Abstract
Background
Proton pump inhibitors (PPIs) are widely used for the long-term management of gastroesophageal reflux disease (GERD). However, concerns about the cost and/or inconvenience of continuous maintenance PPI treatment have led to the evaluation of various alternative approaches.
Aim
To assess the effectiveness of on-demand PPI therapy in the maintenance treatment of nonerosive reflux disease (NERD) or mild erosive esophagitis (EE).
Methods
We searched MEDLINE, EMBASE, Web of Science, and Cochrane Library from inception until October 2, 2017, for randomized controlled trials (RCTs) comparing on-demand PPI versus placebo or daily PPI in the management of NERD or mild EE (Savary-Miller grade 1). Discontinuation of therapy during the trial was used as a surrogate for patient dissatisfaction and failure of symptomatic control. We calculated pooled odds ratios (OR) to evaluate the efficacy of on-demand PPI treatment. Separate analyses were conducted for studies comparing on-demand PPI with daily PPI and with placebo. Subgroup analysis was done based on NERD studies alone and on studies of both NERD and mild EE. These were analyzed using a random effects model.
Results
We included 10 RCTs with 4574 patients. On-demand PPI was superior to daily PPI (pooled OR = 0.50; 95% confidence interval (CI) = 0.35, 0.72). On subgroup analysis in NERD patients only, pooled OR was 0.44 (0.29, 0.66). In studies including patients with NERD and mild EE, pooled OR was 0.76 (0.36, 1.60). For studies comparing on-demand PPI with placebo, pooled OR was 0.21 (0.15, 0.29); subgroup analyses of studies evaluating NERD only and studies conducted in NERD and mild EE showed similar results (pooled OR was 0.22 (0.13, 0.36) and 0.18 (0.11, 0.31), resp.).
Conclusions
On-demand PPI treatment is effective for many patients with NERD or mild EE. Although not FDA-approved, it may be adequate for those patients whose symptoms are controlled to their satisfaction.
1. Introduction
Gastroesophageal reflux disease (GERD) is a common disorder of the upper gastrointestinal tract. The prevalence of reflux symptoms is steadily rising throughout the industrialized world [1]. An estimated 20–40% of Western adult populations report chronic heartburn or regurgitation symptoms [2]. Different manifestations of GERD include nonerosive reflux disease (NERD) and erosive esophagitis (EE). Complications of GERD, which are generally confined to EE patients, include ulceration, stricture, and Barrett's esophagus with attendant risk of esophageal adenocarcinoma [3]. NERD, the most frequent manifestation of GERD, is present in around 70% of patients and characterized by the presence of typical GERD symptoms associated with pathological acid reflux but the absence of demonstrable esophageal mucosal injury on endoscopy [4, 5]. Despite the absence of mucosal injury on endoscopy, many patients with NERD experience severe symptoms and impairment in quality of life that may be equivalent to, or greater than, seen in patients with EE [6, 7]. Acid-suppressive therapy with proton pump inhibitors (PPI) has proved to be the most effective treatment strategy for both NERD and EE [8–10]. PPIs have shown superiority over histamine H2-receptor antagonists for controlling symptoms as well as for healing erosions and preventing relapse [8, 10]. However, up to 75% patients with NERD and up to 90% of patients with EE may experience symptomatic relapse within six months of stopping treatment [11, 12]. Therefore, many patients subsequently receive long-term treatment to maintain adequate symptom control and, for EE patients, healing of erosions. However, this may have led to unnecessary use of these drugs, among NERD patients especially, adding to overall costs [13]. In the United States, the total expenditure for PPI treatment may be over $11 billion annually [14]. Due to the costs of PPI treatment, there have been efforts to develop effective and cost-efficient alternative long-term maintenance strategies for some GERD patients [15, 16], including “on-demand” PPI therapy, with patients taking a daily dose of a PPI when symptoms recur and stopping treatment when symptoms resolve. This is in contrast to intermittent treatment, in which patients take a regular daily dose of a PPI upon symptom relapse and continue it for a prespecified duration (typically 1 or 2 weeks) regardless of symptom response.
To evaluate the effectiveness of on-demand PPI treatment in patients with NERD or mild EE, we conducted a systematic review of randomized controlled trials (RCTs) comparing it with regular daily PPI treatment or placebo.
2. Methods
2.1. Data Sources and Search Strategy
We carried out this systematic review and meta-analysis in accordance with the guidelines of preferred reporting items for systematic review and meta-analysis (PRISMA) [17]. The search strategies were developed in Ovid MEDLINE, and the same keywords and subject headings were applied to Ovid EMBASE, Cochrane, Scopus, and ISI Web of Science databases from inception through November 2, 2016. The search terms included “Esophagitis” OR “Gastroesophageal reflux” OR “GERD” OR “Nonerosive reflux disease” OR “NERD” OR “Erosive esophagitis” OR “EE” AND “Proton pump inhibitors” OR “PPIs” AND “on-demand” OR “on demand” OR “daily” AND “Placebo.” A medical librarian with more than 20 years of experience performed this search.
