Abstract
The type III transforming growth factor-β (TGF-β) receptor (TβRIII), a coreceptor of the TGF-β superfamily, is known to bind TGF-βs and regulate TGF-β signaling. However, the regulatory roles of TβRIII in TGF-β-induced mesenchymal stem cell (MSC) chondrogenesis have not been explored. The present study examined the effect of TβRIII RNA interference (RNAi) on TGF-β3-induced human MSC (hMSC) chondrogenesis and possible signal mechanisms. A lentiviral expression vector containing TβRIII small interfering RNA (siRNA) (SiTβRIII) or a control siRNA (SiNC) gene was constructed and infected into hMSCs. The cells were cultured in chondrogenic medium containing TGF-β3 or control medium. TβRIII RNAi significantly enhanced TGF-β3-induced chondrogenic differentiation of hMSCs, the ratio of type II (TβRII) to type I (TβRI) TGF-β receptors, and phosphorylation levels of Smad2/3 as compared with cells infected with SiNC. An inhibitor of the TGF-β signal, SB431542, not only inhibited TβRIII RNAi-stimulated TGF-β3-mediated Smad2/3 phosphorylation but also inhibited the effects of TβRIII RNAi on TGF-β3-induced chondrogenic differentiation. These results demonstrate that TβRIII RNAi enhances TGF-β3-induced chondrogenic differentiation in hMSCs by activating TGF-β/Smad2/3 signaling. The finding points to the possibility of modifying MSCs by TβRIII knockdown as a potent future strategy for cell-based cartilage tissue engineering.
1. Introduction
Cell-based cartilage tissue engineering provides a feasible way of regenerating damaged cartilage tissue caused by trauma or joint diseases. Mesenchymal stem cells (MSCs), common precursor cells of chondrocytes, are the basis for the development of cartilage and represent promising cells for use in stem cell therapy [1, 2]. However, cartilage tissue formed by MSC-derived chondrocytes is not the same as that of native articular cartilage and has poor functional properties [3, 4]. Understanding the molecular mechanisms that control chondrogenic differentiation of MSCs and enhancing chondrogenic activities of cells are crucial to improve cartilage regeneration by MSCs.
Chondrogenic differentiation is potently induced by growth factors [2, 5]. Transforming growth factor-β3 (TGF-β3), a member of the transforming growth factor-β (TGF-β) superfamily, induces chondrogenic differentiation of MSCs [6]. TGF-β signaling is initiated by the binding of TGF-β to type II TGF-β receptors (TβRII) and then forms heteromeric complexes with type I receptors (TβRI) [7]. These complexes further phosphorylate cytoplasmic effector molecules Smad2 and Smad3, which are translocated to the nucleus, where they modulate the expression of target genes, such as SOX9 and collagen type II (COL II) [8].
In addition to kinase receptors, the type III TGF-β receptor (TβRIII), also known as betaglycan, participates in ligand binding of the TGF-β superfamily and signaling [9, 10]. TβRIII is a membrane-anchored proteoglycan found in many cell types. The main function of this receptor was thought to be binding members of the TGF-β family, including TGF-β isoforms TGF-β1–TGF-β3, and presenting them to type II receptors [11, 12]. However, according to the recent literature, TβRIII seems to play complex roles in cellular processes. For example, studies reported that in some cell lines, TβRIII significantly enhanced the response to TGF-β by increasing the affinity of TβRII for TGF-β [12, 13]. Studies also showed that TβRIII functioned as a potent inhibitor of TGF-β signaling in other types of cells, such as renal epithelial cells [14, 15]. Our previous study demonstrated that human MSCs (hMSCs) expressed abundant TβRIII and that TGF-β3 stimulation clearly repressed TβRIII expression in MSCs and induced MSC chondrogenic differentiation [16]. These results suggested that the effect of TGF-β3 on MSC chondrogenesis might be associated with low expression of TβRIII. However, the role of TβRIII in TGF-β-induced chondrogenic differentiation of MSCs is unknown.
In the present study, we studied the biological effects of TβRIII on TGF-β3-induced MSC chondrogenesis. We demonstrated that TβRIII RNA interference (RNAi) enhanced TGF-β-induced chondrogenic differentiation of hMSCs by activating TGF-β and Smad2/3 signaling.
2. Materials and Methods
2.1. Isolation and Culture of hMSCs
The study was approved by the ethics committee of the First Affiliated Hospital of Sun Yat-sen University in accordance with the Declaration of Helsinki, and all the subjects provided written informed consent. hMSCs were isolated and purified from bone marrow samples of 3 healthy volunteer donors by density gradient centrifugation, as described previously [17]. Briefly, bone marrow samples (8–10 ml) were diluted with 10 ml phosphate-buffered saline (PBS). Cells were then fractionated using a Lymphoprep (MP Biomedicals LLC., Santa Ana, CA, USA) density gradient by centrifugation at 500 ×g for 20 minutes. Interfacial mononuclears were collected and cultured in low-glucose Dulbecco's modified Eagle medium (L-DMEM) (Gibco; Invitrogen Corporation, NY, USA), supplemented with 10% fetal bovine serum (FBS) (Gibco; Invitrogen Corporation, NY, USA) under 37°C and 5% CO2. Cells were passaged when they reached approximately 80% confluence. Passages 3 to 5 cells were used for the experimental protocols.
2.2. TβRIII Small Interfering RNA (siRNA) Design and Lentiviral Vector Construction
The human TβRIII cDNA sequence (GenBank accession number: NM_003243) was searched for siRNA target sequences. Four target sequences were selected, AAGCATGAAGGAACCAAAT, TGCTTTATCTCTCCATATT, ACCTGAAATCGTGGTGTTT, and AGTTGGTAAAGGGTTAATA. A scrambled sequence, TTCTCCGAACGTGTCACGT, was used as a negative control (NC). DNA oligos containing the target sequence were synthesized, annealed, and inserted into a green fluorescent protein (GFP) lentiviral expression vector GV248 (GeneChem, Shanghai, China). The ligated production was transformed into Escherichia coli DH5α cells. Briefly, 100 μl of DH5α cells was mixed with 2 μl ligated product at 42°C for 90 s. The mixtures were added on Luria-Bertani (LB) media (ATCC, USA) containing ampilillin (50 μg/ml) and incubated at 37°C for 16 h. The transformant was identified by polymerase chain reaction (PCR) and DNA sequencing.
