Abstract
The bone marrow is primary site of hematopoiesis and home for hematopoietic stem cells (HSCs) in adult mammals. Prostate cancer commonly metastasizes to bone and forms bone metastases in almost all patients who die of the disease. Prostate cancer bone metastases are thought to develop after rare bone marrow disseminated tumor cells (DTCs) escape a dormant state and reactivate. Prostate cancer DTCs and normal HSCs have been shown to compete for residence in the bone marrow and share many of same regulatory mechanisms for survival, proliferation and homing. In this review, we highlight these parallels in order to help our readers use the literature in HSC and DTC biology to inform their research and generate hypotheses in both fields.
Keywords: prostate cancer, disseminated tumor cell, hematopoietic stem cell, dormancy, recurrence, GAS6, CXCL12, stem cell, niche
Introduction
Prostate cancer (PCa) is a large public health problem with over 180,000 new cases and over 26,000 deaths per year in the United States alone1. Of PCa patients with distant metastases, 90% have metastases to bone2. Prostate cancer cells are thought to spread to the bone marrow early in the disease process, even at or before the time of curative intent surgery or radiation therapy to the prostate. At this time, they are termed disseminated tumor cells (DTCs) or micro-metastases. These cells are found in bone marrow or other tissues rather than circulating in peripheral blood, which most investigators term circulating tumor cells, or CTCs. The presence of bone marrow DTCs in PCa patients at the time of radical prostatectomy has been demonstrated by research groups at three institutions using various techniques including, RT-PCR for prostate specific antigen (PSA), immunohistochemistry for PSA and single cell selection of epithelial cell adhesion molecule (EPCAM) positive cells by single cell isolation after prior negative and positive selection3–7. Furthermore, their presence was shown by all three groups to correlate with PCa recurrence5,8,9. However, recently investigators reported being able to detect these cells in only a small minority of patients with localized PCa10. This report highlights the challenges in conducting this type of research and suggests further investigation.
PCa DTCs can remain viable but dormant for long time periods; as illustrated by the fact that about 20% of PCa recurrences after surgery occur greater than 5 years after patients were thought to be cured11. Reactivation or dormancy escape of bone marrow DTCs is thought to be a major cause of relapse in PCa and other malignancies12. Furthermore, an understanding of the biology of how DTCs or micrometastases are different from macroscopic tumors and how they interact with bone microenvironment has the potential to lend insight into later stages of the disease when metastases are visible on imaging. Therefore, understanding the biology of DTCs in the bone environment is crucial for prevention of relapse or treatment of relapsed disease.
There are two major models which have been invoked to describe tumor heterogeneity, either of which could describe the behavior of DTCs. The cancer stem cell (CSC) model suggests that subpopulations of cancer cells form a hierarchical cluster of tumor initiating cells13. These CSCs often feature parameters present in normal tissue stem cells, and like normal stem cells maintain themselves through self-renewal, but also differentiate into progenitor cell populations and ultimately into mature or non-stem cancer cells (NSCCs). The second major model for tumor development is the stochastic model. In the stochastic model, tumors develop by clonal evolution to progress into heterogeneous populations which are influenced by both intrinsic and extrinsic environmental factors14.
Many tissues which harbor DTCs, including lung, liver and bone marrow, are also sites in which clinical relapse can eventually occur. Each of these organs support stem cell populations and tightly regulates proliferation as a component of normal function. This suggests that the normal activity of the host tissues to regulate stem cell function, be it the induction or maintenance of quiescence, ultimately becomes insufficient to enforce dormancy of DTCs. Yet, whether DTCs are primed for proliferation but constantly kept in check by suppressive signals or rather reprogrammed into a semi-permanent dormant state by the microenvironment remains unclear. In several experimental systems DTCs isolated directly from humans or from preclinical models are difficult to grow in vitro, and only proliferate after extended culture periods12,15. The implication from these studies is that DTCs, at least in these organs, are reprogrammed into a dormant state rather than held in check by the continual presence of negative regulators. Alternatively, there is ample evidence that immune regulation of DTCs plays a major role in controlling DTC proliferation16–19. How the host is able to distinguish normal stem cells from tumor cells, be they CSCs or NSCCs remains unclear.
