Abstract
Opioids are often used for analgesia via continuous intrathecal delivery by implantable devices. A higher concentration and daily dose of opioid have been postulated as risk factors for intrathecal granuloma formation. We present a 42-year-old female patient with chronic abdominal pain from refractory pancreatitis, with an intrathecal drug delivery device implanted 21 years prior, delivering continuous intrathecal morphine. After many years without concerning physical signs or complaints, with gradual increases in daily morphine dose, she presented with rapidly progressive neurologic deficits, including lower extremity, bladder, and bowel symptoms. These symptoms were determined to be secondary to mass effect and local inflammation related to an intrathecal catheter tip granuloma, detected on magnetic resonance imaging of the spine. The mass was urgently resected. On histopathologic examination, this granuloma was found to be unique, in that in addition to the expected inflammatory components, it appeared to contain precipitated nonpolarizable crystals. These were identified as precipitated morphine using liquid extraction surface analysis–tandem mass spectrometry (LESA-MS/MS) and matrix-assisted laser desorption ionization–Fourier transform ion cyclotron resonance–mass spectrometry imaging (MALDI-FTICR-MSI). In addition to the unique finding of precipitated morphine crystals, the long-term follow-up of both morphine concentration and daily dose increases provides insight into the formation of intrathecal granulomas.
Keywords: intrathecal drug delivery device, intrathecal catheter tip granuloma, inflammatory mass, morphine, mass spectrometry imaging
INTRODUCTION
Intrathecal drug delivery devices (IDDDs) are implanted for a number of medical conditions, with indications including chronic nonmalignant pain, chronic malignant pain, and spasticity. IDDDs allow for gradual titration of various medications into the intrathecal space to maximize efficacy with manageable side effect profile. This may ultimately allow for significantly lower doses than usually given orally or even parenterally, while avoiding peaks and troughs in serum concentration associated with intermittent rather than continuous dosing.
However, complications related to IDDDs can be numerous. They can include complications related to the catheter itself, such as migration, fracture, occlusion, puncture, or disconnection from the implanted pump.1 Medication-related complications are related to overdelivery or underdelivery, resulting in overdose or lack of efficacy or withdrawal, respectively. Surgical complications may include hematomas or seromas, or infections, which can be located at the pocket site, epidural space, or intrathecally. Finally, a complication that is unique to IDDDs is the formation of aseptic inflammatory masses, or intrathecal granulomas (ITGs), which spontaneously form at the catheter tip in the intrathecal space. Although rare, they can have devastating consequences, and timely diagnosis and management are crucial.2
The majority of reported cases of catheter tip inflammatory masses in the medical literature are in the context of intrathecal opioid delivery, as monotherapy or in conjunction with other analgesics such as local anesthetics and clonidine. Though the vast majority of reported cases have involved morphine,3 and to a lesser extent hydromorphone,4 ITGs have also been reported in the context of intrathecal delivery of fentanyl,3 sufentanil,5 and tramadol.6
Several risk factors have been described, corroborated both by anecdotal and small retrospective review studies. Identified risk factors have included an IDDD catheter tip located in the thoracic spine,7 previous spinal surgery,7,8 higher morphine concentration,7 higher daily morphine dose,7 and greater yearly dose increase.9 The proposed mechanism is that highly concentrated opioids trigger local meningeal mast cell degranulation, causing fibroblast and inflammatory cell migration.10
The following case report details the presentation of a patient who developed an ITG with a unique feature: precipitated crystals encapsulated within the ITG. We carefully tracked the concentration and dosing increases over 21 years, as our patient’s neurologic deficits presented rather abruptly after many years of intrathecal morphine therapy.