2.3. Lentiviral Production and Infection
A lentivirus TβRIII (LV-TβRIII) siRNA-mix and lentivirus normal control (LV-NC) virus were produced by plasmid cotransfection of 293T cells. Briefly, 293T cells were transfected with DNA mix (pGC-LV vector, 20 μg; pHelper 1.0 vector, 15 μg; and pHelper 2.0 vector, 10 μg) (GeneChem, Shanghai, China) and 100 μl of Lipofectamine 2000 reagent (Invitrogen, NY, USA), according to the manufacturer's instructions. The viral supernatant was harvested 48 hours (h) after transfection, and the concentrated viral titer was determined. The viral supernatant was added into target MSCs at multiplicity of infection (MOI 10). Before infection, 5 × 104/ml of MSCs were seeded onto a 60 cm2 cell culture dish overnight. The cells were then infected with 100 μl of 1 × 108 TU/ml virus and 5 μg/ml of polybrene, following the manufacturer's instructions. Then, 10 h after infection, the cells were incubated with L-DMEM containing 10% FBS. Three days after infection, GFP expression in cells was observed by a fluorescence microscope (Olympus, Japan). TβRIII expression was detected by the real-time polymerase chain reaction (PCR) and Western blot analysis.
2.4. Chondrogenic Differentiation of hMSCs in Micromass Culture
Chondrogenic differentiation of hMSCs was performed using a modified micromass culture system according to a previously described method [18]. Briefly, MSCs being infected with TβRIII siRNA (SiTβRIII) or control siRNA (SiNC), or without infection, were harvested and resuspended at 2 × 107 cells/ml. Cell droplets (4 × 105/20 μl) were placed carefully in each well of 24-well plates for 2 h, followed by the addition of control medium or chondrogenic medium at 37°C/5% CO2. The control medium consisted of high-glucose DMEM (H-DMEM), supplemented with 50 μg/ml of vitamin C, 100 nM dexamethasone, 1 mM sodium pyruvate, 40 μg/ml of proline, and ITS+Universal Culture Supplement Premix (BD Biosciences, NY, USA). The chondrogenic medium consisted of the control medium, added by 10 ng/ml of TGF-β3 (PeproTech, Rocky Hill, USA). After 24 h, the cell droplets became spherical. The medium was changed every 3 days.
Cell pellets from uninfected MSCs were divided into a control group and TGF-β3 group according to the control medium and chondrogenic medium and used for TβRIII analysis. The cells were harvested on day 7 after chondrogenic induction. The pellets from infected MSCs were divided into the following four groups for identification of chondrogenic differentiation and TGF-β/Smad signaling: C-SiNC group (SiNC-infected cells cultured in control medium), C-SiTβRIII group (SiTβRIII-infected cells cultured in control medium), T-SiNC group (SiNC-infected cells cultured in chondrogenic medium), and T-SiTβRIII group (SiTβRIII-infected cells cultured in chondrogenic medium). The cells were harvested on day 14 for identification of chondrogenic differentiation, and on day 7 for TβRI and TβRII analysis. All experiments were performed by using 3 biological replicate samples each group.
2.5. RNA Extraction and Real-Time PCR Analysis
Total RNA was isolated from transfected MSCs or pellets using an RNAsimple Total RNA Kit (Tiangen, Beijing, China). Total RNA was then converted into cDNA using a PrimeScript® RT Reagent Kit (Takara, Osaka, Japan) according to the manufacturer's instructions. Real-Time PCR assay was performed in triplicate in a Real-Time PCR system (Bio-Rad Laboratories, Hercules, CA, USA) by using SYBR Green I Master Mix (Takara, Osaka, Japan). The following genes were examined: COL II, alpha 1 (COL2A1), SRY- (sex determining region Y-) box 9 [SOX9], TβRI, TβRII, and TβRIII. The primer sequences are listed in Table 1. The relative expression levels for each target gene were calculated by referencing to the internal controls glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-actin using the 2–ΔΔCT method.
Table 1.
Primers used for real-time PCR.
| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| GAPDH | 5′-AGAAAAACCTGCCAAATATGATGAC-3′ | 5′-TGGGTGTCGCTGTTGAAGTC-3′ |
| β-Actin | 5′-GACTTAGTTGCGTTACACCCTTTC-3′ | 5′-GCTGTCACCTTCACCGTTCC-3′ |
| Col2A1 | 5′-GGCAATAGCAGGTTCACGTACA-3′ | 5′-CGATAACAGTCTTGCCCCACTT-3′ |
| SOX 9 | 5′-AGCGAACGCACATCAAGAC-3′ | 5′-GCTGTAGTGTGGGAGGTTGAA-3′ |
| TβRI | 5′-ATTACCAACTGCCTTATTATGA-3′ | 5′-CATTACTCTCAAGGCTTCAC-3′ |
| TβRII | 5′-ATGGAGGCCCAGAAAGATG-3′ | 5′-GACTGCACCGTTGTTGTCAG-3′ |
| TβRIII | 5′GTGTTCCCTCCAAAGTGCAAC-3′ | 5′-AGCTCGATGATGTGTACTTCCT-3′ |
GAPDH: glyceraldehyde-3-phosphate dehydrogenase; COL2A1: collagen type II; SOX9: SRY- (sex determining region Y-) box 9; TβRI/II/III: recombinant human transforming growth factor-β receptor type I/II/III.
2.6. Histology and Immunohistochemistry
The pellets were fixed in 4% paraformaldehyde and embedded in paraffin. 4 μm sections were deparaffinized, rehydrated through decreasing concentrations of ethanol, and stained with 0.1% Alcian blue (Sigma-Aldrich, St. Louis, USA) for glycosaminoglycan (GAG) analysis. For immunohistochemistry analysis, the sections were blocked with 1/100 diluted goat serum for 15 min and then reacted overnight at 4°C with the appropriate primary antibody against human COL II polyclonal antibodies (Abzoom Biolabs, Dallas, TX, USA) and human TβRIII antibody (Santa Cruz, Dallas, USA) followed by biotinylated goat anti-rabbit immunoglobulin G (IgG) (EarthOx, SFO, USA) for 30 min. The sections were incubated with peroxide-conjugated streptavidin and stained with 3,3′-diaminobenzidine tetrahydrochloride (DAB) (Jinshan Jinqiao, Beijing, China).
