Abstract
Mouse lemurs are the smallest of the living primates, and are members of the understudied radiation of strepsirrhine lemurs of Madagascar. They are thought to closely resemble the ancestral primates that gave rise to present day primates. Here we have used multiple histological and immunochemical methods to identify and characterize sensory areas of neocortex in four brains of adult lemurs obtained from a licensed breeding colony. We describe the laminar features for the primary visual area (V1), the secondary visual area (V2), the middle temporal visual area (MT) and area prostriata, somatosensory areas S1(3b), 3a, and area 1, the primary motor cortex (M1), and the primary auditory cortex (A1). V1 has “blobs” with “nonblob” surrounds, providing further evidence that this type of modular organization might have evolved early in the primate lineage to be retained in all extant primates. The laminar organization of V1 further supports the view that sublayers of layer 3 of primates have been commonly misidentified as sublayers of layer 4. S1 (area 3b) is proportionately wider than the elongated area observed in anthropoid primates, and has disruptions that may distinguish representations of the hand, face, teeth and tongue. Primary auditory cortex is located in the upper temporal cortex and may include a rostral area, R, in addition to A1. The resulting architectonic maps of cortical areas in mouse lemurs can usefully guide future studies of cortical connectivity and function.
Keywords: Primates, Auditory cortex, Visual Cortex, Somatosensory Cortex, Prosimian Evolution, RRID_AB_177621, RRID_AB_2313581, RRID_AB_2564642, RRID_AB_477329, RRID_AB_2313581
Graphical abstract
Mouse lemurs are the smallest of primates, and members of the understudied radiation of lemurs of Madagascar. Overall, mouse lemurs are thought to resemble early primates. We used a series of histological procedures to identify and characterize visual, auditory, somatosensory and motor areas in the neocortex of mouse lemur brains as a step towards discovering how these brains are similar and different from other primate brains.
INTRODUCTION
“The most primitive existing representatives of a group are likely to give a more reliable indication of the nature of the ancestral type from which the group originated” - Le Gros Clark, 1931
Here we describe the architectonic features and relative location of primary sensory areas S1, A1, and V1 and related cortical areas in a strepsirrhine primate, the mouse lemur (Microcebus murinus). Mouse lemurs are part of the lemur radiation from a few founding lemurs that reached the island of Madagascar by rafting from Africa some 50–80 mya (Kappeler 2000, Horvath and Willard 2007, Horvath et al., 2008). Most branches of lemur evolution diverged from these ancestors between 24 and 40 mya. The mouse lemur radiation occurred more recently, within 9–10 mya, due to environmental fragmention from aridification and human impact (Yoder et al., 2016). As many as 24 species of mouse lemurs have now been identified (Olivier et al., 2017).
Studies of brain organization in mouse lemurs are important for understanding brain evolution for two major reasons. First, as the name implies, mouse lemurs are the smallest of living primates, ranging from 50 to 90 grams, and have the smallest primate brains (about 2.5 grams for M. murinus; Clark, 1931; Mittermlier et al., 1994). Humans have the largest primate brain, about 500 times larger than that of the mouse lemur. As some features of primate brains scale systematically with brain size (e.g.; Herculano-Houzel et al., 2007; Herculano-Houzel, 2016; Horvát et al., 2016) studies of mouse lemur brains will play a key role in refining existing scaling rules for primate brains. In addition, extremely small brains may exhibit unique adaptations including fewer cortical areas than the brains of other mammals in the clade (Catania et al., 1991). There is some evidence that the number of cortical areas scale with brain size in primates (Kaas et al., 2000; Kaskan et al., 2005). Second, mouse lemur membership in the understudied Strepsirrhini clade makes its phylogenetic relation to other primates of particular interest. This clade of wet-nosed primates (the wet nose is a primitive primate feature; haplorrhine primates that diverged from strepsirrhine primates have a dry nose) includes the lemurs, galagos and pottos of Africa, and the lorises of Southeast Asia (Horvath et al., 2008). Relative to body size, strepsirrhines have small brains with less expanded temporal lobes, and closely resemble those of the extinct adaptiform branch of the primate radiation (Dann et al., 2016; Silcox et al., 2009; 2010). As ancestral primates are thought to have been small, around the size of a mouse lemur or slightly larger (Bloch and Boyer, 2002; Sussman et al., 2013), the organization of mouse lemur brains may closely resemble that of early primate brains. Overall, mouse lemurs have been considered a living model of early primates (Gebo, 2004).
