Abstract
Abstract
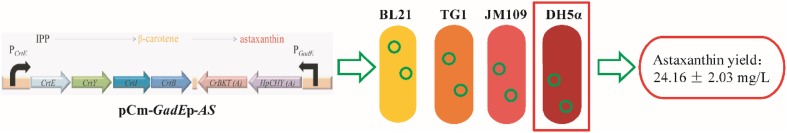
Astaxanthin is a value-added ketocarotenoid with great potential in nutraceutical and pharmaceutical industries. Genetic engineering of heterologous hosts for astaxanthin production has attracted great attention. In this study, we assessed some key factors, including codon usage of the expressed genes, types of promoters, bacterial strains, and culture media, for engineered Escherichia coli to produce astaxanthin. The effect of codon usage was shown to be related to the types of promoters. E. coli DH5α was superior to other strains for astaxanthin production. Different culture media greatly affected the contents and yields of astaxanthin in engineered E. coli. When the expression cassette containing GadE promoter and its driving genes, HpCHY and CrBKT, was inserted into the plasmid pACCAR16ΔcrtX and expressed in E. coli DH5α, the engineered strain was able to produce 4.30 ± 0.28 mg/g dry cell weight (DCW) or 24.16 ± 2.03 mg/L of astaxanthin, which was a sevenfold or 40-fold increase over the initial production of 0.62 ± 0.03 mg/g DCW or 0.61 ± 0.05 mg/L.
Graphical Abstract
Electronic supplementary material
The online version of this article (10.1007/s13659-018-0172-z) contains supplementary material, which is available to authorized users.
Keywords: Astaxanthin, Escherichia coli, Bacterial strains, Codon usage, Medium
Introduction
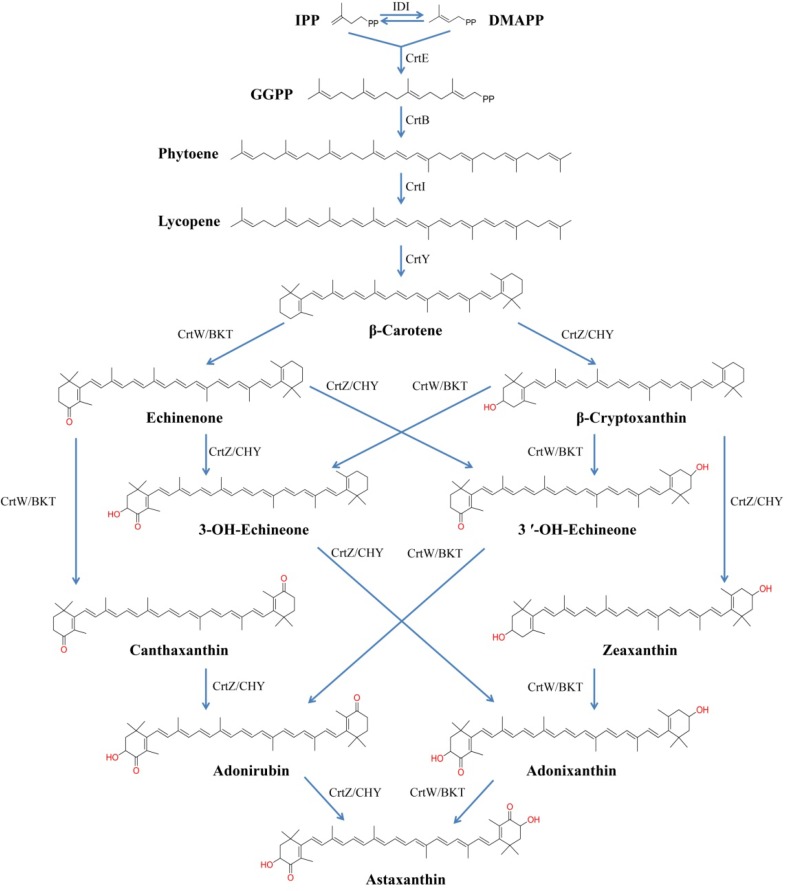
Astaxanthin (3,3′-dihydroxy-β,β-carotene-4,4′-dione) is a red ketocarotenoid with strong antioxidant activity, which has been widely applied in aquaculture, nutraceutical, pharmaceutical and cosmetic industries [1–3]. Currently, commercial natural astaxanthin is mainly extracted from the green alga Haematococcus pluvialis with relatively low productivity [4]. As a result, non-carotenogenic microorganisms, such as Escherichia coli [5–11] and Saccharomyces cerevisiae [12, 13], have been engineered to produce significant amounts of astaxanthin with different strategies, since the biosynthetic pathway of astaxanthin was elucidated at enzyme and gene levels [14]. Engineering an astaxanthin biosynthetic pathway in E. coli requires an exogenous carotenogenic pathway containing phytoene synthase (CrtB), phytoene desaturase (CrtI), lycopene cyclase (CrtY), β-carotene hydroxylase (CrtZ/CHY) and β-carotene ketolase (CrtW/BKT), (Fig. 1). Generally, two or more plasmids consisting of all the genes involved in converting isopentenyl pyrophosphate (IPP) to astaxanthin were co-transformed [6, 7, 9, 10] or the expression