Abstract
Purpose
Rheumatoid arthritis (RA) is associated with adverse body composition profiles, and low muscle density due to accumulation of intramuscular fat. This study assessed associations between muscle density, body composition, muscle strength, and physical functioning in patients with RA and a reference group.
Methods
Patients with RA, ages 18–70 years, and healthy control participants completed whole-body DXA and peripheral quantitative CT (pQCT) to quantify appendicular lean mass (ALMI, kg/m2) and fat mass indices (FMI, kg/m2), visceral fat area, and muscle density. Dynamometry was used to measure hand-grip strength and muscle strength at the knee and lower leg (ft-lbs). Disability and physical functioning were measured with the Health Assessment Questionnaire (HAQ) and the Short Physical Performance Battery (SPPB). Linear regression analyses assessed differences related to RA and associations between muscle density, strength, and function.
Results
The study consisted of 103 RA patients (51 men) and 428 healthy participants. Low muscle density was associated with greater disease activity, CRP, and Interleukin-6, greater total and visceral fat, lower ALMI Z-Scores, physical inactivity, and long-term use of glucocorticoids (>1yr). Patients with low ALMI Z-Scores had lower muscle density Z-Score compared to reference participants with similarly low ALMI. Low muscle density was independently associated with lower muscle strength, higher HAQ, and lower SPPB after adjusting for ALMI and FMI Z-Scores.
Conclusions
Low muscle density observed among patients with RA is observed in association with low muscle mass, excess adiposity, poor strength, and greater disability. Interventions to address poor muscle quality could potentially affect important functional outcomes.
Keywords: Bone structure, rheumatoid arthritis, body composition
Introduction
Intramuscular fat accumulation resulting in low muscle density has been observed with aging and in patients with excess total and visceral adiposity and insulin resistance (1–7). Low muscle density is also observed among individuals with cancer cachexia (8). It has also been linked to a number of adverse long-term outcomes in the general population, including low muscle strength, poor physical function, fracture, cardiovascular disease, and early mortality (2, 3, 8–18).
Low muscle density has been observed in patients with rheumatoid arthritis (RA) and is associated with greater disease activity and Interleukin(IL)-6 levels (19–21). Two previous studies demonstrated that low muscle density was correlated with poorer physical functioning in RA (21, 22). However, there is a strong and well-described link between visceral adiposity and muscle density (6, 7). Total and visceral adiposity are each strongly associated with poor physical function (23, 24). Therefore, total and visceral adiposity are potential confounders between muscle density and physical function. In other words, if adiposity were a confounder, it would suggest that relationships that have been identified in prior studies are unlikely to be causal. Previous studies in RA have not ascertained whether relationships between muscle density and physical function and strength are independent of other relevant (and more easily measured) body composition outcomes, including total and visceral adiposity assessed by whole-body dual-energy absorptiometry (DXA) (15, 25). Few studies have assessed associations between muscle density and physical function independent of estimates visceral fat in other patient populations, but we are aware of one that did demonstrate independent associations (17).
We previously reported low muscle density in this RA cohort. The logical extension of this work was to determine risk factors for low muscle density and to assess relationships with physical function. We hypothesized that low muscle density is related to low lean mass and excess visceral adiposity and independently associated with adverse physical function. We aimed to characterize the relationship between muscle density and disease characteristics, physical activity, physical functioning, and muscle strength, independent of other comprehensive assessments of body composition (lean mass & total and regional adiposity) among patients with RA. Because the etiology and impact of low muscle density was hypothesized to be distinct in RA, we also aimed to compare the relationships observed in RA to those observed in a reference control population.
Methods
Study Setting
RA subjects, ages 18–70 years, who met 2010 American College of Rheumatology classification criteria, were recruited from the University of Pennsylvania and Philadelphia VA Medical Center Rheumatology practices. Subjects with Juvenile Idiopathic Arthritis (or another inflammatory arthritis), active cancer, a history of chronic diseases known to affect bone health (e.g. chronic kidney disease, liver disease, or malabsorption syndromes), pregnancy, or who were unable to perform the muscle density or body composition assessments due to physical limitations, excess weight (>300 pounds), or a calf size that would not fit in the scanner were excluded.
Adults, ages 21 to 78 years (239 male, 261 female) were enrolled as healthy reference participants for multiple bone studies at Penn, as previously described (26). These healthy participants were recruited from Penn internal medicine clinics and the surrounding community. All study subjects (RA and reference participants) underwent scans in the same laboratory using identical equipment and methods. For each subject, all studies were performed at the same study visit. The protocols were approved by the Institutional Review Board at University of Pennsylvania and the Philadelphia Veterans Affairs Medical Center and informed consent was obtained from all participants.
Assessment of Anthropometrics and Race
Weight and height were measured in light clothing and with shoes removed using a digital scale (Scaltronix, White Plains, NY) and stadiometer (Holtain Ltd., Crymych, UK), respectively. Body Mass Index (BMI) was calculated (weight/height2, kg/m2). Participants self-identified race according to National Institute of Health categories.