Due to their limited availability, there have been few studies of brain organization in lemurs and other Strepsirrhini, with the exception of the African galago, which is available in laboratory breeding colonies. For studies on lemurs, a few mouse lemurs (Microcebus murinus) have been available from a colony in France, the Brunoy colony in Paris and another colony in the USA. Previously, there have been two comprehensive architectonic studies of cortical organization in Microcebus murinus. Le Gros Clark, an early leader in the field of primate brain evolution (see Le Gros Clark, 1971), described the external appearance and cortical cytoarchitecture from a single mouse lemur donated by the zoological society at London (Le Gros Clark, 1931). A number of proposed cortical areas were delineated and described using the numbering system of Brodmann (1909). A more recent quantitative study, but still limited to cytoarchitectonics, was from the laboratory of Karl Zilles (Zilles et al., 1979). Proposed areas were named and numbered by location in a brain region (e.g.: Frontal areas Fr1, Fr2, and Fr3). Here, we describe cortical areas from brain sections processed from 4 mouse lemurs obtained from the Paris breeding colony. As a number of histochemical and immunochemical procedures have recently emerged to augment architectonic studies (e.g., Wong and Kaas, 2009), we were able to identify and describe architectonic fields with increased certainty compared to previous descriptions of cortical organization in this species.
METHODS
Cortical hemispheres from four adult mouse lemurs (Microcebus murinus) were obtained from the Brunoy colony (MNHN, FRANCE, license approval N° E91-114-1) Paris, France. All experimental procedures were conducted in accordance with the animal care and use guidelines established by the European Communities Council and National Institutes of Health.
Tissue Acquisition
Animals were euthanized and perfused with 2% or 4% paraformaldehyde (PFA) dissolved in 0.01M phosphate-buffer (PB) in Lyon, France; brains were then extracted and shipped to Vanderbilt University. Two cortical hemispheres were separated from subcortical regions and manually flattened (see Stepniewska et al, 2004), postfixed in 4% PFA/0.01M PB, and cryoprotected in 30% sucrose in 0.1M phosphate buffer (PB). The flattened cortices were then cut into 40μm sections parallel to the pial surface. Remaining hemispheres were blocked coronally, sagittally, or horizontally, and cryoprotected in 30% sucrose in 0.1M phosphate buffer (PB), then cut into 40μm sections in each plane. Alternating series of sections were processed immediately for cytochrome oxidase (CO; Wong-Riley, 1979) or Nissl, or stored in 0.05m TBS/AZ for immunohistochemistry.
Immunohistochemistry
Immunohistochemical methods (IHC) were used to label neuronal nuclei (NeuN), vesicular glutamate transporter 2 (VGLUT2), parvalbumin (PV), or nonphosphorylated neurofilament (SMI-32), as previously described (Balaram et al, 2013). Sections were incubated in blocking solution for 2 hours prior to overnight incubation with the primary antibody, dissolved in blocking solution. Sections were rinsed several times in 0.01M PBS to remove excess primary antibody, incubated for 2 hours with the secondary antibody in a blocking solution, followed by overnight incubation in ABC solution dissolved in horse serum. After additional rinses in 0.01M PBS, sections were mounted on gelatin-subbed slides, dehydrated, and coverslipped with Permount. All antibodies have been previously characterized in architectonic studies of primates and closely related nonprimate species (Wong and Kaas, 2010). Other sections were processed histochemically for cytochrome oxidase (Wong-Riley, 1979) or with thionin for Nissl substance.
Antibody Characterization
Anti-NeuN primary antibody: Neurons were labeled with the anti-NeuN antibody (Mouse anti-NeuN, Catalog no. AB_177621 Millipore, Burlington MA). The immunogen is purified cell nuclei from mouse brain. This primary antibody was used in a concentration of 1:5000 parts.
Anti-VGluT2 primary antibody: The antigen for the vesicular glutamate transporter 2 (VGluT2) was recombinant protein from mouse VGluT 2 (Mouse anti VGluT2, Cat# MAB5504, Millipore, Burlington MA; Immunogen is KLH-conjugated linear peptide). In Western Blots of primate neocortex, the antibody recognizes a 56-kDA bond, the molecular weight of VGluT2 (Baldwin et al, 2013). This primary antibody was used in concentration of 1:5000 parts.