cassettes were integrated into the genomes of the targeted organisms [8, 11]. Strategies including combination of genes [6–8, 11] and their codon optimization [11], gene copy number adjustment [8, 9, 11], and so on, were integrated for improving astaxanthin production. Because these studies used different expression cassettes, strains, and culture conditions, it is not easy to assess the effects of these factors for the biosynthesis of astaxanthin in E. coli.
Fig. 1.
Biosynthetic pathways of astaxanthin. IPP isopentenyl pyrophosphate, DMAPP dimethylallyl diphosphate, IDI IPP isomerase, CrtE geranylgeranyl pyrophosphate synthase, CrtB phytoene synthase, CrtI phytoene desaturase, CrtY lycopene cyclase, CrtW/BKT β-carotene ketolase, CrtZ/CHY β-carotene hydroxylase
In this study we assessed the effects of codon usage, promoters, bacterial strains, and culture media on engineered E. coli for astaxanthin production. The CHY from Haematococcus pluvialis (HpCHY) and the BKT from Chlamydomonas reinhardtii (CrBKT) were adopted to convert β-carotene to astaxanthin based on our previous study [15]. We found bacterial strains and culture media were the most critical factors affecting the production of astaxanthin. When the astaxanthin pathway was constructed in one plasmid and expressed in E. coli DH5α strain and cultured with SBMSN medium containing 0.4% glucose, the engineered strain was able to produce 4.30 ± 0.28 mg/g DCW or 24.16 ± 2.03 mg/L of astaxanthin, which was a sevenfold or 40-fold increase over the initial production of 0.62 ± 0.03 mg/g DCW or 0.61 ± 0.05 mg/L.
Results and Discussion
Codon Usage of HpCHY-CrBKT Toward Astaxanthin Production
We previously found that the CHY from Haematococcus pluvialis (HpCHY) and the BKT from Chlamydomonas reinhardtii (CrBKT) were a better combination for efficiently converting β-carotene to astaxanthin in E. coli [15]. To investigate if codon optimization of the genes could improve astaxanthin production, HpCHY and CrBKT were synthesized based on the codon usage of E. coli [16]. The original algal HpCHY-CrBKT together with another codon optimizing ones based on Arabidopsis thaliana were used as controls. The three pairs of gene combinations were inserted into the plasmid pUC19 under the control of lacZ promoter. These constructs were expressed in β-carotene-producing E. coli JM109. The engineered bacteria cultured in Luria–Bertani (LB) medium with the inducer isopropyl β-D-thiogalactopyranoside (IPTG) demonstrated differential astaxanthin contents and productions (Tables 1, S1).
Table 1.
