Abstract
Objective
To identify rates of overweight (BMI ≥85th percentile) and obesity (BMI ≥95th percentile) at 6-7 years of age and associated risk factors among extremely preterm infants born at < 28 weeks of gestation.
Study design
Anthropometrics, blood pressure, and active and sedentary activity levels were prospectively assessed. Three groups were compared, those with BMI≥85th percentile (overweight or obese for age, height and sex) and ≥95th percentile (obese) versus <85th percentile. Multiple regression analyses estimated the relative risks of BMI ≥85th percentile and ≥95th percentile associated with perinatal and early childhood factors.
Results
Of 388 children, 22% had a BMI≥85th percentile and 10% were obese. Obese children and overweight children compared with normal weight children had higher body fat (subscapular skinfold and triceps skinfold> 85th percentile), central fat (waist circumference> 90th percentile), spent more time in sedentary activity (20.5 versus 18.2 versus 16.7 hours/week), and had either systolic and/or diastolic hypertension (24% versus 26% versus 14 %) respectively. Post discharge weight gain velocities from 36 weeks postmenstrual age (PMA) to 18 months, and 18 months to 6-7 years were independently associated with BMI ≥85th percentile whereas weight-gain velocity from 18 months to 6-7 years was associated with obesity.
Conclusions
One in five former extremely preterm infants is overweight or obese and has central obesity at early school age. Post discharge weight gain velocities were associated with overweight and obesity. These findings suggest the obesity epidemic is spreading to the most extremely preterm infants.
Trial registration ClinicalTrials.gov NCT00063063 and NCT0000
Keywords: preterm, overweight, obese, hypertension, sedentary activity, school age
The current epidemic of obesity and associated cardiovascular morbidities in the United States is a significant public health problem,1–4,4–7 but there have been few longitudinal investigations of very preterm infants to evaluate whether they are at risk for long term effects of overweight and obesity.7–11 Most studies of older PT infants reported that they are shorter and weigh less than term control infants.8, 11, 12–18 However, there has been limited evaluation of rates of overweight and obesity among extremely preterm (EPT) children at early school age born in the post-surfactant era.
Findings of elevated body mass index (BMI) in childhood are of concern because obesity is associated with abnormal levels of lipids, insulin and blood pressure (BP)19, 20 Barker21, 22 first proposed the “fetal origins” of adult disease hypothesis, demonstrating that alterations in fetal nutrition causing intrauterine growth restriction were associated with developmental adaptations that predispose to adult cardiovascular and metabolic disease. The original concept in growth-restricted term infants23 has been expanded to concerns about “rapid early catch-up growth” of preterm infants predisposing to increased cardiovascular risk and obesity.7, 23
The primary objective of this study was to identify the risk for weight-related outcomes at early school age among EPT children born at < 28 weeks of gestation, including rates of overweight or obesity, obesity, central obesity, and associated high BP and hypertension. The second objective was to determine risk factors associated with BMI ≥85th percentile and BMI ≥95th percentile, including weight gain velocities from birth to 36 weeks postmenstrual age (PMA), 36 weeks PMA to 18-22 months corrected age (CA), and 18-22 months CA to 6-7 years. Associations of BMI ≥85th percentile with maternal and neonatal risk factors and 6-7 year BP and activity levels were examined. It was hypothesized that increased early weight gain velocity would be associated with overweight, obesity and central obesity among EPT.
Methods
The obesity study was a selective secondary to the National Institute of Child Health and Human Development (NICHD) Neonatal Network SUPPORT Neuroimaging and Neurodevelopmental Outcomes (NEURO) school age cohort.24 The children were originally enrolled in SUPPORT, a randomized, multicenter trial of ventilation and oxygenation management strategies for infants born at 24-276/7 weeks of gestation (ClinicalTrials.gov: NCT00233324)25, 26 The subset of children with anthropometric data at 6-7 year follow-up was eligible (N=412). Twenty-four children were excluded for severe growth failure associated with short bowel syndrome (N=2), a congenital syndrome or chromosomal abnormality (N=9), and missing weight (N=4) or height data (N=9) needed to compute BMI percentiles. In the final dataset, 388/412 (94%) children were included. Prenatal and neonatal data from the SUPPORT cohort database including demographics, anthropometrics and BP obtained at 6 years, 4 months to 7 years, 2 months were analyzed.
Measurements at birth, 36 weeks PMA, and 18-22 months CA were retrieved for analyses from the NRN database. Fenton27 growth charts were used at birth and 36 weeks PMA and the Centers for Disease Control and Prevention growth charts28, 29 were used at 18 months CA and 6-7 years of age. Standardized methods for measurements were utilized at all sites.30 Duplicate measurements of weight, height, head circumference, waist circumference, subcutaneous skinfold measurements and BP were obtained at 6-7 years of age.
Overweight or obesity was defined as a BMI >85th percentile and obesity as a BMI >95th percentile. BMI was calculated as Wt (Kg)/height (M2)/ A waist circumference > 90th percentile was used as an indicator of central adiposity.31 Skinfolds were categorized as ≥85th percentile (overweight) and ≥95th percentile (obese).32 Measurements obtained to differentiate central adiposity from peripheral adiposity included triceps, subscapular, and abdominal skinfolds which are part of a standard assessment of obesity for children.33
Skinfolds were measured with a Lange Skinfold Caliper (Cambridge Scientific Industries, Inc., Cambridge, MD) using standard techniques. All skinfold measurements were taken twice on the right side. If there was > 2 mm difference between measurements, the measurement was taken a 3rd time and the 2 measurements within 2 mm of each other were recorded. Waist circumference was taken horizontally at the level of the narrowest part of the torso with the child standing and relaxed. Head and waist circumferences were measured twice using an inelastic, flexible, retractable metal tape. The head circumference was the largest occipital-frontal measurement obtained with the tape placed superior to the eyebrows. Weight and height were obtained using a standard upright scale. The head was in the Frankfort plane, with subjects fully erect and in stocking feet. Weight gain velocities were calculated as grams/week from birth to 36 weeks PMA, and kg/month from 36 weeks PMA to 18-22 months CA and 18-22 months CA to 6-7 years of age. Site examiners were trained in all measurement techniques.34 Techniques recommended by the Fourth Task Force on Blood Pressure Control for Children were followed for obtaining BP.35, 36 Children sat in a quiet room with the right arm fully exposed and resting on a supportive surface at heart level. The American Diagnostic Corporation ADC E Sphygmomanometer automatically measured systolic and diastolic BP, and pulse rate. The appropriate-sized cuff to cover approximately 75% of the upper arm between the top of the shoulder to the olecranon was used. BP was measured twice 2 minutes apart.
BP was classified as normal (average systolic and diastolic BP < 90th percentile), high BP (average systolic or diastolic BP of 90-95th percentile), and high BP or hypertension (average systolic or diastolic BP ≥ 95th percentile) using updated definitions and nomograms for BP by age and sex for height developed by the Fourth Task Force Report on Blood Pressure.36
A brief parent questionnaire of physical and sedentary behavior derived from the NICHD study on growth and calcium intake was completed.37 The activity questionnaire includes questions which reflect either sedentary screen activity (eg, television or computer time) or active physical activity (eg, sports or dance). Parents reported the number of minutes or hours/day for weekdays and weekends that the child participates in these activities. All site examiners received reliability training in study procedures. Institutional review board approval was obtained, and all parents provided informed consent.