CASE DESCRIPTION
A 42-year-old female with a history significant for multiple sclerosis and severe, refractory familial pancreatitis, had an IDDD implanted over 20 years previously, replaced 4 times at regular and expected intervals, with programming to deliver continuous intrathecal morphine as a monotherapy. The chronic pancreatitis was of such severity that the patient had undergone pancreatic duct surgery at age 12 years and a Whipple procedure at age 15 years in an attempt to alleviate her symptoms. Ultimately, the IDDD was implanted due to unremitting abdominal pain, after more conventional modes of analgesic therapy had failed to provide adequate pain relief. Her most recent device programming was morphine at a concentration of 50 mg/mL, with a continuous dose of morphine 21 mg/day. The concentration of 50 mg/mL had been constant for the previous 16 years, before which the concentration was 25 mg/mL. The daily dose of morphine was increased in a stepwise fashion when abdominal pain increased, likely as the analgesic effect of intrathecal morphine was attenuated by patient tolerance. The stepwise dose increases typically ranged from 3% to 10% each time, with intervals between increases ranging from 3 months to more than 2 years (Table 1). At each visit for refill, she was asked about any side effects, as well as any new symptoms, such as unusual pain, other sensory changes, or motor weakness.
Table 1.
Log of Adjustments of Morphine Concentration and Daily Dose
| Date of Increase | Concentration (mg/mL) | Daily Dose (mg/day) |
|---|---|---|
| February 2016 | 50 | 21 |
| May 2013 | 50 | 19.2 |
| February 2013 | 50 | 18.7 |
| January 2012 | 50 | 18.5 |
| March 2011 | 50 | 17.5 |
| May 2010 | 50 | 16 |
| October 2007 | 50 | 15.0 |
| September 2007 | 50 | 13.0 |
| October 2006 | 50 | 11.0 |
| July 2006 | 50 | 9.6 |
| November 2005 | 50 | 8.8 |
| February 2005 | 50 | 8 |
| February 2004 | 50 | 6.6 |
| February 2002 | 50 | 6 |
| December 2000 | 50 | 5.5 |
| August 1999 | 25 | 5.3 |
| December 1997 | 25 | 4.8 |
Two months after her final increase from 19.2 mg/day to 21 mg/day (10% increase), she presented with a progressive 1-month history of worsening bilateral lower extremity radiculitis, weakness, constipation, urinary retention, and saddle anesthesia. Her ability to ambulate had degraded to the point of requiring a cane, and then a walker. On hospital presentation, total spine magnetic resonance imaging (MRI) was performed, which provided the diagnosis of a catheter tip mass. Brain MRI was not indicative of new characteristic lesions of multiple sclerosis.
The spine MRI demonstrated an intrathecal extramedullary lesion (1.5 × 1.1 × 1.2 cm) at the superior T11 level with central hypointensity and peripheral enhancement, which displaced and compressed the spinal cord to its left, causing cord edema spanning from T7 to the conus medullaris. These findings were located at the tip of the radiopaque intrathecal catheter (Figure 1).
Figure 1.
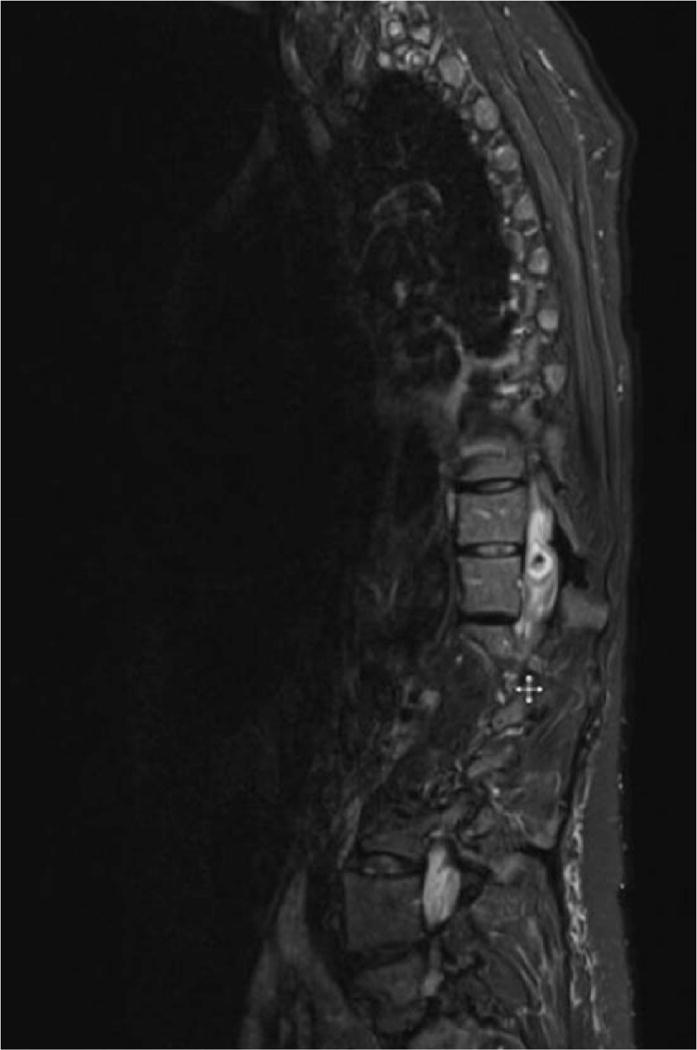
Magnetic resonance image of the thoracic and lumbar spine, demonstrating an intrathecal lesion with central hypointensity and peripheral enhancement at T11.