Astaxanthin production of E. coli JM109 co-transformants (pACCAR16ΔcrtX+pUC-lacZp-HpCHY-CrBKT) cultivated in LB medium with IPTG
| Plasmid | OD600 | Content (mg/g DCW) | Concentration (mg/L) | Ratio (%) |
|---|---|---|---|---|
| pACCAR16ΔcrtX+pUC-lacZp-HpCHY-CrBKT | 3.085 ± 0.428 | 0.374 ± 0.028 | 0.429 ± 0.028 | 34.247 |
| pACCAR16ΔcrtX+pUC-lacZp-HpCHY-CrBKT (E) | 2.590 ± 0.050 | 0.624 ± 0.032 | 0.608 ± 0.050 | 81.578 |
| pACCAR16ΔcrtX+pUC-lacZp-HpCHY-CrBKT (A) | 3.240 ± 0.060 | 0.466 ± 0.006 | 0.567 ± 0.004 | 44.976 |
Each value is shown as mean ± standard deviation (n ≥ 3)
The bacterium expressing the pair of HpCHY-CrBKT (E) with E. coli-based codon usage exhibited best astaxanthin content (0.62 ± 0.03 mg/g DCW) and production (0.61 ± 0.05 mg/L), but with lower biomass, suggesting that effective expression of the astaxanthin pathway driven by the lacZ promoter was detrimental to cell growth. The Arabidopsis thaliana codon optimized genes HpCHY-CrBKT (A) led to 0.47 ± 0.01 mg/g DCW or 0.57 ± 0.004 mg/L of astaxanthin, which is higher than that of the original algal genes HpCHY-CrBKT. These results showed that optimization of codon usage of the expressed genes could benefit astaxanthin production when the genes were driven by the ITPG-induced lacZ promoter.
Assessment of GadE Promoter for Driving the Expression of HpCHY-CrBKT
GadE is a transcriptional activator of acid resistance system [17, 18]. GadE promoter is a stress-response one that was previously shown to improve the final titers of desired products [19]. To achieve the goal of producing high-levels of astaxanthin in the absence of inducer, the GadE promoter in the regulon was cloned 5′ of HpCHY-CrBKT and the resulting constructs were introduced into the β-carotene-producing E. coli JM109 strain.
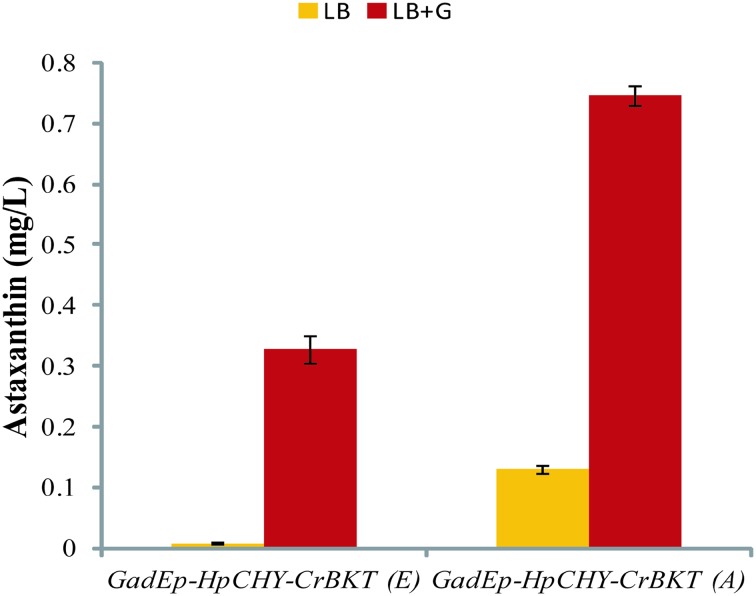
Interestingly, when driven by GadE promoter, the HpCHY-CrBKT (A) demonstrated better effects on astaxanthin production than the HpCHY-CrBKT (E) (Fig. 2 and Table S2). Furthermore, glucose greatly enhanced astaxanthin production, supporting that the expression of GadE was influenced by glucose [20]. The expression of GadE gene was affected by several different environmental cues, including transitions into stationary-phase, concentrations of sodium and magnesium [21], as well as exponential growth in minimal medium adjusted to pH 7 or below [18]. Thus, it is necessary to compare the performance of the astaxanthin pathway driven by GadE promoter in various E. coli strains and culture media.
