Abstract
Rationale & Objective
Abnormal cardiac structure and function is common in chronic kidney disease (CKD) and end-stage renal disease (ESRD) and linked with mortality and heart failure. We examined changes in echocardiogram measures during the transition from CKD to ESRD and their associations with post-ESRD mortality.
Study design
Prospective study.
Setting and participants
We studied 417 participants with CKD in the Chronic Renal Insufficiency Cohort (CRIC) who had research echocardiograms during CKD and ESRD.
Predictor
We measured change in left ventricular mass index (LVMI), ejection fraction (LVEF), diastolic relaxation (normal, mildly abnormal, moderately/severely abnormal),, end systolic (LVESV) and diastolic (LVEDV) volume and left atrial (LA) volume, from CKD to ESRD.
Outcomes
All-cause mortality after dialysis initiation.
Analytical Approach
Cox proportional hazard models were used to test the association of change in each echocardiographic measure with post-dialysis mortality.
Results
Over a mean of 2.9 years between pre- and post-dialysis echocardiograms, there was worsening of mean LVEF (52.5% to 48.6%, p<0.001) and LVESV (18.6 to 20.2 ml/m2.7, p<0.001). during this time, there was improvement in LVMI (60.4 to 58.4 g/m2.7, p=0.005) and diastolic relaxation (11.11% to 4.94% with moderately/severely abnormal; p=0.02). Changes in LA volume (4.09 to 4.15, p=0.08) or LVEDV (38.6 to 38.4 ml/m2.7, p=0.8) were not significant. Worsening from CKD to ESRD of LVEF (adjusted hazard ratio [aHR] for every 1% decline in LVEF, 1.03; 95% CI, 1.00–1.06) and LVESV (aHR for every 1 ml/m2.7 increase, 1.04; 95% CI, 1.02–1.07) were independently associated with greater risk of post-dialysis mortality.
Limitations
Some missing or technically inadequate echocardiograms.
Conclusions
In a longitudinal study of patients with CKD who subsequently initiated dialysis, LVEF and LVESV worsened and were significantly associated with greater risk of post-dialysis mortality. There may be opportunities for intervention during this transition period to improve outcomes.
Keywords: Kidney, Heart Failure, Echocardiogram, Dialysis, CKD to ESRD transition, dialysis initiation, cardiovascular disease (CVD), left ventricular mass index (LVMI), left ventricular ejection fraction (LVEF), diastolic relaxation, left atrial volume, left ventricular end-systolic volume (LVESV), left ventricular end-diastolic volume (LVEDV), cardiac disease, all-cause mortality, subclinical CVD, end-stage renal disease (ESRD)
INTRODUCTION
Patients with chronic kidney disease (CKD) and end-stage renal disease (ESRD) have a substantial burden of cardiovascular disease (CVD), which is associated with high levels of morbidity and mortality.1–3 In CKD and ESRD patients, abnormalities in left ventricular structure and function are common and often precede the onset of clinical CVD, including heart failure, which is one of the most prevalent cardiovascular subtypes in CKD. The majority of incident maintenance dialysis patients have left ventricular hypertrophy (LVH),4,5 which is an independent predictor of CVD and death.6,7 In addition, low left ventricular ejection fraction (LVEF), even without clinical heart failure, has been shown to be a risk factor for cardiovascular and all-cause mortality both among patients with CKD and those with ESRD.8,9
Few studies have examined the evolution of subclinical CVD as CKD progresses to ESRD. Previously, we studied 190 participants from the Chronic Renal Insufficiency Cohort (CRIC) and noted a 3% decline in LVEF but no statistically significant change in left ventricular mass index (LVMI) between stage 4 CKD and ESRD (mean time gap was 2.0 ± 1.0 years).10 In a subset of 182 Initiating Dialysis Early and Late (IDEAL) trial participants who had serial echocardiograms 12 months apart11 (the majority of participants had started dialysis by the second echocardiogram), there was no change in LVMI, left atrial (LA) volume, diastolic dysfunction or LVEF.11 In contrast, substantial improvements in echocardiogram parameters have been reported after kidney transplantation.12,13 Furthermore, other echocardiographic measures, such as left ventricular end-systolic volume (LVESV), a measure of contractility and ventricular remodeling,14–16 and left ventricular end-diastolic volume (LVEDV), a measure of preload and diastolic function,17,18 have not been well described in patients with kidney disease and larger volumes have been shown to be associated with poor outcomes in the general population. Notably, no prior studies have related change in these measures of subclinical CVD as measured by echocardiogram with clinical outcomes in patients with kidney disease
In the current study, we expanded on our prior analysis10 by increasing our sample size, examining change in echocardiographic parameters over a longer time period, broadening our analyses to include additional echocardiographic parameters (such as LA volume, diastolic relaxation, LVESV), and most importantly, testing the association of changes in echocardiographic parameters with mortality after progression to ESRD. We hypothesized that there is significant worsening of echocardiogram measures during the transition from CKD to ESRD, and that this worsening of subclinical cardiovascular disease (assessed by echocardiograms) is associated with greater risk of post-ESRD mortality.
METHODS
Study Participants
We analyzed data from the Chronic Renal Insufficiency Cohort (CRIC) study, an ongoing multi-center prospective cohort study of persons with CKD recruited from seven clinical centers (with 13 enrolling sites). The eligibility criteria and baseline characteristics have been previously published.19,20 The CRIC study initially enrolled CKD patients with estimated glomerular filtration rate (eGFR) 20–70 ml/min/1.73m2 by the MDRD Study equation. Exclusion criteria included New York Heart Association Class III or IV heart failure. IRB approval was obtained from all participating institutions and informed consent to participate in CRIC was obtained from all subjects.
CRIC participants have annual in-person visits and phone contact every six-months. At the annual in-person visits, participants complete questionnaires updating interim medical events and medication use and undergo laboratory testing.
