Abstract
The proximal portion of human chromosome 22q has been implicated in the pathogenesis of a clinically diverse group of conditions including DiGeorge sequence (DGS), velocardiofacial syndrome, and CHARGE association as well as isolated conotruncal heart anomalies. Frequently, overlap in the clinical presentation of these syndromes occurs and, recently, the presence of microdeletions on chromosome 22q11.2 with varying frequencies has been demonstrated in these syndromes. Using fluorescence in situ hybridization (FISH), we assessed 20 consecutive patients who were cytogenetically and clinically evaluated for a suspected syndrome that could be due to a microdeletion of chromosome 22q11.2. After cytogenetic testing and full clinical evaluation, we compared the results by FISH with the final clinical diagnosis and karyotype results. We found that microdeletions of 22q11.2 were detected in three of the five patients who were evaluated for DGS. The three cases with microdeletions appeared clinically to have DGS while the two negative cases were more atypical. High-resolution banding techniques did not detect a microdeletion in any of the cases; however, one of the 20 patients had a translocation between chromosomes 13 and 22. This patient also had a microdeletion of 22q11.2 detected by FISH and clinical features of DGS. None of the patients who were evaluated for disorders related to DGS showed microdeletions. We conclude that FISH is a useful, easily applied technique for the diagnosis of 22q11.2 microdeletion syndromes, particularly DGS. This test may also be useful in genetic counseling and in both prenatal and postnatal diagnoses.
Keywords: DiGeorge sequence, Chromosome 22, Fluorescence in situ hybridization, Microdeletion syndromes.
A number of screening and diagnostic tests have been developed for the evaluation of congenital disorders. The proximal portion of human chromosome 22q has been implicated in the pathogenesis of various developmental disorders, including DiGeorge sequence (DGS) (4–7,14,16), velocardiofacial syndrome (VCFS) (2,7,8,12,13), and isolated congenital conotruncal heart defects (10,17). Overlap in the clinical presentation of these syndromes occurs and, accordingly, microdeletions on chromosome 22q11.2 have been demonstrated in each of these disorders. The acronym CATCH 22 association (Cardiac defects, Abnormal facies, Thymic hypoplasia, Cleft palate, and Hypocalcemia) has been proposed as an encompassing term for this group of disorders (11,14,15). In addition, CHARGE association, (Coloboma, Heart defects, Atresia of choanus, Retardation (growth and/or mental), Genital defects, and Ear abnormalities) has also been shown to be associated with a microdeletion of chromosome 22q11 in rare cases (9).
Microdeletions of chromosome 22q11.2 can be detected occasionally with cytogenetic high-resolution banding techniques (8,16), restriction fragment length polymorphisms (4,6,14), or DNA dosage analysis (6). Recently, the use of cosmid probes has allowed for the detection of microdeletions using fluorescence in situ hybridization (FISH) (5,7,9,13). Although FISH is relatively new, it is simple, rapid, and less work intensive than other techniques. Hence, we report our experience of screening for chromosome 22q11.2 microdeletions in 20 consecutive patients with suspected DGS, CHARGE association, or related disorders by using FISH.
MATERIALS AND METHODS
Patients
Twenty consecutive patients with clinical features suggestive of a 22q11.2 microdeletion syndrome were referred to the cytogenetics laboratory for chromosomal analysis (Table 1). Of these 20 patients, five, seven, and eight patients had suspected DGS, CHARGE association, or a related disorder, respectively.
Table 1.
