Abstract
Objectives
To help guide empiric treatment of infants ≤60 days old with suspected invasive bacterial infection (IBI) by describing pathogens and their antimicrobial susceptibilities.
Study design
Cross-sectional study of infants ≤60 days old with IBI (bacteremia and/or bacterial meningitis) evaluated in the emergency departments (EDs) of 11 children’s hospitals between July 1, 2011 and June 30, 2016. Each site’s microbiology laboratory database or electronic medical record system was queried to identify infants from whom a bacterial pathogen was isolated from either blood or cerebrospinal fluid (CSF). Medical records of these infants were reviewed to confirm the presence of a pathogen and to obtain demographic, clinical, and laboratory data.
Results
Of the 442 infants with IBI, 353 (79.9%) had bacteremia without meningitis, 64 (14.5%) had bacterial meningitis with bacteremia, and 25 (5.7%) had bacterial meningitis without bacteremia. The peak number of cases of IBI occurred in the second week of life; 364 (82.4%) infants were febrile. Group B streptococcus (GBS) was the most common pathogen identified (36.7%), followed by Escherichia coli (30.8%), Staphylococcus aureus (9.7%), and Enterococcus spp. (6.6%). Overall, 96.8% of pathogens were susceptible to ampicillin plus a third-generation cephalosporin, 96.0% to ampicillin plus gentamicin, and 89.2% to third-generation cephalosporins alone.
Conclusions
For most infants ≤60 days old evaluated in a pediatric ED for suspected IBI, the combination of ampicillin plus either gentamicin or a third-generation cephalosporin is an appropriate empiric antimicrobial treatment regimen. Of the pathogens isolated from infants with IBI, 11% were resistant to third-generation cephalosporins alone.
Keywords: bacteremia, meningitis, febrile infant, pathogen
Infants ≤60 days old are at increased risk of bacterial infections due to exposure to bacterial pathogens in the perinatal period and lack of vaccine-induced immunity.1,2 Although viral infections cause most episodes of fever in infants ≤60 days of age,3 2–5% of these infants have bacteremia and/or bacterial meningitis, 4–7 ie, invasive bacterial infection (IBI).7,8 These infants routinely undergo extensive diagnostic evaluation and are frequently hospitalized for treatment with empiric intravenous antimicrobials.9 Understanding the epidemiology of IBI in young infants could inform the selection of empiric antimicrobials while awaiting bacterial culture results in infants with suspected IBI.
Due in large part to broadened screening and perinatal antimicrobial prophylaxis for Group B streptococcus (GBS),2 as well as expanded vaccines for infants in the United States,10–13 the epidemiology of IBI in young infants has changed since the 1990s. Recent multicenter studies of bacteremia and/or bacterial meningitis in young infants ≤90 days of age predominantly reported Escherichia coli as the most common pathogen.14–19 Though ampicillin is effective for the treatment of GBS,20 47- 58% of E. coli and other gram negative pathogens are resistant to ampicillin.15,19 Furthermore, Enterococcus spp. and Listeria monocytogenes, pathogens typically susceptible to ampicillin but intrinsically resistant to third-genergation cephalosporins,21 were uncommon in these prior studies,14–19,22 raising concerns about the need for routine use of ampicillin as empiric therapy in young infants with suspected IBI.15 However, many of the recent studies focused either on infants with bacteremia14–18,23 or with bacterial meningitis19,22 rather than including those with bacteremia and/or bacterial meningitis. Additionally, most of the investigations included older infants (>60 days of age) in whom the rates of these infections are lower 14–16,19,22,23
Given the higher risk of bacteremia and bacterial meningitis and the uncertainty of optimal empiric antimicrobial selection for infants ≤60 days old with suspected IBI, we conducted a large, multicenter investigation of infants with IBI in this younger age group. Our objective was to describe the bacterial pathogens identified and their antimicrobial susceptibilities in infants ≤60 days old with bacteremia and/or bacterial meningitis evaluated in the emergency department (ED).
METHODS
We identified infants ≤60 days of age with bacteremia and/or bacterial meningitis evaluated in the ED at one of 11 geographically diverse children’s hospitals between July 1, 2011 and June 30, 2016. The study was approved by each site’s institutional review board with permission for data sharing.
Study Population
We searched the microbiology laboratory database or the electronic medical record system at each hospital to identify positive blood or cerebrospinal fluid (CSF) cultures obtained in the ED from infants ≤60 days of age. We defined pathogenic bacteria a priori through expert consensus (Appendix 1 (available at www.jpeds.com) for list of pathogens).18,24–26 For eligible infants with growth of a pathogen from culture, we reviewed the medical records and included infants who were documented to have received an antimicrobial treatment course commensurate for an IBI,14,22,23 defined as bacteremia and/or bacterial meningitis. We excluded infants whose positive culture was documented to have been treated as a contaminant by the treating physician and those with bacterial cultures positive for contaminant species.14,22,23 Pathogens that grew only from CSF enrichment broth cultures were considered contaminants if the blood culture had no growth and there was no CSF pleocytosis.26
Data Collection
For each eligible infant, we extracted the following variables: demographics (age, sex), past medical history (prematurity or presence of a complex chronic condition), temperature (at home, in an outpatient clinic, in triage, and highest recorded in the ED), clinical appearance, presence of a clinically apparent infection on physical examination, laboratory data (complete blood count, urinalysis, and CSF cell count), bacterial culture results (urine, blood, CSF), and antimicrobial susceptibilities. Data were entered directly into a Research Electronic Data Capture (REDCap) tool hosted at Yale University.27
Study Definitions
Fever was defined as a documented temperature ≥38.0° C (100.4° F) at home, in an outpatient clinic, or in the ED obtained via any method (e.g., rectal, axillary). Ill-appearance was defined as any of the following words documented on the physical examination in the ED: “ill-appearing,” “toxic,” “limp,” “unresponsive,” “gray,” “cyanotic,” “apnea,” “weak cry,” “poorly perfused,” “grunting,” “listless,” “lethargic,” or “irritable.”28 If none of these terms were documented, the infant was classified as not ill-appearing. In cases with contradictory documentation of appearance between the attending physician and a trainee, the attending physician’s documentation was used. We defined complex chronic conditions as severe medical conditions expected to last ≥12 months, and that involve ≥1 organ system and/or require pediatric specialty care.29,30 CSF pleocytosis was defined as CSF WBC ≥20 cells/mm3 for infants ≤28 days and ≥10 cells/mm3 for infants 29 to 60 days of age.31
Bacterial Infections
Bacteremia and bacterial meningitis were defined a priori as growth of a pathogen from blood or from CSF, respectively.8,18,32 Bacteremia with CSF pleocytosis but negative CSF culture was classified as bacterial meningitis if antimicrobials were administered prior to CSF collection.19,33 Urinary tract infection (UTI) was defined as either 1) a urine culture obtained by catheterization with ≥50,000 colony-forming units (CFUs)/mL of a single pathogen or 10,000–50,000 CFUs/mL of a single pathogen with an abnormal urinalysis (i.e., positive nitrite or leukocyte esterase on urine dipstick or >5 WBCs/hpf on urine microscopy),32,34–36 or 2) ≥100,000 CFUs/mL of a single pathogen on culture obtained from a bagged urine specimen or from an unknown method of collection, if the pathogen was simultaneously identified in the blood.37,38 Clinically apparent infection was defined as the presence of any of the following that were either documented in the ED or confirmed in the inpatient records: cellulitis, abscess, omphalitis, osteomyelitis/septic arthritis, myositis, lymphadenitis, parotitis, surgical site infection, or necrotizing enterocolitis.