Statistical analyses
A power analysis indicated the study would have 91% power to detect differences in means for continuous variables between children who were overweight or obese (BMI ≥85th percentile, n=89) versus not overweight or obese (BMI <85th percentile, n=310) with an alpha of P = .05, assuming a medium-sized effect (Cohen’s d=0.5). Bivariate analyses tested for differences in the characteristics of children with a BMI ≥85th percentile versus <85th percentile and for BMI ≥95th percentile versus <85th percentile at 6-7 years, using chi-square tests and t-tests as appropriate. Median tests were used to compare continuous variables with skewed distributions. The association of physical activity and sedentary activity rates with overweight and obesity were also explored. Regression models with generalized estimating equations were conducted to identify antenatal, neonatal, social and demographic factors that predicted BMI ≥85th percentile and BMI ≥95th percentile, accounting for clustering by study site. Independent variables associated with cardiovascular sequelae were selected based on the literature. Effects of sex, public insurance, minority race, birth weight, antenatal steroids, and weight gain velocity between birth and 36 weeks PMA, 36 weeks PMA to 18-22 months CA, and 18-22 months CA to 6-7 years were examined in bivariate and regression analyses. Relative risks and 95% confidence intervals were computed to quantify the relationship between the independent variables and BMI. Analyses were conducted using SAS statistical software, which were carried out by the NRN Data Coordinating Center, Research Triangle Institute International.
Results
Among the 388 EPT children with BMI data at 6-7 years, 86 (22%) were either overweight or obese (BMI ≥85th percentile), of which 39 (10%) were obese (BMI ≥95th percentile). Maternal and infant characteristics by child BMI categories are shown in Table I. There were no differences between groups in maternal age, race, health insurance, education level, or sex. Cesarean delivery rate was higher in the normal/low weight group. Infant differences identified for those with a BMI ≥85th percentile were higher birthweight and birth weight z-score, lower rates of intrauterine growth restriction, lower rates of bronchopulmonary dysplasia (BPD), and lower rates of receiving steroids for BPD. Table 2 shows that children with a BMI ≥85th percentile were heavier and taller at 36 weeks PMA and 18 months CA and had consistently higher growth velocities from birth to 36 weeks PMA, 36 weeks PMA to 18-22 months CA and 18-22 months CA to 6-7 years of age.
Table 1.
Maternal and Infant Characteristics by BMI
| Characteristic | Normal/Underweight BMI< 85 Percentile | Overweight/Obese BMI ≥ 85 Percentile | P value |
|---|---|---|---|
| N=302 | N=86 | ||
| Male | 162 (54) | 48 (56) | .721 |
| Female | 140 (46) | 38 (44) | |
| Maternal age | 27.8 ± 7 | 28.0 ± 7 | .780 |
| Maternal age < 20 | 38 (13) | 9 (10) | .595 |
| Race Black | 91 (30) | 29 (34) | .089 |
| White | 136 (45) | 27 (31) | |
| Hispanic | 66 (22) | 28 (33) | |
| Other | 9 (3) | 2 (2) | |
| Maternal education | |||
| Less than high school | 73 (25) | 23 (27) | .654 |
| High school graduate or more | 223 (75) | 62 (73) | |
| Hypertension in pregnancy | 77 (23) | 22 (26) | .987 |
| Diabetes in pregnancy | 20 (7) | 8 (9) | .397 |
| Antenatal steroids | 287 (95) | 82 (95) | .905 |
| Cesarean section | 207 (69) | 48 (56) | .028 |
| Birth weight grams | 849 ± 189 | 904 ± 186 | .019 |
| Birth weight below 10th % | 26 (9) | 2 (2) | .047 |
| Birth weight z score | −0.1 ± 0.9 | 0.1 ± 0.9 | .043 |
| Percent weight loss | 12.7 ± 6 | 12.1 ± 6 | .459 |
| Days to regain birth weight | 11.5 ± 5 | 11.5 ± 5 | .952 |
| Human milk in first 28 days | 269 (89) | 77 (90) | .903 |
| Gestation (weeks) | 25.9 ± 1 | 26.0 ± 1 | .250 |
| Adverse finding on early ultrasound | 28 (9) | 8 (9) | 1.000 |
| Adverse finding on Late ultrasound | 18 (6) | 9 (10) | .150 |
| White Matter injury (moderate/severe) | 63 (21) | 14 (16) | .347 |
| BPD | 122 (40) | 20 (23) | .004 |
| Steroids for BPD | 27 (9) | 1 (1) | .014 |
| Late Onset sepsis | 92 (30) | 25 (29) | .804 |
| Proven NEC | 23 (8) | 5 (6) | .569 |
| Severe ROP | 42 (15) | 6 (8) | .104 |
| Days of Hospitalization | 102.7 ± 40 | 95.5 ± 42 | .145 |
Note: N (%) or mean ± SD
Table 2.
Child growth characteristics at 36 weeks and 18 months corrected age
| Characteristic | Normal/Underweight BMI< 85 Percentile | Overweight/Obese BMI ≥ 85 Percentile | P value |
|---|---|---|---|
| N=302 | N=86 | ||
| Weight | |||
| Weight at 36 weeks (kg) | 2.1 ± 0.4 | 2.2 ± 0.4 | .041 |
| Weight % at 36 weeks | 10.8 ± 15 | 14.8 ± 18 | .046 |
| Weight z score at 36 weeks | −1.7 ± 1 | −1.4 ± 1 | .041 |
| Weight at 18 months (kg) | 10.5 ± 1 | 12.3 ± 2 | < .001 |
| Weight % at 18 months | 16.7 ± 21 | 49.7 ± 32 | < .001 |
| Weight z score at 18 months | −1.6 ± 1 | 0.0 ± 1 | < .001 |
| Height | |||
| Height at 18 months (cm) | 81.0 ± 4 | 83.0 ± 4 | < .001 |
| Height % at 18 months | 20.6 ± 22 | 36.0 ± 28 | < .001 |
| Height z score at 18 months | −1.2 ± 1 | −0.5 ± 1 | < .001 |
| Weight gain velocities | |||
| Birth- 36 weeks (g/w) | 133.8 ± 32 | 143.2 ± 38 | .032 |
| Time birth wt regained to 36 weeks (g/w) | 166.7 ± 47 | 178.5 ± 49 | .063 |
| 36 weeks to 18-22 months (kg/yr) | 8.0 ± 2 | 9.9 ± 2 | < .001 |
| 18-22months to 6-7 years (kg/yr) | 2.3 ± .5 | 3.8 ± .9 | < .001 |
Note: mean ± SD
Child anthropometrics and BPs at 6-7 years of age are shown in Table 3. Compared with children with a BMI <85th percentile, children with a BMI ≥85th percentile and ≥95th percentile had increased height, waist circumference >90th percentile, waist-height ratio, and abdominal, triceps, and subscapular skinfolds. Overweight and obese EPT children also had higher systolic and diastolic mean BPs, and higher rates of systolic hypertension. In addition, 86/302 (28%) children in the normal/underweight group had weights <10th percentile and 72/388 (19%) had heights <10th percentile. Five percent of children in the overweight group had heights <10th percentile. We compared children with weights <10th percentile to those with weights >10th percentile on the maternal and infant characteristic shown in Table 1 using chi-square tests. Children with weights <10th percentile at 6-7 years of age were significantly less likely to have mothers who received antenatal steroids (p=0.032) and were more likely to have weight <10th percentile at birth (p < 0.001), an adverse finding on an early cranial ultrasound (CUS) (p=0.035) or a late CUS (p=0.050), moderate/severe white matter injury on magnetic resonance imaging (p < 0.001), BPD (p < 0.001), steroids for BPD (p=0.023), and severe retinopathy of prematurity (p < 0.001). Similar comparisons were conducted based on height percentiles. Children with height <10th percentile at 6-7 years of age were significantly less likely to have mothers who received antenatal steroids (p=0.011) and were more likely to be white (p=0.048) and have human milk in first 28 days (p=0.031), adverse findings on early CUS (p=0.008) or late CUS (p=0.016), and moderate/severe white matter injury (p < 0.001).