Research echocardiograms were performed at the following time points: follow-up visits one and four years after study entry, at the first study visit at which a participant’s eGFR was <20 ml/min/1.73 m2 and at the first follow-up visit after start of maintenance dialysis or receipt of a kidney transplant (defined as ESRD).10,21
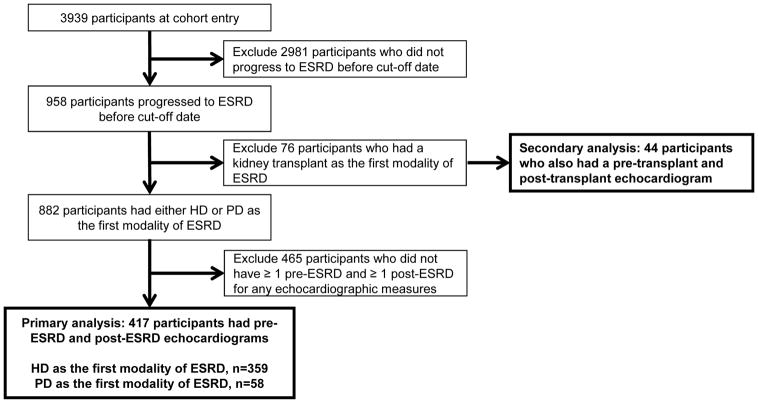
For our primary analysis, we included CRIC participants who initiated maintenance hemodialysis or peritoneal dialysis and had complete echocardiogram performed prior to and after ESRD onset through March 2013 (Figure 1). We analyzed the first technically adequate echocardiogram during the pre-ESRD CKD phase of disease (referred to as the “CKD echocardiogram”), which for the most participants was the Year 1 echocardiogram. We also analyzed the first, technically adequate echocardiogram after initiation of hemodialysis or peritoneal dialysis. Participants on hemodialysis (typically three times a week among CRIC enrollees) were targeted to have their echocardiogram performed one day following a dialysis session (which was achieved in ~80% of participants in our study). A total of 417 participants were included in our sample (Figure 1). In comparing the participants who initiated dialysis who were included versus those who were excluded from the analysis, there were few differences in baseline characteristics or Year 1 echocardiogram measures (Table S1).
Figure 1.
Flowchart of the study population
In secondary analyses, for comparison and because there are reports that that kidney transplantation is associated with substantial improvements in echocardiogram parameters,12,13 we examined the subset of 44 CRIC study participants who had a pre-emptive kidney transplant (i.e. underwent kidney transplantation prior to needing maintenance dialysis) and had paired echocardiograms (pre and post transplantation) performed as part of the CRIC protocol (Figure 1). Kidney transplant recipients included versus excluded in this analyses had similar baseline characteristics (Table S2).
Echocardiogram Measurements
Assessments of cardiac structure and function were performed by echocardiography according to American Society of Echocardiography guidelines.22 Transthoracic echocardiograms were performed at the individual sites in accordance with a standard imaging protocol. Sonographers were initially trained in telephone conference calls and provided with a detailed scanning manual complete with a checklist. The CRIC Central Echocardiography Laboratory at the University of Pennsylvania monitored quality control and adherence to the scanning protocol and provided the sites with evaluations of the quality of the first several hundred echocardiograms. Supplemental training was provided on an as-needed basis. All echocardiograms were then quantified at the CRIC Central Echocardiography Laboratory by a single Registered Diagnostic Cardiac Sonographer who was unaware of the identity of the participants whose echocardiograms were being analyzed.
Left ventricular mass (LVM) was calculated from 2D images of the left ventricular short axis muscle area and apical left ventricular length (LVM = (5/6 area*length)). 2D echocardiography had been selected by the CRIC Steering Committee to be used in all primary analyses of CRIC echocardiographic data.10,23 LVMI was defined using the Cornell Criteria and indexed to height (in meters) raised to the power of 2.7.22 LVEF was calculated using diastolic and systolic left ventricular volumes measured by the single plane Simpson’s rule method: EF= ((Dvol-Svol)/Dvol) *100. Left ventricular geometry was based on LVMI and relative wall thickness (RWT) and divided into four categories: (1) normal (normal LVMI and normal RWT), (2) concentric remodeling (normal LVMI and high RWT), (3) eccentric hypertrophy (elevated LVMI and normal RWT) and (4) concentric hypertrophy (elevated LVMI and elevated RWT). Diastolic relaxation based on traditional spectral Doppler measurements of mitral inflow and pulmonary venous flow. These included E wave to A wave ratio (E/A ratio), isovolumic relaxation time (IVRT), E wave deceleration time and pulmonary venous A wave duration.24 We confirmed these traditional Doppler definitions by comparing to color M-Mode E-wave propagation velocities in a subset of patients. Left atrial (LA) volume was quantified and reported in ml/m2. Left ventricular end-systolic (LVESV) and diastolic volumes (LVEDV) were indexed for height and reported in mL/m2.7.
Reproducibility, inter-reader reliability, intra-reader reliability and reader drift analyses were performed periodically on a 2% random sample. The intra-class correlation coefficients for the echocardiographic measures are: 0.922 for left ventricular mass, 0.848 for diastolic dysfunction, 0.688 for RWT and 0.854 for LVEF.
Post-ESRD all-cause mortality
All-cause mortality after ESRD was the primary outcome. Deaths were identified through report from next of kin, retrieval of death certificates or obituaries, review of hospital records, and linkage with the Social Security Mortality Master File through March 31, 2013.25,26
Covariates
Information on covariates19,20 was obtained from the closest CRIC study visits prior to the CKD and ESRD echocardiograms respectively. Covariates included demographic characteristics, physical examination components, self-reported co-morbid conditions, tobacco use, and medication use within 30 days of study visit. Self-reported history of CVD included coronary heart disease, myocardial infarction or revascularization, heart failure, stroke or peripheral vascular disease and were tallied cumulatively from earlier to later visits. Blood pressure measurement was performed in a quiet, standardized setting. Three seated resting blood pressure readings were obtained at each visit and the average of the three seated resting blood pressure readings were used for this study.21,27–29 Concurrent medication use was ascertained at study visits (i.e., participant medication bottles or updated medication list).
