Abstract
Objectives
Elevated levels of serum homocysteine (Hcy) have been associated with cardiovascular diseases and endothelial dysfunction, conditions closely associated with erectile dysfunction (ED). This meta-analysis was aimed to assess serum Hcy levels in subjects with ED compared to controls in order to clarify the role of Hcy in the pathogenesis of ED.
Methods
Medline, Embase, and the Cochrane Library were searched for publications investigating the possible association between ED and Hcy. Results were restricted by language, but no time restriction was applied. Standardized mean difference (SMD) was obtained by random effect models.
Results
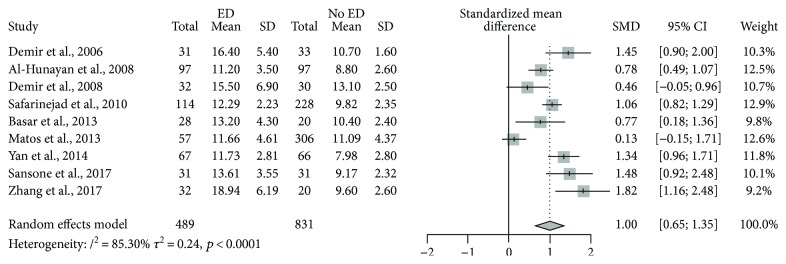
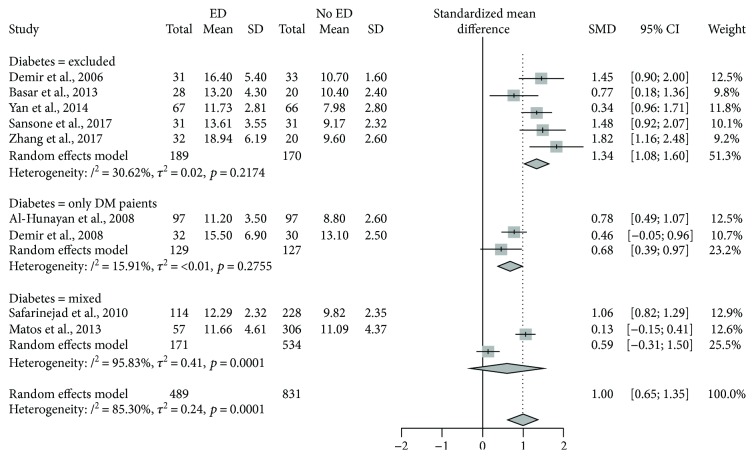
A total of 9 studies were included in the analysis with a total of 1320 subjects (489 subjects with ED; 831 subjects without ED). Pooled estimate was in favor of increased Hcy in subjects with ED with a SMD of 1.00, 95% CI 0.65–1.35, p < 0.0001. Subgroup analysis based on prevalence of diabetes showed significantly higher SMD in subjects without diabetes (1.34 (95% CI 1.08–1.60)) compared to subjects with diabetes (0.68 (95% CI 0.39–0.97), p < 0.0025 versus subgroup w/o diabetes).
Conclusions
Results from our meta-analysis suggest that increased levels of serum Hcy are more often observed in subjects with ED; however, increase in Hcy is less evident in diabetic compared to nondiabetic subjects. This study is registered with Prospero registration number CRD42018087558.
1. Introduction
Prevalence of erectile disorders increases steadily with aging, from 1 to 10% in men younger than 40 years up to 70–100% in men older than 70 years [1, 2]. It is widely accepted that several factors might be involved in the pathogenesis of erectile dysfunction (ED): guidelines suggest investigating organic causes [3], such as endocrine alterations [4, 5], neurological impairment [1, 6], and vascular dysfunction [6] as well as psychological or relational issues [7, 8].
However, given the importance of hemodynamics in allowing adequate erection, it should come as no surprise that ED shares the same risk factors of many cardiovascular (CV) affections [9].
In these regards, hyperhomocysteinemia (HHcy) has sprung into attention for its involvement in endothelial dysfunction [10]. Several meta-analysis studies have confirmed the association with consistently elevated levels of homocysteine (Hcy) and the risk of CV diseases: Boushey and colleagues [11] reported a 1.6 OR (95% CI 1.4–1.7) for coronary artery disease in men following an increase of 5 μmol/l in serum Hcy levels, whereas Clarke et al. [12] described that a reduction of 25% of serum Hcy was associated with an 11% lower risk of ischemic heart disease and with a ~20% lower risk of stroke. However, other reports have suggested that Hcy lowering interventions do not prevent CV events [13], or that Hcy is a marker of unhealthy lifestyles [14], thus being indirectly associated with CV health.