Whole-body Dual-Energy Absorptiometry (DXA)
Subjects underwent whole-body DXA assessment using a Hologic densitometer (Delphi/Discovery Systems, Hologic, Inc., Bedford, MA) to measure appendicular lean mass, as well as total and regional fat mass. Similar to the adjustment of weight for height to estimate BMI, body composition estimates were adjusted for height2 to generate appendicular lean mass index (ALMI, kg/m2) and fat mass index (FMI, kg/m2). The in vitro coefficient of variation for Hologic measurement of lean mass was less than 0.6% and the in vivo coefficient of variation (CV) in adults was less than 1% (27). We utilized a previously validated method to quantify visceral adipose tissue area (VAT) area.(28) The CV for VAT was 5.3%.
Peripheral Quantitative Computed Tomography (pQCT)
Muscle, fat, and bone measures in the left lower leg were obtained by pQCT (Stratec XCT2000 12-detector unit, Orthometrix, Inc.) with a voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/sec. All scans were analyzed with Stratec software version 6.00. Calf muscle and subcutaneous fat CSA (mm2) were assessed 66% proximal to the distal physis using threshold 40 mg/cm3 for fat/lean separation and 711 mg/cm3 for lean/bone separation. The pQCT measure of muscle density (mg/cm3) was used as a composite index of intra and extra-myocellular fat content, as previously described (29, 30). Muscle density measured by pQCT correlates strongly with muscle fiber lipid content on histopathology (1). Edge-detection and threshold techniques were used to separate tissues (fat, muscle, and bone) based on attenuation characteristics that are directly related to tissue composition and density (1, 4). Images were filtered prior to being analyzed using contour mode 3 (−101 mg/cm3) to find skin, and peel mode 2 (40 mg/cm3) to separate adipose and muscle/bone, respectively. Images were filtered subsequently with a combination 3×3, and double 5×5 kernel image filter that clearly defined the edge of the muscle using contour mode 31 (40 mg/cm3). All bone was identified using a threshold of 150 mg/cm3 and mathematically removed to generate results for muscle density. Quality control was monitored daily using a phantom. In our laboratory, the coefficient of variation (CV) for short-term precision has ranged from 0.5 to 1.6% for pQCT outcomes.
Dynamometric Measurement of Muscle Strength
Muscle strength was assessed in several ways using Biodex Multi-Joint System 3 Pro Dynamometer (Biodex Medical Systems, Inc, Shirley, NY). High intra-rater (0.97 to 0.99) and inter-rater (0.93 to 0.96) intra-class correlation coefficients have been reported (31). Peak isokinetic torque (ft-lbs) was measured in triplicate at the knee and lower leg (ankle). For lower leg (ankle) biodex, we report strength as peak isometric torque (ft-lbs) in dorsiflexion (with the foot placed in 20 degrees of plantarflexion) as previously described (32). Peak isometric torque in flexion and extension at the knee was also reported for patients with RA (ft-lbs). Hand-grip strength (lbs) was measured using a hand-grip dynamometer (Takei Scientific Instruments Co., Ltd., Japan).
Assessments of Physical Function and Disability
Physical function was assessed using the Short Physical Performance Battery (SPPB). The SPPB is a simple test to measure lower extremity function using tasks that mimic daily activities. It examines static balance, gait speed, and timed chair-raises. Because SPPB scores are highly skewed, this variable was analyzed as categories as previously defined (0–4, 5–8, and 9–12) (33, 34). Disability was assessed using the Health Assessment Questionnaire (HAQ), a widely used tool in RA.(35) Briefly, eight categories are assessed including dressing and grooming, arising, eating, walking, hygiene, reach, and grip (35).
Physical Activity Questionnaire
Physical activity was assessed using a detailed questionnaire developed for the Multi-Ethnic Study of Atherosclerosis (MESA) (36). We used a definition of intentional exercise (the sum of walking for exercise, sports/dancing, and conditioning MET-hours/week) that has been previously defined (26, 37). The total number of reported sedentary hours per week was also recorded.
Disease Measures, Inflammatory Markers
Erythrocyte Sedementation Rate (ESR) was performed using the Westergren method. C-Reactive Protein (CRP) levels were measured using a Fixed Point Immuno Rate Assay. Medication use was determined by self-report and confirmed in the medical record. Disease activity was measured in a standard fashion using the Disease Activity Score 28 (DAS28) with CRP. Cytokine assays were performed using a V-Plex Plus Proinflammatory Panel 1 kit from Meso Scale Discovery. X-rays of the hands and feet were performed and van der Heidje-Sharp (vdHS) scores were determined by a trained radiologist (E.T.).
Statistical Analysis
Multiple-imputation for missing vdHS scores (N=17) was performed. Body composition (ALMI, FMI), muscle density, and measures of muscle strength (lower leg biodex, handgrip) that were available in the reference population were converted to age, sex, and race-specific Z-Scores based on distributions among the reference population as has been described (20, 23, 38, 39). Z-Scores represent the number of standard deviations above or below the predicted value for a healthy control of the same age, sex, and race.
In order to illustrate relationships between appendicular lean mass and muscle density independent of the effects of fat, the generation of adiposity-adjusted appendicular lean mass index (ALMIFMI) Z-Scores were generated for both RA and reference participants was also performed as previously described (23, 38). The ALMIFMI Z-Score represents the number of standard deviations above or below the predicted value for a reference population [National Health and Nutrition Examination Survey (NHANES)] of the same age, sex, race, and FMI Z-Score. This measure was more closely associated with physical functioning in RA and the elderly compared to the unadjusted ALMI Z-Score (40, 41). Low muscle density and low ALMI were each defined as a respective Z-Score of −1 or lower (equivalent to <16th percentile among the reference group).
