Abstract
Objective
To assess the relationship between overweight (BMI percentile ≥85 and <95) and obesity (BMI ≥95 percentile) and developmental and health outcomes at 10 years of age in a cohort of individuals born extremely preterm (.
Study design
This was an observational cohort study of children born EP and then assessed at age 10 years for neurocognitive function and parent-reported behavior and health outcomes. Participants included 871 10-year-olds. To describe the strength of association between overweight or obesity and outcomes, we used logistic regression models adjusting for confounders. Neurocognitive function, academic achievement, parent-reported health outcome surveys, and height and weight were measured.
Results
BMI category at 10 years of age was not associated with differences in intelligence, language, or academic achievement. Parents of children with obesity were more likely to report their child had asthma (odds ratio (OR): 2.2; 95% confidence interval (CI): 1.4, 3.5), fair/poor general health (OR: 3.2; 95% CI: 1.4, 7.5), and decreased physical function (OR: 1.7; 95% CI: 1.1, 2.9), but less likely to have physician diagnosed Attention Deficit Hyperactivity Disorder (ADHD) (OR: 0.5; 95% CI: 0.3, 0.97) or an individualized education plan (IEP) (odds ratio: 0.6; 95% CI: 0.4, 0.99).
Conclusion
Among children born extremely preterm, an elevated BMI, compared with normal or low BMI, is not associated with a difference in neurocognitive function. However, asthma, fair/poor general health, and decreased physical function were more prevalent among study participants with obesity, and ADHD and IEPs were less prevalent.
Keywords: overweight, obesity, extremely preterm, neurocognitive outcomes, asthma
Infants born extremely preterm () and infants with extremely low birth weight (ELBW) often exhibit growth delay during the first several postnatal months.1,2 As a result of more rapid growth in infancy, children born EP often attain weights similar to those of full-term normal birth weight peers.3,4 Children born EP who exhibit greater growth during infancy have better cognitive outcomes in childhood,5,6 but are also more likely to develop obesity.5,7,8
Childhood obesity is associated with worse school performance7,9 and decreased cognitive functioning,8,10,11 outcomes for which preterm infants are already at high risk.12,13 A potential mechanism for this association is suggested by the observation that in preclinical models, overfeeding is associated with brain inflammation14 and neurocognitive impairment.15,16 Another correlate of childhood obesity is asthma.5,17,18 Potential explanations for this association include overlapping environmental, developmental, and behavioral risk factors as well as obesity-induced immune dysregulation, contributing to asthma risk.19
Given the potential trade-offs associated with rapid infant weight gain after discharge from neonatal intensive care, it is important to know whether individuals born EP who become overweight or obese are more or less likely to have impaired cognitive functioning or other adverse outcomes. In this study, we evaluated the null hypothesis that in a cohort of children born EP, cognitive function does not differ for those children who are overweight or obese at 10 years of age, as compared with those who are healthy weight.
Methods
We evaluated a total of 1506 infants born before the 28th week of gestation and enrolled in the Extremely Low Gestational Age Newborn (ELGAN) study during the years 2002- 2004. The ELGAN study is a multi-center prospective, observational study of EP infants.20 From the original ELGAN cohort, 1198 (80%) children survived to 10 years of age. Because the primary aim of this second phase of the ELGAN study involved relationships between inflammation and outcomes during childhood, 966 surviving members of the EGLAN cohort from whom we had collected blood spots during the first postnatal month for measurement of inflammation-related proteins were actively recruited for a second follow-up evaluation at 10 years of age between February 2012 and April 2015. Height and weight were obtained on 90% (n=871) of these children. These children are the subjects of this report. Anthropometric data were unable to be collected on some children with severe cerebral palsy (n=6), when home visits were conducted and a scale was unavailable (n=5), or when parents did not consent for measurements (n=4). In three children, the reason for missing height and weight measurements was not recorded. Enrollment and consent procedures for this follow up study were approved by the institutional review boards of all participating institutions.
Maternal characteristics for this infant sample, including pre-pregnancy height and weight (converted to body mass index [BMI]), were self-reported within a few days of the delivery. Perinatal characteristics, including reason for preterm delivery, were obtained by maternal chart review shortly after the mother’s discharge.
The birth weight Z-score is the number of standard deviations the infant’s birth weight is above or below the median weight of infants at the same gestational age.21,22 Data reported by Yudkin et al were used for reference because this data set excluded infants born after pregnancies with growth-restricting conditions. Chronic lung disease (bronchopulmonary dysplasia) was defined as supplemental oxygen use at 36 weeks postmenstrual age. Patients discharged home on oxygen prior to 36 weeks postmenstrual age were included as having chronic lung disease.
Families willing to participate were scheduled for one visit during which all the measures reported here were administered. Although the child was tested, the parent or caregiver completed questionnaires regarding the child’s medical status and behavior.
Anthropometric Data
Weight and height were obtained by study personnel. In order to obtain these measurements, all outer garments such as coats and shoes were removed. If children were unable to stand unsupported, either a wheel chair scale or the difference of the parent’s weight plus child’s weight and the parent’s weight alone was utilized for weight measurements. As a substitute for height in these patients, the child’s length was measured while lying down. BMI was then calculated using the following formula: BMI = Weight (in kilograms)/Height (in meters).2 BMI Z-scores and percentiles for age and sex were then determined centrally by the study statistician, using the Statistical Analysis Software program based on current CDC growth charts.23,24
Neurocognitive measures
Neurocognitive ability was assessed with the School-Age Differential Ability Scales-II (DAS-II), Oral and Written Language Scales (OWLS), Developmental NEuroPSYchological Assessment-II (NEPSY-II), and the Wechsler Individual Achievement Test-III (WIAT-III). The Pediatric Quality of Life Inventory (PedsQL) Measurement Model is a modular approach that was used to measure health-related quality of life. Details on the specific subsets of these tests can be found in Appendix 1 (available at www.jpeds.com).
Statistical Analyses
We evaluated the null hypothesis that at age 10 years, neither a BMI percentile between 85 and just less than 95 (overweight) nor a 10-year BMI percentile of 95 or above (obese) is associated with any cognitive, executive, communication or social dysfunction, achievement limitation, or unfavorable parent-reported health outcome. The reference group used was children in this cohort with BMI percentile at 10 years <85. We began by assessing correlates of these BMI percentile groups, including the maternal demographics, pregnancy and newborn characteristics, and educational history at age 10 years.