Antimicrobial Susceptibilities
In vitro antimicrobial susceptibilities were categorized as susceptible or resistant based on microbiology reports.39 Additionally, as in vitro susceptibility testing may not be performed due to assumed susceptibility or resistance for certain pathogen-antimicrobial combinations, Clinical and Laboratory Standards Institute M100-S27 was consulted and used to determine intrinsic resistance, and predictable and inferred susceptibility.21 All isolates from infants with bacterial meningitis were considered resistant to gentamicin due to poor CSF penetration.40
Statistical Analyses
Descriptive analyses were stratified by type of IBI (bacteremia without meningitis or bacterial meningitis [with or without bacteremia]) and, for pathogens and antimicrobial susceptibilities, by age group (≤28 days and 29–60 days of age). We used chi-square tests to compare the distribution of antimicrobial resistance with binary demographic, clinical, and laboratory factors. We then used mixed-effects logistic regression for the adjusted analysis, with variables selected at a p-value ≤0.1 from the unadjusted analysis. Statistical significance was determined as a two-sided p-value <0.05. Statistical analyses were performed using Stata Data Analysis and Statistical Software version 15.0 (StataCorp, Inc, College Station, Texas).
RESULTS
During the 5-year study period, there were 20,896 blood cultures and 10,635 CSF cultures obtained from infants ≤60 days old evaluated in the ED. We identified 497 infants with a blood and/or CSF culture that grew a potential bacterial pathogen. Fifty-five of these infants were excluded: 45 had bacteria that were treated as contaminants (34 from CSF and 11 from blood culture), 7 infants did not have an ED visit, and 3 infants had CSF bacterial detection from broth culture alone with no concurrent CSF pleocytosis. Of the 442 infants with an IBI, 353 (79.9%) had bacteremia without meningitis, 64 (14.5%) had bacterial meningitis with bacteremia, and 25 (5.7%) had bacterial meningitis without bacteremia. Overall, 417/20,896 (2.0%) blood cultures and 76/10,635 (0.7%) CSF cultures demonstrated growth of a pathogen. Thirteen infants had bacteremia and CSF pleocytosis but negative CSF culture after receipt of antimicrobials prior to CSF collection.
Clinical and Laboratory Characteristics of Infants with IBI
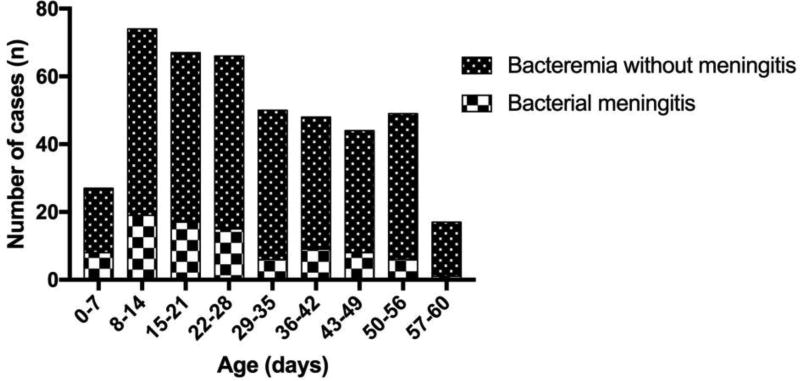
The peak number of cases of IBI occurred in the second week of life (Figure; available at www.jpeds.com). Though IBI declined in the second month of life as compared with the first, the number of infants with bacteremia was similar from the fifth to the eighth week of life. Characteristics of infants with IBI are shown in Table I. Over 80% of infants were febrile at the time of presentation and 29% had a concomitant UTI. Among infants with bacterial meningitis, 20% had an abnormal urinalysis and 9% had a UTI. Three infants with meningitis had ventriculo-peritoneal (VP) shunts.
Figure.
online; Cases of invasive bacterial infection by week of life
Table I.
Characteristics of Infants with Invasive Bacterial Infection
| Characteristic | Total N (%) n=442 |
Bacteremia without meningitis N (%) n=353 |
Bacterial Meningitis1 N (%) n=89 |
|---|---|---|---|
| Demographics | |||
| Age Group | |||
| ≤28 days | 234 (52.9) | 175 (49.6) | 59 (66.3) |
| 29–60 days | 208 (47.1) | 178 (50.4) | 30 (33.7) |
| Male | 256 (57.9) | 204 (57.8) | 52 (58.4) |
| Past Medical History | |||
| Prematurity (<37w0d) | 70 (15.8) | 53 (15.0) | 17 (19.1) |
| Complex Chronic Condition | 64 (14.5) | 55 (15.6) | 9 (10.1) |
| Temperature at the Time of Presentation | |||
| Fever | 364 (82.4) | 291 (82.4) | 73 (82.0) |
| At home only | 77 (17.4) | 62 (17.6) | 15 (16.9) |
| In ED | 287 (64.9) | 229 (64.9) | 58 (65.2) |
| Physical Examination | |||
| Ill-appearing | 148 (33.5) | 101 (28.6) | 47 (52.8) |
| Clinically Apparent Infection | 35 (7.9) | 32 (9.1) | 3 (3.4) |
| Laboratory | |||
| Abnormal Urinalysis3 | 164 (37.1) | 146 (41.4) | 18 (20.2) |
| Peripheral WBC <5K or >15K | 192 (43.4) | 141 (39.9) | 51 (57.3) |
| CSF Cell Count Obtained | 357 (80.8) | 275 (77.9) | 82 (92.1) |
| CSF Pleocytosis4 | 123 (34.5) | 52 (18.9) | 71 (86.6) |
| Urinary Tract Infection | 130 (29.4) | 122 (34.6) | 8 (9.0) |
Infants with bacterial meningitis with or without bacteremia
Includes fever first recorded in the ED
Positive nitrite or leukocyte esterase, or >5 WBC/high-powered field
Percentages reported of infants tested
Abbreviations: CSF, cerebrospinal fluid; ED, emergency department; WBC, white blood cell
Bacteremia Without Meningitis
The bacterial pathogens isolated in infants with bacteremia without meningitis are listed in Table II. E. coli was the most common pathogen overall (33.7%) though GBS accounted for a higher proportion of bacteremia in the second month of life. Of the 119 infants with E. coli bacteremia without meningitis, 98 (82.4%) had a UTI. S. aureus was isolated in 40 (11.3%) infants; 11 (27.5%) of these infants had a clinically apparent infection, including 5 infants with cellulitis, 3 with surgical site infections, 2 with myositis, and 1 with parotitis. Twenty-seven (7.6%) infants had Enterococcus spp. and 12 (3.4%) had Klebsiella spp., including 4 (33.3%) with UTI. Among the 238 febrile infants without a complex chronic condition or a clinically apparent infection, E. coli was the most common pathogen isolated (40.8%). Sixteen (6.7%) of the infants had S. aureus and 15 (6.3%) had Enterococcus spp., 6 (40%) with concomitant UTI.