Anti-PV primary antibody: The mouse monoclonal antibody PV (Mouse anti PV, Cat# P3088, Sigma-Aldrich, StLouis MO; Immunogen is frog muscle parvalbumin) reacts with the calcium binding spot of PV. This primary antibody was used in the concentration of 1:2000 parts.
Anti-SMI-32 primary antibody: The anti-SMI – 32 antibody is a mouse monoclonal antibody that specifically recognizes the 200–kD non phosphorylated epitope in neurofilament H of a tested range of mammalian species (Mouse anti SMI-32, Cat# 801701, BioLegend, San Diego CA; Immunogen is homogenized hypothalami). The SMI-32 antibody labels neuronal cell bodies, dendrites and some thick axons, but not other cells and tissues (Hof and Morrison, 1995; Wong and Kaas, 2009). The concentration of this primary antibody used was 1:2000 parts as well.
Secondary antibody: The secondary against all the above primary antibodies was made in horse (Horse anti-mouse, Cat# BA-2000 also BA2000, Lot#RRID:AB_2313581, Vector Laboratories). The concentration used was 1:300 parts.
Light Microscopy
Digital photomicrographs of cortical regions were acquired using a Nikon DXM1200 camera mounted on a Nikon E800 microscope (Nikon Inc., Melville NY). Images were adjusted for brightness and contrast using Adobe Photoshop (Adobe Systems Inc., San Jose CA) prior to reconstruction.
Cortical reconstruction
Flat sections were projected and traced using a Bausch and Lomb microprojector (Bausch and Lomb, Rochester NY). Adjacent sections were aligned using blood vessel and areal boundaries to generate reconstructions spanning the cortical depth or the length or width of the hemisphere. Cortical areal boundaries were consistently identifiable across CO and VGluT2 labeled flat sections. Coronal, sagittal, and horizontal labeled sections were also aligned to generate reconstructions of rostral and dorsal views of the brain. In these preparations, all six histological and immunohistochemical procedures were used to identified cortical areal boundaries.
RESULTS
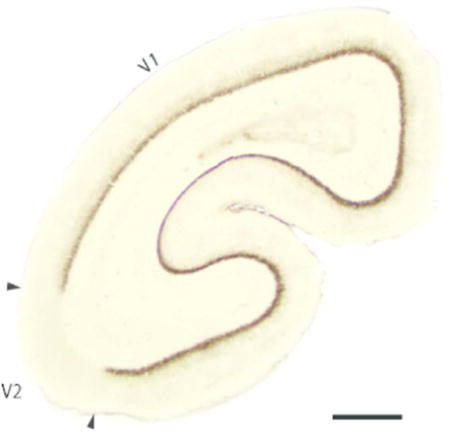
We used multiple histological procedures to optimally define and characterize sensory and motor areas of neocortex in four mouse lemur brains. The locations of areas under consideration are shown in Figure 1. Each view of the brain was reconstructed from serial brain sections cut in a different plane, and imposed on a photograph of a matching view of the brain. Figure 1 a shows cortical areas on the cortical surface that has been separated from the rest of forebrain, manually flattened, and cut sectioned parallel to the surface. This allows most of the cortical surface to be shown in a single view, and the spatial relations of areas to be well appreciated. Although the flattening procedures produce some distortion, this is a relatively minor issue in small brains with few fissures. The small mouse lemur brain has only two fissures, the lateral or sylvian fissure, and the calcarine fissure of primary visual cortex on the medial wall of the hemisphere (Le Gros Clark, 1931). This surface view allows the most accurate reconstruction of areal borders, as maximum extents of borders are visible in a single brain section (Figure 2). As borders are reconstructed over fewer sections, they are based on fewer histological preparations. Reconstructions (Figure 1 b and c) were based on serial coronal or sagittal sections, respectively. Here, we first describe the architecture of visual areas, followed by somatosensory and then auditory cortex.
Figure 1.