To allow for the differences in age at the time of the assessment, and to facilitate a comparison of our findings to those reported for children presumably born very near term, we used Z-scores based on distributions of values reported for the historical normative samples that are described by the authors of the assessments we used.25-27 We created logistic regression models of the risk of a score one or more standard deviations below the normative mean of each assessment. These models, which included potential confounders (including infant’s sex and birth weight Z-score < −1, as well as maternal characteristics of Hispanic ethnicity, education ≤ 12 years, single marital status, and pre-pregnancy BMI <25 and 25 to <30), allowed us to calculate odds ratios (and 95% confidence intervals) of each 10-year characteristic associated with a BMI percentile between 85 and <95 or ≥95. Similar data analysis was also performed excluding children with BMI percentile <5 (underweight).
Results
The children not seen at 10-year follow-up were more likely than those assessed to have a mother who had less formal education, was not married, and was eligible for government-provided (public) health care insurance. The children who returned for the assessment were similar in the frequency of neonatal complications to those not evaluated at age 10, except that those who were assessed at age 10 were more likely to have had chronic lung disease than those not assessed (Table I; available at www.jpeds.com). There were few notable differences between those with BMI available at 10 years and those without measurements. (Table 2; available at www.jpeds.com).
Table 1.
online. Characteristics of children who were eligible for follow up (had some or all follow-up tests/examinations at 2 years) and were seen at 10 years and those eligible for follow up but not seen at 10 years. These are column percents.
| Eligible at 10 years* | Row N |
||||||
|---|---|---|---|---|---|---|---|
| Seen** | Not seen | ||||||
| Maternal characteristics | |||||||
| Racial identity | White | 64 | 50 | 714 | |||
| Black | 26 | 31 | 322 | ||||
| Other | 11 | 19 | 151 | ||||
| Hispanic | Yes | 10 | 19 | 147 | |||
| Age, years | < 21 | 13 | 19 | 170 | |||
| 21-35 | 67 | 66 | 802 | ||||
| > 35 | 20 | 16 | 226 | ||||
| Education, years | ≤ 12 | 41 | 52 | 506 | |||
| > 12, < 16 | 23 | 24 | 270 | ||||
| ≥ 16 | 36 | 24 | 376 | ||||
| Single marital status | Yes | 39 | 52 | 513 | |||
| Public insurance | Yes | 35 | 52 | 464 | |||
| Smoking during pregnancy | Yes | 14 | 16 | 162 | |||
| Passive smoking | Yes | 24 | 28 | 293 | |||
| Pre-pregnancy BMI | < 18.5 | 8 | 8 | 90 | |||
| 18.5, < 30 | 69 | 74 | 809 | ||||
| ≥ 30 | 23 | 18 | 248 | ||||
| Gestational diabetes | Yes | 7 | 8 | 82 | |||
| Perinatal characteristics | |||||||
| Any antenatal steroid | Yes | 89 | 82 | 1073 | |||
| Histologic chorioamnionitis¶ | Yes | 32 | 39 | 411 | |||
| Missing | 8 | 9 | 99 | ||||
| Delivery complication | Preterm labor | 46 | 41 | 534 | |||
| Preterm PROM | 22 | 22 | 363 | ||||
| Preeclampsia | 13 | 13 | 153 | ||||
| Abruption | 10 | 11 | 128 | ||||
| Cervical Insufficiency | 5 | 8 | 72 | ||||
| Fetal indication | 4 | 4 | 49 | ||||
| Cesarean delivery | Yes | 66 | 67 | 795 | |||
| Multifetal pregnancy | Yes | 35 | 27 | 393 | |||
| Newborn characteristics | |||||||
| Sex | Male | 51 | 54 | 621 | |||
| Gestational age, weeks | 23-24 | 21 | 20 | 245 | |||
| 25-26 | 46 | 48 | 553 | ||||
| 27 | 34 | 32 | 400 | ||||
| Birth weight, grams | ≤ 750 | 37 | 35 | 436 | |||
| 751-1000 | 43 | 44 | 520 | ||||
| > 1000 | 20 | 21 | 242 | ||||
| Birth weight Z-score† | < −2 | 6 | 3 | 62 | |||
| ≥ −2, < −1 | 13 | 13 | 153 | ||||
| ≥ −1 | 81 | 85 | 983 | ||||
| Head circumference Z-score† | < −2 | 8 | 6 | 89 | |||
| ≥ −2, < −1 | 21 | 25 | 260 | ||||
| ≥ −1 | 70 | 69 | 806 | ||||
| Postnatal Characteristics | |||||||
| Growth velocity quartile†† | Lowest | 23 | 29 | 290 | |||
| Highest | 25 | 24 | 291 | ||||
| Bacteremia, week 1 | Yes | 10 | 10 | 76 | |||
| Bacteremia, weeks 2-4 | Yes | 30 | 28 | 296 | |||
| Necrotizing enterocolitis‡ | Yes | 8 | 6 | 88 | |||
| Chronic lung disease‡‡ | Yes | 52 | 46 | 598 | |||
| BSID-II MDI < 70 at 2 years | Yes | 26 | 29 | 268 | |||
| Cerebral palsy at 2 years | Yes | 10 | 14 | 119 | |||
| Corrected age at 2 years | < 24 months | 25 | 28 | 276 | |||
| Maximum column N | 871 | 327 | 1198 | ||||
Eligible at 10 years are the 1198 children who survived to 10-years
Seen at 10 years are the 871 children for whom a BMI centile could be calculated (weight and height were collected).
Grades 3 and 4
Yudkin standard
1000 × [(weight day 28 - weight day 7)/weight day 7]/21
Stage IIIa, IIIb, or perforation
Receiving O2 at 36 weeks PCA
Table 2.
online. Characteristics of children who had and did not have measures of weight and height at 10 years. These are column percents.