Table II.
Pathogens Isolated in Infants with Invasive Bacterial Infection
| INFANTS ≤28 DAYS OF AGE | |||
|---|---|---|---|
| Pathogen | Total N (%) (n=2341) |
Bacteremia without meningitis N (%) (n=1751) |
Bacterial Meningitis2 N (%) (n=59) |
| E. coli | 72 (30.8) | 62 (35.4) | 10 (16.9) |
| Group B streptococcus | 71 (30.3) | 41 (23.4) | 30 (50.8) |
| S. aureus | 29 (12.4) | 26 (14.9) | 3 (5.1) |
| Enterococcus spp. | 17 (7.3) | 16 (9.1) | 1 (1.7) |
| Klebsiella spp. | 13 (5.6) | 11 (6.3) | 2 (3.4) |
| Other Gram Negative3 | 9 (3.8) | 8 (4.6) | 1 (1.7) |
| Group A streptococcus | 8 (3.4) | 8 (4.6) | 0 |
| Other Gram Positive4 | 7 (3.0) | 1 (0.6) | 6 (10.2) |
| Enterobacter spp. | 5 (2.1) | 4 (2.3) | 1 (1.7) |
| L. monocytogenes | 4 (1.7) | 0 | 4 (6.8) |
| Salmonella spp. | 2 (0.9) | 1 (0.6) | 1 (1.7) |
| S. pneumoniae | 0 | 0 | 0 |
| INFANTS 29–60 DAYS OF AGE | |||
| Pathogen | Total N (%) (n=2081) | Bacteremia without meningitis N (%) (n=1781) | Bacterial Meningitis2 N (%) (n=30) |
| Group B streptococcus | 91 (44.3) | 73 (41.0) | 18 (60.0) |
| E. coli | 64 (30.8) | 57 (32.0) | 7 (23.2) |
| S. aureus | 14 (6.7) | 14 (7.9) | 0 |
| Enterococcus spp. | 12 (5.8) | 11 (6.2) | 1 (3.3) |
| Other Gram Negative3 | 7 (3.4) | 4 (2.2) | 3 (10.0) |
| Enterobacter spp. | 6 (2.9) | 6 (3.4) | 0 |
| S. pneumoniae | 6 (2.9) | 5 (2.8) | 1 (3.3) |
| Salmonella spp. | 4 (1.9) | 4 (2.2) | 0 |
| Group A streptococcus | 3 (1.4) | 3 (1.7) | 0 |
| Other Gram Positive4 | 2 (1.0) | 2 (1.1) | 0 |
| Klebsiella spp. | 1 (0.5) | 1 (0.6) | 0 |
| L. monocytogenes | 0 | 0 | 0 |
Some cultures grew >1 organism.
Infants with bacterial meningitis with or without bacteremia
Includes Citrobacter spp. (3 infants overall), Pseudomonas aeruginosa (2), Neisseria meningitidis (2), Moraxella spp. (2), Haemophilus influenzae non-typeable (2), Haemophilus parainfluenzae (1), Proteus spp. (1), Serratia spp. (1), Pasteurella spp. (1), Acinetobacter spp. (1)
Includes Streptococcus gallolyticus (4 infants overall), Streptococcus bovis (4), Paenibacillus spp. (1)
Over 96% of infants with bacteremia without meningitis had pathogens susceptible to a combination of ampicillin plus either gentamicin or a third-generation cephalosporin (defined as cefotaxime or ceftriaxone) [Table III]. However, 11.5% (95% confidence interval [CI]: 8.5–15.2% ) had pathogens resistant to third-generation cephalosporins alone, including 10.2% (95% CI: 6.6–15.6%) of those 29–60 days of age. Resistance patterns were similar among febrile and afebrile infants; 8.5% of febrile infants without a chronic condition or a clinically apparent infection had a pathogen resistant to a third-generation cephalosporin.
Table III.
