Abstract
Females across their lifespan and certain male populations are susceptible to urinary tract infections (UTI). The influence of female vs male sex on UTI is incompletely understood, in part because preclinical modeling has been performed almost exclusively in female mice. Here, we employed established and new mouse models of UTI with uropathogenic Escherichia coli (UPEC) to investigate androgen influence on UTI pathogenesis. Susceptibility to UPEC UTI in both male and female hosts was potentiated with 5α-dihydrotestosterone, while males with androgen receptor deficiency and androgenized females treated with the androgen receptor antagonist enzalutamide were protected from severe pyelonephritis. In androgenized females and in males, UPEC formed dense intratubular, biofilm-like communities, some of which were sheltered from infiltrating leukocytes by the tubular epithelium and by peritubular fibrosis. Abscesses were nucleated by small intratubular collections of UPEC first visualized at five days post infection and briskly expanded over the subsequent 24 hours. Male mice deficient in Toll-like receptor 4, which fail to contain UPEC within abscesses, were susceptible to lethal dissemination. Thus, androgen receptor activation imparts susceptibility to severe upper-tract UTI in both female and male murine hosts. Visualization of intratubular UPEC communities illuminates early renal abscess pathogenesis and the role of abscess formation in preventing dissemination of infection. Additionally, our study suggests that androgen modulation may represent a novel therapeutic route to combat recalcitrant or recurrent UTI in a range of patient populations.
Keywords: pyelonephritis, inflammation
Graphical Abstract
Conclusions
Androgenized female hosts exhibit organ higher bacterial loads and harbor kidney bacterial communities (KBCs) that nucleate renal abscess formation
INTRODUCTION
Bacterial infection of the urinary tract represents one of the most common human infectious diseases and imposes a large economic burden.1, 2 Ascension of uropathogens to the kidneys, resulting in pyelonephritis or urosepsis, is associated with mortality and threatens lifelong morbidity, including renal scarring and attendant risks of hypertension and chronic kidney disease, despite appropriate initial antibiotic treatment.3, 4 These contemporary challenges to the human host manifest in an era in which the primary causative agent of UTI, uropathogenic Escherichia coli (UPEC), displays unprecedented global prevalence and breadth of antimicrobial resistance.5
Preclinical modeling of severe pyelonephritis has been constrained by a lack of susceptible female murine models and technical inability to access the male mouse bladder via catheter.6, 7 To begin to decipher sex differences in UTI, we recently developed a minimally invasive surgical technique enabling direct bladder inoculation and comparison of UTI pathogenesis and outcomes in both male and female hosts. After equal intravesical inoculation with UPEC, male mice of multiple genetic backgrounds, compared with females, developed more severe renal infection – including extremely high kidney UPEC burdens and 90% prevalent renal abscess in male C3H/HeN mice.7 The C3H strain is known to exhibit vesicoureteral reflux (VUR)8 – a translationally relevant feature, as VUR is a major risk factor for upper-tract UTI, particularly in affected children. Micro- and macroscopic abscess formation is a pathological feature of pyelonephritis in humans,9 and the severity of inflammation correlates with subsequent renal scarring in several models of renal injury.10, 11
We previously reported that castration of male C3H/HeN mice prior to induction of UTI mitigated the development of chronic cystitis, severe pyelonephritis, and renal abscess, while ovariectomy had no effect on these outcomes in females. Exogenous testosterone replacement in castrated male mice restored severe UTI phenotypes.7 It remained unknown if androgen-driven UTI susceptibility extends to females, in whom UTI is much more prevalent overall.1, 12, 13 Here, we used specific androgen receptor (AR) agonists and antagonists as well as genetic models to conclusively demonstrate the involvement of AR activation in susceptibility to UTI in both female and male hosts. The androgenized phenotypes enabled us to detail the formation of intratubular UPEC communities – dense, biofilm-like bacterial masses filling multiple tubular segments at the center of developing abscesses. These UPEC communities were sheltered from intense neutrophilic infiltrates by intact tubular epithelia, which exhibited peritubular fibrosis. Mice lacking functional TLR4 (the innate immune sensor of bacterial lipopolysaccharide) failed to contain UPEC within abscesses and were prone to mortality from urosepsis. These experiments reveal the importance of androgen exposure in UTI susceptibility across both host sexes, and enable the future dissection of niches and mechanisms exploited by UPEC for growth and persistence in the kidney during pyelonephritis and renal abscess formation.
RESULTS
Androgen exposure aggravates UTI severity in female C3H/HeN mice
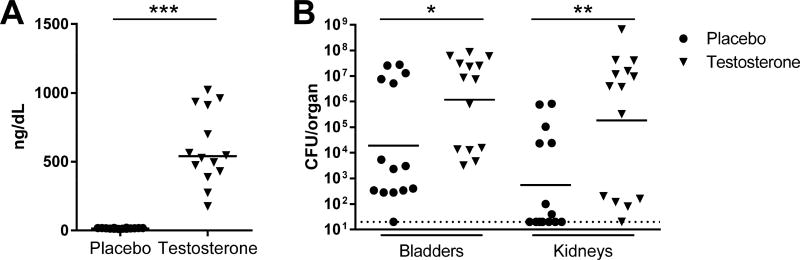
To test whether androgens could amplify UTI severity and enhance abscess development in female mice, as seen previously in males,7 we implanted slow-release subcutaneous testosterone or placebo pellets in female C3H/HeN mice 4 wk prior to mini-surgical bladder inoculation with UPEC. Testosterone pellet implantation significantly increased serum testosterone compared with placebo-treated females (Figure 1A), mirroring serum testosterone levels seen in males of similar age.14, 15 Multiple prior studies show that female C3H/HeN mice develop a bimodal distribution of bladder UPEC titers at 2–4 weeks post infection (wpi), whereby a minority (20–40%) of animals display chronic, active infection with ongoing inflammation and persistently high bladder bacterial loads while the majority of these females resolve infection.6, 7, 16 Consistent with these reports, a minority (36%) of placebo-treated females here exhibited chronic cystitis 2 wpi (i.e., bladder bacterial loads > 104 CFU; Figure 1B, circles). In contrast, 64% of androgenized females exhibited chronic cystitis 2 wpi (Figure 1B, triangles). When CFU burdens were compared numerically, testosterone-treated females exhibited significantly higher bladder (P=0.0102) and kidney (P=0.002) bacterial loads 2 wpi compared to placebo-treated controls (Figure 1B). More strikingly, androgen exposure led to development of grossly visible renal abscess in 9 of 14 females (64%), compared to 0 of 14 placebo controls (P=0.0006). These data illustrate that elevated circulating testosterone predisposes to severe UTI independent of biological sex. Of note, while intravesical inoculation with UPEC does induce acute and chronic prostatitis in C3H/HeN males (Supplementary Figure 1), our data in androgenized females excludes the prostate as a driver of androgen-induced severe UTI.
Figure 1. Androgen exposure aggravates UTI severity in females.
Female C3H/HeN mice were subcutaneously implanted with placebo (filled circles) or long-release testosterone pellets (filled triangles) 4 wk prior to induction of UTI. Testosterone was measured via enzyme immunoassay at time of infection; each sample was measured in duplicate and recorded as the mean. As anticipated, testosterone pellets increased the serum testosterone to levels physiologically relevant to normal males (A, ***P<0.0001 vs placebo). Organ harvest 2 wpi (B) revealed higher bacterial loads in C3H/HeN females receiving testosterone, in both the bladders (*P=0.0102) and kidneys (**P=0.002). Dotted line indicates limit of detection.
Androgen receptor activation drives UTI severity
We next aimed to identify the hormonal pathway(s) by which testosterone acts to induce severe UTI. Testosterone supplementation could perturb levels of other hormones in the hypothalamic-pituitary-gonadal (HPG) axis via its negative feedback on gonadotropin releasing hormone (GnRH), luteinizing hormone (LH), and follicle-stimulating hormone (FSH). In this scenario, increased GnRH, LH, and/or FSH could be protective in castrated males and non-androgenized females, while decreased secretion of these hormones in androgenized hosts could promote UTI susceptibility. Refuting this possibility, we previously found that while orchiectomy dramatically attenuated UTI, ovariectomy in females (which would activate the HPG axis similarly to castration in males17) had no influence on UTI outcome.7 This finding, in conjunction with our ability to facilitate severe UTI in females via androgen treatment (Figure 1B), eliminates alteration in GnRH or gonadotropin levels as a mechanism for increased UTI severity in androgenized hosts.
