Abstract
Objective
To investigate the impact of maternal stress during pregnancy on newborn iron and Stage 1 iron deficiency (ID) at one year of age.
Study design
245 mothers and their newborn infants (52% male; 72% white) were recruited at the Meriter Hospital Birthing Center on the basis of known risk factors for ID. Umbilical cord blood (CB) hemoglobin (Hb) and zinc protoporphyrin/heme (ZnPP/H) were determined to evaluate erythrocyte iron; plasma ferritin (Ferr) to reflect storage iron. Mothers retrospectively reported stress experienced previously during pregnancy on a 25-item questionnaire. Blood was also collected from 79 breastfed infants at one year of age.
Results
Maternal recall of distress and health concerns during pregnancy correlated with CB ZnPP/H indices (r=0.21, P < .01), even in the absence of major traumatic events. When concurrent with other known risks for ID, including maternal adiposity, SES and race, maternal stress had a summative effect, lowering CB iron. At one year, 24% of breastfed infants had moderate ID (Ferr <12 μg/L). Higher CB ZnPP/H was predictive of this moderate ID ([95%CI: 0.26, 1.47], p=.007). When coincident with maternal reports of gestational stress, the likelihood of low Ferr at one year increased 36-fold in breastfed infants as compared with low stress pregnancies ([95%CI: 1.33, 6.83], p=.007).
Conclusions
Maternal recall of stress during pregnancy was associated with with lower iron stores at birth. High CB ZnPP/H, reflecting low erythrocyte iron, was correlated with the likelihood of Stage 1 ID at one year, when rapid growth can deplete storage iron in breastfed infants.
Keywords: Iron deficiency, risk factors, psychosocial, sex
Iron deficiency is prevalent worldwide,1 more common in women,2 and of clinical concern for children.3–6 Several gestational conditions can reduce iron levels in newborn infants, including maternal obesity, gestational diabetes, hypertension and fetal overgrowth, increasing the subsequent risk for infantile ID.7–9 Despite a reduction in clinical anemia, moderate (Stage 1) ID in the US has remained stable at over 7%, especially in infants of younger and poorer mothers.2,10–12 The American Academy of Pediatrics (AAP) Committee on Nutrition and World Health Organization now recommend universal screening for anemic or preanemic ID at one year of age, in addition to earlier screening of high risk infants.1,13 Prevention programs would benefit from a more comprehensive identification of maternal and prenatal risks that contribute to ID.
Iron sequestration is an evolutionarily-conserved response to infection, trauma, and stress, mediated in part by a hepcidin-induced reduction of iron absorption. Although adaptive acutely, sequestration may inhibit placental iron transfer.14–17 Consistent with this view, stress can decrease iron absorption, inhibiting erythropoiesis and hemoglobin (Hb) synthesis.18,19 Daily maternal stress in pregnant monkeys increased the likelihood that infants became anemic.20 Further, pregnant Israeli women living in a war zone during their first trimester delivered infants with lower cord blood ferritin (CB Ferr), a primary iron storage protein.21 Our study evaluated whether these conclusions generalize to subjective experiences of a stressful pregnancy, and included a follow-up determination of the incidence of Stage 1 ID in breastfed infants. We focused on enduring effects in breastfed infants because they would not have access to the extra iron in fortified formula.6 Maternal stress may also synergize with other factors known to affect the placental transfer of iron,9 such as obesity, poverty, and minority background, and thus participants with several of these known risk factors were recruited at delivery.10,22,23
METHODS
This prospective, observational cohort study enrolled women and their healthy newborn infants with risk factors for infantile ID. Known risk factors for insufficient fetal iron allotment included: maternal anemia diagnosed at the initiation of prenatal care, maternal obesity, gestational diabetes, late preterm birth, fetal undergrowth or overgrowth, and maternal ethnic minorities or low socioeconomic status.7,9,24 To evaluate obesity, maternal morphometric measures were used to calculate prepregnancy and delivery body mass indices (BMI). Small-for-gestational age (SGA - birth weight <10th percentile), large-for-gestational age (LGA - birth weight >90th percentile), and maternal diabetes were verified from the digital health records. Assistance from the supplemental program for Women, Infants and Children (WIC) or Medicaid was used as a proxy for designating lower SES. Because male infants are often more vulnerable to gestational stress, and their tendency for faster growth places greater demands on iron,25,26 we considered the additional influence of infant sex.
Upon obtaining informed written consent, women who were >18 years old, fluent in English or Spanish and with a singleton pregnancy participated. Exclusion criteria included fetal congenital anomaly, chromosomal abnormality, neonatal intensive care unit admission or other complications, and birth at <35 weeks of gestation (given that placental iron transfer is greatest in late pregnancy).27 Women were not selected for having experienced extreme stress or major stressful life events. To maximize generalizability, we included infants born at the Meriter Hospital Birthing Center with either vaginal or by cesarean delivery. A subset of the vaginal births, through provider preference, had cord clamping delayed until pulsations had stopped. This study was approved by the Institutional Review Boards of the University of Wisconsin-Madison and Meriter Hospital and recruitment took place between June 2008 and August 2010.
Blood Collection at 1 Year of Age in Breastfed Infants
Follow-up blood collection evaluated infant iron status at one year, focusing on the subset who breastfed for ≥ 6 months to preclude the influence of iron-fortified formulas. Of the 245 participants who met inclusion criteria, completed the stress questionnaire and had CB iron indices at delivery, 136 returned for the later blood tests. Seventy-nine of the 136 infants (32% of original sample) were breastfed until at least 6 months of age, and were included in this follow-up analysis. Stage 1 ID at one year was defined as Ferr <12 μg/L.28 Three children had mildly elevated white blood cell counts (<16.5 109/L) at one year of age. We verified that Ferr did not differ in these infants and found no relationship between cell count and iron indices. Dietary histories were also obtained, including duration of breastfeeding, formula use, and age when solid foods were introduced.