For fluorescent immunohistochemistry, the pellets from transfected MSCs were frozen and then sectioned using a Leica CM1950 microtome (Leica, Germany) on day 7 after chondrogenic induction. Tissue sections that were 4 μm thick were permeabilized with 0.1% Triton X for 5 min and blocked with 5% BSA for 1 h. The sections were then incubated with primary antibodies against TβRI (Santa Cruz, Dallas, USA) and human TβRII antibody (RD Systems, USA), diluted at 1 : 50 at 4°C overnight. FITC-conjugated secondary antibody (K00018968; Dako North America Inc., Dako, Denmark) diluted at 1 : 100 was applied for 1 h. The sections were then stained with 4′-6-diamidino-2-phenylindole (1 mg/ml) and visualized using a Zeiss LSM 710 confocal microscope (Carl Zeiss, Heidelberg, Germany).
2.7. Glycosaminoglycan (GAG) Quantitation
The pellets were harvested on day 14 and papain-digested for 16 h at 65°C. An aliquot of 40 μl lysate was reacted with 1,9-dimethyl-methylene blue (DMMB) (Sigma-Aldrich, St. Louis, USA) for GAG analysis. The absorbance at 525 nm was measured using an Automatic Microplate Reader (BioTek, Winooski, Vermont, USA). Total GAG was calculated by a standard curve obtained with shark chondroitin sulfate (Sigma-Aldrich, MO, USA). The total amount of DNA was quantified by reacting with 0.7 μg/ml Hoechst 33258 solution (Sigma-Aldrich, St. Louis, USA). The reaction product was measured using a Synergy Microplate Reader (BioTek, Winooski, Vermont, USA). The results of GAG quantification were normalized to the DNA content.
2.8. Western Blot
For TβRIII RNAi identification, proteins were extracted from MSCs 72 h after transfection. For detection of Smad2/3 phosphorylation, proteins were extracted from pellets after 24 h of chondrogenic induction. The protein concentration was quantified by a BCA Protein Assay Kit (CWBio, Beijing, China). 100 μg proteins was subjected to 6% SDS-PAGE and electrotransferred onto PVDF membrane (Millipore, Boston, USA) at 250 mV for 100 min. After blocking with 5% skim milk and Tris-buffered saline containing 0.1% Tween-20, the PVDF membranes were incubated with antibodies against human TβRIII antibody (Santa Cruz, Dallas, USA), rabbit anti-phospho-Smad2 (Ser465/467)/Smad3 (Ser423/425) (Cell Signaling, Danvers, USA), rabbit anti-Smad2/3 (Cell Signaling, Danvers, USA), and anti-GAPDH monoclonal antibody (EarthOx, SFO, USA) followed by incubation with HRP-conjugated corresponding secondary antibodies. The signals were detected using SuperSignal West Pico Chemiluminescent Substrate (Pierce, NY, USA). Protein levels in phosphorylated-Smad2/3 (P-Smad2/3) were normalized to those of total Smad2/3 quantities or GAPDH.
2.9. Inhibition of TGF-β/Smad Signaling
The infected cells were treated with or without SB431542 (Sigma-Aldrich, St. Louis, USA), a selective inhibitor of activin receptor-like kinase 5 (ALK5) (TβRI) [19], for 2 h before being cultured in chondrogenic medium or control medium. After 24 h of chondrogenic induction, the cells were collected, and the expression of P-Smad2/3 and that of Smad2/3 was detected by a Western blot. After 14 days of chondrogenic induction, the chondrogenic differentiation ability of hMSCs was assayed by detecting protein and gene expression.
2.10. Statistical Analysis
All quantitative data were presented as mean values ± standard errors (S.E.). All the statistical analysis was performed using SPSS 16.0 statistical software (SPSS, Chicago, IL, USA). For comparisons of two groups, independent student's t-test was performed; for comparisons of multiple groups, one-way ANOVA followed by an LSD t-test was performed. P < 0.05 was chosen as the threshold of significance.
3. Results
3.1. TGF-β3 Inhibited the Expression of TβRIII in hMSCs
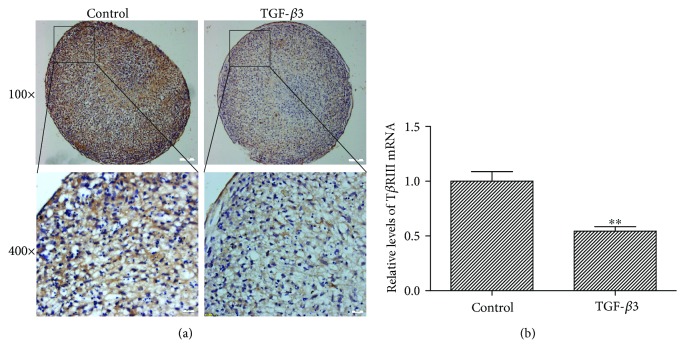
To ascertain whether hMSCs express TβRIII and TGF-β3 could regulate TβRIII expression level, we detected the expression of TβRIII during TGF-β3-induced hMSC chondrogenesis by immunohistochemistry staining and quantitative PCR. The results showed that hMSCs expressed abundant TβRIII protein (Figure 1(a), left panels) and high TβRIII mRNA (P < 0.01, Figure 1(b)). Exogenous TGF-β3 clearly reduced TβRIII expression in hMSCs at protein and mRNA levels (Figures 1(a) and 1(b)).
Figure 1.
TGF-β3 inhibited the expression of TβRIII during chondrogenic differentiation of hMSCs. (a) hMSCs were cultured in chondrogenic medium including TGF-β3 or control medium without TGF-β3 for 7 days and subjected to immunohistochemistry analysis for TβRIII (upper panel, scale bar = 100 μm; lower panel, scale bar = 20 μm; the TβRIII is stained in brown, cell nucleus is stained in blue). (b) qPCR analysis of TβRIII mRNA level in hMSCs cultured in chondrogenic medium or control medium for 7 days. The TβRIII mRNA level was lower in hMSCs cultured in chondrogenic medium containing TGF-β3 as compared with that of cells cultured in control medium. Error bars represent the means ± SD, n = 3. ∗∗P < 0.01 versus control.
