Abstract
The central nervous system is not a static, hard-wired organ. Examples of neuroplasticity, whether at the level of the synapse, the cell, within and between circuits can be found during development, throughout the progression of disease or after injury. One essential component of the molecular, anatomical and functional changes associated with neuroplasticity is the spinal interneuron (SpIN). Here we draw on recent multidisciplinary studies to identify and interrogate subsets of SpINs and their roles in locomotor and respiratory circuits. We highlight some of the recent progress that elucidates the importance of SpINs in circuits affected by spinal cord injury, especially those within respiratory networks, as well as discuss potential ways spinal neuroplasticity can be therapeutically harnessed for recovery.
Keywords: Neuroplasticity, spinal cord injury, spinal interneurons, transplantation
Spinal Interneurons – An essential element for neuroplasticity
The adult mammalian spinal cord is comprised of 4 main neuronal types: lower (or spinal) motoneurons, pre-ganglionic neurons, ascending projection neurons, and spinal interneurons (see Box 1). Although more than 20 spinal interneuron (SpIN) subtypes have been identified by their location, electrophysiological properties and specific transcriptional factors [1], there are likely many more that are yet to be characterized. In the normal spinal cord, SpINs i) receive supraspinal sensorimotor information, ii) transduce sensorimotor information sent from the spinal cord to supraspinal centers by ascending tract neurons, iii) modulate motoneuron activity, iv) relay information between near and distant spinal cord segments (short and long propriospinal neurons, respectively, with ascending or descending projections), and v) send information to the opposite side of the spinal cord (commissural SpINs). This passage of information can be described both anatomically (e.g. ipsi- vs. contralateral, commissural) and/or functionally (e.g. excitatory, inhibitory or modulatory; reviewed in [1–3]). Furthermore, this can occur within a given motor or sensory system (e.g. phrenic motor system [4]), or between distinct systems (e.g. locomotor and respiratory [5])_. In addition to the diversity of these roles, SpINs readily sprout axon collaterals and/or dendrites, reorganize their connectivity [6], and can even switch their phenotype [7]. This has recently been illustrated by evidence of the involvement of SpINs in anatomical reorganization after injury and disease [8, 9], and in functional plasticity resulting in both adaptive [6, 10–13] and maladaptive changes in outcome [14–17].
Box 1. What is a Spinal Interneuron?
In the literal sense of the word, and building on a historical definition, an “interneuron” is a neuron that connects other neurons within the central nervous system. As we continue to learn more about how the nervous system is connected and how it functions, our appreciation for interneurons expands. We now know that they are more than just ‘relay’ cells. They are capable of deciphering inputs they receive and regulating and modulating output of the cells they connect to.
An interneuron is now most often defined as a neuron that projects within a structure. This distinguishes them from ‘projection neurons’, which are those that project outside of the structure that the cell body resides in. Thus, spinal interneurons (SpINs) are those located within the spinal cord, that project to other cells within the spinal cord. The spinal cord is comprised of lower (or spinal) motoneurons (innervating muscles), pre-ganglionic neurons (innervating peripheral ganglia), ascending tract neurons (those that project to ‘supraspinal neurons’) and the SpINs. Note that while the ascending tract neurons are often considered relay neurons which are located in the spinal cord, because they project out of the spinal cord they are not technically considered ‘spinal interneurons’.
SpINs can be divided further into local (or segmental) neurons and propriospinal neurons (not to be confused with ‘proprioceptive’ neurons). Local SpINs integrate neurons within or between networks over short distances. These project either within a spinal segment (intrasegmentally) or between 1–2 spinal segments (intersegmentally). Propriospinal neurons project intersegmentally to integrate quite distinct neuronal circuits over short or long distances. By definition, propriospinal refers to cells contained within the spinal cord (“proprio” derived from the Latin word for “within one’s self”). While this definition is consistent with all SpINs, the term is used to describe neurons that project between spinal segments within the spinal cord. Short propriospinal neurons project between few spinal segments (e.g. within the cervical or thoracic spinal segments), while long propriospinal neurons project between many segments (e.g. form cervical to lumbar spinal cord). Worth noting is that distinguishing between local SpINs and short propriospinal neurons is sometimes quite difficult (size and length of axon is not sufficient), and the term used to describe them may depend more on their functional role.
SpINs have axons that can project on the same side (ipsilaterally) or to the opposite side of the spinal cord (contralaterally). Those with projections that cross the spinal midline to the contralateral side within the same spinal segment as their cell body are known as ‘commissural’ interneurons. These SpINs project via the spinal commissures which are tracts crossing the spinal midline at the level of origin. SpINs may also have axons that cross the spinal midline or ‘decussate’ obliquely between spinal segments (the axon crosses at a level other than its origin). It is worth noting that a single SpIN can have axon collaterals that project bilaterally (innervate cells both ipsi- and contralaterally). See also [137].
Neuroplasticity (see Glossary) can occur following injury or disease, or can be stimulated or enhanced with therapeutic interventions (reviewed in [18]). As is highlighted below, there is a growing experimental and clinical appreciation for adaptive plasticity (e.g. partial motor recovery) and maladaptive plasticity (e.g. neuropathic pain) exhibited by the injured spinal cord (reviewed in [11, 18]). Furthermore, SpINs have been identified as a key component of these neuroplastic changes. The present review highlights the critical role of SpINs in recovery after spinal cord injury (SCI), and treatments designed to therapeutically target them to enhance repair and recovery, and limit maladaptive changes.
Spinal Interneuron Subtypes
The convergence between developmental biology, genetics, physiological and behavioral systems have allowed for interrogation of the specific roles that subsets of SpINs play in generation, modulation and execution of function (reviewed in [3, 19, 20]). Understanding the neuroplastic potential of SpINs will likely elucidate important mechanisms that occur within spinal networks following injury or disease. To appreciate their potential contribution to neuroplasticity, it is important to first highlight interneuronal diversity.
Homeodomain transcription factors expressed early in development have been used to divide interneurons into classes of identifiable populations (Figure 1). The dI1–6 interneurons are born dorsally, although some migrate ventrally during development (reviewed in [1]). The ventrally born interneurons are divided into V0–V3 and Vx, and further divided into subtypes (e.g. V2a and V2b). This division of interneurons based on developmentally expressed transcription factors has provided a means to i) determine transmitter phenotypes, ii) identify general projection patterns (ipsilateral or contralateral), and iii) manipulate subsets of neurons in order to ascribe function within the hindlimb central pattern generator (reviewed in [3, 21]). These same divisions are present throughout the spinal cord and therefore relevant in the consideration of both locomotor and respiratory functions. The focus of prior studies of transcription factor expression has been mostly on locomotor networks at lumbar spinal levels. There is also now evidence to show some differences between lumbar and cervical levels [22]. How some of these distinctions might impact our understanding of cervical and lumbar locomotor networks, or primary respiratory networks that are located at cervical and thoracic levels, is the subject of ongoing investigation.
Figure 1. Identifying subtypes of spinal interneurons (SpINs) in the mammalian spinal cord.
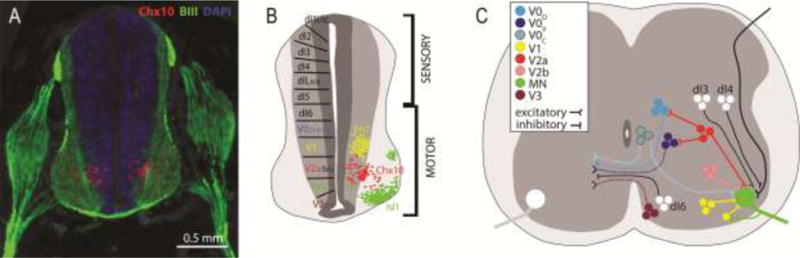
Cross-section through 13-day old embryonic rat spinal cord (A), with schematic diagrams of the embryonic (B) and postnatal (C) mammalian spinal cord, identifying known SpIN progenitors. SpINs can be identified anatomically using markers for transcriptional factors known to be expressed during development. Immunolabeling for Chx10 (A) identifies the ventral distribution of V2a SpINs. Note: beta-III-Tubulin (BIII) labeled spinal roots, dorsal root ganglion and spinal nerve surrounding the developing neural tube. Ventrally-derived progenitors are highlighted in B and C. Note that crossed projections from SpINs (C) can innervate motoneurons directly, or other SpINs.