Table 3.
Child Characteristics at 6-7 years
| Characteristic | Normal/Underweight BMI < 85 Percentile | Overweight/Obese BMI ≥ 85 Percentile | Overweight/Obese versus Normal/Underweight | Obese BMI ≥ 95 Percentile | Obese versus Normal/Underweight |
|---|---|---|---|---|---|
| N=302 | N=86 | p-value | N=39 | p-value | |
| Mean age (years) | 6.3 ± .5 | 6.3 ± .5 | .481 | 6.2 ± 0.4 | .121 |
| Mean BMI | 14.9 ± 1.2 | 19.4 ± 1.8 | < .001 | 20.8 ± 1.7 | < .001 |
| BMI z score | −0.6 ± 1.0 | 1.6 ± 0.4 | < .001 | 2.0 ± 0.3 | < .001 |
| Health Insurance- Public | 174 (58) | 47 (55) | .580 | 21 (54) | .622 |
| Weight (kg) | 20.5 ± 3 | 29.4 ± 4 | < .001 | 31.7 ± 5 | < .001 |
| Weight % | 30.3 ± 25 | 88.6 ± 10 | < .001 | 94.3 ± 6 | < .001 |
| Weight below 10th% | 86 (28) | 0 (0) | < .001 | 0 (0) | < .001 |
| Weight z score | −0.7 ± 1 | 1.4 ± .6 | < .001 | 1.8 ±0.6 | < .001 |
| Height (cm) | 117.2 ± 6 | 123.0 ± 6 | < .001 | 123.3 ± 6 | < .001 |
| Height % | 34.6 ± 28 | 63.0 ± 28 | < .001 | 66.5 ± 29 | < .001 |
| Height below 10th% | 72 (24) | 4 (5) | < .001 | 2 (5) | .008 |
| Height z score | −0.6 ± 1.1 | 0.5 ± 1.0 | < .001 | 0.6 ± 1 | < .001 |
| Waist circumference (cm) | 54.1 ± 4 | 66.3 ± 8 | < .001 | 68.6 ± 6 | < .001 |
| Waist Circumference>90th% | 1 (0) | 28 (33) | < .001 | 19 (50) | < .001 |
| Waist-height ratio | 0.5 ± 0.0 | 0.5 ± 0.1 | < .001 | 0.6 ± 0.0 | < .001 |
| Triceps skinfold (mm) | 8.1 ± 3 | 14.2 ± 4 | < .001 | 15.6 ± 4 | < .001 |
| Triceps skinfold> 85th% | 17 (6) | 50 (62) | < .001 | 29 (81) | < .001 |
| Triceps skinfold> 95th% | 2 (1) | 26 (32) | < .001 | 18 (50) | < .001 |
| Subscapular skinfold (mm) | 5.2 ± 2 | 10.3 ± 4 | < .001 | 11.4 ± 4 | < .001 |
| Subscapular skinfold>85th% | 26 (9) | 60 (74) | < .001 | 31 (86) | < .001 |
| Subscapular skinfold>95th% | 7 (2) | 33 (41) | < .001 | 19 (53) | < .001 |
| Abdominal skinfold (mm) | 6.8 ± 3 | 13.7 ± 7 | < .001 | 15.8 ± 8 | < .001 |
| Systolic blood pressure mm Hg | 99.6 ± 8 | 105.3 ± 9 | < .001 | 105.4 ± 9 | < .001 |
| Systolic blood pressure % | 64.1 ±23 | 71.9 ± 21 | .006 | 72.2 ± 21 | .043 |
| Systolic prehypertension (≥90%)* | 39 (13) | 20 (23) | .019 | 7 (19) | .363 |
| Systolic hypertension (≥95th%) | 17 (6) | 13 (15) | .004 | 5 (14) | .079 |
| Diastolic blood pressure mm Hg | 62.3 ± 8 | 64.7 ± 9 | .013 | 65.0 ± 9 | .045 |
| Diastolic blood pressure % | 68.3 ± 20 | 70.8 ± 20 | .311 | 71.2 ± 20 | .413 |
| Diastolic prehypertension (≥ 90%) | 39 (13) | 15 (17) | .285 | 5 (14) | .985 |
| Diastolic hypertension (≥ 95%) | 17 (6) | 9 (10) | .114 | 4 (11) | .245 |
| Systolic or diastolic prehypertension | 63(21%) | 26(30%) | .068 | 9 (24) | .711 |
| Systolic or diastolic hypertension | 26(9%) | 17(20%) | <.004 | 34 (87) | .017 |
| Heart rate | 81.3 ± 12 | 81.9 ± 13 | .709 | 84.3 ± 16 | .195 |
Note: N (%) or mean ± SD
any blood pressure ≥90th% for age and height
Rates of physical and sedentary activity are shown in Table 4 (available at www.jpeds.com). There were no differences between groups in active physical activity (<1 hour/week for all groups). However, children with a BMI ≥95th percentile and BMI ≥85th percentile versus BMI <85th percentile had a significantly longer duration of mean hours of sedentary activity using electronic devices both during the 5 weekdays and for the total week. Total sedentary activity for screen time for the cohort ranged from 0-40.3 hours per week. Among obese EPT children, 62% had a television and 44% a computer in their bedroom.
Table 4.
Active Physical and sedentary activity rates-online
| Characteristic Activity | Normal/Underweight BMI < 85 Percentile | Overweight/Obese BMI ≥ 85 Percentile | Overweight/Obese versus Normal/Underweight | Obese BMI ≥ 95 Percentile | Obese versus Normal/Underweight |
|---|---|---|---|---|---|
| N=302 | N=86 | p-value | N=39 | p-value | |
| Physical - 5 week days | |||||
| Mean ± SD | 0.5 ± 0.9 | 0.4 ± 0.8 | .584 | 0.4 ± 0.8 | .598 |
| Median (IQR) | 0.0 (0.0-0.6) | 0.0 (0.0-0.6) | .871 | 0.0 (0.0-0.6) | .869 |
| Range | 0.0-5.0 | 0.0-3.6 | 0.0-3.6 | ||
| Physical - Weekend | |||||
| Mean ± SD | 0.2 ± 0.3 | 0.2 ± 0.3 | .584 | 0.2 ± 0.3 | .598 |
| Median (IQR) | 0.0 (0.0-0.3) | 0.0 (0.0-0.3) | .871 | 0.0 (0.0-0.3) | .869 |
| Range | 0.0-2.0 | 0.0-1.4 | 0.0-1.4 | ||
| Physical- Total | |||||
| Mean ± SD | 0.7 ± 1.2 | 0.6 ± 1.1 | .584 | 0.6 ± 1.2 | .598 |
| Median (IQR) | 0.0 (0.0-0.9) | 0.0 (0.0-0.9) | .871 | 0.0 (0.0-0.9) | .869 |
| Range | 0.0-7.0 | 0.0-5.0 | 0.0-5.0 | ||
| Sedentary -5 week days | |||||
| Mean ± SD | 10.9 ± 7.7 | 12.4 ± 7.4 | .108 | 14.0 ± 7.9 | .019 |
| Median (IQR) | 8.8 (3.8-13.8) | 8.8 (8.8-13.8) | .037 | 13.8 (8.8-18.8) | .011 |
| Range | 0.0-28.8 | 3.8-28.8 | 3.8-28.8 | ||
| Sedentary- Weekend | |||||
| Mean ± SD | 5.8 ± 3.5 | 5.7 ± 3.1 | .943 | 6.5 ± 3.1 | .203 |
| Median (IQR) | 5.5 (3.5-7.5) | 5.5 (3.5-7.5) | .285 | 5.5 (3.5-7.5) | .085 |
| Range | 0.0-11.5 | 0.0-11.5 | 1.5-11.5 | ||
| Sedentary - Total | |||||
| Mean ± SD | 16.7 ± 10.3 | 18.2 ± 8.9 | .229 | 20.5 ± 8.7 | .026 |
| Median (IQR) | 14.3 (7.3-23.3) | 17.3 (12.3-21.3) | .009 | 19.3 (14.3- 23.3) | .002 |
| Range | 0.0-40.3 | 3.8-40.3 | 5.3-40.3 | ||
| TV in bedroom N (%) | 160 (53) | 50 (58) | .413 | 24 (62) | .323 |
| Computer in bedroom N (%) | 93 (31) | 31 (36) | .367 | 17 (44) | .111 |
The Figure (available at www.jpeds.com) shows the z-scores for weight and height at 6-7 years by BMI <85th percentile versus ≥85th percentile by sex. In addition to the high scores for weight, all height z-scores for both boys and girls with a BMI ≥85th percentile were above zero and significantly higher (p<.001) than for those with a BMI <85th percentile. For boys with a BMI ≥85th percentile versus <85th percentile, the median height z-scores were 0.44 versus −0.67 and for girls were 0.44 versus −0.54, respectively. There were no sex differences.