3.2. Viral Infection and Suppression of TβRIII Expression at mRNA and Protein Levels
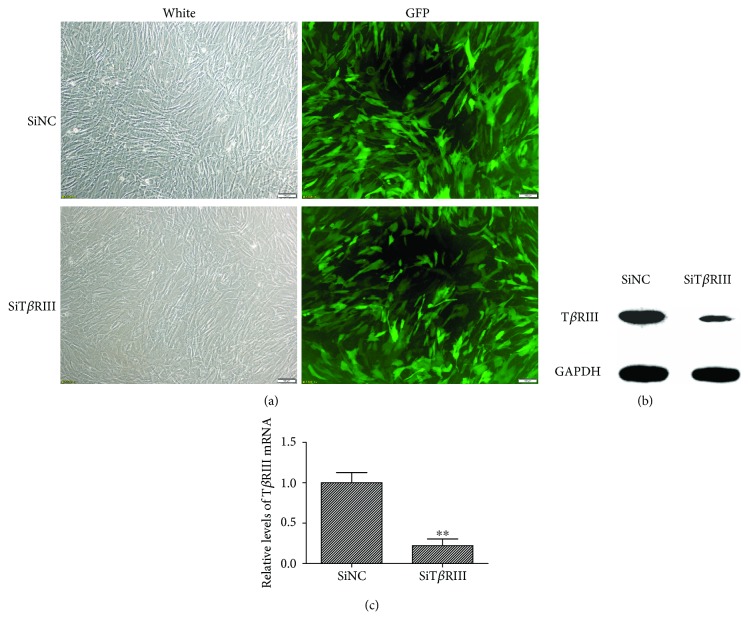
In order to investigate the role of TβRIII in hMSC chondrogenesis, we infected hMSCs with LV-TβRIII siRNA-mix and identified the silencing effect on TβRIII. Following viral infection for 72 h, most of the cells exhibited high GFP expression under fluorescence microscopy (Figure 2(a)). As compared with the control group, the expression profile of TβRIII mRNA and that of the TβRIII protein decreased significantly in the SiTβRIII groups, with mRNA expression decreased by 77.65% (Figures 2(b) and 2(c)).
Figure 2.
Identification and efficiency of lentiviral infection. (a) GFP fluorescence imaging confirmed that the majority of hMSCs were GFP positive 72 h after they were infected by TβRIII siRNA (SiTβRIII) or SiNC virus. Scale bar = 100 μm. (b) Western blot showed that TβRIII siRNA clearly inhibited the expression of the TβRIII protein. (c) qPCR confirmed that the expression profiles of TβRIII mRNA decreased significantly in the SiTβRIII groups as compared with those in the SiNC group. Error bars represent the means ± SD, n = 3. ∗∗P < 0.01 versus SiNC group.
3.3. TβRIII RNAi Enhanced TGF-β3-Induced Chondrogenic Differentiation of hMSCs
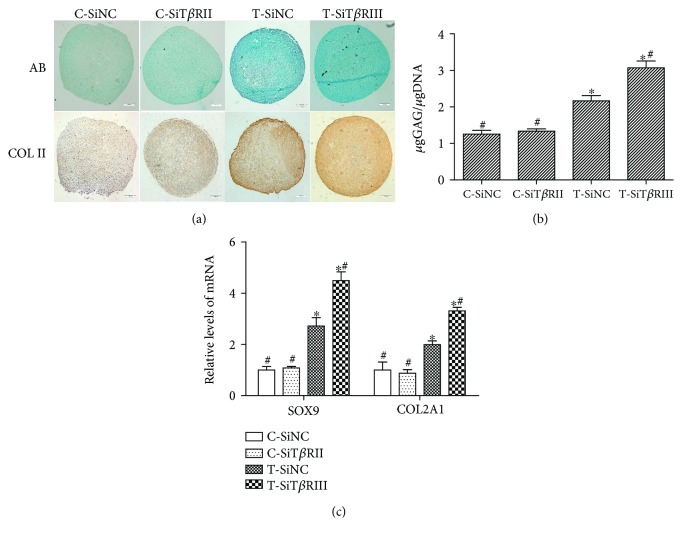
We then investigated the effects of TβRIII RNAi on the TGF-β3-induced chondrogenic differentiation of hMSCs. As shown in Figure 3, TβRIII RNAi had no obvious effects on GAG and COL II secretion (Figures 3(a) and 3(b); P > 0.05) or any noticeable effects on the expression of cartilage-specific genes (SOX9 and COL2A1) when hMSCs were cultured in control medium (Figure 3(c); P > 0.05). However, TβRIII RNAi significantly enhanced TGF-β3-induced chondrogenic differentiation of hMSCs as compared with that of cells infected with SiNC (Figures 3(a)–3(c); P < 0.05).
Figure 3.
TβRIII RNAi enhanced TGF-β3-induced chondrogenic differentiation of hMSCs. SiNC- or SiTβRIII-infected cells were cultured in control medium (C-SiNC group; C-SiTβRIII group) or chondrogenic medium containing TGF-β3 (T-SiNC group; T-SiTβRIII group) for 14 days. (a) Upper panels show glycosaminoglycan (GAG) expression by Alcian blue staining; lower panels show COL II expression by immunohistochemistry. Scale bar = 100 μm. (b) GAG content was quantitatively analyzed and normalized by DNA content. (c) Real-time PCR analysis of COL2A1 and SOX9 mRNA levels in hMSCs from different groups. Error bars represent the means ± SD, n = 3. ∗P < 0.05 versus C-SiNC group; #P < 0.05 versus T-SiNC group.
