Abstract
Little research has examined determinants of newborn telomere length, a potential biomarker of lifetime disease risk impacted by prenatal exposures. No study has examined whether maternal exposures in childhood influence newborn telomere length or whether there are sex differences in the maternal factors that influence newborn telomere length. We tested whether a range of maternal risk and protective factors in childhood and pregnancy were associated with newborn telomere length among 151 sociodemographically diverse mother-infant dyads. We further examined whether the pattern of associations differed by infant sex. Newborn telomere length was assessed from cord blood collected at birth. Risk/protective factors included maternal health (smoking, body mass index), socioeconomic status (education, income), stress exposures, and mental health (depressive and posttraumatic stress disorder symptoms) in pregnancy as well as maternal experiences of abuse (physical, emotional, sexual) and familial emotional support in childhood. When examined within the whole sample, only maternal smoking in pregnancy and familial emotional support in childhood emerged as significant predictors of newborn telomere length. Male and female newborns differed in their pattern of associations between the predictors and telomere length. Among males, maternal smoking, higher body mass index, and elevated depressive symptoms in pregnancy and maternal sexual abuse in childhood were associated with shorter newborn telomere length; higher maternal educational attainment and household income in pregnancy and greater maternal familial emotional support in childhood were associated with longer newborn telomere length. Together, these factors accounted for 34% of the variance in male newborn telomere length. None of the risk/protective factors were associated with female newborn telomere length. The results suggest that male fetuses are particularly susceptible to maternal exposure effects on newborn telomere length. These findings have implications for elucidating mechanisms contributing to sex disparities in health.
Keywords: newborn, telomere length, sex differences, cord blood, maternal exposures
1. Introduction
According to the Developmental Origins of Health and Disease theory, many human disease processes begin as early as the fetal period, even if symptoms do not emerge until late in life (Gluckman et al., 2008). Various maternal exposures have been identified as robust influencers on fetal development and, ultimately, offspring physical and mental health and neurodevelopment (Entringer et al., 2015; Van den Bergh et al., 2017). Importantly, evidence suggests that these exposures may not uniformly affect male and female offspring (Van den Bergh et al., 2017). The mechanisms underlying sex differences in outcomes related to maternal exposures in pregnancy are yet to be elucidated. Ascertaining biomarkers that capture disease vulnerability attributable to such exposures beginning in early life would inform our understanding of the earliest origins of sex differences in lifetime health and disease processes.
Newborn telomere length has emerged as a possible biomarker of the effects of maternal-fetal processes on offspring long-term health (Entringer et al., 2015). Telomeres are repeating nucleotide sequences of variable number that protect against chromosome deterioration and regulate cellular and tissue function (Blackburn and Gall, 1978). Shorter telomere length has been associated with chromosomal instability and is predictive of decreased immunocompetence, the development of chronic disease throughout life (e.g., cardiovascular disease, diabetes, obesity, inflammatory diseases, depression), abnormalities in brain structure and functioning, and earlier mortality (Baragetti et al., 2015; Factor-Litvak et al., 2016; Geronimus et al., 2015; Hochstrasser et al., 2012; Mundstock et al., 2015; Qi Nan et al., 2015; Rode et al., 2015). Telomere length at birth provides an individual’s initial telomere length setting, such that telomere length at any given time point is determined by newborn telomere length and subsequent attrition (Factor-Litvak et al., 2016; Martens et al., 2016). Therefore, processes that shorten newborn telomere length may be critical determinants of lifetime health, and newborn telomere length may be a powerful biomarker of lifetime disease risk. Notably, biomarkers of lifetime health that can be assessed in childhood are rare (Entringer et al., 2011; Entringer et al., 2013).
Limited research has explored determinants of newborn telomere length. Research among adults suggests high heritability, with a large meta-analysis estimating telomere length heritability as 0.70 (Broer et al., 2013). However, many biological variables show increased heritability with advancing age (Sillanpaa et al., 2017), such that heritability of telomere length is likely lower among newborns than adults. Moreover, data suggest that telomere dynamics during early development are largely determinative of relative telomere length for life and that environmental factors have major impact on telomere length at birth (Hjelmborg et al., 2015). To date, a number of environmental influences have been implicated (Broer et al., 2013; Entringer et al., 2015). The extant literature suggests that shorter telomere length at birth may be associated with a variety of maternal factors during pregnancy, including maternal health, socioeconomic status (SES), and stress. For example, studies have linked maternal smoking and increased maternal body mass index (BMI) during pregnancy with shorter newborn telomere length (Drury et al., 2015; Factor-Litvak et al., 2016; Martens et al., 2016). Two studies documented shorter telomere length among newborns of mothers with lower educational attainment (Martens et al., 2016; Wojcicki et al., 2016), although two others failed to find such effects (Drury et al., 2015; Factor-Litvak et al., 2016). Studies have also linked higher maternal self-reported stress during pregnancy to shorter newborn telomere length (Entringer et al., 2013; Marchetto et al., 2016; Salihu et al., 2016; Send et al., 2017). Although not yet directly tested, data suggest that maternal psychological functioning in pregnancy may also predict newborn telomere length. Internalizing symptoms/disorders (depression, anxiety, posttraumatic stress disorder) have been correlated with shorter telomere length and predict more rapid telomere length erosion across time in adults (Revesz et al., 2016; Shalev et al., 2014). Additionally, maternal depression has been associated with shortened telomere length in children, including among psychologically healthy children (Coimbra et al., 2017; Gotlib et al., 2015). Research is needed to determine if maternal mental health during pregnancy predicts newborn telomere length.
Notably, each of these factors (i.e., maternal smoking and BMI in pregnancy; maternal SES; maternal stress and internalizing symptoms/disorders in pregnancy) has been associated with mechanisms hypothesized to contribute to telomere length erosion, including elevated oxidative stress, inflammation, and hypothalamic-pituitary-adrenal axis [HPAA] activity (Choi et al., 2008; Gotlib et al., 2015; Price et al., 2013; Revesz et al., 2016; Shalev, 2012; Shiels et al., 2011; Tomiyama et al., 2012; Van den Bergh et al., 2017; von Zglinicki, 2002; Wikgren et al., 2012; Wolkowitz et al., 2011). Disrupted functioning of these key physiological systems in pregnancy may negatively impact the initial setting and on-going regulation of newborn telomere length via epigenetic and other gene-regulating processes across cells (Entringer et al., 2011; Entringer et al., 2013; Lupien et al., 2009; Shiels et al., 2011; Steptoe et al., 2011; Van den Bergh et al., 2017).
Studies have not examined whether maternal experiences prior to pregnancy predict newborn telomere length. However, several lines of research suggest that adverse maternal exposures during her childhood, specifically maltreatment, may increase risk for shortened newborn telomere length. A number of studies have correlated retrospective reports of childhood maltreatment to shorter leukocyte telomere length in adults (Boeck et al., 2017; Kananen et al., 2010; Kiecolt-Glaser et al., 2011; Price et al., 2013; Shalev et al., 2013; Tyrka et al., 2010; Vincent et al., 2017). In fact, severe stressors in childhood, including maltreatment, have greater impact on telomere length shortening than more recent stress in adults (Tyrka et al., 2010). One study in children prospectively linked exposure to violence, including physical abuse and witnessing domestic violence, to telomere attrition (Shalev et al., 2013). Moreover, childhood maltreatment predicts persistent disruptions to the key physiological systems described above, potentially influencing maternal-fetal processes in pregnancy (Lupien et al., 2009). Furthermore, maternal exposures may influence maternal oocyte telomere length beginning in childhood, thereby impacting offspring telomere length (Ozturk et al., 2014). Thus, a maternal history of maltreatment in childhood may increase risk for shortened newborn telomere length through effects on maternal oocyte telomere biology and maternal physiological stress reactivity in pregnancy. Although studies suggest that different types of maltreatment may have differential effects on telomere biology (Ridout et al., 2017; Tyrka et al., 2010; Vincent et al., 2017), the specific forms of maltreatment likely to have greatest impact on offspring newborn telomere length are unknown.
Examination of protective factors that confer resilience against telomere attrition is lacking (Ridout et al., 2017), and no study has explored whether positive maternal experiences predict longer newborn telomere length. Emotionally supportive parental caregiving in childhood has been shown to promote more optimal development and long-term functioning of stress reactivity systems (Bosquet Enlow et al., 2014). Interestingly, preliminary data suggest that increased oxytocin, a possible result of sensitive caregiving, may protect again telomere attrition (Boeck et al., 2017). Therefore, receiving supportive caregiving in a mother’s childhood may confer a protective influence on her newborn’s telomere biology through programming of key maternal physiological systems that influence maternal oocyte telomere length and maternal-fetal physiological processes. Notably, in adults, including pregnant women, greater current family social support is associated with longer telomere length, providing evidence that family support may provide a buffering effect against telomere attrition (Mitchell et al., 2017).
Although a few studies have tested for sex differences in mean newborn telomere length, with some finding no differences and others finding longer telomere length among females (Factor-Litvak et al., 2016; Martens et al., 2016; Okuda et al., 2002; Wojcicki et al., 2016), there has yet to be an examination of whether maternal factors differentially impact telomere length among male versus female infants. A body of research suggests that males and females may differ in the patterns of associations between prenatal exposures and telomere length at birth. An extensive review of studies examining the developmental effects of prenatal stress exposures found that, although both male and female fetuses are susceptible to prenatal stress effects, there are sex differences in the response to specific types of stress at different periods of gestation for different developmental outcomes (Van den Bergh et al., 2017). Also, male and female fetuses experience different patterns of cortisol exposure during gestation (DiPietro, Costigan et al., 2011), which has implications for telomere biology. Further, changes in epigenetic regulation of factors affecting fetal cortisol exposure show sex-specific effects (Gabory et al., 2009; Ostlund et al., 2016). In children and adults, sex differences in the effects of various risk factors (e.g., internalizing disorders, parental caregiving quality, family violence exposure) on telomere attrition have been documented, with males more susceptible in many, but not all studies (Drury et al., 2015; Enokido et al., 2014; Moller et al., 2009; Shalev et al., 2014; Zalli et al., 2014). Together, these data suggest that the effects of specific maternal factors on newborn telomere biology may vary by sex, but this hypothesis requires investigation.