Excitatory SpINs
The majority of ventrally derived locomotor-related excitatory SpINs come from the V0V, V2a, and V3 ventral populations. The V0V population is exclusively commissural and maintains alternation at higher locomotor speeds, through connections with inhibitory neurons, which likely project to both motoneurons and rhythm generating SpINs (reviewed in [3]). The excitatory V3 population is mainly commissural and can be further divided based on neuronal size and location [23]. The V2a SpINs are exclusively ipsilaterally projecting and rhythmically active during locomotion [24–26], and they are implicated in left-right coordination, particularly at higher locomotor speeds, via input to excitatory V0V population [27, 28]. A subpopulation of V2a interneurons expressing the transcription factor Shox2 does not appear to be involved in left-right coordination, but instead is involved in the activation of ipsilateral motoneurons [29]. Importantly, this latter role could mean that recruitment of such SpINs after injury could facilitate enhanced motoneuron activity. This highlights the fact that even within the V2a subtype of SpINs, there are additional subclasses of interneurons that are still being defined. RNA-sequencing has revealed type I and II V2a neurons within cervical and lumbar spinal cord, that can be distinguished based on a molecular, anatomical and functional basis [22]. Type I V2as appear to make short local connections, continue expressing Chx10 into adulthood and are abundant throughout the spinal levels. Type II have either local or supraspinal projections, downregulate Chx10 expression with development and are most abundant at cervical spinal levels. V2as can also be divided into medial and lateral pools of SpINs in the intermediate-ventral grey matter, each with distinct gene expression [22].
Locomotor rhythm generating neurons have been found to include at least two more populations of excitatory, ipsilaterally projecting SpINs: the Shox2+ nonV2a SpINs [29] and the Hb9 SpINs [30]. The small ventromedial group of Hb9, or “Vx”, SpINs have received a lot of attention as they have many of the hallmarks expected of a rhythm generating population [31]. However, neither the Shox2+ nonV2a nor Vx SpINs are likely to be the only rhythm generating neurons [29, 30].
Studies of neuroplasticity involving specific SpIN subtypes are in their infancy. V2a SpINs are perhaps the most well characterized. Like spinal motoneurons, V2a SpINs display a super-sensitivity to serotonin after SCI, at least partly due to an increased clustering of 5-HT2C receptors on these neurons [32]. Although alterations in connectivity within locomotor networks post-SCI have not been described, the V2a SpINs are an attractive target for mediating circuit changes as many of the V2a SpINs have long ascending and/or descending axonal branches [24] and have the potential to enhance trans-lesional coordination. As outlined below, they also appear to play a role in respiratory plasticity post-SCI [8].
Inhibitory SpINs
Inhibitory SpINs are essential to regulation of excitability of other interneurons and lower motoneurons. They play a key role in the alternation of both flexors-extensors and left-right sides of the body. The spinal circuitry controlling this in rodents is the intrasegmental dual inhibitory system, which is comprised of two pathways that are: i) mediated by glycinergic/GABAergic commissural SpINs that have projections onto contralateral motoneurons, and ii) mediated by glutamatergic commissural SpINs, resulting in the indirect inhibition of contralateral motoneurons [33]. The inhibitory V0D and excitatory V0V commissural SpINs make up the direct and indirect inhibitory pathways, respectively, and have been shown to underlie different alternating gaits in rodents [34].
While the V1 interneurons comprise more than a third of inhibitory SpINs, their diversity and distribution is still being elucidated [35]. Renshaw and Ia SpINs comprise 30% of the V1s, which are a class of glycinergic/GABAergic and ipsilaterally projecting interneurons derived from Engrailed 1 (En1) expressing developing cells. Although the V1 class of SpINs are implicated in rhythmic inhibition of locomotor activity, genetic ablation of the entire V1 population results in consistent slowing of the locomotor frequency, whilst leaving rhythmic inhibition intact [36]. Thus, rhythmic inhibition of motoneurons is generated by several groups of molecularly defined interneurons, including V1 SpINs, non-reciprocal Ia SpINs and other subtypes such as Gata2/3-expressing V2b SpINs [37].
SpIN Subtypes in Respiratory Networks
Anatomical and electrophysiological characterization of respiratory SpINs has led to a growing appreciation for their involvement in respiration and plasticity after injury (Table 1). The focus of previous work has been on SpINs innervating the phrenic and intercostal motor systems, although there are likely interneurons that innervate other respiratory systems. These respiratory SpINs are bilaterally distributed throughout the ventral, intermediate and dorsal grey matter, and innervate phrenic and intercostal motoneurons via ipsi- or contralateral projections. Dual transneuronal tracing also reveals that subsets of SpINs in the intermediate grey matter anatomically integrate i) the phrenic circuitry on each side of the spinal cord, and ii) phrenic and intercostal circuitry [4]. This is consistent with electrophysiological data suggesting that SpINs integrate and modulate activity in respiratory motoneuron pools.
Table 1.
Respiratory spinal interneurons in mammals. These assessments were made in adult animals unless specified. Modified from Lane [104]. References to specific examples are indicated with an asterisk.
| Innervating | Spinal level | Subtype | Rostro-caudal distribution | Species | References | |
|---|---|---|---|---|---|---|
| Electrophysiological demonstration | Phrenic motor system | Cervical | C1–C2 | Cat | [105–109] | |
| Excitatory* | C1–C2 | Rat | [110–114] | |||
| Inhibitory* | C3–C5 | Cat | [115–118] | |||
| C3–C5 | Rabbit | [119, 120] | ||||
| Excitatory | C4–5 | Rat | [38, 40] | |||
| C4–C6 | Guinea Pig | [121] | ||||
| Intercostal motor system | Cervical | C1–C2 | Cat | [105, 106] | ||
| Excitatory* | C1–C2 | Rat | [113, 122] | |||
| Thoracic | Excitatory | – | Cat | [123–128] | ||
| Expiratory motoneurons | Cervical | C1–C2 | Cat | [129] | ||
| C3–T9 | Cat | [129] | ||||
| Anatomical demonsration | Phrenic motoneurons | Cervical | C1–T4 | Cat | [130] | |
| C1–C7 | Ferret | [131] | ||||
| CholinergicV0C | Neonatal Mouse | [41] | ||||
| Excitatory | Neonatal Mouse | [45] | ||||
| Excitatory *V2a | Mouse | [8, 132]* | ||||
| C1–C7 | Rat | [4, 47, 133] | ||||
| Inhibitory | C3–C5 | Rat | [43, 44] | |||
| Intercostal motoneurons | Cervical | Rat | [4] | |||
| Abdominal motoneurons | Abdominal | T4–L4 | Ferret | [134–136] |
Respiratory SpINs within the cervical spinal cord are often divided into those at upper- and mid-cervical levels (Table 1). The anatomical distribution of pre-phrenic SpINs [4], and their distinct firing patterns [38], suggest that there is substantial heterogeneity among respiratory interneurons. It is also possible that distinct subsets of SpINs are recruited under different conditions (e.g. physiological demands). For example, interneurons within the mid-cervical level can exhibit altered activity when the animals are exposed to hypoxia [39, 40] and altered connectivity after SCI [8] (see below).
While the role of SpINs in control and modulation of respiration remains a subject of ongoing investigation, their potential contribution to neuroplastic changes and reorganization is of increasing interest to those studying SCI or disease. Although respiratory drive and pattern generation is known to originate within the medulla, several lines of evidence suggest that the spinal cord retains the capacity to drive respiratory activity without supraspinal input. While the identity of spinal neurons that contribute to this phenomenon has been elusive, recent studies of the injured spinal cord have started to provide important insights.
The use of transgenic models and molecular genetics has meant that the roles specific interneuronal subtypes play in generation and modulation of respiration can now be explored more extensively. Most research effort has focused on the neuronal phenotypes within the respiratory brainstem nuclei, but SpINs within respiratory networks are now also being identified (e.g. V0 [41]; V2a [42]). Aside from their reported contribution to respiratory control, V2a SpINs have been implicated to play a crucial role in compensatory plasticity that occurs with early stages of amyotrophic lateral sclerosis. More specifically, the V2a class of interneurons located both in the brainstem and spinal cord are crucial to the recruitment of inspiratory accessory respiratory muscles, activity of which maintains ventilation during disease progression [9].
Although much less is known about inhibitory SpINs within the spinal respiratory network, some have been identified within the mid-cervical spinal cord which were shown to modulate phrenic motor output [43, 44]. Specific SpIN subtypes involved in these neuroplastic mechanisms, however, are currently unknown. Interestingly, disinhibition of inhibitory SpINs and/or descending supraspinal inputs via pharmacological manipulation (i.e. antagonists of glycinergic/GABAergic inputs such as picrotoxin and strychnine) unmasks the potential for activation of respiratory patterns mediated by glutamatergic SpINs [45]. Pharmacological disinhibition may therefore enhance the potential for restorative plasticity and may open a therapeutic window for the use of additional treatments that can enhance plasticity and recovery.