Fig. 2.
Astaxanthin production of E. coli JM109 co-transformants (pACCAR16ΔcrtX+pUC-GadEp-HpCHY-CrBKT (E)/(A)) cultivated in LB with or without glucose induction. Data represent averages from three or more replicate cultures; error bars show standard deviation
Assessment of E. coli Strains for Astaxanthin Production
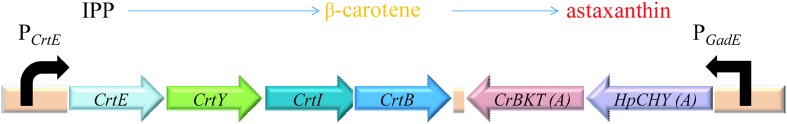
A low-copy-number pACYC-derived plasmid was previously shown to be superior to a pUC-derived plasmid for the production of zeaxanthin in E. coli [22]. In addition, multi-plasmid transformation generally decreased the final production [22]. To alleviate cellular burden, we constructed a plasmid contained the whole astaxanthin pathway by inserting the expression cassette of the HpCHY-CrBKT (A) driven by GadE promoter into the plasmid pACCAR16ΔcrtX (Fig. 3).
Fig. 3.
Schematic diagram of the plasmid pCm-GadEp-AS
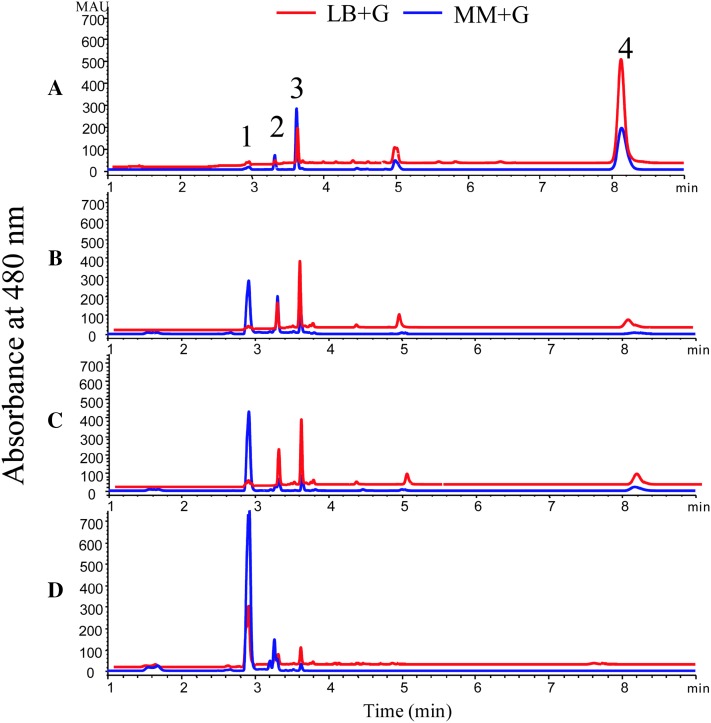
Previously, heterologous formation of carotenoids was shown to be related to E. coli strains [23]. Thus, the resulting construct was introduced into four E. coli strains so as to assess the relationship between strains and astaxanthin production. When cultured in LB+G medium (LB medium with 0.2% glucose) and MM+G medium (minimal medium with 0.6% glucose), the strains demonstrated various efficiency of astaxanthin biosynthesis (Fig. 4). Of the four strains, DH5α exhibited the best one for astaxanthin production in the context of contents, yields, and percentage (Fig. 4d). Compared with DH5α, the strains JM109 and TG1 shared similar carotenoid profiles in MM+G medium, but different in LB+G medium. The strains DH5α, JM109 and TG1 are derivatives of E. coli K-12, while they may have different precursor pools or different cellular environments for functional assembly of enzymes [23], or different membrane storage capacity for astaxanthin [5, 22], which may resulted in different productivity of astaxanthin. Furthermore, multi-omics analysis showed that, the strain DH5α was predicted to be the best producer for most constructs because of its higher expression of the biosynthetic pathways for tyrosine and phenylalanine [24].