Despite the prevalence of ED and its known association with endothelial dysfunction [15, 16], its link with serum Hcy has not been adequately addressed. HHcy is associated with increased arterial stiffness [17] and with impaired nitric oxide synthase activity [18]. Several studies have assessed whether subjects suffering from ED have increased serum Hcy compared to controls, but most of these studies have been performed in small populations and generally do not provide solid evidence in these regards.
2. Materials and Methods
2.1. Methods
This meta-analysis was performed in line with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) reporting guideline [19] (see Supplementary file 1). The protocol of this study (CRD42018087558) was published on the website of the University of York (Centre for Reviews and Dissemination) and can be accessed at the following address: http://www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42018087558.
2.2. Search Strategy
An extensive Medline, Embase, and Cochrane search was performed, including the following words: (“erectile dysfunction”[MeSH Terms] OR (“erectile”[All Fields] AND “dysfunction”[All Fields]) OR “erectile dysfunction”[All Fields]) OR ((“penile erection”[MeSH Terms] OR (“penile”[All Fields] AND “erection”[All Fields]) OR “penile erection”[All Fields] OR “erectile”[All Fields]) AND (“physiology”[Subheading] OR “physiology”[All Fields] OR “function”[All Fields] OR “physiology”[MeSH Terms] OR “function”[All Fields])) OR ((“sexual behavior”[MeSH Terms] OR (“sexual”[All Fields] AND “behavior”[All Fields]) OR “sexual behavior”[All Fields] OR “sexual”[All Fields]) AND (“physiopathology”[Subheading] OR “physiopathology”[All Fields] OR “dysfunction”[All Fields])) AND (“homocysteine”[MeSH Terms] OR “homocysteine”[All Fields]). The search, which accrued data up to February 28th 2018, was restricted to articles written in English, Italian, or French and studies including human participants. The identification of relevant studies was performed independently by two of the authors (SA and SM), and conflicts were resolved by a different investigator (CA). We did not employ search software but hand-searched bibliographies of retrieved papers for additional references. The main source of information was derived from published articles.
2.3. Study Selection
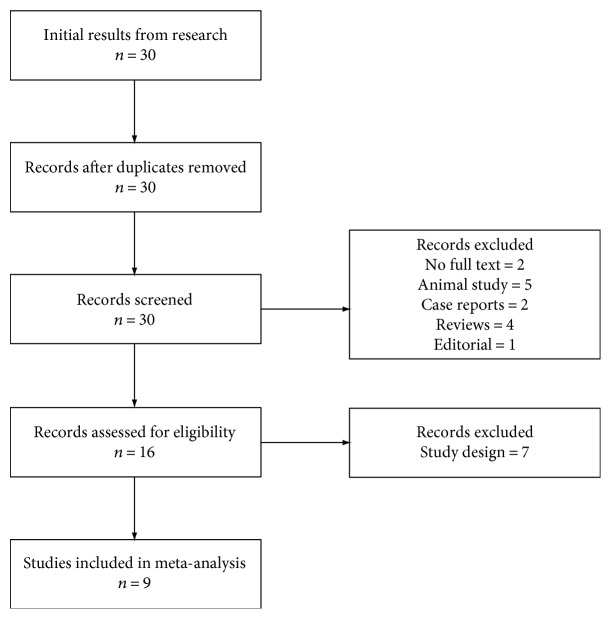
All studies reporting serum Hcy levels in patients with ED and control population were included. Case reports were excluded from the analysis (see Figure 1).
Figure 1.
Flowchart detailing all phases of data retrieval.
2.4. Outcome and Quality Assessment
The principal outcome was the serum concentration of Hcy in subjects with and without erectile dysfunction. The quality of studies included was assessed using the Cochrane criteria [20]. Difference in Hcy levels between subjects with and without ED was measured with standardized mean difference (SMD) as different kits and tools have been used in different studies (Table 1). All measures of ED were included in the analysis, including the International Index of Erectile Function (IIEF), a 15-item questionnaire extensively used for clinical and research purposes [21], and its abridged, 5-item version (IIEF-5) [22], as well as assessment of ED by means of a single question.