This study sought to address the gaps noted above to help elucidate the determinants of newborn telomere length. The overall goal of this study was to test whether there are sex differences in the risk and protective factors associated with newborn telomere length. Risk factors examined included factors previously linked to newborn telomere length, including maternal physical health (smoking during pregnancy, maternal pre-pregnancy BMI), maternal SES in pregnancy (educational attainment, household income), and maternal stress exposures during pregnancy. Risk factors examined also included factors not studied to date, including maternal mental health in pregnancy (depressive and posttraumatic stress disorder symptoms) and maternal abuse in her childhood (physical, emotional, sexual). Protective factors examined included maternal familial emotional support in her childhood, also not previously studied. Finally, we examined the independent and joint contributions of risk/protective factors across categories in predicting male and female newborn telomere length.
2. Materials and methods
2.1. Participants
Participants were pregnant women enrolled in the PRogramming of Intergenerational Stress Mechanisms (PRISM) study, a prospective pregnancy cohort originally designed to recruit N = 276 mother-child dyads to examine the role of maternal and child stress exposures on child development. Between July 2011 and November 2013, pregnant women were recruited from a prenatal clinic in an urban hospital in the Northeast of the United States during the first or second trimester (27 ± 8 weeks of gestation). Eligibility criteria included: 1) English- or Spanish-speaking; 2) age ≥ 18 years at enrollment; 3) single gestation birth. Exclusion criteria included: 1) maternal endorsement of drinking ≥ 7 alcoholic drinks/week during pregnancy; 2) maternal positive HIV status, which would influence/confound biomarkers of interest. Approximately 9 months into recruitment, additional funding was obtained to collect cord blood at delivery. Among women enrolled in PRISM who had not yet delivered when this additional funding was obtained, 151 provided usable cord blood. Based on screening data, there were no differences in race/ethnicity, education, or income between women who enrolled in PRISM and those who declined. Within the PRISM cohort, compared to mothers who were not included in the current analyses, mothers who were included were more likely to be White, Black, or Other race and less likely to be Hispanic and had on average higher educational attainment and annual income during pregnancy, ps < .001. There were no differences between families who did and did not participate on maternal age at enrollment or infant sex, gestational age, or birthweight, ps > .10.
2.2. Measures
2.2.1. Maternal physical health during pregnancy (predictors).
Maternal pregnancy physical health variables included maternal cigarette smoking during pregnancy and maternal pre-pregnancy BMI. Smoking during pregnancy was categorized as yes/no based on maternal self-report of smoking at recruitment and/or in the third trimester. BMI was calculated by dividing maternal self-reported pre-pregnancy weight (kg) by height squared (meter), a validated method for estimating pre-pregnancy BMI (Wright et al., 2013).
2.2.2. Maternal socioeconomic characteristics during pregnancy (predictors).
Mothers self-reported their highest level of education and annual household income during pregnancy. Education level was scored as an ordinal variable as follows: completion of high school/GED or less, some college, college degree, and graduate degree. Annual household income was scored as an ordinal variable as follows: less than $20,000, $20,000-$34,999, $35,000-$59,999, $60,000-$99,999, and $100,000 or more.
2.2.3. Maternal mental health during pregnancy (predictors).
2.2.3.1. Posttraumatic stress disorder (PTSD) symptoms.
Maternal PTSD symptoms during pregnancy were assessed using the Posttraumatic Stress Disorder Checklist-Civilian Version (PCL-C) (Weathers et al., 1993), a 17-item self-report measure that reflects DSM-IV criteria for PTSD. Mothers rated each symptom on a five-point Likert scale in terms of how much the symptom had bothered them in the past month, with item scores ranging from 1 (not at all) to 5 (extremely). The PCL-C provides a single symptom score based on number and severity of PTSD symptoms (possible range 17–85). The PCL-C has high internal consistency for the total scale and good test-retest reliability and convergent validity with a number of other PTSD scales and with the Clinician-Administered PTSD Scale, a structured clinical interview for PTSD.
2.2.3.2. Depressive symptoms.
Maternal depressive symptoms over the previous 7 days were assessed using the Edinburgh Postnatal Depression Scale (EPDS) (Gibson et al., 2009). The EPDS is a 10-item self-report questionnaire specifically designed to measure the presence of depressive symptoms in mothers during the perinatal period. Each EPDS item is scored for severity from 0 to 3 and then summed to provide a total score (possible range 0–30). Additionally, a previously validated (Gibson et al., 2009) cutoff score of 13 was used to obtain a likely clinical diagnosis of major depression, categorizing mothers into a “non-elevated” (score < 12) or an “elevated” (score ≥ 13) depressive symptoms group. The non-elevated group (n = 121) had a mean total EPDS score of 4.54, SD = 3.99, and the elevated group (n = 23) had a mean score of 16.78, SD = 3.36. The EPDS has demonstrated high internal consistency and validity for detecting major depression in the perinatal period (Gibson et al., 2009).
2.2.4. Maternal stress exposures during childhood and pregnancy (predictors).
2.2.4.1. Childhood abuse exposures.
Mothers’ experiences of abuse during her childhood were assessed using the Childhood Trauma Questionnaire (CTQ)-short form (Bernstein et al., 1994),which inquires about experiences up to age 11 years. The CTQ has demonstrated excellent test-retest reliability and validity, including among racially/ethnically mixed community samples (Scher et al., 2001).Two items assess emotional abuse, three items assess physical abuse, and two items assess sexual abuse. Items were answered on a five-point scale including 0 (never), 1 (rarely), 2 (sometimes), 3 (often), and 4 (very often). Relevant item scores were summed to create total subscale scores for emotional abuse, physical abuse, and sexual abuse to be tested separately, given that data among adults suggest different types of maltreatment may have differential effects on telomere biology (Ridout et al., 2017; Tyrka et al., 2010; Vincent et al., 2017). In addition, all item scores were summed to create a total child abuse exposure score, given research suggesting that exposure to more types of childhood adversity may be associated with larger telomere length effects (Price et al., 2013; Ridout et al., 2017; Shalev et al., 2013). Higher scores indicate greater exposure to abuse.
2.2.4.2. Stress exposures during pregnancy.
Maternal stress exposures during pregnancy were assessed using the Crisis in Family Systems-Revised (CRISYS-R) survey, which inquires about exposure to negative life events during the prior six months (Berry et al., 2001). The CRISYS-R is suitable for sociodemographically diverse populations, has good test/retest reliability, has been validated in English and Spanish in samples of parents, and has been utilized in several studies as a measure of prenatal stress (Enlow et al., 2017). The 80-item survey encompasses 11 domains (financial, legal, career, stability in relationships, medical issues pertaining to self, medical issues pertaining to others, safety in the community, safety in the home, housing problems, difficulty with authority, discrimination), with multiple items assessing each domain. Mothers rated endorsed items as positive, negative, or neutral events. Research suggests increased vulnerability when exposed to negative events across multiple domains, including for telomere biology (Enlow et al., 2017; Ridout et al., 2017). Thus, the number of domains with one or more negative events endorsed was summed to create a negative life events domain score (possible range 0–11), as done in prior research (Enlow et al., 2017). Higher scores indicate greater exposure to stressors.
2.2.5. Maternal familial emotional support during childhood (predictor).
Mothers’ experiences of familial emotional support during their childhood (up to age 11 years) were assessed using the CTQ-short form (Bernstein et al., 1994) (described in 2.2.4.1). The five family support items inquire about the respondent’s feelings of being emotionally supported and close with family members during childhood. Items were rated on a five-point scale including 0 (never), 1 (rarely), 2 (sometimes), 3 (often), and 4 (very often). Item scores were summed to create a total childhood familial emotional support score (possible range 0–20). Higher scores indicate greater familial emotional support in childhood.
2.2.6. Cord blood telomere length (outcome).
Newborn telomere length was assessed from banked cord blood leukocyte DNA, a valid index of newborn telomere length (Entringer et al., 2013; Okuda et al., 2002). Cord blood samples were collected at delivery in EDTA-tubes, centrifuged to obtain buffy coat fraction, and stored at −80°C until DNA extraction.DNA extraction was conducted using the Promega Wizard DNA extraction system (Madison, WI, USA).Samples were assayed at the University of Milan (PI Bollati). Telomere length was determined using real-time polymerase chain reaction (PCR), which requires a small amount of DNA. A recent meta-analysis determined that this method is a valid technique for quantifying telomere length (Ridout et al., 2017). Telomere length was measured using the quantitative real-time method described by Cawthon (Cawthon, 2002, 2009). For each sample, the amplification of telomere and β-globin (gene present in single copy) were run in triplicate in two plates. Telomere length is represented by the ratio between the average of three values obtained from telomere amplification and from β-globin amplification (T/S ratio). Details regarding the telomere assaying procedure are provided in the Supplementary Material, Telomere Assaying Procedures. Because PCR provides a relative measure of telomere length, the acronym rTL (for “relative telomere length”) is utilized when describing the current analyses.
2.2.7. Covariates.
Maternal age, race/ethnicity, pregnancy health conditions (preeclampsia/eclampsia, gestational diabetes), and newborn gestational age and birthweight were considered as potential covariates in analyses. Maternal age was treated as a continuous variable. Maternal self-reported race/ethnicity was categorized as White, Black, Hispanic, or other. Maternal diagnoses of preeclampsia, eclampsia, and gestational diabetes were extracted from medical records and coded as present/absent. Newborn gestational age and birthweight were extracted from medical records and treated as continuous variables.