Fig. 4.
UHPLC analysis of carotenoids in different E. coli strains harboring plasmid pCm-GadEp-AS. A BL21; B TG1; C JM109; D DH5a. 1 astaxanthin; 2 adonixanthin; 3 canthaxanthin; 4 β-carotene
In contrast, BL21 (DE3) showed the worst one for astaxanthin production due to the accumulation of intermediates, β-carotene and canthaxanthin (Fig. 4a). The genome of the strain BL21 lacks rcsB and dsrA genes, which are involved in the activation of GadE [25], possibly leading to the lower expression of HpCHY-CrBKT (A). In addition, the accumulation of di-keto carotenoid canthaxanthin indicated that CrBKT had higher affinity to β-carotene than HpCHY.
Assessment of Culture Media for Astaxanthin Production
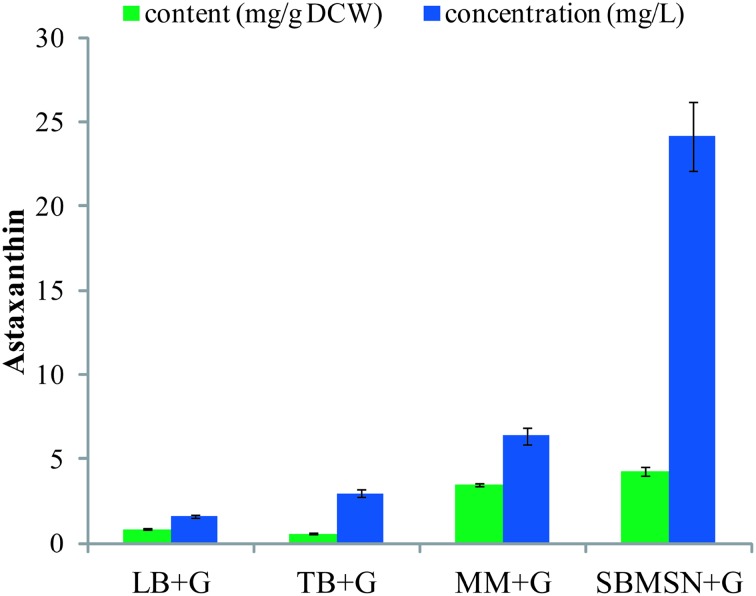
The popular complex LB medium allows the growth of E. coli up to a cell density of 1 g/L DCW, whereas a defined medium that contains the maximum non-inhibitive concentration of nutrients up to 15 g/L [26]. Therefore we cultivated the astaxanthin-producing DH5α cells in LB, TB, MM, and SBMSN media to investigate their effects on astaxanthin accumulation. The best concentrations of glucose were set to be 0.2% for LB and TB media, 0.6 and 0.4% for MM and SBMSN, respectively based on a pilot test (Table S3). The highest production of astaxanthin was achieved by the cells cultured in SBMSN+G medium, which reached 4.30 ± 0.28 mg/g DCW or 24.16 ± 2.03 mg/L (Fig. 5 and Table S4). The cells cultured in MM+G medium produced a similar content of astaxanthin (3.49 ± 0.09 mg/g DCW) to that in SBMSN+G, but a much lower yield of astaxanthin (6.39 ± 0.51 mg/L) due to its low biomass. SBMSN is a nutrient-rich and expensive medium containing high amounts of tryptone and yeast extract. In contrast, minimal medium is a defined and cheaper one. Hence, it is possible to achieve a much higher yield of astaxanthin by cultivating the cells in MM medium with further optimization of culture conditions, e.g., adjusting C:N ratios and adding Mg2+, Fe2+, Cu2+ to stimulate the activity of key enzymes.