We and others have hypothesized that DTCs reside in similar environments or “niches” as hematopoietic stem cells (HSCs) and compete for occupancy of the bone marrow microenvironment20. Likely because of shared interactions with stromal cell types, research over the past one or two decades has discovered shared regulatory mechanisms between DTCs and HSCs. In this review, we use HSC biology to frame a discussion of PCa bone marrow DTC regulation in hopes that we will help our readers gain intuition into DTC biology and help them generate new hypotheses by drawing on the more extensive HSC literature. We concentrate here on PCa, but note that many of the same concepts will apply to breast cancer and other malignancies which metastasize to bone. The mechanisms discussed below are summarized in Figure 1.
Figure 1.
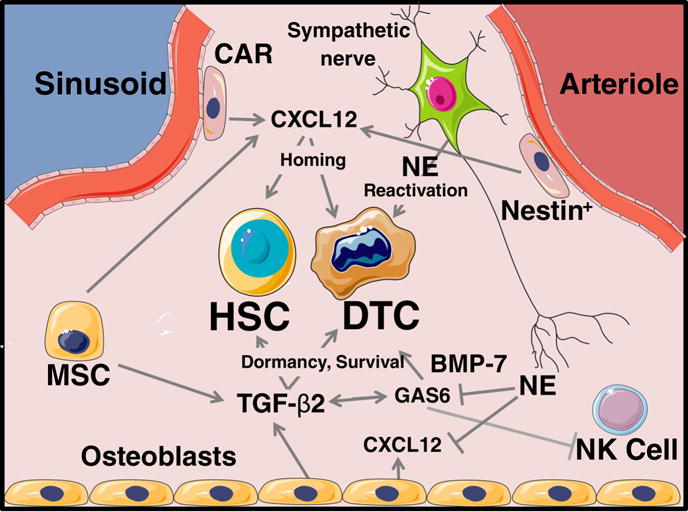
Key cells and secreted factors regulating HSCs and prostate cancer DTCs. HSC; hematopoietic stem cell, DTC; disseminated tumor cell, NE; norepinephrine, CAR; Cxcl12 abundant reticular cell, MSC; mesenchymal stem cell, NK Cell; natural killer cell.
Homing
Perhaps the most established and robust parallel between DTCs and HSCs is in homing to the bone marrow, much of which involves the cytokine CXCL12 (SDF-1). Over a decade ago, CXCL12 was suggested to be important for PCa bone metastasis21. Subsequently, blocking CXCL12 was shown to inhibit transit of PCa cells to the bone marrow. CXCL12 and was also shown to strongly co-localize with metastatic PCa cells – principally in the metaphysis of long bones in the mouse models used for these studies22. There is an extensive literature on the role of CXCL12 in the HSC niche and homing. The niche constituent Cxcl12 abundant reticular cell (CAR) cell is defined by the high expression of CXCL12 as measured by a fluorescent reporter gene23. Furthermore, the effect of other cells on homing and the HSC niche are in part through effects on CXCL12 – namely that the effects of the sympathetic nervous system on the HSC niche are proposed to be through modulation of CXCL12 expression in peri-vascular cells and osteoblasts24,25. Most recently, the pre-metastatic niche for both PCa and breast cancer was proposed to consist of vasculature associated mesenchymal stromal cells. Similarly, our group found a role for the adhesion molecule, Annexin 2, in homing of both HSCs and PCa DTCs to bone marrow26,27. In keeping with the central role of CXCL12 for homing of both cell types, Annexin 2 appeared to act by stabilization of CXCL1228.
Location
We and others have shown that HSCs and DTCs functionally compete for residency in bone marrow20. In an analogous fashion to its function of maintaining HSCs in a pluripotent state, the bone marrow environment appears to cause PCa cells to assume a more primitive or cancer stem cell phenotype. Although this was long hypothesized, Shiozawa and colleagues recently showed a rapid assumption of a stem-like phenotype as defined by increased percentage of CD133+/CD44+ positive PCa cells29.
However, the precision location and cellular makeup of the HSC and especially DTC niches remains much less well defined. There has been much more work published on the location of the HSC niche than the precise location of DTCs in bone marrow. However, even for HSCs, the publication of multiple high impact papers has not brought clarity to this discussion. After decades of study, multiple cell types have been implicated as constituents of the bone marrow HSC niche, most of which are derived from mesenchyme. Earlier studies predominantly suggested an endosteal and osteoblast associated location of the HSC niche30–32. However, more recent studies using mouse genetic manipulations have found perivascular locations to be more important33–35. Among the peri-vascular cell types that are proposed to support HSCs are Cxcl12 abundant reticular (CAR) cells lining sinusoids and nestin positive cells adjacent to arterioles23,36. Nerve fibers are often associated with vasculature in bone marrow as in other parts of the body. More recently than some other cell types, the sympathetic nervous system has also been reported to regulate bone marrow HSCs and more recently DTCs as well24,37.