2.3. Procedures
Participant sociodemographics were assessed shortly following recruitment (M = 19.4 weeks gestation, SD = 8.6 weeks gestation). Within two weeks of enrollment, trained research assistants administered the study questionnaires as in-person interviews. Cord blood samples were collected at delivery. Study procedures were approved by the relevant institutions’ human studies ethics committees (Brigham and Women’s Hospital/Partners HealthCare, Beth Israel Deaconess Medical Center). Mothers provided written informed consent in their preferred language.
2.4. Data Analytic Plan
Data analyses proceeded in several steps. First, descriptive data were calculated. Next, analyses tested for sex differences in cord blood rTL and the risk and protective predictor factors. Then, associations between the predictors and cord blood rTL were examined in the sample as a whole and stratified by infant sex. Differences in the correlation estimates between male and female infants were determined using correlation coefficient difference tests via the Fisher-to-z transformation. Finally, a series of predictive models, via structural equation modeling (SEM), were run to examine independent and joint contributions of risk/protective factors on cord blood rTL for male versus female infants. Specifically, data for both males and females were estimated simultaneously using two steps: (a) freely estimated parameters and (b) parameters constrained to be equivalent between the sexes. In the latter instance, chi-square difference tests were estimated, with significant differences signaling model misfit and, consequently, significant differences in the predictive estimates between male and female infants (Byrne, 1989). To increase confidence regarding the stability of estimated parameters, all estimates of slopes were bootstrapped using 1,000 replications (Efron, 1979). Due to the non-normality of some of the data distributions, robust standard errors were estimated using maximum likelihood. Pairwise deletion was adopted, as the number of missing cases was, on average, only 5 per model run. Analyses were conducted using SPSS v23 and Mplus v8.
3. Results
Table 1 details the sample characteristics. The sample was sociodemographically diverse in terms of maternal and child race/ethnicity, maternal educational attainment, annual household income, and maternal marital status. The infants were primarily of normal birthweight (M = 3347 grams, SD = 492 grams; 93% born greater than 2500 grams) and born full-term (M = 39.19 weeks, SD = 1.50 weeks; 93% born 37 weeks or later); 5.3% and 9.3% of the mothers were diagnosed with preeclampsia/eclampsia and gestational diabetes, respectively. The mean cord blood T/S ratio for the full sample was 2.48 (SD = 0.75); the values were normally distributed with no outliers. Cord blood rTL did not differ between male (M = 2.45, SD = 0.74) and female (M = 2.52, SD = 0.76) infants, t(149) = −0.62, p = .538. There were also no significant differences between male and female infants on any of the predictor variables, gestational age, birthweight, sociodemographic characteristics, or potential covariates, all ps ≥ .05. Table 2 presents the inter-correlations among the predictor variables.
Table 1.
Sample characteristics (N = 151)
| na | % | M | SD | |
|---|---|---|---|---|
| Maternal age (years) | 31.53 | 5.00 | ||
| Maternal race/ethnicity | ||||
| White |
68 | 45 | ||
| Black/Haitian | 51 | 34 | ||
| Hispanic | 13 | 9 | ||
| Otherb | 19 | 13 | ||
| Child race/ethnicity | ||||
| White | 57 | 38 | ||
| Black/Haitian | 54 | 36 | ||
| Hispanic | 18 | 12 | ||
| Otherb | 22 | 15 | ||
| Maternal relationship status | ||||
| Married | 102 | 68 | ||
| Living together | 16 | 11 | ||
| Otherc | 31 | 21 | ||
| Maternal education | ||||
| High school/GED or less | 17 | 11 | ||
| Some College | 35 | 23 | ||
| College Degree | 41 | 27 | ||
| Graduate Degree | 56 | 37 | ||
| Annual household income | ||||
| < $20,000 | 27 | 18 | ||
| $20,000-$34,999 | 14 | 9 | ||
| $35,000-$59,999 | 27 | 18 | ||
| $60,000-$99,999 | 26 | 17 | ||
| $100,000+ | 53 | 35 | ||
| Maternal pre-pregnancy body mass index (BMI), kg/m2 | 25.72 | 6.55 | ||
| Maternal cigarette smoking in pregnancy | 32 | 21 | ||
| Maternal posttraumatic stress symptoms in pregnancy (PCL-C) | 23.45 | 11.49 | ||
| Maternal depressive symptoms in pregnancy (continuous, EPDS) | 6.49 | 5.95 | ||
| Maternal elevated depressive symptoms in pregnancy (categorical, EPDS) | 23 | 15 | ||
| Maternal physical abuse in her childhood (CTQ-SF) | 1.52 | 2.54 | ||
| Maternal emotional abuse in her childhood (CTQ-SF) | 2.25 | 2.03 | ||
| Maternal sexual abuse in her childhood (CTQ-SF) | 0.36 | 1.16 | ||
| Maternal total abuse in her childhood (CTQ-SF) | 4.11 | 4.84 | ||
| Maternal stress exposures during pregnancy (CRISYS-R) | 2.34 | 1.95 | ||
| Maternal familial emotional support in her childhood (CTQ-SF) | 14.52 | 2.42 | ||
| Newborn sex (male) | 83 | 55 | ||
| Cord blood relative telomere length (T/S ratio)d | 2.48 | 0.75 |
Note. PCL-C= Posttraumatic Stress Disorder Checklist-Civilian Version; EPDS = Edinburgh Postnatal Depression Scale; CTQ-SF = Childhood Trauma Questionnaire, Short Form; CRISYS-R = Crisis in Family Systems-Revised.
Data were missing for 0 to 11 participants across study variables.
The majority categorized as “other” race/ethnicity were identified by self/mother as Asian or multi-racial.
Other included never married (n = 23), divorced (n = 2), separated (n = 1), and other relationship status (n = 5).
Telomere length is represented by the ratio between the average of three values obtained from telomere amplification and from β-globin amplification (T/S ratio).
Table 2.
Correlation coefficients among predictor variables (maternal characteristics) for entire sample (N = 151)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Maternal smoking during pregnancy | -- | ||||||||||||
| 2. Maternal body mass index (BMI), kg/m2 | .03 | -- | |||||||||||
| 3. Maternal educational attainment | −.21* | −23** | -- | ||||||||||
| 4. Annual household income in pregnancy | −.07 | −.24** | .68*** | -- | |||||||||
| 5. Posttraumatic stress symptoms (PCL-C) | .09 | .20* | −.18* | −.28** | -- | ||||||||
| 6. Depressive symptoms (continuous, EPDS) | .05 | .07 | −.21* | 27** | 31*** | -- | |||||||
| 7. Elevated depressive symptoms (categorical, EPDS) | .09 | .21* | −.15 | −.13 | 27** | 76*** | -- | ||||||
| 8. Physical abuse in childhood (CTQ-SF) | .03 | .09 | −23** | 33*** | 2*** | .18* | .04 | -- | |||||
| 9. Emotional abuse in childhood (CTQ-SF) | .06 | .04 | −.13 | −.19* | 27** | .14 | .13 | .54*** | -- | ||||
| 10. Sexual abuse in childhood (CTQ-SF) | −.02 | .13 | −.18* | −.21* | .34*** | .06 | .02 | 35 *** | .24** | -- | |||
| 11. Total abuse in childhood (CTQ-SF) | .04 | .08 | −.19* | −29** | 37*** | .18* | .07 | .79*** | .88*** | .43*** | -- | ||
| 12. Stress exposures in pregnancy (CRISYS) | .08 | .12 | .32*** | .45*** | .31*** | .33*** | .23** | .35*** | .32*** | .19* | .37*** | -- | |
| 13. Familial emotional support in childhood (CTQ-SF) | .03 | −.11 | .20* | .16 | .06 | −.11 | −.10 | −.17* | −.27** | −.12 | −.24** | −.07 | -- |
| 14. Maternal age | .02 | .10 | .25** | .36*** | −.13 | −.17* | −.09 | −.07 | −.06 | .04 | −.08 | −.16* | −.03 |
Note. PCL-C= Posttraumatic Stress Disorder Checklist-Civilian Version; EPDS = Edinburgh Postnatal Depression Scale; CTQ-SF = Childhood Trauma Questionnaire, Short Form; CRISYS-R = Crisis in Family Systems-Revised. Pearson’s correlation coefficients were calculated when both variables were continuous and normally distributed; Spearman’s correlation coefficients when both variables were continuous and at least one was non-normally distributed and/or ordinal; point biserial correlations when one variable was dichotomous and the other was continuous; and phi correlation when both variables were dichotomous.
p < .05.
p < .01.
p < .001.
When the sample was considered as a whole, cord blood rTL was negatively associated with maternal smoking in pregnancy and positively associated with maternal familial emotional support in childhood (Table 3). Cord blood rTL was not associated with maternal pre-pregnancy BMI or indicators of maternal SES, mental health in pregnancy, or stress exposures in childhood or pregnancy. Cord blood rTL was also not associated with any of the covariates (maternal age, race/ethnicity, preeclampsia/eclampsia, or gestational diabetes).
Table 3.