3.4. TβRIII RNAi Increased the Ratio of TβRII to TβRI
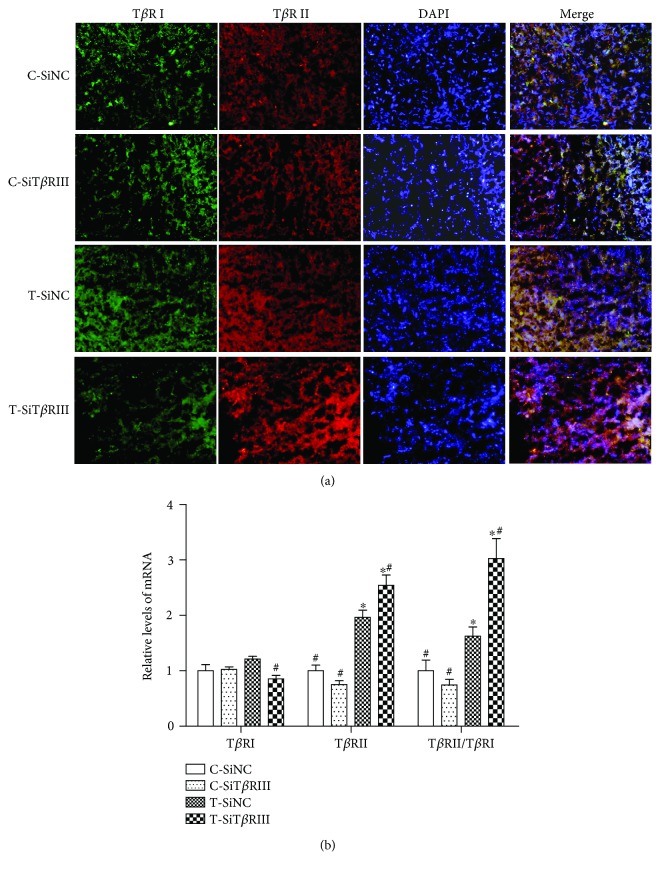
To explore the mechanism on TβRIII RNAi regulating TGF-β3-induced chondrogenic differentiation of hMSCs, we further analyzed the effect of TβRIII RNAi on TβRI and TβRII expression and downstream Smad2/3 signaling during TGF-β3-induced chondrogenesis. Analysis of TβRI and TβRII expression revealed that both TβRI mRNA levels and protein expression had no difference between the cells in the C-SiNC, C-SiTβRIII, and T-SiNC groups (Figures 4(a) and 4(b); P > 0.05). However, TβRI mRNA and protein expression levels were decreased in the T-SiTβRIII group as compared with those in the other groups (Figures 4(a) and 4(b); P < 0.05). Neither the expression of the TβRII gene (Figure 4(a)) nor that of the protein (Figure 4(b)) was increased in the C-SiTβRIII group as compared with that in the C-SiNC group (P > 0.05). However, as compared with the C-SiNC group, both the T-SiNC and T-SiTβRIII groups had enhanced mRNA levels of TβRII (Figure 4(a); P < 0.05) and TβRII expression (Figure 4(b)). The expression of the TβRII gene, as well as that of the TβRII protein, was higher in the T-SiTβRIII group as compared with that in the T-SiNC group. The ratio of TβRII to TβRI in the T-SiNC and T-SiTβRIII groups was higher than that in the C-SiNC and C-SiTβRIII groups (Figure 4(a); P < 0.05 and P < 0.01, resp.). TβRII/TβRI levels increased dramatically in the T-SiTβRIII groups as compared with those in other groups (Figure 4(a); P < 0.05).
Figure 4.
TβRIII RNAi increased the ratio of TβRII to TβRI. SiNC- or SiTβRIII-infected cells were cultured in control medium (C-SiNC group; C-SiTβRIII group) or chondrogenic medium containing TGF-β3 (T-SiNC group; T-SiTβRIII group) for 7days (n = 3). (a) The expression of TβRI and that of TβRII were visualized by immunofluorescence staining using anti-TβRI (green) and anti-TβRII (red) antibodies. Nuclei were counterstained using DAPI (blue). The far right panels show merged images. Scale bar = 20 μm. (b) mRNA expression of TβRI and TβRII and the ratio of TβRII to TβRI by real-time RT-PCR. ∗P < 0.05 versus C-SiNC group; #P < 0.05 versus T-SiNC group.
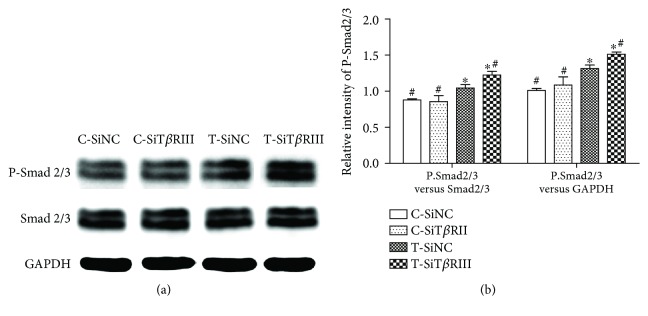
3.5. TβRIII RNAi Strengthened TGF-β3-Mediated Phosphorylation of Smad2/3
Analysis of phosphorylation of Smad2/3 revealed that TβRIII-RNAi did not affect the expression of P-Smad2/3 in control medium. However, phosphorylation of Smad2/3 was obviously activated in T-SiNC and T-SiTβRIII groups. Interestingly, when cells were infected with SiTβRIII, the activation of P-Smad2/3 was further enhanced (Figures 5(a) and 5(b)).
Figure 5.
TβRIII RNAi strengthened TGF-β3-mediated Smad2/3 phosphorylation. SiNC- or SiTβRIII-infected cells were cultured in control medium (C-SiNC group; C-SiTβRIII group) or chondrogenic medium containing TGF-β3 (T-SiNC group; T-SiTβRIII group) and harvested after 24 h. (a) A Western blot of protein levels of P-Smad2/3, total Smad2/3, and GAPDH. (b) Quantification of protein levels of P-Smad2/3 normalized to total levels of Smad2/3 or GAPDH. Error bars represent the means ± SD, n = 3. ∗P < 0.05 versus C-SiNC group; #P < 0.05 versus T-SiNC group.
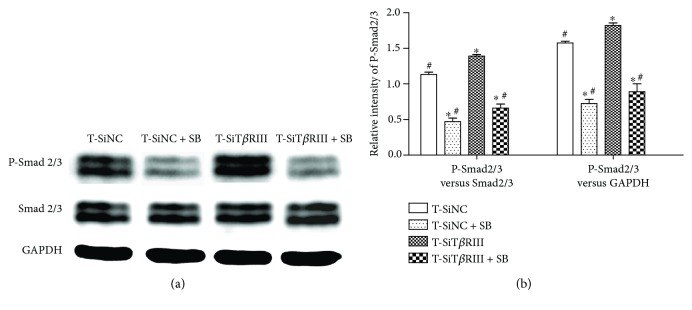
3.6. SB431542 Blocked TβRIII RNAi-Activated TGF-β3-Mediated Phosphorylation of Smad2/3
SB431542 was identified as a specific inhibitor of TβRI and TGF-β signaling [19]. Therefore, we tested whether SB431542 blocked TβRIII RNAi-activated TGF-β signaling. The results showed that SB431542 inhibited both TGF-β3-activated Smad2/3 phosphorylation and TβRIII RNAi-activated TGF-β3-mediated Smad2/3 phosphorylation (Figure 6(a)). The results of the statistical analysis revealed decreased ratios of P-Smad2/3 to Smad2/3 and P-Smad2/3 to GAPDH in the T-SiNC + SB and T-SiTβRIII + SB groups as compared with those in the T-SiNC and T-SiTβRIII groups (P < 0.05), as shown in Figure 6(b). There was no statistical difference in P-Smad2/3 levels in the T-SiNC + SB group versus those in the T-SiTβRIII + SB group (P > 0.05) (Figure 6(b)). These data showed that SB431542 completely inhibited TGF-β3-mediated Smad2/3 phosphorylation, activated by TβRIII RNAi.