Statistical Considerations
We compared differences in participant characteristics between the CKD and ESRD visits. We then compared the CKD echocardiogram parameters with the ESRD echocardiogram parameters. We used paired t-test models to compare normally distributed variables and the Wilcoxon signed rank sum test for non-normally distributed variables. For categorical variables, we used the McNemar’s test for 2-level variables and the Bowler’s test of symmetry for >2-level variables. Missing echocardiogram measures were not included in the analyses.
We then performed multivariable Cox models to test the association of risk of post-ESRD mortality with (1) each ESRD measure and (2) change in each echocardiogram measure from CKD to ESRD. For these time-to-event analyses, start time was always the date of the ESRD echocardiogram in order to keep the follow-up duration constant. Changes in LVMI, LVEF, LVESV, LVEDV and left atrial volume were considered as continuous variables. Change in left ventricular geometry was defined as stable (no change in categories); worsened (changing from normal to any abnormal category or changing from concentric remodeling to either eccentric or concentric hypertrophy); or improved (changing from eccentric/concentric hypertrophy to either concentric remodeling or normal; or changing from concentric remodeling to normal). Change in diastolic relaxation was defined as stable (no change in category); worsening or improving (based on categories of severity). Models were adjusted for: demographic characteristics, modality of ESRD (PD or HD), history of CVD, diabetes, smoking status, level of systolic blood pressure, body mass index (BMI) and number of anti-hypertensive drug classes (self-report). We confirmed that there were no violations in the proportional hazards assumption by testing a time*exposure interaction term.
To complement our main analysis, we also examined whether patient characteristics at CKD and changes in characteristics from CKD to ESRD predicted change in echocardiogram measures from CKD to ESRD. We chose characteristics based on a priori hypotheses and biological plausibility. Models were adjusted for the corresponding echocardiogram measure at CKD.
In a secondary analysis, we repeated our analyses in the subgroup who had received a pre-emptive kidney transplant as their first ESRD treatment modality (i.e. did not transition from CKD to maintenance hemodialysis or peritoneal dialysis).
All analyses were done using SAS 9.2, Cary, NC.
RESULTS
Characteristics of study participants
Among our 417 participants, mean (±SD) age was 57 (±11) years at the time of the CKD echocardiogram; 41% were female and 56% were non-Hispanic black (Table 1). Mean eGFR at the time of the CKD echocardiogram was 25 (±10) ml/min/1.73 m2.
Table 1.
Characteristics of study participants at CKD and at initiation of HD or PD
| Variable | CKD1 | ESRD1* | P value2 |
|---|---|---|---|
| Age, year, mean±SD | 56.97±11.10 | 59.88±11.18 | |
| Male sex | 245 (58.75%) | . | |
| Race, N(%) | |||
| Non-Hispanic white | 82 (19.66%) | . | |
| Non-Hispanic black | 234 (56.12%) | . | |
| Hispanic | 85 (20.38%) | . | |
| Other | 16 (3.84%) | . | |
| Current smoker, N(%) | 55 (13.19%) | 42 (10.07%) | 0.02 |
| eGFR, ml/min/1.73m2, mean±SD | 24.59±9.75 | . | |
| Body mass index, kg/m2, mean±SD | 32.92±8.24 | 30.94±7.51 | <.001 |
| Systolic blood pressure, mmHg, mean±SD | 139.73±23.89 | 132.20±25.41 | <.001 |
| Diastolic blood pressure, mmHg, mean±SD | 73.13±13.52 | 67.13±13.38 | <.001 |
| LDL cholesterol, mg/dl, mean±SD | 98.83±37.55 | 84.71±32.32 | <.001 |
| HDL cholesterol, mg/dl, mean±SD | 46.11±14.97 | 46.62±15.91 | 0.6 |
| Triglycerides, mg/dl, mean±SD | 144.00 [94.00–207.00] | 132.00 [98.00–173.00] | 0.6 |
| Total cholesterol, mg/dl, mean±SD | 184.07±51.40 | 167.99±47.06 | 0.001 |
| Hemoglobin, g/dl, mean±SD | 11.49±1.68 | 12.18±1.60 | <.001 |
| HbA1c, %, mean±SD | 7.27±1.71 | 6.43±1.40 | <.001 |
| Serum albumin, g/dl, mean±SD | 3.68±0.51 | 3.73±0.50 | 0.1 |
| Hypertension, N(%) | 411 (98.56%) | 415 (99.52%) | 0.05 |
| Cardiovascular disease, N(%) | 186 (44.60%) | 263 (63.07%) | <.001 |
| Heart failure, N (%) | 63 (15.1%) | 120 (28.8%) | <0.001 |
| Chronic obstructive pulmonary disease, N(%) | 16 (3.88%) | 42 (10.17%) | <.001 |
| Diabetes, N(%) | 290 (69.54%) | 300 (71.94%) | 0.002 |
| β-blockers, N(%) | 253 (61.11%) | 291 (71.50%) | 0.001 |
| ACEi/ARB | 281 (67.87%) | 199 (48.89%) | <.001 |
| Calcium channel blockers, N(%) | 254 (61.35%) | 229 (56.27%) | 0.08 |
| Diuretics, N(%) | 316 (76.33%) | 163 (40.05%) | <.001 |
| No. of anti-hypertensive medication classes, N(%) | 3.43±1.50 | 2.89±1.50 | <.001 |
| Erythropoiesis-stimulating agents | 65 (15.70%) | 88 (21.62%) | 0.02 |
| Dialysis access, N(%)3 | |||
| Venous catheter | NA | 246 (59.7) | |
| Arteriovenous fistula | NA | 81 (19.7) | |
| Arteriovenous graft | NA | 27 (6.6) | |
| Peritoneal dialysis catheter | NA | 58 (14.1) | |
| Unknown | NA | 5 (1.2) |
N=417.
For categorical variables, n (%) is reported. For continuous variables, mean±sd is reported if normally distributed, and median [quartile 1-quartile 3) is reported if non-normally distributed.