Correlation coefficients between predictor variables and cord blood relative telomere length for entire sample and split by infant sex
| Entire Sample N = 151 |
Males n = 83 |
Females n = 68 |
|||||
|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | Z-test | |
| Maternal health in pregnancy | |||||||
| Maternal smoking during pregnancy | −.18 | .025 | −.28 | .011 | −.05 | .706 | −1.423 |
| Maternal body mass index (BMI), kg/m2 | −.12 | .155 | −.28 | .011 | .09 | .501 | −2.263* |
| Socioeconomic status in pregnancy | |||||||
| Maternal educational attainment | .14 | .096 | .37 | .001 | −.12 | .335 | 3.048* |
| Annual household income in pregnancy | .07 | .436 | .32 | .004 | −.22 | .072 | 3.325* |
| Maternal mental health in pregnancy | |||||||
| Posttraumatic stress symptoms (PCL-C) | −.12 | .166 | −.22 | .056 | −.02 | .855 | −1.220 |
| Depressive symptoms (continuous, EPDS) | .06 | .504 | −.08 | .470 | .21 | .099 | −1.757 |
| Elevated depressive symptoms (categorical, EPDS) | −.08 | .317 | −.24 | .030 | .16 | .206 | −2.432* |
| Maternal stress exposures in her childhood and in pregnancy | |||||||
| Physical abuse in childhood (CTQ-SF) | −.03 | .710 | −.03 | .797 | −.04 | .772 | 0.060 |
| Emotional abuse in childhood (CTQ-SF) | −.05 | .569 | −.05 | .657 | −.06 | .658 | 0.060 |
| Sexual abuse in childhood (CTQ-SF) | −.13 | .143 | −.31 | .007 | .05 | .718 | −2.219* |
| Total abuse in childhood (CTQ-SF) | −.07 | .388 | −.11 | .337 | −.04 | .770 | −0.422 |
| Stress exposures in pregnancy (CRISYS-R) | .05 | .516 | −.03 | .817 | −.02 | .855 | −0.060 |
| Maternal support in her childhood | |||||||
| Familial emotional support in childhood (CTQ-SF) | .24 | .004 | .34 | .003 | .13 | .284 | 1.338 |
| Control variables | |||||||
| Maternal age | −.11 | .171 | −.06 | .618 | −.18 | .132 | 0.730 |
Note. PCL-C= Posttraumatic Stress Disorder Checklist-Civilian Version; EPDS = Edinburgh Postnatal Depression Scale; CTQ-SF = Childhood Trauma Questionnaire, Short Form; CRISYS-R = Crisis in Family Systems-Revised. Telomere length was normally distributed. Pearson’s correlation coefficients were calculated when both variables were continuous and normally distributed; Spearman’s correlation coefficients when both variables were continuous and at least one was non-normally distributed and/or ordinal; point biserial correlations when one variable was dichotomous and the other was continuous; and phi correlation when both variables were dichotomous. Bolded correlation coefficient values are significant at p < .05, two-tailed. Z-test is a result of the transformation of the difference between the correlation coefficients for male and female infants using the Fisher-to-z transformation;
indicates that the magnitude of the correlation coefficients was significantly different between male and female infants using a two-tailed test.
When the sample was stratified by infant sex, significant correlations with cord blood rTL emerged for several predictors among male infants (Table 3). Maternal smoking in pregnancy, greater maternal pre-pregnancy BMI, elevated maternal depressive symptoms (categorical) in pregnancy, and more severe maternal sexual abuse in childhood were each associated with shorter cord blood rTL; greater maternal education, higher household income during pregnancy, and greater maternal familial emotional support in childhood were each associated with longer cord blood rTL. The association between greater maternal PTSD symptoms in pregnancy and shorter cord blood rTL approached significance. Among female infants, cord blood rTL was not significantly associated with any of the predictor variables. Using the Fisher-to-z transformation, significant differences in correlation coefficients were observed between male and female infants on maternal pre-pregnancy BMI, educational attainment, household income during pregnancy, depressive symptoms (categorical) in pregnancy, and sexual abuse in childhood; in all instances, the correlation coefficients were significantly more pronounced among male infants.
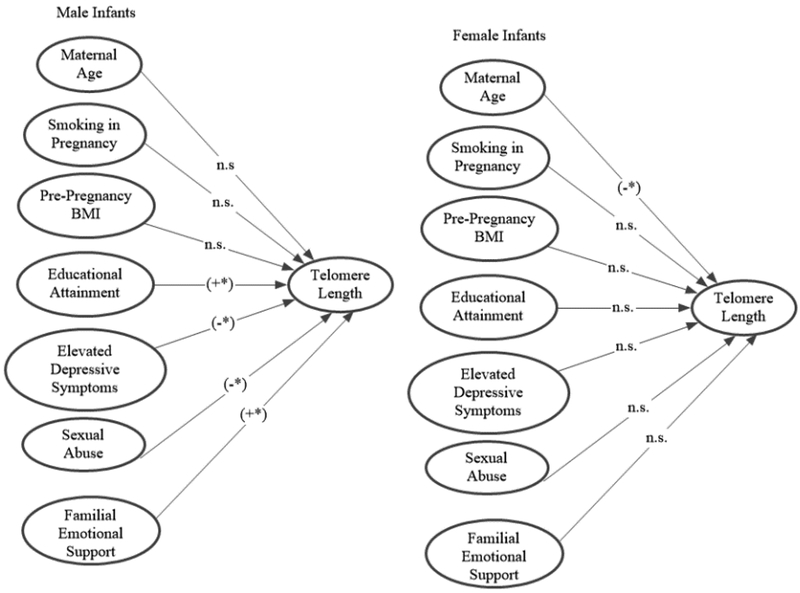
A series of predictive SEM models using robust standard errors that controlled for maternal age1 tested the independent effects of the significant predictors in the bivariate analyses on cord blood rTL among male and female infants. Table 4a presents the detailed results for males and Table 4b for females, including chi-square tests of invariance. Steps (termed Models in Tables 4a and 4b) were ordered from variables hypothesized to have the most direct effect on newborn rTL (maternal pregnancy health) to the most indirect effect (maternal childhood exposures). In the first step, maternal age was included as a control variable. The second step included the pregnancy health variables, maternal smoking and pre-pregnancy BMI, which resulted in a significant prediction for males, R2=14%, but not for females, R2=5%. Chi-square tests of invariance2 indicated that males’ and females’ coefficients were associated with differential model fit in a one-tailed test. The third step included maternal SES in pregnancy. Because maternal educational attainment and annual household income were highly associated (rs = .68), raising concerns about multi-collinearity, only maternal educational attainment was included in this step. Educational attainment was chosen over income given that education has been described as an indicator of an individual’s long-term SES trajectory and thus may represent a cumulative, robust measure of SES across time compared to a “snapshot” measure such as current income (Steptoe et al., 2011). Adding maternal educational attainment to the model resulted in a significant prediction among males, R2 = 23%, and no change among females, R2 = 5%. The fourth step added maternal mental health in pregnancy, specifically elevated depressive symptoms (categorical); this model increased the prediction for males by another 3% and for females 4% to a total R2 of 26% among males and 9% among females. For females, the inclusion of maternal depressive symptoms was associated with a significant trend when using a one-tailed test. Chi-square tests of invariance indicated that males’ and females’ coefficients were associated with differential model fit in a two-tailed test. The fifth step added maternal abuse in childhood, specifically sexual abuse, which resulted in an overall prediction for males of 30% and for females of 8%. Sexual abuse was a significant predictor for male but not for female infants. Again, invariance between males and females was not supported, with the chi-square test positing equal slopes between males and females showing significant misfit. The sixth and final step added maternal familial emotional support in childhood, increasing the predictive ability of the model for males to explaining 34% of the variance in cord blood rTL; the respective percentage for females was 10%. The sex difference in the slopes in this model was significant using the chi-square fixed slopes discrepancy test. Pictorial representations of the final model (Model 6) and all models (Model 1–6) are presented in Figure 1 and in the Supplementary Material, Figure S1, respectively. All significant effects of slope estimates were associated with 95% confidence intervals that did not contain the value of zero; thus, the present findings are likely robust and reflective of true population effects.
Table 4a.
Summary of structural equation models for maternal variables predicting cord blood telomere length among male infants
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | β | p | B | SE B | β | p | B | SE B | β | p | |
| 1. Maternal age | −.002 | .02 | −.02 | .889 | −.011 | .02 | −.07 | .481 | −.03 | .02 | −.23 | .025* |
| 2. Pregnancy health | ||||||||||||
| 2a. Smoking in pregnancy | −.44 | .18 | −.25 | .012* | −.22 | .18 | −.13 | .205 | ||||
| 2b. Pre-pregnancy BMI | −.03 | .01 | −.25 | .012* | −.02 | .01 | −.19 | .067† | ||||
| 3. Socioeconomic status in pregnancy | ||||||||||||
| Educational attainment | .23 | .08 | .32 | .004* | ||||||||
| 4. Mental health in pregnancy | ||||||||||||
| Elevated depressive symptoms | ||||||||||||
| 5. Stress exposures in childhood | ||||||||||||
| Sexual abuse | ||||||||||||
| 6. Support in childhood | ||||||||||||
| Familial emotional support | ||||||||||||
| Adjusted R2 | .00 | .14 | .23 | |||||||||
| Chi-square test of invariance by infant sex | 1.332 | 6.343† | 9.347† | |||||||||
| Model 4 | Model 5 | Model 6 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | β | P | B | SE B | β | p | B | SE B | β | p | |
| 1. Maternal age | −.03 | .02 | −.22 | .030* | −.03 | .02 | −.22 | .043* | −.03 | .02 | −.18 | .100 |
| 2.Pregnancy health | ||||||||||||
| 2a. Smoking in pregnancy | −.17 | .18 | −.10 | .322 | −.19 | .17 | −.12 | .254 | −.19 | .16 | −.12 | .237 |
| 2b. Pre-pregnancy BMI | −.01 | .01 | −.12 | .272 | −.01 | .01 | −.13 | .222 | −.01 | .01 | −.10 | .360 |
| 3. Socioeconomic status in pregnancy | ||||||||||||
| Educational attainment | .26 | .08 | .36 | .001* | .20 | .08 | .29 | .015* | .17 | .08 | .25 | .037* |
| 4. Mental health in pregnancy | ||||||||||||
| Elevated depressive symptoms | −.34 | .18 | −.19 | .060† | −.38 | .19 | −.21 | .038* | −.36 | .18 | −.20 | .044* |
| 5. Stress exposures in childhood | ||||||||||||
| Sexual abuse | −.22 | .07 | −.32 | .001* | −.19 | .07 | −.27 | .005* | ||||
| 6. Support in childhood | ||||||||||||
| Familial emotional support | .06 | .03 | .20 | .048* | ||||||||
| Adjusted R2 | .26 | .30 | .34 | |||||||||
| Chi-square test of invariance by infant sex | 15.108* | 19.297* | 38.645* | |||||||||
Note: BMI = body mass index. Estimates and standard errors were bootstrapped using 1,000 replications. Symmetric confidence intervals were computed at 95%, provided that the variables were normally distributed. Whenever the 95% confidence interval around a given slope did not include the value of zero, it was concluded that the observed coefficient was significantly different from zero.
p < .05, one-tailed significance.
p < .05, two-tailed significance.