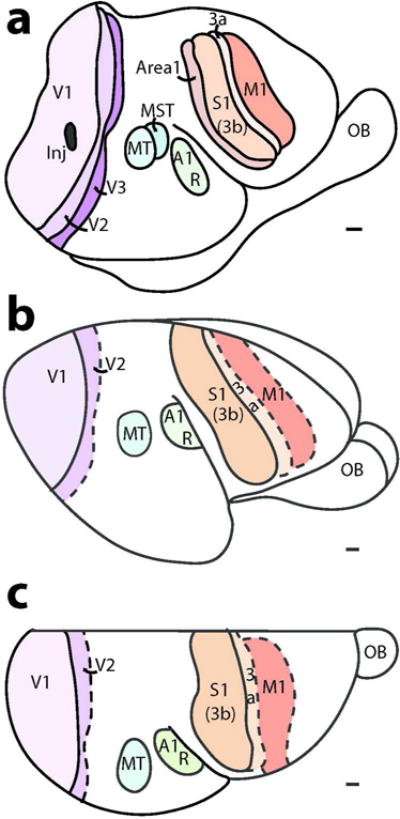
The locations of sensory and motor areas of neocortex in mouse lemur as defined by six different architectonic procedures. (a) A surface view of cortex based on brain sections cut parallel to the surface of flattened cortex; (b) a lateral view based on coronal sections; and (c) a dorsal view based on sagittal sections. V1, V2 and V3, visual areas 1–3; MST and MT, middle temporal visual areas and medial superior temporal area; R and A1, auditory area 1 and the rostral auditory area; S1, 3b, 3a and 1;somatosensory areas S1(3b), 3a and 1; M1, primary motor cortex; OB, olfactory bulb. Inj, a damaged region due to an injection of a tracer. Scale bar = 1 mm.
Figure 2.
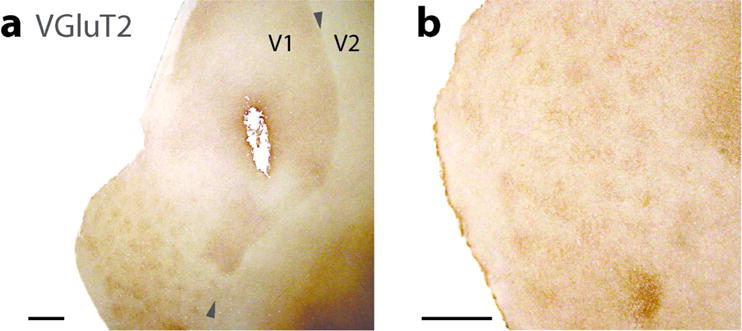
Caudal parts of brain sections cut parallel to flattened cortex and processed for vesicular glutamate transporter 2 (VGluT2). (a) Lower magnification shows a clear border between V1 and V2. The darker parts along upper border reflect the lateral geniculate nucleus (LGN) projection terminations in layer 4, while the pattern of dots in the lower part of the section reflect the layer 3 that are characterized by LGN inputs. (b) A higher magnification of the part of the section with blobs. Scale bar = 1 mm.
Visual areas V1 (area 17), V2 (area 18), Prostriata, and MT
The primary visual area, V1, as in other primates, is proportionately large (Frahm et al., 1984; Cooper at al., 1979) and very distinct in histological preparations. One of the most revealing preparations is to react for the VGluT2 protein which is concentrated in the synaptic terminals of thalamocortical axons (see Balaram et al., 2013) of the driving type that activates primary cortical areas (Sherman and Guillery., 2011). Studies in other primates indicate that dense concentrations of VGluT2 protein identify layer 4 and the layer 3 blobs of V1 (e.g., Wong and Kaas, 2010; Balaram et al., 2013; Balaram and Kaas, 2014). Thus, the border of V1–V2 is most apparent in brain sections of flattened cortex in the section containing layer 4 (dark regions in Figure 2 a) or the dark dot like pattern of layer 3 blobs (lower left part of Figure 2 a). The arrangement of VGluT2 dense blobs (in layer 4 of V1) is shown at higher magnifications (Figure 2 B). The dense concentration of VGluT2 is readily apparent in layer 4 of brain sections cut in the coronal plane (Figure 3 a) and the sagittal plane (not shown). The VGluT2 expression is almost totally absent from V2, as V2 receives relatively few inputs from the dorsal lateral geniculate nucleus (e.g., Bullier and Kennedy, 1983). In this way, the border appears very sharp. The V1 blobs are much less evident in this coronal section, as the geniculate projections to blobs are much less dense than those to layer 4. Sections processed for the calcium binding protein, parvalbumin (PV) likewise densely stain layer 4 of V1, and reveal a lighter blob pattern (Figure 3 b). The PV antibody also labels the geniculate afferent terminals in V1 of other primates and mammals (e.g., Wong and Kaas, 2009). In addition, V1 is readily distinguished from V2 by antibody SMI-32 against the non-phosphorylated neurofilament protein, which labels mostly pyramidal neurons and their dendritic arbors in lower layer 3 and layer 5 (e.g., Hof and Marrison, 1995; Baldauf, 2005; Homman-Ludiye et al., 2010). The densely stained neurons in layers 3 and layer 5 are more broadly distributed in V1 than in V2 (Figure 3 c). Because of the paucity of pyramidal neurons, the SMI-32 label is very sparse in layer 4 of both V1 and V2. The dense SMI-32 label in layer 3c is expected, but layer 3c of V1 in primates is often identified as layer 4b.