Identifying Spinal Interneurons after Spinal Cord Injury
Advances in transneuronal tracing and their applications to the spinal cord have improved our understanding of the diverse populations of SpINs that exist in the intact and injured spinal cord. Transneuronal tracing, for example, has allowed the mapping of neuronal networks in the intact or injured spinal cord, that mediate muscle [4, 6, 8, 46, 47] or organ function [14, 48, 49]. Combining retrograde and anterograde tracing techniques also allows the supraspinal, spinal or primary afferent inputs to SpINs to be explored in the naïve or injured spinal cord (e.g. [4, 50]).
In addition to anatomically mapping the reorganization of interneuronal networks post-injury, immunohistochemical staining for immediate early genes (e.g. c-fos, zif268, Arc) has enabled anatomical assessment of alterations in neuronal activity [49, 51]. Increased c-fos reactivity could be related to changes in motor or sensory plasticity, and possibly across multiple circuits [52]. By combining the use of neuronal activity markers with anatomical tracers [15], neuronal activity within specific circuits of interest can be visualized, providing a more specified functional insight.
The development of opto- and chemogenetic methods has enhanced the resolution by which we can target SpIN activity in the naïve or injured spinal cord. Capitalizing on this, Alilain et al. [53] used optogenetic techniques to demonstrate the functional influence of spinal phrenic motor circuitry on diaphragm activity, by selectively stimulating transduced moto- and interneurons immediately caudal to a high cervical SCI [53]. Furthermore, genetic strategies, including transgenic animals and viral vectors, to fluorescently tag and specifically manipulate subsets of interneurons allows for their identification and functional analysis [54, 55]. These strategies are now being applied to preclinical studies of SCI, to assess how specific subsets of SpINs contribute to neural plasticity after injury [8, 32, 56].
Spinal Interneurons following Spinal Cord Injury
SpINs comprise neuronal networks that can provide new anatomical pathways capable of facilitating functional recovery [6, 8, 57–59]. Most traumatic SCIs are anatomically incomplete, even when considered functionally or neurologically complete, thus sparing some tissue at the site of injury. Tissue sparing likely means that some SpINs retain synaptic connections with neurons around the injury site. Even if normally latent, these connections can become activated or strengthened following injury, providing a ‘by-pass’ pathway around the injury (Figure 2). SpINs may also undergo axonal sprouting, allowing interneurons that were not previously part of a neuronal circuit to be recruited into a new neuronal circuit (Figure 2). Thus, whether they are i) part of an existing network with connections around a partial injury, or ii) they are recruited into newly formed neuronal pathways that develop around the injury over time, SpINs can relay information from the brain, around the injury, to motoneurons below the injury, at least partially restoring control to muscles. Even following an anatomically complete SCI, spared tissue in denervated neuronal networks caudal to injury can be therapeutically re-activated.
Figure 2.
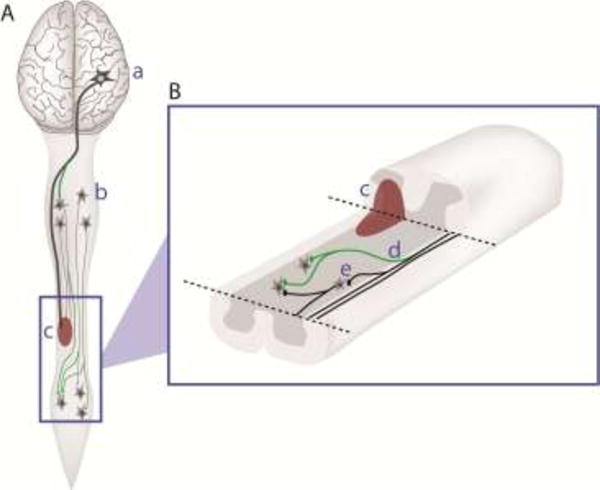
Anatomical plasticity after spinal cord injury
This schematic diagram offers an overview of the entire mammalian central nervous system (A), showing axonal projections from supraspinal neurons in the brain (a) and propriospinal interneurons (b), to neurons at lumbar spinal levels. A spinal cord injury (SCI), which partially disrupts these axons, is shown in red (c). After SCI, the intact axons can undergo some limited axonal growth (or ‘sprouting’) that can lead to the formation of new axonal projections and pathways (green, d in B), some of which may project around the site of injury, by-passing the damaged area. It is not clear whether the ability to undergo axonal sprouting differs between SpIN subtypes (i.e. do some interneurons have more axonal sprouting than others). A close-up of the spinal cord (B), with the dorsal tissue cut-away to reveal the intermediate grey matter, shows crossed-pathways from the uninjured side of the spinal cord, to neurons below the site of injury. While some of these pathways are thought to be newly formed via axonal sprouting (d), others may pre-exist (b) and become more active or strengthened after injury. These examples of plasticity can also be therapeutically enhanced. Figure is modified from Reier et al. [103].
The loss of supraspinal and propriospinal inputs to neurons caudal to SCI also results in altered synaptic properties of connected neurons. Sub-acutely following a complete thoracic spinal transection, Skup et al. [60] report a reduction in local cholinergic inputs to lumbar motoneurons. However, this reduction can be attenuated with locomotor training. Chronic SCI also affects neuronal networks and connectivity. For example, studies on deep dorsal horn interneurons revealed considerable spontaneous plasticity in both intrinsic and synaptic mechanisms that occur after SCI, which take at least 10 weeks after SCI to stabilize [58].
An important consideration is that not all newly formed connections will be beneficial (reviewed in [17, 61]). A maladaptive increase in connectivity between inhibitory SpINs and spinal motor networks post-SCI may limit potential motor recovery. Alternatively, excessive recruitment of excitatory SpINs into sensory networks may enhance pain or spasticity. Increased excitatory input to lower motoneurons without regulation by inhibitory SpINs can also lead to hyper-excitability, neuronal damage and functional loss, as seen in amyotrophic lateral sclerosis (reviewed in [62]). Thus, treatments used to harness the neuroplastic potential of SpINs need to take possible adverse effects into account, with some consideration given to the SpIN subtypes that respond to treatment.
Spinal Interneurons in Respiration following Cervical Spinal Cord Injury
Given that respiratory networks are distributed throughout the cervical, thoracic and lumbar spinal cord (Table 1), injury at nearly any spinal level can affect respiratory muscle activity. The most devastating consequences arise following cervical level injuries which not only compromise supraspinal input to all respiratory networks, but can also compromise spinal phrenic circuitry which controls function of the primary respiratory muscle – the diaphragm. Impaired diaphragm function often necessitates assisted ventilation and increases the risk of morbidity and mortality (reviewed in [63, 64]). Despite these devastating consequences, there are many examples of endogenous spinal respiratory neuroplasticity post-SCI (reviewed in [63, 65]) that lead to some functional improvement, albeit limited. Improvements in our understanding of the anatomical changes that contribute to this functional recovery are allowing for the development and refinement of treatments to enhance neuroplasticity. One of the best pre-clinical models of respiratory neuroplasticity is the high cervical (C2) lateral hemisection, in which ipsilateral phrenic motoneuron and diaphragm activity can be induced shortly after injury (crossed phrenic phenomenon, CPP) or arise spontaneously weeks post-injury (termed as spontaneous CPP, sCPP) [66]; also reviewed in [18, 63]). More recent studies have shown that contusion/compression injury that results in clinically comparable neuropathological deficits, also exhibits spontaneous respiratory plasticity, despite gray matter damage typical of contusive injuries (reviewed in [18, 63]).
Recovery observed with the CPP and sCPP has been attributed to spared (uninjured) mono- and polysynaptic bulbospinal pathways within the spinal cord contralateral to the injury [13, 47]. It has also been shown that some of these pathways comprise glutamatergic inputs to phrenic motoneurons which are essential for restorative plasticity [67–69]. While some glutamatergic input is derived from supraspinal projections, more recent work has also shown that this post-injury excitatory input comes in part from glutamatergic SpINs. Not only are glutamatergic SpINs capable of driving rhythmic phrenic output in the absence of supraspinal drive [45], but their input to spinal phrenic networks is increased post-SCI [8], and silencing them attenuates recovery.
Transneuronal tracing has revealed changes in interneuronal connectivity to phrenic circuitry following contusion [59] or hemisection injury [47], identifying excitatory V2a SpINs as contributors to anatomical plasticity post-injury [8]. However, how other SpIN subtypes contribute to respiratory function post-injury remains unknown. The heterogenous population of local SpINs that are connected with the spinal phrenic network likely have quite diverse functional roles, which may change under different physiological conditions [39, 40], and may not all be beneficial for recovery, as the example with inhibitory SpINs potentially limiting the extent of recovery post-SCI [70]. Whether specific SpIN phenotypes can be therapeutically harnessed to maintain adaptive, and avoid maladaptive plasticity, remains of significant interest to the field.
