Abstract
Primary tumoral calcinosis is a rare autosomal recessive disorder characterized by ectopic calcified tumoral masses. Mutations in 3 genes (GALNT3, FGF23, and KL) have been linked to this human disorder. We describe a case of a 28-year-old man with a history of painful firm masses over his right and left gluteal region, right clavicle region, knees, and left elbow. Biochemical analysis disclosed hyperphosphatemia (phosphate, 9.0 mg/dL) and normocalcemia (calcium, 4.8 mg/dL), with normal kidney function and fractional excretion of phosphate of 3%. Parathyroid hormone was suppressed (15 pg/mL), associated with a low-normal 25-hydroxyvitamin D (26 ng/mL) concentration but high 1,25-dihydroxyvitamin D concentration (92 pg/mL). Serum intact FGF-23 (fibroblast growth factor 23) was undetectable. Genetic analysis revealed tumoral calcinosis due to a compound heterozygous mutation in FGF23, c.201G>C (p.Gln67His) and c.466C>T (p.Gln156*). Due to lack of other treatment options and because the patient was facing severe vascular complications, we initiated a daily hemodialysis program even in the setting of normal kidney function. This unusual therapeutic option successful controlled hyperphosphatemia and reduced metastatic tumoral lesions. This is a report of a new mutation in FGF23 in which dialysis was an effective treatment option for tumoral calcinosis with normal kidney function.
Primary tumoral calcinosis is a rare autosomal recessive metabolic disorder that features ectopic calcified tumoral masses, dental abnormalities, and periarticular soft tissue and vascular calcifications. Biochemical characteristics of tumoral calcinosis include hyperphosphatemia, increased renal tubular phosphate reabsorption, and inappropriately normal or elevated 1,25-dihydroxyvitamin D concentrations.1
We report on a case of a 28-year-old man with tumoral calcinosis with novel mutations in the fibroblast growth factor 23 gene (FGF23) and an unusual treatment option in an attempt to effectively control serum phosphate concentrations and consequently the calcified lesions.
Case Report
A 28-year-old man presented with a 10-year history of painful firm masses over his gluteal and right clavicle regions, knees, and left elbow that spontaneously drained whitish material several times (Fig 1A–C). These lesions slowly increased in size, resulting in difficulties with locomotion and positioning. He had no other medical conditions. There was no family history of kidney stones, calcifications, systemic illness, or consanguineous marriages. Physical examination revealed hard, tender, irregular masses as described. Tenderness of the left hip abductor muscles limited active abduction. The patient had undergone surgery several times to reduce the masses, but they increased in size again later on. Examination also revealed reduced femoral pulses.
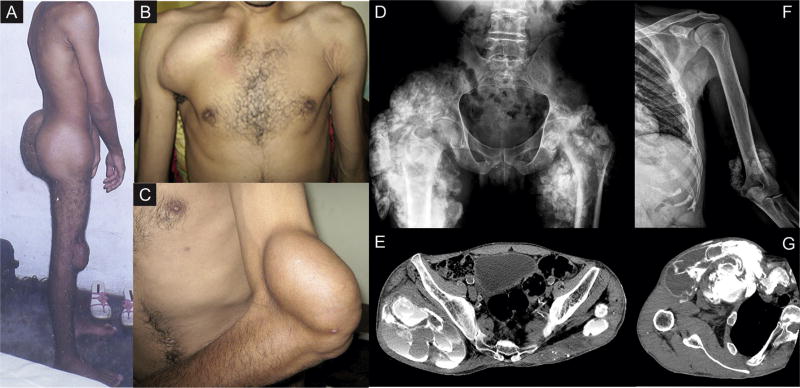
Figure 1.
Metastatic calcifications presenting as firm solid masses over the patient’s (A) gluteal region and knees, (B) right clavicle, and (C) left elbow regions. Image diagnostic methods including radiographs and computed tomography revealed giant calcified masses in the (D) right hip, (E) thigh, (F) left elbow, and (G) near the right clavicle, as well as vascular calcifications.
Biochemical analysis disclosed hyperphosphatemia (phosphate, 9.0 mg/dL); serum ionized calcium concentration of 4.8 mg/dL, which is within the reference range; normal kidney function; and inappropriately low urinary fractional excretion of phosphate of 3%. Intact parathyroid hormone was suppressed (15 pg/mL) and associated with a low-normal 25-hydroxyvitamin D concentration (26 ng/mL) but high 1,25-dihydroxyvitamin D concentration (92 pg/mL). Serum intact FGF-23 was undetectable (the reference range is 26 ± 8.4 pg/mL; Kainos Laboratories), whereas carboxy-terminal FGF-23 concentration was 950 RU/mL (reference range, 50 ± 51 RU/mL; Immutopics).