2.2. Study Selection and Inclusion and Exclusion Criteria
Two authors (Z.K. and Y.A.) searched for original studies based on the previously defined search strategy. We searched for RCTs comparing on-demand PPI treatment with either placebo or daily PPI in the maintenance treatment of NERD and/or mild EE. NERD was defined as the presence of classic GERD symptoms in the absence of esophageal mucosal injury during upper endoscopy (Savary-Miller Grade 0 and LA class M). Mild EE was defined as having esophagitis with Savary-Miller Grade 1 or LA class A. The main outcome measure used to assess treatment efficacy was discontinuation of therapy during the trial. Continuation of therapy during a trial was used as a surrogate for patient satisfaction and control of GERD symptoms. Hence, the proportion of patients who discontinued therapy during a trial was taken as an indirect measure of failure of symptomatic control of GERD. Studies were excluded if they did not contain raw or usable data or were published only in abstract form. We also excluded duplicate publications, expert opinion, and letters. We also searched bibliographies of retrieved articles to enhance the yield of our search strategy. All articles were downloaded into Endnote 7.0, a bibliographic database manager, and any duplicate citations were identified and removed.
2.3. Data Extraction and Quality Assessment
Two reviewers (Z.K. and Y.A.) assessed the eligibility of selected studies and extracted data using customized data extraction forms. Any disagreement between reviewers was discussed with a third reviewer (M.A.K.), and agreement was reached by consensus. Extracted data included study design, the year and country of publication, inclusion and exclusion criteria, PPI regimen used for on-demand and continuous groups, classification of esophagitis, outcome studied (number of patients discontinuing on-demand treatment due to inadequate symptom control), follow-up duration, and patient demographics.
We used the Cochrane tool for assessing risk of bias for RCTs. Two reviewers (Z.K. and Y.A.) performed quality assessment with any disagreement to be discussed with a third reviewer (M.A.K.). We used the GRADE framework to interpret our findings [18].
2.4. Data Synthesis and Statistical Analysis
Our main outcome of interest was the effectiveness of on-demand PPI treatment versus placebo or daily PPI in the management of NERD and/or mild EE. The primary efficacy endpoint used was the premature discontinuation of treatment. We analyzed pooled data using a random effects model and calculated odds ratios (ORs) with their 95% confidence interval (CI). We conducted separate analyses for studies comparing on-demand PPI with daily PPI and with placebo. We performed additional subgroup analyses based on NERD studies alone and on studies including patients with either NERD or mild EE. Cochrane's Q test and I 2 statistics were used to assess heterogeneity among studies. A P value of <0.1 for the Cochrane Q test or an I 2 value of >50% signified the presence of heterogeneity.
We constructed funnel plots and used Egger's precision test to assess publication bias. Statistical analysis was performed using RevMan, version 5.3 for Windows (Cochrane Collaboration, the Nordic Cochrane Center, Copenhagen, Denmark, 2014).
3. Results
3.1. Search Strategy Yield and Identification of Studies
The search strategy identified 409 articles, of which 35 were removed as duplicates. Of the remaining 374 articles, 347 were removed after title and abstract review. The remaining 27 full-text articles were reviewed, of which 10 RCTs [19–28] with 4574 patients were included in the meta-analysis as shown in PRISMA flowchart (Supplementary Materials (available here)). Among the patients, 2797 received on-demand PPI, 843 received daily PPI and 934 received placebo. Four RCTs [19–22] compared a daily PPI regimen with on-demand treatment. Three of these were confined to patients with NERD [19–21], while the fourth [22] also included patients with mild EE. Six trials compared on-demand treatment with placebo [23–28]; four only included patients with NERD [23–26], and two included both patients with NERD or mild EE [27, 28]. Tables 1 –3 highlight the characteristics of included studies.
Table 1.
Characteristics of studies comparing on-demand PPI with daily PPI.