The relatively small number of studies examining the location of PCa DTCs in mouse models with microscopy have placed them predominantly at the metaphyses or otherwise adjacent to the growth plates38,39. These are also the locations where macroscopic bone metastases most commonly ultimately form. DTCs at the metasphasyses are predominantly within a few cell diameters or less than about 100 μm from the endosteal surface, which suggests an endosteal or osteoblastic location for PCa DTCs. Some have suggested that cancer associated fibroblasts are also important for tumor development in these endosteal locations39. However, it is also important to note that the metasphasyses of mice are very heterogenous with a high vessel density, and many other cell types including hematopoietic precursors and immune cells. Furthermore, because the growth plate closes in humans but not rodents, the location of DTCs in humans might also differ. In breast cancer, investigator have shown the importance of a perivascular niche for breast cancer bone marrow DTCs and that vessel outgrowth promotes dormancy escape40. Alagous micro-anatomy and mechanisms are plausible in PCa but to our knowledge have not be directly studied.
Stress Response and Survival
A commonly proposed reason for “why” DTCs become dormant is as a survival mechanism41. After hematogenous spread to a new location such as the bone marrow, DTCs might not have the same proliferative signals present in the primary tumor and therefore stop cycling, which has been shown to correlate with resistance to chemotherapy and other causes of apoptosis. Prominent molecular mediators of this survival signaling include TGF-β2, p38 MAPK and the endoplasmic reticulum stress response6,41–43. Even more recently, the importance of transcription factors best known for induction of pluripotency in embryonic stem cells has become apparent. These include SOX2, SOX9, NANOG, and retinoic acid receptor β44,45.
Dormancy vs. Proliferation
Many of the best characterized regulators of PCa dormancy have analogous roles in regulation of HSC quiescence vs. proliferation. TGF-β2 is perhaps the best characterized factor maintaining dormancy in DTCs from PCa and other cancers42. Similarly, the TGFβ family member BMP-7 also maintains PCa DTC dormancy46,47. TGF-β family members have analogous effects for HSCs. TGF-β2 maintains HSCs in a quiescent state but increases their ability to engraft. Conversely, TGF-β1 inhibits HSC reconstituting ability48.
It is interesting to consider further that the bone marrow microenvironment is a rich source of growth factors and cytokines with capacity to regulate DTC growth. Numerous molecules present in the marrow including basic fibroblast growth factors (bFGF), insulin-like growth factors I and II (IGF-I and –II), platelet-derived growth factor (PDGF), epidermal growth factor (EGF), and transforming growth factor alpha (TGF-α) may serve as mitogens for DTCs49. In fact, even some forms of TGF-β may stimulate the growth of DTCs50–53. Stromal-derived factor-1 or CXCL12 is well established as a critical regulator of DTC homing and mobilization54–56. However, inhibition of CXCL12 signaling demonstrates anti-mitogen activities, which in part is due to autocrine signaling but could also be due to stromal control of DTC activiites28,57.
In a similar way, bone morphogenetic protein 7 (BMP7) produced by bone marrow stromal cells triggers dormancy of PCa cells. When PCa cells were co-cultured with bone marrow stromal cells, prostate cancer cells entered dormancy through the activation of the cell cycle inhibitor p21, the metastasis suppressor gene N-myc downstream-regulated gene 1 (NDRG1), and p38 MAPK phosphorylation46. Additionally, BMP7 dramatically suppresses the ERK MAPK pathway46. These observations are consistent with previous studies by Aguirre-Ghiso’s group, proposing that the ratio of ERK and p38 MAPK pathways plays a pivotal role in the determination of tumor dormancy42. Dormancy is prevented when BMP7 secreted by bone marrow stromal cells is blocked by shRNA or pan-BMP inhibitor Noggin. Moreover, the effects of BMP7 on dormancy of PCa cells both in vitro and in vivo are attenuated when BMP receptor 2 (BMPR2) on PCa is down-regulated46.