Multiple radiographs and computed tomographic scans revealed giant calcified masses near the right clavicle, right hip, and left elbow, as well as vascular calcifications. Surgical biopsy showed milky fluid with calcifications. Histologic evaluation was characteristic of tumoral calcinosis.
Genetic analysis was initiated by sequencing the entire GALNT3 coding region and the intron-exon junctions because most reported cases of primary (familial) tumoral calcinosis are associated with mutations in this gene, which encodes N-acetylgalactosaminyltransferase 3. No variant predicted to be pathogenic was detected in GALNT3, leading us to search for potentially deleterious variants in FGF23. Analysis of this gene resulted in the identification of 2 novel FGF23 variants that are very likely to be disease causing: c.201G>C (a guanine to cystosine substitution at nucleotide 201 of the coding sequence, leading to a predicted glutamine to histidine substitution at amino acid 67 [p.Gln67His]) and c.466C>T (a cytosine to thymine substitution at nucleotide 466 of the coding sequence, leading to a predicted substitution of the codon for the glutamine at amino acid 156 by a termination codon [p.Gln156*]). Analysis with PolyPhen-2 software2 yielded a pathogenicity score of 0.998 (for which 0 corresponds to benign, and 1.0, to damaging) for the first variant while the presence of a stop codon established the second mutation as definitely pathogenic. The patient’s mother was heterozygous for c.466C>T, but showed no variant at codon 67; the father was not available for analysis.
Despite 4 surgeries for tumoral resection and medical treatment with phosphate binders (aluminum hydroxide, 2 tablespoons, 3 times daily, and sevelamer, 3,200 mg, 3 times daily) and acetazolamide, 250 mg, twice a day, lesions continued to progress, as shown in Fig 1D to G. In addition, the patient developed clinical signs of left femoral artery obstruction, requiring a surgical arterial bypass. In the scenario of refractory hyperphosphatemia with severe clinical complications despite the use of different classes of drugs, we decided to approach his severe condition in an unconventional way, starting the patient on hemodialysis therapy even in the context of normal kidney function (serum creatinine, 0.89 mg/dL, corresponding to estimated glomerular filtration rate using the Chronic Kidney Disease Epidemiology Collaboration [CKD-EPI] equation of 116 mL/min/1.73 m2). A tunneled hemodialysis catheter was placed in the left internal jugular vein, due to a giant tumoral calcification in the right clavicle region. His prescription included 6-times-weekly hemodialysis sessions, each of 2 hours’ duration, with use of a 2.0-m2, high-efficiency, high-flux, polysulfone filter (Diacap HI PS20; BBraun), blood flow of 350 mL/min, dialysate flow of 800 mL/min, and dialysate with calcium, 1.25 mmol/L; potassium, 4 mEq/L; sodium, 138 mEq/L; and bicarbonate, 26 mmol/L. Unfractionated heparin (10,000 IU/session) was used for anticoagulation, and there was no need for ultrafiltration.
This strategy resulted in better serum phosphate control after the initial months of therapy (Fig 2A) and was followed 24 months later by a substantial decrease in tumoral mass volume without a surgical procedure, which was accompanied by mobility recovery (Fig 2B–D).
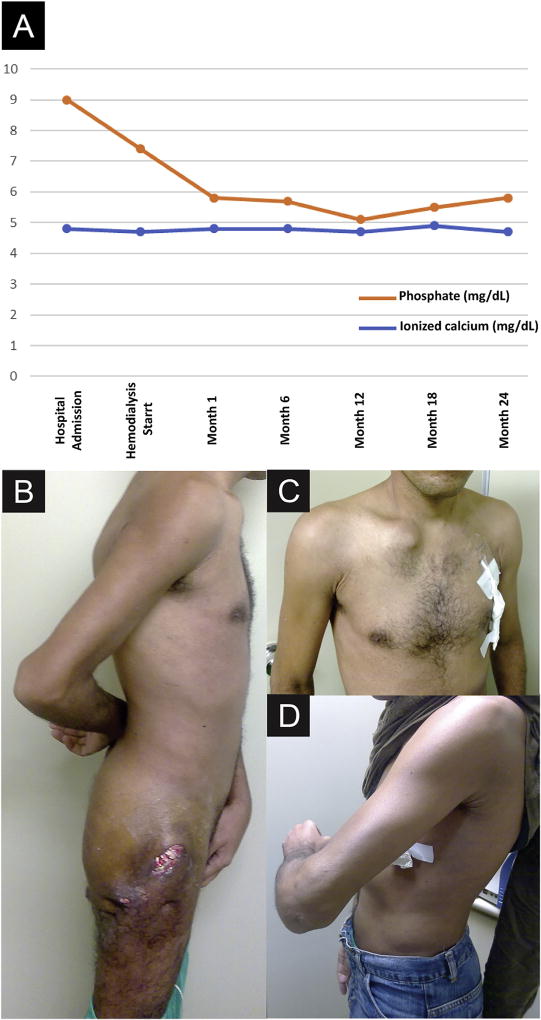
Figure 2.
