Abstract
The overexpression of ABCB1 in cancer cells is a major factor contributing to the development of multidrug resistance (MDR) and treatment failure in cancer patients. Therefore, re-sensitization of MDR cancer cells to anticancer drugs remains an important aspect in chemotherapy. The progress in developing clinically applicable synthetic inhibitors of ABCB1 has been slow, mostly due to complications associated with intrinsic toxicities and unforeseen drug-drug interactions. Here, we explored the drug-repositioning approach for cancer therapy by targeting ABCB1-mediated MDR in human cancer cells. We found that DPI-201106, a positive inotropic agent, selectively inhibits the drug efflux function of ABCB1, and in doing so, re-sensitizes ABCB1-overexpressing MDR cancer cells to conventional anticancer drugs. Furthermore, the ATPase activity of ABCB1 and docking analysis of DPI-201106 in the drug-binding pocket of ABCB1 were determined to confirm the interaction between DPI-201106 and ABCB1 protein. In summary, we revealed an additional action and a potential clinical application of DPI-201106 to reverse ABCB1-mediated MDR in human cancer cells, which may be beneficial for cancer patients who have developed multidrug resistance and no longer respond to conventional chemotherapy, and should be further investigated.
Keywords: ABCB1, Multidrug resistance, Modulator, Sodium Channel, DPI-201106
1. Introduction
The occurrence of multidrug resistance (MDR) remains a major obstacle to successful cancer chemotherapy [30]. The overexpression of ATP-Binding Cassette (ABC) transporter ABCB1 (P-glycoprotein/ MDR1) often contributes, at least in part, to the MDR phenotype in tumors that results in treatment failure and cancer relapse [15, 43]. ABCB1 is a typical mammalian ABC transport protein, composed of two transmembrane domains (TMD) and two nucleotide-binding domains (NBD) that utilizes energy derived from ATP hydrolysis to actively transport a wide range of therapeutic agents that are structurally and mechanically unrelated, out of cancer cells [1, 16]. As a result, the intracellular concentration and cytotoxicity of ABCB1 substrate drugs are significantly reduced in these ABCB1-overexpressing cancer cells, rendering chemotherapy ineffective [43]. Therefore, it is not surprising that the overexpression of ABCB1 has been linked to the development of MDR phenotype in blood cancer and solid tumors [27, 31, 33, 36, 40, 47]. Moreover, ABCB1 is highly expressed in cells forming the blood-brain and blood-tissue barrier sites, capable of altering the absorption, distribution, metabolism, and elimination of most drugs, thereby affecting the therapeutic outcome [9, 29]. For these reasons, modulating the function and/or protein expression level of ABCB1 has clinical importance.
At present, direct modulation of the drug efflux function of ABCB1 is still considered by many to be the most effective approach to re-sensitize MDR cancer cells to chemotherapeutic agents [50]. The basic concept is to use a compound that has the ability to transiently block the function of ABCB1 at non-toxic concentrations, thus potentiating the efficacy of co-administered anticancer drugs in ABCB1-overexpressing MDR cancer cells [42, 43]. Unfortunately, there is currently no synthetic reversing agent that can be applied clinically to treat MDR cancer, mostly due to complications associated with selectivity, high toxicity and unforeseen drug-drug interactions [42]. Consequently, instead of developing novel synthetic compounds, many research groups, including our own, have adopted the drug repurposing (drug repositioning) approach and examined the chemosensitization effect of therapeutic agents with known pharmacological and toxicological profiles on MDR cancer cells [42].
In the present study, we investigated the potency and selectivity of DPI-201106 on ABCB1-mediated MDR in cancer cells. DPI-201106 is a positive inotropic agent that has been used frequently as a standard cardioselective modulator of voltage-gated sodium channels (VGSCs) [12, 13, 28, 32, 34, 49], and in patients who have undergone coronary arterial bypass grafting (CABG) [12]. DPI-201106 has also been proposed as a treatment option for patients with heart failure [14, 20, 26]. Our data demonstrated that DPI-201106 is capable of inhibiting the transport function of ABCB1, enhancing drug-induced apoptosis and reversing MDR in ABCB1-overexpressing cancer cells at non-toxic nanomolar concentrations. More importantly, we found that DPI-201106 selectively interacts with ABCB1 as a high-affinity substrate similar to cyclosporine A or as a modulator compared to ABCC1 and ABCG2.
2. Materials and methods
2.1. Chemicals
RPMI medium, Iscove’s modified Dulbecco’s medium (IMDM), Dulbecco’s Modified Eagle’s medium (DMEM), fetal calf serum (FCS), Phosphate-buffered saline (PBS), trypsin-EDTA, penicillin and streptomycin were purchased from Gibco, Invitrogen (CA, USA). DPI-201106 was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Tools Cell Counting (CCk-8) Kit was purchased from Biotools Co., Ltd (Taipei, Taiwan). Verapamil, MK-571, Ko143 and all other chemicals were purchased from Sigma (St. Louis, MO, USA), unless stated otherwise. Annexin V : FITC Apoptosis Detection Kit was purchased from BD Pharmingen (San Diego, CA, USA).
2.2. Cell culture conditions
The human KB-3–1 epidermal cancer cell line and the ABCB1-overexpressing variant KB-V-1, human OVCAR-8 ovarian cancer cell line and the ABCB1-overexpressing variant NCI-ADR-RES, mouse fibroblast NIH3T3 and ABCB1-transfected NIH3T3-G185 cells, as well as pcDNA3.1-HEK293, ABCB1-transfected MDR19-HEK293, ABCC1-transfected MRP1-HEK293 and ABCG2-transfected R482-HEK293, were cultured in DMEM. KB-V-1 cells were maintained in 1 mg/mL vinblastine [41], NIH3T3-G185 cells were cultured in the presence of 60 ng/mL colchicine [11], whereas HEK293 and HEK293 transfected cells were maintained in media containing 2 mg/mL G418 [52]. All cell lines were maintained in medium supplemented with 10% FCS, 2 mM L-glutamine and 100 units of penicillin/streptomycin/mL, at 37 °C in 5% CO2 humidified air and placed in drug-free medium 7 days prior to assay.
2.3. Fluorescent drug accumulation assay
The intracellular accumulation of calcein and pheophorbide A (PhA) was recorded using a FACScan flow cytometer (BD Biosciences) and analyzed using CellQuest software (Becton-Dickinson) according to the method described by Gribar et al [17]. Fluorescent calcein (excitation and emission wavelengths of 485 and 535 nm) generated by intracellular esterases from calcein-AM was used to monitor ABCB1- and ABCC1-mediated efflux, whereas pheophorbide A (PhA) (excitation and emission wavelengths of 395 and 670 nm) was used to monitor ABCG2-mediated efflux. Cells were harvested by trypsinization and resuspended in 4 mL of IMDM supplemented with 5 % FCS, 0,5 μM of calcein-AM or 1 μM of PhA was added to 3 × 105 cells suspended in IMDM in the presence or absence of DPI-201106 or a reference inhibitor of ABCB1, ABCC1 or ABCG2 as described previously [38].