| BMI centile available at 10 years | Row N |
|||
|---|---|---|---|---|
| Yes | No | |||
| Maternal characteristics | ||||
| Racial identity | White | 64 | 44 | 562 |
| Black | 26 | 22 | 227 | |
| Other | 11 | 33 | 98 | |
| Hispanic | Yes | 10 | 6 | 86 |
| No | 90 | 94 | 801 | |
| Age, years 39 |
< 21 | 13 | 33 | 115 |
| 21-35 | 67 | 39 | 594 | |
| > 35 | 20 | 28 | 180 | |
| Education, years | ≤ 12 | 41 | 44 | 367 |
| > 12, < 16 | 23 | 33 | 210 | |
| ≥ 16 | 36 | 22 | 312 | |
| Single marital status | Yes | 39 | 56 | 353 |
| No | 61 | 44 | 536 | |
| Public insurance | Yes | 35 | 39 | 314 |
| No | 65 | 61 | 575 | |
| Pre-pregnancy BMI | < 25 | 58 | 76 | 497 |
| 25, < 30 | 19 | 18 | 166 | |
| ≥ 30 | 23 | 3 | 194 | |
| Perinatal characteristics | ||||
| Any antenatal corticosteroids | Yes | 89 | 83 | 788 |
| No | 11 | 17 | 100 | |
| Delivery complication | PE/FI | 17 | 22 | 151 |
| Spontaneous | 83 | 78 | 738 | |
| Cesarean delivery | Yes | 66 | 18 | 590 |
| No | 34 | 22 | 299 | |
| Inflammation of chorionic plate of placenta | Yes | 32 | 33 | 288 |
| No | 59 | 67 | 530 | |
| Missing | 8 | 0 | 71 | |
| Newborn characteristics | ||||
| Sex | Male | 51 | 67 | 455 |
| Female | 49 | 33 | 434 | |
| Gestational age, weeks | 23-24 | 21 | 39 | 187 |
| 25-26 | 46 | 17 | 400 | |
| 27 | 34 | 44 | 302 | |
| Birth weight, grams | ≤ 750 | 37 | 56 | 332 |
| 751-1000 | 43 | 33 | 382 | |
| > 1000 | 20 | 11 | 175 | |
| Birth weight Z-score | < −2 | 6 | 0 | 53 |
| ≥ −2, < −1 | 13 | 44 | 120 | |
| ≥ −1 | 81 | 56 | 716 | |
| Maximum column N | 871 | 18 | 889 | |
Sample characteristics
A higher percentage of women who identified as Hispanic and, who at the time of delivery, were less than 21 years of age, had a child who was overweight or obese at 10-years (Table 3; available at www.jpeds.com). The higher the mother’s pre-pregnancy BMI, and the higher the newborn’s birth weight Z-score, the higher the prevalence of obesity.
Table 3.
online. Sample characteristics among children classified by BMI centile at 10 years. These are row percents.
| Child’s BMI centile at 10 years | Row N |
||||
|---|---|---|---|---|---|
| < 85 | 85, < 95 | ≥ 95 | |||
| Maternal characteristics | |||||
| Racial identity | White | 79 | 10 | 11 | 554 |
| Black | 74 | 13 | 13 | 223 | |
| Other | 67 | 21 | 12 | 92 | |
| Hispanic | Yes | 62 | 21 | 16 | 85 |
| No | 78 | 11 | 11 | 784 | |
| Age, years | < 21 | 70 | 17 | 14 | 109 |
| 21-35 | 76 | 12 | 11 | 587 | |
| > 35 | 79 | 9 | 11 | 175 | |
| Education, years | ≤ 12 | 73 | 14 | 14 | 359 |
| > 12, < 16 | 76 | 13 | 11 | 204 | |
| ≥ 16 | 81 | 10 | 9 | 308 | |
| Single marital status | Yes | 72 | 15 | 13 | 343 |
| No | 79 | 10 | 11 | 528 | |
| Public insurance | Yes | 75 | 13 | 12 | 307 |
| No | 77 | 12 | 11 | 564 | |
| Pre-pregnancy BMI | < 25 | 83 | 10 | 7 | 484 |
| 25, < 30 | 69 | 14 | 15 | 163 | |
| ≥ 30 | 67 | 12 | 20 | 193 | |
| Perinatal characteristics | |||||
| Any antenatal corticosteroids | Yes | 76 | 12 | 12 | 773 |
| No | 74 | 18 | 8 | 97 | |
| Delivery complication | PE/FI | 81 | 12 | 7 | 147 |
| Spontaneous | 75 | 12 | 12 | 724 | |
| Cesarean delivery | Yes | 77 | 13 | 10 | 576 |
| No | 74 | 11 | 15 | 295 | |
| Inflammation of chorionic plate of placenta | Yes | 74 | 11 | 16 | 282 |
| No | 78 | 12 | 9 | 518 | |
| Missing | 72 | 17 | 11 | 71 | |
| Newborn characteristics | |||||
| Sex | Male | 79 | 11 | 10 | 443 |
| Female | 73 | 14 | 13 | 428 | |
| Gestational age, weeks | 23-24 | 79 | 12 | 9 | 180 |
| 25-26 | 76 | 11 | 13 | 397 | |
| 27 | 74 | 15 | 11 | 294 | |
| Birth weight, grams | ≤ 750 | 82 | 10 | 7 | 322 |
| 751-1000 | 73 | 12 | 15 | 376 | |
| > 1000 | 71 | 16 | 13 | 173 | |
| Birth weight Z-score | < −2 | 85 | 8 | 8 | 53 |
| ≥ −2, < −1 | 84 | 10 | 6 | 112 | |
| ≥ −1 | 74 | 13 | 13 | 706 | |
| Maximum column N | 664 | 106 | 101 | 871 | |
Childhood neurodevelopmental outcomes
Cognitive
Children across the three categories of BMI percentiles had similar prevalences of low and very low scores on measures of IQ, academic achievement, language, working memory, and most indicators of executive function (Table 4 and Figure 1).
Table 4.
Distribution of intelligence, executive function, language, achievement test scores in each category of BMI centile at 10 years. These are column percents.