Sample Collection and Laboratory Tests
Umbilical CB was collected at delivery, stored at 4 °C in EDTA anticoagulant tubes, and assayed within 8 days. After washing to remove interfering pigments, ZnPP/H was measured using a Front-Face Hematofluorometer (Aviv Biomedical Co, Lakewood, NJ).29 ZnPP/H is a measure of zinc substitution for unavailable iron in red blood cells and known to be sensitive to moderate ID. The top fraction or reticulocyte-enriched (RE) ZnPP/H was measured in the immature, lightest 6.25% of cells, in a fashion analogous to reticulocyte Hb. Δ-ZnPP/H was calculated as the difference between the washed and RE-ZnPP/H, and is especially sensitive for identifying impaired erythrocyte iron delivery in neonates.29 Ferr was quantified with a commercial human ELISA kit by Bio-Quant (San Diego, CA) in duplicate, with intra-assay coefficient of variation < 7.5%
Perceived Maternal Stress
Women completed the Perceived Stress During Pregnancy (PSP) questionnaire within 48 hours after delivery. Because pregnancy-specific anxiety rather than general anxiety has been more related to birth outcomes and neuroendocrine activation,30,31 our questionnaire focused on perceived stress during gestation. It was adapted from the Prenatal Social Environment Inventory (PSEI),32 employed by others.33,34 The adapted questionnaire probed stressors specific to pregnancy health and wellbeing concerns, and stress associated with finances, parenting, familial and partner relationships, housing, and employment (see Appendix for final questionnaire and validation [available at www.jpeds.com]). All items were scored on a 0–10 Likert scale, ranging from 0 (did not happen) to 10 (extremely disturbing), with the lowest total score being 0 and 250 the highest score. Total scores were used, and a stress score greater ≥32 (mean score) was considered to be indicative of having experienced some stress during pregnancy.
Statistical Analyses
Statistical tests were conducted using SPSS, Version 23.0 (IBM SPSS, Chicago, IL) and R (R Core Team, 2015). For the questionnaire evaluation, internal-consistency reliability was examined with the Cronbach α and item-total correlation. Factor analysis with principal axis factoring and varimax rotation was conducted to explore factor structure and to determine construct validity. The normality of the distribution for each continuous study measure was tested using the Shapiro-Wilk test, and log-transformed as appropriate. To limit the influence of extreme outliers, one RE-ZnPP/H and Δ-ZnPP/H value was winsorized and set at the upper 3 SD point.35 Analysis of covariance and linear regression were employed to test the association between maternal stress and the infants’ iron status. Post hoc testing was based on planned orthogonal contrasts. Logistic regression was used to assess the likelihood of ID at 12 months of age. Given our aim to examine the association between maternal stress in the context of established risk factors, covariates known to affect perinatal iron status were identified.7 For ease of interpretation, non-transformed continuous variables are shown, but log transformed variables were used in all statistical analyses. Significance was set at p< 0.05.
RESULTS
Table I provides descriptive statistics for maternal demographic, clinical, and delivery characteristics. Enrolled neonates were 52.8% male and 71.8% white. Because diabetes was a selection criterion, 34.5% were LGA, with 12.2% SGA. Seven newborns (0.03%) were ≤37 weeks of gestation. Most infants (76%) were delivered vaginally. Compared with mothers delivering vaginally, mothers with cesarean deliveries did not differ by ethnicity, nor did they report more stress during pregnancy.
Table 1.
Sociodemographic and clinical characteristics for mothers birthing male or female infants.
| Mean ± SD (Range) or % (N) | ||
|---|---|---|
| Female (N=115) | Male (N=130) | |
| Maternal Age (yr) | 28.32 ± 5.44 (18–39) | 29.07 ± 5.15 (18–39) |
| Maternal BMI at Delivery | 31.46 ± 6.26 (22.66– 52.87) | 32.75 ± 6.64 (21.40–52.62) |
| Ethnicity | ||
| Caucasian | 32% (79) | 40% (97) |
| Black | 5% (13) | 6% (15) |
| Hispanic | 6% (15) | 5% (11) |
| Other | 3% (8) | 3% (7) |
| Maternal Diabetes | 12% (28) | 15% (36) |
| Maternal Anemia | 19% (47) | 18% (44) |
| Prenatal Vitamins | 39% (96) | 48% (117) |
| Iron Supplements | 14% (33) | 9% (21)* |
| Insurance | ||
| Private Insurance | 29% (72) | 36% (87) |
| WIC or Medicaid | 18% (43) | 18% (43) |
| Gestational Age (wk) | 39.37 ± 1.25 (35.5– 41.4) | 39.35 ± 1.22 (35–42.2) |
| Mode of Delivery | ||
| Vaginal | 36% (88) | 40% (98) |
| Cesarean | 11% (27) | 13% (32) |
| Delayed Cord Clamping | 13% (32) | 15% (37) |
| Birth Weight (kg) | 3.58 ± .6 (2.1–4.95) | 3.67 ± .62 (2.09–4.7) |
| Ponderal Index | 2.93 ± .39 (2.02–3.77) | 2.91 ± .37 (2.02–3.83) |
Demographic variables of all enrollees at birth are stratified by infant sex.
p <.05 from 2-sided t test or chi square comparing female and male infants.
Delivery mode did not affect CB zinc indices; however, CB Ferr levels were lower in newborns from caesarean deliveries (F1,243=7.93, p=.005). This difference reflected that overweight, diabetic and older mothers were more likely to deliver via caesarean section (r=0.22, p<.001; r=0.23, p<.001; r=0.22, p<.001, respectively). Delayed cord clamping, which occurred after 62% of the vaginal deliveries, was not predictive of CB ZnPP/H indices, but was associated with higher CB Ferr levels as compared with traditional cord clamping at <1 min (F1,243=6.94, p=.009). The prevalence of obesity and gestational diabetes did not differ across ethnic groups, but anemia during early pregnancy was more common in white women (41% in white vs. 20% in Hispanic women; χ2(1) = 4.51, p=.034). Black and Hispanic women were more likely to be of lower socioeconomic status (79% and 73% vs. 24%; χ2(3) = 64.23, p<.001) and were younger than the white women (F3,241=6.61, p<.001).