Neurosurgical consultation was then requested, and due to her significant neurologic deficits, she was taken to the operating room for urgent decompression. The patient underwent T10–T11 laminectomy and careful resection of the intradural granuloma.
Under direct visualization, a large intradural right-sided mass was clearly identified, with several nerve rootlets entangled within the mass. The catheter tip was identified, buried inside the mass. The medial border of the mass appeared to blend into the spinal cord itself, with a significant amount of scar tissue. The mass was dark, firm, and calcified. After it was incised, yellow puslike material exuded from the lesion. The sample was sent for histopathologic examination.
Grossly, the specimen consisted of an aggregate of 3 green-tan, necrotic, diffusely cauterized, ragged tissue fragments (1.3 × 0.9 × 0.3 cm in aggregate). The specimen was serially sectioned to exhibit pink-black cut surfaces. Hematoxylin and eosin staining revealed fibrous tissue with acute and chronic inflammation, extensive reactive changes, hemosiderin, necrosis, and nonpolarizable crystalline foreign material of uncertain nature (Figure 2), with confirmation of granulomatous inflammation by presence of histiocytes by PU.1 staining. Special stains (acid-fast bacillus, Gram, methamine silver) were all negative for bacteria and fungus. The foreign crystalline material was of particular interest, as we hypothesized that it could be morphine crystals, which has not previously been described to be present within an ITG.
Figure 2.
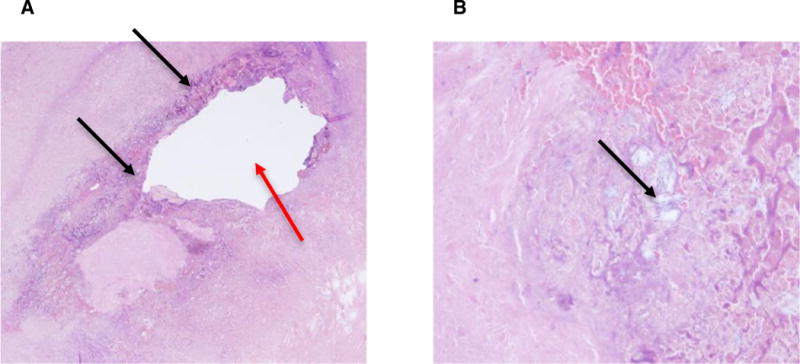
(A) Hematoxylin and eosin staining shows near circumferential granulomatous inflammation surrounding the previous catheter tip site (red arrow) and multifocal deposition of nonpolarizable precipitated foreign material (black arrows). (B) Higher power view of the nonpolarizable, precipitated foreign material (black arrow).