Fig. 5.
Astaxanthin production of E. coli DH5α transformants (pCm-GadEp-AS) cultivated in different media. Data represent averages from three or more replicate cultures; error bars show standard deviation
In summary, we have assessed the most critical factors involved in astaxanthin biosynthesis in E. coli. By using the glucose-inducing GadE promoter to drive the codon optimized CrBKT-HpCHY (A) genes and constructing the whole astaxanthin pathway in a low copy number plasmid, a 40-fold increase of astaxanthin production was achieved by E. coli DH5α cells cultivated in the SBMSN+G medium. This study provides insight into the construction of much more robust strains for astaxanthin production in the future.
Experimental Section
Bacterial Strains, Culture Media and Plasmid
Escherichia coli JM109 [endA1, gyrA96, hsdR17 (r−k, m+k), recA1, relA1, supE44, thi-1, Δ (lac-proAB), F′ (traD36, proAB+, lacIq lacZΔM15)] was used for DNA mainipulations and carotenoids production. E. coli strains TG1 [Δ(hsdMS-mcrB)5, Δ(lac-proAB), supE, thi-1, F′ (traD36, proAB+, lacIq lacZΔM15)], DH5α [deoR, endA1, gyrA96, hsdR17 (r−k, m+k)), recA1, relA1, supE44, thi-1, Δ (lac)U169 (ϕ80 lacZΔM15), F−], and BL21 [E. coli B, F−, dcm, ompT, hsdS (rB− mB−), gal, λ (DE3)] were also used for carotenoids production. The carotenogenic plasmid pACCAR16ΔcrtX was used for E. coli to produce β-carotene [14].
The LB medium (pH 7.0) contains 10 g/L tryptone, 5 g/L yeast extract and 10 g/L NaCl. The TB medium (pH 7.0) contains 12 g/L tryptone, 24 g/L yeast extract, 4 g/L glycerol, 2.31 g/L KH2PO4 and 16.41 g/L K2HPO4·3H2O. The minimal medium (pH 7.0) used had the following composition: 2 g/L Glucose, 13.3 g/L KH2PO4, 4 g/L (NH4)2HPO4, 1.2 g/L MgCl2·6H2O, 1.86 g/L citric acid monohydrate, 10 mL 100 × Trace. The SBMSN (pH 7.0) contains 12 g/L tryptone, 24 g/L yeast extract, 1.7 g/L KH2PO4, 14.96 g/L K2HPO4·3H2O, 1 g/L MgCl2·6H2O, 1.42 g/L ammonium oxalate, and 2 g/L Tween-80.
Genetic Methods
The genes CHY from Haematococcus pluvialis (HpCHY) and BKT from Chlamydomonas reinhardtii (CrBKT) were synthesized and cloned into pUC19 (SalI-BamHI) by Shanghai Generay Biotech according to our previous study [15] and “31 codon folding optimization” method reported by Boël et al. [16] for coexpression and codon optimization of the two genes.
The GadE promoter was amplified by PCR using genomic DNA of JM109 as the template and the primers GadEp F and R (Table S5). The product was inserted between HindIII and SalI of pUC19. Then the GadE promoter was cloned 5′of HpCHY-CrBKT, resulting in plasmids pUC-GadEp-HpCHY-CrBKT (E) and pUC-GadEp-HpCHY-CrBKT (A).
The plasmid pCm-GadEp-AS was constructed by inserting the fragment GadEp-HpCHY-CrBKT (A) into the pACCAR16ΔcrtX using PCR-based and seamless connection approaches.
PCR amplifications were performed using Prime STAR Max premix (Takara) and a thermal cycler (Eppendorf). Restriction enzymes were purchased from New England BioLabs. Hieff Clone™ Plus One Step Cloning Kit was purchased from Shanghai Yeasen Biotech. All the primers used in this study were listed in Table S5.