| IQ | Z-score | Child’s BMI centile at 10 years | Row N |
||
|---|---|---|---|---|---|
| < 85 | 85, < 95 | ≥ 95 | |||
| DAS-II Verbal reasoning | ≤ −2 | 18 | 16 | 14 | 146 |
| > −2, ≤ −1 | 18 | 18 | 19 | 158 | |
| DAS-II Nonverbal reasoning | ≤ −2 | 15 | 12 | 16 | 126 |
| > −2, ≤ −1 | 25 | 26 | 20 | 209 | |
| Executive Function | |||||
| DAS-II Working memory | ≤ −2 | 18 | 18 | 14 | 152 |
| > −2, ≤ −1 | 18 | 14 | 16 | 147 | |
| NEPSY-II Auditory Attention | ≤ −2 | 22 | 25 | 23 | 186 |
| > −2, ≤ −1 | 22 | 17 | 18 | 175 | |
| NEPSY-II Auditory Response Set | ≤ −2 | 19 | 19 | 22 | 165 |
| > −2, ≤ −1 | 28 | 22 | 32 | 231 | |
| NEPSY-II Inhibition Inhibition | ≤ −2 | 35 | 25 | 33 | 281 |
| > −2, ≤ −1 | 22 | 28 | 26 | 198 | |
| NEPSY-II Inhibition Switching | ≤ −2 | 27 | 27 | 32 | 226 |
| > −2, ≤ −1 | 29 | 24 | 32 | 239 | |
| NEPSY-II Animal Sorting | ≤ −2 | 27 | 28 | 35 | 239 |
| > −2, ≤ −1 | 31 | 30 | 25 | 258 | |
| Processing Speed | |||||
| NEPSY-II Inhibition Naming | ≤ −2 | 31 | 27 | 32 | 262 |
| > −2, ≤ −1 | 18 | 22 | 28 | 169 | |
| Visual Perception | |||||
| NEPSY-II Arrows | ≤ −2 | 25 | 23 | 33 | 218 |
| > −2, ≤ −1 | 23 | 24 | 22 | 193 | |
| NEPSY-II Geometric Puzzles | ≤ −2 | 17 | 13 | 15 | 138 |
| > −2, ≤ −1 | 22 | 23 | 23 | 191 | |
| Fine Motor Function | |||||
| NEPSY-II Visuomotor Precision | ≤ −2 | 19 | 18 | 27 | 172 |
| > −2, ≤ −1 | 36 | 32 | 31 | 300 | |
| Language | |||||
| OWLS Listening Comprehension | ≤ −2 | 19 | 16 | 17 | 158 |
| > −2, ≤ −1 | 26 | 28 | 31 | 229 | |
| OWLS Oral Expression | ≤ −2 | 20 | 15 | 17 | 160 |
| > −2, ≤ −1 | 23 | 21 | 23 | 189 | |
| Academic Achievement | |||||
| WIAT-III Word reading | ≤ −2 | 13 | 10 | 9 | 102 |
| > −2, ≤ −1 | 17 | 17 | 18 | 146 | |
| WIAT-III Pseudoword decoding | ≤ −2 | 14 | 13 | 17 | 122 |
| > −2, ≤ −1 | 16 | 14 | 21 | 142 | |
| WIAT-III Spelling | ≤ −2 | 11 | 10 | 9 | 91 |
| > −2, ≤ −1 | 16 | 17 | 13 | 133 | |
| WIAT-III Numeric operations | ≤ −2 | 16 | 14 | 14 | 134 |
| > −2, ≤ −1 | 23 | 23 | 24 | 198 | |
| Maximum column N | 653 | 106 | 101 | 860 | |
Figure 1.
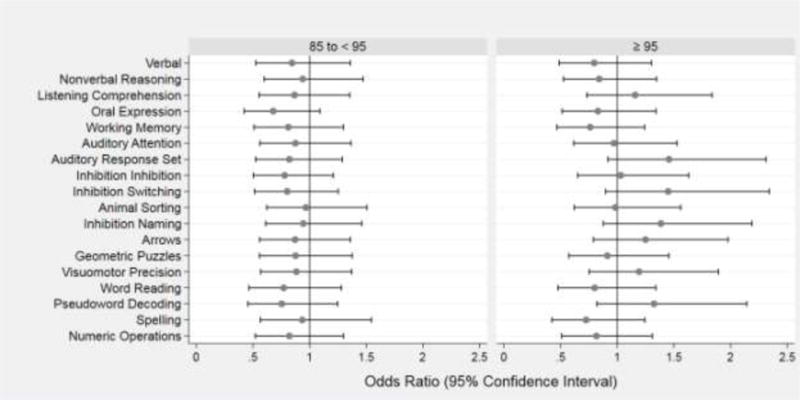
Forest plots of odds ratios (ORs) and 95% confidence intervals of a Z-score ≤ −1 on each DAS-II and NEPSY-II neurocognitive assessment at age 10 associated with BMI centile at 10 years 85 to < 95 (left panel) and ≥ 95 (right panel). The reference group is children from the same cohort with BMI centile at 10 years <85. Odds ratios are adjusted for maternal Hispanic ethnicity, education ≤ 12 years, single marital status, and pre-pregnancy BMI < 25 and 25 to < 30; and child’s sex and birth weight Z-score < −1. Statistically significant items are bolded.
Health Outcomes
Children who were overweight had a lower prevalence of physician-diagnosed Attention Deficit Hyperactivity Disorder (ADHD) (OR: 0.5; 95% CI: 0.3, 0.97) than normal or underweight peers, and those who were obese were less likely to be prescribed an ADHD medication (OR: 0.5; 95% CI: 0.3, 0.97) (Table 5 and Figure 2). Overweight children were also less likely to have an individual education plan (OR: 0.6; 95% CI: 0.4, 0.99). In contrast, children who were obese had a higher prevalence of an asthma diagnosis and were more likely than their peers to be prescribed a drug for asthma symptoms (OR: 2.2; 95% CI: 1.4, 3.5). Parents of children who were obese were also more likely than parents of healthy weight children to report that their child’s quality of life was very low in the physical function domain (OR: 1.7; 95% CI: 1.1, 2.9) and that their child’s general health was “fair” to “poor” as opposed to “good” or better (OR: 3.2; 95% CI: 1.4, 7.5). BMI groups did not differ in the number of school days missed for respiratory illness, surgery, or other illness.
Table 5.
The percent of children classified by BMI centile at 10 years who also had the listed health or quality of life characteristics. These are column percents.
| Child’s BMI centile at 10 years | Row N |
||||
|---|---|---|---|---|---|
| < 85 | 85, < 95 | ≥ 95 | |||
| Had an individual education plan | Yes | 56 | 46 | 45 | 404 |
| Repeated a grade | Yes | 19 | 15 | 17 | 162 |
| Placed in a special remedial class | Yes | 22 | 18 | 16 | 183 |
| Any seizure (algorithm) | Yes | 11 | 15 | 12 | 103 |
| Epilepsy (algorithm) | Yes | 8 | 7 | 7 | 64 |
| Physician diagnosis of: | ADHD | 26 | 16 | 20 | 207 |
| Asthma | 34 | 44 | 55 | 329 | |
| Currently receiving medication for: | ADHD | 18 | 13 | 11 | 146 |
| Seizures | 5 | 4 | 6 | 44 | |
| Asthma | 19 | 18 | 33 | 177 | |
| School days missed for respiratory illness | ≥ 2 | 32 | 29 | 29 | 270 |
| School days missed for surgery | ≥ 1 | 7 | 7 | 9 | 63 |
| School days missed for other illness | ≥ 2 | 33 | 28 | 30 | 282 |
| General health | < good | 3 | 5 | 10 | 36 |
| Dean handedness Inventory | < −10 (L) | 16 | 23 | 13 | 143 |
| −10 to 10 | 5 | 6 | 12 | 47 | |
| > 10 (R) | 79 | 72 | 74 | 657 | |
| Manual ability classification system | ≥ 3 | 9 | 11 | 11 | 83 |
| Gross motor function* | ≥ 3 | 5 | 2 | 7 | 40 |
| Communication function classification system system | 3 | 12 | 5 | 15 | 99 |
| 4-5 | 9 | 8 | 12 | 83 | |
| Peds QoL inventory | |||||
| Physical functioning | < 70 | 16 | 17 | 26 | 150 |
| ≥ 70, < 85 | 15 | 8 | 16 | 125 | |
| Emotional functioning | < 70 | 26 | 23 | 30 | 224 |
| ≥ 70, < 85 | 25 | 29 | 18 | 214 | |
| Social functioning | < 70 | 25 | 21 | 30 | 216 |
| ≥ 70, < 85 | 19 | 15 | 15 | 153 | |
| School functioning | < 70 | 41 | 35 | 40 | 341 |
| ≥ 70, < 85 | 24 | 23 | 22 | 202 | |
| Psychosocial Functioning | < 70 | 30 | 26 | 32 | 258 |
| ≥ 70, < 85 | 30 | 27 | 27 | 252 | |
| Maximum column N | 664 | 106 | 101 | 871 | |
Gross motor function classification system
Figure 2.