Table 1.
Studies included in the meta-analysis. Data expressed as means ± standard deviation.
| Study | Country | Method | Subjects without ED | Subjects with ED | ||||
|---|---|---|---|---|---|---|---|---|
| N | Hcy | Age | N | Hcy | Age | |||
| Demir et al. [24] | Turkey | Immunoassay | 33 | 10.7 ± 1.6 | 44.5 ± 4.7 | 31 | 16.4 ± 5.4 | 55.6 ± 8.4 |
| Al-Hunayan et al. [29] | Kuwait | HPLC | 97 | 8.8 ± 2.6 | 47.6 ± 10.1 | 97 | 11.2 ± 3.5 | 46.6 ± 9.9 |
| Demir et al. [30] | Turkey | Immunoassay | 30 | 13.1 ± 2.5 | 53.6 ± 6.5 | 32 | 15.5 ± 6.9 | 54.2 ± 7.3 |
| Safarinejad et al. [32] | Iran | HPLC | 228 | 9.82 ± 2.35 | 31.7 ± 6.6 | 114 | 12.29 ± 2.32 | 32.2 ± 6.4 |
| Basar et al. [25] | Turkey | HPLC | 20 | 10.4 ± 2.4 | 38.6 ± 8.2 | 28 | 13.2 ± 4.3 | 40.2 ± 7.5 |
| Matos et al. [31] | Brazil | HPLC | 306 | 11.09 ± 4.37 | 38.6 ± 13.99 | 57 | 11.66 ± 4.61 | 54.1 ± 12.83 |
| Yan et al. [26] | China | Immunoassay | 66 | 7.98 ± 2.8 | 29.41 ± 4.42 | 67 | 11.73 ± 2.81 | 29.12 ± 4.41 |
| Sansone et al. [27] | Italy | HPLC | 31 | 9.17 ± 2.32 | 49.14 ± 13.63 | 31 | 13.61 ± 3.55 | 52.83 ± 11.89 |
| Zhang et al. [28] | China | Immunoassay | 20 | 9.6 ± 2.6 | 32.8 ± 7.8 | 32 | 18.94 ± 6.19 | 34.08 ± 11.99 |
2.5. Statistical Analysis
Data from retrieved study were extracted and entered twice by two authors (SA and CA) into two separate standard Excel templates which were then cross-checked by the other author. Extracted data included data sources, eligibility, methods, participant characteristics, and results. Heterogeneity in serum Hcy levels was assessed using I 2 statistics. Even when low heterogeneity was detected, a random effect model was applied, because the validity of tests of heterogeneity can be limited with a small number of component studies. We used funnel plots and the Begg adjusted rank correlation test to estimate possible publication or disclosure bias (Begg et al., 1994); however, undetected bias may still be present because these tests have low statistical power when the number of trials is small. Statistical analysis was performed independently by two authors (SA and CA) with the meta package on the statistical software R (version 3.4.2) [23].
3. Results
Out of 30 retrieved articles, 9 were included in the study (Table 1). The study flow is summarized in Figure 1. The characteristics of the retrieved studies, including assessment of their overall quality, are reported in Table 1 and Supplementary file 1. Retrieved trials included 1320 subjects with a mean age of 39.52 ± 2.11 years. Most of the studies did not provide information in regard to ED severity (missing in 7 studies), BMI of the subjects (missing in 3 studies), smoking habits (missing in 3 studies), and prevalence of diabetes (missing in 1 study).