Of the 308 recruited women, 245 completed the PSP and had CB iron indices determined at delivery; 47 left ≥1 question(s) empty and 16 had incomplete iron measures. Demographics of the excluded participants did not differ significantly from those with all measures.
Psychological Stress Reported by Mothers
The mean score was 31.8 (± 29.8) with a range from 0 to 169. For specific items, the mean response was 1.3 (± 2.1) with a range from 0 to 3.2, indicating that most women reported low-to-moderate stress during pregnancy. The highest scored item was: You were not able to sleep well or get enough sleep while pregnant. Black women had higher total stress scores than Hispanic or white mothers (F3,241=3.12, p=.027). Perceived stress levels were not associated with infant sex.
CB Iron Indices at Delivery
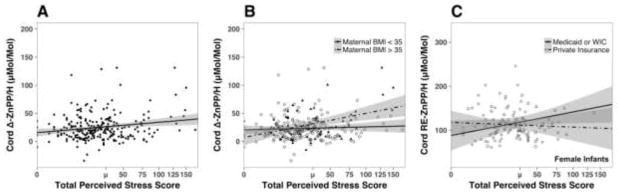
More perceived stress during pregnancy was positively correlated with higher CB RE-ZnPP/H (r=0.19, p=.003) and Δ-ZnPP/H (r=0.21, p<.01; Figure 1, A). A similar trend was evident for CB ZnPP/H (r=0.12, p=.075). The effect of maternal stress on CB RE-ZnPP/H and Δ-ZnPP/H was influenced by newborn sex, with above average maternal stress being more predictive of higher CB RE-ZnPP/H and Δ-ZnPP/H in male than female infants (F1,241=4.92, p=.028; F1,241=4.89, p=.028, respectively). Overall, ZnPP/H and RE-ZnPP/H were significantly higher in the CB of male than female newborns (F1,243=9.23, p=.003; F1,243=5.21, p=.023, respectively; Table 2). Although perceived stress predicted the ZnPP/H indices, it did not further account for lower CB Ferr beyond the influence of other risk factors.
Figure 1.
(A) Correlation of delta ZnPP/H in cord blood with maternal perceived stress scores (r = 0.22, p< .001). (B) Correlation of delta ZnPP/H with maternal stress was significantly higher in infants born to mothers who had a BMI greater than or equal to 35 at the time of delivery (p< .01). (C) Correlation of maternal stress and CB RE-ZnPP/H in female infants born to mothers of low socioeconomic status (p =.01).
Table 2.
Cord blood iron status indices in male and female infants from mothers reporting low and high stress during pregnancy.
| Iron Statues Indices | Low Stress | High Stress | 2-way ANCOVA | |
|---|---|---|---|---|
| N | 156 | 89 | 245 | |
| ZnPP/H μMol/Mol | ||||
| Male | 130 | 99.99 (37.77) | 108.95 (33.11) | Sex (p=0.003) |
| Female | 115 | 91.15 (24.20) | 90.91 (25.13) | |
|
| ||||
| RE-ZnPP/H μMol/Mol | Sex (p=0.006) | |||
| Male | 130 | 120.63 (46.01) | 142.62 (49.31)* | Stress (p=0.048) |
| Female | 115 | 115.66 (36.01) | 115.70 (44.45) | Sex X Stress (p=0.028) |
|
| ||||
| Δ-ZnPP/H μMol/Mol | ||||
| Male | 130 | 20.65 (21.21) | 33.67 (27.91)* | Stress (p=0.036) |
| Female | 115 | 24.51 (21.22) | 30.63 (60.67) | Sex X Stress (p=0.028) |
|
| ||||
| Ferritin μg/L | ||||
| Male | 130 | 147.70 (87.98) | 164.36 (111.54) | None |
| Female | 115 | 145.39 (78.96) | 151.53 (111.70) | |
Values are Mean (SD). High stress based on questionnaire scores at or above the mean value of 32.
Indicates significantly different from low stress males at p<0.05.
Maternal BMI at delivery was positively correlated with CB RE-ZnPP/H and Δ-ZnPP/H, and negatively correlated with CB Ferr (r=0.12, p=.053; r=0.12, p=.047; and r=−0.16, p=.011, respectively). Further, maternal adiposity accentuated the influence of perceived stress during pregnancy, resulting in a significantly larger effect of total stress on both CB RE-ZnPP/H (F1,241=5.98, p=.015) and CB Δ-ZnPP/H (F1,241=5.98, p=.002). Specifically, the effect of maternal stress was greater in women with a BMI 35 at delivery (Figure 1, B). Maternal adiposity accentuated the influence of perceived stress on size at birth, as reflected by a larger Ponderal Index, but only for male newborns (F1,127=5.16, p=.025). The BMI of mothers who delivered sons was significantly greater than for mothers of daughters (F1,243=4.65, p=.032).
Conversely, the influence of maternal stress on the iron status of female infants was related to sociodemographic factors. CB ZnPP/H, RE-ZnPP/H, and Δ-ZnPP/H were higher in female infants if their mothers reported more stress and were of black or Hispanic ethnicity (F1,103=2.91, p=.08; F1,103=4.53, p=.036; and F1,103=7.70, p=.007, respectively). The influence of lower SES was more evident if the women delivered a daughter. Higher CB RE-ZnPP/H levels were found in female infants born to low SES women reporting greater stress (F1,111=4.52, p=.036; Figure 1, C). These differences in iron status remained significant when maternal age, delivery BMI, gestational diabetes, maternal anemia, prenatal iron supplement or vitamin use, and gestational age at delivery were controlled in the analyses.