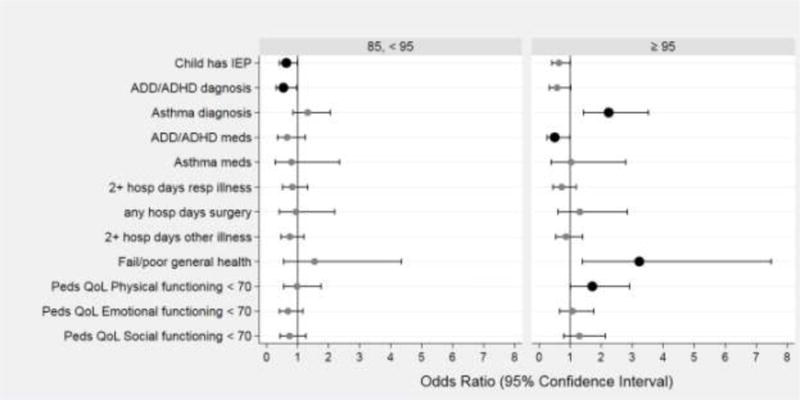
Forest plots of odds ratios (ORs) and 95% confidence intervals of several educational and health characteristics associated with BMI centile at 10 years 85 to < 95 (left panel) and ≥ 95 (right panel). The reference group is children from the same cohort with BMI centile at 10 years <85. Odds ratios are adjusted for maternal Hispanic ethnicity, education ≤ 12 years, single marital status, and pre-pregnancy BMI < 25 and 25 to < 30; and child’s sex and birth weight Z-score < −1. Statistically significant items are bolded.
Analyses excluding children with BMI below the fifth percentile
Only 34 children (3.9%) had a BMI percentile <5 (underweight). Analyses that excluded these underweight children produced findings similar to those of analyses involving the entire sample.
Discussion
In this cohort of 10-year-old children born extremely preterm, the health and neurodevelopmental outcomes of children who were overweight or obese were similar to those of peers with a healthy weight, except that children who were obese were more likely to have asthma, fair/poor general health, and decreased physical function, but were less likely to have ADHD or an IEP. The combined prevalence of overweight and obesity in this cohort of children born extremely preterm was 24%, lower than the 35% of children in the US, studied from 1999-2010.28 Only 4% of the cohort was underweight (<5th percentile).
Epidemiologic studies of the relationship of obesity to cognitive function provide conflicting results. In a large population-based sample of school-aged children, overweight was associated with worse cognitive functioning.11 However, in another sample of school-aged children, drawn from the United States, Holland, and Australia, no association was found between BMI, modeled as a continuous variable, and cognitive function.29 The current study adds that in a sample of infants born EP, there also does not appear to be a cross-sectional relationship between BMI and neurocognitive function at 10 years of age.
Our finding, that children born with EP who had obesity at 10 years of age were less likely to have been diagnosed with ADHD or have an IEP, is consistent with prior studies.30 Both low birth weight and intrauterine growth restriction seen in infants born EP have been shown to be risk factors for ADHD.31-33 Birth weight z-score was adjusted in our analysis, but interestingly, more recent research on the temporal relationship between obesity and ADHD would suggest that ADHD symptoms in childhood are an independent risk factor for obesity later in life.34,35 Similarly, our finding that children with obesity were more likely to have asthma is also congruent with previous studies in samples unselected for prematurity.18,36,37 Low birth weight has been associated with asthma, and excess body mass later in life may amplify the asthma risk.38 The reason for the links between obesity and asthma remain obscure, but likely explanations for the link between obesity and asthma invoke inflammatory phenomena (eg, with roles for adiponectin,39 the gut microbiome,40 or Th17 cells41). Others have also reported an association between increasing child BMI and parents’ perception of poor general health of their children.18,42,43
The strengths of this study include the relatively large and diverse sample of children whom were born EP and followed until age 10 years. We broadly assessed neurocognitive and academic function and controlled for many relevant confounders. The assessment was done by examiners who were unaware of the study objectives. The primary limitation of this study is that direct measures of health, such as pulmonary function testing, were not obtained. Parents fail to report physician-diagnosed asthma in about 25% of cases.44 Obesity is associated with metabolic and cardiovascular complications, which were not assessed in this sample. In addition, the measure of adiposity fat that we used, ie, BMI, is a relatively crude measure of body fat, although the correlation of BMI and body fat in prepubertal children is high.45,46 This was also a cross-sectional study, and as such, did not assess timing of excess weight gain and how the timing may contribute to the presence of overweight/obesity and the described associated outcomes at 10 years.
Contrary to our hypothesis, children born extremely preterm who are overweight or obese at 10 years of age had similar neurocognitive skills and abilities as their peers with healthy weights. Despite a higher prevalence of parent-reported asthma, decreased physical functioning, and fair/poor general health among children who are obese in the ELGAN cohort, this study provides tentative reassurance that children born EP who then go on to be overweight or obese in childhood do not have worse neurocognitive outcomes than their healthy weight peers and in fact have a lower prevalence of ADHD.
Supplementary Material
Acknowledgments
Acknowledgments available at www.jpeds.com (Appendix 2).
Supported by grants from the National Institute of Neurologic Disorders and Stroke (5U01NS040069-05, 2R01NS040069-06A2) and the Office of the Director, National Institutes of Health under Award Number 1UG3OD023348-01. The study sponsors had no role in the study design, the collection, analysis, and interpretation of data, the writing of the report, or the decision to submit the paper for publication.