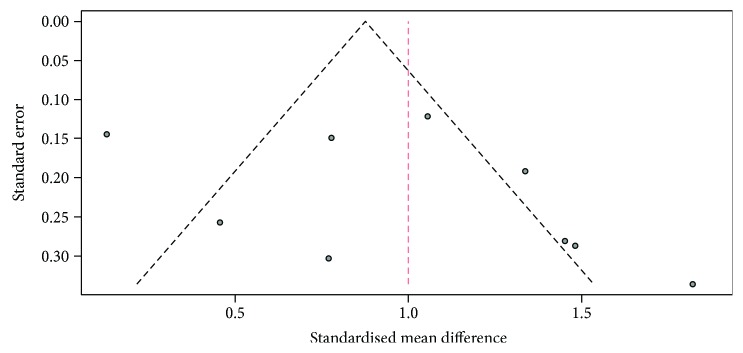
The I 2 in studies assessing serum Hcy in men with or without ED was 85.3% (p < 0.0001, (95% CI 73.9%–91.7%)). Serum Hcy was significantly higher among subjects with ED compared to controls (SMD 1.00, p < 0.0001, (95% CI 0.65–1.35)) (Figure 2). A funnel plot and Begg adjusted rank correlation test (Kendall's τ: 1.147; p = 0.29) suggested no publication bias (Figure 3).
Figure 2.
Forest plot for the base model.
Figure 3.
Funnel plot for all studies included in the meta-analysis.
Sensitivity analysis was then performed based on prevalence of diabetes. Five studies [24–28] had diabetes as an exclusion factor, whereas 2 included only subjects with diabetes [29, 30], one [31] did not report actual prevalence of diabetes, and another one [32] reported 4.7% of diabetes among controls and 3.5% among ED patients. The resulting forest plot is shown in Figure 4. Association between Hcy and ED appears steeper in nondiabetic subjects. Indeed, absence of ED is associated with a SMD of 1.34 (95% CI 1.08–1.60) in subjects without diabetes, while SMD is significantly lower in subjects with diabetes (0.68 (95% CI 0.39–0.97); Q 12.00, p < 0.0025 versus subgroup w/o diabetes). Despite remaining higher in the subgroup with “mixed” prevalence of diabetes, heterogeneity is remarkably reduced in both other subgroups.
Figure 4.
Forest plot by subgroup.
4. Discussion
The results of our meta-analysis suggest that Hcy is significantly associated with ED, showing a mean increase of 1.00 standard deviation between controls and ED patients. Among patients with ED, sensitivity analysis has shown that when stratified for presence of diabetes, subjects without diabetes are more likely to have increased levels of serum Hcy compared to diabetic subjects; conversely, this association appears evident in patients with diabetes even for small variation of Hcy, or rather, it could be argued that in diabetic patients, a small increase of Hcy is enough to negatively affect erectile function. In fact, the difference between diabetic and nondiabetic patients might suggest that in the presence of comorbidities, small changes in serum Hcy might negatively be involved in erectile function, based on the premise that multiple risk factors might act in a synergistic, rather than additive, fashion [33, 34]. This observation seems to be rather counterintuitive, as Hcy is a risk factor for cardiovascular events, whose prevalence is higher in diabetic people, and also a sign of metabolic derangement, featured in diabetes [35]. Indeed, different studies observed higher serum level of Hcy both in type 1 and in type 2 diabetes mellitus [36]. More in detail, serum Hcy level appears to be higher in patients affected by T1DM suffering with retinopathy and/or nephropathy [36]. Interestingly, antidiabetic treatment may be considered as an important factor involved in regulation of level of Hcy in diabetic patients [37]: a recent meta-analysis showed both detrimental and beneficial effects of several drugs commonly used in metabolic syndrome and in type 2 diabetes mellitus [38]. Although there seems to be no significant effect of antidiabetic treatments on Hcy levels as a whole (SMD −0.53 (95% CI −1.60 to 0.53)), combination treatment with thiazolidinediones and diguanides or meglitinide and thiazolidinediones has shown significant effects on lowering serum Hcy (SMD −1.67 (95% CI −2.85 to −0.50) and SMD −4.40 (95% CI −4.94 to −3.86), resp.). Following on, metformin appears to increase serum Hcy levels decreasing serum B12 and folate level, although the exact mechanism is not known. Unexpectedly, the improved sensitivity to insulin associated with metformin has been associated to an increase in plasma Hcy [39, 40]; conversely, rosiglitazone as well as pioglitazone showed interesting properties in lowering serum Hcys level [37, 41, 42]. It has been suggested that insulin may stop homocysteine catabolic transformations resulting in an increase in the amount of homocysteine and its blood level [43]. Nevertheless, it should be considered that in T2DM insulin-induced increments of methionine transmethylation, homocysteine transsulfuration, and clearance were markedly reduced [44]. On the contrary, in T1DM, insulin deprivation brings about an increase in Hcy, whereas insulin treatment normalized transsulfuration and remethylation of Hcy, therefore decreasing its serum levels. Furthermore, in the same study, plasma homocysteine concentrations were observed to be lower in T1DM compared to healthy controls [45]. Similarly, in healthy humans, insulin seems to increase in vivo homocysteine clearance [46].