Growth Condition of Astaxanthin Production
Single colonies were picked from the plates and inoculated into 12 mL tubes containing 3 mL LB medium. Chloramphenicol (34 mg/L) and ampicillin (100 mg/L) were added to the culture medium as required. The culture was grown to OD600 = 2.0 at 37 °C in a rotary shaking incubator set to 220 rpm. Then, 210 µL of seed-culture was inoculated into new 12 mL tubes containing 3 mL medium, with appropriate antibiotics and grown at 28 °C, 220 rpm for 48 h [22]. For lacZ promoter to drive gene expression, 1 mM (final concentration) of IPTG was added at OD600 = 1. After 48 h growth, cells were collected for measurement of carotenoids production. Cell growth was measured at the optical density of OD600 nm and the cell density was converted into DCW (g/L) using standard curves (Fig. S1).
Extraction and Analysis of Carotenoids
Carotenoids analysis was performed as described previously [27]. Briefly, 1 mL cells were harvested by centrifugation and the cell pellets were used for the extraction of pigments. The cells were extracted with acetone until the color of the sample faded.
The extracts were filtered through a 0.22 μm Millipore organic membrane, and then analyzed by an Agilent Ultra High Performance Liquid Chromatography (UHPLC) 1290 Infinity, which is equipped with an Agilent Eclipse plus C18 RRHD 1.8 μm column (2.1 × 50 mm). The mobile phase consisted of elution A (20% water, 60% acetonitrile, 5% isopropanol and 15% methanol) and elution B (80% acetonitrile, 5% isopropanol and 15% methanol). The extracts were eluted at a flow rate of 0.5 mL/min with following process: 100% A for 1 min, and then a liner gradient from 100% A to 100% B within 1 min, followed by 100% B for 8 min. Individual pigment was identified by comparing the retention times and absorption spectra to standard carotenoids, and the concentrations of pigments were determined by standard curve and peak areas (Fig. S2).
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (DOCX 107040 kb)
Acknowledgements
This study was supported by a research grant from Department of Economic Plants and Biotechnology, Yunnan Key Laboratory for Wild Plant Resources, Kunming Institute of Botany, Chinese Academy of Sciences.
Compliance with Ethical Standards
Conflict of interest
The authors declare that there is no conflict of interest.
References
- 1.Ambati RR, Phang SM, Ravi S, Aswathanarayana RG. Mar. Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan JP, Peng J, Yin K, Wang JH. Mol. Nutr. Food Res. 2011;55:150–165. doi: 10.1002/mnfr.201000414. [DOI] [PubMed] [Google Scholar]
- 3.Higuera-Ciapara I, Félix-Valenzuela L, Goycoolea FM. Crit. Rev. Food Sci. Nutr. 2006;46:185–196. doi: 10.1080/10408690590957188. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Zhu DL, Niu JF, Shen SD, Wang GC. Biotechnol. Adv. 2011;29:568–574. doi: 10.1016/j.biotechadv.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 5.Wang CW, Oh MK, Liao JC. Biotechnol. Bioeng. 1999;62:235–241. doi: 10.1002/(SICI)1097-0290(19990120)62:2<235::AID-BIT14>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 6.Choi SK, Matsuda S, Hoshino T, Peng X, Misawa N. Appl. Microbiol. Biotechnol. 2006;72:1238–1246. doi: 10.1007/s00253-006-0426-2. [DOI] [PubMed] [Google Scholar]
- 7.Scaife MA, Burja AM, Wright PC. Biotechnol. Bioeng. 2009;103:944–955. doi: 10.1002/bit.22330. [DOI] [PubMed] [Google Scholar]