Abbreviations list
- EP
extremely preterm
- ADHD
Attention Deficit Hyperactivity Disorder
- IEP
individualized education plan
- ELBW
extremely low birth weight
- BMI
body mass index
- ELGAN
Extremely Low Gestational Age Newborn study
Appendix 1 - Neurocognitive assessments
General cognitive ability (or IQ) was assessed with the School-Age Differential Ability Scales–II (DAS-II) Verbal and Nonverbal Reasoning scales.25 Expressive and receptive language skills were evaluated with the Oral and Written Language Scales (OWLS), which assess semantic, morphological, syntactic, and pragmatic production and comprehension of elaborated sentences.26
Attention and executive function were assessed with both the DAS-II25 and the NEPSY- II (A Developmental NEuroPSYchological Assessment-II).27 The DAS-II Recall of Digits Backward and Recall of Sequential Order measured verbal working memory, while the NEPSY-II Auditory Attention and Response Set measured auditory attention, set switching and inhibition, the NEPSY-II Inhibition and Inhibition Switching measured simple inhibition and inhibition in the context of set shifting, respectively, and the NEPSY-II Animal Sorting measured visual concept formation and set shifting.
Speed of processing was assessed with NEPSY-II Inhibition Naming, which provides a baseline measure of processing speed and has no inhibitory component. Visual perception and motor function were assessed with NEPSY-II Arrows and Geometric Puzzles & Visuomotor Precision and Fingertip Tapping respectively. Academic Function was assessed with The Wechsler Individual Achievement Test-III (WIAT-III [C]) which provides standard scores in word recognition and decoding, spelling, and numeric operations.47
The Pediatric Quality of Life Inventory™ (PedsQL™) Measurement Model is a modular approach to measuring health-related quality of life (HRQOL) in healthy children and adolescents and those with acute and chronic health conditions. The PedsQL Measurement Model integrates seamlessly both generic core scales and disease- specific modules into one measurement system.48 The 23-item PedsQL Generic Core Scales were designed to measure the core dimensions of health: physical functioning (8 items), emotional functioning (5 items), social functioning (5 items), and school functioning (5 items). For ease of interpretability, items are reversed scored and linearly transformed to a 0-100 scale, so that higher scores indicate better HRQOL.
Appendix 2: Study Group Members
The authors gratefully acknowledge the contributions of their subjects, and their subjects’ families, as well as those of their colleagues listed below.
Project Lead for ELGAN-2: Julie V. Rollins, MA, University of North Carolina at Chapel Hill, Chapel Hill, NC.
Site Principal Investigators
Baystate Medical Center, Springfield, MA: Bhahvesh Shah, MD; Rachana Singh, MD, MS
Boston Children’s Hospital, Boston, MA: Linda Van Marter, MD, MPH and Camilla Martin, MD, MPH; Janice Ware, PhD
Tufts Medical Center, Boston, MA: Cynthia Cole, MD; Ellen Perrin, MD
University of Massachusetts Medical School, Worcester, MA: Frank Bednarek, MD; Jean Frazier, MD
Yale University School of Medicine, New Haven, CT: Richard Ehrenkranz, MD; Jennifer Benjamin, MD
University of North Carolina, Chapel Hill, NC: Carl Bose, MD; Diane Warner, MD, MPH
East Carolina University, Greenville, NC: Steve Engelke, MD
Helen DeVos Children’s Hospital, Grand Rapids, MI: Mariel Poortenga, MD; Steve Pastyrnak, PhD
Sparrow Hospital, East Lansing, MI: Padu Karna, MD; Nigel Paneth, MD, MPH; Madeleine Lenski, MPH
University of Chicago Medical Center, Chicago, IL: Michael Schreiber, MD; Scott Hunter, PhD; Michael Msall, MC
William Beaumont Hospital, Royal Oak, MI: Danny Batton, MD; Judith Klarr, MD
Site Study Coordinators
Baystate Medical Center, Springfield, MA: Karen Christianson, RN; Deborah Klein, BSM, RN
Boston Children’s Hospital, Boston MA: Maureen Pimental, BA; Collen Hallisey, BA; Taryn Coster, BA
Tufts Medical Center, Boston, MA: Ellen Nylen, RN; Emily Neger, MA; Kathryn Mattern, BA
University of Massachusetts Medical School, Worcester, MA: Lauren Venuti, BA; Beth Powers, RN; Ann Foley, EdM
Yale University School of Medicine, New Haven, CT: Joanne Williams, RN; Elaine Romano, APRN
Wake Forest University, Winston-Salem, NC: Debbie Hiatt, BSN (deceased); Nancy Peters, RN; Patricia Brown, RN; Emily Ansusinha, BA
University of North Carolina, Chapel Hill, NC: Gennie Bose, RN; Janice Wereszczak, MSN; Janice Bernhardt, MS, RN
East Carolina University, Greenville, NC: Joan Adams (deceased); Donna Wilson, BA, BSW
Nancy Darden-Saad, BS, RN
Helen DeVos Children’s Hospital, Grand Rapids, MI: Dinah Sutton, RN; Julie Rathbun, BSW, BSN
Sparrow Hospital, East Lansing, MI: Karen Miras, RN, BSN; Deborah Weiland, MSN
University of Chicago Medical Center, Chicago, IL: Grace Yoon, RN; Rugile Ramoskaite, BA; Suzanne Wiggins, MA; Krissy Washington, MA; Ryan Martin, MA; Barbara Prendergast, BSN, RN
William Beaumont Hospital, Royal Oak, MI: Beth Kring, RN
Psychologists
Baystate Medical Center, Springfield, MA: Anne Smith, PhD; Susan McQuiston, PhD
Boston Children’s Hospital: Samantha Butler, PhD; Rachel Wilson, PhD; Kirsten McGhee, PhD; Patricia Lee, PhD; Aimee Asgarian, PhD; Anjali Sadhwani, PhD; Brandi Henson, PsyD
Tufts Medical Center, Boston MA: Cecelia Keller, PT, MHA; Jenifer Walkowiak, PhD; Susan Barron, PhD
University of Massachusetts Medical School, Worcester MA: Alice Miller, PT, MS; Brian Dessureau, PhD; Molly Wood, PhD; Jill Damon-Minow, PhD
Yale University School of Medicine, New Haven, CT: Elaine Romano, MSN; Linda Mayes, PhD; Kathy Tsatsanis, PhD; Katarzyna Chawarska, PhD; Sophy Kim, PhD; Susan Dieterich, PhD; Karen Bearrs, PhD
Wake Forest University Baptist Medical Center, Winston-Salem NC: Ellen Waldrep, MA; Jackie Friedman, PhD; Gail Hounshell, PhD; Debbie Allred, PhD
University Health Systems of Eastern Carolina, Greenville, NC: Rebecca Helms, PhD; Lynn Whitley, PhD Gary Stainback, PhD
University of North Carolina at Chapel Hill, NC: Lisa Bostic, OTR/L; Amanda Jacobson, PT; Joni McKeeman, PhD; Echo Meyer, PhD
Helen DeVos Children’s Hospital, Grand Rapids, MI: Steve Pastyrnak, PhD
Sparrow Hospital, Lansing, MI: Joan Price, EdS; Megan Lloyd, MA, EdS
University of Chicago Medical Center, Chicago, IL: Susan Plesha-Troyke, OT; Megan Scott, PhD
William Beaumont Hospital, Royal Oak, MI: Katherine M. Solomon, PhD; Kara Brooklier, PhD; Kelly Vogt, PhD
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reprint requests: Olivia Linthavong, MD, MS
The authors declare no conflicts of interest.