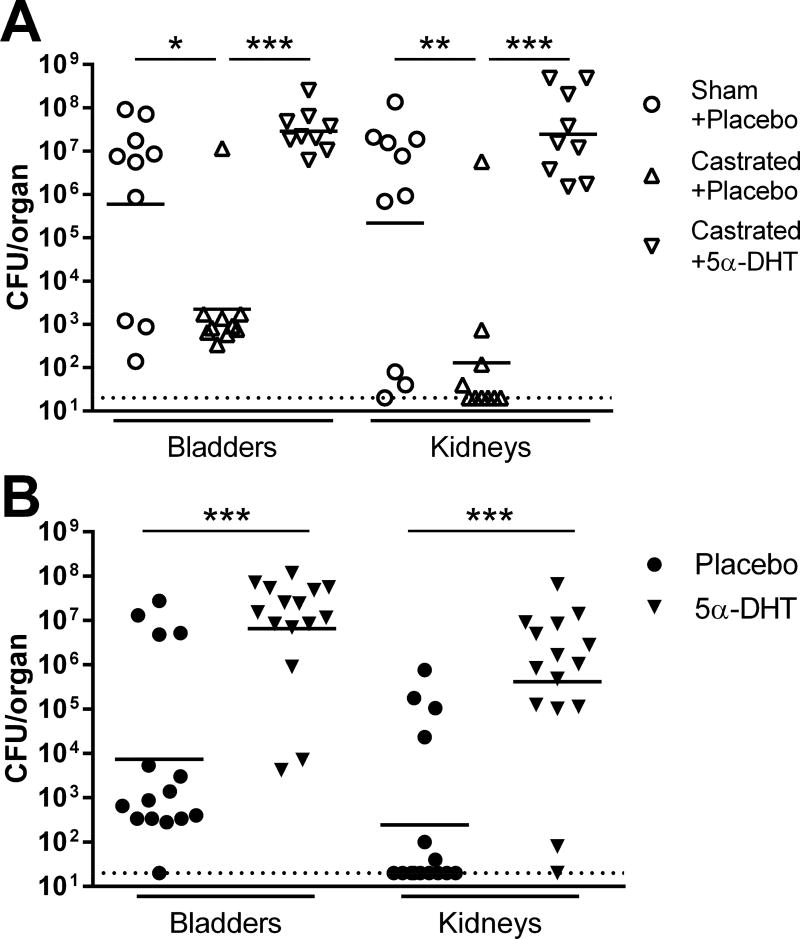
We next focused on the two canonical pathways of testosterone activity and relevant receptors. First, testosterone can undergo peripheral conversion by aromatase into estradiol, which could then activate the estrogen receptor. We therefore tested whether an aromatase-resistant and (compared with testosterone) more potent and specific AR agonist, 5α-dihydrotestosterone (DHT), would enhance UTI severity. We implanted slow-release DHT or placebo pellets 4 d after castration or sham operation in C3H/HeN males, then surgically infected mice 4 wk later. Consistent with our previous results, castration attenuated bladder (P=0.041) and kidney (P=0.0063) bacterial burdens 2 wpi in placebo-treated males (Figure 2A). Treatment with exogenous DHT reversed the effects of castration (Figure 2A), recapitulating the complementation seen with testosterone.7 Organ bacterial burdens in castrated, DHT-complemented males were similar to those in sham-operated, placebo-treated males, with most animals in both groups developing bilateral renal abscess. Likewise, infection of DHT-treated female C3H/HeN mice led to significantly higher bladder bacterial burdens (P<0.0001) and frequency of chronic cystitis (87% vs 27%; P=0.0025) 2 wpi, compared with placebo-treated females (Figure 2B). DHT treatment also induced gross abscess formation in 73% of these females (vs 0% with placebo, P<0.0001) and dramatically increased kidney titers (Figure 2B, P<0.0001). Thus, peripheral aromatization of testosterone does not mediate susceptibility to severe UTI. Of additional note, in female-only experiments we replicated these results with UPEC inoculations via the traditional catheter route, demonstrating that the observed severe UTI phenotypes and their androgen dependence are not attributable to the mini-surgical infection methodology.
Figure 2. Testosterone influence on UTI severity is not mediated via aromatization.
Bladder and kidney bacterial burdens were measured 2 wpi (A) in sham-operated C3H/HeN males treated with placebo pellets (open circles), castrated males treated with placebo pellets (open triangles), and castrated males receiving pellets of 5α-dihydrotestosterone (DHT), a potent androgen not susceptible to conversion to estrogen via the action of aromatase (inverted triangles). As seen previously, castration was protective against severe UTI (*P<0.041 in bladder, **P<0.0063 in kidney vs sham-operated). Treatment of castrated males with exogenous DHT reversed this effect (***P<0.0001 in both organs vs castrated males receiving placebo); DHT-complemented males exhibited organ bacterial burdens equivalent to sham-operated, placebo-treated males (A) and similarly developed gross renal abscess. (B) In an analogous way, treatment of C3H/HeN females with DHT prior to initiation of UTI was associated with significantly higher bladder and kidney bacterial burdens 2 wpi (***P<0.0001 vs placebo). Dotted lines indicate limit of detection.
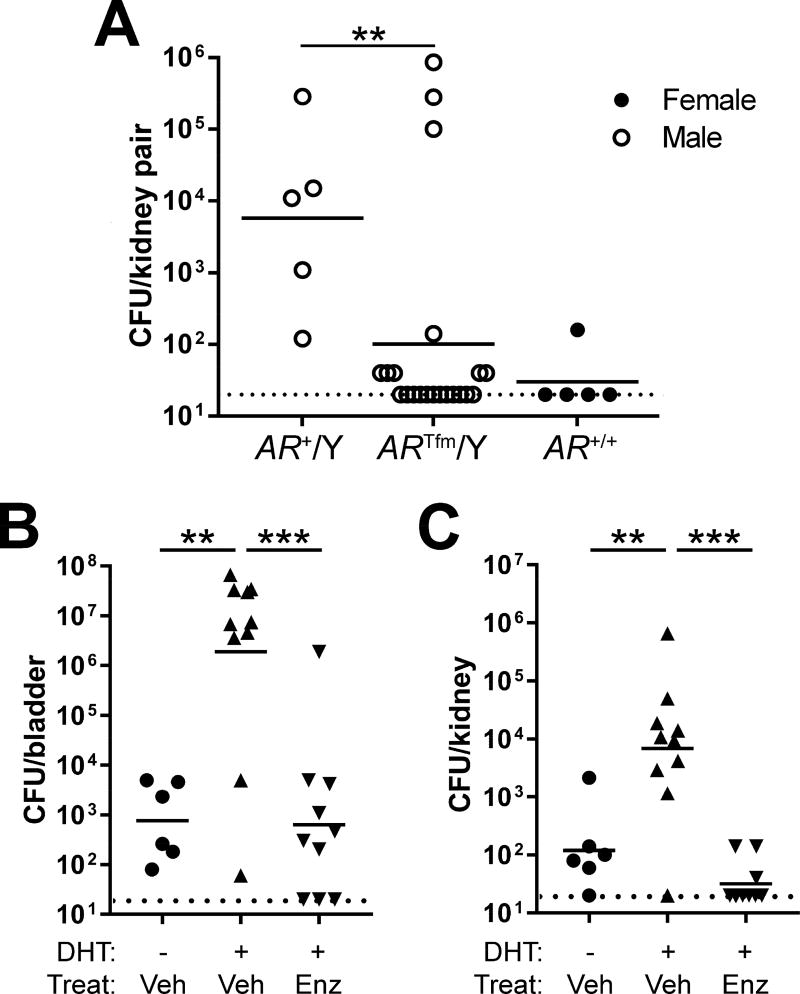
Independent of aromatization, androgens classically engage the AR, though alternative signaling pathways independent of AR have recently emerged.18, 19 We confirmed AR expression in both tubular epithelium and infiltrating leukocyte populations 2 wpi in male C3H kidneys (Supplementary Figure 2). To demonstrate AR involvement in our observed phenotypes, we first adopted a genetic approach using C57BL/6 Aw-j testicular feminization (Tfm) mice, which encode a frameshift mutation disrupting the steroid-binding domain of the AR, rendering these animals insensitive to classical AR signaling.20, 21 Wild-type (WT) males exhibited kidney titers significantly higher than WT females (Figure 3A; P=0.016), as we previously observed.7 Importantly, the majority of Tfm males resolved renal infection (Figure 3A), with kidney bacterial burdens significantly lower than WT males (P=0.0043) but indistinguishable from WT females (P=0.50). The Tfm mutation did not have a significant effect on bacterial loads in the bladder 24 hpi (Supplementary Figure 3).
Figure 3. Androgen receptor activation enhances severity of UTI.
(A) Renal bacterial loads 2 wpi in genotypes comprising males (open circles) or females (filled circles). Included strains are WT males (AR+/Y), androgen receptor-deficient (Tfm) males (ARTfm/Y), and WT females (AR+/+). The renal bacterial loads of functionally AR-deficient (TFM) males were significantly lower than in WT males (**P<0.01) and were equivalent to those in WT females. In fact, the kidneys of most AR-deficient males were sterile 2 wpi (below the limit of detection, indicated by the dotted line). (B, C) Female C57BL/6 mice were implanted 2 wk prior to infection with a long-release subcutaneous pellet of DHT or placebo; 3 d later (11 d prior to infection), oral gavage with enzalutamide (Enz) or vehicle (Veh) was initiated and continued until sacrifice 2 wpi. As observed in C3H mice (Figures 1 and 2), DHT treatment of C57BL/6 females conferred susceptibility to severe UTI, as evidenced by significantly higher bladder (B) and kidney (C) bacterial loads 2 wpi compared with non-androgenized females (**P<0.01). In addition, treatment of DHT-treated mice with Enz sharply reduced bacterial loads in both bladder and kidneys compared with those receiving vehicle (***P<0.001).