To compare characteristics between CKD and HD/PD, the P values are generated using the McNemar’s test for binary variables, and the paired t test or the Wilcoxon signed rank sum test for continuous variables.
NA= not applicable
ACEi/ARB, Angiotensin converting enzyme inhibitors/Angiotensin receptor blockers; LDL, ____; HDL, _____; eGFR, ______; CKD, _____; PD, ____; HD, _____; HbA1c, ____.
Defined as hemodialysis or peritoneal dialysis.
During progression from CKD to dialysis initiation, study participants on average had a reduction in BMI, had lower blood pressure and higher hemoglobin (associated with an increased prevalence in use of erythropoiesis stimulating agents). Prevalence of CVD, COPD and diabetes increased over time. Use of β-blockers increased, use of ACE inhibitors/ARBs and diuretics decreased; and overall number of anti-hypertensive medications decreased (Table 1). The proportion of patients taking calcium channel blockers did not significantly change from CKD to ESRD. Of the participants who were taking calcium channel blockers, the majority were taking dihydropyridines (81.1% at CKD and 87.3% at ESRD).
Change in echocardiograms from CKD to dialysis initiation
The mean time between the CKD and post-dialysis echocardiogram was 2.9 years (and 0.89 years after the initiation of dialysis). Comparing echocardiograms performed at CKD to those done after dialysis initiation, there was a 4% worsening (decrease) in LVEF (<0.001), a mean 2 g/m2.7 improvement (reduction) in LVMI (p=0.005), and improvement in diastolic relaxation (p=0.02)(Table 2). There was also a worsening (increase) of LVESV (p=<0.001). Although not statistically significant, we observed nominally improved LV geometry but nominally worsened (increasing) LA volume. LVEDV was unchanged.
Table 2.
Echocardiographic measures at CKD and at initiation of HD or PD
| Variable | No. of pairs of measureme nts within a person | CKD | ESRD* | P value1 |
|---|---|---|---|---|
| Left ventricular mass index, g/m2.7 | 340 | 60.36±15.58 | 58.37±13.70 | 0.005 |
| Ejection fraction, % | 387 | 52.45±9.03 | 48.59±9.61 | <.0001 |
| Left ventricular geometry | 305 | 0.09 | ||
| Normal | 27 (8.85%) | 38 (12.46%) | ||
| Concentric remodeling | 47 (15.41%) | 39 (12.79%) | ||
| Eccentric hypertrophy | 62 (20.33%) | 74 (24.26%) | ||
| Concentric hypertrophy | 169 (55.41%) | 154 (50.49%) | ||
| Diastolic relaxation | 324 | 0.02 | ||
| Normal relaxation | 72 (22.22%) | 73 (22.53%) | ||
| Mildly abnormal relaxation | 216 (66.67%) | 235 (72.53%) | ||
| Moderately & Severely abnormal relaxation | 36 (11.11%) | 16 (4.94%) | ||
| Left atrial volume (ml/m2) | 368 | 4.09±0.63 | 4.15±0.68 | 0.08 |
| Left ventricular end-systolic volume (ml/m2.7) | 366 | 18.60±6.89 | 20.21±8.92 | <0.001 |
| Left ventricular end-diastolic volume (ml/m2.7) | 372 | 38.59±9.36 | 38.44±10.48 | 0.8 |
Values shown are mean±sd or n (%)
Bold= statistically significant
N=417.
To compare echocardiographic measures between CKD and dialysis initiation, the P values are generated using the paired t test for continuous variables and the Bowker’s test of symmetry for more than two levels of categorical variables.
Defined as hemodialysis or peritoneal dialysis.
Participants who were women, who had higher systolic blood pressure at CKD and who used calcium channel blockers at CKD and who were treated with peritoneal dialysis were less likely to have a decline in LVEF from CKD to ESRD (Table S3). Congruently, women, those taking calcium channel blockers or taking a higher number of anti-hypertensives, and those who initiated peritoneal dialysis were less likely to have a worsening (increase) of LVESV from CKD to ESRD.
Association of dialysis echocardiogram measures with post-dialysis mortality
Among the 417 participants, there were 105 deaths after ESRD over a mean follow-up time of 3.2 years. The rate of death overall was 79.3 per 1000 person-years. Lower LVEF, higher LVMI, higher LA volume, higher LVESV and higher LVEDV from the echocardiogram performed after dialysis initiation were independent risk factors for mortality in multivariable models (Table S4).
Association of change in echocardiogram measures from CKD to dialysis initiation with post-dialysis mortality
We tested the association of change in each echocardiogram parameter with risk of all-cause mortality after dialysis initiation. Decline in LVEF from CKD to ESRD was significantly associated with greater risk of post-dialysis mortality in both unadjusted and adjusted models (Table 3). Every 1% decline in LVEF was associated with a 3% greater risk of mortality after ESRD (HR, 1.03; 95% CI, 1.00–1.06). Additionally, increase in LVESV (another measure of worsening contractility) was also significantly associated with greater risk of post-dialysis mortality. In adjusted models, every 1 ml/m2.7 increase in LVESV was associated with a 4% greater risk of mortality after ESRD (HR, 1.04; 95% CI, 1.02–1.07).
Table 3.