As previously stated, HHcy is closely associated with endothelial dysfunction. Studies in animal models have proven that HHcy markedly inhibits NO formation in rabbit isolated corpus cavernosum [47]. NO acts as a key regulator for endothelial function: activation of guanylyl cyclase by NO leads to increased levels of cyclic GMP (cGMP), which in turn allow for smooth muscle relaxation in the corpora cavernosa [48]. High levels of serum Hcy have been associated with uncoupling of the endothelial NO synthase enzyme (eNOS), therefore reducing availability of NO while at the same time increasing production of reactive oxygen species (ROS). HHcy has also been proven an independent risk factor for atherosclerosis [49], providing further proof of a causative role for Hcy in the pathogenesis of ED.
Our study has some limitations, most notably the small number of studies involved and the lack of a clear definition of ED. A single study [31] assessed presence of ED by means of a single question (“How would you describe your ability to get and keep an erection that is adequate for satisfactory intercourse?”). The remaining studies used validated questionnaires: in detail, four studies [24, 25, 30, 32] used the IIEF and four studies [26–29] used the IIEF-5 [22]. However, most studies did not report separate measurements of serum Hcy based on the degree of severity of ED.
5. Conclusions
Results from our meta-analysis suggest that increased levels of serum Hcy are more often observed in subjects with ED: based on existing literature on this topic, a causative role for HHCy as an independent risk factor for ED can be postulated, although confirmation would require interventional studies aimed to decrease serum Hcy levels considering erectile function as primary outcome. Actually, only in rat model of HHcy has been observed an improvement in erectile function after being treated with a demethylation agent [50]. We also reported significantly higher levels of Hcy in subjects without diabetes, compared to diabetic men: while we can assume that this is further proof of a multifactorial pathogenesis for ED, it is also a clear indication that future research in this field should investigate the possible association with other known risk factors—such as smoking habit and obesity—in order to adequately address the possible effects of different variates.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Supplementary Materials
Characteristics and outcomes of the studies included in the meta-analysis. Y = yes; NA = not applicable; N = no.
References
- 1.Shamloul R., Ghanem H. Erectile dysfunction. Lancet. 2013;381(9861):153–165. doi: 10.1016/S0140-6736(12)60520-0. [DOI] [PubMed] [Google Scholar]
- 2.Lewis R. W., Fugl-Meyer K. S., Corona G., et al. Definitions/epidemiology/risk factors for sexual dysfunction. The Journal of Sexual Medicine. 2010;7(4):1598–1607. doi: 10.1111/j.1743-6109.2010.01778.x. [DOI] [PubMed] [Google Scholar]
- 3.Hackett G., Kirby M., Wylie K., et al. British Society for Sexual Medicine guidelines on the management of erectile dysfunction in men—2017. The Journal of Sexual Medicine. 2018;15(4):430–457. doi: 10.1016/j.jsxm.2018.01.023. [DOI] [PubMed] [Google Scholar]
- 4.Sansone A., Romanelli F., Gianfrilli D., Lenzi A. Endocrine evaluation of erectile dysfunction. Endocrine. 2014;46(3):423–430. doi: 10.1007/s12020-014-0254-6. [DOI] [PubMed] [Google Scholar]
- 5.Isidori A. M., Buvat J., Corona G., et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment-a systematic review. European Urology. 2014;65(1):99–112. doi: 10.1016/j.eururo.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 6.Yafi F. A., Jenkins L., Albersen M., et al. Erectile dysfunction. Nature Reviews Disease Primers. 2016;2, article 16003 doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rastrelli G., Maggi M. Erectile dysfunction in fit and healthy young men: psychological or pathological? Translational Andrology and Urology. 2017;6(1):79–90. doi: 10.21037/tau.2016.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corona G., Ricca V., Bandini E., et al. SIEDY scale 3, a new instrument to detect psychological component in subjects with erectile dysfunction. The Journal of Sexual Medicine. 2012;9(8):2017–2026. doi: 10.1111/j.1743-6109.2012.02762.x. [DOI] [PubMed] [Google Scholar]