We also demonstrated direct AR involvement via pharmacological inhibition with enzalutamide,22 a second-generation antiandrogen with higher potency and improved specificity over earlier agents.23, 24 We implanted C57BL/6 females with DHT or placebo pellets 2 wk prior to UTI, and 3 days later initiated enzalutamide or vehicle treatment. Enzalutamide administration conferred strong protection against severe UTI outcomes, with significantly reduced bacterial loads in the kidneys and bladder (P<0.0001 vs vehicle; Figure 3B, C). In total, these data specify that AR activation induces susceptibility to severe UTI in both host sexes and across multiple genetic backgrounds.
Severe upper-tract UTI in the androgenized host manifests as abscess nucleated by intratubular UPEC communities
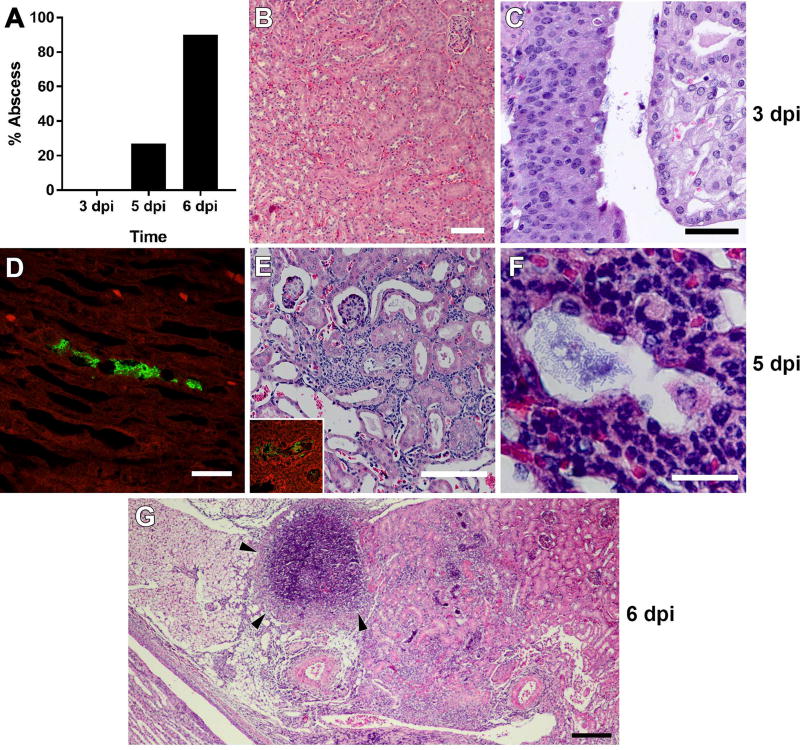
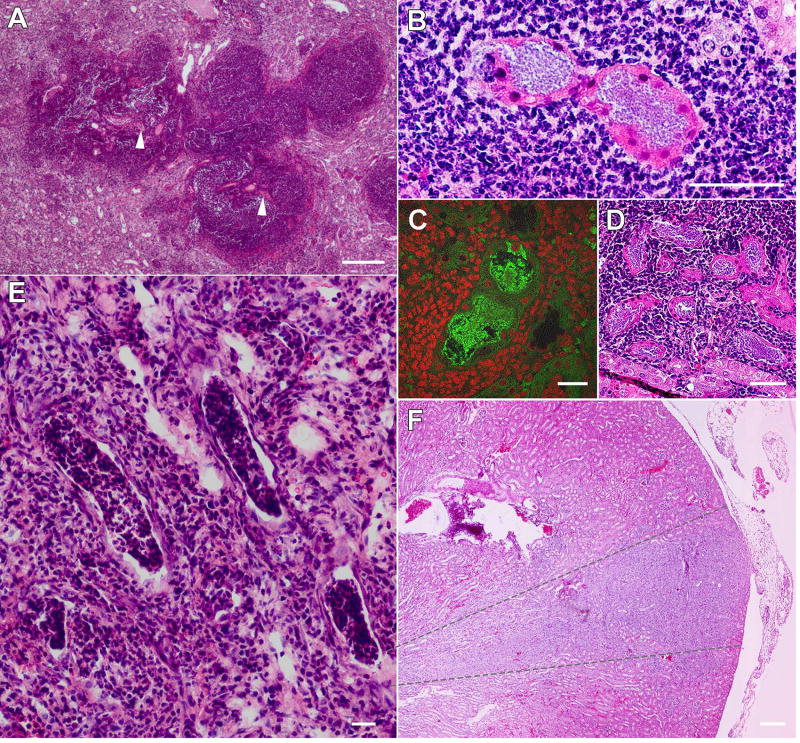
As modeling of UTI has until now been limited to female mice, and females of most strains are resistant to severe pyelonephritis and renal abscess formation,6, 7, 25, 26 the field lacks a detailed understanding of the pathogenesis of ascending renal abscesses. However, the high incidence of abscess formation in androgenized C3H/HeN hosts7 (Figure 1B, 2B) now allows interrogation of the anatomic and temporal details of abscess development following intravesical UPEC inoculation. Histological examination of kidneys 3 days post infection (dpi) from mice exhibiting urine and bladder bacterial titers >104 CFU (predictive of pyelonephritis7) demonstrated only scant neutrophilic inflammation near the renal pelvis, with no visible abscess (Figure 4A) and no apparent tissue alteration in the renal cortex or medulla (Figure 4B). Visualized bacteria were limited to the renal pelvis and calyces (Figure 4C). By 5 dpi, a minority of infected mice (4 of 15, 27%; Figure 4A) exhibited gross abscess at necropsy. Sectioning of entire kidneys revealed infrequent small, intraluminal collections of bacillary UPEC distributed from the renal medulla (Figure 4D) to the cortex (Figure 4E and Supplementary Figure 4). The tubules housing these UPEC communities reflected cellular injury and were surrounded by small numbers of infiltrating neutrophils, without intraluminal inflammation (Figure 4E, F). By 6 dpi, 90% of infected males exhibited renal abscess at necropsy (Figure 4A, G), equivalent to the frequency observed 2 wpi.7 Thus, ascending renal abscesses in this model develop precipitously between 5 and 6 dpi, signifying rapid UPEC replication and neutrophil recruitment in this interval.
Figure 4. Timeline of renal abscess formation in C3H/HeN mice.
(A) Proportion of male C3H/HeN mice exhibiting gross abscess formation at necropsy at the indicated intervals (n = 8–15 per time point). At 3 dpi, histology demonstrates normal renal architecture (B) and bacteria within the renal pelvis (C). At 5 dpi, immunofluorescence and H&E staining identify small collections of bacillary UPEC in the urinary space from the medulla (D; anti-E. coli green) to the cortex (E, F; anti-E. coli green in E inset). By 6 dpi, histologic evidence of abscess is fully established, with necrotic lesions, loss of tubular architecture and replacement by neutrophilic infiltrate (G, arrowheads). Scale bars: B, 100 µm; C, 50 µm; D, 25 µm; E, 100 µm; F, 20 µm; G, 200 µm.
Kidney sections from C3H/HeN males 2 wpi revealed UPEC populations in the centers of dense neutrophilic lesions appearing predominantly in the cortex (Figure 5A, B and Supplementary Figure 5). At this advanced stage, visible UPEC were located within the intratubular space, adopting coccobacillary morphology in tightly packed communities (Figure 5B, C), in contrast to their more loosely associated appearance 5 dpi (Figure 4F). Recruited neutrophils had largely destroyed or replaced much of the surrounding tubular architecture (Figure 5A–D) and also formed casts within surrounding tubules (Figure 5E), as seen in human pyelonephritis and as reported in other mouse models.8, 27, 28 We often observed multiple, discrete intratubular bacterial collections in close proximity within the same neutrophilic lesion (Figure 5D), and at low power, parenchymal involvement distinctly followed a wedge-shaped pattern while remote areas of kidney were spared (Figure 5F). In conjunction with findings at earlier time points (Figure 4), and consonant with recently published data in C3H/HeOuJ mice,27 these results indicate that ascending UPEC colonize an individual papillary collecting duct pyramid and multiple interconnected nephrons via robust intraluminal replication to nucleate abscess formation within a given segment of the kidney. Consistent with our prior data,29 serum BUN was not significantly elevated in these C3H males 2 wpi (Supplementary Figure 6). Additionally, abscesses rarely develop in non-androgenized female C3H/HeN mice,6, 7, 30 but we did identify an abscess with similar bacterial intratubular community morphology in one such mouse (out of >30 examined 2 wpi; Supplementary Figure 7).