Correlations between muscle density Z-Scores and disease characteristics, demographics, and whole-body DXA measures of body composition were assessed in univariate and multivariate linear regression models. Initial multivariable models examining factors associated with muscle density Z-Scores included factors associated in univariate analyses (p<0.05). ALMI Z-Score was forced into multivariable models given that univariate relationships of ALMI with muscle density may be confounded by fat mass. Associations of muscle density and body composition Z-Scores with physical function and muscle strength outcomes were assessed in multivariable linear and ordinal regression models, including tests for interactions by disease status These tests for interactions assess if the associations among patients with RA differe from those observed in the reference participants.
Analyses were performed with STATA 14.1 (StataCorp, LP, College Station, TX).
Results
Characteristics of the RA study population are described in Table 1. Comparisons between RA patients and the reference population have been previously published from this cohort (19). Patients with RA had substantially lower muscle density compared to the reference group as evidenced by low (negative) muscle density Z-Scores, consistent with greater intramuscular fat (Table 1). Modest deficits in ALMI and ALMIFMI were also previously reported. Patients with RA also had excessive visceral adiposity, with 32% of patients being characterized as having a “high” VAT area (>160 cm) (42). The characteristics of the reference population have been previously described elsewhere (15, 19, 20).
Table 1.
Basic characteristics of study participants.
| N | 103 |
| Age (yrs) | 55.5 (12.8) |
| Female, N (%) | 52 (50%) |
| Black, N (%) | 32 (31%) |
| Body Mass Index, (kg/m2) | 27.8 (6.1) |
| ALMI Z-Score | −0.095 (1.17) |
| FMI Z-Score | 0.28 (1.19) |
| Muscle Density Z-Score | −0.78 (1.13) |
| Visceral Fat Area (cm) | 131.9 (74.2) |
| High Visceral Fat Area, N (%) | 33 (32%) |
| Lower Leg Strength Z-Score | −0.29 (1.10) |
| Hand Grip Z-Score | −1.10 (1.25) |
| Knee Extension Strength (ft-lbs) | 57.7 (30.1) |
| Knee Flexion Strength (ft-lbs) | 28.3 (16.1) |
| RA Disease Characteristics | |
| DAS28 (CRP) | 4.00 (1.19) |
| HAQ Score | 0.74 (0.61) |
| SPPB | 11 (9, 12) |
| ACPA Positive, N (%) | 84 (82%) |
| vdHS Score (N=92) | 13.5 (3, 60) |
| Disease Duration, yrs | 8.3 (2.6, 18.6) |
| Current Methotrexate, N (%) | 68 (66%) |
| Current Biologic Therapy, N (%) | 53 (51%) |
| Current Prednisone, N (%) | 49 (48%) |
| Ever Methotrexate, N (%) | 87 (86%) |
| Ever Biologic Therapy, N (%) | 68 (66%) |
| Ever Prednisone, N (%) | 81 (79%) |
Results displayed as Mean(SD) or Median (IQR) for skewed data.
Mean and SD of all Z-Scores in controls are 0 +/− 1, by definition.
Abbreviations: ALMI= Appendicular Lean Mass Index; FMI= Fat Mass Index; RA= Rheumatoid Arthritis; DAS28(CRP)= Disease Activity Score of 28 joints including C-Reactive Protein; HAQ= Health Assessment Questionnaire; SPPB= Short Physical Performance Battery; ACPA= Anti-Citrullinated Peptide Antibody; vdHS= van der Heidje-Sharpe.
Factors associated with muscle density Z-Scores
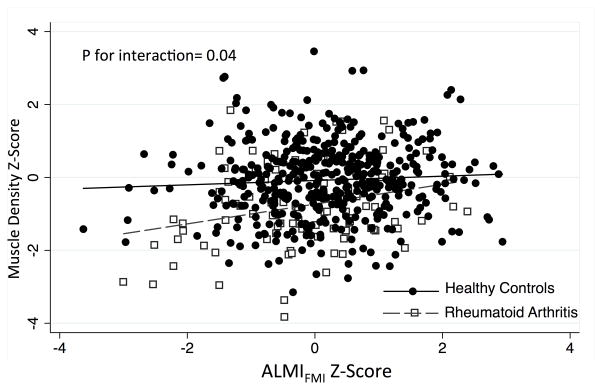
In univariate analyses, factors that were associated with lower muscle density Z-Scores among patients with RA included greater DAS28(CRP), greater CRP levels, greater IL-6 levels, greater sedentary time, current smoking, use of prednisone (>1 year), greater FMI Z-Score, and greater VAT area (Table 2). In the reduced multivariable model, only lower ALMI Z-Score, greater FMI Z-Score, current smoking, and greater CRP were independently associated with lower muscle density. Figure 1 demonstrates that the group differences in muscle density Z-Scores in RA between RA and reference participants were more pronounced among those with low ALMIFMI Z-Scores (p for interaction = 0.04).
Table 2.