Figure 1. Weight and Height Z-Scores by Gender and BMI - online.
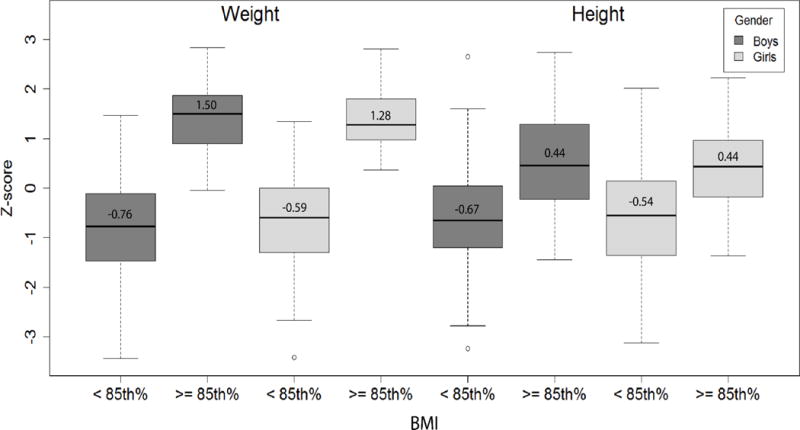
Note: Comparisons of BMI < 85th% vs. BMI >= 85th % significant at p < 0.001
The regression models to predict BMI ≥85th percentile and BMI >95th percentile are shown in Table 5. Significant predictors of overweight were weight gain velocities from 36 weeks PMA to 18-22 months CA and from 18-22 months CA to 6-7 years. Only weight gain velocity between 18-22 months and 6-7 years was significantly associated with obesity. Antenatal steroids were protective of overweight and borderline protective of obesity. The risk ratio for obesity contributed by total sedentary activities was 1.03 (0.99 to 1.06)
Table 5.
Regression Analyses to Predict Overweight and Obesity
| Variable | Overweight/Obese BMI ≥85 Percentile | Obese BMI ≥95 Percentile | ||
|---|---|---|---|---|
| Risk Ratio (95% CI) |
P value | Risk Ratio (95% CI) |
P value | |
| Male | 0.94 (0.65, 1.35) | .730 | 1.43 (0.84, 2.43) | .192 |
| Public Insurance | 0.89 (0.60, 1.32) | .553 | 0.65 (0.35, 1.20) | .166 |
| Non-White | 1.21 (0.84, 1.73) | .305 | 1.19 (0.66, 2.13) | .569 |
| Birth weight (g) | 1.00 (1.00, 1.00) | .743 | 1.00 (1.00, 1.00) | .118 |
| Antenatal Steroids | 0.43 (0.23, 0.79) | .006 | 0.33 (0.09, 1.24) | .100 |
| Sedentary activities @6-7 years (hrs) | 1.00 (0.98, 1.02) | .951 | 1.03 (0.99, 1.06) | .106 |
| Birth- 36 weeks (g/week) | 1.00 (0.99, 1.00) | .451 | 1.00 (0.99, 1.01) | .940 |
| 36 weeks to 18-22months (kg/yr) | 1.32 (1.18, 1.48) | < .001 | 1.23 (0.95, 1.60) | .123 |
| 18-22months to 6-7 years (kg/yr) | 2.36 (2.07, 2.70) | < .001 | 3.25 (2.48, 4.26) | < .001 |
Note: Models also control for center
Discussion
Consistent with our study objective, we identified that 22% of EPT children were overweight or obese and 10% were obese at early school age. In the EPICURE study of EPT infants ≤ 25 weeks of gestation from the United Kingdom and Ireland born in 1995, at a median age of 6 years, children had a mean BMI z-score of −0.88±1.3.38 The BMI z-scores in our cohort at the same age ranged from −0.6 for children with a BMI <85% to 2.0 for the obese children. Our more contemporary cohort was enrolled between February 2005 and 2009 with subsequent follow-up during a period of increasing rates of overweight and obesity in children and adolescents reported worldwide. Hack et al reported that rates of obesity in a cohort of extremely low birth weight (ELBW, birth weights < 1000 grams) born from 1992-1995 increased from 12% at 8 years to 19% at 14 years.7, 9 A number of studies have reported increased rates of overweight and obesity among preterm children in adolescence and early adulthood.10, 39 The most recent National Center for Health Statistics obesity rates for the United States (US) are 8.9% for ages 2-5 years and 17.5% for 6-11 years.2 The preterm children in our cohort had an obesity rate of 10% at a mean age of 6.8 years. The prevalence of obesity in this cohort of EPT children is lower than the prevalence in population data but higher than expected for EPT children. The SUPPORT cohort children were born in 2005-2009 and evaluated in 2012 to 2015. Obesity rates have been increasing in the general pediatric population between 2005 and 2015. We addressed this by comparing the cohort to US population data for obesity trends for 2013-2014.3 The weighted prevalence obesity rate for 6-11 year old children was 17.5 (15.2-20.1). There were no sex differences. The US data for 2015-2016 indicates the rate is continuing to increase for children and is now 18.4 (6-11) for children 6-11 years. Longer follow-up of anthropometrics is needed to determine the extent of impact as rates of obesity increase with increasing age.