- 8.Lemuth K, Steuer K, Albermann C. Microb. Cell Fact. 2011;10:1–12. doi: 10.1186/1475-2859-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zelcbuch L, Antonovsky N, Bar-Even A, Levin-Karp A, Barenholz U, Dayagi M, Liebermeister W, Flamholz A, Noor E, Amram S, Brandis A, Bareia T, Yofe I, Jubran H, Milo R. Nucleic Acids Res. 2013;41:e98–e98. doi: 10.1093/nar/gkt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma T, Zhou YJ, Li XW, Zhu FY, Cheng YB, Liu Y, Deng ZX, Liu TG. Biotechnol. J. 2016;11:228–237. doi: 10.1002/biot.201400827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu Q, Bu YF, Liu JZ. Mar. Drugs. 2017;15:296. doi: 10.3390/md15100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou PP, Ye LD, Xie WP, Lv XM, Yu HW. Appl. Microbiol. Biotechnol. 2015;99:8419–8428. doi: 10.1007/s00253-015-6791-y. [DOI] [PubMed] [Google Scholar]
- 13.Zhou PP, Xie WP, Li AP, Wang F, Yao Z, Bian Q, Zhu YQ, Yu HW, Ye LD. Enzyme Microb. Technol. 2017;100:28–36. doi: 10.1016/j.enzmictec.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W. J. Bacteriol. 1995;177:6575–6584. doi: 10.1128/jb.177.22.6575-6584.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang JC, Zhong YJ, Liu J, Sandmann G, Chen F. Metab. Eng. 2013;17:59–67. doi: 10.1016/j.ymben.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 16.Boël G, Letso R, Neely H, Price WN, Wong KH, Su M, Luff JD, Valecha M, Everett JK, Acton TB, Xiao R, Montelione GT, Aalberts DP, Hunt JF. Nature. 2016;529:358–363. doi: 10.1038/nature16509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hommais F, Krin E, Coppe´e JY, Lacroix C, Yeramian E, Danchin A, Bertin P. Microbiology. 2004;150:61–72. doi: 10.1099/mic.0.26659-0. [DOI] [PubMed] [Google Scholar]
- 18.Ma Z, Gong SM, Richard H, Tucker DL, Conway T, Foster JW. Mol. Microbiol. 2003;49:1309–1320. doi: 10.1046/j.1365-2958.2003.03633.x. [DOI] [PubMed] [Google Scholar]
- 19.Dahl RH, Zhang FZ, Alonso-Gutierrez J, Baidoo E, Batth TS, Redding-Johanson AM, Petzold CJ, Mukhopadhyay A, Lee TS, Adams PD, Keasling JD. Nat. Biotechnol. 2013;31:1039–1046. doi: 10.1038/nbt.2689. [DOI] [PubMed] [Google Scholar]
- 20.Sayed AK, Foster JW. Mol. Microbiol. 2009;71:1435–1450. doi: 10.1111/j.1365-2958.2009.06614.x. [DOI] [PubMed] [Google Scholar]
- 21.Richard H, Foster JW. Microbiology. 2007;153:3154–3161. doi: 10.1099/mic.0.2007/007575-0. [DOI] [PubMed] [Google Scholar]
- 22.Ruther A, Misawa N, Böger P, Sandmann G. Appl. Microbiol. Biotechnol. 1997;48:162–167. doi: 10.1007/s002530051032. [DOI] [PubMed] [Google Scholar]
- 23.Chae HS, Kim KH, Kim SC, Lee PC. Appl. Biochem. Biotechnol. 2010;162:2333–2344. doi: 10.1007/s12010-010-9006-0. [DOI] [PubMed] [Google Scholar]
- 24.Monk JM, Koza A, Campodonico MA, Machado D, Seoane JM, Palsson BO, Herrgård MJ, Feist AM. Cell Syst. 2016;3:238–251. doi: 10.1016/j.cels.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Woo JM, Kim JW, Song JW, Blank LM, Park JB. PLoS ONE. 2016;11:e0163265. doi: 10.1371/journal.pone.0163265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiloach J, Fass R. Biotechnol. Adv. 2005;23:345–357. doi: 10.1016/j.biotechadv.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 27.Huang WP, Ye JR, Zhang JJ, Lin Y, He MX, Huang JC. Algal Res. 2016;17:236–243. doi: 10.1016/j.algal.2016.05.015. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material 1 (DOCX 107040 kb)