guide to the stages of chick heart embryology. Developmental Dynamics. 2005;233:1217–1237. doi: 10.1002/dvdy.20468. [DOI] [PubMed] [Google Scholar]
- Martinsen BJ, Lohr JL. Cardiac Development. In: AIaizzo P, editor. Handbook of Cardiac Anatomy, Physiology, and Devices. Totowa, NJ: Humana Press; 2005. pp. 15–23. [Google Scholar]
- Martinsen BJ, Lohr JL. Cardiac Development. In: AIaizzo P, editor. Handbook of Cardiac Anatomy, Physiology, and Devices. Cham: Springer International Publishing; 2015. pp. 23–33. [Google Scholar]
- McQuinn TC, Bratoeva M, deAlmeida A, Remond M, Thompson RP, Sedmera D. High-frequency ultrasonographic imaging of avian cardiovascular development. Developmental Dynamics. 2007;236:3503–3513. doi: 10.1002/dvdy.21357. [DOI] [PubMed] [Google Scholar]
- Menon V, Eberth J, Goodwin R, Potts J. Altered Hemodynamics in the Embryonic Heart Affects Outflow Valve Development. Journal of Cardiovascular Development and Disease. 2015;2(2):108. doi: 10.3390/jcdd2020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgett M, Chivukula VK, Dorn C, Wallace S, Rugonyi S. Blood flow through the embryonic heart outflow tract during cardiac looping in HH13–HH18 chicken embryos. Journal of The Royal Society Interface. 2015;12(111) doi: 10.1098/rsif.2015.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgett M, Goenezen S, Rugonyi S. Blood flow dynamics reflect degree of outflow tract banding in Hamburger-Hamilton stage 18 chicken embryos. Journal of the Royal Society Interface. 2014;11(100):20140643. doi: 10.1098/rsif.2014.0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgett M, López CS, David L, Maloyan A, Rugonyi S. Increased Hemodynamic Load in Early Embryonic Stages Alters Endocardial to Mesenchymal Transition. Frontiers in Physiology. 2017a;8(56) doi: 10.3389/fphys.2017.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgett M, Rugonyi S. Congenital heart malformations induced by hemodynamic altering surgical interventions. Frontiers in Physiology. 2014;5 doi: 10.3389/fphys.2014.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midgett M, Thornburg KL, Rugonyi S. Blood Flow Patterns Underlie Developmental Heart Defects. American Journal of Physiology - Heart and Circulatory Physiology. 2017b;312(3):H632–H642. doi: 10.1152/ajpheart.00641.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miquerol L, Kelly RG. Organogenesis of the vertebrate heart. Wiley Interdisciplinary Reviews: Developmental Biology. 2013;2(1):17–29. doi: 10.1002/wdev.68. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, et al. Heart Disease and Stroke Statistics—2015 Update. A Report From the American Heart Association. 2015;131(4):e29–e322. doi: 10.1161/cir.0000000000000152. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Clark EB, Hu N, Wispe J. Effect of Environmental Hypothermia on Vitelline Artery Blood Pressure and Vascular Resistance in the Stage 18, 21, and 24 Chick Embryo. Pediatric Research. 1985;19:651. doi: 10.1203/00006450-198507000-00003. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Miyagawa S, Ohno T, Miura S, Takao A. Developmental Hemodynamic Changes in Rat Embryos at 11 to 15 Days of Gestation: Normal Data of Blood Pressure and the Effect of Caffeine Compared to Data from Chick Embryo. Pediatric Research. 1988;23:200. doi: 10.1203/00006450-198802000-00015. [DOI] [PubMed] [Google Scholar]
- Nakazawa M, Miyagawa S, Takao A, Clark EB, Hu N. Hemodynamic Effects of Environmental Hyperthermia in Stage 18, 21, and 24 Chick Embryos. Pediatric Research. 1986;20(1213) doi: 10.1203/00006450-198612000-00001. [DOI] [PubMed] [Google Scholar]