2.4. Cytotoxicity assay
Cell Counting Kit-8 (CCK-8) and MTT cytotoxicity assays were carried out to determine the cytotoxicity of therapeutic drugs in various cell lines according to the method described by Ishiyama et al [23]. Briefly, 5000 cells were plated in each well of 96-well plates containing 100 μL of culture medium and maintained at 37 °C for 24 h before an additional 100 μL of various drug regimen was added to each well to make a final volume of 200 μL and incubated for an additional 72 h, HEK293 cells and HEK293 cells stably transfected with human ABCB1, ABCC1 or ABCG2 were developed with CCK reagent, whereas attached cancer cells were developed with MTT reagent, IC50 values were calculated from fitted concentration-response curves obtained from at least three independent experiments, For the drug resistance reversal assays, a nontoxic concentration of DPI-201106 or a reference inhibitor of ABCB1, ABCC1 or ABCG2, was added to the cytotoxicity assays, The extent of reversal was determined based on the calculated relative resistance (RR) values as described previously [52].
2.5. Immunoblotting
Cells were treated with various concentrations of DPI-201106 for 72 h before harvesting and subjected to SDS-PAGE electrophoresis. Primary antibodies C219 (1: 3000) and α-tubulin (1:100000) were used in Western blot immunoassay to detect ABCB1 and positive loading control tubulin, respectively. The horseradish peroxidase-conjugated goat anti-mouse IgG (1:100000) was used as the secondary antibody. Signals were detected as described previously [52].
2.6. Apoptosis assay
The percentage of apoptotic cells in the total cell population induced by DPI-201106, colchicine or in combinations, was determined using the conventional annexin V-FITC and propidium iodide (PI) staining method, as described previously [22]. The labeled cells (10000 per sample) were analyzed by FACScan using CellQuest software (BD Biosciences). Phosphatidylserine (PS)-positive and PI-negative cells (lower right dot-plot quadrant ) were counted as early apoptotic cells with intact plasma membranes, whereas PS-positive and PI-positive cells (upper right dot-plot quadrant ) are considered as either necrotic or late apoptotic with leaky membranes [5].
2.7. ATPase assay
The effect of DPI-201106 on vanadate (Vi)-sensitive ATPase activity of ABCB1 was determined using membrane vesicles of High-Five cells expressing ABCB1 based on the endpoint Pi assay as described previously [1].
2.8. Docking of DPI-201106 in the drug-binding pocket of human ABCB1
The three dimensional structure of ABCB1 was predicted using an automated protein homology-modeling server SWISS-MODEL. The templates were searched with BLAST and HHBlits against SWISS-MODEL template library. For each identified template, the template’s quality was predicted from features of the target-template alignment. The templates with the highest quality were then selected and built based on the target-template alignment using ProMod3 [6–8]. The energy was minimized for human ABCB1 homology modeled structure using Acclerys Discovery Studio 4.0. Ligand preparation and docking was performed by the CDOCKER module of the same software.
2.9. Statistical analysis
Experimental data and IC50 values are presented as mean ± standard deviation or where indicated, the values are given as mean ± standard error of the mean (SEM) calculated from at least three independent experiments. KaleidaGraph (Reading, PA, USA) and GraphPad Prism (La Jolla, CA, USA) software were used for curve plotting and statistical analysis. The improvement in fit was analyzed by two-sided Student’s t-test and labeled “statistically significant” if the probability,p, was less than 0.05.
3. Results
3.1. DPI-201106 selectively inhibits the transport activity of ABCB1
The selectivity and the potential interaction of DPI-201106 with major MDR-linked ABC transporters were first examined by monitoring the inhibitory effect of DPI-201106 on fluorescent substrate transport mediated by ABCB1, ABCC1 or ABCG2. Paired drug-sensitive and drug-resistant cells overexpressing either ABCB1, ABCC1 or ABCG2 were treated with DMSO (solid lines), 10 μM of DPI-201106 (shaded, solid lines), or 10 μM of ABCB1 reference inhibitor verapamil (A, D and E, dotted lines) or 25 μM of ABCC1 reference inhibitor MK-571 (B, dotted lines) or 1 μM of Ko143 (C, dotted lines), and analyzed as described in Materials and methods. As shown in Fig. 1A, DPI-201106 significantly increased the accumulation of calcein, a known substrate drug of ABCB1[21] in HEK293 cells transfected with human ABCB1 (MDR19-HEK293). In contrast, DPI-201106 had no major effect on the accumulation of calcein and PhA, known fluorescent substrates of ABCC1 and ABCG2 [38] in HEK293 cells transfected with human ABCC1 (MRP1-HEK293) (Fig. 1B) or human ABCG2 (R482-HEK293), respectively (Fig. 1C). In order to eliminate the potential cell-type-specific responses, we examined the effect of DPI-201106 on the accumulation of calcein in human KB-3–1 epidermal cancer cells and the ABCB1-overexpressing variant KB-V-1cells, as well as in human OVCAR-8 ovarian cancer cells and the ABCB1-overexpressing variant NCI-ADR-RES cells. Comparable to the results obtained in MDR9-HEK293 cells, ABCB1-mediated transport of calcein from KB-V-1 (Fig. 1D, right panel) and NCI-ADR-RES cells (Fig. 1E, right panel) was fully inhibited by DPI-201106. Moreover, DPI-201106 inhibited ABCB1-mediated transport of calcein-AM in MDR19-HEK293 cells in a concentration-dependent manner, with a calculated IC50 value of approximately 0.13 μM. In comparison, the calcium channel blocker verapamil inhibited the transport function of ABCB1 with a considerably higher IC50 value of approximately 10 μM (Fig. 1F). Of note, DPI-201106 had no significant effect on the accumulation of fluorescent drugs in HEK293 cells or any of the drug-sensitive parental cells (Fig. 1A–1E, left panels).
Fig. 1. DPI-201106 selectively inhibits ABCB1-mediated drug efflux.

The accumulation of fluorescent calcein in HEK293 cells (A and B, left panels), HEK293 cells transfected with human ABCB1 (A, right panel) or human ABCC1 (B, right panel), human KB-3–1 epidermal cancer cells (D, left panel) and ABCB1-overexpressing KB-V-1 cancer cells (D, right panel), as well as in human OVCAR-8 ovarian cancer cells (E, left panel) and ABCB1-overexpressing NCI-ADR/RES cancer cells (E, right panel), or fluorescent PhA in HEK293 cells (C, left panel) and HEK293 cells transfected with human ABCG2 (C, right panel), was measured in the absence (solid lines) or presence of 500 nM DPI-201106 (shaded, solid lines) or 20 μM verapamil, a reference inhibitor for ABCB1 (A, D and E, dotted lines), 25 μM MK-571, a reference inhibitor for ABCC1 (B, dotted lines) or 3 μM Ko143, a reference inhibitor for ABCG2 (C, dotted lines), and analyzed immediately by flow cytometry as described previously [52] Representative histograms of three independent experiments are shown. (F) The concentration-dependent inhibition of ABCB1-mediated calcein-AM efflux by DPI-201106 (empty circles) or verapamil (filled circles) was determined in ABCB1-transfected MDR19-HEK293 cells. Values are presented as mean ± SD calculated from at least three independent experiments.