Therapeutic targeting of SpINs after SCI
The neuroplastic potential of SpINs after injury or disease makes these cells an attractive target for therapeutic interventions. Such interventions include activity-based therapies, such as exercise and rehabilitation [51, 71] (reviewed in [72]); neural interfacing, such as epidural stimulation [51] (reviewed in [73–75]); and cell therapies, such as transplantation of SpIN precursors [76–78]. The current challenge is to understand how to access, stimulate, and/or enhance these spinal networks, establishing sustainable excitability of damaged/denervated circuits. Furthermore, the goal is to do so in a way that enhances recovery while limiting maladaptive effects.
Pharmacological, electrical, and – in animal models – chemogenetic targeting of SpIN populations can be used to disinhibit, amplify or ‘prime’ spared spinal networks. Even the isolated spinal cord has the capacity to generate locomotor patterns if stimulated [71] (reviewed in [74, 79]). Such stimulation can be derived from activity-based therapies with the appropriate pattern and duration of repetitive activity or direct neural stimulation (Figure 3). Task-specific, activity dependent plasticity can be elicited in the functionally isolated spinal cord, months to even years after SCI when neuronal networks are stimulated appropriately. There is some evidence to suggest that this effect is at least in part mediated by cholinergic SpINs [60, 80]. Harkema [72] suggested that this input may then recruit SpIN networks that play a significant role in generating locomotor patterns. Recent pre-clinical studies also suggest that SpINs are recruited by respiratory training (e.g. intermittent hypoxia [38, 40]), and may be activated by direct neural stimulation of the denervated cervical spinal cord (reviewed in [75]).
Figure 3. Therapeutic strategies to target spinal interneuronal (SpIN) plasticity.
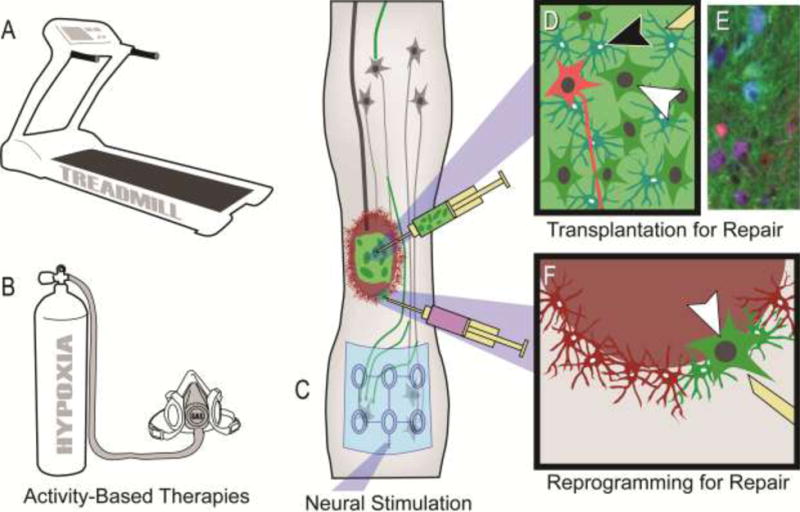
Neuroplasticity within spinal networks can be enhanced by increasing activity within neurons spared by injury or repairing injured neural substrates. Strategies targeting neurons and circuitry spared by injury can typically be divided into 1) activity-based therapies that stimulate activity and afferent feedback to the spinal cord, and 2) more invasive electrical stimulation of central or peripheral neural pathways and neurons. A range of activity-based therapies exist, that can target specific aspects of function, such as locomotor training (A) or respiratory training with intermittent exposure to low-oxygen (hypoxia; B). Neural stimulation can be applied almost anywhere in the periphery, spinal cord (e.g. schematic of an epidural stimulating array electrode in C) or brain. The limitation of such approaches is that they rely on neural pathways spared by the injury. In contrast, some repair strategies, such as cell transplantation (D,E) or in vivo cellular reprogramming (F), can provide new sources of spinal neurons that can form novel pathways and contribute to plasticity. Transplantation of neuronal and glial progenitors (white and black arrowheads in D, respectively) provides the building blocks for repair, and these cells can be tagged to track their distribution (e.g. with a green dye as shown). An important consideration with such repair strategies is the type of neuron being provided. Cellular engineering is now being used to direct the development of donor neuronal progenitors towards specific SpIN subtypes which can be tagged with separate markers (shown in red in D, white arrowhead). An example of recent work using this approach in the injured rat spinal cord is shown in E (one month following transplantation of neuronal lineage-restricted progenitors in blue-cyan, enriched with V2a neurons in red-purple). Image reproduced with permission from [77]. Alternatively, viral injection into a spinal contusion cavity 1 week after mid-cervical injury in the adult rat, can selectively target cells (e.g. astrocytes shown in red in E) and convert them into neurons (converting cells shown in green. Arrowhead in indicates a converted neuron. *Please note that treadmill training for individuals with SCI is usually performed with body-weight support as illustrated in Reier et al. [103]. For respiratory training with intermittent hypoxia individuals are given a small mask that covers their mouth and nose, and intermittently exposed to low-oxygen.
The contribution of SpINs to recovery after activity-based treatments has been demonstrated in the injured spinal cord using markers of neuronal activity (e.g. c-fos) to anatomically map activity within SpIN populations [52], and anatomical tracing to identify changes in spinal network connectivity [51]. By combining these outcome measures with genetic labeling of SpIN subtypes, Bui et al. [56] identified that recovery following activity-based therapies is at least in part dependent on a specific excitatory, ipsilaterally projecting population of SpINs (dI3 neurons). A limitation of stimulation or activity-based therapies is that they primarily rely on spared neural pathways to be activated and/or recruited to promote plasticity. In contrast, strategies designed to enhance anatomical repair may provide a greater capacity for plasticity.
Harnessing SpINs to Repair the Injured Spinal Cord
A discussion of the vast range of strategies developed for repair of the injured spinal cord, and their targeted effects on SpINs, is beyond the scope of this review. Regenerative compounds, biomaterials, tissue or cellular transplants have been used to enhance neurite outgrowth and facilitate tissue regeneration [81] (reviewed in [82, 83]). Many of these approaches have shown that SpINs are recruited into the repaired neuronal networks (e.g. [51, 84]). Among these, transplantation of interneuronal precursors represents one strategy with a strong emphasis on harnessing SpINs. Thus, the present discussion focuses on the transplantation of neuronal precursor cells with the goal of delivering new populations of SpINs to the injured spinal cord (Figure 3) (reviewed in [85]). Transplantation of neural precursor cells obtained from i) acutely dissected fetal spinal cord tissue (FSC), ii) FSC’s more selected in vitro expanded counterpart (lineage restricted neural progenitor cells (NPCs) devoid of extracellular and non-neural components), or iii) even neural stem cells, have been shown to survive, integrate with the injured adult spinal cord, and alter functional outcome (reviewed in [83, 85–88]). Despite the therapeutic benefit seen with transplantation, some caveats remain [78] (reviewed in [85]). Among these caveats is donor heterogeneity.
Theoretically, while donor FSC tissue can produce any of the SpIN subtypes defined above, it is important to consider that refining donor cells through cell-culturing may alter that potential [77]. Even more importantly, not all donor SpINs will be beneficial for repair and functional recovery. Thus, identifying which of these cells survive isolation/preparation, dissociation, and transplantation, and then proliferate, differentiate, and mature within the injured host spinal cord will be crucial in future preclinical studies. One quite remarkable feature of transplanted NPCs, however, is that even once transplanted into the injured adult spinal cord the surviving cells can retain their long-term fate once transplanted [89–91]. When transplanting dorsally vs ventrally derived FSC tissue, micro-dissected from the extreme alar and basal plates, White et al. [90] found that recipients of dorsally-derived tissue showed impaired recovery and attenuated phrenic plasticity. This indicates that some dorsally derived spinal precursors can limit phrenic motor recovery. While donor neurons can become synaptically integrated with injured phrenic networks, Spruance et al. [78] recently demonstrated that the extent of recovery is incomplete and not all donor neurons become synaptically integrated with the host. Donor cholinergic interneurons, for instance, show minimal integration with the host phrenic network ipsilateral to injury. This may be due in part to the commissural projections these SpINs have in the normal spinal cord. In contrast, V1 and V2a SpINs normally have ipsilaterally projecting axons, and may therefore be better donor candidates for innervating the circuitry caudal to injury.
Identifying donor neuronal subtypes and determining their contribution to function post-SCI becomes important for future cellular therapies. It is likely that no single SpIN subtype alone will be optimal for promoting recovery post-SCI, but identifying those cells that contribute to adaptive plasticity offers some insight into which may be beneficial (e.g. dI3 [56] and V2a [8]). In addition, while excitatory neurons may be viable candidates for enhancing motor recovery post-SCI, inhibitory neurons may be more effective for the treatment of spasticity, bladder dysfunction or pain [92]. Through cellular engineering, specific neuronal subtypes can be derived from neural stem cells or progenitors for transplantation.