Figure 5. UPEC establish biofilm-like communities in a protected intraluminal niche.
Neutrophilic abscesses were seen 2 wpi predominantly in the cortex (A), centered on a nidus of infected tubules (examples at A arrowheads, and B). Infected tubules at this time point were filled with coccobacillary UPEC, as seen by immunofluorescence with anti-E. coli antibodies (green in C; host nuclei are stained red). In many infected kidneys, multiple infected tubules were identified within a single large abscess lesion (D). The lumina of some tubules were occupied by neutrophil casts (E). Overall, the pattern of inflammation followed that of a wedge of nephrons associated with an infected papillary collecting duct (duct of Bellini), generally outlined by the gray dashed lines in (F). Scale bars: A, 200 µm; B, 50 µm; C, 25 µm; D, 50 µm; E, 25 µm; F, 200 µm.
Abscess formation prevents lethal dissemination but shelters UPEC intratubular communities
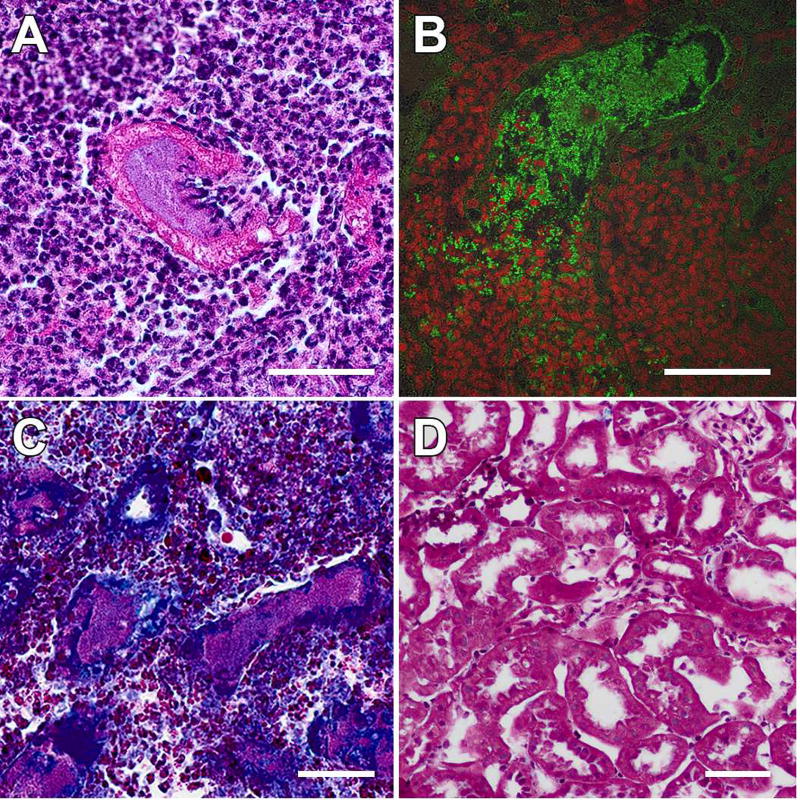
In the abscessed region of kidney 2 wpi, renal parenchyma was largely replaced by intense neutrophilic infiltrate (Figure 5). Light and fluorescence microscopy captured breaching of the UPEC-harboring tubules by neutrophils (Figure 6A, B), and trichrome staining of male C3H/HeN kidneys 2 wpi indicated that the infected tubules were fibrotic (Figure 6C, D). These findings suggest that for at least some duration, the tubular structure and subsequent UPEC-provoked peritubular fibrosis shield the intratubular UPEC community from phagocytic attack.
Figure 6. Tubular epithelium and peritubular fibrosis restrict infection but protect UPEC from infiltrating phagocytes.
The process by which tubular architecture is destroyed within the abscess by 2 wpi in C3H/HeN males was captured in images showing infiltrating neutrophils breaching the epithelium of tubules harboring UPEC communities (A, H&E; B, anti-E. coli in green, nuclear stain in red). Gomori trichrome staining 2 wpi revealed that infected tubules were fibrotic (C), compared with kidneys from mock-infected C3H/HeN males at the same time point (D). Scale bars, 50 µm.
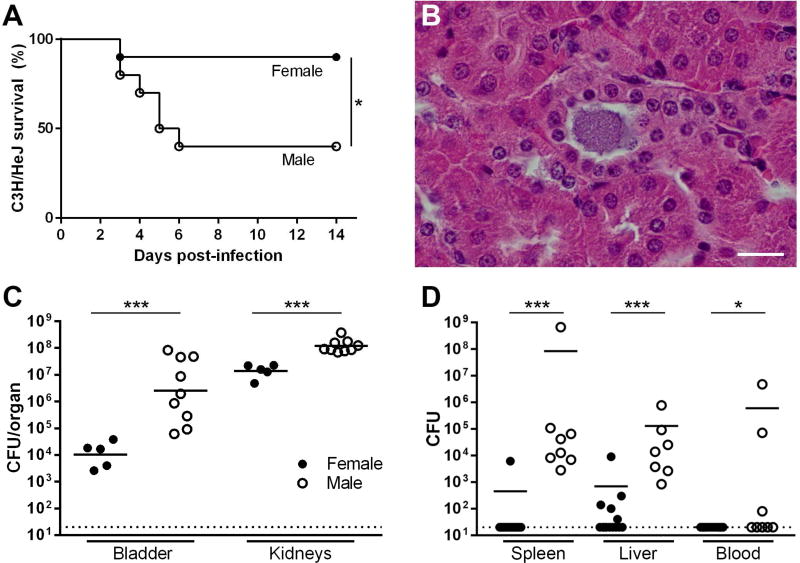
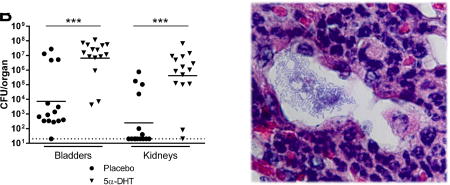
It was previously reported that mice with functional deficiency of Toll-like receptor 4 (TLR4), which exhibit markedly delayed and diminished innate responses to UTI,31, 32 do not develop renal abscesses even following direct renal injection of UPEC.6, 33, 34 We therefore tested how TLR4 deficiency would impact sex differences in renal infection by surgically infecting male and female C3H/HeJ mice, isogenic with C3H/HeN except for a TLR4 mutation that renders HeJ mice insensitive to lipopolysaccharide.35 Most infected C3H/HeJ females survived through the period of observation, while many infected C3H/HeJ males succumbed to infection between 3 and 7 dpi (P=0.0274; Figure 7A). Interestingly, the timing of demise in these C3H/HeJ males coincided with the window of abscess development we observed in C3H/HeN males (Figure 4). Abscess was not observed in C3H/HeJ males at necropsy despite the presence of intratubular UPEC communities (Figure 7B). In addition, at 3 dpi (preceding any deaths), infected male C3H/HeJ mice displayed significantly higher renal (P=0.001) and bladder (P=0.001) bacterial burdens compared to female C3H/HeJ mice (Figure 7C).
Figure 7. UPEC-infected TLR4-deficient males fail to form renal abscesses and are vulnerable to lethal dissemination.
(A) Neutrophil recruitment and abscess formation were necessary for containment of infection; when the TLR4-deficient C3H/HeJ strain was used, male mice were much more likely to succumb to infection (n=10 per group, *P=0.0274), an outcome not seen in immunocompetent C3H/HeN mice. Surviving C3H/HeJ males failed to form abscesses surrounding UPEC-infected tubules (B; scale bar, 20 µm). Male C3H/HeJ developed significantly higher bladder and kidney titers than females 3 dpi (C, ***P=0.001). In addition, male C3H/HeJ mice were significantly more susceptible than females to dissemination, the likely cause of death, as evidenced by bacterial loads 3 dpi in spleen, liver and blood (D, ***P<0.001, *P<0.05). Dotted lines indicate limit of detection.
These outcomes in C3H/HeJ males are in sharp contrast to infected C3H/HeN males, which uniformly survive despite persistence (for at least 20 wpi) of high renal bacterial burdens and abscesses.29 To further assess the cause of this sex difference in mortality, we infected male or female C3H/HeJ mice and cultured spleen, liver and blood 3 dpi (preceding any deaths). Dissemination of infection was significantly more prevalent in males, as reflected by higher bacterial loads in spleen (P<0.001), liver (P<0.001), and blood (P<0.05) (Figure 7D). Persistence of observed sex differences in UTI outcomes in the absence of functional TLR4 suggests that these differences are not attributable to androgen effects on TLR4 signaling, expression, or activation. Collectively, these data support a model in which abscess formation with peritubular fibrosis enables the immunocompetent host to contain renal infection and prevent lethal systemic disease; however, this host strategy also permits UPEC to maintain a sheltered intratubular niche for replication and survival following ascension into the kidney.