3.2. DPI-201106 reverses ABCB1-mediated drug resistance
Providing that DPI-201106 selectively inhibits the function of ABCB1, we examined the reversal effect of DPI-201106, at non-toxic concentrations, on ABCB1-mediated multidrug resistance in ABCB1-overexpressing cancer cells. First, we discovered that without affecting the proliferation of drug-sensitive parental KB-3–1 and OVCAR-8 cells, DPI-201106 re-sensitized KB-V-1 and NCI-ADR-RES multidrug resistant cancer cells to paclitaxel (Fig. 2A and 2B, right panels), a well-established drug substrate of ABCB1 [24], in a concentration-dependent manner. Similarly, ABCB1-mediated resistance to ABCB1 drug substrates doxorubicin and colchicine [24] was also reversed by DPI-201106 at nanomolar concentrations (Table 1). The reversal effect of DPI-201106 on ABCB1-mediated MDR in respective cell lines is presented as the relative resistance (RR) value [52], which corresponds to the extent of drug resistance of respective MDR cells to a particular therapeutic drug in the presence or absence of a reversing agent. At the highest tested concentration of 500 nM, DPI-201106 significantly re-sensitized KB-V-1 and NCI-ADR-RES cells to multiple therapeutic agents, providing approximately 23 to 350-fold and 20 to 96-fold increase in sensitivity, respectively (Table 1). In addition, the chemosensitization effect of DPI-201106 was further tested in MDR19-HEK293, MRP1-HEK293 cells and R482-HEK293 cells. As summarized in Table 2, DPI-201106 substantially restored the sensitivity of MDR19-HEK293 cells to paclitaxel, doxorubicin and colchicine by approximately 104-fold, 7-fold and 8-fold, respectively. In contrast, DPI-201106 was unable to reverse ABCC1-mediated etoposide resistance in MRP1-HEK293 cells or ABCG2-mediated topotecan resistance in R482-HEK293 cells, which is consistent with the results of the drug accumulation assays (Fig. 1). Of note, verapamil, MK-571 and Ko143 were used as positive controls for the reversal of drug resistance mediated by ABCB1, ABCC1 and ABCG2. Our data indicate that DPI-201106 is selective for ABCB1 relative to ABCC1 and ABCG2.
Fig. 2. DPI-201106 reverses ABCB1-mediated paclitaxel resistance in ABCB1-overexpressing MDR cell lines.

Drug-sensitive human KB-3–1 epidermal cancer cells (A, left panel) and ABCB1-overexpressing variant KB-V-1 cancer cells (A, right panel), drug-sensitive human OVCAR-8 ovarian cancer cells (B, left panel) and ABCB1-overexpressing variant NCI-ADR/RES cancer cells (B, right panel), as well as parental HEK293 cells (C, left panel) and HEK293 cells transfected with human ABCB1 (C, right panel) were treated with paclitaxel in the presence of DMSO (empty circles), or DPI-201106 at 50 nM (filled circles), 100 nM (empty squares), 200 nM (filled squares), 500 nM (empty triangles) or 5 μM of verapamil (filled triangles) as a positive control. Points, mean values from at least three independent experiments; bars; SEM.
Table 1.
Reversal effect of DPI-201106 on drug resistance mediated by ABCB1 in human cancer cell lines.
| Treatment | Concentration (nM) |
Mean IC50 ± SD† | RR‡ | |
|---|---|---|---|---|
| KB-3-1 (parental) |
KB-V-1 (resistant) |
|||
| [nM] |
[nM] |
|||
| Paclitaxel | - | 1.93 ± 0.63 | 2213.20 ± 168.58 | 1147 |
| + DPI-201106 | 50 | 2.04 ± 0.71 | 752.09 ± 109.68*** | 369 |
| + DPI-201106 | 100 | 1.88 ± 0.64 | 145.06 ± 20.56*** | 77 |
| + DPI-201106 | 200 | 1.93 ± 0.62 | 35.87 ± 3.68*** | 19 |
| + DPI-201106 | 500 | 1.91 ± 0.63 | 6.20 ± 0.55*** | 3 |
| + Verapamil | 5000 | 1.78 ± 0.45 | 64.14 ± 7.90*** | 36 |
| [nM] |
[μM] |
|||
| Doxorubicin | - | 25.13 ± 7.03 | 6.94 ± 1.40 | 359 |
| + DPI-201106 | 50 | 24.82 ± 4.33 | 1.42 ± 0.29** | 57 |
| + DPI-201106 | 100 | 23.76 ± 4.29 | 0.68 ± 0.13** | 29 |
| + DPI-201106 | 200 | 25.32 ± 4.40 | 0.25 ± 0.04** | 10 |
| + DPI-201106 | 500 | 24.22 ± 4.08 | 0.12 ± 0.02** | 5 |
| + Verapamil | 5000 | 6.38 ± 1.82* | 0.59 ± 0.09** | 14 |
| [nM] |
[nM] |
|||
| Colchicine | - | 7.63 ± 2.64 | 445.30 ± 27.69 | 58 |
| + DPI-201106 | 50 | 8.21 ± 2.83 | 144.85 ± 10.40*** | 18 |
| + DPI-201106 | 100 | 8.27 ± 2.72 | 62.57 ± 6.92*** | 8 |
| + DPI-201106 | 200 | 8.12 ± 2.78 | 42.71 ± 5.42*** | 5 |
| + DPI-201106 | 500 | 8.07 ± 2.83 | 18.97 ± 3.84*** | 2 |
| + Verapamil | 5000 | 4.53 ± 1.54 | 72.38 ± 14.21*** | 16 |
| Treatment |
Concentration (nM) |
OVCAR-8 (parental) |
NCI-ADR-RES (resistant) |
RR |
| [nM] |
[μM] |
|||
| Paclitaxel | - | 5.79 ± 1.09 | 5.13 ± 0.82 | 886 |
| + DPI-201106 | 50 | 11.14 ± 3.86 | 4.32 ± 0.93 | 388 |
| + DPI-201106 | 100 | 9.11 ± 2.96 | 0.77 ± 0.13*** | 85 |
| + DPI-201106 | 200 | 10.78 ± 2.95 | 0.17 ± 0.03*** | 16 |
| + DPI-201106 | 500 | 9.93 ± 2.80 | 53.60 ± 9.80 (nM) *** | 5 |
| + Verapamil | 5000 | 3.85 ± 0.80 | 0.20 ± 0.05*** | 52 |
| [μM] |
[μM] |
|||
| Doxorubicin | - | 0.20 ± 0.03 | 15.92 ± 1.12 | 80 |
| + DPI-201106 | 50 | 0.21 ± 0.03 | 4.47 ± 0.42*** | 21 |
| + DPI-201106 | 100 | 0.20 ± 0.02 | 1.92 ± 0.19*** | 10 |
| + DPI-201106 | 200 | 0.20 ± 0.03 | 1.11 ± 0.12*** | 6 |
| + DPI-201106 | 500 | 0.19 ± 0.03 | 0.78 ± 0.06*** | 4 |
| + Verapamil | 5000 | 0.17 ± 0.02 | 1.59 ± 0.17*** | 9 |
| [nM] |
[μM] |
|||
| Colchicine | - | 23.68 ± 6.63 | 2.78 ± 0.61 | 117 |
| + DPI-201106 | 50 | 39.84 ± 11.12 | 1.27 ± 0.24* | 32 |
| + DPI-201106 | 100 | 40.36 ± 10.70 | 0.49 ± 0.09** | 12 |
| + DPI-201106 | 200 | 37.56 ± 9.79 | 0.24 ± 0.06** | 6 |
| + DPI-201106 | 500 | 35.61 ± 8.69 | 0.08 ± 0.02** | 2 |
|
+ Verapamil |
5000 | 16.64 ± 4.77 | 0.46 ± 0.14** | 28 |
Abbreviation: RR, relative-resistance.