To determine the chemical identity of the crystals, orthogonal mass spectrometry methodologies were utilized. High mass resolution matrix-assisted laser desorption ionization mass spectrometry (MALDI-MS) using a 9.4-Tesla mass spectrometer was performed on formalin-fixed, paraffin-embedded tissue sections from the intradural mass. A strong signal at mass-to-charge ratio (m/z) of 286.144 ± 0.001, corresponding to the exact mass of morphine, was detected in multiple fragments of the tissue. The crystal region itself was estimated to be approximately 1 mm in length, so the 50-lm spatial resolution of the MS images confirmed the nature of the crystalline structure as being morphine. The distribution of the morphine within the tissue section was coincident with regions of granulomatous inflammation but also very high in the largest fragment, where the catheter tip likely resided. Deuterated (D3) morphine internal standard (m/z 289.163) was added to the matrix solution, demonstrating no increased overall signal for this molecule in the regions with highest morphine content. To further confirm the identity of morphine, we used liquid extraction surface analysis mass spectrometry (LESA-MS) on a serial section of the tissue, which demonstrated a number of characteristic morphine fragment peaks when the precursor ion was subjected to fragmentation by collision-induced dissociation. Taken together, these analyses confirmed the presence of morphine throughout this tissue, including the areas around the granulomatous inflammation, indicative that the crystalline material in the tissue was indeed morphine (Figure 3).
Figure 3.
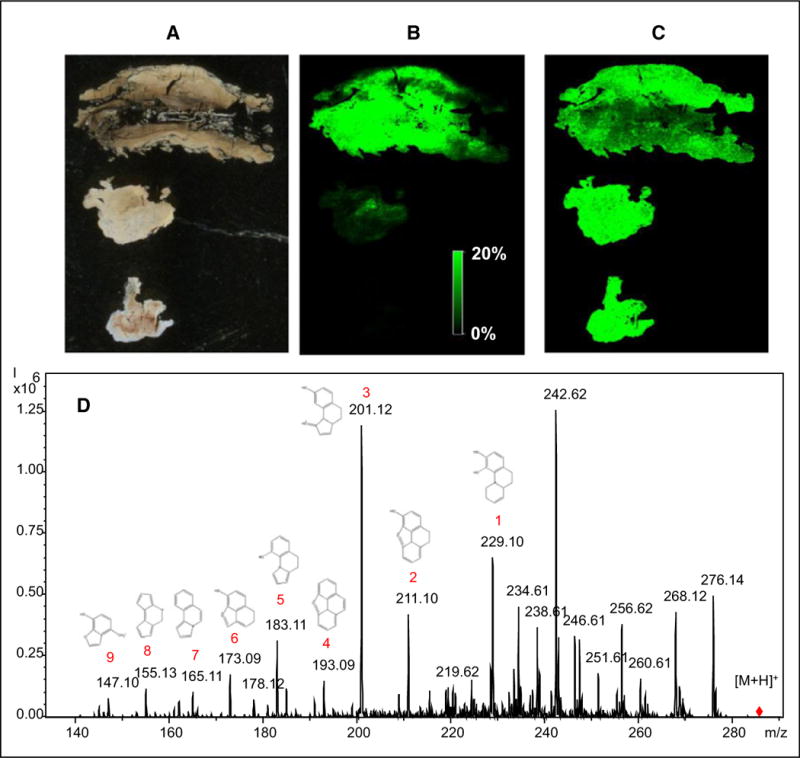
Mass spectral analysis of intradural granuloma tissue fragments. (A) Unstained tissue fragments. (B) Matrix-assisted laser desorption ionization–mass spectrometry imaging (MALDI-MSI) for 286.144 ± 0.001 Daltons (m/z), corresponding to morphine. (C) MALDI-MSI of 289.163 m/z ± 0.001, corresponding to morphine-D3 internal standard. (D) Liquid extraction surface analysis–tandem mass spectrometry of intradural granuloma tissue. Product ion mass spectrum from parent mass 286.1 (m/z) using collision-induced dissociation along with proposed chemical structures corresponding to mass spectral peaks.
After surgical decompression, the patient had improvement of her lower extremity strength, and she noted that the numbness and heaviness of her legs improved. The IDDD infusion was restarted on postoperative day 1, as the catheter tip was still in the intrathecal space, despite 0.9 cm of the catheter tip being removed during surgery. After uneventful and satisfactory neurologic improvement and postoperative recovery, she was discharged to a rehabilitation facility on postoperative day 8, and has continued to do well to date.