Table 4b.
Summary of structural equation model for maternal variables predicting cord blood telomere length among female infants
| Model 1 | Model 2 | Model 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | β | p | B | SE B | β | p | B | SE B | β | p | |
| 1. Maternal age | −.032 | .02 | −.20 | .081† | −.034 | .02 | −.22 | .070† | −.03 | .02 | −.21 | .108 |
| 2.Pregnancy health | ||||||||||||
| 2a. Smoking in pregnancy | −.03 | .24 | −.02 | .888 | −.04 | .25 | −.02 | .866 | ||||
| 2b. Pre-pregnancy BMI | .01 | .01 | .05 | .704 | .01 | .01 | .04 | .753 | ||||
| 3. Socioeconomic status in pregnancy | ||||||||||||
| Educational attainment | −.02 | .09 | −.03 | .835 | ||||||||
| 4. Mental health in pregnancy | ||||||||||||
| Elevated depressive symptoms | ||||||||||||
| 5. Stress exposures in childhood | ||||||||||||
| Sexual abuse | ||||||||||||
| 6. Support in childhood | ||||||||||||
| Familial emotional support | ||||||||||||
| Adjusted R2 | .04 | .05 | .05 | |||||||||
| Chi-square test of invariance by infant sex | 1.332 | 6.343† | 9.347† | |||||||||
| Model 4 | Model 5 | Model 6 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | SE B | β | P | B | SE B | β | p | B | SE B | β | p | |
| 1. Maternal age | −.04 | .02 | −.24 | .072† | −.04 | .02 | −.26 | .059† | −.04 | .02 | −.28 | .041* |
| 2. Pregnancy health | ||||||||||||
| 2a. Smoking in pregnancy | .04 | .24 | .02 | .872 | .05 | .24 | .03 | .831 | .01 | .24 | .01 | .962 |
| 2b. Pre-pregnancy BMI | .01 | .01 | .02 | .876 | .01 | .01 | .01 | .920 | .01 | .01 | .02 | .863 |
| 3. Socioeconomic status in pregnancy | ||||||||||||
| Educational attainment | .03 | .10 | .04 | .792 | .05 | .10 | .07 | .638 | .04 | .10 | .06 | .702 |
| 4. Mental health in pregnancy | ||||||||||||
| Elevated depressive symptoms | .48 | .29 | .21 | .096† | .45 | .31 | .18 | .146 | .47 | .31 | .19 | .128 |
| 5. Stress exposures in childhood | ||||||||||||
| Sexual abuse | .04 | .07 | .07 | .621 | .04 | .07 | .08 | .549 | ||||
| 6. Support in childhood | ||||||||||||
| Familial emotional support | .04 | .04 | .12 | .377 | ||||||||
| Adjusted R2 | .09 | .08 | .10 | |||||||||
| Chi-square test of invariance by infant sex | 15.108* | 19.297* | 38.645* | |||||||||
Note: BMI = body mass index. Estimates and standard errors were bootstrapped using 1,000 replications. Symmetric confidence intervals were computed at 95%, provided that the variables were normally distributed. Whenever the 95% confidence interval around a given slope did not include the value of zero, it was concluded that the observed coefficient was significantly different from zero.
p < .05, one-tailed significance.
p < .05, two-tailed significance.
Figure 1.
Paths in the prediction of cord blood telomere length by infant sex for final predictive structural equation model (Model 6) for male and female infants. Paths marked by (+*) were significant and positively associated with cord blood telomere length in the final model. Paths marked by (-*) were significant and negatively associated with cord blood telomere length in the final model. Paths marked by “n.s.” were not significant in the final model.
4. Discussion
The overall goal of this study was to examine whether maternal exposures are differentially associated with newborn telomere length, assessed from cord blood, between male and female infants. Maternal exposures tested comprised a range of domains hypothesized to be associated with newborn telomere length, including maternal health behaviors, SES, and stress exposures in pregnancy. An additional goal of this study was to consider maternal exposures not yet considered in the newborn telomere literature, including maternal mental health in pregnancy and maternal childhood experiences. We tested associations of newborn telomere length with different types of maternal abuse in childhood (physical, emotional, sexual). We also considered maternal familial emotional support in childhood as a potential protective factor on newborn telomere length; to date, studies have focused almost exclusively on risk factors and neglected consideration of resilience factors.
There were several significant findings. First, maternal exposures across risk domains were associated with cord blood rTL among male but not female newborns. Specifically, maternal smoking in pregnancy, greater maternal pre-pregnancy BMI, lower SES (educational attainment, household income), elevated depressive symptoms in pregnancy, and more severe sexual abuse in childhood were each associated with shorter cord blood rTL, and greater maternal familial emotional support in childhood was associated with longer cord blood rTL among male infants. Among female infants, none of the identified risk or protective factors were associated with cord blood rTL. Moreover, the magnitude of the correlation coefficients between the majority of these risk/protective factors and cord blood rTL was significantly more pronounced among male compared to female infants, providing further evidence of sex differences in the impact of maternal exposures on newborn telomere length. Notably, when the sample was considered as a whole (i.e., male and females together), only maternal smoking during pregnancy and familial emotional support in childhood emerged as significant predictors of cord blood rTL, highlighting the importance of considering sex differences in environmental exposure effects on newborn telomere length. Lack of consideration of sex differences in prior research on newborn telomere length determinants may have contributed to inconsistencies in findings across studies.
A series of SEM models tested the independent and joint effects of the significant predictors on cord blood rTL among male and female infants. The final model, which included maternal age (control variable), maternal smoking in pregnancy, pre-pregnancy BMI, educational attainment, elevated depressive symptoms in pregnancy, sexual abuse in childhood, and familial emotional support in childhood, accounted for 34% of the variance in cord blood rTL among male infants and 10% among female infants. The sex difference in the slopes in this model was significant, indicating that the model performed differently between males and females. In the final model, maternal SES (i.e., educational attainment), elevated depressive symptoms in pregnancy, sexual abuse in childhood, and familial emotional support in childhood were independent significant predictors among males; among females, only maternal age (control variable) was significant. The results suggest that male fetuses are acutely susceptible to the effects of a variety of maternal exposures on telomere biology. These findings are consistent with other studies that have documented sex differences in fetal responses to prenatal exposures and highlight the particular vulnerability of male fetuses (Doyle et al., 2015; Van den Bergh et al., 2017). The findings are also consistent with the limited studies in adults indicating that males experience more accelerated telomere attrition in response to psychological distress, reduced social support, and early life adversity (Shalev et al., 2014; Zalli et al., 2014).
The mechanisms via which the examined exposures influence newborn telomere length are as yet unknown. Potential mechanisms include disruptions to maternal HPAA reactivity and increased oxidative stress and inflammation, epigenetic changes resulting in altered gene expression, mitochondrial dysfunction, and telomerase inactivation due to heightened and prolonged stress signaling (Entringer et al., 2011; Entringer et al., 2013; Lupien et al., 2009; Shiels et al., 2011; Steptoe et al., 2011; Van den Bergh et al., 2017). Importantly, each of the risk factors identified in this study has been associated with disruptions in stress physiology systems, including during pregnancy, and thus may have contributed to the observed effects. Research is needed to elucidate the likely varied biological mechanisms that contribute to newborn telomere biology and link maternal exposures to offspring telomere attrition.
Findings from a number of lines of research suggest several factors that may contribute to the increased male vulnerability to maternal exposures observed in this study. First, studies suggest sex differences in the effects of maternal stress reactivity during pregnancy on various aspects of fetal development (Davis et al., 2013; Doyle et al., 2015; Gabory et al., 2009; Ostlund et al., 2016). Specifically, fetal sex appears to moderate the production of maternal cortisol over the second half of pregnancy (DiPietro et al., 2011), and male and female fetuses show different strategies for adapting to exposure to stress hormones that result in sex differences across a range of outcomes (Davis et al., 2013). Importantly, exposure to increased cortisol has been implicated as a mechanism in accelerated telomere shortening (Choi et al., 2008; Entringer et al., 2011). Therefore, factors that influence functioning of the maternal HPAA, including the variables studied in the current analyses, may have sex-specific effects on fetal telomere biology. There is also evidence of sex-specific differences in placental responsivity to maternal distress, with males less likely to make adaptations to adverse in utero environments in placental gene and protein expression (Bale et al., 2010; Clifton, 2010; Doyle et al., 2015; Mueller and Bale, 2008; Stark et al., 2009). Some suggest that these processes contribute to greater male vulnerability across a range of fetal and child outcomes (Clifton, 2010; Doyle et al., 2015). Research is needed to determine if such processes apply to telomere biology. In addition, epigenetic processes occurring early in embryonic development may contribute to sex-specific patterns of telomere attrition on sex chromosomes. For example, in a study utilizing umbilical cord blood lymphocytes, Perner and colleagues (Perner et al., 2003) found significant sex differences in the lengths of the Xq-telomeres, derived from the maternal germline, with males’ telomeres 1100 base pairs shorter than that of females. However, research has been mixed as to whether telomere length is maternally inherited via an X-linked mechanism (Broer et al., 2013). Also, because the method used in the current analyses to quantify telomere length provides an average measure of telomere length across all chromosomes within all cells present in the sample, the proportion of variance in telomere length explained by varying length of the telomeres of sex chromosomes is likely small. Future studies should aim to explicate the biological mechanisms that contribute to sex differences in vulnerability to maternal exposures on newborn telomere attrition.