Tailoring Therapies with Cellular Engineering
The traditional method of differentiating pluri- or multipotent stem cells to cells of the central nervous system involves recapitulating the internal milieu of the developing spinal cord in vitro by introducing growth factors and morphogens at specific time points. Doing so results in the generation of spinal neuronal and glial cells [93, 94], and can drive cells toward specific cell fates [95]. However, challenges generating large numbers of high purity interneurons persist. Cellular engineering efforts are focused in using transgenic selectable and reporter mouse embryonic stem cell lines that enable the identification and/or purification of large numbers of SpIN populations [96, 97]. Highly enriched subsets of SpINs can now be generated for use as donor populations for transplantation [76, 77, 95, 98]. An important consideration with such developments, however, is that donor neuron populations will likely still need to be combined with donor glia [93] and/or other supportive matrices (e.g. [81, 98]) for optimal survival and integration with the host spinal cord.
Since the development of cellular reprogramming technology, cell transplantation studies have also begun exploring the development of NPCs derived from other easily obtainable cells (e.g. skin cells) (reviewed in [99]). The opportunity then exists to not only bank human NPCs, but in the future also derive autologous donor NPCs from patients. To take this technology one step further, in vivo direct reprogramming strategies are being developed pre-clinically to convert host cells directly into neurons (Figure 3). Direct conversion from mouse embryonic fibroblasts to parvalbumin-expressing interneurons was recently achieved by a single neurogenic transcription factor, Ascl1 and a PKA activator, forskolin [100]. In vivo conversion studies have also paved the way for this technology after SCI [101]. Subsequent studies have extended this direct conversion technology to human cultures, and towards a variety of cellular phenotypes [102]. Despite this, to date there are no established protocols that guide direct conversion to specific SpIN phenotypes.
Concluding remarks
Spinal interneurons not only play crucial roles in the modulation and regulation of motor and sensory activity within the uninjured spinal cord, but they contribute to plasticity following injury or disease. Given their integration of spinal networks and ability to adapt to changing conditions, they also serve as important therapeutic targets for treatments designed to enhance neuroplasticity and/or promote repair. Progress in genetics, developmental neurobiology and cellular engineering continue to advance our understanding of SpIN phenotypes, their neuroplastic potential, and how they can be harnessed to promote adaptive and restorative neuroplasticity and contribute to spinal cord repair.
Highlights.
Spinal interneurons (SpINs) are key cellular elements for plasticity following spinal cord injury.
Advances in molecular genetics are allowing scientists to characterize populations of SpINs, integrated with motor and sensory functions.
As SpIN subtypes are identified, their contribution to neuronal networks in the normal and injured spinal cord, and their role in plasticity can explored.
Understanding how specific SpINs contribute to adaptive or maladaptive plasticity will enable the development of more targeted treatments for spinal cord injury.
There is an increased scientific and clinical interest in the contribution of SpINs to respiratory function following spinal cord injury (i.e. cervical) or disease (i.e. amyotrophic lateral sclerosis).
The present review highlights some of these concepts drawing on some recent examples from locomotor and respiratory networks.
Outstanding Questions.
How many distinct functional tasks do each of the identified spinal interneurons contribute to? To what extent do they switch functional tasks under different conditions (altered physiological state, traumatic injury and degenerative disease)?
Do all subtypes of SpINs display neuroplasticity (anatomical or functional) after spinal cord injury, or are some subtypes more “stable”? Does this change with the type of injury (i.e. transection, contusion, compression)? Does this change with time after injury (i.e. acute, sub-acute, chronic)?
It is unlikely that there is a single SpIN optimal for repair of multiple motor and/or sensory circuits. Can SpINs be beneficial to function (adaptive) in one network, but limit function (maladaptive) in another? How can treatments be tailored to target SpIN subtypes for repair, while limiting their contribution to maladaptive change?
Ongoing studies are identifying SpIN subtypes that can be engineered for transplantation. Which SpINs, however, will survive in the harsh internal milieu of the injured spinal cord? If they survive, will they connect to the right networks spontaneously, or are additional interventions required? If they do form appropriate connections, will they contribute to functional improvement as intended?
While cellular engineering has enabled pre-clinical advances in cell therapies, what are the specific adjustments in this technology needed for it to become translational?
Acknowledgments
The authors are supported by the NINDS, NIH R01 NS081112 (Lane), R01 NS095366 (Dougherty), NIH R01 NS090617 (Sakiyama-Elbert), the Craig H. Neilsen Foundation (#338432, Lane; #381793, Qiang), the Pennsylvania Department of Health CURE program to Drexel University College of Medicine (Qiang), the Lisa Dean Moseley Foundation (Lane), the United States Department of Defense (CDMRP #SC140038; Marchenko), the Drexel Deans Fellowship for Collaborative or Themed Research (Zholudeva) and the Spinal Cord Research Center at Drexel University, College of Medicine (NIH, P01 NS 055976). The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the funding organizations.
Glossary
- Activity-based therapy
non-electrical means of stimulating neural substrate. Examples include persistent, task-specific activity such as locomotor and respiratory training, which strengthen musculature, stimulate afferent feedback and drive contextual neural plasticity
- Direct reprogramming
conversion of one cellular phenotype into a different phenotype without undergoing a pluripotent state via overexpression of transcriptional factors directly responsible for cell specification
- Neural Interfacing
technology operating at the intersection of the central or peripheral nervous system and exogenous devices (e.g. brain computer interfacing, muscle stimulation, cochlear implants, transcranial magnetic stimulation, epidural stimulation)
- Neuroplasticity
the ability of the nervous system to make anatomical and/ord to persistent alterations in sensory-motor function
- Spinal Cord Injury (SCI)
damage to the cells and axons comprising the spinal cord; can be traumatic or atraumatic.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lu DC, et al. Molecular and cellular development of spinal cord locomotor circuitry. Front Mol Neurosci. 2015;8:25. doi: 10.3389/fnmol.2015.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gosgnach S, et al. Delineating the Diversity of Spinal Interneurons in Locomotor Circuits. J Neurosci. 2017;37(45):10835–10841. doi: 10.1523/JNEUROSCI.1829-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci. 2016;17(4):224–38. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lane MA, et al. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008;511(5):692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le Gal JP, et al. Bimodal Respiratory-Locomotor Neurons in the Neonatal Rat Spinal Cord. J Neurosci. 2016;36(3):926–37. doi: 10.1523/JNEUROSCI.1825-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bareyre FM, et al. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7(3):269–77. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- 7.Nitzan-Luques A, et al. Genotype-selective phenotypic switch in primary afferent neurons contributes to neuropathic pain. Pain. 2011;152(10):2413–26. doi: 10.1016/j.pain.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Zholudeva LV, et al. Anatomical Recruitment of Spinal V2a Interneurons into Phrenic Motor Circuitry after High Cervical Spinal Cord Injury. J Neurotrauma. 2017;34(21):3058–3065. doi: 10.1089/neu.2017.5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romer SH, et al. Accessory respiratory muscles enhance ventilation in ALS model mice and are activated by excitatory V2a neurons. Exp Neurol. 2016 doi: 10.1016/j.expneurol.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 10.Courtine G, et al. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat Med. 2008;14(1):69–74. doi: 10.1038/nm1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fouad K, Tse A. Adaptive changes in the injured spinal cord and their role in promoting functional recovery. Neurol Res. 2008;30(1):17–27. doi: 10.1179/016164107X251781. [DOI] [PubMed] [Google Scholar]