DISCUSSION
Of particular interest is the role of opioid concentration and daily dose in the development of ITGs, as these are the most modifiable risk factors under the clinician’s control. In one retrospective study by Kratzsch et al.7, the average morphine concentration in 13 patients who developed ITGs was 30.3 mg/mL (average total daily dose of 12.5 mg) vs. 19.5 mg/mL (average total daily dose of 6.2 mg) in 54 patients who did not develop ITGs. In a small canine study, Allen et al.11 observed ITG formation in 100% of dogs receiving intrathecal morphine at a concentration of 12 mg/mL (total daily dose of 12 mg), and in 25% of dogs receiving intrathecal morphine at a concentration of 1.5 mg/mL (total daily dose of 12 mg). Finally, a systematic literature review by Duarte et al.12 showed an average morphine concentration of 18.12 mg/mL (average total daily dose of 13.06 mg) in patients who developed ITGs vs. the control group, whose average concentration was 6.94 mg/mL (average total daily dose of 2.89 mg). In these studies, average morphine concentration and daily dose did vary significantly, though a definite trend toward higher concentrations and daily doses seem to correlate with ITG formation. It should be noted that in the Polyanalgesic Consensus Conference (PACC) in 2012 for management of intrathecal drug delivery, maximum concentration and dose recommendations were published for morphine (20 mg/mL, 15 mg/day), hydromorphone (15 mg/mL, 10 mg/day), fentanyl (10 mg/mL, no known upper limit for daily dose), and sufentanil (5 mg/mL, no known upper limit for daily dose).13 The morphine concentration and dosing of our patient’s IDDD were higher than these guidelines, but were consistent with standard practice at the time of initiation.14 Upon publication of the PACC concentration and dosing guidelines in 2012, it was not feasible to reduce the morphine concentration, as refill intervals would become too short if the daily dose was maintained. Another consideration that potentially could have reduced our patient’s morphine daily dose was addition of adjuvant analgesics such as bupivacaine or clonidine, which was not done as she had tolerated her morphine monotherapy well.
It is difficult to explain why an ITG had manifested for the first time after more than 20 years of continuous intrathecal morphine therapy, as stepwise 3% to 10% increases in daily dose occurred regularly over many years. Her presentation did occur soon after a final 10% increase in daily dose, which introduces the question of whether a critical threshold had been surpassed or if an inflammatory mass had been slowly increasing in size over time. Inflammatory masses have been shown to form and recede rather quickly with the introduction and removal of concentrated morphine therapy, within days.11 Therefore, it is possible that the ITG developed quickly after the last dose increase. The ITG in this case report was unique, as it encapsulated crystalline structures, which were confirmed to be precipitated morphine. We postulate that the highly concentrated morphine caused local inflammation, leading to the formation of a semi-enclosed space, where more morphine was locally trapped and increasingly concentrated, leading to precipitation of crystals and expansion of the granulomatous inflammation.
Consensus guidelines support gradual titration of intrathecal opioid therapy, especially as higher concentrations and daily doses are utilized.13 Though studies have demonstrated a clear trend toward ITG formation with higher concentrations and daily doses, the ranges present in ITG formation vary widely, so interpatient variability is likely expected. Appropriate clinical suspicion and timely diagnosis is imperative when a patient with IDDD in situ presents with new neurologic changes and deficits. Very careful symptomatic monitoring is necessary, such as a thorough neurologic examination at each visit, particularly when dosing changes are made, even if the IDDD has been present for many years, as demonstrated in this report. This could help with earlier detection of the ITG.
Acknowledgments
There were no additional sources of technical help, and no financial or material sources supported the work.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to report. Nathalie Agar is co-founder and advisor to Bayesian Diagnostics, and advisor to inviCRO.