- 9.Miner M., Parish S. J., Billups K. L., Paulos M., Sigman M., Blaha M. J. Erectile dysfunction and subclinical cardiovascular disease. Sexual Medicine Reviews. 2018 doi: 10.1016/j.sxmr.2018.01.001. In press. [DOI] [PubMed] [Google Scholar]
- 10.Cheng Z.-J., Yang X., Wang H. Hyperhomocysteinemia and endothelial dysfunction. Current Hypertension Reviews. 2009;5(2):158–165. doi: 10.2174/157340209788166940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boushey C. J., Beresford S. A., Omenn G. S., Motulsky A. G. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274(13):1049–1057. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 12.Clarke R., Lewington S., Landray M. Homocysteine, renal function, and risk of cardiovascular disease. Kidney International. Supplement. 2003;63:S131–S133. doi: 10.1046/j.1523-1755.63.s84.7.x. [DOI] [PubMed] [Google Scholar]
- 13.Martí-Carvajal A. J., Solà I., Lathyris D., Salanti G. Homocysteine lowering interventions for preventing cardiovascular events. Cochrane Database of Systematic Reviews. 2009;4, article CD006612 doi: 10.1002/14651858.cd006612.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cleophas T. J., Hornstra N., van Hoogstraten B., van der Meulen J. Homocysteine, a risk factor for coronary artery disease or not? A meta-analysis. The American Journal of Cardiology. 2000;86(9):1005–9. doi: 10.1016/S0002-9149(00)01137-1. A8. [DOI] [PubMed] [Google Scholar]
- 15.Guay A. T. ED2: erectile dysfunction = endothelial dysfunction. Endocrinology and Metabolism Clinics of North America. 2007;36(2):453–463. doi: 10.1016/j.ecl.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 16.Aversa A., Bruzziches R., Francomano D., Natali M., Gareri P., Spera G. Endothelial dysfunction and erectile dysfunction in the aging man. International Journal of Urology. 2010;17(1):38–47. doi: 10.1111/j.1442-2042.2009.02426.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen L., Wang B., Wang J., et al. Association between serum total homocysteine and arterial stiffness in adults: a community-based study. Journal of Clinical Hypertension. 2018;20(4):686–693. doi: 10.1111/jch.13246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuhlinger M. C., Tsao P. S., Her J. H., Kimoto M., Balint R. F., Cooke J. P. Homocysteine impairs the nitric oxide synthase pathway: role of asymmetric dimethylarginine. Circulation. 2001;104(21):2569–2575. doi: 10.1161/hc4601.098514. [DOI] [PubMed] [Google Scholar]
- 19.Moher D., Shamseer L., Clarke M., et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Systematic Reviews. 2015;4(1):p. 1. doi: 10.1186/2046-4053-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins J. P. T., Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley-Blackwell; 2008. [DOI] [Google Scholar]
- 21.Rosen R. C., Riley A., Wagner G., Osterloh I. H., Kirkpatrick J., Mishra A. The international index of erectile function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822–830. doi: 10.1016/S0090-4295(97)00238-0. [DOI] [PubMed] [Google Scholar]
- 22.Rosen R. C., Cappelleri J. C., Smith M. D., Lipsky J., Peña B. M. Development and evaluation of an abridged, 5-item version of the International Index of Erectile Function (IIEF-5) as a diagnostic tool for erectile dysfunction. International Journal of Impotence Research. 1999;11(6):319–326. doi: 10.1038/sj.ijir.3900472. [DOI] [PubMed] [Google Scholar]
- 23.Schwarzer G. meta: an R package for meta-analysis. R News. 2007;7(3):40–45. [Google Scholar]