- 12.Flynn JR, et al. The role of propriospinal interneurons in recovery from spinal cord injury. Neuropharmacology. 2011;60(5):809–22. doi: 10.1016/j.neuropharm.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 13.Sandhu MS, et al. Respiratory recovery following high cervical hemisection. Respir Physiol Neurobiol. 2009;169(2):94–101. doi: 10.1016/j.resp.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ueno M, et al. Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat Neurosci. 2016;19(6):784–7. doi: 10.1038/nn.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hou S, et al. Plasticity of lumbosacral propriospinal neurons is associated with the development of autonomic dysreflexia after thoracic spinal cord transection. J Comp Neurol. 2008;509(4):382–99. doi: 10.1002/cne.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dougherty KJ, Hochman S. Spinal cord injury causes plasticity in a subpopulation of lamina I GABAergic interneurons. J Neurophysiol. 2008;100(1):212–23. doi: 10.1152/jn.01104.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwak YS, Hulsebosch CE. GABA and central neuropathic pain following spinal cord injury. Neuropharmacology. 2011;60(5):799–808. doi: 10.1016/j.neuropharm.2010.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hormigo KM, et al. Enhancing neural activity to drive respiratory plasticity following cervical spinal cord injury. Exp Neurol. 2017;287(Pt 2):276–287. doi: 10.1016/j.expneurol.2016.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McLean DL, Dougherty KJ. Peeling back the layers of locomotor control in the spinal cord. Curr Opin Neurobiol. 2015;33:63–70. doi: 10.1016/j.conb.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grillner S, Jessell TM. Measured motion: searching for simplicity in spinal locomotor networks. Curr Opin Neurobiol. 2009;19(6):572–86. doi: 10.1016/j.conb.2009.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goulding M. Circuits controlling vertebrate locomotion: moving in a new direction. Nat Rev Neurosci. 2009;10(7):507–18. doi: 10.1038/nrn2608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi M, et al. Graded Arrays of Spinal and Supraspinal V2a Interneuron Subtypes Underlie Forelimb and Hindlimb Motor Control. Neuron. 2018;97(4):869–884 e5. doi: 10.1016/j.neuron.2018.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Borowska J, et al. Functional subpopulations of V3 interneurons in the mature mouse spinal cord. J Neurosci. 2013;33(47):18553–65. doi: 10.1523/JNEUROSCI.2005-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dougherty KJ, Kiehn O. Functional organization of V2a-related locomotor circuits in the rodent spinal cord. Ann N Y Acad Sci. 2010;1198:85–93. doi: 10.1111/j.1749-6632.2010.05502.x. [DOI] [PubMed] [Google Scholar]
- 25.Dougherty KJ, Kiehn O. Firing and cellular properties of V2a interneurons in the rodent spinal cord. J Neurosci. 2010;30(1):24–37. doi: 10.1523/JNEUROSCI.4821-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong G, et al. Neuronal activity in the isolated mouse spinal cord during spontaneous deletions in fictive locomotion: insights into locomotor central pattern generator organization. J Physiol. 2012;590(19):4735–59. doi: 10.1113/jphysiol.2012.240895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crone SA, et al. In mice lacking V2a interneurons, gait depends on speed of locomotion. J Neurosci. 2009;29(21):7098–109. doi: 10.1523/JNEUROSCI.1206-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Crone SA, et al. Genetic ablation of V2a ipsilateral interneurons disrupts left-right locomotor coordination in mammalian spinal cord. Neuron. 2008;60(1):70–83. doi: 10.1016/j.neuron.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 29.Dougherty KJ, et al. Locomotor rhythm generation linked to the output of spinal shox2 excitatory interneurons. Neuron. 2013;80(4):920–33. doi: 10.1016/j.neuron.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Caldeira V, et al. Spinal Hb9::Cre-derived excitatory interneurons contribute to rhythm generation in the mouse. Sci Rep. 2017;7:41369. doi: 10.1038/srep41369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brownstone RM, Wilson JM. Strategies for delineating spinal locomotor rhythm-generating networks and the possible role of Hb9 interneurones in rhythmogenesis. Brain Res Rev. 2008;57(1):64–76. doi: 10.1016/j.brainresrev.2007.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Husch A, et al. Spinal cord injury induces serotonin supersensitivity without increasing intrinsic excitability of mouse V2a interneurons. J Neurosci. 2012;32(38):13145–54. doi: 10.1523/JNEUROSCI.2995-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Quinlan KA, Kiehn O. Segmental, synaptic actions of commissural interneurons in the mouse spinal cord. J Neurosci. 2007;27(24):6521–30. doi: 10.1523/JNEUROSCI.1618-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Talpalar AE, et al. Dual-mode operation of neuronal networks involved in left-right alternation. Nature. 2013;500(7460):85–8. doi: 10.1038/nature12286. [DOI] [PubMed] [Google Scholar]
- 35.Sweeney LB, et al. Origin and Segmental Diversity of Spinal Inhibitory Interneurons. Neuron. 2018;97(2):341–355 e3. doi: 10.1016/j.neuron.2017.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gosgnach S, et al. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440(7081):215–9. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- 37.Zhang J, et al. V1 and v2b interneurons secure the alternating flexor-extensor motor activity mice require for limbed locomotion. Neuron. 2014;82(1):138–50. doi: 10.1016/j.neuron.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sandhu MS, et al. Midcervical neuronal discharge patterns during and following hypoxia. J Neurophysiol. 2015;113(7):2091–101. doi: 10.1152/jn.00834.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lane MA, et al. Spinal circuitry and respiratory recovery following spinal cord injury. Respir Physiol Neurobiol. 2009;169(2):123–32. doi: 10.1016/j.resp.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Streeter KA, et al. Intermittent Hypoxia Enhances Functional Connectivity of Midcervical Spinal Interneurons. J Neurosci. 2017;37(35):8349–8362. doi: 10.1523/JNEUROSCI.0992-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu J, et al. A V0 core neuronal circuit for inspiration. Nat Commun. 2017;8(1):544. doi: 10.1038/s41467-017-00589-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Crone SA, et al. Irregular Breathing in Mice following Genetic Ablation of V2a Neurons. J Neurosci. 2012;32(23):7895–906. doi: 10.1523/JNEUROSCI.0445-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Marchenko V, et al. The role of spinal GABAergic circuits in the control of phrenic nerve motor output. Am J Physiol Regul Integr Comp Physiol. 2015;308(11):R916–26. doi: 10.1152/ajpregu.00244.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marchenko V, Rogers RF. GABAAergic and glycinergic inhibition in the phrenic nucleus organizes and couples fast oscillations in motor output. J Neurophysiol. 2009;101(4):2134–45. doi: 10.1152/jn.91030.2008. [DOI] [PubMed] [Google Scholar]
- 45.Cregg JM, et al. A Latent Propriospinal Network Can Restore Diaphragm Function after High Cervical Spinal Cord Injury. Cell Rep. 2017;21(3):654–665. doi: 10.1016/j.celrep.2017.09.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepien AE, et al. Monosynaptic rabies virus reveals premotor network organization and synaptic specificity of cholinergic partition cells. Neuron. 2010;68(3):456–72. doi: 10.1016/j.neuron.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 47.Buttry JL, Goshgarian HG. Injection of WGA-Alexa 488 into the ipsilateral hemidiaphragm of acutely and chronically C2 hemisected rats reveals activity-dependent synaptic plasticity in the respiratory motor pathways. Exp Neurol. 2014;261:440–50. doi: 10.1016/j.expneurol.2014.07.016. [DOI] [PubMed] [Google Scholar]