DISCUSSION
In this study, we show that AR activation in either host sex underlies increased susceptibility to pyelonephritis and renal abscess formation, a paradigm with significant translational implications. We further demonstrate that UPEC establishes intraluminal communities within kidney tubules, arising rapidly in the androgenized host within a narrow temporal window to nucleate nascent renal abscesses. A subset of these kidney bacterial communities (KBCs) were protected from phagocytosis, as the fibrotic tubular epithelium encapsulating these communities appeared to hinder phagocyte entry.
Abscesses were focal or multifocal, occurring within regions of pyelonephritis that followed the pattern of a collecting duct unit27 and located adjacent to other, uninvolved segments of renal parenchyma. This observation suggests that among UPEC that reach the renal pelvis, only a fraction are ultimately successful in accessing and persisting within selected nephrons. Beyond the process of uropathogen ascension through the urethra or from the bladder to the kidney, it is equally important to illuminate this “third ascension” of uropathogens from the renal pelvis and calyces to more proximal segments of the nephron. Although some studies have implicated immunologic factors influencing ascension and persistence in the collecting duct,36–38 the virulence mechanisms and host factors which enable tubular ascension and intraluminal replication are incompletely defined.
Even in a single micrograph of a developing abscess, one can see areas of complete destruction of cortical and tubular architecture, infected tubules that have been invaded partially or completely by neutrophils (consistent with neutrophil casts seen in other recent studies8, 27), and intratubular KBCs that have not yet been successfully breached by phagocytes. These persisting KBCs are apparently shielded for a more prolonged time by tubular epithelium and peritubular fibrosis. These images thus offer snapshots of various stages of the infiltration and phagocytic activity of host immune cells en route to a fully formed abscess. In other disease models, including ischemic kidney injury, transmigration of neutrophils across the tubule epithelium appears to be unimpaired.39–41 Fruitful future investigations in the present model will illuminate pathogen strategies or host factors (e.g., local ischemia,42, 43 anatomic features such as the basement membrane, or specific cellular programs that promote post-infection fibrosis) that impede neutrophil migration into the abscess community. Importantly, while male C3H/HeN mice are unable to resolve renal infection, their survival indicates that abscess formation successfully restricts the dissemination of infection. In contrast, congenic males with impaired innate responses fail to form abscesses surrounding bacterial communities and ultimately succumb to disseminated infection. We therefore propose a model whereby the pathology of abscess formation is a double-edged sword: bacteria are contained via inflammation and fibrosis, preventing overwhelming systemic disease, but some tubules represent a privileged site for bacterial replication where, at least for a time, phagocytes within the abscess lesion are unable to readily access the expanding UPEC community.
These KBCs display notable parallels to earlier descriptions of intracellular bacterial communities within bladder epithelium,44–47 though the microenvironment is presumably quite different in these two niches. The thematic similarity of UPEC community morphology in bladder and kidney hints that UPEC may use conserved programming to proliferate and to ultimately subvert the innate cellular response through biofilm formation within these niches.48 Beyond the recognized advantage of biofilms in limiting antibiotic diffusion, the location of KBCs also likely impairs antibiotic action, perhaps by occluding local transit of antibiotics in the urinary space, inducing restriction of blood flow to UPEC-infected areas,42, 43 or limiting diffusion from the blood space through injured and fibrotic tubular epithelia. These features correlate with the clinical need for prolonged antibiotic therapy in severe pyelonephritis and renal abscess.
AR activation clearly induced susceptibility to severe pyelonephritis and abscess development in both host sexes; genetic or pharmacologic inhibition of AR signaling attenuated severe UTI. Further investigations will specify the AR-regulated gene networks that mediate UTI susceptibility in androgenized hosts, in renal epithelium and/or local or circulating immune cells. Androgen signaling has been implicated in immune cell function in other systems. For example, monocyte migration and production of TNFα were amplified by androgen exposure in a mouse model of wound healing;49 overall, mouse models and in vitro studies of neutrophils and macrophages have not yet painted a complete and unified picture regarding androgen influence.50–53 Further, the persistence of a sex difference in C3H/HeJ mice, which lack functional TLR4 (the primary instigator of inflammatory responses in the kidney), suggests that the effect of androgens on UTI pathogenesis does not reside entirely in the hematopoietic compartment.
We previously saw no appreciable phenotype following estrogen depletion,7 and here have ruled out a contribution from peripheral aromatization. This is in contrast to a current paradigm, supported by some human and mouse studies, in which estrogen levels are believed to influence UTI pathogenesis.54–58 Instead, we argue that even modest elevations in circulating testosterone (which often parallel increases in estradiol59–61) in female and susceptible male patients may have a greater effect on frequency or severity of UTI. Certainly, the human population with the highest circulating testosterone, adolescent males,62 exhibit the lowest rates of UTI,12 but the urogenital anatomy (compared with that of women) comprises the key defense against UTI in otherwise healthy men.7 In fact, when these anatomic barriers are compromised or bypassed, epidemiologic data reflect increased morbidity and mortality in men who do develop complicated UTI, as compared with women.63–66 In a related vein, male infants (e.g., those under 6 months of age) presenting with UTI outnumber their female counterparts, with male UTI rates falling steadily from the neonatal period to late infancy.67–75 This epidemiologic phenomenon closely parallels the postnatal surge in testosterone in male infants that reaches pubertal levels shortly after birth, then steadily wanes to a prepubertal baseline by 6–9 months of age.76–79 Thus, while UTI risk in male infants is commonly ascribed to indistinct “urodynamic immaturity,” we speculate that testosterone activity may actually underlie this risk, especially in the two thirds of such infants who (after first febrile UTI) lack demonstrable VUR or obstruction.80
The present finding that androgen-mediated UTI receptivity affects females (as well as males) extends the translational implications of our work. In murine models of polycystic ovarian syndrome (PCOS; a common hyperandrogenic state in young women), mice overexpressing LH (and consequently testosterone) develop spontaneous pyelonephritis and associated renal damage.81–84 Most clinical studies of PCOS have not specifically ascertained UTI incidence in these women; however, where data are available, women with PCOS exhibited increased UTI rates compared to healthy females,85–87 and these rates fell in affected women following antiandrogen therapy.85 An untold number of women without overt hyperandrogenism might be at higher UTI risk because of circulating testosterone levels near the upper limits of the “normal” range, a potential causative relationship that mandates further study. We present proof of concept that the course of severe UTI can be mitigated by AR antagonism, suggesting that antiandrogen therapy may represent an avenue for adjunctive therapy in treatment-refractory cases of complicated UTI.
METHODS
Bacteria
Uropathogenic E. coli strain UTI89, a type 1 piliated clinical cystitis isolate,88 was grown in static Luria-Bertani (LB) broth for 16–18 h at 37°C. Cultures were centrifuged for 10 min at 7,500 × g at 4°C before resuspension in sterile phosphate-buffered saline (PBS) to a final density of ∼4 × 108 CFU/mL.
Surgical and catheterization murine models of UTI
All animal protocols received prior approval from the Washington University Institutional Animal Care and Use Committee. Experiments were conducted in C3H/HeN (Envigo, Indianapolis, IN), C3H/HeJ, C57BL/6 Aw-J/Aw-J (or its isogenic Tfm mutant), or C57BL/6 (all from Jackson Laboratories, Bar Harbor, ME) strains. Mini-surgical bladder inoculations were carried out as described previously.7 Mice aged 8–9 wk were anesthetized with inhaled 3% isoflurane, and the abdomen was shaved and sterilized with 2% chlorhexidine solution. A 3-mm vertical, midline incision was made over the bladder through the skin and peritoneum. The bladder was aseptically emptied before injection of 50 µL containing 1–2 × 107 CFU into the bladder lumen over 10 s. The bladder was allowed to expand for an additional 10 s before the needle was removed, and the peritoneum and skin were closed with sutures. Certain female-only experiments, as indicated, were replicated with bladder inoculation by catheter.89–91
Castration and androgen treatment
For surgical castration, male mice aged 4 wk received preoperative subcutaneous buprenorphine SR (0.05 mg/kg) prior to inhaled 3% isoflurane anesthesia. The scrotum was depilated with Nair (Church & Dwight Co., Ewing, NJ) and the operative field was disinfected with 2% chlorhexidine solution. A 1-cm ventral, midline incision was made in the scrotum, the tunica was pierced, and the testis and vas deferens were mobilized. The spermatic cord on each side was clamped and ligated with 4-0 Vicryl above the epididymis, and the testis and vas deferens were removed. The skin incision was closed with Vetbond (3M Animal Care Products, St. Paul, MN). Four days following castration or sham operation, 60-day extended-release pellets containing 25 mg testosterone, 25 mg DHT, or placebo (Innovative Research of America, Sarasota, FL) were implanted in sterile fashion at the posterior neck, using a small incision and blunt dissection to create a subcutaneous pocket. UTI was induced 4 weeks later (i.e., at 8–9 weeks of age).