- Oosterbaan AM, Ursem NTC, Struijk PC, Bosch JG, van der Steen AFW, Steegers EAP. Doppler flow velocity waveforms in the embryonic chicken heart at developmental stages corresponding to 5–8 weeks of human gestation. Ultrasound in Obstetrics and Gynecology. 2009;33(6):638–644. doi: 10.1002/uog.6362. [DOI] [PubMed] [Google Scholar]
- Peterson LM, Gu S, Karunamuni G, Jenkins MW, Watanabe M, Rollins AM. Embryonic aortic arch hemodynamics are a functional biomarker for ethanol-induced congenital heart defects [Invited] Biomedical Optics Express. 2017;8(3):1823–1837. doi: 10.1364/BOE.8.001823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phoon CK, Turnbull DH. Ultrasound biomicroscopy-Doppler in mouse cardiovascular development. Physiological Genomics. 2003;14(1):3–15. doi: 10.1152/physiolgenomics.00008.2003. [DOI] [PubMed] [Google Scholar]
- Poelma C, Van der Heiden K, Hierck BP, Poelmann RE, Westerweel J. Measurements of the wall shear stress distribution in the outflow tract of an embryonic chicken heart. Journal of The Royal Society Interface. 2010;7(42):91–103. doi: 10.1098/rsif.2009.0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie M, Stovall S, Carson J, Danilchik M, Thornburg K, Rugonyi S. Hemodynamics Modify Collagen Deposition in the Early Embryonic Chicken Heart Outflow Tract. Journal of Cardiovascular Development and Disease. 2017;4(4):24. doi: 10.3390/jcdd4040024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rugonyi S. Genetic and flow anomalies in congenital heart disease. AIMS Genetics. 2016;3(3):157–166. doi: 10.3934/genet.2016.3.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Runyan RB, Potts JD, Weeks DL, Sharma RV, Loeber CL, Chiang JJ, et al. Tissue Interaction and Signal Transduction in the Atrioventricular Canal of the Embryonic Hearta. Annals of the New York Academy of Sciences. 1990;588(1):442–443. doi: 10.1111/j.1749-6632.1990.tb13257.x. [DOI] [Google Scholar]
- Samsa LA, Givens C, Tzima E, Stainier DYR, Qian L, Liu J. Cardiac contraction activates endocardial Notch signaling to modulate chamber maturation in zebrafish. Development. 2015;142(23):4080–4091. doi: 10.1242/dev.125724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedmera D, Pexieder T, Rychterova V, Hu N, Clark EB. Remodeling of chick embryoniv ventricular myoarchitecture under experimentally changed loading conditions. The Anatomical Record. 1999;254:238–252. doi: 10.1002/(SICI)1097-0185(19990201)254:2<238::AID-AR10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Sehnert AJ, Huq A, Weinstein BM, Walker C, Fishman M, Stainier DYR. Cardiac troponin T is essential in sarcomere assembly and cardiac contractility. Nature Genetics. 2002;31:106. doi: 10.1038/ng875. https://www.nature.com/articles/ng875#supplementary-information. [DOI] [PubMed] [Google Scholar]
- Shi L, Goenezen S, Haller S, Hinds MT, Thornburg KL, Rugonyi S. Alterations in pulse wave propagation reflect the degree of outflow tract banding in HH18 chicken embryos. American Journal of Physiology - Heart and Circulatory Physiology. 2013;305:H386–H396. doi: 10.1152/ajpheart.00100.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slough J, Cooney L, Brueckner M. Monocilia in the embryonic mouse heart imply a direct role for cilia in cardiac morphogenesis. Developmental dynamics : an official publication of the American Association of Anatomists. 2008;237(9):2304–2314. doi: 10.1002/dvdy.21669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stainier DY, Lee RK, Fishman MC. Cardiovascular development in the zebrafish. I. Myocardial fate map and heart tube formation. Development. 1993;119(1):31–40. doi: 10.1242/dev.119.1.31. [DOI] [PubMed] [Google Scholar]