Our findings provide evidence for increased central obesity in the preterm population. At early school age, 27% of the EPT children with a BMI ≥85th percentile and 39% with a BMI ≥95th percentile had a waist circumference >90th percentile. In addition, abdominal skinfolds and waist-height ratios were significantly greater in the children who were overweight and obese. This is an important finding because central obesity has been shown to be a better predictor than overall obesity of subsequent risk of type II diabetes, cardiovascular disease, and hypertension.19,40–42
The finding of height z-scores above zero (0.44) for both boys and girls with a BMI ≥85th percentile at 6-7 years of age suggests that a subgroup of preterm survivors is beginning to catchup in height to mean scores for term children by early school age. Our findings are in contrast to those of Doyle et al, who reported that although a cohort of ELBW infants were relatively heavy for their height, their height z-scores were significantly below zero at ages 2, 5, 8, 14, and 20 years.8 Preterm children who were enrolled in the indomethacin for intracranial hemorrhage prevention trial39 were shorter and weighed less than term controls at 16 years of age; however, they had a high rate of overweight or obesity (31%) and obesity (16%) at 16 years of age. A recent review43 of studies reporting adult outcomes of preterm birth confirms that preterm survivors are at increased risk of numerous adverse health outcome including cardiovascular disease and hypertension.
The significant predictor of BMI >85th percentile in our cohort was rapid catch-up growth that began in the hospital and accelerated after discharge from the hospital as reflected by weight gain velocities from 36 weeks PMA to 18-22 months CA and from 18-22 months CA to 6-7 years of age. This is consistent with results from the indomethacin cohort39 in which weight gain velocity between birth and 36 months significantly predicted obesity at 16 years of age. The Avon longitudinal study in the United Kingdom in a general population also found that increased catch-up growth between birth and 3 years was associated with increased obesity and central obesity at 5 years of age.23 Hack et al reported that the rate of growth in childhood and maternal BMI rather than perinatal risk factors predicted obesity at 14 years among former ELBW infants.7 Finally, children in low SES families are known to be at increased risk of obesity.44, 45 However, in this cohort there were no differences in level of education, race, or health insurance of mothers at the time of enrollment as shown in Table 1.
In addition, children in the SUPPORT cohort with a BMI ≥85th percentile were significantly more likely to have higher systolic and diastolic BP and higher rates of systolic hypertension or either systolic or diastolic hypertension (P<0.0001). Evidence of elevated BP has been reported as early as 1 year of age among former preterm infants.46 The finding in association with overweight and obesity is of particular concern and is consistent with findings of increasing obesity and hypertension in the general US population.3
The only maternal characteristic that was protective in our regression model was maternal antenatal steroids. Overweight and obese children also had a higher birth weight z-score, were less like to have BPD, and were less likely to receive postnatal steroids for BPD. We propose that overweight and obese 6 to 7-year-old children may have had a more optimal in-utero environment and had somewhat decreased early neonatal illness severity. In addition, the infants who were growth restricted at birth were less likely to have received ANS, more likely to have had multiple neonatal morbidities, and more likely to have weight <10th percentile at school age. This may account for some of the apparent protective effects of ANS that were identified in our regression analysis to predict overweight and obesity.
In addition, the infants who were growth restricted at birth continued to have evidence of slow growth. They were less likely to receive ANS and more likely to experience neonatal morbidities, which may account for some of the protective effects of ANS on obesity that were identified in our regression analysis. Finally, although extended feeding of human milk has been associated with decreased rates of obesity47, we only have data on human milk in the first 28 days with rates of 89 and 90% for the children with BMI <85th percentile and ≥85th percentile respectively.
Low activity levels and increased screen time have been associated with childhood obesity.48, 49 In 2015, the American Academy of Pediatrics updated their recommendations to 60 minutes/day of physical activity for children ≥6 years of age. We identified that children in our cohort spent an average of <1 hour per week in active physical activity, although sedentary screen activity including television, tablets, smart phones and electronic games was 16.7 hours/week for children with BMI <85th percentile, 18.2 hours/week for children with a BMI ≥85th percentile and 20.5 hours/week for obese children. In addition, children who were overweight or obese spent more time on weekdays participating in sedentary screen activity. As of 10/21/2016 the American Academy of Pediatrics recommends that children ages 2-5 years limit screen use to 1 hour per day of high-quality programs and that for children ages ≥6 years, parents place consistent limits on the time spent using media. (https://healthychildren.org/English/news/Pages/AAP-Announces-New-Recommendations-for-Childrens-Media-Use.aspx) Some children in our study groups spent up to 40 hours/week on screen time. Although we are living in the electronic media age, there is increasing evidence that increased screen time has deleterious effects on children including obesity, decreased physical activity, learning problems and absenteeism.48–51 A recent study of children at 8-10 years of age who were evaluated twice over two years found that higher levels of moderate-to-vigorous physical activity and lower screen time were beneficial to insulin sensitivity in part through their effect on adiposity levels.49 Studies have shown that young adult former preterm infants spend less leisure time in physical activity.52 A limitation is that our activity data were obtained by parent report which may be either over or underestimated, rather than an objective assessment such as actigraphy or the 6 minute walk test.53–55
Although there is a body of evidence showing a relationship between low socioeconomic status and higher rates of obesity in middle-school-age children, adolescents and adults,44, 56, 57,44, 45 we did not find a significant relationship between Medicaid insurance and risk of overweight or obesity at 6-7 years. However, in our cohort there were no differences in level of education, race, or health insurance of mothers at the time of delivery. There is also evidence that food insecurity and problems associated with food acquisition, preparation and knowledge are associated with overweight.58 Further evaluation of these aspects of nutritional intake over time is needed. There is moderate evidence that interventions with families and in schools can have beneficial effects on reducing rates of obesity in children and are in fact cost effective.59, 60 Use of parent mentors is one cost effective approach which has been shown to have benefits.61 Of equal importance is that the growth outcomes reflect a bell shaped curve in which 22% of the cohort were overweight or obese and an equal 22% were below the 10th percentile for weight at early school age. Current data suggest the percent of preterm infants with poor growth at followup continues to improve.62
Strengths of this study are the availability of comprehensive perinatal, neonatal, maternal, and infant data, and longitudinal anthropometric and BP data. Limitations include the lack of a full-term control population and data on maternal pre-pregnancy weight, weight gain in pregnancy, nutrition intake in the NICU and family diet.
In summary, the epidemic of overweight and obesity appears to be spreading to even the most extreme preterm infants. The findings of increased post-discharge weight gain velocities as a predictor of BMI ≥85th percentile, and the associated low physical activity and increased screen time may in part be related to changes in the US culture. Of particular concern are the findings at early school age of associated central adiposity and elevated BP which are highly associated with subsequent metabolic abnormalities and cardiovascular disease. These findings should be a wake-up call for both pediatricians and the internists who are currently caring for or will be managing these former preterm future adults.
Acknowledgments
Supported by the National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development, which provided grant support for the Neonatal Research Network’s Generic Database and Follow-up Studies.
Abbreviations
- BMI
body mass index
- BP
blood pressure
- IVH
intraventricular hemorrhage
- PVL
periventricular hemorrhage
- VLBW
very low birth weight
Appendix
List of additional members from the Eunice Kennedy Shriver National Institute of Child Health and Development Neonatal Research Network
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Heart, Lung, and Blood Institute (NHLBI) provided grant support for the Neonatal Research Network’s Extended Follow-up at School Age for the SUPPORT Neuroimaging and Neurodevelopmental Outcomes (NEURO) Cohort through cooperative agreements. While NICHD staff had input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of the NICHD.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Drs. Abhik Das (DCC Principal Investigator), Marie Gantz, Lisa Wrage, and Helen Cheng (DCC Statisticians) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chairs: Alan H. Jobe, MD PhD, University of Cincinnati (2003-2006); Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine (2006-2011); Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University, (2011-present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Abbot R. Laptook, MD; Angelita M. Hensman, MS RNC-NIC; Elisabeth C. McGowan, MD; Elisa Vieira, RN BSN; Emilee Little, RN BSN; Katharine Johnson, MD; Barbara Alksninis, PNP; Mary Lenore Keszler, MD; Andrea M. Knoll; Theresa M. Leach, MEd CAES; Victoria E. Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children’s Hospital (U10 HD21364, M01 RR80) – Michele C. Walsh, MD MS; Avroy A. Fanaroff, MD; Deanne E. Wilson-Costello, MD; Allison Payne, MD MSCR; Nancy S. Newman, RN; H. Gerry Taylor, PhD; Bonnie S. Siner, RN; Arlene Zadell, RN; Julie DiFiore, BS; Monika Bhola, MD; Harriet G. Friedman, MA; Gulgun Yalcinkaya, MD.