Figure 3.
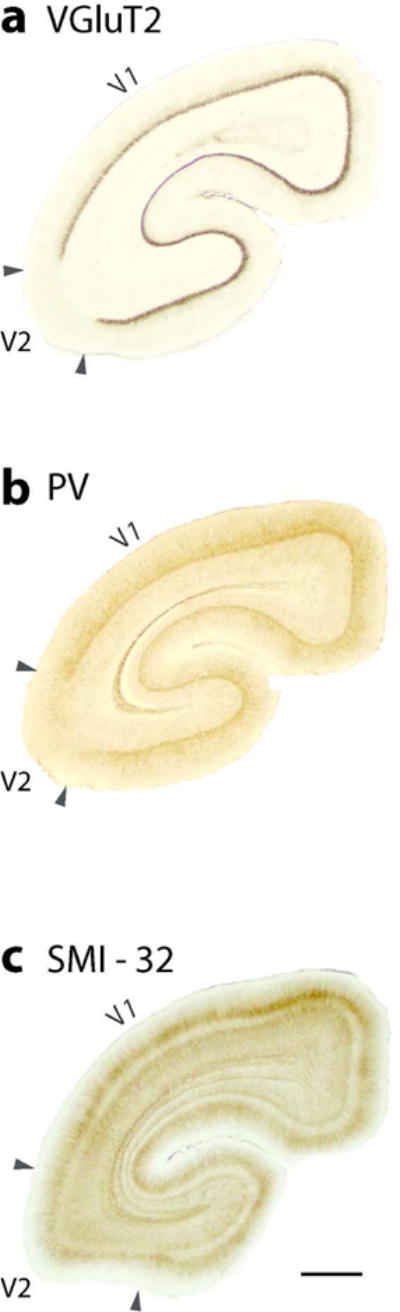
Coronal brain sections from occipital cortex showing the architectonic features of V1 (area17) and V2 (area18). The sections have been processed for, (a) vesicular glutamate transporter 2 (VGlut2), (b) Parvalbumin (PV) and (c) SMI-32. Scale bar = 1 mm.
The classical view of lamination in V1 (area 17) of primates is largely based on Nissl preparations (e.g., Brodmann, 1909). In Nissl section through V1 of mouse lemurs, the laminar pattern of V1 is marked, and it clearly distinguishes V1 from V2 (Figure 4). However, as a nocturnal strepsirrhine primate, the laminar pattern in V1 is less distinct than in diurnal monkeys (Paxinos et al., 2012; Balaram and Kaas, 2014). What is evident is that layer 6 is densely packed with cells, layer 5 has sparsely scattered cells and layers 4 and 3 are densely packed, and not easy to subdivide. On careful inspection, it can be seen that layer 4 includes a deeper sublayer (4b) that is narrow and more cell dense (Figure 4 a). Just above sublayer 4b, a thin, light septum or 4b sublayer may be apparent. More superficially, a somewhat broader sublayer (4a) is distinguished as composed of less darkly stained cells. Just superficial to layer 4, a sublayer of layer 3, is apparent as a lighter band of less densely packed cells. This is layer 3c of Hassler’s (1966) terminology for V1 of primates, and it is not always obvious throughout V1 of mouse lemurs. However, in the boundary region of 17/18, the sublayer 3c of V1 continues into V2 as a similar deep part of layer 3, and the three sublayers of layer 4 continue from area 17 to form a homogenous layer 4 in area 18. The significance of this interpretation of layers and sublayers in area 17 is considered further in the discussion.
Figure 4.