Univariate and multivariable associations between muscle density Z-Scores and patient characteristics among patients with RA.
| Univariate Associations | P value | Multivariable Model | P value | |
|---|---|---|---|---|
| Age (per yr) | −0.011 (−0.028, 0.006) | 0.21 | -- | |
| Female | 0.19 (−0.26, 0.63) | 0.41 | -- | |
| Black | 0.22 (−0.25, 0.70) | 0.36 | -- | |
| BMI (kg/m2) | −0.026 (−0.062, 0.010) | 0.16 | -- | |
| Current Smoking | −0.82 (−1.40, −0.25) | 0.005 | −0.58 (−1.03, −0.12) | 0.02 |
| Ever Smoker | −0.38 (−0.89, 0.12) | 0.13 | -- | |
| DAS28(CRP) | −0.24 (−0.42, −0.060) | 0.01 | -- | |
| Ln(CRP) (mg/dL) | −0.54 (−0.90, −0.19) | 0.003 | −0.48 (−0.82, −0.15) | 0.005 |
| Ln(IL-6 Level) (pg/mL) | −0.25 (−0.48, 0.024) | 0.03 | -- | |
| Disease Duration | −0.012 (−0.032, 0.009) | 0.26 | -- | |
| ACPA Positive | −0.36 (−0.93, 0.20) | 0.21 | -- | |
| vdHS Score (per 1 unit) | −0.040 (−0.17, 0.095) | 0.56 | -- | |
| Current Prednisone | −0.30 (−0.75, 0.15) | 0.19 | -- | |
| Ever Prednisone | −0.51 (−1.04, 0.018) | 0.06 | -- | |
| Prednisone >1 year | −0.48 (−0.93, −0.030) | 0.04 | -- | |
| ALMI Z-Score | 0.024 (−0.17, 0.21) | 0.80 | 0.31 (0.078, 0.54) | 0.009 |
| FMI Z-Score | −0.21 (−0.39, −0.022) | 0.03 | −0.40 (−0.63, −0.18) | 0.001 |
| VAT area (per 10 cm) | −0.005 (−0.007, −0.002) | 0.002 | -- | |
| Ln(Exercise) (MET-hrs) | 0.088 (−0.040, 0.22) | 0.17 | -- | |
| Ln(Sedentary Time) (Hrs) | −0.34 (−0.61, −0.069) | 0.01 | -- | -- |
Abbreviations: BMI=Body Mass Index; DAS28(CRP)= Disease Activity Score in 28 Joints with C-Reactive Protein; CRP= C-Reactive Protein; IL=Interleukin; ACPA= Anti-Cyclic Citrullinated Peptide; vdHS= van der Heidje-Sharpe; ALMI=Appendicular Lean Mass Index; FMI=Fat Mass Index; VAT=Visceral Adipose Tissue; MET= Metabolic Equivalent of Task.
Figure 1.
Relationships between adiposity-adjusted lean mass and muscle density in RA and controls.
Low muscle density Z-Scores are observed among RA patients with low ALMIFMI (lean mass scores adjusted for adiposity). The relationship is significantly stronger in RA such that RA patients with low lean mass have the greatest deficits in muscle density compared to reference participants with similarly low lean mass.
Abbreviations: ALMIFMI= Adiposity-adjusted Appendicular Lean Mass Index
Independent associations of muscle density with physical functioning
In univariate analyses, greater muscle density Z-Score was associated with less disability as measured by HAQ and with greater physical functioning as measured by the SPPB (Table 3). In multivariable models, greater muscle density remained associated with lower HAQ scores and a lower odds of being in a low SPPB category after adjustment for whole-body DXA estimates including ALMI and FMI Z-Scores (Table 3). ALMI and FMI Z-Scores were not significantly associated with function in these models. Further adjustment for CRP and swollen joint count partially attenuated associations between muscle density and HAQ scores [β: −0.097 (−0.20, 0.10) p=0.08] and SPPB [OR: 0.70 (0.43, 1.14) p=0.15].
Table 3.
Independent associations between appendicular lean mass, visceral fat area, and muscle density with physical functioning.
| Health Assessment Questionnaire (N=103) | Lower SPPB Category (N=83) | |||
|---|---|---|---|---|
|
| ||||
| β (95% CI) | p | OR (95%CI) | p | |
|
|
||||
| Model 1 | ||||
| Muscle Density Z | −0.18 (−0.28, −0.085)) | <0.001 | 0.38 (0.21, 0.68) | 0.001 |
| Model 2 | ||||
| Muscle Density Z | −0.16 (−0.26, −0.054) | 0.003 | 0.43 (0.23, 0.80) | 0.008 |
| ALMI Z-Score | −0.099 (−0.23, 0.036) | 0.16 | 0.76 (0.39, 1.46) | 0.41 |
| FMI Z-Score | 0.072 (−0.067, 0.21) | 0.31 | 1.50 (0.72, 3.12) | 0.28 |
Models adjusted for age, sex, and race.
Abbreviations: SPPB= Short Physical Performance Battery; ALMI=Appendicular Lean Mass Index; FMI=Fat Mass Index
Associations of muscle density and muscle mass with muscle strength
In multivariable models adjusting for age, sex, race, height, ALMI and FMI Z-Score, muscle density Z-Scores were independently associated with hand-grip, knee extension/flexion, and lower leg strength (Table 4). These associations were somewhat attenuated for hand grip strength [β: 1.23 (−0.31, 2.77) p=0.12] and knee extension [β: 4.10 (−0.73, 8.93) p=0.10] when adjusting for swollen joint count and CRP, but remained significant for knee flexion [β: 3.15 (0.53, 5.77) p=0.02] and lower leg strength [β: 1.77 (0.37, 3.17) p=0.01].