We have reported that growth arrest specific-6 (GAS6) expression from osteoblasts in the bone marrow environment plays a critical role in establishing prostate tumor cell dormancy58. GAS6 signaling inhibits PCa proliferation, as it does with hematopoietic progenitor cells59 suggesting once DTCs enter the niche, interactions between GAS6 and its receptors may regulate PCa dormancy58. Interestingly, GAS6 expression is not uniform amongst different bone locations as significantly higher expression of the protein can be detected in murine femurs vs humeri60. These observations correlate well with the prevalence of human PCa metastasis in immune deficient mice were the predicted probability across all animal models was 7% (range 5-10%) for the forearms and 33% (range 28-38%) for the leg bones60. GAS6 signals predominately through the TAM family of receptor tyrosine kinases Tyro3, Axl and Mer tyrosine kinase (MERTK). Subsequently, we have shown that DTCs recovered from marrow of immune deficient mice differ in their expression of two of the major GAS6 receptors such that a balance between the expression of Axl and Tyro3 may serve as a molecular switch between a dormant (predominatly Axl expression) and a proliferative phenotype (Tyro3 expression) in PCa bone metastases61. Similarly, further investigations found that MERTK signaling stimulates dormancy escape and stimulates formation of a CSC phenotype29,45. Although, there is scant literature for a role of GAS6 in normal HSC function, it is intriguing to note that AXL signaling was recently found to regulate the self-renewal of malignant HSCs in chronic myelogenous leukemia62. This was found to be due to β-catenin signaling, which has a well-established role in the function of CSCs for PCa and other solid tumors63.
It is not surprising that HSC quiescence is tightly regulated given the importance of these cells in maintaining normal homeostasis, or in response to injury. In fact multiple pathways are thought to intersect for the purpose of providing redundancy and for maintaining stem cell activities64–68. Thus it should come as no surprise that DTCs, as molecular parasites of the HSC niche, are likely to be maintained in a dormant state by many intersecting signals. As an example, when DTCs come into close proximity to niche osteoblasts they upregulate their expression of Axl43. Recently we found that expression of both TGF-ß and its receptors were regulated by Axl expression in PCa cells, while blockade of TGF-ß signaling limits the ability of the osteoblasts to induce dormancy of PCa cells43. Importantly, both Gas6 and Axl are required for TGF-ß2-mediated cell growth suppression43. Together, these data suggest that a feedback loop between GAS6 and TGF-ß signaling is likely to provide intersecting and redundant systems critical to maintaining PCa cell dormancy (Illustrated in Figure 2).
Figure 2.
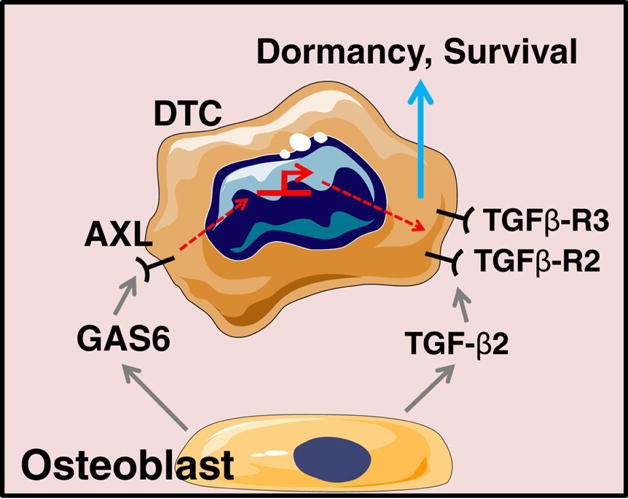
Crosstalk between TAM and TGF-β pathways in dormancy induction and maintenance.
From the preceding discussions it is implied that DTCs are tumor-initiating and are kept in check by proliferative inhibitory signals emanating from the host tissue12. At the same time, clinical observations suggest that a loss of immune function is associated with resurgence of tumor progression16–19. Recently Malladi et al demonstrated that a distinct class of stem-like DTCs are primed to enter quiescence and evade innate immunity69. As part of the phenotype of a tumor-initiating stem/progenitor population, the cells through production of DKK1, enter a dormant like state which is predicated to counteract WNT signaling69. Excitingly, in this state the DTCs are able to evade NK cell surveillance and thus acquire a long-term survival advantage which poises the population for proliferation as immune function changes over time69. Furthermore, in keeping with the studies discussed above on the role of GAS6 in maintaining PCa DTC cellular dormancy, AXL signaling was recently shown to induce development of natural killer cells70.