(A) Evolutive laboratory parameters of serum phosphate and ionized calcium concentrations from hospital admission to the last month of hemodialysis therapy. After 24 months of hemodialysis treatment, there was a substantial decrease in tumoral mass volume of the (B) gluteal region and knees, (C) right clavicle, and (D) left elbow regions.
Discussion
Tumoral calcinosis is a condition characterized by solitary or multiple periarticular calcified masses. The first description of this entity dates to 1898 and 1899, when Giard3 and Duret4 described this disease independently. Unaware of these publications, Inclan et al,5 in 1943, first used the term “tumoral calcinosis.” This pivotal article defined specific criteria for disease diagnosis, including hyperphosphatemia with normocalcemia, and also differentiated the primary or familial forms of the disease from the secondary conditions also associated with tumoral calcinosis, such as connective tissue diseases, endocrinologic disorders, and the calcinosis occasionally associated with metabolic bone disease in CKD. Later, detailed radiologic features of tumoral calcinosis were described by Martinez et al.6
The tumoral lesions are usually densely calcified masses confined to soft tissue, composed of calcium hydroxyapatite crystals with calcium phosphate and calcium carbonate.7,8 These lesions typically occur at the extensor surfaces of the joints and are most commonly observed at the hip, elbow, shoulder, foot, and wrist.9 Lesions can be multiple, bilateral, and commonly symmetrical. Younger male patients and individuals of African ancestry are more frequently affected. This autosomal recessive disorder is genetically heterogeneous and is caused by mutations in GALNT3, FGF23, or KL (encoding Klotho).10–14
Most reported tumoral calcinosis cases are caused by mutations in the GALNT3 gene. The GALNT3 product is a glycosyltransferase responsible for initiating mucin-O-glycosylation, a posttranslational modification that prevents proteolytic processing of FGF-23, resulting in limited amounts of intact FGF-23 production. Mutations in FGF23 make the FGF-23 protein susceptible to destruction, explaining the undetectable concentrations observed in the present case. Mutations in KL in turn result in resistance to FGF-23 action because they impair assembly of the ternary complex comprising FGF-23, FGFR1c (the FGF receptor 1c), and Klotho.14
Typically, laboratory studies show hyperphosphatemia (though not frequent, normal serum phosphate concentrations can occur), high-normal calcium concentrations, low-normal or suppressed parathyroid hormone and alkaline phosphatase concentrations, and elevated 1,25-dihydroxyvitamin D concentrations.8 The hyperabsorption of phosphate in the proximal tubule of the kidneys in the setting of low bioactive FGF-23 concentrations is thought to cause hyperphosphatemia. However, phosphate has a tendency to complex with calcium, causing hypocalcemia, which seems to be compensated by elevated calcitriol production and increased intestinal calcium absorption. The hyperphosphatemia together with normocalcemia results in high calcium-phosphate product concentrations and consequent deposition in the skin and subcutaneous tissues.
There is limited clinical evidence to guide the treatment of tumoral calcinosis. Due to the rarity of the disease, all available treatment information is derived from case reports or case series.11 Many patients undergo surgical treatment because tumoral calcinosis lesions often become very large, causing pain, deformity, and movement restriction. Although surgery may relieve symptoms, unfortunately, these lesions classically recur8,15 due to persistence of the underlying metabolic defect. Medical treatment regimens involve altering the biochemical profile to counter the consequences of insufficient FGF-23 action, therefore allowing resolution of the lesions. Lowering phosphate concentrations with dietary restriction, antacids, and/or phosphate binders is the current approach. Acetazolamide can also be used to increase urinary phosphate excretion.16,17 A decrease in phosphate concentrations has been associated with lesion regression,18 but this approach does not consistently succeed in treating the lesions.15
Our patient underwent dietary phosphate restriction and received aluminum hydroxide, sevelamer, and acetazolamide, with no clinical improvement. Although not included in the therapeutic arsenal to tumoral calcinosis, we used hemodialysis as a final attempt to improve his clinical condition. He was admitted to a short daily hemodialysis program, scheduled for 6 days a week and delivered through a tunneled catheter. Serum phosphate concentrations started to decrease, and after 24 months, the lesions significantly decreased in size. The patient recovered most of his mobility and regained quality of life. Ultimately he was able to return to his previous work.