Figure 6.
SB431542 blocked TβRIII RNAi-activated TGF-β3-mediated phosphorylation of Smad2/3. SiNC- or SiTβRIII-infected hMSCs were cultured in chondrogenic medium, supplemented with TGF-β3 (T-SiNC group or T-SiTβRIII group) and exposed to SB431542 treatment for 2 h before treatment with TGF-β3 (T-SiNC + SB group or T-SiTβRIII + SB group). Samples were harvested after 24 h. (a) Western blot of protein levels of P-Smad2/3, total Smad2/3, and GAPDH. (b) Quantification of protein levels of P-Smad2/3 normalized to total levels of Smad2/3 and GAPDH. Error bars represent the means ± SD, n = 3. ∗P < 0.05 versus T-SiNC group, #P < 0.05 versus T-SiTβRIII group.
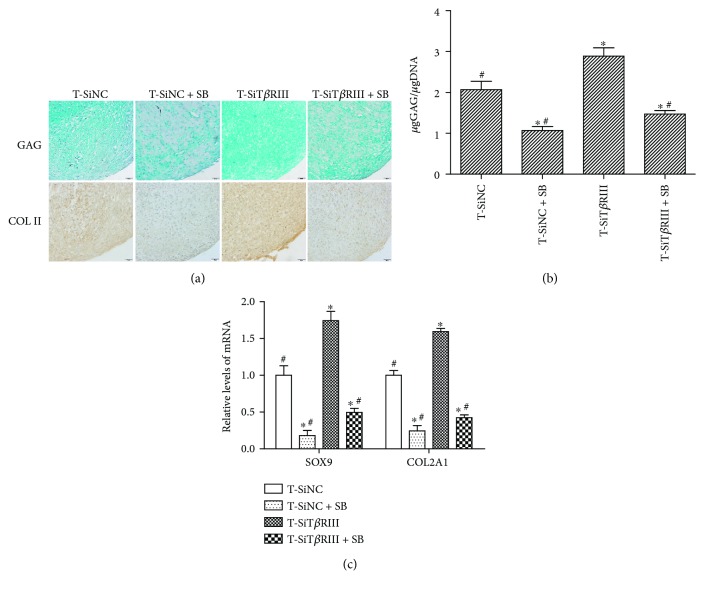
3.7. SB431542 Inhibited TβRIII RNAi-Enhanced TGF-β3-Induced Chondrogenic Differentiation of hMSCs
We have shown that SB431542 blocked TβRIII RNAi-activated TGF-β signaling. We next investigated whether it was sufficient to inhibit the ability of TβRIII RNAi-enhanced TGF-β3-induced chondrogenic differentiation of hMSCs. As shown in Figure 7, GAG and COL II secretion increased significantly in the T-SiTβRIII group (Figures 7(a) and 7(b)), as well as mRNA levels of cartilage-specific genes (SOX9 and COL2A1), as compared with that in the T-SiNC group (Figure 7(c); P < 0.05). Cartilage-specific protein and gene expression decreased in the T-SiNC + SB and T-SiTβRIII + SB groups as compared with cartilage-specific protein and gene expression in the T-SiNC and T-SiTβRIII groups (Figures 7(a)–7(c); P < 0.05).
Figure 7.
SB431542 inhibited TβRIIIRNAi-enhanced TGF-β3-induced chondrogenic differentiation of hMSCs. Cells from T-SiNC, T-SiNC + SB, T-SiTβRIII, and T-SiTβRIII + SB groups were cultured for 14 days. (a) Alcian blue staining for GAG expression and immunohistochemistry staining for COL II expression. n = 3, scale bar = 50 μm. (b) GAG content was quantitatively analyzed and normalized by DNA content. (c) mRNA levels of SOX9 and COL2A1 were measured by real-time PCR. Error bars represent the means ± SD, n = 3. ∗P < 0.05 versus T-SiNC group, #P < 0.05 versus T-SiTβRIII group.
4. Discussion
TβRIII is an abundant TGF-β receptor, which is present in many cell types [9, 13]. We previously demonstrated that hMSCs expressed high levels of TβRIII and that TGF-β3 reduced the expression of TβRIII at mRNA and protein levels [15]. Other studies demonstrated the specific role of TGF-β1 in decreasing TβRIII mRNA and protein levels in cancer cells [20, 21]. These findings were similar to those of our own study. Hempel et al. [21] showed the TGF-β1-mediated downregulation of TβRIII mRNA expression by exerting effects on the ALK5/Smad2/3 pathway of the TGFβR3 gene proximal promoter. Three highly homologous isoforms of TGF-β in humans (TGF-β1, TGF-β2, and TGF-β3) share a similar receptor complex and signaling pathway [20]. Therefore, the mechanism underlying the suppression by TGF-β3 on TβRIII may be the same as that involved in TGF-β1-mediated downregulation of TβRIII.
In recent years, many studies have investigated the effects of altering the expression level of TβRIII and its roles on mediating cell migration, invasion, growth, and differentiation in several cell types, including cancer and epithelial cells [13, 20, 22, 23]. However, the regulatory roles of TβRIII in MSCs have not been explored. RNAi is a powerful tool for studying protein function [24]. Lentiviral vectors encoding short hairpin RNAs (shRNAs) or microRNAs (miRNAs) can be used to specifically knock down target mRNAs [25]. Besides the ability to transfer vectors in primary and nondividing cells, the reverse-transcribed lentiviral vector is integrated in the genome, allowing stable expression of the gene or shRNA of interest [26]. We used GV248-based lentiviral vectors for delivery of shRNAs, precursors of TβRIII siRNA, into MSCs to suppress TβRIII gene expression. This vector coexpressed enhanced GFP, a reporter gene, permitting infected cells to be detected by fluorescence microscopy. Three days after infection with Lenti-siTβRIII or Lenti-NC, fluorescence microscopy images revealed efficient infection of MSCs with lentiviral vectors. The data also demonstrated that the lentiviral vector-based shRNA vector effectively downregulated mRNA and protein expression of TβRIII. These data indicated that Lenti-siTβRIII MSCs could be used as a powerful tool for studying the effect of the siTβRIII gene on MSC chondrogenesis.