Table 4.
Multivariable model assessing independent associations between muscle and fat outcomes with measures of muscle strength (ft-lbs).
| Hand Grip | Knee extension | Knee flexion | Lower Leg Strength | |
|---|---|---|---|---|
| Model 1 | ||||
| Muscle Density Z | 2.16 (0.61, 3.72)** | 2.35 (0.88, 3.82)** | 4.11 (1.70, 6.53)** | 1.93 (0.59, 3.28)** |
| Model 2 | ||||
| Muscle Density Z-Score | 1.85 (0.24, 3.47) * | 6.47 (1.71, 11.24) ** | 4.54 (1.95, 7.12) ** | 1.81 (0.48, 3.14) ** |
| ALMI Z-Score | 1.97 (0.092, 3.85) * | 2.68 (−3.37, 8.73) | 1.00 (−2.28, 4.27) | 2.52 (0.83, 4.21) ** |
| FMI Z-Score | −0.56 (−2.49, 1.38) | 3.98 (−2.30, 10.25) | 1.65 (−1.75. 5.04) | −0.18 (−1.90, 1.51) |
p<0.05;
p<0.01
Models adjusted for age, sex, race, and height.
Abbreviations: ALMI=Appendicular Lean Mass Index; FMI=Fat Mass Index
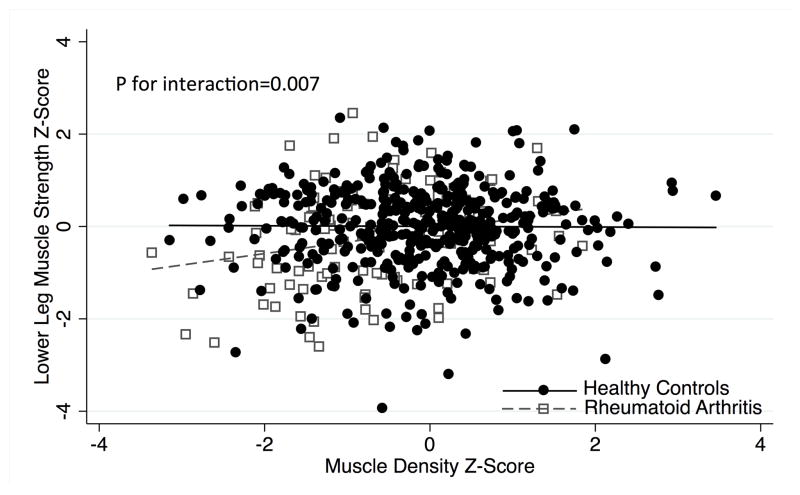
The deficits in lower leg strength in RA participants compared to the reference group were most pronounced among those with lower muscle density Z-Scores (p for interaction = 0.007) (Figure 2). This interaction was not significant for handgrip strength (p=0.12). Among all participants with normal ALMI and normal muscle density (N=326), RA had similar lower leg strength to the reference group [β: 0.002 (−0.30, 0.30) p=0.99]. Among all participants with low muscle density Z-Scores (Z ≤ −1; N=120), RA patients had significantly poorer lower leg strength Z-Scores compared to the reference group [β: −0.56 (−0.92, −0.10) p=0.003]. Similarly, among all participants with low lean mass (ALMI Z ≤ −1; N=90), RA patients had greater deficits in lower leg strength Z-Scores compared to the reference group [β: −0.45 (−0.86, −0.037) p=0.03].
Figure 2.
Relationships between muscle density and lower leg strength Z-Scores among RA and controls.
Lower muscle strength Z-Scores at the lower leg are observed among RA patients with muscle density. The relationship is not present in the reference group. Thus, the figure illustrates that RA patients with lower muscle density have the greatest deficits in muscle density compared to reference participants with similarly low muscle density.
Discussion
The current study describes relationships between intramuscular fat accumulation (low muscle density) and body composition, muscle strength, and physical functioning in patients with RA. Low muscle density at the calf was strongly associated with low lean mass and greater total and visceral adiposity. Muscle density was also independently associated with several measures of strength and physical functioning. This study builds on previous literature by demonstrating that associations between muscle density and physical functioning and strength in this population are independent of total lean mass and total and regional adiposity as measured by whole-body DXA (21, 22). In other words, these results provide further support for the hypothesis that fat infiltration of muscle contributes to muscle dysfunction among patients with RA, over and above the effects of low muscle mass and excess total and visceral adiposity.
The implications of this finding are several-fold. Firstly, these observations further support a causal relationship between muscle density and physical function. If causal, assessment of muscle density represents a potential surrogate outcome. Clinical interventions or therapies that result in improvements in muscle density might therefore be reasonably expected to result in improvements in physical function.
Previous studies in the elderly and other at-risk populations have also demonstrated significant associations between low muscle density (greater intramuscular fat accumulation) and poor strength, poor physical functioning, and risk of falls and hospitalization (2, 3, 17, 43). For example, Hicks et al. previously demonstrated correlations between muscle density and physical functioning among the elderly from the Healthy Aging and Body Composition study (44). In our study, low muscle density among healthy participants was not associated with lower leg strength, suggesting that low muscle density may be of greater relevance to muscle function in aging and in chronic disease. In other words, the novel interactions identified in the current study suggest that patients with RA who have low muscle density experience greater deficits in strength compared to what would be expected for a the reference population. Lower-than-expected strength could suggest that the mechanisms leading to low muscle density in RA are relevant. In other words, intramuscular fat accumulation due to cachexia or aging may have more pathologic implications. Alternatively, other RA-specific factors may lead to low muscle strength in RA patients with low muscle density.