The sympathetic nervous system has been proposed to stimulate HSC proliferative activity and bone marrow regeneration. For example, Lucas et al showed that hematopoietic regeneration was inhibited by damage to bone marrow sympathetic nerve fibers from chemotherapy drugs, especially cisplatin71. Similarly, Heidt et al showed that chronic variable stress increased proliferative activity of HSCs72. The resultant increased production of inflammatory cells elevated the risk of myocardial infarction and stroke in an animal model. Likewise, in PCa the sympathetic nervous system stimulates the development of the primary tumor73. Our group recently showed that sympathetic neurotransmitter norepinephrine stimulates escape from dormancy in a PCa model. This effect of norepinephrine was through direct action on PCa cells and also through decreased expression of the well know dormancy associated molecule GAS6 by osteoblast lineage cells37.
Conclusions and Perspective
Over the prior 20 years, we have seen remarkable parallels develop in our understanding of the biology of PCa DTCs and normal HSCs. This includes the study of localization, homing, survival, and proliferation. Frequently, the advances in normal hematology have preceded concurrent findings in solid tumor biology. This has provided enormous opportunities for hypothesis generation for investigators studying the biology of PCa DTCs. We are confident that these research parallels will continue in the future. This will provide fertile ground for both hematology and PCa researchers to the benefit of their respective patients.
Highlights.
Prostate cancer disseminated tumor cells (DTCs) and hematopoietic stem cells (HSCs) have analogous functions.
Both home to the bone marrow, for which CXCL12 is critically important.
HSCs and DTCs share a home or “niche” as shown in part by competition experiments.
The niche regulates survival and dormancy vs. proliferation of both cell types.
Acknowledgments
Financial Support: Direct funding was provided by the NIH/NCI P01-CA093900, the NIH/NCI Tumor Microenvironment Network U54-CA163124 and supplement, Department of Defense W81XWH-14-1-0403. And Prostate Cancer Foundation Challenge award 16CHAL05. R.T. receives support as the Major McKinley Ash Colligate Professor. F.C. receives support from a Career Enhancement Award from the NIH/NCI Prostate Cancer Specialized Program in Research Excellence (SPORE) #F048931, sub-award of #F036250 at the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.SEER Cancer Statistics Factsheets: Prostate Cancer. National Cancer Institute; Bethesda, MD: https://seer.cancer.gov/statfacts/html/prost.html (accessed December 9, 2016. [Google Scholar]
- 2.Bubendorf L, Schopfer A, Wagner U, et al. Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol. 2000;31(5):578–83. doi: 10.1053/hp.2000.6698. [DOI] [PubMed] [Google Scholar]
- 3.Wood DP, Jr, Banks ER, Humphreys S, McRoberts JW, Rangnekar VM. Identification of bone marrow micrometastases in patients with prostate cancer. Cancer. 1994;74(9):2533–40. doi: 10.1002/1097-0142(19941101)74:9<2533::aid-cncr2820740922>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 4.Melchior SW, Corey E, Ellis WJ, et al. Early tumor cell dissemination in patients with clinically localized carcinoma of the prostate. Clin Cancer Res. 1997;3(2):249–56. [PubMed] [Google Scholar]
- 5.Morgan TM, Lange PH, Porter MP, et al. Disseminated tumor cells in prostate cancer patients after radical prostatectomy and without evidence of disease predicts biochemical recurrence. Clin Cancer Res. 2009;15(2):677–83. doi: 10.1158/1078-0432.CCR-08-1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chery L, Lam HM, Coleman I, et al. Characterization of single disseminated prostate cancer cells reveals tumor cell heterogeneity and identifies dormancy associated pathways. Oncotarget. 2014;5(20):9939–51. doi: 10.18632/oncotarget.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murray NP, Reyes E, Tapia P, Badinez L, Orellana N. Differential expression of matrix metalloproteinase-2 expression in disseminated tumor cells and micrometastasis in bone marrow of patients with nonmetastatic and metastatic prostate cancer: theoretical considerations and clinical implications-an immunocytochemical study. Bone Marrow Res. 2012;2012:259351. doi: 10.1155/2012/259351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wood DP, Jr, Banerjee M. Presence of circulating prostate cells in the bone marrow of patients undergoing radical prostatectomy is predictive of disease-free survival. J Clin Oncol. 1997;15(12):3451–7. doi: 10.1200/JCO.1997.15.12.3451. [DOI] [PubMed] [Google Scholar]