Stage 1 ID at 1 Year of Age in Breastfed Infants
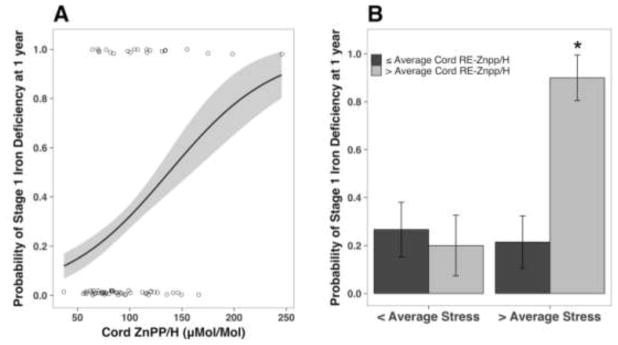
The longitudinal analyses focused on 79 infants breastfed for at ≥ 6 months who returned for blood collection at one year of age. The demographics of these infants not differ significantly from the 109 infants assessed only at birth nor did they differ from the 57 formula-fed infants with follow-up iron indices. However, mothers who breastfed for ≥ 6 months did report lower stress on average than the mothers of formula-fed infants (F1,243=4.35, p=.038). Nevertheless, among the breastfed infants, 19 (24%) had Ferr levels <12 μg/L at one year, indicative of a moderate non-anemic ID.11,28 Twelve were white, 2 were black, 4 were Hispanic, and 1 was of mixed ethnicity. Overall, having Stage 1 ID at one year was associated with having had higher CB ZnPP/H indices at delivery. Figure 2, A shows the prognostic value of a higher CB ZnPP/H (SE= 0.30, χ2(1) = 8.46, p=.004). The odds of having low Ferr at 1 year increased by a factor of 2.30 for every point increase in CB ZnPP/H ([95% CI: 0.26, 1.47], p=.007). A higher CB Δ-ZnPP/H was also associated with low Ferr at one year (SE= 0.23, χ2(1) = 4.49, p< .034). The odds of having low Ferr increased by 61% for each point increase in CB Δ-ZnPP/H ([95% CI: 0.03, 0.95], p=.044). Further, higher reported maternal stress and a high CB RE-ZnPP/H increased the probability of low Ferr at one year, (SE= 1.63, χ2(1) = 8.87, p=.003) (Figure 2, B). Reporting higher maternal stress and delivering an infant with above average CB RE-ZnPP/H increased the odds of a low Ferr at one year by 36-fold as compared with low stress pregnancies ([95% CI: 1.33, 6.83], p=.007).
Figure 2.
A) The predicted probability (and 95% confidence intervals) of low Ferr indicative of depleted iron stores (<12 μg/L) at 1 year of age, estimated with logistic regression from CB ZnPP/H values at birth. Jittered circles denote each individual infant. The odds of having lower Ferr increased significantly by 1.03 for every unit increase in ZnPP/H at birth. (B) Probability of stage I iron deficiency at 1 year by iron stores at birth (assessed using Re-ZnPP/H) and pregnancy stress reported by mothers. * indicates significant difference between groups.
DISCUSSION
Our study concurs with previous reports indicating that maternal psychological stress can worsen newborn iron status and also shows a subsequent influence on infantile ID at one year of age. This effect was evident even though the participating mothers reported experiencing only low-to-moderate levels of distress. The cohort was not selected for any major traumatic life events, but evinced an influence of stress on iron biology similar to the impact of more intense and sustained stressors evaluated in animal models.36 Maternal stress also appeared to summate with other risk factors known to affect newborn iron status, including maternal obesity, minority status, and being low SES.9 A high CB ZnPP/H, reflecting lower newborn erythrocyte iron, proved to be a sensitive predictor of the likelihood of Stage 1 ID at one year of age in breastfed infants. This finding is in keeping with other studies demonstrating that low iron stores at birth increase the risk for low Ferr at 9 months of age.37 Although breast milk usually provides optimal nutrition, the iron content of human milk is actually relatively low (0.2–0.4 mg/L), and breastfeeding alone cannot compensate for an inadequate fetal iron endowment.6,28,38 Maternal stress also added to the effects of maternal obesity, resulting in poorer newborn erythrocyte iron and an increased likelihood of Stage 1 ID at one year of age in the breastfed infants. Although not a frank clinical anemia, even this type of moderate ID has been linked to neurodevelopmental concerns for infants and later for adolescents.39–42
Psychological stress can act on many of the same pathways as physiological stressors, including on nutritional processes such as iron availability.43 Maternal stress specifically may impact iron biology at several levels. First, hepcidin-mediated sequestering of iron in maternal tissue could decrease the transfer across the placenta. Additionally, in a manner similar to obesity, the maternal response to a stressful pregnancy activates inflammatory processes,44 potentially interfering with the necessary four-fold rise in intestinal absorption of iron and functionally impeding placental iron transfer.14,15 Stress-induced increases in fetal oxidative metabolism and iron utilization may also occur, especially in the context of an obese or diabetic pregnancy. Other physiological systems can also be affected by stress or physical insults during pregnancy, including the pituitary-adrenal axis, as well as the documented influences on oxygen and nutrient delivery.45,46 Further, it has long been known that a rapid and acute decrease in circulating transport iron along with increased storage iron (ferritin) are components of the acute phase response to infection and trauma even in non-pregnant individuals.