| References | Country | Center (multi, single) |
Study design | Inclusion criteria | Exclusion criteria | PPI regimen for continuous therapy | PPI regimen for on-demand therapy | Outcome studied | Follow-up period | Esophagitis class |
|---|---|---|---|---|---|---|---|---|---|---|
| [19] | UK (92 general practices, 28 hospitals) | Multicenter | Single-blind (investigator), randomized, parallel group study | NERD with resolution of heartburn after 2 to 4 weeks of esomeprazole 20 | Patients with persistent heartburn and structural diseases | Lansoprazole 15 mg PO once daily | Esomeprazole 20 mg | Time to discontinuation due to unwillingness to continue | 6 months | Not applicable |
|
| ||||||||||
| [20] | Single university hospital Japan | Single center | Prospective parallel randomized open-label study | Patients with modified LA class M after having 8-week treatment with PPIs | Patients with cancer, serious liver disease, kidney disease, heart disease, a hematological disorder, gastric ulcers, and/or duodenal ulcers | Omeprazole 20 mg | Omeprazole 20 mg | Symptom relief at 4, 8, 16, and 24 weeks in each study group with relief from symptoms as the primary endpoint | 6 months | Modified LA class M |
|
| ||||||||||
| [21] | Austria, France, Germany, South Africa, and Spain (61 centers) | Multicenter | Open-label, randomized, parallel group | NERD who were heartburn-free after 4-week treatment with esomeprazole 20 mg daily | Reflux esophagitis | Esomeprazole 20 mg | Esomeprazole 20 mg | Discontinuation due to unsatisfactory treatment | 6 months | Not applicable |
|
| ||||||||||
| [22] | 58 active centers: 29 in Germany, 12 in France, 11 in Switzerland, and 6 in Hungary | Multicenter | Open-label, randomized, parallel group | NERD + mild esophagitis treated with pantoprazole 20 mg PO daily for 4 weeks | Patients with persistent symptoms and heartburn, erosive esophagitis | Pantoprazole 20 mg | Pantoprazole 20 mg | The symptoms (as assessed in the patient's diary) were considered controlled until the time of failure, which was defined as the first point at which one of the following events occurred: (1) GERD symptoms of at least moderate severity were present for 3 or more consecutive days despite medication (event time = the first of these 3 days); (2) use of >1 tablet of study medication on >3 consecutive days (event time = the first of these 3 days); or (3) premature withdrawal from the study due to lack of efficacy (event time = the date of withdrawal) | 6 months | Savary-Miller grade 0 or 1 |
Table 2.
Characteristics of studies comparing on-demand PPI with placebo.
| References | Country | Center (multi, single) | Study design | Inclusion criteria | Exclusion criteria | PPI regimen for continuous therapy | PPI regimen for on-demand therapy | Primary outcome studied | Follow-up period | Esophagitis class |
|---|---|---|---|---|---|---|---|---|---|---|
| [23] | Sweden & Denmark (25 centers) | Multicenter | Double-blind, randomized, placebo controlled | NERD with resolution of heartburn after short-term treatment (4 to 8 weeks) | Erosive, ulcerative PUD | N/A | Omeprazole 20, omeprazole 10, placebo | Discontinuation of medicine due to unwillingness to continue | 6 months | N/A |
|
| ||||||||||
| [24] | 65 centers in Denmark, Finland, Norway, and Sweden | Multicenter | Randomized, double-blind, parallel group | Endoscopy-negative GERD treated with 4 weeks of omep 20 or Eso 20 | Patients requiring concomitant drugs, NSAIDS, quinidine, etc. excluded | N/A | Esomeprazole 20 mg on demand, placebo on demand | Time to discontinuation of on-demand therapy due to unwillingness to continue | 6 months | N/A |
|
| ||||||||||
| [25] | 116 centers in the UK, the Republic of Ireland, and Canada | Multicenter | Randomized, double-blind, parallel-group study | Patients with NERD treated with Eso 40, 20, or Omep 20 for 4 weeks | Patients requiring concomitant drugs, NSAIDS, quinidine, etc. excluded | N/A | Esomeprazole 40 mg on demand, esomeprazole 20 mg on demand, placebo on demand | Time to study discontinuation due to unwillingness to continue for any reason | 6 months | N/A |
|
| ||||||||||
| [26] | International (Greece, Italy, the Netherlands, Spain, France, Portugal, Sweden, Denmark, Ireland, Belgium, United Kingdom, Russia, Poland, and Lithuania) | Multicenter | Randomized, double-blind, placebo-controlled, withdrawal study | NERD treated with rabeprazole 10 mg PO daily for 4 weeks | Patients with erosive disease and no relief of heartburn in acute 4-week phase | N/A | Rabeprazole 10 mg on demand, placebo on demand | The proportion of patients discontinuing treatment in the on-demand phase because of inadequate heartburn control | 6 months | N/A |
|
| ||||||||||
| [27] | Germany 36 centers | Multicenter | Randomized, double-blind, placebo-controlled, parallel-group comparison | Nonerosive GERD or reflux esophagitis grade 1 according to Savary–Miller classification underwent 4 weeks of pantoprazole 20 mg PO OD for 4 weeks | Patients symptomtic after acute phase or nonerosive GERD or reflux esophagitis grade 2 to 4 according to Savary–Miller classification | N/A | Pantoprazole 40 mg on demand, pantoprazole 20 mg on demand, placebo on demand | Discontinuation rate due to insufficient control of heartburn | 6 months | Savary-Miller grade 0 or 1 |
| [28] | 40 centers in Germany and five in Lithuania | Multicenter | Randomized, double-blind, placebo-controlled parallel-group comparison | Nonerosive GERD or reflux esophagitis grade 1 according to Savary–Miller classification underwent 4 weeks of pantoprazole 20 mg PO OD for 4 weeks | Patients symptomtic after acute phase or nonerosive GERD or reflux esophagitis grades 2 to 4 according to Savary–Miller classification | N/A | Pantoprazole 20 mg on demand, placebo on demand | The number of patients unwilling to continue the therapy and the corresponding reasons for it were analyzed using the Kaplan–Meier analysis | 7 months | Savary-Miller grade 0 or 1 |
Table 3.