Clinical features and laboratory findings in twenty consecutive patients presenting to the cytogenetics laboratory with features of syndromes associated with microdeletions of chromosome 22
| Patient (age, gender) |
Reason for referral |
Abnormal clinical features |
High-resolution chromosome results |
22q11 FISH results |
Final clinical diagnosis |
|---|---|---|---|---|---|
| 1 M 2 d | r/o DGS | Leukocytopenia, CHD | 46, XY | Deletion | DGS |
| 2 M 2 d | r/o DGS | Hypocalcemia, CHD, hypertelorism | 45, XY, −13, −22, +der (13)t(13;22)(q33;q11) | Deletion | DGS |
| 3 M 5 y | r/o DGS | VSD, hypocalcemia, ear anomalies, lymphocytopenia, umbilical hernia | 46, XY | Deletion | DGS |
| 4 F 14 m | r/o DGS | CHD | 46, XX | Normal | No recognizable syndrome |
| 5 F 1 m | r/o DGS | CHD, multiple endocrine defects | 46, XX | Normal | Septo-optic dysplasia with panhypopituitarism and CHD |
| 6 F 3 d | r/o CHARGE | Bilateral colobomata, left cataract, ASD, choanal atresia, external ear deformities, hearing deficit | 46, XX | Normal | CHARGE |
| 7 F 2 y 9 m | r/o CHARGE | Choanal atresia, ASD, bilateral optic coloboma, hearing deficit, microphthalmia, esotropia | 46, XX | Normal | CHARGE |
| 8 M 1 d | r/o CHARGE | Duodenal atresia, ASD, PDA, choanal atresia, low-set ears, syndactyly | 47, XY, +21 | Normal | Down syndrome and acrocephalosyndactyly type I |
| 9 F 7 d | r/o CHARGE | ASD, growth retardation, external ear abnormalities | 46, XX | Normal | CHARGE |
| 10 F9 m | r/o CHARGE | Bilateral ocular coloboma | 46, XX | Normal | No recognizable syndrome |
| 11 M 20 d | r/o CHARGE | Hypospadias, micrognathia, inguinal hernia, cardiomegaly | 46, XY | Normal | No recognizable syndrome |
| 12 M 19 d | r/o CHARGE | Cleft lip, bilateral coloboma, PDA, unusual ear shape | 46, XY | Normal | No recognizable syndrome |
| 13 F 21 m | r/o 22q deletion | TOF, PDA | 46, XX | Normal | Multifactorial CHD |
| 14 M 3 d | r/o 22q deletion | TOF, abnormal facies | 46, XY | Normal | VATER |
| 15 M 1 d | r/o 22q deletion | Pulmonary atresia, TGV, VSD with tricuspid valve override, PDA | 46, XY | Normal | Multifactorial CHD |
| 16 M 1 d | r/o 22q deletion | Micrognathia, cleft palate | 46, XY | Normal | No recognizable syndrome |
| 17 F 3 d | r/o 22q deletion | VSD, TGV, PDA, pulmonary atresia with VSD | 46, XX | Normal | Multifactorial CHD |
| 18 M 19 m | r/o VCFS | Cleft palate and lip, microcephaly, abnormal facies | 46, XY | Normal | No recognizable syndrome |
| 19 F 1 d | r/o VATER | VSD, ASD, PDA, coarctation of aorta, esophageal atresia, duodenal atresia, tracheo-esophageal fistula | 46, XX | Normal | No recognizable syndrome |
| 20 F 1 d | r/o VATER | Esophageal atresia, TOF, duodenal atresia, tracheo-esophageal fistula | 46, XX | Normal | No recognizable syndrome |
ASD, atrial septal defect; CHARGE, coloboma, heart defects, atresia of choanus, retardation (growth and/or mental), genital defects, and ear abnormalities; CHD, congenital heart disease; DGS, DiGeorge sequence; PDA, patent ductus arteriosus; TGV, transposition of great vessels; TOF, tetralogy of Fallot; VATER, vertebral defects, anal atresia, tracheo-esophageal fistula with esophageal atresia, and renal dysplasia; VCFS, velo-cardiofacial syndrome; and VSD, ventricular septal defect.