- 9.Murray NP, Aedo S, Fuentealba C, Reyes E, Salazar A. Minimum Residual Disease in Patients Post Radical Prostatectomy for Prostate Cancer: Theoretical Considerations, Clinical Implications and Treatment Outcome. Asian Pac J Cancer Prev. 2018;19(1):229–36. doi: 10.22034/APJCP.2018.19.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chalfin HJ, Glavaris SA, Malihi PD, et al. Prostate Cancer Disseminated Tumor Cells are Rarely Detected in the Bone Marrow of Localized Patients Undergoing Radical Prostatectomy Across Multiple Rare Cell Detection Platforms. J Urol. 2018 doi: 10.1016/j.juro.2018.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC. Natural history of progression after PSA elevation following radical prostatectomy. JAMA. 1999;281(17):1591–7. doi: 10.1001/jama.281.17.1591. [DOI] [PubMed] [Google Scholar]
- 12.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer. 2014;14(9):611–22. doi: 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shackleton M, Quintana E, Fearon ER, Morrison SJ. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138(5):822–9. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Plaks V, Kong N, Werb Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell. 2015;16(3):225–38. doi: 10.1016/j.stem.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sosa MS, Avivar-Valderas A, Bragado P, Wen HC, Aguirre-Ghiso JA. ERK1/2 and p38alpha/beta signaling in tumor cell quiescence: opportunities to control dormant residual disease. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(18):5850–7. doi: 10.1158/1078-0432.CCR-10-2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sheller B, D’Alessandro D. Analysis of a cancer dormancy model and control of immuno-therapy. Math Biosci Eng. 2015;12(5):1037–53. [PubMed] [Google Scholar]
- 17.Gonzalez H, Robles I, Werb Z. Innate and Acquired Immune Surveillance in the Post-Dissemination Phase of Metastasis. FEBS J. 2017 doi: 10.1111/febs.14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gelao L, Criscitiello C, Fumagalli L, et al. Tumour dormancy and clinical implications in breast cancer. Ecancermedicalscience. 2013;7:320. doi: 10.3332/ecancer.2013.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baxevanis CN, Perez SA. Cancer Dormancy: A Regulatory Role for Endogenous Immunity in Establishing and Maintaining the Tumor Dormant State. Vaccines (Basel) 2015;3(3):597–619. doi: 10.3390/vaccines3030597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shiozawa Y, Pedersen EA, Havens AM, et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4):1298–312. doi: 10.1172/JCI43414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62(6):1832–7. [PubMed] [Google Scholar]
- 22.Sun YX, Schneider A, Jung Y, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20(2):318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 23.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25(6):977–88. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Mendez-Ferrer S, Lucas D, Battista M, Frenette PS. Haematopoietic stem cell release is regulated by circadian oscillations. Nature. 2008;452(7186):442–7. doi: 10.1038/nature06685. [DOI] [PubMed] [Google Scholar]
- 25.Lucas D, Bruns I, Battista M, et al. Norepinephrine reuptake inhibition promotes mobilization in mice: potential impact to rescue low stem cell yields. Blood. 2012;119(17):3962–5. doi: 10.1182/blood-2011-07-367102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jung Y, Wang J, Song J, et al. Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood. 2007;110(1):82–90. doi: 10.1182/blood-2006-05-021352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung Y, Shiozawa Y, Wang J, et al. Annexin-2 is a regulator of stromal cell-derived factor-1/CXCL12 function in the hematopoietic stem cell endosteal niche. Exp Hematol. 2011;39(2):151–66 e1. doi: 10.1016/j.exphem.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jung Y, Wang J, Lee E, et al. Annexin 2-CXCL12 interactions regulate metastatic cell targeting and growth in the bone marrow. Mol Cancer Res. 2015;13(1):197–207. doi: 10.1158/1541-7786.MCR-14-0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shiozawa Y, Berry JE, Eber MR, et al. The marrow niche controls the cancer stem cell phenotype of disseminated prostate cancer. Oncotarget. 2016 doi: 10.18632/oncotarget.9251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taichman RS, Emerson SG. Human osteoblasts support hematopoiesis through the production of granulocyte colony-stimulating factor. J Exp Med. 1994;179(5):1677–82. doi: 10.1084/jem.179.5.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calvi LM, Adams GB, Weibrecht KW, et al. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425(6960):841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 32.Nilsson SK, Johnston HM, Coverdale JA. Spatial localization of transplanted hemopoietic stem cells: inferences for the localization of stem cell niches. Blood. 2001;97(8):2293–9. doi: 10.1182/blood.v97.8.2293. [DOI] [PubMed] [Google Scholar]