Although other studies have documented that extremely traumatic events can affect pregnancy outcomes, including increasing the risk for prematurity and low birth weight, the findings on adverse outcomes following moderate stress are more mixed.47,48 These inconsistencies may be due in part to the assessment strategy; some found that quantifying the number of actual stressful life events is more sensitive, whereas others focused on perceived subjective distress.49 Studies relying on the Perceived Stress Scale,50 which does not probe for distress specifically associated with pregnancy, may not be sufficiently sensitive enough to capture maternal anxiety during pregnancy. The Perceived Stress Scale was previously found to be unrelated to cortisol levels in maternal plasma or amniotic fluid, nor was it predictive of neonatal measures.51,52 Measures of stress that focus on pregnancy-related concerns seem to be better predictors of preterm birth risk than measures of general anxiety.49,53 For these reasons, we adapted a questionnaire to record the mothers’ views about their health, finances, parenting, familial and partner relationships, housing, and employment, as well as their anticipatory concerns about their infants’ health and delivery complications.
In general, mothers who bore male offspring had a slightly larger BMI and their infant’s CB ZnPP/H was higher. Maternal adiposity appeared to compound the influence of perceived stress on iron status in male infants. Sex differences in fetal growth rates and in response to maternal prenatal conditions have been reported by others.54,55 Male fetuses tend to show a greater sensitivity to maternal metabolic state and glucoregulation,56 and are more likely than females to be LGA or macrosomic if their mothers had a high pregestational BMI, or were diabetic during pregnancy.57 Unexpectedly, we found the dual influence of maternal stress and sociodemographic factors was actually greater on the iron status of female neonates. Mothers from low SES or ethnic minority backgrounds reported more stress and had a higher CB ZnPP/H if their newborn infant was a female. The reason for this sex specificity is not known, and warrants further investigation in other populations given our relatively small sample size: only 24% of the 115 female infants were delivered by women from an ethnic minority.
One strength of our study was that the follow-up evaluation focused on infants breastfed for longer than 6 months, in order to diminish the corrective influence of iron-fortified formulas. In addition, our analyses considered the type of delivery, because cesarean deliveries were more common in obese women. However, some limitations should also be acknowledged. The women were recruited after delivery; thus, our assessment of maternal stress during pregnancy was retrospective. Delivery outcomes could potentially influence questionnaire responses,58 but we excluded delivery complications and any premature births that necessitated special care of the newborn. It should also be highlighted that the questionnaire was administered during the hospital stay in a comfortable and supportive Birthing Center, rather than after the stressful return home. In addition, given that 75% of fetal iron is acquired during the last trimester,27 maternal recall at delivery may be best for measuring the extent to which stress during those preceding few months can affect iron transfer. Studying erythrocyte iron is important because 80% of newborn iron is contained in erythrocytes and iron is prioritized for hemoglobin, even over vital tissues, such as the brain.59,60 The erythrocyte iron-related measure, ZnPP/H, reflects recent red blood cell physiology, especially the Δ-ZnPP/H indicative of the most recently-produced cells.29 Many animal and human studies of gestational stress have indicated even larger effects of stress during early pregnancy,20,61,62 so it is now important to conduct a larger prospective analysis with serial reporting of perceived stress during all three trimesters, considering the sex of the infant, and serially collecting health measures postnataly. Finally, our assessment of long-term outcomes was limited to a smaller number of breastfed infants because iron-fortified formulas would protect from ID.28 We focused on a moderate ID at one year of age – evinced by low Ferr in the context of normal hematology – because more severe clinical anemia is less prevalent among well-nourished American infants. However, NHANES indicates that 7–10% of American infants still experience this type of Stage 1 ID,12 and some studies suggest that even moderate iron deficiency can adversely influence neurodevelopment.39–42
Our findings indicate that maternal stress during pregnancy can affect iron transfer and the iron status of infants at delivery. In addition, poorer erythrocyte iron in the context of self-reported maternal stress increased the likelihood of Stage 1 ID at one year of age. From a practice perspective, the results concur with the consensus view that these otherwise healthy infants with common risk factors, including maternal obesity and gestational diabetes, should be screened at a younger age of 6–9 months, especially if the parents opt for prolonged breastfeeding. Preemptive prevention strategies with iron fortification would be effective if initiated early.63 Identifying maternal stress as an additional risk factor for ID is likely an accentuated concern for certain at-risk women during pregnancy. Our findings also highlight the importance of focusing on psychological wellbeing as a key component of personalized prenatal care.64
Perceived Stress During Pregnancy questionnaire validation.
The questionnaire originally had 35 questions, but 10 items were dropped due to low inter-item correlation. An exploratory principal component factor analysis was conducted on the remaining 25 items with orthogonal rotation (varimax). The Kaiser-Meyer-Olkin measure verified the sampling adequacy for the analysis, KMO = 0.87, and all KMO values for individual items were greater than 0.73, well above the accepted limit of 0.5.66 The adjusted questionnaire had high reliability, Cronbach’s α ≥ 0.87, and the intraclass correlation for absolute agreement was 0.91. Analysis of the questionnaire items indicated high internal validity; Cronbach’s α was 0.90.
Acknowledgments
Funded by the National Institutes of Health (NIH) (1 ULRR026011 UW CTSA Program), Meriter Foundation (to P.K.), Wisconsin Partnership Collaborative Health Sciences Program Grant (to P.K. and C.C.), Thrasher Research Fund (to P.K.), UW Graduate School Competition (to P.K.), HD080201 (to C.C.).
We thank participating families, Meriter Hospital Birthing Center and Blood Bank, Beth Fisher, PhD, Deb Krumpos, RN, Sue Shafranski, RN, Patricia Green-Sotos, RN, Melinda E. Chen, MD, Ryan J. Baxter, MD, Vidya Sridhar, MBBS, Mary E. Bacsik, BS, Murray L. Katcher, MD, PhD, Sheena Hirschfield, and members of the Kling Laboratory Research Team.