Antimicrobial Susceptibilities of Isolates
| ALL INFANTS12 | |||
|---|---|---|---|
| Antimicrobial(s) | Total N (%)3 | Bacteremia without meningitis N (%) |
Bacterial Meningitis4 N (%) |
| Individual | |||
| Ampicillin | 306/429 (71.3) | 233/344 (67.7) | 73/85 (85.9) |
| 3rd generation cephalosporin | 388/435 (89.2) | 309/349 (88.5) | 79/86 (91.9) |
| Combination | |||
| Ampicillin/gentamicin | 411/428 (96.0)5 | 338/343 (98.5) | 73/85 (85.9)5 |
| Ampicillin/3rd generation cephalosporin | 422/436 (96.8) | 337/350 (96.3) | 85/86 (98.8) |
| Vancomycin/ampicillin/gentamicin | 429/434 (98.9)5 | 345/349 (98.9) | 84/85 (98.8)5 |
| Vancomycin/3rd generation cephalosporin | 424/432 (98.2) | 341/348 (98.0) | 83/84 (98.8) |
| INFANTS ≤28 DAYS OF AGE2 | |||
| Antimicrobial(s) | Total N (%)3 | Bacteremia without meningitis N (%) | Bacterial Meningitis4 N(%) |
| Individual | |||
| Ampicillin | 152/229 (66.4) | 105/173 (60.7) | 47/56 (83.9) |
| 3rd generation cephalosporin | 202/229 (88.2) | 151/173 (87.3) | 51/56 (91.1) |
| Combination | |||
| Ampicillin/gentamicin | 217/228 (95.2)5 | 170/172 (98.8) | 47/56 (83.9)5 |
| Ampicillin/3rd generation cephalosporin | 224/230 (97.4) | 168/174 (96.6) | 56/56 (100) |
| Vancomycin/ampicillin/gentamicin | 227/230 (98.7)5 | 172/174 (98.9) | 55/56 (98.2)5 |
| Vancomycin/3rd generation cephalosporin | 225/227 (99.1) | 171/173 (98.8) | 54/54 (100) |
| INFANTS 29–60 DAYS OF AGE2 | |||
| Antimicrobial(s) | Total N (%)3 | Bacteremia without meningitis N (%) | Bacterial Meningitis4 N(%) |
| Individual | |||
| Ampicillin | 154/200 (77.0) | 128/171 (74.9) | 26/29 (89.7) |
| 3rd generation cephalosporin | 186/206 (90.3) | 158/176 (89.8) | 28/30 (93.3) |
| Combination | |||
| Ampicillin/gentamicin | 194/200 (97.0)5 | 168/171 (98.3) | 26/29 (89.7)5 |
| Ampicillin/3rd generation cephalosporin | 198/206 (96.1) | 169/176 (96.0) | 29/30 (96.7) |
| Vancomycin/ampicillin/gentamicin | 202/204 (99.0)5 | 173/175 (98.9) | 29/29 (100)5 |
| Vancomycin/3rd generation cephalosporin | 199/205 (97.1) | 170/175 (97.1) | 29/30 (96.7) |
5 infants had missing antimicrobial susceptibilities (3 with bacteremia without meningitis, 2 with bacterial meningitis)
Denominators represent infants with available susceptibility testing
N (%) susceptible
Infants with bacterial meningitis with or without bacteremia
Gentamicin has poor cerebrospinal fluid penetration; pathogen considered ampicillin/gentamicin resistant if infant had bacterial meningitis and pathogen was ampicillin-resistant
Bacterial Meningitis
Among infants with bacterial meningitis, GBS was the most common pathogen isolated in both age groups (Table II). Four febrile infants aged 11 to 24 days from 3 different study sites had meningitis due to Listeria monocytogenes. One infant (who was ill-appearing) had concomitant bacteremia, and none had a complex chronic condition. Among the 13 infants with bacteremia and CSF pleocytosis but negative CSF culture after receipt of antimicrobials prior to CSF collection, GBS was the most common pathogen isolated (46.2%) followed by E. coli (23.1%).
All but one infant with bacterial meningitis had pathogens susceptibile to a combination of ampicillin plus a third-generation cephalosporin (Table III). This infant was a febrile 53-day old infant with a ventriculo-peritoneal shunt and Pseudomonas aeruginosa meningitis.
Resistance to Third-Generation Cephalosporins
Across sites, the median proportion of infants with a cephalosporin-resistant pathogen was 13.3% (range 0–17%). Resistance to third-generation cephalosporins was predominantly due to Enterococcus spp., and to a lesser extent Enterobacter spp. and S. aureus (Table IV; available at www.jpeds.com). Four of 43 (9.3%) S. aureus isolates were methicillin-resistant. Of the 9 infants with bacteremia without meningitis who had pathogens susceptible to a combination of ampicillin plus gentamicin but resistant to ampicillin plus a third-generation cephalosporin, 5 (55.6%) had Enterobacter spp. Resistance to third-generation cephalosporins occurred more commonly among infants with complex chronic conditions (Table V); this association persisted on adjusted analysis (odds ratio 3.8; 95% CI: 1.9–7.5).
Table IV.
Pathogen Susceptibilities to Common Empiric Antimicrobial Regimens
| Pathogen | Ampicillin/Gentamicin N (%)123 | 3rd generation cephalosporin N (%)12 |
|---|---|---|
| Group B streptococcus | 162/162 (100) | 162/162 (100) |
| E. coli | 124/135 (91.9) | 132/135 (97.8) |
| S. aureus4 | 35/37 (94.6) | 39/43 (90.7) |
| Enterococcus spp.4 | 28/28 (100) | 0/29 (0) |
| Other Gram Negative5 | 10/11 (90.9) | 9/11 (81.8) |
| Klebsiella spp. | 12/14 (85.7) | 12/14 (85.7)V |
| Enterobacter spp. | 10/11 (90.9) | 6/11 (54.5) |
| Group A streptococcus | 11/11 (100) | 11/11 (100) |
| Other Gram Positive6 | 8/8 (100) | 8/8 (100) |
| Salmonella spp. | 6/6 (100) | 6/6 (100) |
| S. pneumoniae | 6/6 (100) | 6/6 (100) |
| L. monocytogenes | 4/4 (100) | 0/4 (0) |
| Total | 416/433 (96.1) | 391/440 (88.9) |
Denominators represent isolates with available susceptibility testing
Some cultures grew >1 organism
Gentamicin has poor cerebrospinal fluid penetration; pathogen considered ampicillin/gentamicin resistant if infant had bacterial meningitis and pathogen was ampicillin-resistant
6 isolates of S. aureus and 1 isolate of Enterococcus spp. had available susceptibility testing to third-generation cephalosporins but not to ampicillin/gentamicin
Includes Citrobacter spp. (3), Pseudomonas aeruginosa (2), Neisseria meningitidis (2), Moraxella spp. (2), Haemophilus influenzae non-typeable (2), Haemophilus parainfluenzae (1), Proteus spp. (1), Serratia spp. (1), Pasteurella spp. (1), Acinetobacter spp. (1)
Includes Streptococcus gallolyticus (4), Streptococcus bovis (4), Paenibacillus spp. (1)
Table V.
Distribution of Resistance to Third-Generation Cephalosporins by Demographic, Clinical, and Laboratory Factors
| Proportion Resistant to 3rd Generation Cephalosporins1 N (%) |
P-value | |
|---|---|---|
| Age Group | 0.49 | |
| ≤28 days | 27/229 (11.8) | |
| 29–60 days | 20/206 (9.7) | |
| Gestational Age2 | 0.58 | |
| Preterm (<37w0d) | 6/69 (8.7) | |
| Term | 38/348 (10.9) | |
| Complex Chronic Condition | <0.001 | |
| Yes | 16/64 (25.0) | |
| No | 31/371 (8.4) | |
| Fever | 0.11 | |
| Yes | 35/359 (9.8) | |
| No | 12/76 (15.8) | |
| Clinical Appearance | 0.83 | |
| Ill-Appearing | 15/145 (10.3) | |
| Not Ill-Appearing | 32/290 (11.0) | |
| Abnormal Urinalysis34 | 0.52 | |
| Yes | 17/163 (10.4) | |
| No | 19/223 (8.5) | |
| Peripheral WBC <5K or >15K5 | 0.42 | |
| Yes | 18/188 (9.6) | |
| No | 29/241 (12.0) |
435 infants had isolates with available susceptibility testing to third-generation cephalosporins
18 infants had missing data for gestational age
Positive nitrite or leukocyte esterase, or >5 WBC/high-powered field
49 infants had no urinalysis results
6 infants had no peripheral WBC results
Abbreviations: WBC, white blood cell
DISCUSSION
In this multicenter study of infants ≤60 days of age with IBI evaluated in the ED, the overall prevalence of IBI was similar to previous studies.15,18 GBS accounted for a greater proportion of all cases of IBI including in the second month of life.14–19,41–43 We also found a higher prevalence of Enterococcus spp., and a similar proportion of cases due to E. coli.14–19,22,41–43 Overall, nearly 11% of isolates were resistant to third-generation cephalosporins.