Our study is the first to demonstrate that TβRIII knockdown in MSCs enhanced TGF-β3-induced chondrogenic differentiation, indicating a role for TβRIII as a negative modulator of MSC chondrogenesis. Previous research reported that the functional impact of TβRIII on TGF-β ligand signaling was cell-type dependent [9]. Li et al. [13] demonstrated that transfection of TβRIII-containing plasmid DNA dramatically promoted a TGF-β1-induced decrease in cell viability, apoptosis, and cell arrest. On the other hand, Eickelberg et al. indicated that the expression of TβRIII in renal epithelial LLC-PK1 cells resulted in inhibition of TGF signaling [27]. They also suggested that TβRIII with larger proteoglycans negatively modulated TGF-β1-induced cellular responses, whereas TβRIII containing smaller proteoglycans enhances TGF-β1-induced cellular responses [27]. These results suggested that TβRIII in MSCs may contain larger proteoglycans.
TGF-βs (TGF-β1, TGF-β2, and TGF-β3, resp.) are small secreted signaling proteins, each of which signals through TβRI and TβRII. In contrast to TβRI and TβRII, TβRIII is a membrane-anchored nonsignaling receptor, and its function is to bind and concentrate TGF-β isoforms on the cell surface and promote the binding of TβRII and recruitment of TβRI [11]. Chen et al. [15] demonstrated that TβRI was recruited to form TβRIII/TβRII/TβRI ternary complexes that it had two forms: complex I and complex II. Complex I contained more TβRII than TβRI, underwent clathrin-mediated endocytosis, and transduced signals in endosomes. Complex II, which contained more TβRI than TβRII, underwent caveolae/lipid-raft-mediated endocytosis and rapid degradation. The formation of these complexes was regulated by the proteoglycan moiety of TβRIII and altered the expression of TβRII or TβRI [15]. Herein, we demonstrated that TβRIII RNAi decreased TβRI expression and increased TβRII expression in the T-SiTβRIII group when MSCs were cultured with TGF-β3 as compared with the T-SiNC group (Figure 4). The data, combined with the findings of previous reports [15], indicated that TβRIII RNAi increased the ratio of TβRII to TβRI of the receptor complex and formed complex I, therefore enhancing TGF-β3 signaling in MSC chondrogenesis.
TGF-β signals predominantly bind to the ALK5 receptor (TβRI) and subsequently activate C-terminal Smad 2/3 phosphorylation. This signaling route stimulates the production of matrix components [19]. The present study also showed that TβRIII RNAi further enhanced the TGF-β3-activated downstream Smad2/3 signaling pathway (Figure 5). In addition, SB431542, a TβRI inhibitor, as well as dominant negative Smad2/3 specifically [28], significantly reversed TβRIII RNAi-mediated increases in TGF-β3-induced MSC chondrogenesis (Figures 6 and 7). These data indicated that TβRIII knockdown enhanced TGF-β3-induced MSC chondrogenesis via the Smad2/3 signaling pathway.
5. Conclusions
This study demonstrated that TGF-β3 inhibited the expression of TβRIII. TβRIII RNAi enhanced TGF-β3-induced chondrogenic differentiation of hMSCs by increasing the ratio of TβRII to TβRI in the receptor complex and further activating downstream Smad2/3 signaling. These findings contribute to the understanding of the molecular mechanisms that control chondrogenic differentiation of MSCs and provide a potential strategy (i.e., modification of MSCs by TβRIII knockdown for cell-based cartilage tissue engineering).
Acknowledgments
This research was supported by grants from the National Natural Science Foundation of China (nos. 81472039, 81772302, 816018988, and 1572091) and the Guangzhou Science and Technology Program key projects (201704020120).
Contributor Information
Peiqiang Su, Email: supq@mail.sysu.edu.cn.
Caixia Xu, Email: xucx3@mail.sysu.edu.cn.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contributions
Shuhui Zheng and Hang Zhou contributed equally to this work.
References
- 1.Wang Y., Yuan M., Guo Q.-Y., Lu S.-B., Peng J. Mesenchymal stem cells for treating articular cartilage defects and osteoarthritis. Cell Transplantation. 2015;24(9):1661–1678. doi: 10.3727/096368914x683485. [DOI] [PubMed] [Google Scholar]
- 2.Huh S. W., Shetty A. A., Ahmed S., Lee D. H., Kim S. J. Autologous bone-marrow mesenchymal cell induced chondrogenesis (MCIC) Journal of Clinical Orthopaedics and Trauma. 2016;7(3):153–156. doi: 10.1016/j.jcot.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yamasaki S., Mera H., Itokazu M., Hashimoto Y., Wakitani S. Cartilage repair with autologous bone marrow mesenchymal stem cell transplantation: review of preclinical and clinical studies. Cartilage. 2014;5(4):196–202. doi: 10.1177/1947603514534681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan A. R., Hung C. T. Concise review: mesenchymal stem cells for functional cartilage tissue engineering: taking cues from chondrocyte-based constructs. Stem Cells Translational Medicine. 2017;6(4):1295–1303. doi: 10.1002/sctm.16-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoshiya S., Dhawan A. Cartilage repair techniques in the knee: stem cell therapies. Current Reviews in Musculoskeletal Medicine. 2015;8(4):457–466. doi: 10.1007/s12178-015-9302-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang Q. O., Shakib K., Heliotis M., et al. TGF-β3: a potential biological therapy for enhancing chondrogenesis. Expert Opinion on Biological Therapy. 2009;9(6):689–701. doi: 10.1517/14712590902936823. [DOI] [PubMed] [Google Scholar]
- 7.Nickel J., ten Dijke P., Mueller T. D. TGF-β family co-receptor function and signaling. Acta Biochimica et Biophysica Sinica. 2018;50(1):12–36. doi: 10.1093/abbs/gmx126. [DOI] [PubMed] [Google Scholar]