Antiandrogen treatment
Female C57BL/6 mice, 5 weeks of age, received subcutaneous DHT or placebo pellets as described above. Beginning 3 d later, mice received enzalutamide 50 mg/kg daily for 6 d per wk by oral gavage, or vehicle (1% carboxymethylcellulose, 0.1% Tween-80, 5% DMSO). Ten days later, UTI was induced via catheter inoculation, as described above. Treatment continued until sacrifice 2 wpi.
Serum analyte measurements
Blood was collected by submandibular puncture, retroorbital aspiration, or cardiac puncture (if collection was coincident with euthanasia) into Microtainer serum separation tubes (Becton Dickinson, Franklin Lakes, NJ). Samples were allowed to clot for 90 min at room temperature (RT) before centrifugation at 10,000 × g. Serum testosterone was measured by enzyme immunoassay at the Ligand Assay and Analysis Core, University of Virginia Center for Research in Reproduction (Charlottesville, VA). Blood urea nitrogen (BUN) was measured by the veterinary laboratory at Washington University School of Medicine.
Determination of urine and tissue bacterial loads
Where indicated, we obtained post-infection, clean-catch urine samples using gentle suprapubic pressure to enumerate CFU/mL urine. At the indicated time points, mice were euthanized via CO2 asphyxiation, and bladders and kidney pairs were aseptically removed and homogenized in 1 ml or 0.800 ml sterile PBS, respectively. To ascertain prostate bacterial loads, the male urogenital system was removed en bloc and the prostate was micro-dissected under a dissecting microscope as previously described.92 Serial dilutions of tissue homogenates or urine were plated on LB agar to enumerate bacterial loads. Where indicated, cystitis in C3H/HeN mice was classified as “chronic” if all urine and endpoint bladder titers contained >104 CFU/mL.
Tissue histopathology, immunofluorescence, and immunohistochemistry
Infected bladders and kidneys were bisected and fixed in 10% neutral buffered formalin for 24 h. Fixed tissues were embedded in paraffin, sectioned, and stained with H&E or Gomori trichrome. Alternatively, kidneys were frozen, sectioned with a cryostat, and similarly stained. For immunofluorescence, unstained slides were deparaffinized in xylenes and rehydrated in isopropanol, washed in water, and boiled in 10 mM sodium citrate. Slides were blocked in 1% BSA, 0.3% Triton-X 100 in PBS for 30 min at RT, and incubated with rabbit anti-E. coli antibody (E3500-06C, US Biological, Salem, MA) for 1 h at RT or overnight at 4°C. After washing in PBS, sections were stained with AlexaFluor 488-conjugated goat anti-rabbit IgG (Life Technologies, Grand Island, NY) and SYTO 61 red fluorescent nucleic acid stain (Molecular Probes, Eugene, OR). Images were acquired on an Olympus FV1200 confocal microscope. For immunohistochemistry, unstained slides were deparaffinized in xylenes, rehydrated in a series of ethanol washes, boiled in 10mM sodium citrate, and blocked in 1% BSA, 0.3% Triton-X 100 in PBS for 1 h at RT. Slides were incubated with rat anti-Ly6G antibody (BE0075-1, BioXCell, West Lebanon, NH) for 2 h at RT, then stained with biotinylated goat anti-rat IgG (BA-9400, Vector Laboratories, Burlingame, CA) for 1 h at RT; or incubated with rabbit anti-AR antibody (ab74272, Abcam, Cambridge, MA) for 2 h at RT, followed by biotinylated goat anti-rabbit IgG (BA-1000, Vector Laboratories) for 30 min at RT. For each target, slides were then treated for 30 min with Vectastain Elite ABC reagent and developed using DAB substrate kit (Vector Laboratories). Slides were then counterstained with hematoxylin, rehydrated, mounted, and visualized.
Statistics
Organ bacterial loads and numerical data were compared by the nonparametric Mann-Whitney U test. Survival analysis was performed with the Mantel-Cox log-rank test. Fisher exact test was used for 2×2 comparisons. P values <0.05 were considered significant.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants P50-DK064540 (and associated supplement) to DAH, T32-AI007172 to PDO, F30-DK104446 to PDO, and F32-DK007126 to TNH; and National Science Foundation grant DGE-1143954 and a Mr. and Mrs. Spencer T. Olin Fellowship for Women in Graduate Study to LKM. We thank V. Arora for enzalutamide, and K. Tiemann and D. Rosen for technical contributions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
DAH serves on the Board of Directors of BioVersys AG, Basel, Switzerland. All other authors have no potential conflicts of interest to disclose.
References
- 1.Foxman B, Brown P. Epidemiology of urinary tract infections: transmission and risk factors, incidence, and costs. Infect Dis Clin North Am. 2003;17:227–241. doi: 10.1016/s0891-5520(03)00005-9. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg M. Pharmacoeconomics of treating uncomplicated urinary tract infections. Int J Antimicrob Agents. 1999;11:247–251. doi: 10.1016/s0924-8579(99)00024-2. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson SH, Eklöf O, Eriksson CG, et al. Development of hypertension and uraemia after pyelonephritis in childhood: 27 year follow up. Brit Med J. 1989;299:703. doi: 10.1136/bmj.299.6701.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levey AS, Coresh J. Chronic kidney disease. Lancet. 2012;379:165–180. doi: 10.1016/S0140-6736(11)60178-5. [DOI] [PubMed] [Google Scholar]
- 5.Zowawi HM, Harris PN, Roberts MJ, et al. The emerging threat of multidrug-resistant Gram-negative bacteria in urology. Nat Rev Urol. 2015;12:570–584. doi: 10.1038/nrurol.2015.199. [DOI] [PubMed] [Google Scholar]
- 6.Hannan TJ, Mysorekar IU, Hung CS, et al. Early severe inflammatory responses to uropathogenic E. coli predispose to chronic and recurrent urinary tract infection. PLoS Pathog. 2010;6:e1001042. doi: 10.1371/journal.ppat.1001042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Olson PD, Hruska KA, Hunstad DA. Androgens enhance male urinary tract infection severity in a new model. J Am Soc Nephrol. 2016;27:1625–1634. doi: 10.1681/ASN.2015030327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowen SE, Watt CL, Murawski IJ, et al. Interplay between vesicoureteric reflux and kidney infection in the development of reflux nephropathy in mice. Dis Model Mech. 2013;6:934–941. doi: 10.1242/dmm.011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schrier RW. Diseases of the Kidney & Urinary Tract. 8. Wolters Kluwer Health/Lippincott Williams & Wilkins; Philadelphia: 2007. [Google Scholar]
- 10.Anders HJ, Schaefer L. Beyond tissue injury-damage-associated molecular patterns, Toll-like receptors, and inflammasomes also drive regeneration and fibrosis. J Am Soc Nephrol. 2014;25:1387–1400. doi: 10.1681/ASN.2014010117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suarez-Alvarez B, Liapis H, Anders HJ. Links between coagulation, inflammation, regeneration, and fibrosis in kidney pathology. Lab Invest. 2016;96:378–390. doi: 10.1038/labinvest.2015.164. [DOI] [PubMed] [Google Scholar]
- 12.Foxman B. The epidemiology of urinary tract infection. Nat Rev Urol. 2010;7:653–660. doi: 10.1038/nrurol.2010.190. [DOI] [PubMed] [Google Scholar]
- 13.Foxman B. Epidemiology of urinary tract infections: incidence, morbidity, and economic costs. Dis Mon. 2003;49:53–70. doi: 10.1067/mda.2003.7. [DOI] [PubMed] [Google Scholar]
- 14.Angele MK, Ayala A, Cioffi WG, et al. Testosterone: the culprit for producing splenocyte immune depression after trauma hemorrhage. Am J Physiol Cell Physiol. 1998;274:C1530–C1536. doi: 10.1152/ajpcell.1998.274.6.C1530. [DOI] [PubMed] [Google Scholar]
- 15.Brouillette J, Rivard K, Lizotte E, et al. Sex and strain differences in adult mouse cardiac repolarization: importance of androgens. Cardiovasc Res. 2005;65:148–157. doi: 10.1016/j.cardiores.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 16.Nicholson TF, Watts KM, Hunstad DA. OmpA of uropathogenic Escherichia coli promotes postinvasion pathogenesis of cystitis. Infect Immun. 2009;77:5245–5251. doi: 10.1128/IAI.00670-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couse JF, Yates MM, Walker VR, et al. Characterization of the hypothalamic-pituitary-gonadal axis in estrogen receptor (ER) null mice reveals hypergonadism and endocrine sex reversal in females lacking ERα but not ERβ. Mol Endocrinol. 2003;17:1039–1053. doi: 10.1210/me.2002-0398. [DOI] [PubMed] [Google Scholar]