Due to increasing antimicrobial resistance15,19,44 and potential detrimental effects of antimicrobials on the infantile gut microbiome,45 clinicians increasingly need to practice antimicrobial stewardship, even for the youngest infants. Infants at low-risk of IBI do not warrant empiric antimicrobial therapy.46,47 For the empiric treatment of infants ≤60 days of age with suspected IBI, clinicians should select the narrowest spectrum antimicrobial therapy with the most tolerable side effect profile. Our study informs this important issue by identifying the most prevalent pathogens and their antimicrobial susceptibilities. Ampicillin plus gentamicin has traditionally been used for the empiric treatment of IBI in young infants.48 However, concerns about gentamicin toxicity,49 sub-optimal therapy with gentamicin alone in the setting of an ampicillin-resistant pathogen (up to 35% of isolates in this population),15,19,39,44 and the low prevalence of Listeria monocytogenes14,50,51 have all contributed to the common use of third-generation cephalosporins as empiric antimicrobial therapy for young infants, particularly in the second month of life.9 However, we found that 11% of isolates, including those from infants in the second month of life, were resistant to third-generation cephalosoporins. Our finding that Enterococcus spp. accounted for a greater proportion of bacteremia than prior reports14,15,18 partially explains this level of resistance to third-generation cephalosporins. Additionally, 10 infants had bacteremia due to Enterobacter spp. and 3 had Citrobacter spp.; both of these pathogens can have inducible beta-lactamases.52 Although resistance to third-generation cephalosporins was more common in children with complex chronic conditions, two-thirds of infants with a cephalosporin-resistant pathogen did not have a complex chronic condition. Therefore, our findings support the empiric use of ampicillin plus gentamicin for most infants with suspected bacteremia while awaiting bacterial culture results, particularly given the lower risk of toxicity with once daily dosing49 and the association of third-generation cephalosporin use with development of resistant bacteria.53 When a pathogen is identified, which frequently occurs within 24 hours,23 the antimicrobial regimen can be adjusted to provide definitive therapy.
However, in vitro susceptibilities do not necessarily correlate with in vivo effectiveness,39 and it is unknown if discordant empiric antimicrobial selection in the ED is associated with adverse clinical outcomes.54,55 Therefore, despite the high rate of in vitro pathogen susceptibility to a combination of ampicillin plus gentamicin in infants with bacteremia without meningitis, there are several circumstances in which an alternative empiric antimicrobial regimen would be more appropriate. First, we identified S. aureus as the pathogen for 11% of infants with bacteremia. Though most S. aureus had in vitro susceptibility to a combination of ampicillin plus gentamicin, this regimen would not provide appropriate in vivo coverage, particularly for methicillin-resistant S. aureus.54 Clinician suspicion of S. aureus should prompt broadening of empiric coverage to include vancomycin or another anti-staphylococcal antimicrobial. Suspicion for S. aureus may be elicited by the presence of Gram-positive cocci in clusters on blood and/or CSF culture or by a clinically apparent infection, although unlike older children,56 only 27.5% of infants with S. aureus had a clinically apparent infection.
For cases in which the clinical suspicion for bacterial meningitis is high (i.e., ill-appearance, bacteria identified on CSF Gram stain, or CSF pleocytosis with a neutrophil predominance),57,58 an alternative presumptive antimicrobial regimen with better CSF penetration and a broader spectrum of coverage is necessary. As 99% of infants with bacterial meningitis had a pathogen susceptible to the combination of ampicillin plus a third-generation cephalosporin, our findings support the use of this antimicrobial regimen for most infants with bacterial meningitis, though local antimicrobial susceptibilities may better guide empiric therapy, particularly when meningitis due to a gram-negative pathogen is suspected.19,39 Clinicians may also initiate empiric antimicrobial therapy for infants with suspected IBI prior to the availability of CSF cell counts. In this scenario, initial treatment in the ED with ampicillin plus a third-generation cephalosporin would provide adequate empiric coverage for most infants.
Our study has several limitations. First, we classified a priori defined pathogens as either contaminants or pathogens based on treatment by the medical team, a definition used in prior investigations.14,22,23 It is possible that some isolates classified as pathogens would have been eradicated without treatment. However, the time to detection on blood and/or CSF culture was significantly shorter for pathogens vs. contaminants, and the proportion of pathogens detected within 24 hours, including Enterococcus spp. and Klebsiella spp., was similar to a prior multicenter study (data not shown).23 Additionally, although Enterococcus spp. may sometimes be considered a contaminant, the proportion of febrile infants with Enterococcal bacteremia with associated UTI was similar to prior investigations.14,39,41
We relied on medical records for historical features and physical examination findings of prematurity, complex chronic conditions, and ill-appearance. Although we used an established definition of ill-appearance to mitigate the potentially subjective nature of medical record documentation, this definition may not accurately reflect clinical appearance in young infants with IBI.28 Our antimicrobial susceptibility data is partially based on intrinsic resistance patterns and inferred susceptibility for certain pathogen-antimicrobial combinations.21 Fourth, limiting inclusion to the ED setting likely underrepresents IBI in the 0–7 day age range, as some infants with IBI would have been identified prior to hospital discharge, particularly if premature.59 As 47 infants had pathogens resistant to third-generation cephalosporins, our study may have been underpowered to find an association between certain clinical or laboratory factors and resistance to third-generation cephalosporins. The current study was designed to inform the selection of empiric antimicrobial therapy for infants ≤60 days old with suspected IBI, not susceptibility-derived definitive antimicrobial therapy after bacterial culture results are available. While our results and recommendations are focused on bacteremia and bacterial meningitis, the optimal antimicrobial regimen may differ for more common infections such as UTI. Lastly, our study was limited to EDs at children’s hospitals, rendering our findings less generalizable to other clinical settings.