IC50 values are mean ± SD calculated from dose-response curves obtained from three independent experiments using cytotoxicity assay as described in Materials and methods.
RR values were calculated by dividing IC50 values of ABCBl-overexpressing MDR cancer cells by the IC50 values of drug-sensitive parental cells treated with the same regimen.
P < 0.05;
P < 0.01;
P < 0.001.
Table 2.
Chemosensitization effect of DPI-201106 on drug resistance mediated by ABCB1, ABCC1 or ABCG2.
| Treatment | Concentration (nM) |
Mean IC50 ± SD† | RR‡ | |
|---|---|---|---|---|
| pcDNA-HEK293 [nM] |
MDR19-HEK293 [nM] |
|||
| Paclitaxel | - | 1.86 ± 0.37 | 559.28 ± 86.87 | 301 |
| + DPI-201106 | 50 | 1.38 ± 0.28 | 194.56 ± 18.22** | 141 |
| + DPI-201106 | 100 | 1.37 ± 0.23 | 8.76 ± 0.43*** | 6 |
| + DPI-201106 | 200 | 1.23 ± 0.24 | 2.72 ± 0.56*** | 2 |
| + DPI-201106 | 500 | 1.18 ± 0.21 | 5.38 ± 1.10*** | 5 |
| + Verapamil | 5000 | 1.20 ± 0.21 | 8.38 ± 2.34*** | 7 |
| Doxorubicin | - | 7.49 ± 2.18 | 185.57 ± 15.97 | 25 |
| + DPI-201106 | 50 | 6.54 ± 1.35 | 74.49 ± 8.41*** | 11 |
| + DPI-201106 | 100 | 8.17 ± 2.47 | 39.15 ± 2.44*** | 5 |
| + DPI-201106 | 200 | 7.55 ± 2.07 | 38.53 ± 4.12*** | 5 |
| + DPI-201106 | 500 | 7.76 ± 1.84 | 28.45 ± 4.12*** | 4 |
| + Verapamil | 5000 | 5.44 ± 1.04 | 25.50 ± 3.59*** | 5 |
| Colchicine | - | 3.75 ± 1.45 | 82.50 ± 18.95 | 22 |
| + DPI-201106 | 50 | 3.60 ± 1.39 | 34.83 ± 8.59* | 10 |
| + DPI-201106 | 100 | 3.08 ± 1.12 | 15.43 ± 4.14** | 5 |
| + DPI-201106 | 200 | 3.53 ± 1.34 | 15.77 ± 6.02** | 4 |
| + DPI-201106 | 500 | 2.84 ± 1.00 | 10.18 ± 3.71** | 4 |
| + Verapamil | 5000 | 3.15 ± 1.26 | 1.96 ± 0.59** | 1 |
|
pcDNA-HEK293 [μM] |
MRP1-HEK293 [μM] |
|||
| Etoposide | - | 0.15 ± 0.03 | 59.61 ± 11.89 | 397 |
| + DPI-201106 | 500 | 0.12 ± 0.02 | 47.58 ± 10.31 | 397 |
| + MK-571 | 25000 | 0.06 ± 0.01** | 8.49 ± 1.36** | 142 |
|
pcDNA-HEK293 [nM] |
R482-HEK293 [nM] |
|||
| Topotecan | - | 22.90 ± 4.90 | 696.00 ± 107.42 | 30 |
| + DPI-201106 | 500 | 21.75 ± 5.30 | 502.99 ± 103.08 | 23 |
| + Ko143 | 1000 | 20.97 ± 4.56 | 23.12 ± 4.67*** | 1 |
Abbreviation: RR, relative-resistance.
IC50 values are mean ± SD calculated from dose-response curves obtained from three independent experiments using cytotoxicity assay as described in Materials and methods.
RR values were calculated by dividing IC50 values of HEK293 cells transfected with ABCB1, ABCC1 or ABCG2 by the IC50 values of parental HEK293 cells treated with the same regimen.
P < 0.05;
P < 0.01;
P < 0.001.
3.3. DPI-201106 does not affect the protein expression of ABCB1
Considering that drug-induced transient down-regulation of ABCB1 can re-sensitize ABCBl-overexpressing MDR cancer cells to chemotherapeutic agents [10, 35, 48], we examined the effect of DPI-201106 on the protein expression of ABCB1 in ABCB1-overexpressing cancer cells. Human KB-V-1 and human NCI-ADR-RES cancer cells were exposed to increasing concentrations of DPI-201106 (0 – 0.5 μM) for 72 h, harvested and processed by immunoblotting as described in Materials and methods. We found that DPI-201106 did not significantly alter the protein expression of ABCB1 in KB-V-1 (Fig. 3A) or NCI-ADR-RES cancer cells (Fig. 3B) over a period of 72 h. Our results indicate that DPI-201106 reverses ABCB1-mediated MDR by inhibiting the drug function of ABCB1, and not by down-regulating the protein expression of ABCB1.
Fig. 3. Effect of DPI-201106 on ABCB1 protein expression in human KB-V-1 epidermal cancer cells.