Data Statement
Data will be made available on request.
References
- 1.Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003;111:986–90. doi: 10.1542/peds.111.5.986. [DOI] [PubMed] [Google Scholar]
- 2.Farooqi A, Hagglof B, Sedin G, Gothefors L, Serenius F. Growth in 10- to 12- year-old children born at 23 to 25 weeks’ gestation in the 1990s: a Swedish national prospective follow-up study. Pediatrics. 2006;118:e1452–65. doi: 10.1542/peds.2006-1069. [DOI] [PubMed] [Google Scholar]
- 3.Hack M, Schluchter M, Margevicius S, Andreias L, Taylor HG, Cuttler L. Trajectory and correlates of growth of extremely-low-birth-weight adolescents. Pediatr Res. 2014;75:358–66. doi: 10.1038/pr.2013.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saigal S, Stoskopf B, Streiner D, Paneth N, Pinelli J, Boyle M. Growth trajectories of extremely low birth weight infants from birth to young adulthood: a longitudinal, population-based study. Pediatr Res. 2006;60:751–8. doi: 10.1203/01.pdr.0000246201.93662.8e. [DOI] [PubMed] [Google Scholar]
- 5.Belfort MB, Gillman MW, Buka SL, Casey PH, McCormick MC. Preterm infant linear growth and adiposity gain: trade-offs for later weight status and intelligence quotient. J Pediatr. 2013;163:1564–9 e2. doi: 10.1016/j.jpeds.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 7.Kark M, Hjern A, Rasmussen F. Poor school performance is associated with a larger gain in body mass index during puberty. Acta Paediatr. 2014;103:207–13. doi: 10.1111/apa.12471. [DOI] [PubMed] [Google Scholar]
- 8.Miller AL, Lee HJ, Lumeng JC. Obesity-associated biomarkers and executive function in children. Pediatr Res. 2015;77:143–7. doi: 10.1038/pr.2014.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kipping RR, Jago R, Lawlor DA. Obesity in children. Part 1: Epidemiology, measurement, risk factors, and screening. BMJ. 2008;337:a1824. doi: 10.1136/bmj.a1824. [DOI] [PubMed] [Google Scholar]
- 10.Luciano R, Barraco GM, Muraca M, Ottino S, Spreghini MR, Sforza RW, et al. Biomarkers of Alzheimer disease, insulin resistance, and obesity in childhood. Pediatrics. 2015;135:1074–81. doi: 10.1542/peds.2014-2391. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Dai Q, Jackson JC, Zhang J. Overweight is associated with decreased cognitive functioning among school-age children and adolescents. Obesity (Silver Spring) 2008;16:1809–15. doi: 10.1038/oby.2008.296. [DOI] [PubMed] [Google Scholar]
- 12.Johnson S, Hennessy E, Smith R, Trikic R, Wolke D, Marlow N. Academic attainment and special educational needs in extremely preterm children at 11 years of age: the EPICure study. Arch Dis Child Fetal Neonatal Ed. 2009;94:F283–9. doi: 10.1136/adc.2008.152793. [DOI] [PubMed] [Google Scholar]
- 13.Joseph RM, O’Shea TM, Allred EN, Heeren T, Hirtz D, Jara H, et al. Neurocognitive and Academic Outcomes at Age 10 Years of Extremely Preterm Newborns. Pediatrics. 2016:137. doi: 10.1542/peds.2015-4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guillemot-Legris O, Muccioli GG. Obesity-Induced Neuroinflammation: Beyond the Hypothalamus. Trends Neurosci. 2017;40:237–53. doi: 10.1016/j.tins.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Ziko I, De Luca S, Dinan T, Barwood JM, Sominsky L, Cai G, et al. Neonatal overfeeding alters hypothalamic microglial profiles and central responses to immune challenge long-term. Brain Behav Immun. 2014;41:32–43. doi: 10.1016/j.bbi.2014.06.014. [DOI] [PubMed] [Google Scholar]
- 16.De Luca SN, Ziko I, Sominsky L, Nguyen JC, Dinan T, Miller AA, et al. Early life overfeeding impairs spatial memory performance by reducing microglial sensitivity to learning. J Neuroinflammation. 2016;13:112. doi: 10.1186/s12974-016-0578-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belfort MB, Cohen RT, Rhein LM, McCormick MC. Preterm infant growth and asthma at age 8 years. Arch Dis Child Fetal Neonatal Ed. 2016;101:F230–4. doi: 10.1136/archdischild-2015-308340. [DOI] [PubMed] [Google Scholar]
- 18.Cockrell Skinner A, Perrin EM, Steiner MJ. Healthy for now? A cross-sectional study of the comorbidities in obese preschool children in the United States. Clin Pediatr (Phila) 2010;49:648–55. doi: 10.1177/0009922810362098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frey U, Latzin P, Usemann J, Maccora J, Zumsteg U, Kriemler S. Asthma and obesity in children: current evidence and potential systems biology approaches. Allergy. 2015;70:26–40. doi: 10.1111/all.12525. [DOI] [PubMed] [Google Scholar]
- 20.O’Shea TM, Allred EN, Dammann O, Hirtz D, Kuban KC, Paneth N, et al. The ELGAN study of the brain and related disorders in extremely low gestational age newborns. Early Hum Dev. 2009;85:719–25. doi: 10.1016/j.earlhumdev.2009.08.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yudkin PL, Aboualfa M, Eyre JA, Redman CW, Wilkinson AR. New birthweight and head circumference centiles for gestational ages 24 to 42 weeks. Early Hum Dev. 1987;15:45–52. doi: 10.1016/0378-3782(87)90099-5. [DOI] [PubMed] [Google Scholar]
- 22.Leviton A, Paneth N, Reuss ML, Susser M, Allred EN, Dammann O, et al. Maternal infection, fetal inflammatory response, and brain damage in very low birthweight infants. Pediatric Research. 1999;46:566–75. doi: 10.1203/00006450-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 23.http://www.cdc.gov/growthcharts/html_charts/bmiagerev.htm