- 18.Walker WH. Non-classical actions of testosterone and spermatogenesis. Philos Trans R Soc Lond B Biol Sci. 2010;365:1557–1569. doi: 10.1098/rstb.2009.0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman F, Christian HC. Non-classical actions of testosterone: an update. Trends Endocrinol Metab. 2007;18:371–378. doi: 10.1016/j.tem.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 20.Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227:1217–1219. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- 21.Yeh S, Tsai M-Y, Xu Q, et al. Generation and characterization of androgen receptor knockout (ARKO) mice: an in vivo model for the study of androgen functions in selective tissues. Proc Natl Acad Sci USA. 2002;99:13498–13503. doi: 10.1073/pnas.212474399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tran C, Ouk S, Clegg NJ, et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science. 2009;324:787–790. doi: 10.1126/science.1168175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fenton MA, Shuster TD, Fertig AM, et al. Functional characterization of mutant androgen receptors from androgen-independent prostate cancer. Clin Cancer Res. 1997;3:1383–1388. [PubMed] [Google Scholar]
- 24.Wong CI, Kelce WR, Sar M, et al. Androgen receptor antagonist versus agonist activities of the fungicide vinclozolin relative to hydroxyflutamide. J Biol Chem. 1995;270:19998–20003. doi: 10.1074/jbc.270.34.19998. [DOI] [PubMed] [Google Scholar]
- 25.Svensson M, Yadav M, Holmqvist B, et al. Acute pyelonephritis and renal scarring are caused by dysfunctional innate immunity in mCxcr2 heterozygous mice. Kidney Int. 2011;80:1064–1072. doi: 10.1038/ki.2011.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaillon S, Moalli F, Ragnarsdottir B, et al. The humoral pattern recognition molecule PTX3 is a key component of innate immunity against urinary tract infection. Immunity. 2014;40:621–632. doi: 10.1016/j.immuni.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 27.Li B, Haridas B, Jackson AR, et al. Inflammation drives renal scarring in experimental pyelonephritis. Am J Physiol Renal Physiol. 2017;312:F43–F53. doi: 10.1152/ajprenal.00471.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stamm WE. Measurement of pyuria and its relation to bacteriuria. Am J Med. 1983;75:53–58. doi: 10.1016/0002-9343(83)90073-6. [DOI] [PubMed] [Google Scholar]
- 29.Olson PD, McLellan LK, Liu A, et al. Renal scar formation and kidney function following antibiotic-treated murine pyelonephritis. Dis Model Mech. 2017;10:1371–1379. doi: 10.1242/dmm.030130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rosen DA, Hung CS, Kline KA, et al. Streptozocin-induced diabetic mouse model of urinary tract infection. Infect Immun. 2008;76:4290–4298. doi: 10.1128/IAI.00255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagberg L, Hull R, Hull S, et al. Difference in susceptibility to Gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun. 1984;46:839–844. doi: 10.1128/iai.46.3.839-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahin RD, Engberg I, Hagberg L, et al. Neutrophil recruitment and bacterial clearance correlated with LPS responsiveness in local Gram-negative infection. J Immunol. 1987;138:3475–3480. [PubMed] [Google Scholar]
- 33.Schilling JD, Mulvey MA, Vincent CD, et al. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J Immunol. 2001;166:1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- 34.Schilling JD, Martin SM, Hung CS, et al. Toll-like receptor 4 on stromal and hematopoietic cells mediates innate resistance to uropathogenic Escherichia coli. Proc Natl Acad Sci USA. 2003;100:4203–4208. doi: 10.1073/pnas.0736473100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vogel SN, Johnson D, Perera PY, et al. Cutting edge: functional characterization of the effect of the C3H/HeJ defect in mice that lack an Lpsn gene: in vivo evidence for a dominant negative mutation. J Immunol. 1999;162:5666–5670. [PubMed] [Google Scholar]
- 36.Chassin C, Hornef MW, Bens M, et al. Hormonal control of the renal immune response and antibacterial host defense by arginine vasopressin. J Exp Med. 2007;204:2837–2852. doi: 10.1084/jem.20071032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chassin C, Vimont S, Cluzeaud F, et al. TLR4 facilitates translocation of bacteria across renal collecting duct cells. J Am Soc Nephrol. 2008;19:2364–2374. doi: 10.1681/ASN.2007121273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paragas N, Kulkarni R, Werth M, et al. α-Intercalated cells defend the urinary system from bacterial infection. J Clin Invest. 2014;124:2963–2976. doi: 10.1172/JCI71630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joannidis M, Truebsbach S, Bijuklic K, et al. Neutrophil transmigration in renal proximal tubular LLC-PK1 cells. Cell Physiol Biochem. 2004;14:101–112. doi: 10.1159/000076931. [DOI] [PubMed] [Google Scholar]
- 40.Bijuklic K, Sturn DH, Jennings P, et al. Mechanisms of neutrophil transmigration across renal proximal tubular HK-2 cells. Cell Physiol Biochem. 2006;17:233–244. doi: 10.1159/000094128. [DOI] [PubMed] [Google Scholar]
- 41.Awad AS, Rouse M, Huang L, et al. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009;75:689–698. doi: 10.1038/ki.2008.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mansson LE, Melican K, Boekel J, et al. Real-time studies of the progression of bacterial infections and immediate tissue responses in live animals. Cell Microbiol. 2007;9:413–424. doi: 10.1111/j.1462-5822.2006.00799.x. [DOI] [PubMed] [Google Scholar]
- 43.Mansson LE, Melican K, Molitoris BA, et al. Progression of bacterial infections studied in real time--novel perspectives provided by multiphoton microscopy. Cell Microbiol. 2007;9:2334–2343. doi: 10.1111/j.1462-5822.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- 44.Rosen DA, Hooton TM, Stamm WE, et al. Detection of intracellular bacterial communities in human urinary tract infection. PLoS Med. 2007;4:e329. doi: 10.1371/journal.pmed.0040329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosen DA, Pinkner JS, Jones JM, et al. Utilization of an intracellular bacterial community pathway in Klebsiella pneumoniae urinary tract infection and the effects of FimK on type 1 pilus expression. Infect Immun. 2008;76:3337–3345. doi: 10.1128/IAI.00090-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schwartz DJ, Chen SL, Hultgren SJ, et al. Population dynamics and niche distribution of uropathogenic Escherichia coli during acute and chronic urinary tract infection. Infect Immun. 2011;79:4250–4259. doi: 10.1128/IAI.05339-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson GG, Palermo JJ, Schilling JD, et al. Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301:105–107. doi: 10.1126/science.1084550. [DOI] [PubMed] [Google Scholar]
- 48.Olson PD, Hunstad DA. Subversion of host innate immunity by uropathogenic Escherichia coli. Pathogens. 2016;5:5010002. doi: 10.3390/pathogens5010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lai JJ, Lai KP, Chuang KH, et al. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-α expression. J Clin Invest. 2009;119:3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chignalia AZ, Oliveira MA, Debbas V, et al. Testosterone induces leucocyte migration by NADPH oxidase-driven ROS- and COX2-dependent mechanisms. Clin Sci. 2015;129:39–48. doi: 10.1042/CS20140548. [DOI] [PubMed] [Google Scholar]
- 51.Chuang KH, Altuwaijri S, Li G, et al. Neutropenia with impaired host defense against microbial infection in mice lacking androgen receptor. J Exp Med. 2009;206:1181–1199. doi: 10.1084/jem.20082521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fijak M, Schneider E, Klug J, et al. Testosterone replacement effectively inhibits the development of experimental autoimmune orchitis in rats: evidence for a direct role of testosterone on regulatory T cell expansion. J Immunol. 2011;186:5162–5172. doi: 10.4049/jimmunol.1001958. [DOI] [PubMed] [Google Scholar]
- 53.Filgueira FP, Lobato NS, DosSantos RA, et al. Endogenous testosterone increases leukocyte-endothelial cell interaction in spontaneously hypertensive rats. Life Sci. 2012;90:689–694. doi: 10.1016/j.lfs.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Perrotta C, Aznar M, Mejia R, et al. Oestrogens for preventing recurrent urinary tract infection in postmenopausal women. Cochrane Database Syst Rev. 2008:CD005131. doi: 10.1002/14651858.CD005131.pub2. [DOI] [PubMed] [Google Scholar]
- 55.Lüthje P, Brauner H, Ramos NL, et al. Estrogen supports urothelial defense mechanisms. Sci Transl Med. 2013;5:190ra180. doi: 10.1126/scitranslmed.3005574. [DOI] [PubMed] [Google Scholar]