- Stankunas K, Ma GK, Kuhnert FJ, Kuo CJ, Chang C-P. VEGF Signaling has Distinct Spatiotemporal Roles During Heart Valve Development. Developmental biology. 2010;347(2):325–336. doi: 10.1016/j.ydbio.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart DE, Kirby ML, Sulik KK. Hemodynamic changes in chick embryos precede heart defects after cardiac neural crest ablation. Circulation Research. 1986;59(5):545–550. doi: 10.1161/01.res.59.5.545. [DOI] [PubMed] [Google Scholar]
- Stovall S, Midgett M, Thornburg K, Rugonyi S. Changes in dynamic embryonic heart wall motion in response to outflow tract banding measured using video densitometry. SPIE; p. 11. (Year) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suga H, Sagawa K, Shoukas AA. Load independence of the instantaneous pressure-volume ratio of the canine left ventricle and effects of epinephrine and heart rate on the ratio. Circulation Research. 1973;32:314–321. doi: 10.1161/01.res.32.3.314. [DOI] [PubMed] [Google Scholar]
- Tan H, Biechler S, Junor L, Yost MJ, Dean D, Li J, et al. Fluid flow forces and rhoA regulate fibrous development of the atrioventricular valves. Developmental Biology. 2013;374(2):345–356. doi: 10.1016/j.ydbio.2012.11.023. [DOI] [PubMed] [Google Scholar]
- Tobita K, Garrison JB, Liu LJ, Tinney JP, Keller BB. Three-dimensional myofiber architecture of the embryonic left ventricle during normal development and altered mechanical loads. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2005;283A(1):193–201. doi: 10.1002/ar.a.20133. [DOI] [PubMed] [Google Scholar]
- van den Berg G, Moorman AFM. Concepts of Cardiac Development in Retrospect. Pediatric Cardiology. 2009;30(5):580–587. doi: 10.1007/s00246-008-9369-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vennemann P, Kiger KT, Lindken R, Groenendijk BC, Stekelenburg-de Vos S, ten Hagen TL, et al. In vivo micro particle image velocimetry measurements of blood-plasma in the embryonic avian heart. J Biomech. 2006;39(7):1191–1200. doi: 10.1016/j.jbiomech.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Vermot J, Forouhar AS, Liebling M, Wu D, Plummer D, Gharib M, et al. Reversing Blood Flows Act through klf2a to Ensure Normal Valvulogenesis in the Developing Heart. PLOS Biology. 2009;7(11):e1000246. doi: 10.1371/journal.pbio.1000246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, et al. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Webb S, Qayyum SR, Anderson RH, Lamers WH, Richardson MK. Septation and separation within the outflow tract of the developing heart. Journal of Anatomy. 2003;202(4):327–342. doi: 10.1046/j.1469-7580.2003.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiford BC, Subbarao VD, Mulherm KM. Noncompaction of the ventricular myocardium. Circulation. 2004;109:2965–2971. doi: 10.1161/01.CIR.0000132478.60674.D0. [DOI] [PubMed] [Google Scholar]
- Wiederhielm CA, Woodbury JW, Kirk S, Rushmer RF. Pulsatile pressures in the microcirculation of frog's mesentery. Am J Physiol. 1964;207:173–176. doi: 10.1152/ajplegacy.1964.207.1.173. [DOI] [PubMed] [Google Scholar]
- Zhang H, von Gise A, Liu Q, Hu T, Tian X, He L, et al. Yap1 Is Required for Endothelial to Mesenchymal Transition of the Atrioventricular Cushion. The Journal of Biological Chemistry. 2014;289(27):18681–18692. doi: 10.1074/jbc.M114.554584. [DOI] [PMC free article] [PubMed] [Google Scholar]