Patients with RA have a number of hypothesized reasons to accumulate intramuscular fat including the direct effects of chronic systemic inflammation, physical inactivity, excess total and visceral adiposity, and use of medications such as glucocorticoids (21). The current study identified a number of factors associated with low muscle density, including greater disease activity and systemic inflammation, smoking, low muscle mass, and excess total and visceral adiposity. Long-term prednisone use (greater than 1 year) and excess sedentary time were also associated with low muscle density in univariate analyses.
Of note, patients with RA with low lean mass had substantially lower muscle density, however, this relationship was not observed in the reference group. This observation has implications in understanding the mechanisms of intramuscular adipose tissue accumulation in this disease. We propose that deficits in muscle density may occur in parallel with loss of muscle mass in RA. Other studies have suggested that cachexia may impact both muscle mass and muscle quality in other settings (45, 46). We hypothesize that, since these mechanisms are not likely to be at play in healthy individuals, there is a lack of as similar relationship between lean mass and muscle density in the healthy participants.
The observational and cross-sectional nature of the current study limits its ability to prove causal associations. Whether low muscle density is truly in the causal pathway between RA and poor physical functioning or simply an independent marker of more severe and longstanding disease is not clear. However, the relationships observed in this study among patients with RA are consistent with what has been seen in in the elderly (2, 3, 14, 17, 43). Longitudinal studies with long-term follow-up are necessary to clarify the relationships between disease features and muscle deficits. While pQCT can assess intramuscular fat content, it does not characterize the distribution of lipids within the muscle nor can it directly assess the metabolic and molecular processes affected. Furthermore, the population studied here may not be entirely generalizable to all other populations of RA patients. The strengths of the study include the well-validated measures of body composition, strength, and function, and the large and well characterized control population allowing for the assessment of altered relationships in the disease state.
In conclusion, intramuscular fat accumulation and resulting low muscle density is observed in RA and associated with systemic inflammation, sedentary lifestyle, smoking, long-term prednisone use, and low muscle mass. Low muscle density is associated with poor physical function and low muscle strength independent of total or regional adiposity and may be directly implicated in poor muscle function.
Key Messages.
Adverse body composition, systemic inflammation, sedentary lifestyle, smoking, and long-term prednisone use are associated with low muscle density in rheumatoid arthritis.
Low muscle density is associated with reduced muscle strength and physical functioning over and above the effects of low muscle mass and excess adiposity.
The association between muscle density and muscle strength in patients with RA is stronger compared to that observed in healthy controls.
Acknowledgments
Dr. Baker would like to acknowledge the support of a Veterans Affairs Clinical Science Research & Development Career Development Award (IK2 CX000955). The contents of this work do not represent the views of the Department of the Veterans Affairs or the United States Government.
Funding: Dr. Baker is supported by a Veterans Affairs Clinical Science Research and Development Career Development Award (IK2 CX000955). This work was also supported by the University of Pennsylvania Clinical and Translational Research Center (UL1 RR024134).
Footnotes
Conflicts of Interest
The authors have nothing to disclose.
References
- 1.Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol. 2000;89(1):104–10. doi: 10.1152/jappl.2000.89.1.104. [DOI] [PubMed] [Google Scholar]
- 2.Cawthon PM, Fox KM, Gandra SR, Delmonico MJ, Chiou CF, Anthony MS, et al. Do muscle mass, muscle density, strength, and physical function similarly influence risk of hospitalization in older adults? J Am Geriatr Soc. 2009;57(8):1411–9. doi: 10.1111/j.1532-5415.2009.02366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malkov S, Cawthon PM, Peters KW, Cauley JA, Murphy RA, Visser M, et al. Hip Fractures Risk in Older Men and Women Associated With DXA-Derived Measures of Thigh Subcutaneous Fat Thickness, Cross-Sectional Muscle Area, and Muscle Density. J Bone Miner Res. 2015;30(8):1414–21. doi: 10.1002/jbmr.2469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelley DE, Slasky BS, Janosky J. Skeletal muscle density: effects of obesity and non-insulin-dependent diabetes mellitus. Am J Clin Nutr. 1991;54:509–15. doi: 10.1093/ajcn/54.3.509. [DOI] [PubMed] [Google Scholar]