High-Resolution Chromosomal Analysis and Fluorescence in situ Hybridization (FISH)
Metaphase chromosome spreads were prepared from peripheral blood lymphocytes harvested routinely. Chromosomal karyotyping and high-resolution banding were performed following pretreatment with actinomycin C as previously described (3). FISH was performed using a commercially available digoxigenin-labeled DNA kit (Oncor, Gaithersburg, MD, U.S.A.), according to manufacturer’s protocols. Briefly, chromosome spreads were cohybridized with two digoxigenin-labeled cosmid probes. One cosmid probe was directed at the site of the deletion (chromosome 22q11.2, locus D22S75) (6), and the other probe was directed to another region of chromosome 22 (22q13.3 locus D22S39) (1) and served as an internal control. Fluorescein-isothiocyanate-labeled anti-digoxigenin monoclonal antibodies were used for detection, and samples were counterstained with propidium iodide. At least 20 chromosome spreads were scored in each case by using an Olympus BH-2 fluorescent photomicroscope (Olympus Optical, Tokyo, Japan). Ektachromatic 400HC color-slide film (Kodak, Rochester, NY, U.S.A.) was used throughout this study.
RESULTS
DiGeorge Sequence
In the present study, five patients were evaluated for possible DGS. Four of the five had a normal karyotype by standard or high-resolution banding techniques. However, FISH detected a 22q11.2 microdeletion in three of the five (Fig. 1 and Table 1). Two of these three patients with a microdeletion of chromosome 22q11.2 had a normal karyotype. The third patient with a microdeletion had a chromosome translocation involving chromosomes 13 and 22 [45,XY, −13, −22, + der13 t(13:22)(q33;q11).
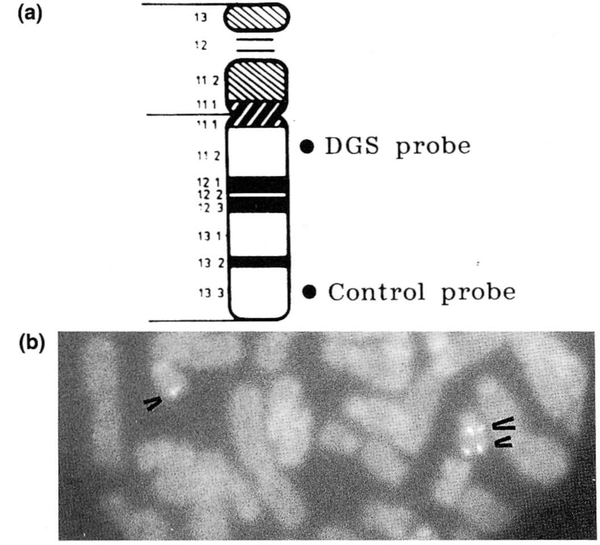
FIG. 1.
a: Chromosome 22 idiogram with the location of the hybridization signals for the DiGeorge sequence (DGS) and control probes. b: Fluorescence in situ hybridization using a cosmid probe (D22S75) from chromosome 22q11.2 for the detection of microdeletions in DGS. The DGS probe and an internal control probe (D22S39) are shown cohybridized to chromosome 22 from a partial metaphase of patient 3. The control probe (D22S39) is shown on the long arm in both chromosome 22s (small arrows) while the DGS probe (D22S75) is hybridized to only the normal chromosome 22 (large arrow), indicating a microdeletion of chromosome 22q11.2 in one of the chromosomes.
Since the karyotype and FISH studies were carried out on patients in which a diagnosis had not been firmly established, we compared the results of the FISH analysis with the clinical diagnosis after a complete clinical evaluation (Table 1). The three patients with microdeletions of chromosome 22 of the 20 patients studied had features of DGS, including hypocalcemia, thymic hypoplasia, heart defects, and facial anomalies. Two patients (numbers 4 and 5, Table 1) who did not have a microdeletion of chromosome 22q11.2 did not have a clinical picture entirely compatible with DGS. Patient 4 was identified with only isolated tetralogy of Fallot, and patient 5 had congenital complex heart abnormalities and multiple endocrine defects associated with panhypopituitarism.