Patient characteristics and demographics of included trials.
| Study | N, on demand/cont | Mean age ± SD | Male (%) |
|---|---|---|---|
| Tsai et al. [19] | (1) Eso 20 mg on demand = 311 | 51 ± 13.8 | 46% |
| (2) Lanso 15 mg continuous = 311 | 51 ± 13.8 | 41.8% | |
|
| |||
| Nagahara et al. [20] | Omeprazole 20 mg continuous = 18 | 56.2 ± 12.8 | 21/35 = 60% |
| Omeprazole 20 mg on demand = 17 | |||
|
| |||
| Bayerdörffer et al. [21] | (1) Eso 20 mg on demand = 301 | 48.2 ± 13.6 | 40.5% |
| (2) Eso 20 mg continuous = 297 | 47.6 ± 15.1 | 43.8% | |
|
| |||
| Janssen et al. [22] | Pantoprazole 20 mg on demand = 215 | 50.4 (SD 13.6) | 46.5% |
| Pantoprazole 20 mg continuous = 217 | 51.8 (SD 13.5) | 47.5% | |
|
| |||
| Lind et al. [23] | (1) Omeprazole 20 on demand = 139 (n) | 52 (19–79) R | 38.1% |
| (2) Omeprazole 10 on demand = 142 | 51 (20–81) R | 45.8% | |
| (3) Placebo = 143 | 48 (20–79) R | 42.7% | |
|
| |||
| Talley et al. [24] | (1) Eso 20 mg on demand = 170 | 49 (19–78) R | 55% |
| (2) Placebo on demand = 172 | 49 (21–79) R | 57% | |
|
| |||
| Talley et al. [25] | (1) Esomeprazole 40 mg on demand = 293 | 48 | 46.1% |
| (2) Esomeprazole 20 mg on demand = 282 | 48.4 | 47.9% | |
| (3) Placebo on demand = 146 | 48.2 | 39.7% | |
|
| |||
| Bytzer et al. [26] | (1) Rabeprazole 10 mg on demand = 279 | 47 (0.81 SE) | 44% |
| (2) Placebo on demand = 139 | 48 (1.23 SE) | 41% | |
|
| |||
| Scholten et al. [27] | Pantoprazole 40 mg on demand = 218 | 54 ± 14 | 47.3% |
| Pantoprazole 20 mg on demand = 217 | 52 ± 14 | 52.5% | |
| Placebo on demand = 108 | 52 ± 14 | 53.7% | |
|
| |||
| Kaspari et al. [28] | Pantoprazole 20 mg on demand = 213 | 50.7 ± 13.7 years | 46% |
| Placebo on demand = 226 | 51.0 ± 14.5 years | 43.3% | |
3.2. Meta-Analysis
3.2.1. On Demand versus Daily PPI
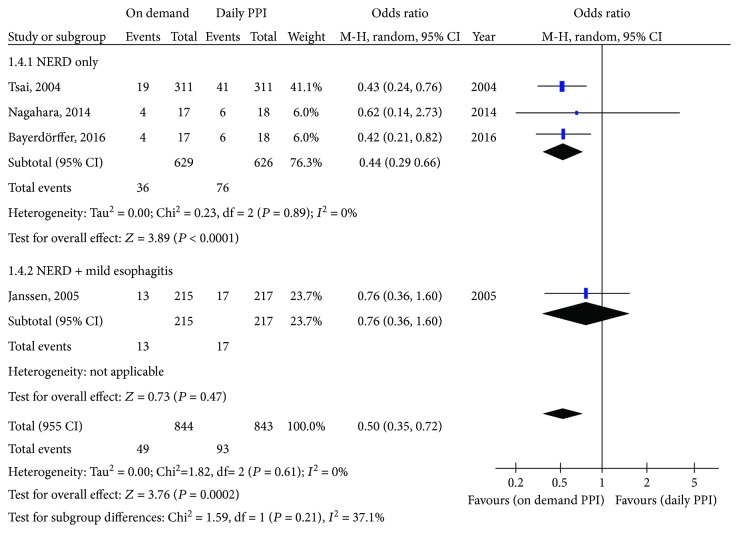
For studies comparing on-demand PPI therapy with daily PPI therapy, 5.8% of patients discontinued treatment in the on-demand group compared to 11.0% in the daily PPI group. The pooled OR with 95% confidence interval (CI) was 0.50 (0.35, 0.72), with no heterogeneity (I 2 = 0%) (Figure 1). On subgroup analysis of the three RCTs that included only NERD patients, results were similar; 5.7% of patients in the on-demand PPI group and 12.1% of patients in the daily PPI group discontinued treatment prematurely (OR = 0.44; 95% CI = 0.29 to 0.66). However, on subgroup analysis of the study that included patients with either NERD or mild EE, there was no significant difference between treatments (OR = 0.76; 95% CI = 0.36 to 1.60). With on-demand treatment, 6.0% of patients discontinued treatment prematurely compared with 7.8% on daily PPI treatment.
Figure 1.
Forest plot comparing on-demand PPI with daily PPI.