- 48.Duale H, et al. Spinal cord injury reduces the efficacy of pseudorabies virus labeling of sympathetic preganglionic neurons. J Neuropathol Exp Neurol. 2009;68(2):168–78. doi: 10.1097/NEN.0b013e3181967df7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Duale H, et al. Noxious colorectal distention in spinalized rats reduces pseudorabies virus labeling of sympathetic neurons. J Neurotrauma. 2010;27(8):1369–78. doi: 10.1089/neu.2010.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nair J, et al. Histological identification of phrenic afferent projections to the spinal cord. Respir Physiol Neurobiol. 2017;236:57–68. doi: 10.1016/j.resp.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van den Brand R, et al. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336(6085):1182–5. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- 52.Houle JD, Cote MP. Axon regeneration and exercise-dependent plasticity after spinal cord injury. Ann N Y Acad Sci. 2013;1279:154–63. doi: 10.1111/nyas.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Alilain WJ, et al. Light-induced rescue of breathing after spinal cord injury. J Neurosci. 2008;28(46):11862–70. doi: 10.1523/JNEUROSCI.3378-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Azim E, et al. Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature. 2014;508(7496):357–63. doi: 10.1038/nature13021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pocratsky AM, et al. Reversible silencing of lumbar spinal interneurons unmasks a task-specific network for securing hindlimb alternation. Nat Commun. 2017;8(1):1963. doi: 10.1038/s41467-017-02033-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bui TV, et al. Spinal microcircuits comprising dI3 interneurons are necessary for motor functional recovery following spinal cord transection. Elife. 2016;5 doi: 10.7554/eLife.21715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Courtine G, et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci. 2009;12(10):1333–42. doi: 10.1038/nn.2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rank MM, et al. Electrophysiological characterization of spontaneous recovery in deep dorsal horn interneurons after incomplete spinal cord injury. Exp Neurol. 2015;271:468–78. doi: 10.1016/j.expneurol.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 59.Lane MA, et al. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp Neurol. 2012;235(1):197–210. doi: 10.1016/j.expneurol.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Skup M, et al. Different effects of spinalization and locomotor training of spinal animals on cholinergic innervation of the soleus and tibialis anterior motoneurons. Eur J Neurosci. 2012;36(5):2679–88. doi: 10.1111/j.1460-9568.2012.08182.x. [DOI] [PubMed] [Google Scholar]
- 61.Grau JW, et al. Metaplasticity and behavior: how training and inflammation affect plastic potential within the spinal cord and recovery after injury. Front Neural Circuits. 2014;8:100. doi: 10.3389/fncir.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramirez-Jarquin UN, Tapia R. Excitatory and Inhibitory Neuronal Circuits in the Spinal Cord and Their Role in the Control of Motor Neuron Function and Degeneration. ACS Chem Neurosci. 2018;9(2):211–216. doi: 10.1021/acschemneuro.7b00503. [DOI] [PubMed] [Google Scholar]
- 63.Hoh DJ, et al. Respiration following spinal cord injury: evidence for human neuroplasticity. Respir Physiol Neurobiol. 2013;189(2):450–64. doi: 10.1016/j.resp.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lane MA, et al. Respiratory neuroplasticity and cervical spinal cord injury: translational perspectives. Trends Neurosci. 2008;31(10):538–47. doi: 10.1016/j.tins.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Goshgarian HG. The crossed phrenic phenomenon and recovery of function following spinal cord injury. Respir Physiol Neurobiol. 2009;169(2):85–93. doi: 10.1016/j.resp.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bezdudnaya T, et al. Spontaneous respiratory plasticity following unilateral high cervical spinal cord injury in behaving rats. Exp Neurol. 2018;305:56–65. doi: 10.1016/j.expneurol.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Alilain WJ, Goshgarian HG. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol. 2008;212(2):348–57. doi: 10.1016/j.expneurol.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Alilain WJ, Goshgarian HG. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med. 2007;30(4):346–54. doi: 10.1080/10790268.2007.11753950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mantilla CB, et al. Phrenic motoneuron expression of serotonergic and glutamatergic receptors following upper cervical spinal cord injury. Exp Neurol. 2012;234(1):191–9. doi: 10.1016/j.expneurol.2011.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ghali MG, Marchenko V. Patterns of Phrenic Nerve Discharge after Complete High Cervical Spinal Cord Injury in the Decerebrate Rat. J Neurotrauma. 2016;33(12):1115–27. doi: 10.1089/neu.2015.4034. [DOI] [PubMed] [Google Scholar]
- 71.Ichiyama RM, et al. Locomotor training maintains normal inhibitory influence on both alpha- and gamma-motoneurons after neonatal spinal cord transection. J Neurosci. 2011;31(1):26–33. doi: 10.1523/JNEUROSCI.6433-09.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Harkema SJ. Plasticity of interneuronal networks of the functionally isolated human spinal cord. Brain Res Rev. 2008;57(1):255–64. doi: 10.1016/j.brainresrev.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edgerton VR, et al. Training locomotor networks. Brain Res Rev. 2008;57(1):241–54. doi: 10.1016/j.brainresrev.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Taccola G, et al. And yet it moves: Recovery of volitional control after spinal cord injury. Prog Neurobiol. 2018;160:64–81. doi: 10.1016/j.pneurobio.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.DiMarco AF, Kowalski KE. Activation of inspiratory muscles via spinal cord stimulation. Respir Physiol Neurobiol. 2013;189(2):438–49. doi: 10.1016/j.resp.2013.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Butts JC, et al. Differentiation of V2a interneurons from human pluripotent stem cells. Proc Natl Acad Sci U S A. 2017;114(19):4969–4974. doi: 10.1073/pnas.1608254114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zholudeva LV, et al. Transplantation of Neural Progenitors and V2a Interneurons after Spinal Cord Injury. J Neurotrauma. 2018 doi: 10.1089/neu.2017.5439. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Spruance VM, et al. Integration of Transplanted Neural Precursors with the Injured Cervical Spinal Cord. J Neurotrauma. 2018 doi: 10.1089/neu.2017.5451. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Harkema S, et al. Evidence-based therapy for recovery of function after spinal cord injury. Handb Clin Neurol. 2012;109:259–74. doi: 10.1016/B978-0-444-52137-8.00016-4. [DOI] [PubMed] [Google Scholar]
- 80.Jordan LM, et al. Cholinergic mechanisms in spinal locomotion-potential target for rehabilitation approaches. Front Neural Circuits. 2014;8:132. doi: 10.3389/fncir.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thompson R, Sakiyama-Elbert S. Using biomaterials to promote pro-regenerative glial phenotypes after nervous system injuries. Biomed Mater. 2018;13(2):024104. doi: 10.1088/1748-605X/aa9e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sakiyama-Elbert S, et al. Scaffolds to promote spinal cord regeneration. Handb Clin Neurol. 2012;109:575–94. doi: 10.1016/B978-0-444-52137-8.00036-X. [DOI] [PubMed] [Google Scholar]
- 83.Bonner JF, Steward O. Repair of spinal cord injury with neuronal relays: From fetal grafts to neural stem cells. Brain Res. 2015;1619:115–23. doi: 10.1016/j.brainres.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ropper AE, et al. Defining recovery neurobiology of injured spinal cord by synthetic matrix-assisted hMSC implantation. Proc Natl Acad Sci U S A. 2017 doi: 10.1073/pnas.1616340114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lane MA, et al. Improving the therapeutic efficacy of neural progenitor cell transplantation following spinal cord injury. Expert Rev Neurother. 2017;17(5):433–440. doi: 10.1080/14737175.2017.1270206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Jakeman L, Reier P. Fetal Spinal Cord Transplantation after Spinal Cord Injury: Around and Back Again. In: So K-F, Xu X-M, editors. Neural regeneration. Academic Press is an imprint of Elsevier; Academic Press Beijing; 2015. pp. 351–368. [Google Scholar]
- 87.Lu P. Stem cell transplantation for spinal cord injury repair. Prog Brain Res. 2017;231:1–32. doi: 10.1016/bs.pbr.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 88.Assinck P, et al. Cell transplantation therapy for spinal cord injury. Nat Neurosci. 2017;20(5):637–647. doi: 10.1038/nn.4541. [DOI] [PubMed] [Google Scholar]
- 89.Jakeman LB, et al. Differentiation of substantia gelatinosa-like regions in intraspinal and intracerebral transplants of embryonic spinal cord tissue in the rat. Exp Neurol. 1989;103(1):17–33. doi: 10.1016/0014-4886(89)90181-7. [DOI] [PubMed] [Google Scholar]
- 90.White TE, et al. Neuronal progenitor transplantation and respiratory outcomes following upper cervical spinal cord injury in adult rats. Exp Neurol. 2010;225(1):231–6. doi: 10.1016/j.expneurol.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dulin JN, et al. Injured adult motor and sensory axons regenerate into appropriate organotypic domains of neural progenitor grafts. Nat Commun. 2018;9(1):84. doi: 10.1038/s41467-017-02613-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fandel TM, et al. Transplanted Human Stem Cell-Derived Interneuron Precursors Mitigate Mouse Bladder Dysfunction and Central Neuropathic Pain after Spinal Cord Injury. Cell Stem Cell. 2016;19(4):544–557. doi: 10.1016/j.stem.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 93.Cai J, et al. Properties of a fetal multipotent neural stem cell (NEP cell) Dev Biol. 2002;251(2):221–40. doi: 10.1006/dbio.2002.0828. [DOI] [PubMed] [Google Scholar]