- 56.Sonnex C. Influence of ovarian hormones on urogenital infection. Sex Transm Infect. 1998;74:11–19. doi: 10.1136/sti.74.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang C, Symington JW, Ma E, et al. Estrogenic modulation of uropathogenic Escherichia coli infection pathogenesis in a murine menopause model. Infect Immun. 2013;81:733–739. doi: 10.1128/IAI.01234-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stamm WE, Raz R. Factors contributing to susceptibility of postmenopausal women to recurrent urinary tract infections. Clin Infect Dis. 1999;28:723–725. doi: 10.1086/515209. [DOI] [PubMed] [Google Scholar]
- 59.Adly L, Hill D, Sherman ME, et al. Serum concentrations of estrogens, sex hormone-binding globulin, and androgens and risk of breast cancer in postmenopausal women. Int J Cancer. 2006;119:2402–2407. doi: 10.1002/ijc.22203. [DOI] [PubMed] [Google Scholar]
- 60.Kaaks R, Berrino F, Key T, et al. Serum sex steroids in premenopausal women and breast cancer risk within the European Prospective Investigation into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2005;97:755–765. doi: 10.1093/jnci/dji132. [DOI] [PubMed] [Google Scholar]
- 61.Braunstein GD, Johnson BD, Stanczyk FZ, et al. Relations between endogenous androgens and estrogens in postmenopausal women with suspected ischemic heart disease. J Clin Endocrinol Metab. 2008;93:4268–4275. doi: 10.1210/jc.2008-0792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ross R, Bernstein L, Judd H, et al. Serum testosterone levels in healthy young black and white men. J Natl Cancer Inst. 1986;76:45–48. [PubMed] [Google Scholar]
- 63.Foxman B, Klemstine KL, Brown PD. Acute pyelonephritis in US hospitals in 1997: hospitalization and in-hospital mortality. Ann Epidemiol. 2003;13:144–150. doi: 10.1016/s1047-2797(02)00272-7. [DOI] [PubMed] [Google Scholar]
- 64.Efstathiou SP, Pefanis AV, Tsioulos DI, et al. Acute pyelonephritis in adults: prediction of mortality and failure of treatment. Arch Intern Med. 2003;163:1206–1212. doi: 10.1001/archinte.163.10.1206. [DOI] [PubMed] [Google Scholar]
- 65.Nicolle LE, Friesen D, Harding GK, et al. Hospitalization for acute pyelonephritis in Manitoba, Canada, during the period from 1989 to 1992; impact of diabetes, pregnancy, and aboriginal origin. Clin Infect Dis. 1996;22:1051–1056. doi: 10.1093/clinids/22.6.1051. [DOI] [PubMed] [Google Scholar]
- 66.Ki M, Park T, Choi B, et al. The epidemiology of acute pyelonephritis in South Korea, 1997–1999. Am J Epidemiol. 2004;160:985–993. doi: 10.1093/aje/kwh308. [DOI] [PubMed] [Google Scholar]
- 67.Ismaili K, Lolin K, Damry N, et al. Febrile urinary tract infections in 0- to 3-month-old infants: a prospective follow-up study. J Pediatr. 2011;158:91–94. doi: 10.1016/j.jpeds.2010.06.053. [DOI] [PubMed] [Google Scholar]
- 68.Park S, Han JY, Kim KS. Risk factors for recurrent urinary tract infection in infants with vesicoureteral reflux during prophylactic treatment: effect of delayed contrast passage on voiding cystourethrogram. Urology. 2011;78:170–173. doi: 10.1016/j.urology.2010.12.023. [DOI] [PubMed] [Google Scholar]
- 69.Wong SN, Tse NK, Lee KP, et al. Evaluating different imaging strategies in children after first febrile urinary tract infection. Pediatr Nephrol. 2010;25:2083–2091. doi: 10.1007/s00467-010-1569-z. [DOI] [PubMed] [Google Scholar]
- 70.Winberg J, Andersen HJ, Bergstrom T, et al. Epidemiology of symptomatic urinary tract infection in childhood. Acta Paediatr Scand Suppl. 1974:1–20. doi: 10.1111/j.1651-2227.1974.tb05718.x. [DOI] [PubMed] [Google Scholar]
- 71.Wettergren B, Jodal U, Jonasson G. Epidemiology of bacteriuria during the first year of life. Acta Paediatr Scand. 1985;74:925–933. doi: 10.1111/j.1651-2227.1985.tb10059.x. [DOI] [PubMed] [Google Scholar]
- 72.Ginsburg CM, McCracken GH., Jr Urinary tract infections in young infants. Pediatrics. 1982;69:409–412. [PubMed] [Google Scholar]
- 73.Kanellopoulos TA, Salakos C, Spiliopoulou I, et al. First urinary tract infection in neonates, infants and young children: a comparative study. Pediatr Nephrol. 2006;21:1131–1137. doi: 10.1007/s00467-006-0158-7. [DOI] [PubMed] [Google Scholar]
- 74.Bonadio W, Maida G. Urinary tract infection in outpatient febrile infants younger than 30 days of age: a 10-year evaluation. Pediatr Infect Dis J. 2014;33:342–344. doi: 10.1097/INF.0000000000000110. [DOI] [PubMed] [Google Scholar]
- 75.Brkic S, Mustafic S, Nuhbegovic S, et al. Clinical and epidemiology characteristics of urinary tract infections in childhood. Med Arhiv. 2010;64:135–138. [PubMed] [Google Scholar]
- 76.Corbier P, Dehennin L, Castanier M, et al. Sex differences in serum luteinizing hormone and testosterone in the human neonate during the first few hours after birth. J Clin Endocrinol Metab. 1990;71:1344–1348. doi: 10.1210/jcem-71-5-1344. [DOI] [PubMed] [Google Scholar]
- 77.de Zegher F, Devlieger H, Veldhuis JD. Pulsatile and sexually dimorphic secretion of luteinizing hormone in the human infant on the day of birth. Pediatr Res. 1992;32:605–607. doi: 10.1203/00006450-199211000-00025. [DOI] [PubMed] [Google Scholar]
- 78.Andersson AM, Toppari J, Haavisto AM, et al. Longitudinal reproductive hormone profiles in infants: peak of inhibin B levels in infant boys exceeds levels in adult men. J Clin Endocrinol Metab. 1998;83:675–681. doi: 10.1210/jcem.83.2.4603. [DOI] [PubMed] [Google Scholar]
- 79.Lamminmaki A, Hines M, Kuiri-Hanninen T, et al. Testosterone measured in infancy predicts subsequent sex-typed behavior in boys and in girls. Horm Behav. 2012;61:611–616. doi: 10.1016/j.yhbeh.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 80.American Academy of Pediatrics. Urinary tract infection: clinical practice guideline for the diagnosis and management of the initial UTI in febrile infants and children 2 to 24 months. Pediatrics. 2011;128:595–610. doi: 10.1542/peds.2011-1330. [DOI] [PubMed] [Google Scholar]
- 81.Risma KA, Clay CM, Nett TM, et al. Targeted overexpression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci USA. 1995;92:1322–1326. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Risma KA, Hirshfield AN, Nilson JH. Elevated luteinizing hormone in prepubertal transgenic mice causes hyperandrogenemia, precocious puberty, and substantial ovarian pathology. Endocrinology. 1997;138:3540–3547. doi: 10.1210/endo.138.8.5313. [DOI] [PubMed] [Google Scholar]
- 83.Rulli SB, Ahtiainen P, Makela S, et al. Elevated steroidogenesis, defective reproductive organs, and infertility in transgenic male mice overexpressing human chorionic gonadotropin. Endocrinology. 2003;144:4980–4990. doi: 10.1210/en.2003-0403. [DOI] [PubMed] [Google Scholar]
- 84.Matzuk MM, DeMayo FJ, Hadsell LA, et al. Overexpression of human chorionic gonadotropin causes multiple reproductive defects in transgenic mice. Biol Reprod. 2003;69:338–346. doi: 10.1095/biolreprod.102.013953. [DOI] [PubMed] [Google Scholar]
- 85.Elkholi DGE, Nagy HM. The endocrine-metabolic disorders and adverse pregnancy outcomes in metabolically obese normal weight women with polycystic ovary syndrome. Womens Health Gynecol. 2016;2:031. [Google Scholar]
- 86.Agrawal R, Chimusoro K, Payne N, et al. Severe ovarian hyperstimulation syndrome: serum and ascitic fluid concentrations of vascular endothelial growth factor. Curr Opin Obstet Gynecol. 1997;9:141–144. [PubMed] [Google Scholar]
- 87.Wang YC, Su HY, Liu JY, et al. Maternal and female fetal virilization caused by pregnancy luteomas. Fertil Steril. 2005;84:509. doi: 10.1016/j.fertnstert.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 88.Chen SL, Hung CS, Pinkner JS, et al. Positive selection identifies an in vivo role for FimH during urinary tract infection in addition to mannose binding. Proc Natl Acad Sci USA. 2009;106:22439–22444. doi: 10.1073/pnas.0902179106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hung CS, Dodson KW, Hultgren SJ. A murine model of urinary tract infection. Nat Protoc. 2009;4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mulvey MA, Lopez-Boado YS, Wilson CL, et al. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science. 1998;282:1494–1497. doi: 10.1126/science.282.5393.1494. [DOI] [PubMed] [Google Scholar]
- 91.Hannan TJ, Hunstad DA. A murine model for Escherichia coli urinary tract infection. Methods Mol Biol. 2016;1333:159–175. doi: 10.1007/978-1-4939-2854-5_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lukacs RU, Goldstein AS, Lawson DA, et al. Isolation, cultivation, and characterization of adult murine prostate stem cells. Nat Protoc. 2010;5:702–713. doi: 10.1038/nprot.2010.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.