In the current study, maternal race/ethnicity was not associated with newborn telomere length in bivariate analyses and did not independently contribute to newborn telomere length in the SEM models. To date, race/ethnicity differences in telomere length have varied widely in nature and degree across studies of infants as well as children and adults (Drury et al., 2015; Factor-Litvak et al., 2016; Martens et al., 2016; Needham et al., 2012; Okuda et al., 2002). Race/ethnicity may contribute to more complex patterns of associations among maternal risk factors and newborn telomere length. Further exploration of the potential nature of race/ethnicity effects on newborn telomere length was beyond the scope of this study but should be pursued in future research. Notably, in the current sample, several of the examined risk factors were present at higher rates among racial/ethnic minority participants, as documented in other studies (Geronimus et al., 2015). Research is needed to disentangle whether any observed differences in telomere length among racial/ethnic groups are attributable to different distributions of risk factors associated with shortened telomere length or to other factors.
This study offers a number of strengths. It is one of a few studies to explore determinants of newborn telomere length and the first to consider maternal mental health during pregnancy and trauma exposures in childhood, specifically different types of childhood abuse. Previous studies have shown links between maternal stress in pregnancy and newborn telomere length (Entringer et al., 2013; Marchetto et al., 2016; Salihu et al., 2016; Send et al., 2017) and, albeit inconsistently, between childhood maltreatment and adult telomere length (Boeck et al., 2017; Kananen et al., 2010; Kiecolt-Glaser et al., 2011; Price et al., 2013; Tyrka et al., 2010; Vincent et al., 2017). This is the first study to demonstrate associations between maternal abuse in childhood and offspring newborn telomere length. Maternal sexual abuse, but not physical or emotional abuse, in childhood was predictive of shorter male newborn telomere length. In adults, the few available studies suggest that risk of telomere attrition differs by type of childhood maltreatment experienced, but the type of maltreatment linked to shortened telomere length has varied across studies (Tyrka et al., 2010; Vincent et al., 2017). This study provides preliminary evidence that a maternal history of sexual abuse in childhood may confer particular risk for shortened telomere length among males at birth. This is also the first study to consider protective factors, specifically maternal familial emotional support in childhood, on newborn telomere length. The findings suggest that a nurturing childhood environment may confer resilience to the next generation via offspring newborn telomere biology. Together, the child maltreatment and familial emotional support findings indicate that maternal lifetime exposures, not just exposures during pregnancy, have implications for newborn telomere length. Other strengths of the study include the relatively large and sociodemographically diverse sample, particularly for a study of newborn telomere length determinants.
Limitations may include the use of peripheral blood mononuclear cells (PBMC) to estimate newborn telomere length. PBMC telomere length is an average across different cell subpopulations (T cells, B cells, NK cells, monocytes); telomere attrition may not be uniform across cell types, and relative ratios of cell types may vary across individuals. However, PBMC telomere length is the metric that has been most commonly linked to morbidity and mortality (Geronimus et al., 2015). Moreover, telomere length from different tissues and cell types from the same individual are highly correlated within individuals from the fetal period through adulthood, suggesting PBMC telomere length is a surrogate parameter for relative telomere length in other tissues (Friedrich et al., 2000; Okuda et al., 2002; Price et al., 2013). Measures of maternal abuse and familial emotional support in childhood relied on retrospective report, introducing possible error due to memory inaccuracies or reporting biases. Prior data suggest that inaccuracies are in the direction of underestimating adverse experiences, which would lead to an underestimation of the magnitude of associations with newborn telomere length (Hardt and Rutter, 2004). Also, maternal reports were collected prior to birth and unlikely to have been reported differentially by cord blood rTL. The current analyses did not include paternal factors, which may contribute to newborn telomere length via various processes, including heritability, paternal characteristics (e.g., paternal age), and assortative mating (Broer et al., 2013). Future studies would be strengthened by considering the role of paternal factors, which have been given minimal attention in the newborn telomere literature to date.
The characteristics of the sample have potential implications for the interpretation of the study findings. The results may be broadly generalizable because the sample was drawn from the community rather than by clinical status or other pre-determined risk factors and closely reflected the demographics and risks of the community at large. For example, the 7% premature birth rate is similar to that of the state in which the study occurred (8.7%). Nearly half of the sample (45%) had household incomes below the median income for the state. Importantly, 15% endorsed depressive symptoms suggestive of a diagnosis of major depression, a rate highly consistent with epidemiological data that indicate a rate of 13% among pregnant and postpartum women (Office on Women’s Health, U.S. Department of Health and Human Services, 2017). Although the current findings indicated an association between elevated maternal depressive symptoms (i.e., symptom suggestive of major depression) and shorter cord blood rTL among males, no association was found between the continuous measure of maternal depressive symptoms and cord blood rTL. The average depression symptom score in this study was low, indicating limited variability in symptoms within the sample, including relatively low numbers of participants experiencing subclinical depression. This lack of variability may have contributed to the non-significant associations between the continuous measure of maternal depressive symptoms and cord blood rTL. The current analyses should be tested in a sample that includes a more varied range of symptoms, including a sufficient representation of subclinical symptoms, to determine if there is a linear association between maternal depressive symptoms and cord blood rTL or if maternal depressive symptoms only have impact when they reach a clinically significant level of severity. This is particularly important given findings that subclinical levels of depressive symptoms in mothers during pregnancy may impact fetal development and child outcomes (e.g., Gustafsson et al., 2018; Kingston et al., 2018). Also of note, maternal mental health symptoms were only assessed at one time point in pregnancy in the current study; the extant literature does not provide guidance as to how the timing and severity of maternal psychopathology during pregnancy may influence newborn telomere biology. Overall, the significant correlation of cord blood rTL with elevated depressive symptoms and the trending association with maternal PTSD symptoms among male infants provide preliminary support for a link between maternal psychological functioning during pregnancy and newborn telomere length. Studies should explore maternal mental health effects on newborn telomere length in a more psychiatrically ill sample, where larger effects and/or a different pattern of results may emerge. Studies should also be conducted to determine whether the pattern of associations between risk/protective factors and newborn telomere length apply to populations with other more extreme characteristics (e.g., premature/low birthweight infants; low income/poverty samples).
The rates of maternal abuse during childhood were also relatively low in this sample. The measure utilized to characterize childhood abuse experiences provided continuous ratings of abusive experiences. A score of at least 1 (i.e., rare exposure) was endorsed by 42% of the sample for physical abuse, 77% for emotional abuse, 10% for sexual abuse, and 81% for any type of abuse. The mean scores on each of these scales were toward the low end of the scales, suggesting that experiences of childhood abuse were infrequent in this sample. Thus, although a significant association between maternal sexual abuse history and cord blood TL was found among males, the lack of significant associations with the other abuse measures should be interpreted cautiously. An extensive literature has demonstrated that a history of childhood maltreatment has large and persistent effects on a wide range of health outcomes across the lifespan. More research is needed with samples with documented histories of severe and chronic childhood abuse to determine the types of childhood maltreatment that may influence newborn telomere length and whether more extreme histories of maltreatment influence both male and female offspring.
4.1. Conclusions
Our findings suggest that maternal lifetime exposures, including physical and mental health and SES in pregnancy and abusive and supportive experiences in childhood, impact newborn telomere length, particularly among males. Such effects may have lifelong health implications given that newborn telomere length provides the initial setting for lifetime telomere length and that regulation of lifetime telomere attrition rate may be programmed in early life (Factor-Litvak et al., 2016; Martens et al., 2016; Tyrka et al., 2010). Therefore, processes that shorten newborn telomere length may be critical determinants of lifetime health, and newborn telomere length may be a powerful biomarker of lifetime disease risk (Martens et al., 2016). Whether telomere length is a determinant of health or a biomarker of the cumulative effects of other health determinants is as yet unknown (Geronimus et al., 2015). Future research should explore whether newborn telomere length is a biomarker that predicts health outcomes or plays an etiological role in linking prenatal exposures with poor health. If the latter, maternal lifetime experiences may be mechanistically involved in the development of a number of offspring disease states into adulthood via fetal programming of telomere length, particularly among male offspring. Studies that elucidate mechanisms responsible for maternal exposures effects on newborn telomere length may inform the development of preventive interventions that optimize offspring lifetime health outcomes.
Supplementary Material
Acknowledgements
We are grateful for the study families whose generous donation of time made this project possible.
Role of Funding Sources
The work was supported by the National Heart, Lung, & Blood Institute (R01HL095606; R01HL114396), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD082078), the National Institute of Environmental Health Sciences (P30ES023515), the Boston Children’s Hospital’s Clinical and Translational Research Executive Committee, and the Program for Behavioral Science in the Department of Psychiatry at Boston Children’s Hospital. None of the funding agencies had any role in the study design, the collection, analysis, or interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication. The content is solely the responsibility of the authors and does not represent the official views of any granting agency.
Footnotes
Competing Interests: None.
Declarations of Interest: None.