- 33.Greenbaum A, Hsu YM, Day RB, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–30. doi: 10.1038/nature11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kunisaki Y, Bruns I, Scheiermann C, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–43. doi: 10.1038/nature12612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Decker AM, Jung Y, Cackowski FC, Yumoto K, Wang J, Taichman RS. Sympathetic signaling re-activates proliferation of dormant disseminated prostate cancer cells in the bone marrow. Cancer Research - Submitted. 2017 doi: 10.1158/1541-7786.MCR-17-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang N, Docherty F, Brown HK, et al. Mitotic quiescence, but not unique “stemness,” marks the phenotype of bone metastasis-initiating cells in prostate cancer. FASEB J. 2015;29(8):3141–50. doi: 10.1096/fj.14-266379. [DOI] [PubMed] [Google Scholar]
- 39.Shahriari K, Shen F, Worrede-Mahdi A, et al. Cooperation among heterogeneous prostate cancer cells in the bone metastatic niche. Oncogene. 2017;36(20):2846–56. doi: 10.1038/onc.2016.436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ghajar CM, Peinado H, Mori H, et al. The perivascular niche regulates breast tumour dormancy. Nat Cell Biol. 2013;15(7):807–17. doi: 10.1038/ncb2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ranganathan AC, Adam AP, Zhang L, Aguirre-Ghiso JA. Tumor cell dormancy induced by p38SAPK and ER-stress signaling: an adaptive advantage for metastatic cells? Cancer Biol Ther. 2006;5(7):729–35. doi: 10.4161/cbt.5.7.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bragado P, Estrada Y, Parikh F, et al. TGF-beta2 dictates disseminated tumour cell fate in target organs through TGF-beta-RIII and p38alpha/beta signalling. Nat Cell Biol. 2013;15(11):1351–61. doi: 10.1038/ncb2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yumoto K, Eber MR, Wang J, et al. Axl is required for TGF-beta2-induced dormancy of prostate cancer cells in the bone marrow. Sci Rep. 2016;6:36520. doi: 10.1038/srep36520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sosa MS, Parikh F, Maia AG, et al. NR2F1 controls tumour cell dormancy via SOX9- and RARbeta-driven quiescence programmes. Nat Commun. 2015;6:6170. doi: 10.1038/ncomms7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cackowski FC, Eber MR, Rhee J, et al. Mer Tyrosine Kinase Regulates Disseminated Prostate Cancer Cellular Dormancy. J Cell Biochem. 2017;118(4):891–902. doi: 10.1002/jcb.25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kobayashi A, Okuda H, Xing F, et al. Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med. 2011;208(13):2641–55. doi: 10.1084/jem.20110840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharma S, Xing F, Liu Y, et al. Secreted Protein Acidic and Rich in Cysteine (SPARC) Mediates Metastatic Dormancy of Prostate Cancer in Bone. J Biol Chem. 2016;291(37):19351–63. doi: 10.1074/jbc.M116.737379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Naka K, Hirao A. Regulation of Hematopoiesis and Hematological Disease by TGF-beta Family Signaling Molecules. Cold Spring Harb Perspect Biol. 2017;9(9) doi: 10.1101/cshperspect.a027987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cooper CR, Chay CH, Gendernalik JD, et al. Stromal factors involved in prostate carcinoma metastasis to bone. Cancer. 2003;97(3 Suppl):739–47. doi: 10.1002/cncr.11181. [DOI] [PubMed] [Google Scholar]
- 50.Newman MJ. Transforming growth factor beta and the cell surface in tumor progression. Cancer metastasis reviews. 1993;12(3–4):239–54. doi: 10.1007/BF00665956. [DOI] [PubMed] [Google Scholar]
- 51.Kloen P, Gebhardt MC, Perez-Atayde A, et al. Expression of transforming growth factor-beta (TGF-beta) isoforms in osteosarcomas: TGF-beta3 is related to disease progression. Cancer. 1997;80(12):2230–9. [PubMed] [Google Scholar]
- 52.Saunier EF, Akhurst RJ. TGF beta inhibition for cancer therapy. Curr Cancer Drug Targets. 2006;6(7):565–78. doi: 10.2174/156800906778742460. [DOI] [PubMed] [Google Scholar]