Associations of changes in echocardiographic measures from CKD to ESRD and risk of all-cause mortality after ESRD
| Variable | Change from CKD to ESRD | |
|---|---|---|
| Unadjusted HR (95% CI) |
Adjusted1 HR (95% CI) |
|
| Left ventricular mass index, per 1g/m2.7 increase | 1.01 (0.99, 1.02) | 1.01 (0.99, 1.03) |
| Ejection fraction, per 1% decline | 1.04 (1.01, 1.05) | 1.03 (1.00, 1.06) |
| Left ventricular geometry | ||
| Stable | 1.00 (reference) | 1.00 (reference) |
| Worsened | 1.14 (0.54, 2.40) | 0.63 (0.25, 1.60) |
| Improved | 0.51 (0.20, 1.28) | 0.70 (0.27, 1.85) |
| Diastolic relaxation | ||
| Stable | 1.00 (reference) | 1.00 (reference) |
| Worsened | 1.24 (0.65, 2.34) | 1.31 (0.60, 2.86) |
| Improved | 0.96 (0.53, 1.72) | 0.95 (0.49, 1.84) |
| Left atrial volume, per 1 ml/m2 increase | 1.21 (0.88, 1.67) | 0.99 (0.68, 1.45) |
| Height-adjusted LVESV, per 1-ml/m2.7 increase | 1.05 (1.02, 1.07) | 1.04 (1.02, 1.07) |
| Height-adjusted LVEDV, per 1-ml/m2.7 increase | 1.03 (1.02, 1.05) | 1.03 (1.01, 1.05) |
Bold=p<0.05
The model is adjusted for age, sex, race, dialysis modality, cardiovascular disease, systolic blood pressure, number of anti-hypertensive med classes, diabetes, current smoker, and BMI. Except for sex, race, and first modality of ESRD, all the variables are from the visit closest to the echocardiographic measures at ESRD.
LVESV, left ventricular end-systolic volume; LVEDV, left ventricular end-diastolic volume
Secondary analysis: participants who received a pre-emptive kidney transplant
Among the 44 participants available for this analysis (Table S5 and S6), there were no statistically significant differences in echocardiogram measures from CKD to post-transplantation (Table S6).
DISCUSSION
We prospectively studied cardiac structure and function in a cohort of participants with CKD prior to and after initiation of dialysis. consistent with our original hypothesis, we noted a worsening (decrease) in LVEF (on average 4%) as well as a worsening (increase) of LVESV (on average 2 ml/m2.7). However, contrary to our original hypothesis, there was a modest but statistically significant improvement (reduction) in LVMI (on average 2 g/m2.7) and improvement in diastolic relaxation. The worsening in LVEF and LVESV, both measures of left ventricular contractility, during CKD progression to dialysis were significantly associated with greater risk of post-dialysis mortality. Thus, clinically meaningful echocardiogram parameters are not fixed, but rather progress or regress even in advanced stages of CKD. These data suggest there are opportunities for intervention during the transition from CKD to dialysis initiation to reduce the risk of mortality and cardiovascular disease. Improving (early) mortality from cardiovascular disease in incident ESRD patients has been identified as a high clinical and public health priority by many, including Health People 2020, which is a program of nationwide health-promotion and disease-prevention goals set by the U.S. Department of Health and Human Services.30
Prior reports have been conflicting on changes in echocardiographic measures during the important physiologic transition from CKD through the initiation of dialysis. In a study of 41 heart failure patients, LVMI improved after initiation of HD.31 In our previous study of stage 4 CKD participants in CRIC, we also noted a decline in LVEF but could not detect a statistically significant change in LVMI. However in the present study, sample size was larger (417 vs. 190) and more time elapsed between echocardiograms (mean of 3 vs. 2 years) which allowed more opportunity for evolution of natural history of disease. Our findings differ from the single-center Hong Kong CASCADE study of stages 3–5 CKD patients, which found that LVMI, LA volume and diastolic dysfunction worsened over 1 year, particularly in participants at more advanced stages of CKD (140 CASCADE enrollees had CKD stages 4 & 5).32 In contrast, in the IDEAL trial conducted in Australia and New Zealand, a subset of 182 participants had serial echocardiograms 12 months apart11 and there was no change in LVMI, LA volume, diastolic dysfunction or LVEF (97% of participants had started dialysis by the second echocardiogram).11 Important differences between our study and the CASCADE study and IDEAL trial are: (1) our study was a multi-center center study which consisted of a diverse U.S. patient population that is larger in size (thus may be greater power to detect differences) and focused exclusively on patients who transitioned to ESRD; (2) 40% of patients in IDEAL initiated PD (vs. HD) which may have affected volume and blood pressure and thus echocardiogram measures; and (3) most of the patients in CASCADE did not initiate dialysis over the course of the study.
In our study, modest worsening in LVEF and LVESV from CKD to dialysis initiation were significantly associated with greater risk of post-dialysis mortality. Very few studies have examined change in echocardiogram measures with important clinical outcomes. One longitudinal study of prevalent HD reported that participants in the top quartile of decline of left ventricular systolic function (over a mean time of 17 months apart) had a 51% increase in cardiovascular events over the subsequent 3 years.33
LVESV is a well-established marked of contractility and ventricular remodeling in the non-CKD population.14–16 In a study of patients with known coronary disease, those with the highest LVESV had a 5-fold increased risk of hospitalization for heart failure.14 Another study found that increases in LVESV in response to exercise was associated with increased risk of mortality among patients with stable coronary disease.34 These data suggest that strategies to improve LVESV may have important prognostic implications.
It is likely that the modest worsening of LVEF and LVESV from CKD to ESRD observed in our study signal early heart failure. Heart failure is one of the most common causes of CVD in patients on dialysis and is associated with numerous complications including increased risk of hospitalizations35 and mortality.36 Furthermore, heart failure is a common cause of abrupt declines in eGFR and may hasten progression to ESRD.37 Patients who initiate dialysis for heart failure/volume overload may have increased risk of mortality after ESRD compared with other indications for dialysis initiation.38 Thus further study of therapies to prevent worsening LVEF and LVESV and reduce the risk of heart failure may significantly improve outcomes during the transition from CKD to ESRD
Our study was novel in that we examined a comprehensive panel of echocardiographic measures including LVEF, LVESV, diastolic relaxation and LA volume. Few prior studies have examined the importance of these other echocardiogram predictors in dialysis patients. An investigation of 230 dialysis patients reported that reduced LVEF (≤ 48%) was a significant predictor of sudden cardiac death.39 In a study of 48 black HD patients, investigators found that global longitudinal strain (an alternative marker of left systolic function), but not LVEF, was associated with greater risk of mortality.40 In another study among 194 incident HD patients, diastolic dysfunction and LA volume were associated with mortality, but not LVEF or LVMI.41 Zoccali et al also reported no association of LVEF with risk of all-cause mortality among 254 dialysis patients.42 However this study was relatively small in size and was conducted over 10 years ago and may not reflect current management of dialysis patients (e.g. in terms of anemia and blood pressure). Our data suggest that LVEF, LVESV and LA volume are important measures of subclinical CVD in incident HD patients. Our study augments the previous body of literature by studying a relatively large, contemporary multi-center cohort of dialysis patients and evaluating several important echocardiographic measures collectively in the same study participants.