Contributors/Authorship
All authors participated in the design of the study and/or acquisition of data and/or analysis and interpretation of data and drafting the manuscript or revising it critically for important intellectual content. All authors provided final approval of the version submitted.
Two supplemental series of predictive models (data not shown) tested potential contributions of demographic and health covariates. In the first series, newborn gestational age and birthweight were included as control variables along with maternal age; the pattern of results did not differ from the analyses presented here. In the second series, maternal race/ethnicity, preeclampsia/eclampsia, and gestational diabetes were included as control variables; these covariates contributed non-significant amounts of variance. For reasons of parsimony, none of these models are shown.
References
- Bale TL, Baram TZ, Brown AS, Goldstein JM, Insel TR, McCarthy MM, . . . Nestler EJ (2010). Early life programming and neurodevelopmental disorders. Biol Psychiatry, 68(4), 314–319. doi: 10.1016/j.biopsych.2010.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baragetti A, Palmen J, Garlaschelli K, Grigore L, Pellegatta F, Tragni E, . . . Talmud PJ (2015). Telomere shortening over 6 years is associated with increased subclinical carotid vascular damage and worse cardiovascular prognosis in the general population. J Intern Med, 277(4), 478–487. doi: 10.1111/joim.12282 [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, Foote J, Lovejoy M, Wenzel K, Sapareto E, Ruggiero J (1994). Initial reliability and validity of a new retrospective measure of child abuse and neglect. Am J Psychiatry, 151(8), 1132–1136. doi: 10.1176/ajp.151.8.1132 [DOI] [PubMed] [Google Scholar]
- Berry C, Shalowitz M, Quinn K, Wolf R (2001). Validation of the Crisis in Family Systems-Revised, a contemporary measure of life stressors. Psychol Rep, 88(3 Pt 1), 713–724. [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Gall JG (1978). A tandemly repeated sequence at the termini of the extrachromosomal ribosomal RNA genes in Tetrahymena. J Mol Biol, 120(1), 33–53. [DOI] [PubMed] [Google Scholar]
- Boeck C, Krause S, Karabatsiakis A, Schury K, Gundel H, Waller C, Kolassa IT (2017). History of child maltreatment and telomere length in immune cell subsets: Associations with stress- and attachment-related hormones. Dev Psychopathol, 1–13. doi: 10.1017/s0954579417001055 [DOI] [PubMed] [Google Scholar]
- Bosquet Enlow M, King L, Schreier HM, Howard JM, Rosenfield D, Ritz T, Wright RJ (2014). Maternal sensitivity and infant autonomic and endocrine stress responses. Early Hum Dev, 90(7), 377–385. doi: 10.1016/j.earlhumdev.2014.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, . . . Boomsma DI (2013). Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet, 21(10), 1163–1168. doi: 10.1038/ejhg.2012.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne BM, Shavelson RJ, Muthen B (1989). Testing for the equivalence of factor covariance and mean structures: The issue of partial measurement invariance. Psychol Bull, 105, 456–466. [Google Scholar]
- Cawthon RM (2002). Telomere measurement by quantitative PCR. Nucl<>eic Acids Res, 30(10), e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawthon RM (2009). Telomere length measurement by a novel monochrome multiplex quantitative PCR method. Nucleic Acids Res, 37(3), e21. doi: 10.1093/nar/gkn1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Fauce SR, Effros RB (2008). Reduced telomerase activity in human T lymphocytes exposed to cortisol. Brain Behav Immun, 22(4), 600–605. doi: 10.1016/j.bbi.2007.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton VL (2010). Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta, 31 Suppl, S33–39. doi: 10.1016/j.placenta.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Coimbra BM, Carvalho CM, Moretti PN, Mello MF, Belangero SI (2017). Stress-related telomere length in children: A systematic review. J Psychiatr Res, 92, 47–54. doi: 10.1016/j.jpsychires.2017.03.023 [DOI] [PubMed] [Google Scholar]
- Davis EP, Sandman CA, Buss C, Wing DA, Head K (2013). Fetal glucocorticoid exposure is associated with preadolescent brain development. Biol Psychiatry, 74(9), 647–655. doi: 10.1016/j.biopsych.2013.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Kivlighan KT, Chen P, Laudenslager ML (2011). Maternal salivary cortisol differs by fetal sex during the second half of pregnancy. Psychoneuroendocrinology, 36(4), 588–591. doi: 10.1016/j.psyneuen.2010.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle C, Werner E, Feng T, Lee S, Altemus M, Isler JR, Monk C (2015). Pregnancy distress gets under fetal skin: Maternal ambulatory assessment & sex differences in prenatal development. Dev Psychobiol. doi: 10.1002/dev.21317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Esteves K, Hatch V, Woodbury M, Borne S, Adamski A, Theall KP (2015). Setting the trajectory: racial disparities in newborn telomere length. J Pediatr, 166(5), 1181–1186. doi: 10.1016/j.jpeds.2015.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efron B (1979). Bootstrap methods: Another look at the jackknife. Ann Stat, 7, 1–26. [Google Scholar]
- Enlow MB, Devick KL, Brunst KJ, Lipton LR, Coull BA, Wright RJ (2017). Maternal lifetime trauma exposure, prenatal cortisol, and infant negative affectivity. Infancy, 22(4), 492–513. doi: 10.1111/infa.12176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enokido M, Suzuki A, Sadahiro R, Matsumoto Y, Kuwahata F, Takahashi N, . . . Otani K (2014). Parental care influences leukocyte telomere length with gender specificity in parents and offsprings. BMC Psychiatry, 14, 277. doi: 10.1186/s12888-014-0277-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD (2015). Prenatal stress, development, health and disease risk: A psychobiological perspective-2015 Curt Richter Award Paper. Psychoneuroendocrinology, 62, 366–375. doi: 10.1016/j.psyneuen.2015.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, Blackburn EH, . . . Wadhwa PD (2011). Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. Proceedings of the National Academy of Sciences, 108(33), E513–E518. doi: 10.1073/pnas.1107759108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH, . . . Wadhwa PD (2013). Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. American Journal of Obstetrics and Gynecology, 208(2), 134.e131–134.e137. doi: 10.1016/j.ajog.2012.11.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Susser E, Kezios K, McKeague I, Kark JD, Hoffman M, . . . Aviv A (2016). Leukocyte telomere length in newborns: Implications for the role of telomeres in human disease. Pediatrics, 137(4). doi: 10.1542/peds.2015-3927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich U, Griese E, Schwab M, Fritz P, Thon K, Klotz U (2000). Telomere length in different tissues of elderly patients. Mech Ageing Dev, 119(3), 89–99. [DOI] [PubMed] [Google Scholar]
- Gabory A, Attig L, Junien C (2009). Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol, 304(1–2), 8–18. doi: 10.1016/j.mce.2009.02.015 [DOI] [PubMed] [Google Scholar]
- Geronimus AT, Pearson JA, Linnenbringer E, Schulz AJ, Reyes AG, Epel ES, . . . Blackburn EH (2015). Race-ethnicity, poverty, urban stressors, and telomere length in a Detroit community-based sample. J Health Soc Behav, 56(2), 199–224. doi: 10.1177/0022146515582100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J, McKenzie-McHarg K, Shakespeare J, Price J, Gray R (2009). A systematic review of studies validating the Edinburgh Postnatal Depression Scale in antepartum and postpartum women. Acta Psychiatr Scand, 119(5), 350–364. doi: 10.1111/j.1600-0447.2009.01363.x [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Cooper C, Thornburg KL (2008). Effect of in utero and early-life conditions on adult health and disease. N Engl J Med, 359(1), 61–73. doi: 10.1056/NEJMra0708473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, . . . Wolkowitz O M. (2015). Telomere length and cortisol reactivity in children of depressed mothers. Mol Psychiatry, 20(5), 615–620. doi: 10.1038/mp.2014.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson HC, Grieve PG, Werner EA, Desai P, Monk C (2018). Newborn electroencephalographic correlates of maternal prenatal depressive symptoms. J Dev Orig Health Dis. Advance online publication. doi: 10.1017/S2040174418000089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardt J, Rutter M (2004). Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry, 45(2), 260–273. [DOI] [PubMed] [Google Scholar]
- Hjelmborg JB, Dalgard C, Moller S, Steenstrup T, Kimura M, Christensen K, . . . Aviv A (2015). The heritability of leucocyte telomere length dynamics. J Med Genet, 52(5), 297–302. doi: 10.1136/jmedgenet-2014-102736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstrasser T, Marksteiner J, Humpel C (2012). Telomere length is age-dependent and reduced in monocytes of Alzheimer patients. Exp Gerontol, 47(2), 160–163. doi: 10.1016/j.exger.2011.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kananen L, Surakka I, Pirkola S, Suvisaari J, Lonnqvist J, Peltonen L, . . . Hovatta I (2010). Childhood adversities are associated with shorter telomere length at adult age both in individuals with an anxiety disorder and controls. PLoS One, 5(5), e10826. doi: 10.1371/journal.pone.0010826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Gouin JP, Weng NP, Malarkey WB, Beversdorf DQ, Glaser R (2011). Childhood adversity heightens the impact of later-life caregiving stress on telomere length and inflammation. Psychosom Med, 73(1), 16–22. doi: 10.1097/PSY.0b013e31820573b6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston D, Kehler H, Austin MP, Mughal MK, Wajid A, Vermeyden L,…Giallo, R. (2018). Trajectories of maternal depressive symptoms during pregnancy and the first 12 months postpartum and child externalizing and internalizing behavior at three years. PLoS One, 13(4):e0195365. doi: 10.1371/journal.pone.0195365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C (2009). Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10(6), 434–445. doi: 10.1038/nrn2639 [DOI] [PubMed] [Google Scholar]
- Marchetto NM, Glynn RA, Ferry ML, Ostojic M, Wolff SM, Yao R, Haussmann MF (2016). Prenatal stress and newborn telomere length. Am J Obstet Gynecol, 215(1), 94 e91–98. doi: 10.1016/j.ajog.2016.01.177 [DOI] [PubMed] [Google Scholar]
- Martens DS, Plusquin M, Gyselaers W, De Vivo I, Nawrot TS (2016). Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med, 14(1), 148. doi: 10.1186/s12916-016-0689-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AM, Kowalsky JM, Epel ES, Lin J, Christian LM (2017). Childhood adversity, social support, and telomere length among perinatal women. Psychoneuroendocrinology, 87, 43–52. doi: 10.1016/j.psyneuen.2017.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller P, Mayer S, Mattfeldt T, Muller K, Wiegand P, Bruderlein S (2009). Sex-related differences in length and erosion dynamics of human telomeres favor females. Aging (Albany NY), 1(8), 733–739. doi: 10.18632/aging.100068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller BR, Bale TL (2008). Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci, 28(36), 9055–9065. doi: 10.1523/jneurosci.1424-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundstock E, Sarria EE, Zatti H, Mattos Louzada F, Kich Grun L, Herbert Jones M, . . . Mattiello R (2015). Effect of obesity on telomere length: Systematic review and meta-analysis. Obesity (Silver Spring), 23(11), 2165–2174. doi: 10.1002/oby.21183 [DOI] [PubMed] [Google Scholar]
- Needham BL, Fernandez JR, Lin J, Epel ES, Blackburn EH (2012). Socioeconomic status and cell aging in children. Social Science & Medicine, 74(12), 1948–1951. doi: 10.1016/j.socscimed.2012.02.019 [DOI] [PubMed] [Google Scholar]
- Office on Women’s Health, U.S. Department of Health and Human Services. (2017). Depression during and after pregnancy. https://www.womenshealth.gov/a-z-topics/depression-during-and-after-pregnancy/ (accessed 27 April 2018).
- Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, . . . Aviv A (2002). Telomere length in the newborn. Pediatr Res, 52(3), 377–381. doi: 10.1203/00006450-200209000-00012 [DOI] [PubMed] [Google Scholar]
- Ostlund BD, Conradt E, Crowell SE, Tyrka AR, Marsit CJ, Lester BM (2016). Prenatal stress, fearfulness, and the epigenome: Exploratory analysis of sex differences in DNA methylation of the glucocorticoid receptor gene. Front Behav Neurosci, 10, 147. doi: 10.3389/fnbeh.2016.00147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozturk S, Sozen B, Demir N (2014). Telomere length and telomerase activity during oocyte maturation and early embryo development in mammalian species. Mol Hum Reprod, 20(1), 15–30. doi: 10.1093/molehr/gat055 [DOI] [PubMed] [Google Scholar]
- Perner S, Bruderlein S, Hasel C, Waibel I, Holdenried A, Ciloglu N, . . . Moller P (2003). Quantifying telomere lengths of human individual chromosome arms by centromere-calibrated fluorescence in situ hybridization and digital imaging. Am J Pathol, 163(5), 1751–1756. doi: 10.1016/s0002-9440(10)63534-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR (2013). Telomeres and early-life stress: an overview. Biol Psychiatry, 73(1), 15–23. doi: 10.1016/j.biopsych.2012.06.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi Nan W, Ling Z, Bing C (2015). The influence of the telomere-telomerase system on diabetes mellitus and its vascular complications. Expert Opin Ther Targets, 19(6), 849–864. doi: 10.1517/14728222.2015.1016500 [DOI] [PubMed] [Google Scholar]
- Revesz D, Verhoeven JE, Milaneschi Y, Penninx BW (2016). Depressive and anxiety disorders and short leukocyte telomere length: mediating effects of metabolic stress and lifestyle factors. Psychol Med, 46(11), 2337–2349. doi: 10.1017/s0033291716000891 [DOI] [PubMed] [Google Scholar]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, Palermo D, . . . Tyrka AR (2017). Early life adversity and telomere length: a meta-analysis. Mol Psychiatry. doi: 10.1038/mp.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L, Nordestgaard BG, Bojesen SE (2015). Peripheral blood leukocyte telomere length and mortality among 64,637 individuals from the general population. J Natl Cancer Inst, 107(6), djv074. doi: 10.1093/jnci/djv074 [DOI] [PubMed] [Google Scholar]
- Salihu HM, King LM, Nwoga C, Paothong A, Pradhan A, Marty PJ, . . . Whiteman VE (2016). Association between maternal-perceived psychological stress and fetal telomere length. South Med J, 109(12), 767–772. doi: 10.14423/smj.0000000000000567 [DOI] [PubMed] [Google Scholar]
- Scher CD, Stein MB, Asmundson GJ, McCreary DR, Forde DR (2001). The childhood trauma questionnaire in a community sample: psychometric properties and normative data. J Trauma Stress, 14(4), 843–857. doi: 10.1023/a:1013058625719 [DOI] [PubMed] [Google Scholar]
- Send TS, Gilles M, Codd V, Wolf I, Bardtke S, Streit F, . . . Witt SH (2017). Telomere length in newborns is related to maternal stress during pregnancy. Neuropsychopharmacology, 42(12), 2407–2413. doi: 10.1038/npp.2017.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I (2012). Early life stress and telomere length: Investigating the connection and possible mechanisms. BioEssays, 34(11), 943–952. doi: 10.1002/bies.201200084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, . . . Caspi A (2014). Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry, 19(11), 1163–1170. doi: 10.1038/mp.2013.183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, . . . Caspi A (2013). Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol Psychiatry, 18(5), 576–581. doi: 10.1038/mp.2012.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiels PG, McGlynn LM, MacIntyre A, Johnson PC, Batty GD, Burns H, . . . Packard, C. J. (2011). Accelerated telomere attrition is associated with relative household income, diet and inflammation in the pSoBid cohort. PLoS One, 6(7), e22521. doi: 10.1371/journal.pone.0022521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sillanpaa E, Sipila S, Tormakangas T, Kaprio J, Rantanen T (2017). Genetic and environmental effects on telomere length and lung function: A twin study. J Gerontol A Biol Sci Med Sci, 72(11), 1561–1568. doi: 10.1093/gerona/glw178 [DOI] [PubMed] [Google Scholar]
- Stark MJ, Wright IM, Clifton VL (2009). Sex-specific alterations in placental 11beta-hydroxysteroid dehydrogenase 2 activity and early postnatal clinical course following antenatal betamethasone. Am J Physiol Regul Integr Comp Physiol, 297(2), R510–514. doi: 10.1152/ajpregu.00175.2009 [DOI] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Butcher L, Lin J, Brydon L, Kivimaki M, . . . Erusalimsky JD (2011). Educational attainment but not measures of current socioeconomic circumstances are associated with leukocyte telomere length in healthy older men and women. Brain Behav Immun, 25(7), 1292–1298. doi: 10.1016/j.bbi.2011.04.010 [DOI] [PubMed] [Google Scholar]
- Tomiyama AJ, O’Donovan A, Lin J, Puterman E, Lazaro A, Chan J, . . . Epel E (2012). Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol Behav, 106(1), 40–45. doi: 10.1016/j.physbeh.2011.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao H-T, Porton B, Marsella SA, Carpenter LL (2010). Childhood maltreatment and telomere shortening: Preliminary support for an effect of early stress on cellular aging. Biological Psychiatry, 67(6), 531–534. doi: 10.1016/j.biopsych.2009.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh BRH, van den Heuvel MI, Lahti M, Braeken M, de Rooij SR, Entringer S, . . . Schwab M (2017). Prenatal developmental origins of behavior and mental health: The influence of maternal stress in pregnancy. Neurosci Biobehav Rev. doi: 10.1016/j.neubiorev.2017.07.003 [DOI] [PubMed] [Google Scholar]
- Vincent J, Hovatta I, Frissa S, Goodwin L, Hotopf M, Hatch SL, . . . Powell TR (2017). Assessing the contributions of childhood maltreatment subtypes and depression case-control status on telomere length reveals a specific role of physical neglect. J Affect Disord, 213, 16–22. doi: 10.1016/j.jad.2017.01.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Zglinicki T (2002). Oxidative stress shortens telomeres. Trends Biochem Sci, 27(7), 339–344. [DOI] [PubMed] [Google Scholar]
- Weathers FW, Husks J, Keane T (1993). The PTSD checklist-Civilian Version (PCL-C), Available from F.W. Weathers Boston, MA: National Center for PTSD, Boston Veterans Affairs Medical Center. [Google Scholar]
- Wikgren M, Maripuu M, Karlsson T, Nordfjall K, Bergdahl J, Hultdin J, . . . Norrback KF (2012). Short telomeres in depression and the general population are associated with a hypocortisolemic state. Biol Psychiatry, 71(4), 294–300. doi: 10.1016/j.biopsych.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Wojcicki JM, Olveda R, Heyman MB, Elwan D, Lin J, Blackburn E, Epel E (2016). Cord blood telomere length in Latino infants: relation with maternal education and infant sex. J Perinatol, 36(3), 235–241. doi: 10.1038/jp.2015.178 [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, . . . Blackburn EH (2011). Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS One, 6(3), e17837. doi: 10.1371/journal.pone.0017837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright RJ, Fisher K, Chiu YH, Wright RO, Fein R, Cohen S, Coull BA (2013). Disrupted prenatal maternal cortisol, maternal obesity, and childhood wheeze. Insights into prenatal programming. Am J Respir Crit Care Med, 187(11), 1186–1193. doi: 10.1164/rccm.201208-1530OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, Blackburn EH, Steptoe A (2014). Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. Proc Natl Acad Sci U S A, 111(12), 4519–4524. doi: 10.1073/pnas.1322145111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.