LIST OF ABBREVIATIONS
- ID
Iron deficiency
- CB
Cord blood
- Hb
Hemoglobin
- Ferr
Ferritin
- ZnPP/H
Zinc protoporphyrin/heme
- RE-ZnPP/H
Reticulocyte-enriched zinc protoporphyrin/heme
- Δ-ZnPP/H
Delta zinc protoporphyrin/heme
- PSP
Perceived Stress During Pregnancy Questionnaire
Appendix
Subject Number________ Date________
The events listed below may or may not have occurred during your pregnancy. If you did not experience one of the events listed below please circle Did Not Happen. If you did experience one of the events listed below, please circle the number most indicative of how distressed you were by that event.
| 1. You were worried about the health of your baby (during the pregnancy). | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 2. Someone close to you was in trouble with police or other government authorities. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 3. You worried about your ability to be a good parent. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 4. You were worried about how your separation from your husband/boyfriend would affect your ability to raise your baby. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 5. You had concerns about not having enough money. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 6. Your house or apartment is too small or crowded for raising a baby. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 7. You have had difficulty raising enough money to pay bills now or in the future. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 8. You recently became separated/divorced from your husband/boyfriend. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 9. A close family member had financial problems and you felt that you should help by giving money to them. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 10. Someone close to you died while you were pregnant. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 11. You were concerned that caring for your baby all the time would interfere with your ability to work or engage in activities important to you. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 12. You have had a difficult relationship with either your family or your husband’s/boyfriend’s family. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 13. Someone close to you has had problems with drugs or alcohol. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 14. You would like to return to school, but do not think you can now. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 15. You were unhappy with your job during pregnancy or had to quit when you didn’t want to. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 16. You were afraid that there might be problems or unbearable pain during a labor and delivery. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 17. Your husband/boyfriend was unemployed from more than one (1) month. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 18. You were unemployed for more than one (1) month (when you wanted to work). | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 19. You were concerned about finding child care for your child after the pregnancy. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 20. You were worried about finding a new job after your pregnancy. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 21. You were not able to sleep well or get enough sleep while pregnant. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 22. You did not like how pregnancy changed your body and appearance. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 23. This was not a pregnancy that you had planned and were looking forward to. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 24. Your boyfriend/husband did not want to have a child at this time. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
| 25. Your husband/boyfriend was not being helpful during your pregnancy. | ||||||||||
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Did Not Happen | Not Upset | Moderately distressed | Extremely disturbed | |||||||
Footnotes
Name of reprint request author: Pamela J. Kling MD
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.WHO. The global prevalence of anaemia in 2011. Geneva: World Heal Organ; 2015. pp. 1–48. [Google Scholar]
- 2.Tussing-Humphreys L, Pustacioglu C, Nemeth E, Braunschweig C. Rethinking iron regulation and assessment in iron deficiency, anemia of chronic disease, and obesity: Introducing hepcidin. J Acad Nutr Diet. 2012;112:391–400. doi: 10.1016/j.jada.2011.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozoff B, Georgieff MK. Iron deficiency and brain development. Semin Pediatr Neurol. 2006;13:158–65. doi: 10.1016/j.spen.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Gorten MK, Hepner R, Workman JB. Iron metabolism in premature infants. Pediatrics. 1963;63:1063–71. doi: 10.1016/s0022-3476(63)80185-7. [DOI] [PubMed] [Google Scholar]
- 5.Lott DG, Zimmerman MB, Labbe RF, Kling PJ, Widness JA. Erythrocyte zinc protoporphyrin is elevated with prematurity and fetal hypoxemia. Pediatrics. 2005;116:414–22. doi: 10.1542/peds.2004-1601. [DOI] [PubMed] [Google Scholar]
- 6.Tsai SF, Chen SJ, Yen HJ, Hung GY, Tsao PC, Jeng MJ, et al. Iron deficiency anemia in predominantly breastfed young children. Pediatr Neonatol. 2014;55:466–9. doi: 10.1016/j.pedneo.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 7.Mclimore HM, Phillips AK, Blohowiak SE, Pham DQ, Coe CL, Fischer BA, et al. Impact of multiple prenatal risk factors on newborn iron status at delivery. J Pediatr Hematol Oncol. 2013;35:473–7. doi: 10.1097/MPH.0b013e3182707f2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rukuni R, Knight M, Murphy MF, Roberts D, Stanworth SJ. Screening for iron deficiency and iron deficiency anaemia in pregnancy: A structured review and gap analysis against UK national screening criteria. BMC Pregnancy Childbirth. 2015;15:269–80. doi: 10.1186/s12884-015-0679-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips AK, Roy SC, Lundberg R, Guilbert TW, Auger AP, Blohowiak SE, et al. Neonatal iron status is impaired by maternal obesity and excessive weight gain during pregnancy. J Perinatol. 2014;34:513–8. doi: 10.1038/jp.2014.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumann-Blackmore NL, Goetz E, Blohowiak SE, Zaka O, Kling PJ. Cord blood zinc protoporphyrin/heme ratio in minority neonates at risk for iron deficiency. J Pediatr. 2008;153:133–6. doi: 10.1016/j.jpeds.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 11.Powers JM, Buchanan GR. Potential for improved screening, diagnosis, and treatment for iron deficiency and iron deficiency anemia in young children. J Pediatr. 2017;188:10–2. doi: 10.1016/j.jpeds.2017.04.069. [DOI] [PubMed] [Google Scholar]