References
- 1.Knight KH, Brand FM, Mchaourab AS, Veneziano G. Implantable intrathecal pumps for chronic pain: highlights and updates. Croat Med J. 2007;48:22–34. [PMC free article] [PubMed] [Google Scholar]
- 2.Hassenbusch S, Burchiel K, Coffey RJ, et al. Management of intrathecal catheter-tip inflammatory masses: a consensus statement. Pain Med. 2002;3:313–323. doi: 10.1046/j.1526-4637.2002.02055.x. [DOI] [PubMed] [Google Scholar]
- 3.Yaksh TL, Hassenbusch S, Burchiel K, Hildebrand KR, Page LM, Coffey RJ. Inflammatory masses associated with intrathecal drug infusion: a review of preclinical evidence and human data. Pain Med. 2002;3:300–312. doi: 10.1046/j.1526-4637.2002.02048.x. [DOI] [PubMed] [Google Scholar]
- 4.Veizi IE, Hayek SM, Hanes M, Galica R, Katta S, Yaksh T. Primary hydromorphone-related intrathecal catheter tip granulomas: is there a role for dose and concentration? Neuromodulation. 2016;19:760–769. doi: 10.1111/ner.12481. [DOI] [PubMed] [Google Scholar]
- 5.Gupta A, Martindale T, Christo PJ. Intrathecal catheter granuloma associated with continuous sufentanil infusion. Pain Med. 2010;11:847–852. doi: 10.1111/j.1526-4637.2010.00860.x. [DOI] [PubMed] [Google Scholar]
- 6.De Andres J, Tatay Vivo J, Palmisani S, Villanueva Perez VL, Minguez A. Intrathecal granuloma formation in a patient receiving long-term spinal infusion of tramadol. Pain Med. 2010;11:1059–1062. doi: 10.1111/j.1526-4637.2010.00885.x. [DOI] [PubMed] [Google Scholar]
- 7.Kratzsch T, Stienen MN, Reck T, Hildebrandt G, Hoederath P. Catheter-tip granulomas associated with intrathecal drug delivery—a two-center experience identifying 13 cases. Pain Physician. 2015;18:E831–E840. [PubMed] [Google Scholar]
- 8.Narouze SN, Casanova J, Souzdalnitski D. Patients with a history of spine surgery or spinal injury may have a higher chance of intrathecal catheter granuloma formation. Pain Pract. 2014;14:57–63. doi: 10.1111/papr.12024. [DOI] [PubMed] [Google Scholar]
- 9.Duarte RV, Raphael JH, Southall JL, Baker C, Hanu-Cernat D. Intrathecal inflammatory masses: is the yearly opioid dose increase an early indicator? Neuromodulation. 2010;13:109–113. doi: 10.1111/j.1525-1403.2009.00259.x. [DOI] [PubMed] [Google Scholar]
- 10.Yaksh TL, Allen JW, Veesart SL, et al. Role of meningeal mast cells in intrathecal morphine-evoked granuloma formation. Anesthesiology. 2013;118:664–678. doi: 10.1097/ALN.0b013e31828351aa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen JW, Horais KA, Tozier NA, et al. Time course and role of morphine dose and concentration in intrathecal granuloma formation in dogs: a combined magnetic resonance imaging and histopathology investigation. Anesthesiology. 2006;105:581–589. doi: 10.1097/00000542-200609000-00024. [DOI] [PubMed] [Google Scholar]
- 12.Duarte RV, Raphael JH, Southall JL, Baker C, Ashford RL. Intrathecal granuloma formation as result of opioid delivery: systematic literature review of case reports and analysis against a control group. Clin Neurol Neurosurg. 2012;114:577–584. doi: 10.1016/j.clineuro.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Deer TR, Prager J, Levy R, et al. Polyanalgesic Consensus Conference 2012: recommendations for the management of pain by intrathecal (intraspinal) drug delivery: report of an interdisciplinary expert panel. Neuromodulation. 2012;15:436–466. doi: 10.1111/j.1525-1403.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 14.Bennett G, Burchiel K, Buchser E, et al. Clinical guidelines for intraspinal infusion: report by an expert panel. Polyanalgesic Consensus Conference 2000. J Pain Symptom Manage. 2000;20:S37–S43. doi: 10.1016/s0885-3924(00)00202-5. [DOI] [PubMed] [Google Scholar]