Department of Diagnostic Imaging and Radiology, Children’s National Medical Center, Washington DC – Dorothy Bulas, MD.
Duke University School of Medicine, University Hospital, and Duke Regional Hospital (U10 HD40492, M01 RR30) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Kathryn E. Gustafson, PhD; Patricia Ashley, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Katherine A. Foy, RN; Sharon F. Freedman, MD; Melody B. Lohmeyer, RN MSN; William F. Malcolm, MD; David K. Wallace, MD MPH.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory Crawford Long Hospital (U10 HD27851, RR25008, M01 RR39) – David P. Carlton, MD; Barbara J. Stoll, MD; Ira Adams-Chapman, MD; Susie Buchter, MD; Anthony J. Piazza, MD; Sheena Carter, PhD; Sobha Fritz, PhD; Ellen C. Hale, RN BS CCRC; Amy K. Hutchinson, MD; Maureen Mulligan LaRossa, RN; Yvonne Loggins, RN, Diane Bottcher, RN.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Brenda B. Poindexter, MD MS; Gregory M. Sokol, MD; Heidi M. Harmon, MD MS; Lu-Ann Papile, MD; Abbey C. Hines, PsyD; Leslie D. Wilson, BSN CCRC; Dianne E. Herron, RN; Lucy Smiley, CCRC.
McGovern Medical School at The University of Texas Health Science Center at Houston and Children’s Memorial Hermann Hospital (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Andrea Freeman Duncan, MD; Allison G. Dempsey, PhD; Janice John, CPNP; Patrick M. Jones, MD MA; M. Layne Lillie, RN BSN; Saba Siddiki, MD; Daniel K. Sperry, RN.
National Heart, Lung, and Blood Institute – Mary Anne Berberich, PhD; Carol J. Blaisdell, MD; Dorothy B. Gail, PhD; James P. Kiley, PhD.
RTI International (U10 HD36790) – Dennis Wallace, PhD; Marie G. Gantz, PhD; Jamie E. Newman, PhD MPH; Jeanette O’Donnell Auman, BS; Jane A. Hammond, PhD; W. Kenneth Poole, PhD (deceased).
Stanford University and Lucile Packard Children’s Hospital (U10 HD27880, UL1 RR25744, M01 RR70) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; Maria Elena DeAnda, PhD; M. Bethany Ball, BS CCRC; Gabrielle T. Goodlin, BAS.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Elisabeth C. McGowan, MD; Anne Furey, MPH; Brenda L. MacKinnon, RNC; Ellen Nylen, RN BSN; Ana Brussa, MS OTR/L; Cecelia Sibley, PT MHA.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN. Vivien A. Phillips, RN BSN; Kirstin J. Bailey, PhD; Fred J. Biasini, PhD; Maria Hopkins, PhD; Kristen C. Johnston, MSN CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Richard V.
Rector, PhD; Leslie Rodriguez, PhD; Amanda Soong, MD; Sally Whitley, MA OTR-L FAOTA; Sheree York, PT DPT MS PCS; Kristy Guest, PhD; Leigh Ann Smith, CRNP.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women (U10 HD40461) – Neil N. Finer, MD; Donna Garey, MD MPH; Maynard R. Rasmussen; MD; Paul R. Wozniak, MD; Yvonne E. Vaucher, MD MPH; Martha G. Fuller, PhD RN; Natacha Akshoomoff, PhD; Wade Rich, BSHS RRT; Kathy Arnell, RNC; Renee Bridge, RN.
University of Iowa (U10 HD53109, UL1 TR442, M01 RR59) – Edward F. Bell, MD; Tarah T. Colaizy, MD; John A. Widness, MD; Jonathan M. Klein, MD; Karen J. Johnson, RN BSN; Michael J. Acarregui, MD; Diane L. Eastman, RN CPNP MA; Tammy L. V. Wilgenbusch, PhD.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997) – Kristi L. Watterberg, MD; Robin K. Ohls, MD; Janell Fuller, MD; Jean Lowe, PhD; Julie Rohr, MSN RNC CNS; Conra Backstrom Lacy, RN; Rebecca Montman, BSN; Sandra Brown, RN BSN.
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children’s Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; Charles R. Rosenfeld, MD; Walid A. Salhab, MD; Luc Brion, MD; Sally S. Adams, MS RN CPNP; James Allen, RRT; Laura Grau, RN; Alicia Guzman; Gaynelle Hensley, RN; Elizabeth T. Heyne, PsyD PA-C; Jackie F. Hickman, RN; Melissa H. Leps, RN; Linda A. Madden, RN CPNP; Melissa Martin, RN; Nancy A. Miller, RN; Janet S. Morgan, RN; Araceli Solis, RRT; Lizette E. Lee, RN; Catherine Twell Boatman, MS CIMI; Diana M Vasil, MSN BSN RNC-NIC.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children’s Medical Center (U10 HD53124, M01 RR64) – Bradley A. Yoder, MD; Roger G. Faix, MD; Sarah Winter, MD; Shawna Baker, RN; Karen A. Osborne, RN BSN CCRC; Carrie A. Rau, RN BSN CCRC; Sean Cunningham, PhD; Ariel Ford, PhD.
Wayne State University, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385) – Seetha Shankaran, MD; Athina Pappas, MD; Beena G. Sood, MD MS; Rebecca Bara, RN BSN; Thomas L. Slovis, MD; Elizabeth Billian, RN MBA; Laura A. Goldston, MA; Mary Johnson, RN BSN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflicts of interest.