3.2.2. On-Demand PPI Treatment versus Placebo
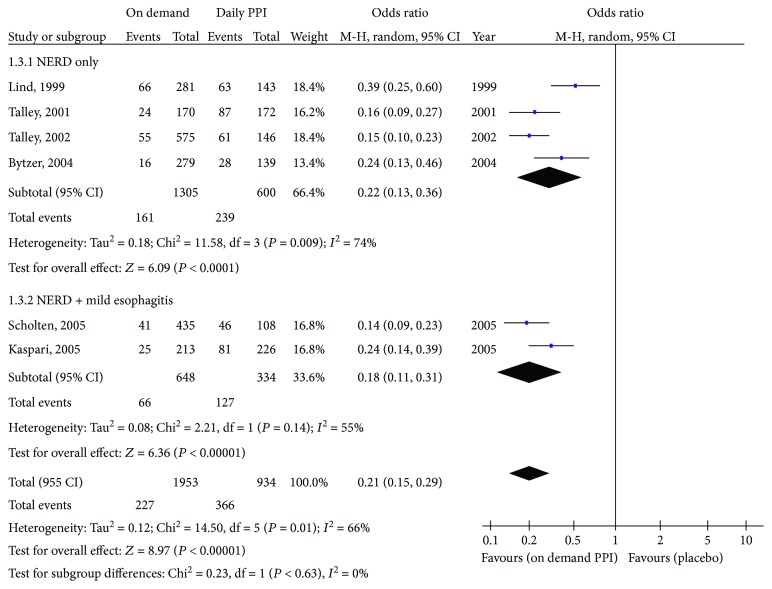
The proportions of patients prematurely discontinuing treatment were 11.6% with on-demand PPI therapy and 39.2% with placebo (pooled OR = 0.21; 95% CI = 0.15 to 0.29). There was significant heterogeneity among studies (I 2 = 66%) (Figure 2). On subgroup analysis of studies conducted only in NERD patients, on-demand PPI treatment was superior to placebo; proportions of patients prematurely discontinuing treatment were 12.3% and 39.8%, respectively (OR = 0.22; 95% CI = 0.13 to 0.36). Among studies evaluating patients with either NERD or mild EE, proportions of patients discontinuing treatment prematurely were 10.2% and 38.0% (OR = 0.18; 95% CI = 0.11 to 0.31), respectively.
Figure 2.
Forest plot comparing on-demand PPI with placebo.
4. Discussion
We found that on-demand PPI therapy was superior to both placebo and daily PPI therapy as maintenance treatment for patients with NERD or mild EE. In general, more patients were willing to continue on-demand PPI treatment than either of the alternatives studied. Furthermore, adherence to treatment and patient satisfaction were higher with on-demand PPI treatment compared to continuous PPI treatment. On-demand treatment may help to improve overall patient satisfaction since patients may feel more in control of their treatment and can take a dose of PPI according to their perceived needs and symptoms [18]. However, the usefulness of on-demand PPI treatment compared to daily PPI was less obvious when patients with mild EE were included in the analysis.
Our analysis is different from two previously conducted analyses. Jiang et al. [29] concluded that on-demand treatment with PPIs is superior to continuous or placebo therapy, but did not perform subgroup analysis of NERD and mild EE. A recently published Cochrane review showed that on-demand deprescribing may lead to an increase in gastrointestinal symptoms (e.g., dyspepsia and regurgitation) in patients with NERD or mild grades of EE (Los Angeles grades A and B or Savary-Miller grades 1 and 2) [30]. Besides having a broad definition for on-demand deprescribing, this review did not differentiate NERD from mild EE; Savary-Miller grading for most of the included patients was >1. In our analysis, the only included were patients with Savary-Miller grade 0 (NERD) or 1 (mild EE). We excluded studies that included EE patients with Savary-Miller grade higher than 1, since those would represent moderate and severe EE.
Since the risk of progression of NERD or mild EE to more advanced disease is low, symptom control is the main objective of management [31–33]. Many patients experience intermittent symptoms of short duration. These symptoms can be effectively managed with on-demand PPI treatment. This symptom-driven approach for the long-term management of NERD also simulates many patients' actual use of these medicines [16]. Many patients who are prescribed daily PPI treatment may actually consume them on an as needed basis. Up to 29% of patients who were prescribed continuous PPI treatment decreased the frequency of use without a recommendation from their providers, and only 21% of patients prescribed continuous PPI treatment fill their prescriptions in a manner to remain fully compliant with the recommended dosing schedule [34, 35]. The most commonly cited reasons for not continuing treatment are inconvenience and cost. However, studies [36–38] evaluating the on-demand strategy versus continuous PPI treatment for EE have shown that the on-demand strategy is inferior. On-demand PPI treatment is, therefore, not appropriate or adequate for patients with EE of Savary-Miller grade 2 or above.