In conclusion, the optimal empiric antimicrobial treatment for IBI in young infants presenting to the ED remains a challenge for the clinician. Our results support the use of a combination of ampicillin plus gentamicin for the empiric treatment of bacteremia in most young infants, though ampicillin plus a third-generation cephalosporin may be used, particularly if bacterial meningitis is suspected. As 11% of isolates were resistant to third-generation cephalosporins, our data highlight potential consequences of using third-generation cephalosporins alone as empiric therapy for infants with suspected IBI. Additional investigation is needed to determine if initially discordant antimicrobial treatment has an impact on clinical outcomes for infants ≤60 days old with IBI.
Acknowledgments
We would like to acknowledge collaborators in the Febrile Young Infant Research Collaborative for their contributions to this study. A list of collaborators is available at www.jpeds.com (Appendix 2).
Supported, in part, by CTSA (KL2 TR001862 [to P.A. and E.S.] and UL1TR0001863 [to E.S.]) from the National Center for Advancing Translational Science (NCATS), a component of the National Institutes of Health (NIH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflicts of interest.
Abbreviations
- CI
Confidence Interval
- CFU
Colony-Forming Unit
- CSF
Cerebrospinal Fluid
- ED
Emergency Department
- GBS
Group B streptococcus
- IBI
Invasive Bacterial Infection
- UTI
Urinary Tract Infection
- VP
Ventriculo-peritoneal
- WBC
White Blood Cell
Appendix 1. Definition of Pathogens (a priori) for Organisms Isolated from Blood and/or Cerebrospinal Fluid Culture
| Any organism isolated from both blood and CSF [exception: coagulase-negative staphylococci] |
| Gram-positive organisms: Enterococcus spp., Listeria monocytogenes, Staphylococcus aureus, Streptococcus agalactiae (GBS), Streptococcus pneumoniae, Streptococcus pyogenes (Group A streptococcus), Streptococcus alactolyticus, Streptococcus gallolyticus, Streptococcus bovis |
| Gram-negative organisms: Acinetobacter spp., Citrobacter spp., Enterobacter spp., Escherichia coli, Haemophilus influenzae, Haemophilus parainfluenzae, Klebsiella spp., Moraxella spp., Neisseria meningitidis, Pasteurella multocida, Proteus mirabilis, Pseudomonas aeruginosa, Salmonella spp., Serratia marcescens |
Organisms treated as contaminants or isolated only from CSF broth cultures were considered contaminants
Abbreviations: CSF: cerebrospinal fluid; GBS: Group B streptococcus
Appendix 2. Collaborators in the Febrile Young Infant Research Collaborative
Elizabeth R. Alpern, MD, MSCE, Division of Emergency Medicine, Ann and Robert H. Lurie Children’s Hospital of Chicago, Northwestern University Feinberg School of Medicine, Chicago, IL
Katie L. Hayes, BS, Division of Emergency Medicine, Department of Pediatrics, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania
Brian R. Lee, PhD, Division of Pediatric Infectious Diseases, Department of Pediatrics, Children’s Mercy Hospital, Kansas City, MO
Catherine E. Lumb, BS, University of Alabama School of Medicine, Birmingham, AL
Christine E. Mitchell, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania
David R. Peaper, MD, PhD, Department of Laboratory Medicine, Yale School of Medicine, New Haven, CT
Sahar N. Rooholamini, MD, MPH, Division of Hospital Medicine, Seattle Children’s Hospital, University of Washington School of Medicine, Seattle, WA, USA
Sarah J. Shin, The Children’s Hospital of Philadelphia, Philadelphia, Pennsylvania
Derek J. Williams, MD, MPH, Division of Hospital Medicine, Department of Pediatrics, Monroe Carell Jr. Children’s Hospital at Vanderbilt, Vanderbilt University School of Medicine, Nashville, TN
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Huebner RE, Mbelle N, Forrest B, Madore DV, Klugman KP. Immunogenicity after one, two or three doses and impact on the antibody response to coadministered antigens of a nonavalent pneumococcal conjugate vaccine in infants of Soweto, South Africa. Pediatr Infect Dis J. 2002;21:1004–7. doi: 10.1097/00006454-200211000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Trends in perinatal group B streptococcal disease - United States, 2000–2006. MMWR Morb Mortal Wkly Rep. 2009;58:109–12. [PubMed] [Google Scholar]
- 3.Pantell RH, Newman TB, Bernzweig J, Bergman DA, Takayama JI, Segal M, et al. Management and outcomes of care of fever in early infancy. JAMA. 2004;291:1203–12. doi: 10.1001/jama.291.10.1203. [DOI] [PubMed] [Google Scholar]
- 4.Baker MD, Bell LM. Unpredictability of serious bacterial illness in febrile infants from birth to 1 month of age. Arch Pediatr Adolesc Med. 1999;153:508–11. doi: 10.1001/archpedi.153.5.508. [DOI] [PubMed] [Google Scholar]
- 5.Baker MD, Bell LM, Avner JR. The efficacy of routine outpatient management without antibiotics of fever in selected infants. Pediatrics. 1999;103:627–31. doi: 10.1542/peds.103.3.627. [DOI] [PubMed] [Google Scholar]
- 6.Hui C, Neto G, Tsertsvadze A, Yazdi F, Tricco AC, Tsouros S, et al. Diagnosis and management of febrile infants (0–3 months) Evid Rep Technol Assess (Full Rep) 2012:1–297. [PMC free article] [PubMed] [Google Scholar]
- 7.Gomez B, Mintegi S, Bressan S, Da Dalt L, Gervaix A, Lacroix L, et al. Validation of the “Step-by-Step” Approach in the Management of Young Febrile Infants. Pediatrics. 2016:138. doi: 10.1542/peds.2015-4381. [DOI] [PubMed] [Google Scholar]
- 8.Milcent K, Faesch S, Gras-Le Guen C, Dubos F, Poulalhon C, Badier I, et al. Use of Procalcitonin Assays to Predict Serious Bacterial Infection in Young Febrile Infants. JAMA Pediatr. 2016;170:62–9. doi: 10.1001/jamapediatrics.2015.3210. [DOI] [PubMed] [Google Scholar]
- 9.Aronson PL, Thurm C, Alpern ER, Alessandrini EA, Williams DJ, Shah SS, et al. Variation in care of the febrile young infant <90 days in US pediatric emergency departments. Pediatrics. 2014;134:667–77. doi: 10.1542/peds.2014-1382. [DOI] [PubMed] [Google Scholar]