Hemodialysis as a treatment for tumoral calcinosis in the scenario of normal kidney function represented an extreme approach, but one that succeeded in decreasing the chronic complications of severe hyperphosphatemia in the case of our patient. In addition, the clinical course of this case illuminates in vivo the role of phosphate in extraosseous calcifications in a genetic disease secondary to FGF23 mutations.
Acknowledgments
Support: None.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Sprecher F. Familial tumoral calcinosis: from characterization of a rare pheynotype to the pathogenesis of ectopic calcification. J Invest Dermatol. 2010;130(3):652–660. doi: 10.1038/jid.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adzhubei IA, Schmidt S, Peshkin L, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giard A. Sur la calcification tibernale. CR Soc Biol. 1898;10:1015–1021. [Google Scholar]
- 4.Duret MH. Tumeurs multiples and singuliers de bourses sereuses. Bull Mém Soc Anat Paris. 1899;74:725–731. [Google Scholar]
- 5.Inclan A, Leon PP, Camejo M. Tumoral calcinosis. J Am Med Assoc. 1943;121:490–495. [Google Scholar]
- 6.Martinez S, Vogler JB, Harrelson JM, Lyles KW. Imaging of tumoral calcinosis: new observations. Radiology. 1990;174(1):215–222. doi: 10.1148/radiology.174.1.2294551. [DOI] [PubMed] [Google Scholar]
- 7.McClatchie S, Bremner AD. Tumoral calcinosis–an unrecognized disease. Br Med J. 1969;1(5637):153–155. doi: 10.1136/bmj.1.5637.142-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lafferty FW, Reynolds ES, Pearson OH. Tumoral calcinosis: a metabolic disease of obscure etiology. Am J Med. 1965;38:105–118. doi: 10.1016/0002-9343(65)90164-6. [DOI] [PubMed] [Google Scholar]
- 9.Olsen KM, Chew FS. Tumoral calcinosis: pearls, polemics, and alternative possibilities. Radiographics. 2006;26(3):871–885. doi: 10.1148/rg.263055099. [DOI] [PubMed] [Google Scholar]
- 10.Folsom LJ, Imel EA. Hyperphosphatemic familial tumoral calcinosis: genetic models of deficient FGF23 action. Curr Osteoporos Rep. 2015;13(2):78–87. doi: 10.1007/s11914-015-0254-3. [DOI] [PubMed] [Google Scholar]
- 11.Topaz O, Shurman DL, Bergman R, et al. Mutations in GALNT3, encoding a protein involved in O-linked glycosylation, cause familial tumoral calcinosis. Nat Genet. 2004;36(6):579–581. doi: 10.1038/ng1358. [DOI] [PubMed] [Google Scholar]
- 12.Benet-Pagès A, Orlik P, Strom TM, Lorenz-Depiereux B. An FGF23 missense mutation causes familial tumoral calcinosis with hyperphosphatemia. Hum Mol Genet. 2005;14(3):385–390. doi: 10.1093/hmg/ddi034. [DOI] [PubMed] [Google Scholar]
- 13.Larsson T, Yu X, Davis SI, et al. A novel recessive mutation in fibroblast growth factor-23 causes familial tumoral calcinosis. J Clin Endocrinol Metab. 2005;90(4):2424–2427. doi: 10.1210/jc.2004-2238. [DOI] [PubMed] [Google Scholar]
- 14.Ichikawa S, Imel EA, Kreiter ML, et al. A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Musculoskelet Neuronal Interact. 2007;7(4):318–319. [PubMed] [Google Scholar]
- 15.Farrow EG, Imel EA, Whitw KE. Hyperphosphatemic familial tumoral calcinosis (FGF23, GALNT3, αKlotho) Best Pract Res Clin Rheumatol. 2011;25(5):735–747. doi: 10.1016/j.berh.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichikawa S, Baujat G, Seyahi A, et al. Clinical variability of familial tumoral calcinosis caused by novel GALNT3 mutations. Am J Med Genet A. 2010;152A(4):896–903. doi: 10.1002/ajmg.a.33337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lufkin EG, Wilson DM, Smith LH, et al. Phosphorus excretion in tumoral calcinosis: response to parathyroid hormone and acetazolamide. J Clin Endocrinol Metab. 1980;50(4):648–653. doi: 10.1210/jcem-50-4-648. [DOI] [PubMed] [Google Scholar]
- 18.Gregosiewicz A, Warda E. Tumoral calcinosis: successful medical treatment. A case report. J Bone Joint Surg Am. 1989;71(8):1244–1249. [PubMed] [Google Scholar]