As well as patient preference and satisfaction for on-demand PPI treatment, cost of long-term treatment is also important. The consistently demonstrated efficacy and the favorable safety profile of PPI treatment have led to its widespread use in GERD patients [13]. There has been a substantial increase in the cost of GERD management with medicines contributing to overall costs. This has led to increasing concern from health care authorities and third-party payers [36]. On-demand PPI treatment might reduce overall consumption by up to one third. Tsai et al. [19], who compared on-demand esomeprazole with daily lansoprazole in 622 patients for six months, found that patients in the esomeprazole on-demand group took treatment on approximately one-third of the days (0.3 times per day) whereas those receiving lansoprazole daily took treatment on approximately 4 of every 5 days (0.8 times per day) despite being instructed to take them every day. Bayerdörffer et al. [21] found similar results with mean daily drug consumption of 0.41 tablets in the on-demand group and 0.91 tablets in the continuous daily group. Therefore, on-demand treatment may be a cost-effective strategy for the long-term management of patients with NERD or mild EE. It can be effective in controlling symptoms and is convenient for, and acceptable to, many patients. Those patients with NERD or mild EE who do not obtain adequate relief with on-demand PPI treatment can be considered for regular, daily PPI treatment in the lowest effective dose.
The studies included in our analysis defined NERD based on symptoms and endoscopic findings without physiological demonstration of abnormal gastroesophageal reflux (acidic or weekly acidic), which could be a limitation. Also, most of the studies used only Savary-Miller grading for classifying esophagitis. The studies that classified esophagitis using the LA classification were very limited and did not differentiate between LA grades A and B. We tried to be conservative while defining mild esophagitis and so confined our analysis to studies with either LA grades M or A and considered LA grade B to be indicative of moderate—rather than mild—esophagitis.
5. Conclusion
On-demand PPI treatment appears to be effective for the long-term management of many patients with NERD or mild EE. After the initial control of symptoms with a course of PPI treatment, on-demand PPI treatment can be appropriately considered for the long-term management of such patients.
Abbreviations
- PPIs:
Proton pump inhibitors
- GERD:
Gastroesophageal reflux disease
- EE:
Erosive esophagitis
- NERD:
Nonerosive reflux disease
- PRISMA:
Preferred reporting items for systematic review and meta-analysis
- RCT:
Randomized clinical trial
- OR:
Odds ratio.
Disclosure
The abstract of this review was presented in the Digestive Disease Week 2017 held in Chicago.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
Zubair Khan helped in the study design, data collection, statistical analysis, manuscript drafting, and final approval of the manuscript. Yaseen Alastal helped in the study design, data collection, statistical analysis, manuscript drafting, critical revision, and final approval of the manuscript. Muhammad Ali Khan helped in the development of search strategies, data collection, manuscript drafting, and final approval of the manuscript. Mohammad Saud Khan helped in the study design, manuscript drafting, and final approval of the manuscript. Basmah Khalil, Shreesh Shrestha, and Faisal Kamal helped in the data collection, manuscript drafting, and final approval of the manuscript. Ali Nawras helped in the manuscript drafting, critical revision for intellectual content, and final approval of the manuscript. Colin W. Howden helped in the statistical analysis, manuscript drafting, critical revision for intellectual content, and final approval of the manuscript.
Supplementary Materials
PRISMA flow chart for study selection.
References
- 1.El–serag H. B. Time trends of gastroesophageal reflux disease: a systematic review. Clinical Gastroenterology and Hepatology. 2007;5(1):17–26. doi: 10.1016/j.cgh.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 2.Frank L., Kleinman L., Ganoczy D., et al. Upper gastrointestinal symptoms in North America: prevalence and relationship to healthcare utilization and quality of life. Digestive Diseases and Sciences. 2000;45(4):809–818. doi: 10.1023/A:1005468332122. [DOI] [PubMed] [Google Scholar]
- 3.Pace F., Porro G. B. Gastroesophageal reflux disease: a typical spectrum disease (a new conceptual framework is not needed) The American Journal of Gastroenterology. 2004;99(5):946–949. doi: 10.1111/j.1572-0241.2004.04164.x. [DOI] [PubMed] [Google Scholar]
- 4.Vakil N., van Zanten S. V., Kahrilas P., Dent J., Jones R., the Global Consensus Group The Montreal definition and classification of gastroesophageal reflux disease: a global evidence-based consensus. American Journal of Gastroenterology. 2006;101(8):1900–1920. doi: 10.1111/j.1572-0241.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- 5.El-Serag H. B. Epidemiology of non-erosive reflux disease. Digestion. 2008;78(1) Supplement 1:6–10. doi: 10.1159/000151249. [DOI] [PubMed] [Google Scholar]