References
- 1.Nader PR, O’Brien M, Houts R, Bradley R, Belsky J, Crosnoe R, et al. Identifying risk for obesity in early childhood. Pediatrics. 2006;118(3):e594–601. doi: 10.1542/peds.2005-2801. [DOI] [PubMed] [Google Scholar]
- 2.Ogden CL, C M, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011–2014. National Center for Health Statistics Data Brief. 2015;219:1–8. [PubMed] [Google Scholar]
- 3.Ogden CL, Carroll MD, Lawman HG, Fryar CD, Kruszon-Moran D, Kit BK, et al. Trends in Obesity Prevalence Among Children and Adolescents in the United States, 1988-1994 Through 2013-2014. JAMA. 2016;315(21):2292–2299. doi: 10.1001/jama.2016.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Umer A, Kelley GA, Cottrell LE, Giacobbi P, Jr, Innes KE, Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. 2017;17(1):683. doi: 10.1186/s12889-017-4691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daniels SR, Morrison JA, Sprecher DL, Khoury P, Kimball TR. Association of body fat distribution and cardiovascular risk factors in children and adolescents. Circulation. 1999;99(4):541–545. doi: 10.1161/01.cir.99.4.541. [DOI] [PubMed] [Google Scholar]
- 6.Young TK, Dean HJ, Flett B, Wood-Steiman P. Childhood obesity in a population at high risk for type 2 diabetes. J Pediatr. 2000;136(3):365–369. doi: 10.1067/mpd.2000.103504. [DOI] [PubMed] [Google Scholar]
- 7.Hack M, Schluchter M, Margevicius S, Andreias L, Taylor HG, Cuttler L. Trajectory and correlates of growth of extremely-low-birth-weight adolescents. Pediatr Res. 2014;75(2):358–366. doi: 10.1038/pr.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doyle LW, Faber B, Callanan C, Ford GW, Davis NM. Extremely low birth weight and body size in early adulthood. Arch Dis Child. 2004;89(4):347–350. doi: 10.1136/adc.2002.025924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hack M, Schluchter M, Andreias L, Margevicius S, Taylor HG, Drotar D, et al. Change in prevalence of chronic conditions between childhood and adolescence among extremely low-birth-weight children. JAMA. 2011;306(4):394–401. doi: 10.1001/jama.2011.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasylyeva TL, Barche A, Chennasamudram SP, Sheehan C, Singh R, Okogbo ME. Obesity in prematurely born children and adolescents: follow up in pediatric clinic. Nutr J. 2013;12(1):150. doi: 10.1186/1475-2891-12-150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farooqi A, Hagglof B, Sedin G, Gothefors L, Serenius F. Growth in 10- to 12-year-old children born at 23 to 25 weeks’ gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics. 2006;118(5):e1452–1465. doi: 10.1542/peds.2006-1069. [DOI] [PubMed] [Google Scholar]
- 12.Hack M. Young adult outcomes of very-low-birth-weight children. Semin Fetal Neonatal Med. 2006;11(2):127–137. doi: 10.1016/j.siny.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Powls A, Botting N, Cooke RW, Pilling D, Marlow N. Growth impairment in very low birthweight children at 12 years: correlation with perinatal and outcome variables. Arch Dis Child Fetal Neonatal Ed. 1996;75:F152–157. doi: 10.1136/fn.75.3.f152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saigal S, Stoskopf B, Streiner D, Paneth N, Pinelli J, Boyle M. Growth trajectories of extremely low birth weight infants from birth to young adulthood: a longitudinal, population-based study. Pediatr Res. 2006;60(6):751–758. doi: 10.1203/01.pdr.0000246201.93662.8e. [DOI] [PubMed] [Google Scholar]
- 15.Saigal S, Stoskopf BL, Streiner DL, Burrows E. Physical growth and current health status of infants who were of extremely low birth weight and controls at adolescence. Pediatrics. 2001;108(2):407–415. doi: 10.1542/peds.108.2.407. [DOI] [PubMed] [Google Scholar]
- 16.Peralta-Carcelen M, Jackson DS, Goran MI, Royal SA, Mayo MS, Nelson KG. Growth of adolescents who were born at extremely low birth weight without major disability. J Pediatr. 2000;136(5):633–640. doi: 10.1067/mpd.2000.104291. [DOI] [PubMed] [Google Scholar]
- 17.Doyle LW, Faber B, Callanan C, Morley R. Blood pressure in late adolescence and very low birth weight. Pediatrics. 2003;111(2):252–257. doi: 10.1542/peds.111.2.252. [DOI] [PubMed] [Google Scholar]
- 18.Pharoah PO, Stevenson CJ, West CR. Association of blood pressure in adolescence with birthweight. Arch Dis Child Fetal Neonatal Ed. 1998;79(2):F114–118. doi: 10.1136/fn.79.2.f114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freedman DS, Mei Z, Srinivasan SR, Berenson GS, Dietz WH. Cardiovascular risk factors and excess adiposity among overweight children and adolescents: the Bogalusa Heart Study. J Pediatr. 2007;150(1):12–17 e12. doi: 10.1016/j.jpeds.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 20.Must A, Strauss RS. Risks and consequences of childhood and adolescent obesity. Int J Obes Relat Metab Disor. 1999;23(Suppl 2):S2–11. doi: 10.1038/sj.ijo.0800852. [DOI] [PubMed] [Google Scholar]
- 21.Barker DJ. Fetal origins of cardiovascular disease. Ann Med. 1999;31(Suppl 1):3–6. [PubMed] [Google Scholar]
- 22.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311(6998):171–174. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ong KK, Ahmed ML, Emmett PM, Preece MA, Dunger DB. Association between postnatal catch-up growth and obesity in childhood: prospective cohort study. BMJ. 2000;320(7240):967–971. doi: 10.1136/bmj.320.7240.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hintz SR, Barnes PD, Bulas D, Slovis TL, Finer NN, Wrage LA, et al. Neuroimaging and neurodevelopmental outcome in extremely preterm infants. Pediatrics. 2015;135(1):e32–42. doi: 10.1542/peds.2014-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finer NN, Carlo WA, Walsh MC, Rich W, Gantz MG, Laptook AR, et al. Early CPAP versus surfactant in extremely preterm infants. N Engl J Med. 2010;362(21):1970–1979. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Network SSGotEKSNNR. Carlo WA, Finer NN, Walsh MC, Rich W, Gantz MG, et al. Target ranges of oxygen saturation in extremely preterm infants. N Engl J Med. 2010;362(21):1959–1969. doi: 10.1056/NEJMoa0911781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fenton TR. A new growth chart for preterm babies: Babson and Benda’s chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109(1):45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Ogden CL, Wei R, Curtin LR, Flegal KM. The 2000 Centers for Disease Control and Prevention growth charts: several insights after 8 years. Nestle Nutr Workshop Ser Pediatr Program. 2010;65:181–193. doi: 10.1159/000281163. discussion 193-185. [DOI] [PubMed] [Google Scholar]
- 30.Seefeldt VD, Harrison GG. Infants, children and youth. In: Lohman TG, Roceh AF, Matorell R, editors. Anthropometric Standardization Reference Manual. Champaign, IL: Human Kinetics Press; 1998. [Google Scholar]
- 31.Fernandez JR, Bohan Brown M, Lopez-Alarcon M, Dawson JA, Guo F, Redden DT, et al. Changes in pediatric waist circumference percentiles despite reported pediatric weight stabilization in the United States. Pediatr Obes. 2017;12(5):347–355. doi: 10.1111/ijpo.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Addo OY, Himes JH. Reference curves for triceps and subscapular skinfold thicknesses in US children and adolescents. Am J Clin Nutr. 2010;91(3):635–642. doi: 10.3945/ajcn.2009.28385. [DOI] [PubMed] [Google Scholar]
- 33.Thompson DR, Obarzanek E, Franko DL, Barton BA, Morrison J, Biro FM, et al. Childhood overweight and cardiovascular disease risk factors: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2007;150(1):18–25. doi: 10.1016/j.jpeds.2006.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Himes JH. Challenges of accurately measuring and using BMI and other indicators of obesity in children. Pediatrics. 2009;124(Suppl 1):S3–22. doi: 10.1542/peds.2008-3586D. [DOI] [PubMed] [Google Scholar]