- 24.http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm
- 25.Elliott CD. Differential Ability Scales. 2nd. San Antonio, TX: Pearson; 2007. [Google Scholar]
- 26.Carrow-Woolfolk E. Oral and Written Language Scales: Written Expression Scale Manual. Circle Pines, MN: American Guidance Service; 1996. [Google Scholar]
- 27.Korkman M, Kirk U, Kemp S. NEPSY: A Developmental Neuropsychological Assessment. New York: The Psychological Corporation; 1998. [Google Scholar]
- 28.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307:483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gunstad J, Spitznagel MB, Paul RH, Cohen RA, Kohn M, Luyster FS, et al. Body mass index and neuropsychological function in healthy children and adolescents. Appetite. 2008;50:246–51. doi: 10.1016/j.appet.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Cortese S, Moreira-Maia CR, St Fleur D, Morcillo-Penalver C, Rohde LA, Faraone SV. Association Between ADHD and Obesity: A Systematic Review and Meta- Analysis. Am J Psychiatry. 2016;173:34–43. doi: 10.1176/appi.ajp.2015.15020266. [DOI] [PubMed] [Google Scholar]
- 31.Sucksdorff M, Lehtonen L, Chudal R, Suominen A, Joelsson P, Gissler M, et al. Preterm Birth and Poor Fetal Growth as Risk Factors of Attention-Deficit/Hyperactivity Disorder. Pediatrics. 2015;136:e599–608. doi: 10.1542/peds.2015-1043. [DOI] [PubMed] [Google Scholar]
- 32.Heinonen K, Raikkonen K, Pesonen AK, Andersson S, Kajantie E, Eriksson JG, et al. Behavioural symptoms of attention deficit/hyperactivity disorder in preterm and term children born small and appropriate for gestational age: a longitudinal study. BMC Pediatr. 2010;10:91. doi: 10.1186/1471-2431-10-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strang-Karlsson S, Raikkonen K, Pesonen AK, Kajantie E, Paavonen EJ, Lahti J, et al. Very low birth weight and behavioral symptoms of attention deficit hyperactivity disorder in young adulthood: the Helsinki study of very-low-birth-weight adults. Am J Psychiatry. 2008;165:1345–53. doi: 10.1176/appi.ajp.2008.08010085. [DOI] [PubMed] [Google Scholar]
- 34.Khalife N, Kantomaa M, Glover V, Tammelin T, Laitinen J, Ebeling H, et al. Childhood attention-deficit/hyperactivity disorder symptoms are risk factors for obesity and physical inactivity in adolescence. J Am Acad Child Adolesc Psychiatry. 2014;53:425–36. doi: 10.1016/j.jaac.2014.01.009. [DOI] [PubMed] [Google Scholar]
- 35.Aguirre Castaneda RL, Kumar S, Voigt RG, Leibson CL, Barbaresi WJ, Weaver AL, et al. Childhood Attention-Deficit/Hyperactivity Disorder, Sex, and Obesity: A Longitudinal Population-Based Study. Mayo Clin Proc. 2016;91:352–61. doi: 10.1016/j.mayocp.2015.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ford ES. The epidemiology of obesity and asthma. J Allergy Clin Immunol. 2005;115:897–909. doi: 10.1016/j.jaci.2004.11.050. quiz 10. [DOI] [PubMed] [Google Scholar]
- 37.Boulet LP. Asthma and obesity. Clin Exp Allergy. 2013;43:8–21. doi: 10.1111/j.1365-2222.2012.04040.x. [DOI] [PubMed] [Google Scholar]
- 38.Lu FL, Hsieh CJ, Caffrey JL, Lin MH, Lin YS, Lin CC, et al. Body mass index may modify asthma prevalence among low-birth-weight children. Am J Epidemiol. 2012;176:32–42. doi: 10.1093/aje/kwr484. [DOI] [PubMed] [Google Scholar]
- 39.Bianco A, Nigro E, Monaco ML, Matera MG, Scudiero O, Mazzarella G, et al. The burden of obesity in asthma and COPD: Role of adiponectin. Pulm Pharmacol Ther. 2017;43:20–5. doi: 10.1016/j.pupt.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Kumari M, Kozyrskyj AL. Gut microbial metabolism defines host metabolism: an emerging perspective in obesity and allergic inflammation. Obes Rev. 2017;18:18–31. doi: 10.1111/obr.12484. [DOI] [PubMed] [Google Scholar]
- 41.Endo Y, Yokote K, Nakayama T. The obesity-related pathology and Th17 cells. Cell Mol Life Sci. 2017;74:1231–45. doi: 10.1007/s00018-016-2399-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dietz WH, Robinson TN. Clinical practice. Overweight children and adolescents. N Engl J Med. 2005;352:2100–9. doi: 10.1056/NEJMcp043052. [DOI] [PubMed] [Google Scholar]
- 43.Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N Engl J Med. 2004;350:2362–74. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- 44.Yang CL, Simons E, Foty RG, Subbarao P, To T, Dell SD. Misdiagnosis of asthma in schoolchildren. Pediatr Pulmonol. 2017;52:293–302. doi: 10.1002/ppul.23541. [DOI] [PubMed] [Google Scholar]
- 45.Stillman CM, Weinstein AM, Marsland AL, Gianaros PJ, Erickson KI. Body-Brain Connections: The Effects of Obesity and Behavioral Interventions on Neurocognitive Aging. Front Aging Neurosci. 2017;9:115. doi: 10.3389/fnagi.2017.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navder KP, He Q, Zhang X, He S, Gong L, Sun Y, et al. Relationship between body mass index and adiposity in prepubertal children: ethnic and geographic comparisons between New York City and Jinan City (China) J Appl Physiol (1985) 2009;107:488–93. doi: 10.1152/japplphysiol.00086.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wechsler D. The Wechsler Individual Achievement Test-III [C] Oxford, UK: Pearson Assessment; 2009. [Google Scholar]
- 48.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39:800–12. doi: 10.1097/00005650-200108000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