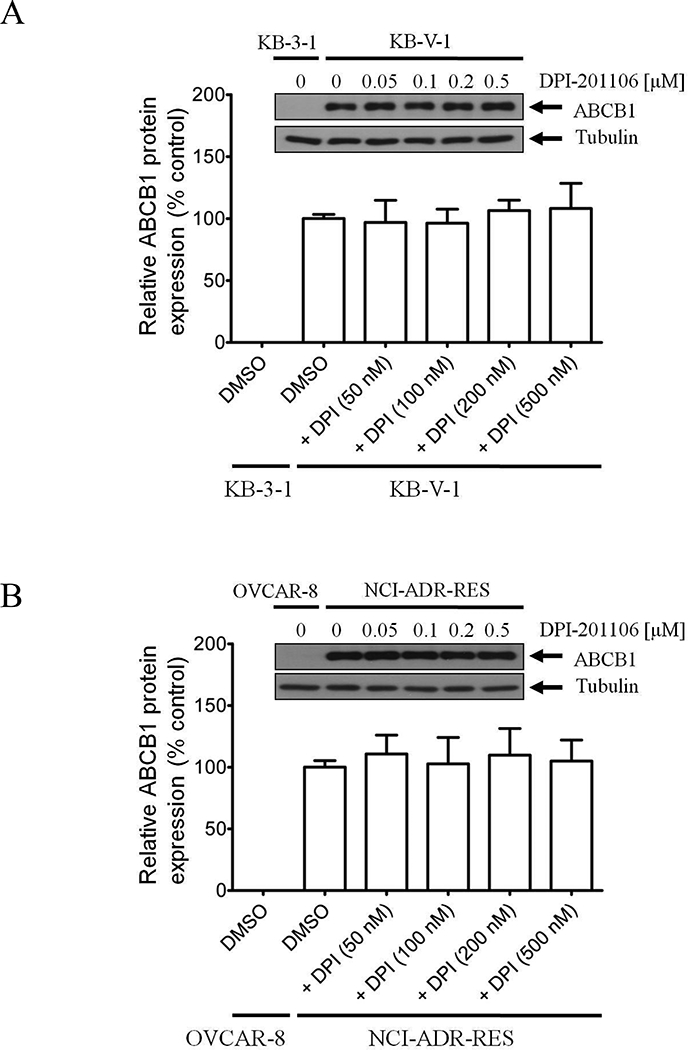
Immunoblot detection and quantification of human ABCB1 in (A) drug-sensitive human KB-3–1 epidermal cancer cells and ABCB1-overexpressing variant KB-V-1 cancer cells or (B) drug-sensitive human OVCAR-8 ovarian cancer cells and ABCB1-overexpressing variant NCI-ADR-RES cancer cells, treated with DMSO (vehicle control) or increasing concentrations (0.05 – 0.5 μM) of DPI-201106 for 72 h as described previously [52]. α-Tubulin was used as an internal loading control. Values are presented as mean ± SD calculated from three independent experiments.
3.4. DPI-201106 enhances drug-induced apoptosis in ABCB1-overexpressing cancer cells
Next, the potentiating effect of DPI-201106 on drug-induced apoptosis was examined in drug-sensitive KB-3–1 cells and drug-resistant variant KB-V-1 cells. As shown in Fig. 4, cells were treated with DMSO, DPI-201106, colchicine or combination of colchicine and DPI-201106 for 48 h and processed as described previously [22]. We found that colchicine, a known drug substrate of ABCB1[37], substantially increased the level of apoptosis in KB-3–1 cells, from 10% basal level to approximately 47% of early and late apoptosis (Fig. 4A, upper panels), but considerably less effective in KB-V-1 cells (Fig. 4A, lower panels). Notably, co-treatment of colchicine and DPI-201106 significantly enhanced the effect of colchicine-induced apoptosis in KB-V-1 cells, from 11% basal level to approximately 63% of early and late apoptosis (Fig. 4B). Of note, the level of apoptosis in KB-3–1 or KB-V-1 cell lines was not affected by DPI-201106 alone (Fig. 4A and 4B).
Fig. 4. DPI-201106 enhances drug-induced apoptosis in ABCB1-overexpressing cancer cells.

(A) Drug-sensitive human epidermal KB-3–1 cancer cells (top panels) and ABCB1-overexpressing MDR variant KB-V-1 cancer cells (lower panels) were treated with either DMSO (control), 500 nM DPI-201106 (+ DPI-201106), 500 nM colchicine (+ colchicine) or a combination of 500 nM colchicine and 500 nM of DPI-201106 (+ colchicine + DPI-201106) for 48 h. Cells were isolated and analyzed by flow cytometry as described previously [22]. Representative dot plots and the mean values of three independent experiments are shown. (B) Quantification of colchicine-induced apoptosis in human epidermal KB cell lines. Values are presented as mean ± SD calculated from three independent experiments. **p < 0.01, versus the same treatment in the absence of DPI-201106.
3.5. DPI-201106 inhibits ATPase activity of ABCB1
To gain insight into the interaction between DPI-201106 and the substrate-biding site of ABCB1, we examined the effect of DPI-201106 on vanadate (Vi)-sensitive ATPase activity of ABCB1. We found that DPI-201106 inhibited ABCB1 ATPase activity in a concentration-dependent manner, producing a 40% maximum inhibition (basal, 33.4 ± 5.1 nmole Pi/min/mg protein), and an IC50 value of approximately 0.5 μM (Fig. 5A). Knowing that substrate transport mediated by ABCB1 is coupled to ATP hydrolysis [3, 4], our result indicates that DPI-201106 inhibits the activity of ABCB1. These data suggest that DPI-201106 is either a weak transport substrate [25] or a modulator. Moreover, in order to understand the binding between ABCB1 protein and compound DPI-201106, docking study was performed using homology model generated from high resolution mouse (Mus musculus) Abcb1a protein crystal structure (4Q9L) as a template. Docking of DPI-201106 with modeled ABCB1 protein structure showed interactions similar to that was reported [44]. Three hydrophobic interactions between two phenyl rings of DPI-201106 and amino acid residues Ala302, Trp232 and Ile306, together with a hydrogen bond between Gln725 and the N-H hydrogen were suggested at the substrate binding site (Fig. 5B).
Fig. 5. DPI-201106 inhibits Vi-sensitive ATPase activity of ABCB1.

(A) The effect of DPI-201106 on ABCB1 ATP hydrolysis was determined as described previously [1]. Points, mean values from at least three independent experiments; bars; SD. (B) Binding modes of DPI-201106 with homology modeled ABCB1 protein structure was predicted by Acclerys Discovery Studio 4.0 software as described in Materials and methods. DPI-201106 is shown as a molecular model with the atoms colored as carbon- gray, hydrogen-light gray, nitrogen-blue and oxygen-red. The same color scheme is used for interacting amino acid residues. Dotted lines indicate proposed interactions.
3.6. ABCB1-overexpressing cells are not resistant to DPI-201106
Next, we examined whether the overexpression of ABCB1 leads to reduced susceptibility of cancer cells to DPI-201106. The cytotoxicity and the IC50 values of DPI-201106 were determine in multiple drug-sensitive and ABCB1-overexpressing MDR cancer cell lines, as well as in cells transfected with human ABCB1 (Table 3). The extent of acquired cellular resistance to DPI-201106 caused by the overexpression of ABCB1 was presented as the resistance factor (RF) value, which is calculated by dividing the IC50 value of DPI-201106 in ABCB1-overexpressing cell lines by the IC50 value of DPI-201106 in the respective drug-sensitive parental lines. Our results show that drug-sensitive cells and ABCB1-overexpressing drug-resistant cells are equally sensitive to DPI-201106, indicating that DPI-201106 is not transported out of cancer cells by ABCB1.
Table 3.