- 6.Wu J. C. Y., Cheung C. M. Y., Wong V. W. S., Sung J. J. Y. Distinct clinical characteristics between patients with nonerosive reflux disease and those with reflux esophagitis. Clinical Gastroenterology and Hepatology. 2007;5(6):690–695. doi: 10.1016/j.cgh.2007.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Kulig M., Leodolter A., Vieth M., et al. Quality of life in relation to symptoms in patients with gastro-oesophageal reflux disease-- an analysis based on the ProGERD initiative. Alimentary Pharmacology & Therapeutics. 2003;18(8):767–776. doi: 10.1046/j.1365-2036.2003.01770.x. [DOI] [PubMed] [Google Scholar]
- 8.Moayyedi P., Talley N. J. Gastro-oesophageal reflux disease. Lancet. 2006;367(9528):2086–2100. doi: 10.1016/S0140-6736(06)68932-0. [DOI] [PubMed] [Google Scholar]
- 9.van Pinxteren B., Sigterman K. E., Bonis P., Lau J., Numans M. E. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database of Systematic Reviews. 2006;(3, article CD002095) doi: 10.1002/14651858.CD002095.pub3. [DOI] [PubMed] [Google Scholar]
- 10.Donnellan C., Sharma N., Preston C., Moayyedi P. Medical treatments for the maintenance therapy of reflux oesophagitis and endoscopic negative reflux disease. Cochrane Database of Systematic Reviews. 2004;2, article CD003245 doi: 10.1002/14651858.CD003245.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Dean B. B., Gano A. D., Jr., Knight K., Ofman J. J., Fass R. Effectiveness of proton pump inhibitors in nonerosive reflux disease. Clinical Gastroenterology and Hepatology. 2004;2(8):656–664. doi: 10.1016/S1542-3565(04)00288-5. [DOI] [PubMed] [Google Scholar]
- 12.Kinoshita Y., Ashida K., Hongo M., The Japan Rabeprazole Study Group for NERD Randomised clinical trial: a multicentre, double-blind, placebo-controlled study on the efficacy and safety of rabeprazole 5 mg or 10 mg once daily in patients with non-erosive reflux disease. Alimentary Pharmacology and Therapeutics. 2011;33(2):213–224. doi: 10.1111/j.1365-2036.2010.04508.x. [DOI] [PubMed] [Google Scholar]
- 13.Heidelbaugh J. J., Kim A. H., Chang R., Walker P. C. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therapeutic Advances in Gastroenterology. 2012;5(4):219–232. doi: 10.1177/1756283X12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaheen N. J., Hansen R. A., Morgan D. R., et al. The burden of gastrointestinal and liver diseases, 2006. The American Journal of Gastroenterology. 2006;101(9):2128–2138. doi: 10.1111/j.1572-0241.2006.00723.x. [DOI] [PubMed] [Google Scholar]
- 15.Metz D. C., Inadomi J. M., Howden C. W., van Zanten S. J. V., Bytzer P. On-demand therapy for gastroesophageal reflux disease. The American Journal of Gastroenterology. 2007;102(3):642–653. doi: 10.1111/j.1572-0241.2006.00998.x. [DOI] [PubMed] [Google Scholar]
- 16.Inadomi J. M., Jamal R., Murata G. H., et al. Step-down management of gastroesophageal reflux disease. Gastroenterology. 2001;121(5):1095–1100. doi: 10.1053/gast.2001.28649. [DOI] [PubMed] [Google Scholar]
- 17.Liberati A., Altman D. G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339(jul21 1, article b2700) doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atkins D., Best D., Briss P. A., et al. Grading quality of evidence and strength of recommendations. BMJ. 2004;328(7454):p. 1490. doi: 10.1136/bmj.328.7454.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai H. H., Chapman R., Shepherd A., et al. Esomeprazole 20 mg on-demand is more acceptable to patients than continuous lansoprazole 15 mg in the long-term maintenance of endoscopy-negative gastro-oesophageal reflux patients: the COMMAND study. Alimentary Pharmacology & Therapeutics. 2004;20(6):657–665. doi: 10.1111/j.1365-2036.2004.02155.x. [DOI] [PubMed] [Google Scholar]
- 20.Nagahara A., Hojo M., Asaoka D., Sasaki H., Watanabe S. A randomized prospective study comparing the efficacy of on-demand therapy versus continuous therapy for 6 months for long-term maintenance with omeprazole 20 mg in patients with gastroesophageal reflux disease in Japan. Scandinavian Journal of Gastroenterology. 2014;49(4):409–417. doi: 10.3109/00365521.2013.878380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bayerdörffer E., Bigard M.-A., Weiss W., et al. Randomized, multicenter study: on-demand versus continuous maintenance treatment with esomeprazole in patients with non-erosive gastroesophageal reflux disease. BMC Gastroenterology. 2016;16(1):p. 48. doi: 10.1186/s12876-016-0448-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssen W., Meier E., Gatz G., Pfaffenberger B. Effects of pantoprazole 20 mg in mildgastroesophageal reflux disease: once-daily treatment in the acute phase, and comparison of on-demand versus continuous treatment in the long term. Current Therapeutic Research, Clinical and Experimental. 