CHARGE Association and Other 22q11.2 Microdeletion Syndromes
Microdeletions of chromosome 22q11.2 were not detected in any of the seven patients referred for CHARGE association. Six of the seven patients had normal chromosome karyotypes. The remaining patient was referred with features of CHARGE association (patient 8, Table 1) but had a trisomy 21. After full clinical evaluation, only two of these seven patients were given a diagnosis of CHARGE association. Subsequently, the remaining five were given a variety of diagnoses (Table 1).
Of the remaining eight cases, two were referred for possible VATER association (Vertebral defects, Anal atresia, Tracheo-esophageal fistula with Esophageal atresia, and Renal dysplasia) or a chromosome 22 microdeletion syndrome, two for VCFS, and five to rule out a chromosome 22 abnormality. All of these patients had normal karyotypes, including the two cases evaluated by high-resolution banding. None of these eight patients had microdeletions of chromosome 22 detected by using FISH. The “rule out” VCFS case had only isolated facial anomalies. The two possible VATER subjects did not fit into any other particular syndrome category, each having complex congenital heart defects with duodenal atresia and tracheo-esophageal fistulas. Three of the five samples sent to rule out a chromosomal 22 abnormality were from patients with complex heart disease. Of the remaining two patients, one was not identified with a specific syndrome after clinical genetics evaluation, and the other patient was thought to have a VATER variant.
DISCUSSION
We have shown that DGS is associated with a microdeletion in chromosome 22 that can be readily detected by FISH. A microdeletion could not be detected by karyotyping using high-resolution chromosome techniques. Early studies in the genetics literature have shown an association among DGS, VCFS, CHARGE, isolated conotruncal defects, and deletions of chromosome 22q11.2. Previous studies have demonstrated the presence of these microdeletions in well-characterized cases. However, there is a paucity of studies of consecutive cases referred to a cytogenetic laboratory because of the clinical suspicion of a syndrome associated with a deletion of chromosome 22q11.2 and reported in the pathology literature. Our study shows that FISH is useful in identifying microdeletions in patients with suspected syndromes associated with a specific chromosome abnormality.
The frequency of microdeletions detected is related to the patient population that is studied. We reported microdeletions in all three of the cases of DGS and, as importantly, microdeletions were not detected in the remaining two subjects suspected to have DGS before detailed clinical evaluation. Our study agrees with previous reports that cases of DGS have a high frequency of microdeletions (7,16).
Microdeletions were not detected in any of the remaining 15 cases that were not referred to rule out DGS. Microdeletions of chromosome 22 apparently occurs much less frequently in syndromes with clinical features similar to those seen in DGS. Hence, none of the remaining cases had a CATCH 22 or CHARGE association after full clinical evaluation and, accordingly, microdeletions were not detected.
FISH has several advantages over classic cytogenetic techniques. As shown in this study, FISH is more sensitive than conventional karyotyping or using high-resolution banding techniques. FISH is also less labor intensive than karyotyping, DNA dosage analysis, or restriction fragment length analysis. In addition, FISH has been useful in prenatal diagnosis using amniotic fluid cells (7).
The observation that a broad spectrum of clinical presentations including DGS (immunologic), VCFS (dysmorphic), and conotruncal anomalies (cardiologic) can have microdeletions of chromosome 22 implies a multifactorial etiology to these syndromes. The precise mechanism by which deletions in chromosome 22q11.2 contribute to these syndromes is not well defined. However, the cosmid probe used for FISH extends over 35 kb of contiguous DNA sequence (6), and such a large span of DNA may encompass several genes. The specific gene(s) within this region is not well characterized. However, a variation in the size and position of the deletion may explain the broad clinical presentation and overlap with several syndromes or congenital defects. The correlation between genotype and phenotype will require continued investigation.