Cytotoxicity of DPI-201106 in drug-sensitive and ABCB1-overexpressing cell lines.
| Cell line | Cancer origin | Transporter expressed |
IC50 [μM] † | RF‡ |
|---|---|---|---|---|
| KB-3-1 | epidermal | - | 6.21 ± 1.48 | 1 |
| KB-V-1 | epidermal | ABCB1 | 5.45 ± 1.35 | 1 |
| OVCAR-8 | ovarian | - | 8.84 ± 2.31 | 1 |
| NCI-ADR-RES | ovarian | ABCB1 | 9.43 ± 2.68 | 1 |
| NIH3T3 | - | - | 3.33 ± 1.10 | 1 |
| NIH3T3-G185 | - | ABCB1 | 4.22 ± 0.84 | 1 |
| pcDNA-HEK293 | - | - | 5.73 ± 1.70 | 1 |
| MDR19-HEK293 | - | ABCB1 | 7.17 ± 1.85 | 1 |
Abbreviation: RF, resistance factor.
IC50 values are mean ± SD calculated from dose-response curves obtained from three independent experiments using cytotoxicity assay as described in Materials and methods.
RF values were calculated by dividing IC50 values of ABCB1-overexpressing cells by IC50 values of respective parental cells.
P < 0.05;
P < 0.01;
P < 0.001.
4. Discussion
The development of MDR phenotype associated with the overexpression of ABCB1 in cancer cells remains a major challenge in cancer treatment [15, 51], most prominently in chronic lymphocytic leukemia (CLL) [33], chronic myeloid leukemia (CML)[31], multiple myeloma (MM) [36, 40, 47] and metastatic breast cancer [27]. ABCB1 is capable of effluxing a wide variety of clinically important conventional anticancer drugs and molecularly targeted agents, including but not limited to anthracyclines, vinca alkaloids and many protein kinase inhibitors, out of cancer cells [18, 45, 46, 51]. Therefore, developing ABCB1-specific modulators to overcome MDR in cancer has clinical significance. Despite a large number of novel inhibitors of ABCB1 that have been developed in recent years, most have failed due to unforeseen toxicity and adverse drug events [42, 43]. We and others have thus explored the drug repurposing approach to identify candidate drugs with known pharmacological and toxicological properties as reversing agents for MDR cancer treatment [42]. However, due to the overlapping substrate specificity of ABCB1, ABCC1 and ABCG2, the task of identifying transporter-selective modulators has been difficult [16, 43].
DPI-201106 is a cardioselective modulator of VGSCs [34] with antiarrhythmic [39] and vasodilation [19] activities. It has been used regularly in in vitro and in vivo pharmacological studies at concentrations ranging from 1 – 100 μM [12, 13, 28, 32, 49], as well as in patients, given by an intravenous (IV) injection of 20 and 40 milligram doses [12]. In this study, we demonstrated that DPI-201106 re-sensitizes ABCB1-overexpressing cells to multiple therapeutic agents at nanomolar concentrations by inhibiting the drug efflux function of ABCB1, without altering the protein expression of ABCB1. Our findings were supported by the result that at a non-toxic concentration, DPI-201106 significantly enhances drug-induced apoptosis in ABCB1-overexpressing cancer cells. More importantly, we found that DPI-201106 is selective for ABCB1 relative to ABCC1 and ABCG2, as it has no significant effect on ABCC1- and ABCG2-mediated drug transport or drug resistance to etoposide or topotecan, which are established substrates for ABCC1 and ABCG2 [43]. Considering that inhibition of ABCB1 ATP hydrolysis is known to be associated with the presence of a high-affinity substrate or inhibitor at the substrate-binding site of ABCB1 [2], the fact that DPI-201106 inhibited ABCB1 ATPase activity in a concentration-dependent manner (Fig. 5A) indicates direct interaction between DPI-201106 and ABCB1. Results of homology modeling and drug docking analysis (Fig. 5B) further support the notion that DPI-201106 interacts with ABCB1 at the substrate-binding site, resulting in direct competition with another drug at the same site. Notably, these data are consistent with the results of the drug accumulation assay that DPI-201106 inhibits the transport function of ABCB1 by directly competing with another drug substrate (Fig. 1). Lastly, we found that ABCB1-overexpressing cancer cells and ABCB1-transfected cells are equally sensitive to DPI-201106 as their respective drug-sensitive parental cells, indicating that ABCB1 does not affect the susceptibility of cancer cells to DPI-201106. It is worth noting that despite encouraging experimental results obtained in our study, favorable experimental results from in vitro or in vivo drug combination studies do not always translate into favorable clinical outcomes [42]. For instance, it is possible that concomitant administration of DPI-201106 and chemotherapeutic agents may lead to altered pharmacokinetics and pharmacodynamics of ABCB1 substrate drugs, causing unforeseen adverse drug reactions in patients. Therefore, the clinical application of DPI-201106 in patients with MDR cancer remains to be determined.
In summary, our results demonstrate that DPI-201106 is a selective modulator of ABCB1, capable of inhibiting the transport function of ABCB1 and restoring chemosensitivity of ABCB1-overexpressing multidrug resistant cancer cells (Fig. 6). Our study suggests that the benefit of combination therapy with DPI-201106 and chemotherapeutic agents for the treatment of MDR tumors should be further evaluated in preclinical animal model studies and in clinical practice.
Fig. 6. A graphic illustration of DPI-201106 attenuating the drug efflux function of ABCB1 in cancer cells.

Anticancer drugs (white circles) are actively transported out of cancer cells by ABCB1, resulting in MDR phenotype (upper panel). In contrast, the presence of DPI-201106 (white rectangle) blocks ABCB1-mediated drug efflux and restores drug accumulation to a sufficient level that causes apoptosis in cancer cells overexpressing ABCB1.
Highlights.
DPI-201106, a positive inotropic agent, inhibits the function of ABCB1.
DPI-201106 re-sensitizes ABCB1-overexpressing cancer cells to anti-cancer drugs.
DPI-201106 enhances drug-induced apoptosis in multidrug-resistant cancer cells.
ABCB1 does not confer resistance to DPI-201106 in cancer cells.
Inclusion of DPI-201106 in the chemotherapy treatment may benefit patients with multidrug-resistant tumors in the clinic.
5. Acknowledgments
This work was supported by funds from the Ministry of Science and Technology of Taiwan (MOST-105–2320-B-182–018 and MOST-106–2320-B-182–017 to CPW; MOST-106–2314-B-182A-146 to THH), Chang Gung Medical Research Program (BMRPC17, CMRPD1D0153 and CMRPD1G0112 to CPW; BMRP688, NMRPG3G0371 and CMRPG3G0281 to THH), Taichung Veterans General Hospital (TCVGH-T1067802 and TCVGH-T1077802 to YSW) and the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research to SVA and MM.
Abbreviations:
- MDR
multidrug resistance
- ABC
ATP-binding cassette
- FCS
fetal calf serum
- CCK-8
Cell Counting Kit-8
- IMDM
Iscove’s Modified Dulbecco’s Medium
- MTT
3-(4,5-dimethylthiazol-yl)-2,5-diphenyllapatinibrazolium bromide
- Vi
sodium orthovanadate
- RF
resistance-factor
- RR
relative-resistance
Footnotes
Conflict of interest
The authors declare no conflict of interest.