2005;66(4):345–363. doi: 10.1016/j.curtheres.2005.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lind T., Havelund T., Lundell L., et al. On demand therapy with omeprazole for the long-term management of patients with heartburn without oesophagitis—a placebo-controlled randomized trial. Alimentary Pharmacology & Therapeutics. 1999;13(7):907–914. doi: 10.1046/j.1365-2036.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- 24.Talley N. J., Lauritsen K., Tunturi-Hihnala H., et al. Esomeprazole 20 mg maintains symptom control in endoscopy-negative gastro-oesophageal reflux disease: a controlled trial of ‘on-demand’ therapy for 6 months. Alimentary Pharmacology & Therapeutics. 2001;15(3):347–354. doi: 10.1046/j.1365-2036.2001.00943.x. [DOI] [PubMed] [Google Scholar]
- 25.Talley N. J., Venables T. L., Green J. R. B., et al. Esomeprazole 40 mg and 20 mg is efficacious in the long-term management of patients with endoscopy-negative gastro-oesophageal reflux disease: a placebo-controlled trial of on-demand therapy for 6 months. European Journal of Gastroenterology & Hepatology. 2002;14(8):857–863. doi: 10.1097/00042737-200208000-00008. [DOI] [PubMed] [Google Scholar]
- 26.Bytzer P., Blum A., de Herdt D., Dubois D., the trial investigators Six-month trial of on-demand rabeprazole 10 mg maintains symptom relief in patients with non-erosive reflux disease. Alimentary Pharmacology and Therapeutics. 2004;20(2):181–188. doi: 10.1111/j.1365-2036.2004.01999.x. [DOI] [PubMed] [Google Scholar]
- 27.Scholten T., Dekkers C. P. M., Schütze K., Körner T., Bohuschke M., Gatz G. On-demand therapy with pantoprazole 20 mg as effective long-term management of reflux disease in patients with mild GERD: the ORION trial. Digestion. 2005;72(2-3):76–85. doi: 10.1159/000087661. [DOI] [PubMed] [Google Scholar]
- 28.Kaspari S., Kupcinskas L., Heinze H., Berghöfer P. Pantoprazole 20 mg on demand is effective in the long-term management of patients with mild gastro-oesophageal reflux disease. European Journal of Gastroenterology & Hepatology. 2005;17(9):935–941. doi: 10.1097/00042737-200509000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Jiang Y. X., Chen Y., Kong X., Tong Y. L., Xu S. C. Maintenance treatment of mild gastroesophageal reflux disease with proton pump inhibitors taken on-demand: a meta-analysis. Hepato-Gastroenterology. 2013;60(125):1077–1082. doi: 10.5754/hge11461. [DOI] [PubMed] [Google Scholar]
- 30.Boghossian T. A., Rashid F. J., Thompson W., et al. Deprescribing versus continuation of chronic proton pump inhibitor use in adults. Cochrane Database of Systematic Reviews. 2017;3, article CD011969 doi: 10.1002/14651858.CD011969.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manabe N., Yoshihara M., Sasaki A., Tanaka S., Haruma K., Chayama K. Clinical characteristics and natural history of patients with low-grade reflux esophagitis. Journal of Gastroenterology and Hepatology. 2002;17(9):949–954. doi: 10.1046/j.1440-1746.2002.02783.x. [DOI] [PubMed] [Google Scholar]
- 32.Labenz J., Nocon M., Lind T., et al. Prospective follow-up data from the ProGERD study suggest that GERD is not a categorial disease. The American Journal of Gastroenterology. 2006;101(11):2457–2462. doi: 10.1111/j.1572-0241.2006.00829.x. [DOI] [PubMed] [Google Scholar]
- 33.Fullard M., Kang J. Y., Neild P., Poullis A., Maxwell J. D. Systematic review: does gastro-oesophageal reflux disease progress? Alimentary Pharmacology & Therapeutics. 2006;24(1):33–45. doi: 10.1111/j.1365-2036.2006.02963.x. [DOI] [PubMed] [Google Scholar]
- 34.Hungin A. P., Rubin G., O'Flanagan H. Factors influencing compliance in long-term proton pump inhibitor therapy in general practice. The British Journal of General Practice. 1999;49(443):463–464. [PMC free article] [PubMed] [Google Scholar]
- 35.Hungin A. P., Rubin G. P., O'Flanagan H. Long-term prescribing of proton pump inhibitors in general practice. The British Journal of General Practice. 1999;49(443):451–453. [PMC free article] [PubMed] [Google Scholar]
- 36.Johnsson F., Moum B., Vilien M., Grove O., Simren M., Thoring M. On-demand treatment in patients with oesophagitis and reflux symptoms: comparison of lansoprazole and omeprazole. Scandinavian Journal of Gastroenterology. 2009;37(6):642–647. doi: 10.1080/00365520212499. [DOI] [PubMed] [Google Scholar]
- 37.Sjostedt S., Befrits R., Sylvan A., et al. Daily treatment with esomeprazole is superior to that taken on-demand for maintenance of healed erosive oesophagitis. Alimentary Pharmacology & Therapeutics. 2005;22(3):183–191. doi: 10.1111/j.1365-2036.2005.02553.x. [DOI] [PubMed] [Google Scholar]
- 38.Pace F., Tonini M., Pallotta S., Molteni P., Porro G. B. Systematic review: maintenance treatment of gastro-oesophageal reflux disease with proton pump inhibitors taken ‘on-demand’. Alimentary Pharmacology & Therapeutics. 2007;26(2):195–204. doi: 10.1111/j.1365-2036.2007.03381.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA flow chart for study selection.