- 35.The Fourth Report on the Diagnosis, Evaluation and Treatment of High Blood Pressure in Children and Adolescents. National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
- 36.Flynn JT, Kaelber DC, Baker-Smith CM, Blowey D, Carroll AE, Daniels SR, et al. Clinical Practice Guideline for Screening and Management of High Blood Pressure in Children and Adolescents. Pediatrics. 2017;140(3) doi: 10.1542/peds.2017-1904. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg DE, Sallis JF, Kerr J, Maher J, Norman GJ, Durant N, et al. Brief scales to assess physical activity and sedentary equipment in the home. Int J Behav Nutr Phys Act. 2010;7:10. doi: 10.1186/1479-5868-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bracewell MA, Hennessy EM, Wolke D, Marlow N. The EPICure study: growth and blood pressure at 6 years of age following extremely preterm birth. Arch Dis Child Fetal Neonatal Ed. 2008;93(2):F108–114. doi: 10.1136/adc.2007.118596. [DOI] [PubMed] [Google Scholar]
- 39.Vohr BR, Allan W, Katz KH, Schneider KC, Ment LR. Early predictors of hypertension in prematurely born adolescents. Acta Paediatr. 2010;99(12):1812–1818. doi: 10.1111/j.1651-2227.2010.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freedman DS, Serdula MK, Srinivasan SR, Berenson GS. Relation of circumferences and skinfold thicknesses to lipid and insulin concentrations in children and adolescents: The Bogolusa Heart Study. Am J Clin Nutr. 1999;107(2):423–426. doi: 10.1093/ajcn/69.2.308. [DOI] [PubMed] [Google Scholar]
- 41.Ortega FB, Ruiz JR, Sjostrom M. Physical activity, overweight and central adiposity in Swedish children and adolescents: the European Youth Heart Study. Int J Behav Nutr Phys Act. 2007;4:61. doi: 10.1186/1479-5868-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fryar CD, C M, Ogden CL. In: Prevalence of overweight and obesity among children and adolescents: United States 1963-1965 through 2011-2012. Statistics NCfH, editor. Atlanta Georgia: Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 43.Raju TNK, Buist AS, Blaisdell CJ, Moxey-Mims M, Saigal S. Adults born preterm: a review of general health and system-specific outcomes. Acta Paediatr. 2017 doi: 10.1111/apa.13880. [DOI] [PubMed] [Google Scholar]
- 44.Springer AE, Li L, Ranjit N, Delk J, Mehta K, Kelder SH. School-level economic disadvantage and obesity in middle school children in central Texas, USA: a cross-sectional study. Int J Behav Nutr Phys Act. 2015;12(Suppl 1):S8. doi: 10.1186/1479-5868-12-S1-S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rogers R, Eagle TF, Sheetz A, Woodward A, Leibowitz R, Song M, et al. The Relationship between Childhood Obesity, Low Socioeconomic Status, and Race/Ethnicity: Lessons from Massachusetts. Child Obes. 2015;11(6):691–695. doi: 10.1089/chi.2015.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duncan AF, Heyne RJ, Morgan JS, Ahmad N, Rosenfeld CR. Elevated systolic blood pressure in preterm very-low-birth-weight infants </=3 years of life. Pediatr Nephrol. 2011;26(7):1115–1121. doi: 10.1007/s00467-011-1833-x. [DOI] [PubMed] [Google Scholar]
- 47.Uwaezuoke SN, Eneh CI, Ndu IK. Relationship Between Exclusive Breastfeeding and Lower Risk of Childhood Obesity: A Narrative Review of Published Evidence. Clin Med Insights Pediatr. 2017;11:1179556517690196. doi: 10.1177/1179556517690196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hansen AR, Pritchard T, Melnic I, Zhang J. Physical activity, screen time, and school absenteeism: self-reports from NHANES 2005-2008. Curr Med Res Opin. 2016;32(4):651–659. doi: 10.1185/03007995.2015.1135112. [DOI] [PubMed] [Google Scholar]
- 49.Henderson M, Benedetti A, Barnett TA, Mathieu ME, Deladoey J, Gray-Donald K. Influence of Adiposity, Physical Activity, Fitness, and Screen Time on Insulin Dynamics Over 2 Years in Children. JAMA Pediatr. 2016;170(3):227–235. doi: 10.1001/jamapediatrics.2015.3909. [DOI] [PubMed] [Google Scholar]
- 50.Community Preventive Services Task F. Reducing Children’s Recreational Sedentary Screen Time: Recommendation of the Community Preventive Services Task Force. American journal of preventive medicine. 2016;50(3):416–418. doi: 10.1016/j.amepre.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 51.Wilson PB, Haegele JA, Zhu X. Mobility Status as a Predictor of Obesity, Physical Activity, and Screen Time Use among Children Aged 5-11 Years in the United States. J Pediatr. 2016;176:23–29 e21. doi: 10.1016/j.jpeds.2016.06.016. [DOI] [PubMed] [Google Scholar]
- 52.Kaseva N, Wehkalampi K, Strang-Karlsson S, Salonen M, Pesonen AK, Raikkonen K, et al. Lower conditioning leisure-time physical activity in young adults born preterm at very low birth weight. PLoS One. 2012;7(2):e32430. doi: 10.1371/journal.pone.0032430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corder K, Crespo NC, van Sluijs EM, Lopez NV, Elder JP. Parent awareness of young children’s physical activity. Prev Med. 2012;55(3):201–205. doi: 10.1016/j.ypmed.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Melo HN, Stoots SJ, Pool MA, Carvalho VO, Almeida LOC, Aragao MLC, et al. Physical activity level and performance in the six-minute walk test of children and adolescents with sickle cell anemia. Rev Bras Hematol Hemoter. 2017;39(2):133–139. doi: 10.1016/j.bjhh.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soares AAA, Barros CM, Santos CGC, Dos Santos MRA, Silva JRS, Silva WMD, Junior, et al. Respiratory muscle strength and pulmonary function in children with rhinitis and asthma after a six-minute walk test. J Asthma. 2017:1–7. doi: 10.1080/02770903.2017.1326133. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y, Liang H, Tussing L, Braunschweig C, Caballero B, Flay B. Obesity and related risk factors among low socio-economic status minority students in Chicago. Public Health Nutr. 2007;10(9):927–938. doi: 10.1017/S1368980007658005. [DOI] [PubMed] [Google Scholar]
- 57.Swinburn BA, Sacks G, Hall KD, McPherson K, Finegood DT, Moodie ML, et al. The global obesity pandemic: shaped by global drivers and local environments. Lancet. 2011;378(9793):804–814. doi: 10.1016/S0140-6736(11)60813-1. [DOI] [PubMed] [Google Scholar]
- 58.Vedovato GM, Surkan PJ, Jones-Smith J, Steeves EA, Han E, Trude AC, et al. Food insecurity, overweight and obesity among low-income African-American families in Baltimore City: associations with food-related perceptions. Public Health Nutr. 2016;19(8):1405–1416. doi: 10.1017/S1368980015002888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gortmaker SL, Long MW, Resch SC, Ward ZJ, Cradock AL, Barrett JL, et al. Cost Effectiveness of Childhood Obesity Interventions: Evidence and Methods for CHOICES. American journal of preventive medicine. 2015;49(1):102–111. doi: 10.1016/j.amepre.2015.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Y, Cai L, Wu Y, Wilson RF, Weston C, Fawole O, et al. What childhood obesity prevention programmes work? A systematic review and meta-analysis. Obes Rev. 2015;16(7):547–565. doi: 10.1111/obr.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Foster BA, Aquino C, Gil M, Flores G, Hale D. A randomized clinical trial of the effects of parent mentors on early childhood obesity: Study design and baseline data. Contemp Clin Trials. 2015 doi: 10.1016/j.cct.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wells N, Stokes TA, Ottolini K, Olsen CH, Spitzer AR, Hunt CE. Anthropometric trends from 1997 to 2012 in infants born at 28 weeks’ gestation or less. J Perinatol. 2017;37(5):521–526. doi: 10.1038/jp.2016.244. [DOI] [PubMed] [Google Scholar]