Antihypertensive medication use during the transition from CKD to ESRD among our study participants is notable in that ACE inhibitor/ARB and diuretic use decreased substantially, while β-blocker use increased. A recent study among over 32,000 U.S. Veterans found that lower utilization of cardiovascular medications in the year prior to dialysis initiation was independently associated with greater risk of all-cause and CVD mortality after dialysis initiation.43 Another recent study from USRDS examined CVD medication use during the transition from CKD to ESRD and found that ACE inhibitor/ARB use was only 40%, even among patients who may have clinical indications, such as systolic heart failure.44 In our study population, use of calcium channel blockers was associated with less decline in LVEF. Although we conducted exploratory analyses regarding correlations between starting and stopping different classes of antihypertensive medications and changes in echocardiographic parameters (Table S2), due to confounding by indication and other potential biases, these results should be interpreted with caution. Nevertheless, data from CRIC and others suggest that appropriate cardiovascular care during CKD may improve subclinical cardiovascular disease as well as improve clinical outcomes around the time of transition from CKD to dialysis initiation.
We had hypothesized that patients with advanced CKD who received a pre-emptive kidney transplant vs. started maintenance dialysis would fare better. However we did not note a statistically significant difference between these two groups, perhaps related to the relatively small number of transplant patients. Not surprisingly, the echocardiographic parameters at CKD are generally better among patients treated with pre-emptive kidney transplantation than those initiated on dialysis. Systolic function was largely preserved at CKD among our study participants which may be one reason we did not find substantial improvements in LVEF after transplantation, which have been reported from prior studies.1213 But those studies preferentially enrolled patients with decreased pre-transplant LVEF13 or relied on serial echocardiograms obtained in the course of routine clinical care (when physicians may be more likely to order follow-up echocardiograms in patients with lower LVEF or other clinical indications).12
Our study had numerous strengths. We utilized a unique, relatively large, multi-center prospective longitudinal cohort of patients with CKD. Serial research-grade echocardiograms were performed at key points of transition (eg, CKD and after ESRD) and were quantified by a single reader in a central laboratory, which is less prone to bias compared with echocardiograms performed for clinical indications. We were able to adjust for a variety of covariates obtained at study visits. We recognize some limitations as well. Although this is to our knowledge the largest study to date, the overall sample size was limited due to missing or technically inadequate echocardiograms among CRIC study participants. We may thus be underpowered for some of our analyses, including examining the transplant population. Although we did not observe large differences between patients who were included vs. excluded in our study (on the basis of missing echocardiograms), selection bias cannot be ruled out. The CRIC study excluded patients with advanced heart failure on cohort entry; thus this may limit the generalizability of our findings. However, study participants who subsequently developed heart failure, including around time of transition to ESRD, were included (approximately 29% of patients had HF by ESRD). To quantify diastolic function, more “traditional” parameters were used due to the fact that a majority of centers were not using tissue Doppler when the CRIC protocol was finalized. We were not able to study the contribution of valvular disease on our outcomes of interest due to limited number of patients with valvular disorders. The CRIC study did not systematically collect measures of residual renal function nor achieved dry weight in ESRD patients, thus we were not able to examine these variables in our analysis. We chose here not examine subtypes of clinical outcomes such as heart failure after ESRD due to limited number of events. This was a study of research volunteers, so results may not be generalizable to all CKD patients.
In conclusion, in a longitudinal study of contemporary CKD patients who subsequently initiated dialysis, our study found that LVEF and LVESV worsened from CKD to ESRD, while LVMI and diastolic dysfunction improved. Even modest changes in LVEF and LVESV from CKD to dialysis initiation were significantly associated with greater risk of post-ESRD mortality. Collectively these data support the need for further studies to test CVD interventions during the transition from CKD to dialysis initiation to reduce post-dialysis mortality.
Supplementary Material
Table S1. Baseline characteristics of participants who initiated dialysis who were included versus excluded in the analysis.
Table S2. Baseline characteristics of participants who had a kidney transplant as the first modality of ESRD who were included versus excluded in the analysis.
Table S3. Association of CKD characteristics with changes in echocardiographic measures from CKD to ESRD.
Table S4. Associations of echocardiographic measures at ESRD and risk of subsequent all-cause mortality.
Table S5. Characteristics at CKD and ESRD for participants who had a kidney transplant as the first modality of ESRD.
Table S6. Echocardiographic measures at CKD and ESRD for participants who had a kidney transplant as the first modality of ESRD.