- 5.Taaffe DR, Henwood TR, Nalls MA, Walker DG, Lang TF, Harris TB. Alterations in muscle attenuation following detraining and retraining in resistance-trained older adults. Gerontology. 2009;55(2):217–23. doi: 10.1159/000182084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, et al. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33(4):863–70. doi: 10.1161/ATVBAHA.112.301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schafer AL, Vittinghoff E, Lang TF, Sellmeyer DE, Harris TB, Kanaya AM, et al. Fat infiltration of muscle, diabetes, and clinical fracture risk in older adults. J Clin Endocrinol Metab. 2010;95(11):E368–72. doi: 10.1210/jc.2010-0780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antoun S, Lanoy E, Iacovelli R, Albiges-Sauvin L, Loriot Y, Merad-Taoufik M, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer. 2013;119(18):3377–84. doi: 10.1002/cncr.28218. [DOI] [PubMed] [Google Scholar]
- 9.Reinders I, Murphy RA, Brouwer IA, Visser M, Launer L, Siggeirsdottir K, et al. Muscle Quality and Myosteatosis: Novel Associations With Mortality Risk: The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. Am J Epidemiol. 2016;183(1):53–60. doi: 10.1093/aje/kwv153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank AW, Farthing JP, Chilibeck PD, Arnold CM, Olszynski WP, Kontulainen SA. Community-dwelling female fallers have lower muscle density in their lower legs than non-fallers: evidence from the Saskatoon Canadian Multicentre Osteoporosis Study (CaMos) cohort. J Nutr Health Aging. 2015;19(1):113–20. doi: 10.1007/s12603-014-0476-6. [DOI] [PubMed] [Google Scholar]
- 11.Zhao Q, Zmuda JM, Kuipers AL, Jonnalagadda P, Bunker CH, Patrick AL, et al. Greater skeletal muscle fat infiltration is associated with higher all-cause mortality among men of African ancestry. Age Ageing. 2016;45(4):529–34. doi: 10.1093/ageing/afw062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miljkovic I, Kuipers AL, Cauley JA, Prasad T, Lee CG, Ensrud KE, et al. Greater Skeletal Muscle Fat Infiltration Is Associated With Higher All-Cause and Cardiovascular Mortality in Older Men. J Gerontol A Biol Sci Med Sci. 2015 doi: 10.1093/gerona/glv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, et al. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care. 2012;35(8):1672–9. doi: 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akazawa N, Okawa N, Tamura K, Moriyama H. Relationships between intramuscular fat, muscle strength and gait independence in older women: A cross-sectional study. Geriatr Gerontol Int. 2016 doi: 10.1111/ggi.12869. [DOI] [PubMed] [Google Scholar]
- 15.Baker JF, Davis M, Alexander R, Zemel BS, Mostoufi-Moab S, Shults J, et al. Associations between body composition and bone density and structure in men and women across the adult age spectrum. Bone. 2013;53(1):34–41. doi: 10.1016/j.bone.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robles PG, Sussman MS, Naraghi A, Brooks D, Goldstein RS, White LM, et al. Intramuscular Fat Infiltration Contributes to Impaired Muscle Function in COPD. Med Sci Sports Exerc. 2015;47(7):1334–41. doi: 10.1249/MSS.0000000000000556. [DOI] [PubMed] [Google Scholar]
- 17.Therkelsen KE, Pedley A, Hoffmann U, Fox CS, Murabito JM. Intramuscular fat and physical performance at the Framingham Heart Study. Age (Dordr) 2016;38(2):31. doi: 10.1007/s11357-016-9893-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akazawa N, Okawa N, Tamura K, Moriyama H. Relationships between intramuscular fat, muscle strength and gait independence in older women: A cross-sectional study. Geriatr Gerontol Int. 2017;17(10):1683–8. doi: 10.1111/ggi.12869. [DOI] [PubMed] [Google Scholar]
- 19.Baker JF, Long J, Mostoufi-Moab S, Denburg M, Jorgenson E, Sharma P, et al. Muscle Deficits in Rheumatoid Arthritis Contribute to Inferior Cortical Bone Structure and Trabecular Bone Mineral Density. Journal of Rheumatology. 2017 doi: 10.3899/jrheum.170513. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baker JF, Von Feldt J, Mostoufi-Moab S, Noaiseh G, Taratuta E, Kim W, et al. Deficits in muscle mass, muscle density, and modified associations with fat in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2014;66(11):1612–8. doi: 10.1002/acr.22328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kramer HR, Fontaine KR, Bathon JM, Giles JT. Muscle density in rheumatoid arthritis: Associations with disease features and functional outcomes. Arthritis Rheum. 2012;2012(5):34464. doi: 10.1002/art.34464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khoja SS, Moore CG, Goodpaster BH, Delitto A, Piva SR. Skeletal muscle fat and its association with physical function in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2017 doi: 10.1002/acr.23278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baker JF, Giles JT, Weber D, Leonard MB, Zemel BS, Long J, et al. Assessment of muscle mass relative to fat mass and associations with physical functioning in rheumatoid arthritis. Rheumatology (Oxford) 2017 doi: 10.1093/rheumatology/kex020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santanasto AJ, Newman AB, Strotmeyer ES, Boudreau RM, Goodpaster BH, Glynn NW. Effects of Changes in Regional Body Composition on Physical Function in Older Adults: A Pilot Randomized Controlled Trial. J Nutr Health Aging. 2015;19(9):913–21. doi: 10.1007/s12603-015-0523-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang P, Peterson M, Su GL, Wang SC. Visceral adiposity is negatively associated with bone density and muscle attenuation. Am J Clin Nutr. 2015;101(2):337–43. doi: 10.3945/ajcn.113.081778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Baker JF, Davis M, Alexander R, Zemel BS, Mostoufi-Moab S, Shults J, et al. Associations between Body Composition and Bone Density and Structure in Men and Women across the Adult Age Spectrum. Bone. 2013;53(1):34–41. doi: 10.1016/j.bone.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34(6):1044–52. doi: 10.1016/j.bone.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 28.Rothney MP, Xia Y, Wacker WK, Martin FP, Beaumont M, Rezzi S, et al. Precision of a new tool to measure visceral adipose tissue (VAT) using dual-energy X-Ray absorptiometry (DXA) Obesity (Silver Spring) 2013;21(1):E134–6. doi: 10.1002/oby.20140. [DOI] [PubMed] [Google Scholar]