- 12.Gupta PM, Perrine CG, Mei Z, Scanlon KS. Iron, anemia, and iron deficiency anemia among young children in the United States. Nutrients. 2016;8:10–3. doi: 10.3390/nu8060330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baker RD, Greer FR. Diagnosis and prevention of iron deficiency and iron-deficiency anemia in infants and young children (0–3 years of age) Pediatrics. 2010;126:1040–50. doi: 10.1542/peds.2010-2576. [DOI] [PubMed] [Google Scholar]
- 14.Ganz T, Nemeth E. Iron sequestration and anemia of inflammation. Semin Hematol. 2009;46:387–93. doi: 10.1053/j.seminhematol.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Collins JF, Wessling-Resnick M, Knutson MD. Hepcidin regulation of iron transport. J Nutr. 2008;138:2284–8. doi: 10.3945/jn.108.096347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koenig MD, Tussing-Humphreys L, Day J, Cadwell B, Nemeth E. Hepcidin and iron homeostasis during pregnancy. Nutrients. 2014;6:3062–83. doi: 10.3390/nu6083062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lozoff B, Beard J, Connor J, Barbara F, Georgieff M, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infancy. Nutr Rev. 2006;64:S34–S91. doi: 10.1037/a0013262.Open. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen J, Shen H, Chen C, et al. The effect of psychological stress on iron absorption in rats. BMC Gastroenterol. 2009;9:83–9. doi: 10.1186/1471-230X-9-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wei C, Zhou J, Huang X, Li M. Effects of psychological stress on serum iron and erythropoiesis. Int J Hematol. 2008;88:52–6. doi: 10.1007/s12185-008-0105-4. [DOI] [PubMed] [Google Scholar]
- 20.Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res. 2007;61:520–24. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- 21.Armony-Sivan R, Aviner S, Cojocaru L, Fytlovitch S, Ben-Alon D, Eliassy A, et al. Prenatal maternal stress predicts cord-blood ferritin concentration. J Perinat Med. 2013;41:259–65. doi: 10.1515/jpm-2012-0125. [DOI] [PubMed] [Google Scholar]
- 22.Stringhini S, Sabia S, Shipley M, Brunner E, Nabi H, Kivimaki M, et al. Association of socioeconomic position with health behaviors and mortality. Jama-Journal Am Med Assoc. 2010;303:1159–66. doi: 10.1001/jama.2010.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brotanek JM, Gosz J, Weitzman M, et al. Iron deficiency in early childhood in the United States: Risk factors and racial/ethnic disparities. Pediatrics. 2007;120:568–575. doi: 10.1542/peds.2007-0572. [DOI] [PubMed] [Google Scholar]
- 24.Phillips AK, Fischer BA, Baxter RJ, Shafranski SA, Coe CL, Kling PJ. Recruiting Latina families in a study of infant iron deficiency: a description of barriers, study adjustments and review of the literature. WMJ. 2011;110:26–31. [PMC free article] [PubMed] [Google Scholar]
- 25.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–65. doi: 10.1523/jneurosci.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bale TL, Epperson CN. Sex differences and stress across the lifespan. Nat Neurosci. 2015;18:1413–20. doi: 10.1038/nn.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bothwell TH. Iron requirements in pregnancy and strategies to meet them. Am J Clin Nutr. 2000;72:257S–64S. doi: 10.3389/fphar.2014.00155. [DOI] [PubMed] [Google Scholar]
- 28.Chandyo RK, Henjum S, Ulak M, Thorne-Lyman AL, Ulvik RJ, Shrestha PS, et al. The prevalence of anemia and iron deficiency is more common in breastfed infants than their mothers in Bhaktapur, Nepal. Eur J Clin Nutr. 2016;70:456–62. doi: 10.1038/ejcn.2015.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blohowiak SE, Chen ME, Repyak KS, Carlton DP, Michael K, Crenshaw TD, et al. Reticulocye enrichment and improved sensitivity of zinc protoporphyrin/heme ratios in human cord blood and suckling rats. Pediatr Res. 2008;64:63–7. doi: 10.1203/PDR.0b013e31817328e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rini C, Dunkel-Schetter C, Wadhwa P, Sandman C. Psychological adaptation and birth outcomes: The role of personal resources, stress, and sociocultural context in pregnancy. Heal Psychol. 1999;18:333–45. doi: 10.1037/0278-6133.18.4.333. [DOI] [PubMed] [Google Scholar]
- 31.Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: A prospective investigation. Am J Obstet Gynecol. 1993;169:858–65. doi: 10.1016/0002-9378(93)90016-C. [DOI] [PubMed] [Google Scholar]
- 32.Orr S, James S, Casper R. Psychosocial stressors and low birth weight: Development of a questionnaire. J Dev Behav Pediatr. 1992;13:343–7. [PubMed] [Google Scholar]
- 33.Nelson DB, Grisso JA, Joffe MM, Brensinger C, Shaw L, Datner E. Does stress influence early pregnancy loss? Ann Epidemiol. 2003;13:223–9. doi: 10.1016/s1047-2797(02)00419-2. [DOI] [PubMed] [Google Scholar]
- 34.Dole N, Savitz DA, Siega-Riz AM, Hertz-Picciotto I, McMahon MJ, Buekens P. Psychosocial factors and preterm birth among African American and white women in central North Carolina. Am J Public Health. 2004;94:1358–65. doi: 10.2105/ajph.94.8.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hastings C, Mosteller F, Tuckey JW, Winsor CP. Low moments for small samples: A comparative study of order statistics. Ann Math Stat. 1947;18:413–26. [Google Scholar]
- 36.Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatr Res. 2007;6:520–4. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- 37.Georgieff MK, Wewerka SW, Nelson CA, DeRegnier RA. Iron status at 9 months of infants with low iron stores at birth. J Pediatr. 2002;141:405–9. doi: 10.1067/mpd.2002.127090. [DOI] [PubMed] [Google Scholar]