- 24.Demir T., Çomlekçi A., Demir O., et al. Hyperhomocysteinemia: a novel risk factor for erectile dysfunction. Metabolism. 2006;55(12):1564–1568. doi: 10.1016/j.metabol.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 25.Basar M. M., Ozkan Y., Kisa U., Simsek B. Serum homocysteine levels and sildenafil 50 mg response in young-adult male patients without vascular risk factors. Indian Journal of Biochemistry & Biophysics. 2013;50(3):215–220. [PubMed] [Google Scholar]
- 26.Yan W. J., Yu N., Yin T. L., Zou Y. J., Yang J. A new potential risk factor in patients with erectile dysfunction and premature ejaculation: folate deficiency. Asian Journal of Andrology. 2014;16(6):902–6. doi: 10.4103/1008-682X.135981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sansone M., Sansone A., Romano M., Seraceno S., di Luigi L., Romanelli F. Folate: a possible role in erectile dysfunction? The Aging Male. 2017;21(2):116–120. doi: 10.1080/13685538.2017.1404022. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z., Xu Z., Dai Y., Chen Y. Elevated serum homocysteine level as an independent risk factor for erectile dysfunction: a prospective pilot case-control study. Andrologia. 2017;49(6, article e12684) doi: 10.1111/and.12684. [DOI] [PubMed] [Google Scholar]
- 29.Al-Hunayan A., Thalib L., Kehinde E. O., Asfar S. Hyperhomocysteinemia is a risk factor for erectile dysfunction in men with adult-onset diabetes mellitus. Urology. 2008;71(5):897–900. doi: 10.1016/j.urology.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 30.Demir T., Cömlekci A., Demir O., et al. A possible new risk factor in diabetic patients with erectile dysfunction: homocysteinemia. Journal of Diabetes and its Complications. 2008;22(6):395–399. doi: 10.1016/j.jdiacomp.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 31.Matos G., Hirotsu C., Alvarenga T. A., et al. The association between TNF-α and erectile dysfunction complaints. Andrology. 2013;1(6):872–878. doi: 10.1111/j.2047-2927.2013.00136.x. [DOI] [PubMed] [Google Scholar]
- 32.Safarinejad M. R., Safarinejad S., Shafiei N. Role of methylenetetrahydrofolate reductase gene polymorphisms (C677T, A1298C, and G1793A) in the development of early onset vasculogenic erectile dysfunction. Archives of Medical Research. 2010;41(6):410–422. doi: 10.1016/j.arcmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 33.Mirone V., Imbimbo C., Bortolotti A., et al. Cigarette smoking as risk factor for erectile dysfunction: results from an Italian epidemiological study. European Urology. 2002;41(3):294–297. doi: 10.1016/S0302-2838(02)00005-2. [DOI] [PubMed] [Google Scholar]
- 34.Lubin J. H., Couper D., Lutsey P. L., Yatsuya H. Synergistic and non-synergistic associations for cigarette smoking and non-tobacco risk factors for cardiovascular disease incidence in the Atherosclerosis Risk In Communities (ARIC) Study. Nicotine & Tobacco Research. 2017;19(7):826–835. doi: 10.1093/ntr/ntw235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kilicdag E. B., Bagis T., Tarim E., et al. Administration of B-group vitamins reduces circulating homocysteine in polycystic ovarian syndrome patients treated with metformin: a randomized trial. Human Reproduction. 2005;20(6):1521–1528. doi: 10.1093/humrep/deh825. [DOI] [PubMed] [Google Scholar]
- 36.Feng Y., Shan M. Q., Bo L., Zhang X. Y., Hu J. Association of homocysteine with type 1 diabetes mellitus: a meta-analysis. International Journal of Clinical and Experimental Medicine. 2015;8(8):12529–12538. [PMC free article] [PubMed] [Google Scholar]
- 37.Sahin M., Tutuncu N. B., Ertugrul D., Tanaci N., Guvener N. D. Effects of metformin or rosiglitazone on serum concentrations of homocysteine, folate, and vitamin B12 in patients with type 2 diabetes mellitus. Journal of Diabetes and its Complications. 2007;21(2):118–123. doi: 10.1016/j.jdiacomp.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 38.Ntaios G., Savopoulos C., Chatzopoulos S., Mikhailidis D., Hatzitolios A. Iatrogenic hyperhomocysteinemia in patients with metabolic syndrome: a systematic review and metaanalysis. Atherosclerosis. 2011;214(1):11–19. doi: 10.1016/j.atherosclerosis.2010.08.045. [DOI] [PubMed] [Google Scholar]