- 10.Recommended childhood immunization schedule--United States. Centers for Disease Control and Prevention. MMWR Recomm Rep. 1995;44:1–9. [PubMed] [Google Scholar]
- 11.Centers for Disease Control (CDC) General recommendations on immunization. MMWR Morb Mortal Wkly Rep. 1989;38:205–14. 219-27. [PubMed] [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC) Recommended childhood immunization schedule--United States, 2001. MMWR Morb Mortal Wkly Rep. 2001;50:7–10. 19. [PubMed] [Google Scholar]
- 13.Poehling KA, Talbot TR, Griffin MR, Craig AS, Whitney CG, Zell E, et al. Invasive pneumococcal disease among infants before and after introduction of pneumococcal conjugate vaccine. JAMA. 2006;295:1668–74. doi: 10.1001/jama.295.14.1668. [DOI] [PubMed] [Google Scholar]
- 14.Biondi E, Evans R, Mischler M, Bendel-Stenzel M, Horstmann S, Lee V, et al. Epidemiology of bacteremia in febrile infants in the United States. Pediatrics. 2013;132:990–6. doi: 10.1542/peds.2013-1759. [DOI] [PubMed] [Google Scholar]
- 15.Greenhow TL, Hung YY, Herz AM. Changing epidemiology of bacteremia in infants aged 1 week to 3 months. Pediatrics. 2012;129:e590–6. doi: 10.1542/peds.2011-1546. [DOI] [PubMed] [Google Scholar]
- 16.Greenhow TL, Hung YY, Herz AM, Losada E, Pantell RH. The changing epidemiology of serious bacterial infections in young infants. Pediatr Infect Dis J. 2014;33:595–9. doi: 10.1097/INF.0000000000000225. [DOI] [PubMed] [Google Scholar]
- 17.Watt K, Waddle E, Jhaveri R. Changing epidemiology of serious bacterial infections in febrile infants without localizing signs. PloS One. 2010;5:e12448. doi: 10.1371/journal.pone.0012448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Powell EC, Mahajan PV, Roosevelt G, Hoyle JD, Jr, Gattu R, Cruz AT, et al. Epidemiology of Bacteremia in Febrile Infants Aged 60 Days and Younger. Ann Emerg Med. 2018;71:211–6. doi: 10.1016/j.annemergmed.2017.07.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ouchenir L, Renaud C, Khan S, Bitnun A, Boisvert AA, McDonald J, et al. The Epidemiology, Management, and Outcomes of Bacterial Meningitis in Infants. Pediatrics. 2017:140. doi: 10.1542/peds.2017-0476. [DOI] [PubMed] [Google Scholar]
- 20.Boyer KM. Neonatal group B streptococcal infections. Curr Opin Pediatr. 1995;7:13–8. doi: 10.1097/00008480-199502000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Clinical and Laboratory Standards Institute. M100-S27: Performance Standards for Antimicrobial Susceptibility Testing: Twenty Seventh informational supplement. CLSI; Wayne PA: 2017. [Google Scholar]
- 22.Leazer R, Erickson N, Paulson J, Zipkin R, Stemmle M, Schroeder AR, et al. Epidemiology of Cerebrospinal Fluid Cultures and Time to Detection in Term Infants. Pediatrics. 2017:139. doi: 10.1542/peds.2016-3268. [DOI] [PubMed] [Google Scholar]
- 23.Biondi EA, Mischler M, Jerardi KE, Statile AM, French J, Evans R, et al. Blood culture time to positivity in febrile infants with bacteremia. JAMA Pediatr. 2014;168:844–9. doi: 10.1001/jamapediatrics.2014.895. [DOI] [PubMed] [Google Scholar]
- 24.Gerber JS, Glas M, Frank G, Shah SS. Streptococcus bovis Infection in young Infants. Pediatr Infect Dis J. 2006;25:1069–73. doi: 10.1097/01.inf.0000240334.91713.48. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy GJ, Kavanagh KL, Kashimawo LA, Cripe PJ, Steele RW. An Unlikely Cause of Neonatal Sepsis. Clinical Pediatr (Phila) 2015;54:1017–20. doi: 10.1177/0009922815591894. [DOI] [PubMed] [Google Scholar]
- 26.Shah SS, Hines EM, McGowan KL. Cerebrospinal fluid enrichment broth cultures rarely contribute to the diagnosis of bacterial meningitis. Pediatr Infect Dis J. 2012;31:318–20. doi: 10.1097/INF.0b013e318243e502. [DOI] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Baskin MN, Goh XL, Heeney MM, Harper MB. Bacteremia risk and outpatient management of febrile patients with sickle cell disease. Pediatrics. 2013;131:1035–41. doi: 10.1542/peds.2012-2139. [DOI] [PubMed] [Google Scholar]
- 29.Feudtner C, Hays RM, Haynes G, Geyer JR, Neff JM, Koepsell TD. Deaths attributed to pediatric complex chronic conditions: national trends and implications for supportive care services. Pediatrics. 2001;107:E99. doi: 10.1542/peds.107.6.e99. [DOI] [PubMed] [Google Scholar]
- 30.Feudtner C, Feinstein JA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kestenbaum LA, Ebberson J, Zorc JJ, Hodinka RL, Shah SS. Defining cerebrospinal fluid white blood cell count reference values in neonates and young infants. Pediatrics. 2010;125:257–64. doi: 10.1542/peds.2009-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahajan P, Kuppermann N, Mejias A, Suarez N, Chaussabel D, Casper TC, et al. Association of RNA Biosignatures With Bacterial Infections in Febrile Infants Aged 60 Days or Younger. JAMA. 2016;316:846–57. doi: 10.1001/jama.2016.9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nigrovic LE, Malley R, Macias CG, Kanegaye JT, Moro-Sutherland DM, Schremmer RD, et al. Effect of antibiotic pretreatment on cerebrospinal fluid profiles of children with bacterial meningitis. Pediatrics. 2008;122:726–30. doi: 10.1542/peds.2007-3275. [DOI] [PubMed] [Google Scholar]
- 34.Hoberman A, Wald ER. Urinary tract infections in young febrile children. Pediatr Infect Dis J. 1997;16:11–7. doi: 10.1097/00006454-199701000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Thomson J, Cruz AT, Nigrovic LE, Freedman SB, Garro AC, Ishimine PT, et al. Concomitant Bacterial Meningitis in Infants With Urinary Tract Infection. Pediatr Infect Dis J. 2017;36:908–10. doi: 10.1097/INF.0000000000001626. [DOI] [PubMed] [Google Scholar]