- 29.Farr JN, Funk JL, Chen Z, Lisse JR, Blew RM, Lee VR, et al. Skeletal muscle fat content is inversely associated with bone strength in young girls. J Bone Miner Res. 2011;(4):414. doi: 10.1002/jbmr.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lang T, Cauley JA, Tylavsky F, Bauer D, Cummings S, Harris TB. Computed tomographic measurements of thigh muscle cross-sectional area and attenuation coefficient predict hip fracture: the health, aging, and body composition study. J Bone Miner Res. 2010;25(3):513–9. doi: 10.1359/jbmr.090807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leggin BG, Neuman RM, Iannotti JP, Williams GR, Thompson EC. Intrarater and interrater reliability of three isometric dynamometers in assessing shoulder strength. J Shoulder Elbow Surg. 1996;5(1):18–24. doi: 10.1016/s1058-2746(96)80026-7. [DOI] [PubMed] [Google Scholar]
- 32.Wetzsteon RJ, Kalkwarf HJ, Shults J, Zemel BS, Foster BJ, Griffin L, et al. Volumetric bone mineral density and bone structure in childhood chronic kidney disease. J Bone Miner Res. 2011 doi: 10.1002/jbmr.427. doi:10./jbmr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mihalko SL, Wickley KL, Sharpe BL. Promoting physical activity in independent living communities. Med Sci Sports Exerc. 2006;38(1):112–5. doi: 10.1249/01.mss.0000183230.08341.6b. [DOI] [PubMed] [Google Scholar]
- 34.Corsonello A, Lattanzio F, Pedone C, Garasto S, Laino I, Bustacchini S, et al. Prognostic significance of the short physical performance battery in older patients discharged from acute care hospitals. Rejuvenation Res. 2012;15(1):41–8. doi: 10.1089/rej.2011.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wolfe F, Michaud K, Pincus T. Development and Validation of the Health Assessment Questionnaire II. Arthritis and Rheumatism. 2004;50(10):3296–305. doi: 10.1002/art.20549. [DOI] [PubMed] [Google Scholar]
- 36.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-ethnic study of atherosclerosis: objectives and design. Am J Epidemiol. 2002;156(9):871–81. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 37.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, et al. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169(4):444–54. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Weber D, Long J, Leonard MB, Zemel B, Baker JF. Development of Novel Methods to Define Deficits in Appendicular Lean Mass Relative to Fat Mass. PLoS One. 2016;11(10):e0164385. doi: 10.1371/journal.pone.0164385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelly TL, Wilson KE, Heymsfield SB. Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS One. 2009;4(9):e7038. doi: 10.1371/journal.pone.0007038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baker JF, Giles JT, Weber D, Leonard MB, Zemel BS, Long J, et al. Assessment of muscle mass relative to fat mass and associations with physical functioning in rheumatoid arthritis. Rheumatology (Oxford) 2017;56(6):981–8. doi: 10.1093/rheumatology/kex020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Baker JF, Long J, Leonard MB, Harris T, Delmonico MJ, Santanasto A, et al. Estimation of Skeletal Muscle Mass Relative to Adiposity Improves Prediction of Physical Performance and Incident Disability. J Gerontol A Biol Sci Med Sci. 2017 doi: 10.1093/gerona/glx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pickhardt PJ, Jee Y, O’Connor SD, del Rio AM. Visceral adiposity and hepatic steatosis at abdominal CT: association with the metabolic syndrome. AJR Am J Roentgenol. 2012;198(5):1100–7. doi: 10.2214/AJR.11.7361. [DOI] [PubMed] [Google Scholar]
- 43.Anderson DE, Quinn E, Parker E, Allaire BT, Muir JW, Rubin CT, et al. Associations of Computed Tomography-Based Trunk Muscle Size and Density With Balance and Falls in Older Adults. J Gerontol A Biol Sci Med Sci. 2016;71(6):811–6. doi: 10.1093/gerona/glv185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, et al. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: the moderating role of back pain. J Gerontol A Biol Sci Med Sci. 2005;60(11):1420–4. doi: 10.1093/gerona/60.11.1420. [DOI] [PubMed] [Google Scholar]
- 45.Malietzis G, Johns N, Al-Hassi HO, Knight SC, Kennedy RH, Fearon KC, et al. Low Muscularity and Myosteatosis Is Related to the Host Systemic Inflammatory Response in Patients Undergoing Surgery for Colorectal Cancer. Ann Surg. 2016;263(2):320–5. doi: 10.1097/SLA.0000000000001113. [DOI] [PubMed] [Google Scholar]
- 46.Fujiwara N, Nakagawa H, Kudo Y, Tateishi R, Taguri M, Watadani T, et al. Sarcopenia, intramuscular fat deposition, and visceral adiposity independently predict the outcomes of hepatocellular carcinoma. J Hepatol. 2015;63(1):131–40. doi: 10.1016/j.jhep.2015.02.031. [DOI] [PubMed] [Google Scholar]