- 39.Homocysteine Studies Collaboration. Homocysteine and risk of ischemic heart disease and stroke: a meta-analysis. JAMA. 2002;288(16):2015–2022. doi: 10.1001/jama.288.16.2015. [DOI] [PubMed] [Google Scholar]
- 40.Fonseca V. A., Fink L. M., Kern P. A. Insulin sensitivity and plasma homocysteine concentrations in non-diabetic obese and normal weight subjects. Atherosclerosis. 2003;167(1):105–109. doi: 10.1016/S0021-9150(02)00386-6. [DOI] [PubMed] [Google Scholar]
- 41.Derosa G., Cicero A. F., D'Angelo A., et al. Effects of 1 year of treatment with pioglitazone or rosiglitazone added to glimepiride on lipoprotein (a) and homocysteine concentrations in patients with type 2 diabetes mellitus and metabolic syndrome: a multicenter, randomized, double-blind, controlled clinical trial. Clinical Therapeutics. 2006;28(5):679–688. doi: 10.1016/j.clinthera.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 42.Derosa G., D'Angelo A., Ragonesi P. D., et al. Metformin-pioglitazone and metformin-rosiglitazone effects on non-conventional cardiovascular risk factors plasma level in type 2 diabetic patients with metabolic syndrome. Journal of Clinical Pharmacy and Therapeutics. 2006;31(4):375–383. doi: 10.1111/j.1365-2710.2006.00756.x. [DOI] [PubMed] [Google Scholar]
- 43.Borowska M., Dworacka M., Winiarska H., Krzyżagórska E. Homocysteine as a non-classical risk factor for atherosclerosis in relation to pharmacotherapy of type 2 diabetes mellitus. Acta Biochimica Polonica. 2017;64(4):603–607. doi: 10.18388/abp.2016_1489. [DOI] [PubMed] [Google Scholar]
- 44.Tessari P., Coracina A., Kiwanuka E., et al. Effects of insulin on methionine and homocysteine kinetics in type 2 diabetes with nephropathy. Diabetes. 2005;54(10):2968–2976. doi: 10.2337/diabetes.54.10.2968. [DOI] [PubMed] [Google Scholar]
- 45.Abu-Lebdeh H. S., Barazzoni R., Meek S. E., Bigelow M. L., Persson X. M. T., Nair K. S. Effects of insulin deprivation and treatment on homocysteine metabolism in people with type 1 diabetes. The Journal of Clinical Endocrinology and Metabolism. 2006;91(9):3344–3348. doi: 10.1210/jc.2006-0018. [DOI] [PubMed] [Google Scholar]
- 46.Tessari P., Kiwanuka E., Coracina A., et al. Insulin in methionine and homocysteine kinetics in healthy humans: plasma vs. intracellular models. American Journal of Physiology Endocrinology and Metabolism. 2005;288(6):E1270–6. doi: 10.1152/ajpendo.00383.2004. [DOI] [PubMed] [Google Scholar]
- 47.Jones R. W. A., Jeremy J. Y., Koupparis A., Persad R., Shukla N. Cavernosal dysfunction in a rabbit model of hyperhomocysteinaemia. BJU International. 2005;95(1):125–130. doi: 10.1111/j.1464-410X.2004.05263.x. [DOI] [PubMed] [Google Scholar]
- 48.Burnett A. L. The role of nitric oxide in erectile dysfunction: implications for medical therapy. Journal of Clinical Hypertension. 2006;8(12 Suppl 4):53–62. doi: 10.1111/j.1524-6175.2006.06026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao J., Chen H., Liu N., et al. Role of hyperhomocysteinemia and hyperuricemia in pathogenesis of atherosclerosis. Journal of Stroke and Cerebrovascular Diseases. 2017;26(12):2695–2699. doi: 10.1016/j.jstrokecerebrovasdis.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Zhang Z., Zhu L. L., Jiang H. S., Chen H., Chen Y., Dai Y. T. Demethylation treatment restores erectile function in a rat model of hyperhomocysteinemia. Asian Journal of Andrology. 2016;18(5):763–768. doi: 10.4103/1008-682X.163271. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characteristics and outcomes of the studies included in the meta-analysis. Y = yes; NA = not applicable; N = no.