Acknowledgment:
We thank Lora Hedges for technical assistance and Jean McClure for her excellent preparation of the manuscript.
REFERENCES
- 1.Budarf ML, McDermid HE, Sellinger B, Emanuel BS. Isolation and regional localization of 5 unique anonymous DNA markers for human chromosome 22. Genomics 1991;10:996–1001. [DOI] [PubMed] [Google Scholar]
- 2.Burn J, Takao A, Wilson D, et al. Conotruncal anomaly face syndrome is associated with a deletion with chromosome 22q11. J Med Genet 1993;30:822–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butler MG, Meaney FJ, Palmer CG. Clinical and cytogenetic survey of 39 individuals with Prader–Labhart–Willi syndrome. Am J Med Genet 1986;23:793–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carey AH, Kelly D, Halford S, et al. Molecular genetic study of the frequency of monosomy 22q11 in DiGeorge syndrome. Am J Hum Genet 1992;51:964–70. [PMC free article] [PubMed] [Google Scholar]
- 5.Desmaze C, Scambler P, Prieur M, et al. Routine diagnosis of DiGeorge syndrome by fluorescent in situ hybridization. Hum Genet 1993;90:663–5. [DOI] [PubMed] [Google Scholar]
- 6.Driscoll DA, Budarf ML, Emanuel BS. A genetic etiology for DiGeorge syndrome: consistent deletions and microdeletions of 22q11. Am J Hum Genet 1992;50:924–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Driscoll DA, Salvin J, Seilinger B, et al. Prevalence of 22q11 microdeletions in DiGeorge and velocardiofacial syndromes: implications for genetic counseling and prenatal diagnosis. J Med Genet 1993;30:813–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driscoll DA, Spinner NB, Budarf ML, et al. Deletions and microdeletions of 22q11.2 in velo-cardio-facial syndrome. Am J Med Genet 1992;44:261–8. [DOI] [PubMed] [Google Scholar]
- 9.Emanual BS, Budarf ML, Seilinger B, Goldmuntz E, Driscoll DA. Detection of microdeletions of 22q11.2 with fluorescence in situ hybridization (FISH): diagnosis of DiGeorge syndrome (DGS), velo-cardio-facial syndrome (VCF), CHARGE association and conotruncal cardiac malformations [Abstract]. Am J Hum Genet 1992;51:A3. [Google Scholar]
- 10.Goldmuntz E, Driscoll D, Budarf ML, et al. Microdeletions of chromosomal region 22q11 in patients with congenital conotruncal cardiac defects. J Med Genet 1993;30:807–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catch Hall J. 22. J Med Genet 1993;30:8–9. [Google Scholar]
- 12.Holder SE, Winter RM, Kamath S, Scrambler PJ. Velocar-diofacial syndrome in a mother and daughter: variability of clinical phenotype. J Med Genet 1993;30:825–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly D, Goldberg R, Wilson D, et al. Confirmation that the velo-cardio-facial syndrome is associated with haplo-insufficiency of genes at chromosome 22q11. Am J Med Genet 1993;45:308–12. [DOI] [PubMed] [Google Scholar]
- 14.Scambler PJ, Carey AH, Wyse RKH, et al. Microdeletions within 22q11 associated with sporadic and familial DiGeorge syndrome. Genomics 1991;10:201–6. [DOI] [PubMed] [Google Scholar]
- 15.Wilson DI, Burn J, Scambler P, Goodship J. DiGeorge syndrome: part of CATCH 22. J Med Genet 1993;30:852–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilson DI, Cross IE, Goodship JA, et al. A prospective cytogenetic study of 36 cases of DiGeorge syndrome. Am J Hum Genet 1992;51:957–63. [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson DI, Goodship JA, Burn J, Cross IE, Scambler PJ. Deletions within chromosome 22q11 in familial congenital heart disease. Lancet 1992;340:573–4. [DOI] [PubMed] [Google Scholar]