7. References
- [1].Ambudkar SV, Drug-stimulatable ATPase activity in crude membranes of human MDR1-transfected mammalian cells, Methods Enzymol, 292 (1998) 504–514. [DOI] [PubMed] [Google Scholar]
- [2].Ambudkar SV, Cardarelli CO, Pashinsky I, Stein WD, Relation between the turnover number for vinblastine transport and for vinblastine-stimulated ATP hydrolysis by human P-glycoprotein, J Biol Chem, 272 (1997) 21160–21166. [DOI] [PubMed] [Google Scholar]
- [3].Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM, Biochemical, cellular, and pharmacological aspects of the multidrug transporter, Annual review of pharmacology and toxicology, 39 (1999) 361–398. [DOI] [PubMed] [Google Scholar]
- [4].Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM, P-glycoprotein: from genomics to mechanism., Oncogene, 22 (2003) 7468–7485. [DOI] [PubMed] [Google Scholar]
- [5].Anderson HA, Maylock CA, Williams JA, Paweletz CP, Shu H, Shacter E, Serum-derived protein S binds to phosphatidylserine and stimulates the phagocytosis of apoptotic cells, Nature immunology, 4 (2003) 87–91. [DOI] [PubMed] [Google Scholar]
- [6].Arnold K, Bordoli L, Kopp J, Schwede T, The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling, Bioinformatics, 22 (2006) 195–201. [DOI] [PubMed] [Google Scholar]
- [7].Benkert P, Biasini M, Schwede T, Toward the estimation of the absolute quality of individual protein structure models, Bioinformatics, 27 (2011) 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Biasini M, Bienert S, Waterhouse A, Arnold K, Studer G, Schmidt T, Kiefer F, Gallo Cassarino T, Bertoni M, Bordoli L, Schwede T, SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information, Nucleic acids research, 42 (2014) W252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bodo A, Bakos E, Szeri F, Varadi A, Sarkadi B, The role of multidrug transporters in drug availability, metabolism and toxicity, Toxicol Lett, 140–141 (2003)133–143. [DOI] [PubMed] [Google Scholar]
- [10].Cuestas ML, Castillo AI, Sosnik A, Mathet VL, Downregulation of mdr1 and abcg2 genes is a mechanism of inhibition of efflux pumps mediated by polymeric amphiphiles, Bioorg Med Chem Lett, 22 (2012) 6577–6579. [DOI] [PubMed] [Google Scholar]
- [11].Currier SJ, Kane SE, Willingham MC, Cardarelli CO, Pastan I, Gottesman MM, Identification of residues in the first cytoplasmic loop of P-glycoprotein involved in the function of chimeric human MDR1-MDR2 transporters, J Biol Chem, 267 (1992) 25153–25159. [PubMed] [Google Scholar]
- [12].Davis ME, Feneck RO, Jones CJ, Lunnon MW, Walesby RK, Effects of the inotrope DPI 201–106 on cardiac performance following cardiac surgery, International journal of cardiology, 29 (1990) 229–237. [DOI] [PubMed] [Google Scholar]
- [13].Doggrell S, Hoey A, Brown L, Ion channel modulators as potential positive inotropic compound for treatment of heart failure, Clinical and experimental pharmacology & physiology, 21 (1994) 833–843. [DOI] [PubMed] [Google Scholar]
- [14].Flesch M, Erdmann E, Na+ channel activators as positive inotropic agents for the treatment of chronic heart failure, Cardiovascular drugs and therapy, 15 (2001) 379–386. [DOI] [PubMed] [Google Scholar]
- [15].Gillet JP, Gottesman MM, Mechanisms of multidrug resistance in cancer, Methods Mol Biol, 596 (2010) 47–76. [DOI] [PubMed] [Google Scholar]
- [16].Gottesman MM, Fojo T, Bates SE, Multidrug resistance in cancer: role of ATP-dependent transporters, Nat Rev Cancer, 2 (2002) 48–58. [DOI] [PubMed] [Google Scholar]
- [17].Gribar JJ, Ramachandra M, Hrycyna CA, Dey S, V Ambudkar S, Functional characterization of glycosylation-deficient human P-glycoprotein using a vaccinia virus expression system, J Membr Biol, 173 (2000) 203–214. [DOI] [PubMed] [Google Scholar]
- [18].Hamidovic A, Hahn K, Kolesar J, Clinical significance of ABCB1 genotyping in oncology, J Oncol Pharm Pract, 16 (2010) 39–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Hof RP, Hof A, Mechanism of the vasodilator effects of the cardiotonic agent DPI 201–106, Journal of cardiovascular pharmacology, 7 (1985) 1188–1192. [DOI] [PubMed] [Google Scholar]
- [20].Hogan JC, Greenbaum RA, Lunnon MW, Hilson AJ, Evans TR, Haemodynamic effects of DPI 201–106, following single intravenous dose administration to patients with moderate cardiac failure, European heart journal, 9 (1988) 498–502. [DOI] [PubMed] [Google Scholar]
- [21].Hollo Z, Homolya L, Davis CW, Sarkadi B, Calcein accumulation as a fluorometric functional assay of the multidrug transporter, Biochimica et biophysica acta, 1191 (1994) 384–388. [DOI] [PubMed] [Google Scholar]
- [22].Hsiao SH, Lu YJ, Li YQ, Huang YH, Hsieh CH, Wu CP, Osimertinib (AZD9291) Attenuates the Function of Multidrug Resistance-Linked ATP-Binding Cassette Transporter ABCB1 in Vitro, Mol Pharm, (2016). [DOI] [PubMed] [Google Scholar]
- [23].Ishiyama M, Tominaga H, Shiga M, Sasamoto K, Ohkura Y, Ueno K, A combined assay of cell viability and in vitro cytotoxicity with a highly water-soluble tetrazolium salt, neutral red and crystal violet, Biol Pharm Bull, 19 (1996) 1518–1520. [DOI] [PubMed] [Google Scholar]
- [24].Kartner N, Riordan JR, Ling V, Cell surface P-glycoprotein associated with multidrug resistance in mammalian cell lines, Science, 221 (1983) 1285–1288. [DOI] [PubMed] [Google Scholar]
- [25].Kerr KM, Sauna ZE, Ambudkar SV, Correlation between steady-state ATP hydrolysis and vanadate-induced ADP trapping in Human P-glycoprotein. Evidence for ADP release as the rate-limiting step in the catalytic cycle and its modulation by substrates, J Biol Chem, 276 (2001) 8657–8664. [DOI] [PubMed] [Google Scholar]
- [26].Kostis JB, Lacy CR, Raia JJ, Dworkin JH, Warner RG, Casazza LA, DPI 201–106 for severe congestive heart failure, The American journal of cardiology, 60 (1987) 1334–1339. [DOI] [PubMed] [Google Scholar]
- [27].Kovalev AA, Tsvetaeva DA, Grudinskaja TV, Role of ABC-cassette transporters (MDR1, MRP1, BCRP) in the development of primary and acquired multiple drug resistance in patients with early and metastatic breast cancer, Experimental oncology, 35 (2013) 287–290. [PubMed] [Google Scholar]