Acknowledgments
Support: This work was supported by the following grants: K23 DK088865 (Bansal), R01 DK70939 (Hsu), K24 DK92291 (Hsu). Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). This work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003 and K01 DK092353 (Anderson), Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the NIH and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago CTSA UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente NIH/NCRR UCSF-CTSI UL1 RR-024131. The funders did not have a role in study design, data collection, analysis, interpretation of the data, and writing of the manuscript.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
Authors’ Contributions: Research idea and study design: NB, JR, CYH; data acquisition: EF, ASG, JH, MK, MR, CYH; data analysis/interpretation: NB, JR, HYC, RD, MD, MF, EF, ASG, JH, MK, JWK, EM, SDN, MR, CYH; statistical analysis: NB, JR, HYC, CYH. EM died while the manuscript was being revised; NB affirms that he contributed to data analysis/interpretation and vouches for his coauthorship status; all other authors approved the final author list. Except as noted, each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Herzog CA, Ma JZ, Collins AJ. Poor long-term survival after acute myocardial infarction among patients on long-term dialysis. N Engl J Med. 1998;339(12):799–805. doi: 10.1056/NEJM199809173391203. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351(13):1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 3.Chan KE, Maddux FW, Tolkoff-Rubin N, Karumanchi SA, Thadhani R, Hakim RM. Early outcomes among those initiating chronic dialysis in the United States. Clin J Am Soc Nephrol. 2011;6(11):2642–2649. doi: 10.2215/CJN.03680411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE. Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int. 1996;49(5):1379–1385. doi: 10.1038/ki.1996.194. [DOI] [PubMed] [Google Scholar]
- 5.Parfrey PS, Foley RN, Harnett JD, Kent GM, Murray DC, Barre PE. Outcome and risk factors for left ventricular disorders in chronic uraemia. Nephrol Dial Transplant. 1996;11(7):1277–1285. [PubMed] [Google Scholar]
- 6.Foley RNPP, Harnett JD, Kent GM, Murray DC, Barre PE. The prognostic importance of left ventricular geometry in uremic cardiomyopathy. J Am Soc Nephrol. 1995;5(12):2024–2031. doi: 10.1681/ASN.V5122024. [DOI] [PubMed] [Google Scholar]
- 7.Silberberg JS, Barre PE, Prichard SS, Sniderman AD. Impact of left ventricular hypertrophy on survival in end-stage renal disease. Kidney Int. 1989;36(2):286–290. doi: 10.1038/ki.1989.192. [DOI] [PubMed] [Google Scholar]
- 8.Yamada S, Ishii H, Takahashi H, et al. Prognostic Value of Reduced Left Ventricular Ejection Fraction at Start of Hemodialysis Therapy on Cardiovascular and All-Cause Mortality in End-Stage Renal Disease Patients. Clin J Am Soc Nephrol. 2010;5(10):1793–1798. doi: 10.2215/CJN.00050110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu IW, Hung MJ, Chen YC, et al. Ventricular function and all-cause mortality in chronic kidney disease patients with angiographic coronary artery disease. J Nephrol. 2010;23(2):181–188. [PubMed] [Google Scholar]
- 10.Bansal N, Keane M, Delafontaine P, et al. A longitudinal study of left ventricular function and structure from CKD to ESRD: the CRIC study. Clin J Am Soc Nephrol. 2013;8(3):355–362. doi: 10.2215/CJN.06020612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Whalley GA, Marwick TH, Doughty RN, et al. Effect of early initiation of dialysis on cardiac structure and function: results from the echo substudy of the IDEAL trial. Am J Kidney Dis. 2013;61(2):262–270. doi: 10.1053/j.ajkd.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 12.Hawwa N, Shrestha K, Hammadah M, Yeo PS, Fatica R, Tang WH. Reverse Remodeling and Prognosis Following Kidney Transplantation in Contemporary Patients With Cardiac Dysfunction. J Am Coll Cardiol. 2015;66(16):1779–1787. doi: 10.1016/j.jacc.2015.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wali RK, Wang GS, Gottlieb SS, et al. Effect of kidney transplantation on left ventricular systolic dysfunction and congestive heart failure in patients with end-stage renal disease. J Am Coll Cardiol. 2005;45(7):1051–1060. doi: 10.1016/j.jacc.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 14.McManus DD, Shah SJ, Fabi MR, Rosen A, Whooley MA, Schiller NB. Prognostic value of left ventricular end-systolic volume index as a predictor of heart failure hospitalization in stable coronary artery disease: data from the Heart and Soul Study. J Am Soc Echocardiogr. 2009;22(2):190–197. doi: 10.1016/j.echo.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76(1):44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- 16.Burns RJ, Gibbons RJ, Yi Q, et al. The relationships of left ventricular ejection fraction, end-systolic volume index and infarct size to six-month mortality after hospital discharge following myocardial infarction treated by thrombolysis. Journal of the American College of Cardiology. 2002;39(1):30–36. doi: 10.1016/s0735-1097(01)01711-9. [DOI] [PubMed] [Google Scholar]
- 17.Bauters C, Dubois E, Porouchani S, et al. Long-term prognostic impact of left ventricular remodeling after a first myocardial infarction in modern clinical practice. PLoS One. 2017;12(11):e0188884. doi: 10.1371/journal.pone.0188884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okada K, Kaga S, Abiko R, et al. Novel echocardiographic method to assess left ventricular chamber stiffness and elevated end-diastolic pressure based on time-velocity integral measurements of pulmonary venous and transmitral flows. European heart journal cardiovascular Imaging. 2017 doi: 10.1093/ehjci/jex305. epub Dec 2017. [DOI] [PubMed] [Google Scholar]
- 19.Feldman HI, Appel LJ, Chertow GM, et al. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14(7 Suppl 2):S148–153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 20.Lash JP, Go AS, Appel LJ, et al. Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline Characteristics and Associations with Kidney Function. Clin J Am Soc Nephrol. 2009;4(8):1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bansal N, McCulloch CE, Rahman M, et al. Blood pressure and risk of all-cause mortality in advanced chronic kidney disease and hemodialysis: the chronic renal insufficiency cohort study. Hypertension. 2015;65(1):93–100. doi: 10.1161/HYPERTENSIONAHA.114.04334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 23.Navaneethan SD, Roy J, Tao K, et al. Prevalence, Predictors, and Outcomes of Pulmonary Hypertension in CKD. J Am Soc Nephrol. 2016;27(3):877–886. doi: 10.1681/ASN.2014111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Appleton CP, Firstenberg MS, Garcia MJ, Thomas JD. The echo-Doppler evaluation of left ventricular diastolic function. A current perspective. Cardiology clinics. 2000;18(3):513–546. ix. doi: 10.1016/s0733-8651(05)70159-4. [DOI] [PubMed] [Google Scholar]
- 25.Deo R, Shou H, Soliman EZ, et al. Electrocardiographic Measures and Prediction of Cardiovascular and Noncardiovascular Death in CKD. J Am Soc Nephrol. 2016;27(2):559–569. doi: 10.1681/ASN.2014101045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Isakova T, Xie H, Yang W, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muntner P, Anderson A, Charleston J, et al. Hypertension awareness, treatment, and control in adults with CKD: results from the Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 55(3):441–451. doi: 10.1053/j.ajkd.2009.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bansal N, McCulloch CE, Lin F, et al. Blood Pressure and Risk of Cardiovascular Events in Patients on Chronic Hemodialysis: The CRIC Study (Chronic Renal Insufficiency Cohort) Hypertension. 2017;70(2):435–443. doi: 10.1161/HYPERTENSIONAHA.117.09091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bansal N, McCulloch CE, Lin F, et al. Different components of blood pressure are associated with increased risk of atherosclerotic cardiovascular disease versus heart failure in advanced chronic kidney disease. Kidney Int. 2016;90(6):1348–1356. doi: 10.1016/j.kint.2016.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chronic Kidney Disease. [Accessed February 7, 2018];Healthy People 2020. https://www.healthypeople.gov/2020/topics-objectives/topic/chronic-kidney-disease/objectives.