- 38.Li C, Solomons NW, Scott ME, Koski KG. Minerals and trace elements in human breast milk are associated with Guatemalan infant anthropometric outcomes within the first 6 months. J Nutr. 2016;146:2067–74. doi: 10.3945/jn.116.232223. [DOI] [PubMed] [Google Scholar]
- 39.Akman M, Cebeci D, Okur V, Angin H, Abali O, Akman AC. The effects of iron deficiency on infants’ developmental test performance. Acta Paediatr Int J Paediatr. 2004;93:1391–6. doi: 10.1080/08035250410030946. [DOI] [PubMed] [Google Scholar]
- 40.Oski F, Honig A, Helu B, Howanitz P. Effect of iron therapy on behavior performance in nonanemic, iron-deficient infants. Pediatrics. 1983;71:877–80. [PubMed] [Google Scholar]
- 41.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive. J Nutr. 2001;131:649–68. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 42.Shafir T, Angulo-Barroso R, Calatroni A, Jimenez E, Lozoff B. Effects of iron deficiency in infancy on patterns of motor development over time. Hum Mov Sci. 2006;25:821–38. doi: 10.1038/nrm2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Monk C, Georgieff MK, Osterholm EA. Maternal prenatal distress and poor nutrition–mutually influencing risk factors affecting infant neurocognitive development. J Child Psychol Psychiatry. 2013;54:115–30. doi: 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, et al. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci. 2012;109:5995–9. doi: 10.1073/pnas.1118355109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandman CA, Davis EP. Neurobehavioral risk is associated with gestational exposure to stress hormones. Expert Rev Endocrinol Metab. 2012;7:445–59. doi: 10.1586/eem.12.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belkacemi L, Nelson DM, Desai M, Ross MG. Maternal undernutrition influences placental-fetal development. Biol Reprod. 2010;83:325–31. doi: 10.1095/biolreprod.110.084517. [DOI] [PubMed] [Google Scholar]
- 47.Dunkel Schetter C, Tanner L. Anxiety, depression and stress in pregnancy. Curr Opin Psychiatry. 2012;25:141–8. doi: 10.1097/YCO.0b013e3283503680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.DiPietro JA. Maternal stress in pregnancy: Considerations for fetal development. J Adolesc Health. 2013;51:S3–S8. doi: 10.1016/j.jadohealth.2012.04.008.Maternal. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lobel M, Cannella DL, Graham JE, DeVincent C, Schneider J, Meyer BA. Pregnancy-specific stress, prenatal health behaviors, and birth outcomes. Heal Psychol. 2008;27:604–615. doi: 10.1037/a0013242. [DOI] [PubMed] [Google Scholar]
- 50.Cohen S, Kamarck T, Mermelstein R. A Global Measure of Perceived Stress. J Health Soc Behav. 1983;24:385–96. [PubMed] [Google Scholar]
- 51.Baibazarova E, Van De Beek C, Cohen-Kettenis PT, Buitelaar J, Shelton KH, van Goozen SHM. Influence of prenatal maternal stress, maternal plasma cortisol and cortisol in the amniotic fluid on birth outcomes and child temperament at 3 months. Psychoneuroendocrinology. 2013;38:907–15. doi: 10.1016/j.psyneuen.2012.09.015. [DOI] [PubMed] [Google Scholar]
- 52.Wing DA, Ortega-Villa AM, Grobman WA, Hediger ML, Grewal J, Pugh SJ, et al. Maternal stress and neonatal anthropometry: the NICHD Fetal Growth Studies. Am J Obstet Gynecol. 2017;217:82.e1–e7. doi: 10.1016/j.ajog.2017.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kramer MS, Lydon J, Séguin L, Goulet L, Kahn SR, McNamara H, et al. Stress pathways to spontaneous preterm birth: The role of stressors, psychological distress, and stress hormones. Am J Epidemiol. 2009;169:1319–26. doi: 10.1093/aje/kwp061. [DOI] [PubMed] [Google Scholar]
- 54.Lampl M, Gotsch F, Kusanovic JP, Gomez R, Nien JK, Frongillo EA, et al. Sex differences in fetal growth responses to maternal height and weight. Am J Hum Biol. 2010;22:431–43. doi: 10.1002/ajhb.21014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis EP, Pfaff D. Sexually dimorphic responses to early adversity: Implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology. 2014;49:11–25. doi: 10.1016/j.psyneuen.2014.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of cardiovascular disease. Gend Med. 2008;5:S121–32. doi: 10.1016/j.genm.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ricart W, Lopez J, Mozas J, Pericot A, Sancho MA, Gonzalez N, et al. Maternal glucose tolerance status influences the risk of macrosomia in male but not in female fetuses. J Epidemiol Community Heal. 2009;63:64–8. doi: 10.1136/jech.2008.074542. [DOI] [PubMed] [Google Scholar]
- 58.Dekel S, Stuebe C, Dishy G. Childbirth induced posttraumatic stress syndrome: A systematic review of prevalence and risk factors. Front Psychol. 2017;8:1–10. doi: 10.3389/fpsyg.2017.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zamora TG, Guiang SF, Widness JA, Georgieff MK. Iron is prioritized to red blood cells over the brain in phlebotomized anemic newborn lambs. Pediatr Res. 2016;79:922–8. doi: 10.1038/pr.2016.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rao R, Georgieff MK. Iron in fetal and neonatal nutrition. Semin Fetal Neonatal Med. 2007;12:54–63. doi: 10.1016/j.siny.2006.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Entringer S, Buss C, Shirtcliff EA, Cammack AL, Yim IS, Chicz-Demet A, et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010;13:258–68. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Roesch SC, Dunkel-Schetter C, Woo G, Hobel CJ. Modeling the types and timing of stress in pregnancy. Anxiety Stress Coping. 2004;17:87–102. doi: 10.1080/1061580031000123667. [DOI] [Google Scholar]
- 63.Petry N, Olofin I, Boy E, Donahue Angel M, Rohner F. The effect of low dose iron and zinc intake on child micronutrient status and development during the first 1000 days of Life: A systematic review and meta-analysis. Nutrients. 2016;8:1–22. doi: 10.3390/nu8120773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Glover V. Maternal depression, anxiety and stress during pregnancy and child outcome; What needs to be done. Best Pract Res Clin Obstet Gynaecol. 2014;28:25–35. doi: 10.1016/j.bpobgyn.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 65.Kaiser H. An index of factorial simplicity. Psychometrika. 1974;39:31–36. [Google Scholar]