- 36.Tzimenatos L, Mahajan P, Dayan PS, Vitale M, Linakis JG, Blumberg S, et al. Accuracy of the Urinalysis for Urinary Tract Infections in Febrile Infants 60 Days and Younger. Pediatrics. 2018:141. doi: 10.1542/peds.2017-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schroeder AR, Newman TB, Wasserman RC, Finch SA, Pantell RH. Choice of urine collection methods for the diagnosis of urinary tract infection in young, febrile infants. Arch Pediatr Adolesc Med. 2005;159:915–22. doi: 10.1001/archpedi.159.10.915. [DOI] [PubMed] [Google Scholar]
- 38.Etoubleau C, Reveret M, Brouet D, Badier I, Brosset P, Fourcade L, et al. Moving from bag to catheter for urine collection in non-toilet-trained children suspected of having urinary tract infection: a paired comparison of urine cultures. J Pediatr. 2009;154:803–6. doi: 10.1016/j.jpeds.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 39.Byington CL, Rittichier KK, Bassett KE, Castillo H, Glasgow TS, Daly J, et al. Serious bacterial infections in febrile infants younger than 90 days of age: the importance of ampicillin-resistant pathogens. Pediatrics. 2003;111:964–8. doi: 10.1542/peds.111.5.964. [DOI] [PubMed] [Google Scholar]
- 40.Sullins AK, Abdel-Rahman SM. Pharmacokinetics of antibacterial agents in the CSF of children and adolescents. Paediatr Drugs. 2013;15:93–117. doi: 10.1007/s40272-013-0017-5. [DOI] [PubMed] [Google Scholar]
- 41.Mischler M, Ryan MS, Leyenaar JK, Markowsky A, Seppa M, Wood K, et al. Epidemiology of Bacteremia in Previously Healthy Febrile Infants: A Follow-up Study. Hosp Pediatr. 2015;5:293–300. doi: 10.1542/hpeds.2014-0121. [DOI] [PubMed] [Google Scholar]
- 42.Yarden-Bilavsky H, Ashkenazi-Hoffnung L, Livni G, Amir J, Bilavsky E. Month-by-month age analysis of the risk for serious bacterial infections in febrile infants with bronchiolitis. Clinical Pediatr (Phila) 2011;50:1052–6. doi: 10.1177/0009922811412949. [DOI] [PubMed] [Google Scholar]
- 43.Sadow KB, Derr R, Teach SJ. Bacterial infections in infants 60 days and younger: epidemiology, resistance, and implications for treatment. Arch Pediatr Adolesc Med. 1999;153:611–4. doi: 10.1001/archpedi.153.6.611. [DOI] [PubMed] [Google Scholar]
- 44.Feldman EA, McCulloh RJ, Myers AL, Aronson PL, Neuman MI, Bradford MC, et al. Empiric Antibiotic Use and Susceptibility in Infants With Bacterial Infections: A Multicenter Retrospective Cohort Study. Hosp Pediatr. 2017 doi: 10.1542/hpeds.2016-0162. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tanaka S, Kobayashi T, Songjinda P, Tateyama A, Kiyohara C, Shirakawa T, et al. Influence of antibiotic exposure in the early postnatal period on the development of intestinal microbiota. FEMS Immunol Med Microbiol. 2009;56:80–7. doi: 10.1111/j.1574-695X.2009.00553.x. [DOI] [PubMed] [Google Scholar]
- 46.Baker MD, Bell LM, Avner JR. Outpatient management without antibiotics of fever in selected infants. N Engl J Med. 1993;329:1437–41. doi: 10.1056/NEJM199311113292001. [DOI] [PubMed] [Google Scholar]
- 47.Mintegi S, Gomez B, Martinez-Virumbrales L, Morientes O, Benito J. Outpatient management of selected young febrile infants without antibiotics. Arch Dis Child. 2017;102:244–9. doi: 10.1136/archdischild-2016-310600. [DOI] [PubMed] [Google Scholar]
- 48.Ashkenazi-Hoffnung L, Livni G, Amir J, Bivalsky E. Serious bacterial infections in hospitalized febrile infants aged 90 days or younger: the traditional combination of ampicillin and gentamicin is still appropriate. Scand J Infect Dis. 2011;43:489–94. doi: 10.3109/00365548.2011.555918. [DOI] [PubMed] [Google Scholar]
- 49.Greenhow TL, Cantey JB. The Disputed Champion: Ampicillin and Gentamicin for Febrile Young Infants. Hosp Pediatr. 2017 doi: 10.1542/hpeds.2017-0101. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 50.Leazer R, Perkins AM, Shomaker K, Fine B. A Meta-analysis of the Rates of Listeria monocytogenes and Enterococcus in Febrile Infants. Hosp Pediatr. 2016;6:187–95. doi: 10.1542/hpeds.2015-0187. [DOI] [PubMed] [Google Scholar]
- 51.Lee B, Newland JG, Jhaveri R. Reductions in neonatal listeriosis: “Collateral benefit” of Group B streptococcal prophylaxis? J Infect. 2016;72:317–23. doi: 10.1016/j.jinf.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 52.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 53.de Man P, Verhoeven BA, Verbrugh HA, Vos MC, van den Anker JN. An antibiotic policy to prevent emergence of resistant bacilli. Lancet. 2000;355:973–8. doi: 10.1016/s0140-6736(00)90015-1. [DOI] [PubMed] [Google Scholar]
- 54.Thaden JT, Ericson JE, Cross H, Bergin SP, Messina JA, Fowler VG, Jr, et al. Survival Benefit of Empirical Therapy for Staphylococcus aureus Bloodstream Infections in Infants. Pediatr Infect Dis J. 2015;34:1175–9. doi: 10.1097/INF.0000000000000850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ivady B, Kenesei E, Toth-Heyn P, Kertesz G, Tarkanyi K, Kassa C, et al. Factors influencing antimicrobial resistance and outcome of Gram-negative bloodstream infections in children. Infection. 2016;44:309–21. doi: 10.1007/s15010-015-0857-8. [DOI] [PubMed] [Google Scholar]
- 56.Greenhow TL, Hung YY, Herz A. Bacteremia in Children 3 to 36 Months Old After Introduction of Conjugated Pneumococcal Vaccines. Pediatrics. 2017:139. doi: 10.1542/peds.2016-2098. [DOI] [PubMed] [Google Scholar]
- 57.Nigrovic LE, Kuppermann N, Malley R. Development and validation of a multivariable predictive model to distinguish bacterial from aseptic meningitis in children in the post-Haemophilus influenzae era. Pediatrics. 2002;110:712–9. doi: 10.1542/peds.110.4.712. [DOI] [PubMed] [Google Scholar]
- 58.Nigrovic LE, Kuppermann N, Macias CG, Cannavino CR, Moro-Sutherland DM, Schremmer RD, et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA. 2007;297:52–60. doi: 10.1001/jama.297.1.52. [DOI] [PubMed] [Google Scholar]
- 59.Weston EJ, Pondo T, Lewis MM, Martell-Cleary P, Morin C, Jewell B, et al. The burden of invasive early-onset neonatal sepsis in the United States, 2005–2008. Pediatr Infect Dis J. 2011;30:937–41. doi: 10.1097/INF.0b013e318223bad2. [DOI] [PMC free article] [PubMed] [Google Scholar]