- 94.Rao MS. Multipotent and restricted precursors in the central nervous system. Anat Rec. 1999;257(4):137–48. doi: 10.1002/(SICI)1097-0185(19990815)257:4<137::AID-AR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 95.Gupta S, et al. Deriving Dorsal Spinal Sensory Interneurons from Human Pluripotent Stem Cells. Stem Cell Reports. 2018;10(2):390–405. doi: 10.1016/j.stemcr.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Iyer NR, et al. Generation of highly enriched V2a interneurons from mouse embryonic stem cells. Exp Neurol. 2016;277:305–316. doi: 10.1016/j.expneurol.2016.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Xu H, et al. A puromycin selectable cell line for the enrichment of mouse embryonic stem cell-derived V3 interneurons. Stem Cell Res Ther. 2015;6:220. doi: 10.1186/s13287-015-0213-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Thompson RE, et al. Effect of Hyaluronic Acid Hydrogels Containing Astrocyte-Derived Extracellular Matrix and/or V2a Interneurons on Histologic Outcomes following Spinal Cord Injury. Biomaterials. 2018 doi: 10.1016/j.biomaterials.2018.02.013. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Doulames VM, Plant GW. Induced Pluripotent Stem Cell Therapies for Cervical Spinal Cord Injury. Int J Mol Sci. 2016;17(4):530. doi: 10.3390/ijms17040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shi Z, et al. Conversion of Fibroblasts to Parvalbumin Neurons by One Transcription Factor, Ascl1, and the Chemical Compound Forskolin. J Biol Chem. 2016;291(26):13560–70. doi: 10.1074/jbc.M115.709808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Su Z, et al. In vivo conversion of astrocytes to neurons in the injured adult spinal cord. Nat Commun. 2014;5:3338. doi: 10.1038/ncomms4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Qiang L, et al. Instant neurons: directed somatic cell reprogramming models of central nervous system disorders. Biol Psychiatry. 2014;75(12):945–51. doi: 10.1016/j.biopsych.2013.10.027. [DOI] [PubMed] [Google Scholar]
- 103.Reier PJ, et al. Spinal Cord Injury: Repair, Plasticity and Rehabilitation, Encyclopedia of Life Science. John Wiley & Sons, Ltd; Chichester: 2017. [Google Scholar]
- 104.Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol. 2011;179(1):3–13. doi: 10.1016/j.resp.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 105.Douse MA, et al. Role of upper cervical inspiratory neurons studied by cross-correlation in the cat. Exp Brain Res. 1992;90(1):153–62. doi: 10.1007/BF00229267. [DOI] [PubMed] [Google Scholar]
- 106.Lipski J, Duffin J. An electrophysiological investigation of propriospinal inspiratory neurons in the upper cervical cord of the cat. Exp Brain Res. 1986;61(3):625–37. doi: 10.1007/BF00237589. [DOI] [PubMed] [Google Scholar]
- 107.Anker AR, et al. Vestibular inputs to propriospinal interneurons in the feline C1-C2 spinal cord projecting to the C5-C6 ventral horn. Exp Brain Res. 2006;170(1):39–51. doi: 10.1007/s00221-005-0186-8. [DOI] [PubMed] [Google Scholar]
- 108.Nakazono Y, Aoki M. Excitatory connections between upper cervical inspiratory neurons and phrenic motoneurons in cats. J Appl Physiol (1985) 1994;77(2):679–83. doi: 10.1152/jappl.1994.77.2.679. [DOI] [PubMed] [Google Scholar]
- 109.Nonaka S, Miller AD. Behavior of upper cervical inspiratory propriospinal neurons during fictive vomiting. J Neurophysiol. 1991;65(6):1492–500. doi: 10.1152/jn.1991.65.6.1492. [DOI] [PubMed] [Google Scholar]
- 110.Tian GF, Duffin J. Connections from upper cervical inspiratory neurons to phrenic and intercostal motoneurons studied with cross-correlation in the decerebrate rat. Exp Brain Res. 1996;110(2):196–204. doi: 10.1007/BF00228551. [DOI] [PubMed] [Google Scholar]
- 111.Oku Y, et al. Respiratory neuron group in the high cervical spinal cord discovered by optical imaging. Neuroreport. 2008;19(17):1739–43. doi: 10.1097/WNR.0b013e328318edb5. [DOI] [PubMed] [Google Scholar]
- 112.Okada Y, et al. Anatomical architecture and responses to acidosis of a novel respiratory neuron group in the high cervical spinal cord (HCRG) of the neonatal rat. Adv Exp Med Biol. 2009;648:387–94. doi: 10.1007/978-90-481-2259-2_44. [DOI] [PubMed] [Google Scholar]
- 113.Lu F, et al. Chemical activation of C1–C2 spinal neurons modulates intercostal and phrenic nerve activity in rats. Am J Physiol Regul Integr Comp Physiol. 2004;286(6):R1069–76. doi: 10.1152/ajpregu.00427.2003. [DOI] [PubMed] [Google Scholar]
- 114.Lipski J, et al. Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Exp Brain Res. 1993;95(3):477–87. doi: 10.1007/BF00227141. [DOI] [PubMed] [Google Scholar]
- 115.Douse MA, Duffin J. Axonal projections and synaptic connections of C5 segment expiratory interneurones in the cat. J Physiol. 1993;470:431–44. doi: 10.1113/jphysiol.1993.sp019867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bellingham MC. Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol. 1999;82(3):1224–32. doi: 10.1152/jn.1999.82.3.1224. [DOI] [PubMed] [Google Scholar]
- 117.Grelot L, et al. Respiratory interneurons of the lower cervical (C4–C5) cord: membrane potential changes during fictive coughing, vomiting, and swallowing in the decerebrate cat. Pflugers Arch. 1993;425(3–4):313–20. doi: 10.1007/BF00374181. [DOI] [PubMed] [Google Scholar]
- 118.Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res. 1990;533(1):141–6. doi: 10.1016/0006-8993(90)91807-s. [DOI] [PubMed] [Google Scholar]
- 119.Palisses R, Viala D. Existence of respiratory interneurons in the cervical spinal cord of the rabbit. C R Acad Sci III. 1987;305(8):321–4. [PubMed] [Google Scholar]
- 120.Palisses R, et al. Evidence for respiratory interneurones in the C3–C5 cervical spinal cord in the decorticate rabbit. Exp Brain Res. 1989;78(3):624–32. doi: 10.1007/BF00230250. [DOI] [PubMed] [Google Scholar]
- 121.Cleland CL, Getting PA. Respiratory-modulated and phrenic afferent-driven neurons in the cervical spinal cord (C4–C6) of the fluorocarbon-perfused guinea pig. Exp Brain Res. 1993;93(2):307–11. doi: 10.1007/BF00228399. [DOI] [PubMed] [Google Scholar]
- 122.Qin C, et al. Chemical activation of C(1)–C(2) spinal neurons modulates activity of thoracic respiratory interneurons in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283(4):R843–52. doi: 10.1152/ajpregu.00054.2002. [DOI] [PubMed] [Google Scholar]
- 123.Kirkwood PA, et al. Functional identities of thoracic respiratory interneurones in the cat. J Physiol. 1993;461:667–87. doi: 10.1113/jphysiol.1993.sp019535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schmid K, et al. Contralateral projections of thoracic respiratory interneurones in the cat. J Physiol. 1993;461:647–65. doi: 10.1113/jphysiol.1993.sp019534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kirkwood PA, et al. Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol. 1988;395:161–92. doi: 10.1113/jphysiol.1988.sp016913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Merrill EG, Lipski J. Inputs to intercostal motoneurons from ventrolateral medullary respiratory neurons in the cat. J Neurophysiol. 1987;57(6):1837–53. doi: 10.1152/jn.1987.57.6.1837. [DOI] [PubMed] [Google Scholar]
- 127.Sumi T. Spinal Respiratory Neurons and Their Reaction to Stimulation of Intercostal Nerves. Pflugers Arch Gesamte Physiol Menschen Tiere. 1963;278:172–80. doi: 10.1007/BF00362689. [DOI] [PubMed] [Google Scholar]
- 128.Ford TW, et al. Functional plasticity in the respiratory drive to thoracic motoneurons in the segment above a chronic lateral spinal cord lesion. J Neurophysiol. 2016;115(1):554–67. doi: 10.1152/jn.00614.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hoskin RW, et al. Projections from upper cervical inspiratory neurons to thoracic and lumbar expiratory motor nuclei in the cat. Exp Neurol. 1988;99(3):544–55. doi: 10.1016/0014-4886(88)90171-9. [DOI] [PubMed] [Google Scholar]
- 130.Lois JH, et al. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol (1985) 2009;106(1):138–52. doi: 10.1152/japplphysiol.91125.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yates BJ, et al. Transneuronal tracing of neural pathways controlling activity of diaphragm motoneurons in the ferret. Neuroscience. 1999;90(4):1501–13. doi: 10.1016/s0306-4522(98)00554-5. [DOI] [PubMed] [Google Scholar]
- 132.Qiu K, et al. The phrenic motor nucleus in the adult mouse. Exp Neurol. 2010;226(1):254–8. doi: 10.1016/j.expneurol.2010.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347(1):64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- 134.Billig I, et al. Transneuronal tracing of neural pathways controlling abdominal musculature in the ferret. Brain Res. 2001;912(1):24–32. doi: 10.1016/s0006-8993(01)02597-5. [DOI] [PubMed] [Google Scholar]
- 135.Billig I, et al. Definition of neuronal circuitry controlling the activity of phrenic and abdominal motoneurons in the ferret using recombinant strains of pseudorabies virus. J Neurosci. 2000;20(19):7446–54. doi: 10.1523/JNEUROSCI.20-19-07446.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Billig I, et al. Transneuronal tracing of neural pathways controlling an abdominal muscle, rectus abdominis, in the ferret. Brain Res. 1999;820(1–2):31–44. doi: 10.1016/s0006-8993(98)01320-1. [DOI] [PubMed] [Google Scholar]
- 137.Jankowska E. Spinal Interneurons. In: Pfaff DW, editor. Neuroscience in the 21st Century: From Basic to Clinical. Springer; New York: 2013. pp. 1063–1099. [Google Scholar]