- [28].Krafte DS, Davison K, Dugrenier N, Estep K, Josef K, Barchi RL, Kallen RG, Silver PJ, Ezrin AM, Pharmacological modulation of human cardiac Na+ channels, Eur J Pharmacol, 266 (1994) 245–254. [DOI] [PubMed] [Google Scholar]
- [29].Leslie EM, Deeley RG, Cole SP, Multidrug resistance proteins: role of P-glycoprotein, MRP1, MRP2, and BCRP (ABCG2) in tissue defense, Toxicol Appl Pharmacol, 204 (2005) 216–237. [DOI] [PubMed] [Google Scholar]
- [30].Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, Louis DN, Christiani DC, Settleman J, Haber DA, Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib, N Engl J Med, 350 (2004) 2129–2139. [DOI] [PubMed] [Google Scholar]
- [31].Maia RC, Vasconcelos FC, Souza PS, Rumjanek VM, Towards Comprehension of the ABCB1/P-Glycoprotein Role in Chronic Myeloid Leukemia, Molecules, 23 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Marinov BS, A possible role of the redox interactions in the dual, activatory and inhibitory, action of DPI 201–106 on the potential-dependent Na+ channels, Membrane & cell biology, 10 (1997) 707–715. [PubMed] [Google Scholar]
- [33].Matthews C, Catherwood MA, Larkin AM, Clynes M, Morris TC, Alexander HD, MDR-1, but not MDR-3 gene expression, is associated with unmutated IgVH genes and poor prognosis chromosomal aberrations in chronic lymphocytic leukemia, Leuk Lymphoma, 47 (2006) 2308–2313. [DOI] [PubMed] [Google Scholar]
- [34].Mevissen M, Denac H, Schaad A, Portier CJ, Scholtysik G, Identification of a cardiac sodium channel insensitive to synthetic modulators, Journal of cardiovascular pharmacology and therapeutics, 6 (2001) 201–212. [DOI] [PubMed] [Google Scholar]
- [35].Natarajan K, Bhullar J, Shukla S, Burcu M, Chen ZS, Ambudkar SV, Baer MR, The Pim kinase inhibitor SGI-1776 decreases cell surface expression of P-glycoprotein (ABCB1) and breast cancer resistance protein (ABCG2) and drug transport by Pim-1-dependent and -independent mechanisms, Biochem Pharmacol, 85 (2013)514–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pilarski LM, Belch AR, Intrinsic expression of the multidrug transporter, P-glycoprotein 170, in multiple myeloma: implications for treatment, Leuk Lymphoma, 17 (1995) 367–374. [DOI] [PubMed] [Google Scholar]
- [37].Riordan JR, Ling V, Purification of P-glycoprotein from plasma membrane vesicles of Chinese hamster ovary cell mutants with reduced colchicine permeability, J Biol Chem, 254 (1979) 12701–12705. [PubMed] [Google Scholar]
- [38].Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, Mistry P, Bates SE, Pheophorbide a is a specific probe for ABCG2 function and inhibition, Cancer Res, 64 (2004) 1242–1246. [DOI] [PubMed] [Google Scholar]
- [39].G Scholtysik FM Williams, Antiarrhythmic effects of DPI 201–106, Br J Pharmacol, 89 (1986) 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Schwarzenbach H, Expression of MDR1/P-glycoprotein, the multidrug resistance protein MRP, and the lung-resistance protein LRP in multiple myeloma, Medical oncology, 19 (2002) 87–104. [DOI] [PubMed] [Google Scholar]
- [41].Shen DW, Fojo A, Chin JE, Roninson IB, Richert N, Pastan I, Gottesman MM, Human multidrug-resistant cell lines: increased mdr1 expression can precede gene amplification, Science, 232 (1986) 643–645. [DOI] [PubMed] [Google Scholar]
- [42].Shukla S, Wu CP, Ambudkar SV, Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges, Expert Opin Drug Metab Toxicol, 4 (2008) 205–223. [DOI] [PubMed] [Google Scholar]
- [43].Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM, Targeting multidrug resistance in cancer, Nature reviews, 5 (2006) 219–234. [DOI] [PubMed] [Google Scholar]
- [44].Y Tajima H Nakagawa A Tamura O Kadioglu K Satake Y Mitani H Murase LO Regasini S Bolzani Vda T Ishikawa G Fricker T Efferth, Nitensidine A, a guanidine alkaloid from Pterogyne nitens, is a novel substrate for human ABC transporter ABCB1, Phytomedicine : international journal of phytotherapy and phytopharmacology, 21 (2014) 323–332. [DOI] [PubMed] [Google Scholar]
- [45].Tamaki A, Ierano C, Szakacs G, Robey RW, Bates SE, The controversial role of ABC transporters in clinical oncology, Essays Biochem, 50 (2011) 209–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Tang C, Schafranek L, Watkins DB, Parker WT, Moore S, Prime JA, White DL, Hughes TP, Tyrosine kinase inhibitor resistance in chronic myeloid leukemia cell lines: investigating resistance pathways, Leuk Lymphoma, 52 (2011) 2139–2147. [DOI] [PubMed] [Google Scholar]
- [47].Tsubaki M, Satou T, Itoh T, Imano M, Komai M, Nishinobo M, Yamashita M, Yanae M, Y Yamazoe S Nishida, Overexpression of MDR1 and survivin, and decreased Bim expression mediate multidrug-resistance in multiple myeloma cells, Leuk Res, 36 (2012) 1315–1322. [DOI] [PubMed] [Google Scholar]
- [48].Wang SQ, Liu ST, Zhao BX, Yang FH, Wang YT, Liang QY, Sun YB, Liu Y, Song ZH, Cai Y, Li GF, Afatinib reverses multidrug resistance in ovarian cancer via dually inhibiting ATP binding cassette subfamily B member 1, Oncotarget, 6 (2015) 26142–26160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wang YJ, Lin MW, Lin AA, Peng H, Wu SN, Evidence for state-dependent block of DPI 201–106, a synthetic inhibitor of Na+ channel inactivation, on delayed-rectifier K+ current in pituitary tumor (GH3) cells, Journal of physiology and pharmacology : an official journal of the Polish Physiological Society, 59 (2008) 409–423. [PubMed] [Google Scholar]
- [50].Wu CP, Calcagno AM, Ambudkar SV, Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies, Current molecular pharmacology, 1 (2008) 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Wu CP, Hsieh CH, Wu YS, The emergence of drug transporter-mediated multidrug resistance to cancer chemotherapy, Mol Pharm, 8 (2011) 1996–2011. [DOI] [PubMed] [Google Scholar]
- [52].Wu CP, Shukla S, Calcagno AM, Hall MD, Gottesman MM, Ambudkar SV, Evidence for dual mode of action of a thiosemicarbazone, NSC73306: a potent substrate of the multidrug resistance linked ABCG2 transporter, Mol Cancer Ther, 6 (2007) 3287–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]