- 53.Miyazono K. Tumour promoting functions of TGF-beta in CML-initiating cells. J Biochem. 2012;152(5):383–5. doi: 10.1093/jb/mvs106. [DOI] [PubMed] [Google Scholar]
- 54.Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK. Use of the Stromal Cell-derived Factor-1/CXCR4 Pathway in Prostate Cancer Metastasis to Bone. Cancer Res. 2002;62:1832–7. [PubMed] [Google Scholar]
- 55.Sun YX, Wang J, Shelburne CE, et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. Journal of cellular biochemistry. 2003;89(3):462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 56.Sun YX, Schneider A, Jung Y, et al. Skeletal Localization and Neutralization of the SDF-1(CXCL12)/CXCR4 Axis Blocks Prostate Cancer Metastasis and Growth in Osseous Sites In Vivo. Journal of Bone & Mineral Research. 2005;(2):318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 57.Sun YX, Schneider A, Jung Y, et al. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research. 2005;20(2):318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 58.Shiozawa Y, Pedersen EA, Ziegler A, et al. GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Submitted. 2009 doi: 10.1593/neo.91384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dormady SP, Zhang XM, Basch RS. Hematopoietic progenitor cells grow on 3T3 fibroblast monolyers that overexpress growth arrest-specific gene-6 (GAS6) Proceedings of the National Academy of Sciences of the United States of America. 2000;97(22):12260–5. doi: 10.1073/pnas.97.22.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jung Y, Shiozawa Y, Wang J, et al. Prevalence of prostate cancer metastases after intravenous inoculation provides clues into the molecular basis of dormancy in the bone marrow microenvironment. Neoplasia. 2012;14(5):429–39. doi: 10.1596/neo.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taichman RS, Patel LR, Bedenis R, et al. GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS One. 2013;8(4):e61873. doi: 10.1371/journal.pone.0061873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jin Y, Nie D, Li J, et al. Gas6/AXL Signaling Regulates Self-Renewal of Chronic Myelogenous Leukemia Stem Cells by Stabilizing beta-Catenin. Clin Cancer Res. 2017;23(11):2842–55. doi: 10.1158/1078-0432.CCR-16-1298. [DOI] [PubMed] [Google Scholar]
- 63.Katoh M. Canonical and non-canonical WNT signaling in cancer stem cells and their niches: Cellular heterogeneity, omics reprogramming, targeted therapy and tumor plasticity (Review) Int J Oncol. 2017;51(5):1357–69. doi: 10.3892/ijo.2017.4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yu VW, Scadden DT. Heterogeneity of the bone marrow niche. Curr Opin Hematol. 2016;23(4):331–8. doi: 10.1097/MOH.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang W, Yu S, Zimmerman G, et al. Notch Receptor-Ligand Engagement Maintains Hematopoietic Stem Cell Quiescence and Niche Retention. Stem cells. 2015;33(7):2280–93. doi: 10.1002/stem.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Park D, Sykes DB, Scadden DT. The hematopoietic stem cell niche. Front Biosci (Landmark Ed) 2012;17:30–9. doi: 10.2741/3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mendez-Ferrer S, Scadden DT, Sanchez-Aguilera A. Bone marrow stem cells: current and emerging concepts. Annals of the New York Academy of Sciences. 2015;1335:32–44. doi: 10.1111/nyas.12641. [DOI] [PubMed] [Google Scholar]
- 68.Lane SW, Wang YJ, Lo Celso C, et al. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood. 2011;118(10):2849–56. doi: 10.1182/blood-2011-03-345165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Malladi S, Macalinao DG, Jin X, et al. Metastatic Latency and Immune Evasion through Autocrine Inhibition of WNT. Cell. 2016;165(1):45–60. doi: 10.1016/j.cell.2016.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kim EM, Lee EH, Lee HY, et al. Axl signaling induces development of natural killer cells in vitro and in vivo. Protoplasma. 2017;254(2):1091–101. doi: 10.1007/s00709-016-1016-5. [DOI] [PubMed] [Google Scholar]
- 71.Lucas D, Scheiermann C, Chow A, et al. Chemotherapy-induced bone marrow nerve injury impairs hematopoietic regeneration. Nat Med. 2013;19(6):695–703. doi: 10.1038/nm.3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Heidt T, Sager HB, Courties G, et al. Chronic variable stress activates hematopoietic stem cells. Nat Med. 2014;20(7):754–8. doi: 10.1038/nm.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Magnon C, Hall SJ, Lin J, et al. Autonomic nerve development contributes to prostate cancer progression. Science. 2013;341(6142):1236361. doi: 10.1126/science.1236361. [DOI] [PubMed] [Google Scholar]