- 31.Ganda A, Weiner SD, Chudasama NL, et al. Echocardiographic changes following hemodialysis initiation in patients with advanced chronic kidney disease and symptomatic heart failure with reduced ejection fraction. Clin Nephrol. 2012;77(5):366–375. doi: 10.5414/cn107169. [DOI] [PubMed] [Google Scholar]
- 32.Cai QZ, Lu XZ, Lu Y, Wang AY. Longitudinal changes of cardiac structure and function in CKD (CASCADE study) J Am Soc Nephrol. 2014;25(7):1599–1608. doi: 10.1681/ASN.2013080899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zoccali C, Benedetto FA, Tripepi G, et al. Left ventricular systolic function monitoring in asymptomatic dialysis patients: a prospective cohort study. J Am Soc Nephrol. 2006;17(5):1460–1465. doi: 10.1681/ASN.2005111240. [DOI] [PubMed] [Google Scholar]
- 34.Turakhia MP, McManus DD, Whooley MA, Schiller NB. Increase in end-systolic volume after exercise independently predicts mortality in patients with coronary heart disease: data from the Heart and Soul Study. Eur Heart J. 2009;30(20):2478–2484. doi: 10.1093/eurheartj/ehp270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Trespalacios FC, Taylor AJ, Agodoa LY, Bakris GL, Abbott KC. Heart failure as a cause for hospitalization in chronic dialysis patients. Am J Kidney Dis. 2003;41(6):1267–1277. doi: 10.1016/s0272-6386(03)00359-7. [DOI] [PubMed] [Google Scholar]
- 36.Banerjee D, Ma JZ, Collins AJ, Herzog CA. Long-term survival of incident hemodialysis patients who are hospitalized for congestive heart failure, pulmonary edema, or fluid overload. Clin J Am Soc Nephrol. 2007;2(6):1186–1190. doi: 10.2215/CJN.01110307. [DOI] [PubMed] [Google Scholar]
- 37.Hsu RK, Chai B, Roy JA, et al. Abrupt Decline in Kidney Function Before Initiating Hemodialysis and All-Cause Mortality: The Chronic Renal Insufficiency Cohort (CRIC) Study. Am J Kidney Dis. 2016;68(2):193–202. doi: 10.1053/j.ajkd.2015.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rivara MB, Chen CH, Nair A, Cobb D, Himmelfarb J, Mehrotra R. Indication for Dialysis Initiation and Mortality in Patients With Chronic Kidney Failure: A Retrospective Cohort Study. Am J Kidney Dis. 2017;69(1):41–50. doi: 10.1053/j.ajkd.2016.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang AY, Lam CW, Chan IH, Wang M, Lui SF, Sanderson JE. Sudden cardiac death in end-stage renal disease patients: a 5-year prospective analysis. Hypertension. 2010;56(2):210–216. doi: 10.1161/HYPERTENSIONAHA.110.151167. [DOI] [PubMed] [Google Scholar]
- 40.Pressman GS, Seetha Rammohan HR, Romero-Corral A, Fumo P, Figueredo VM, Gorcsan J., 3rd Echocardiographic strain and mortality in Black Americans with end-stage renal disease on hemodialysis. Am J Cardiol. 2015;116(10):1601–1604. doi: 10.1016/j.amjcard.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 41.Han JH, Han JS, Kim EJ, et al. Diastolic dysfunction is an independent predictor of cardiovascular events in incident dialysis patients with preserved systolic function. PLoS One. 2015;10(3):e0118694. doi: 10.1371/journal.pone.0118694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zoccali C, Benedetto FA, Mallamaci F, et al. Prognostic value of echocardiographic indicators of left ventricular systolic function in asymptomatic dialysis patients. J Am Soc Nephrol. 2004;15(4):1029–1037. doi: 10.1097/01.asn.0000117977.14912.91. [DOI] [PubMed] [Google Scholar]
- 43.Molnar MZ, Gosmanova EO, Sumida K, et al. Predialysis Cardiovascular Disease Medication Adherence and Mortality After Transition to Dialysis. Am J Kidney Dis. 2016;68(4):609–618. doi: 10.1053/j.ajkd.2016.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang TI, Zheng Y, Montez-Rath ME, Winkelmayer WC. Antihypertensive Medication Use in Older Patients Transitioning from Chronic Kidney Disease to End-Stage Renal Disease on Dialysis. Clin J Am Soc Nephrol. 2016;11(8):1401–1412. doi: 10.2215/CJN.10611015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of participants who initiated dialysis who were included versus excluded in the analysis.
Table S2. Baseline characteristics of participants who had a kidney transplant as the first modality of ESRD who were included versus excluded in the analysis.
Table S3. Association of CKD characteristics with changes in echocardiographic measures from CKD to ESRD.
Table S4. Associations of echocardiographic measures at ESRD and risk of subsequent all-cause mortality.
Table S5. Characteristics at CKD and ESRD for participants who had a kidney transplant as the first modality of ESRD.
Table S6. Echocardiographic measures at CKD and ESRD for participants who had a kidney transplant as the first modality of ESRD.