- 8.Marcu K. B., de Kroon L. M. G., Narcisi R., et al. Activin receptor-like kinase receptors ALK5 and ALK1 are both required for TGFβ-induced chondrogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2015;10(12, article e0146124) doi: 10.1371/journal.pone.0146124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bilandzic M., Stenvers K. L. Betaglycan: a multifunctional accessory. Molecular and Cellular Endocrinology. 2011;339(1-2):180–189. doi: 10.1016/j.mce.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 10.López-Casillas F., Payne H. M., Andres J. L., Massagué J. Betaglycan can act as a dual modulator of TGF-beta access to signaling receptors: mapping of ligand binding and GAG attachment sites. Journal of Cell Biology. 1994;124(4):557–568. doi: 10.1083/jcb.124.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Villarreal M. M., Kim S. K., Barron L., et al. Binding properties of the transforming growth factor-β coreceptor betaglycan: proposed mechanism for potentiation of receptor complex assembly and signaling. Biochemistry. 2016;55(49):6880–6896. doi: 10.1021/acs.biochem.6b00566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLean S., Di Guglielmo G. M. TGFβ (transforming growth factor β) receptor type III directs clathrin-mediated endocytosis of TGFβ receptor types I and II. Biochemical Journal. 2010;429(1):137–145. doi: 10.1042/bj20091598. [DOI] [PubMed] [Google Scholar]
- 13.Li D., Xu D., Lu Z., Dong X., Wang X. Overexpression of transforming growth factor type III receptor restores TGF-β1 sensitivity in human tongue squamous cell carcinoma cells. Bioscience Reports. 2015;35(4, article e00243) doi: 10.1042/bsr20150141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tazat K., Hector-Greene M., Blobe G. C., Henis Y. I. TβRIII independently binds type I and type II TGF-β receptors to inhibit TGF-β signaling. Molecular Biology of the Cell. 2015;26(19):3535–3545. doi: 10.1091/mbc.e15-04-0203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen C.-L., Huang S. S., Huang J. S. Cellular heparan sulfate negatively modulates transforming growth factor-β1(TGF-β1) responsiveness in epithelial cells. Journal of Biological Chemistry. 2006;281(17):11506–11514. doi: 10.1074/jbc.m512821200. [DOI] [PubMed] [Google Scholar]
- 16.Chen J., Wang Y., Chen C., et al. Exogenous heparan sulfate enhances the TGF-β3-induced chondrogenesis in human mesenchymal stem cells by activating TGF-β/Smad signaling. Stem Cells International. 2016;2016:10. doi: 10.1155/2016/1520136.1520136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C., Zheng Z., Fang L., et al. Phosphatidylserine enhances osteogenic differentiation in human mesenchymal stem cells via ERK signal pathways. Materials Science and Engineering: C. 2013;33(3):1783–1788. doi: 10.1016/j.msec.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 18.Zhang L., Su P., Xu C., Yang J., Yu W., Huang D. Chondrogenic differentiation of human mesenchymal stem cells: a comparison between micromass and pellet culture systems. Biotechnology Letters. 2010;32(9):1339–1346. doi: 10.1007/s10529-010-0293-x. [DOI] [PubMed] [Google Scholar]
- 19.van Caam A., Madej W., Garcia de Vinuesa A., et al. TGFβ1-induced SMAD2/3 and SMAD1/5 phosphorylation are both ALK5-kinase-dependent in primary chondrocytes and mediated by TAK1 kinase activity. Arthritis Research & Therapy. 2017;19(1):p. 112. doi: 10.1186/s13075-017-1302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang S. E. N., Sun W.-Y., Wu J.-J., Gu Y.-J., Wei W. E. I. Decreased expression of the type III TGF-β receptor enhances metastasis and invasion in hepatocellullar carcinoma progression. Oncology Reports. 2016;35(4):2373–2381. doi: 10.3892/or.2016.4615. [DOI] [PubMed] [Google Scholar]
- 21.Hempel N., How T., Cooper S. J., et al. Expression of the type III TGF-β receptor is negatively regulated by TGF-β. Carcinogenesis. 2008;29(5):905–912. doi: 10.1093/carcin/bgn049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu D., Li D. U. O., Lu Z., Dong X., Wang X. Type III TGF-β receptor inhibits cell proliferation and migration in salivary glands adenoid cystic carcinoma by suppressing NF-κB signaling. Oncology Reports. 2016;35(1):267–274. doi: 10.3892/or.2015.4390. [DOI] [PubMed] [Google Scholar]
- 23.Jenkins L. M., Singh P., Varadaraj A., et al. Altering the proteoglycan state of transforming growth factor β type III receptor (TβRIII)/Betaglycan modulates canonical Wnt/β-catenin signaling. Journal of Biological Chemistry. 2016;291(49):25716–25728. doi: 10.1074/jbc.m116.748624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L., Liu H.-j., Li T.-j., et al. Lentiviral vector-mediated siRNA knockdown of SR-PSOX inhibits foam cell formation in vitro. Acta Pharmacologica Sinica. 2008;29(7):847–852. doi: 10.1111/j.1745-7254.2008.00823.x. [DOI] [PubMed] [Google Scholar]
- 25.Liu Y. P., Vink M. A., Westerink J. T., et al. Titers of lentiviral vectors encoding shRNAs and miRNAs are reduced by different mechanisms that require distinct repair strategies. RNA. 2010;16(7):1328–1339. doi: 10.1261/rna.1887910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albrecht C., Hosiner S., Tichy B., Aldrian S., Hajdu S., Nürnberger S. Comparison of lentiviral packaging mixes and producer cell lines for RNAi applications. Molecular Biotechnology. 2015;57(6):499–505. doi: 10.1007/s12033-015-9843-8. [DOI] [PubMed] [Google Scholar]
- 27.Eickelberg O., Centrella M., Reiss M., Kashgarian M., Wells R. G. Betaglycan inhibits TGF-β signaling by preventing type I-type II receptor complex formation: glycosaminoglycan modifications alter betaglycan function. Journal of Biological Chemistry. 2002;277(1):823–829. doi: 10.1074/jbc.m105110200. [DOI] [PubMed] [Google Scholar]
- 28.You H. J., Bruinsma M. W., How T., Ostrander J. H., Blobe G. C. The type III TGF-β receptor signals through both Smad3 and the p38 MAP kinase pathways to contribute to inhibition of cell proliferation. Carcinogenesis. 2007;28(12):2491–2500. doi: 10.1093/carcin/bgm195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.