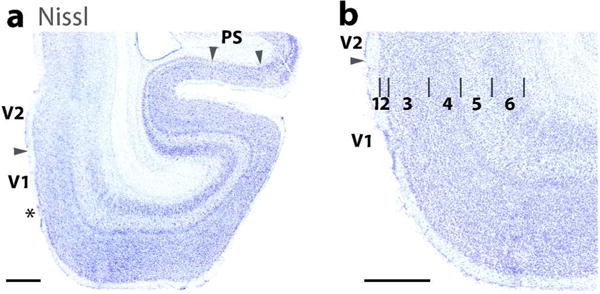
The laminar characteristics of areas 17 (V1), 18 (V2), and Prostriata (PS) in a horizontal brain section stained for Nissl substance. (a) A lower magnification photo of ventromedial V1 (17) and V2 (18). Arrows mark the 17–18 boundary and the 17/prostriata boundary (PS). A transition zone (marked by asterisk) is apparent in area 17 near the area 18 boundary where some of the laminar features of area 17 are less apparent. Prostriata (arrow heads) is also prominent in the section. (b) A higher magnification of the laminar pattern of area 17. Note the sublaminar pattern in layer 4. Scale bar = 1 mm.
The laminar pattern of V1 is shown at higher magnification for five brain preparations (Figure 5). VGluT2 positive axon terminals are densely labeled in layer 4b and less so in layer 4a, reflecting the differences in the projections of parvocellular and magnocellular lateral geniculate layers to lower and upper layer 4, respectively. These differences are also apparent in PV and CO preparations where the darker staining is in layer 4b due to differences in the terminals from the parvocellular and magnocellular layers of the lateral geniculate nucleus. The PV preparations also show the cell bodies of PV positive inhibitory neurons that are scattered across layers. SMI-32 preparations best reveal the cell bodies and dendritic arbours of the larger pyramidal neurons that are located in layers 3b, 3c and 5. Finally, the NeuN preparation reveals a layer 4 that is densely packed with small neurons, while a narrow layer 3c has less densely packed neurons, as does layer 5.
Figure 5.
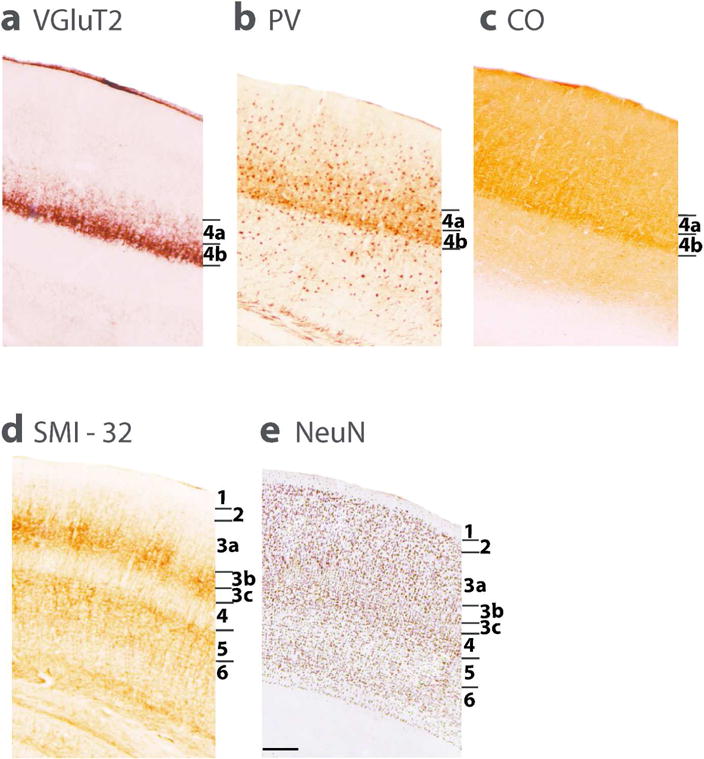
Laminar characteristics of area 17 (V1) in a coronal brain sections processed for VGluT2 (a), Parvalbumin (b), Cytochrome oxidase (c), SMI-32 (d) and NeuN (e). A, B, and C, show two subdivsions of layer 4. SMI-32 (d) stains pyramidal neurons in layer 3 and 5. In NeuN preparations (e), the darkly stained neuron cell bodies mark layers 4,6,3 and 2 as darker than layers 5 and 3 c.
Figure 4 also shows a second area that borders area 17 of primates, area prostriata. In Figure 4 a, it can be seen that the thickness of area 17 becomes thinner in the calcarine fissure of the medial wall, most notably in the upper bank of the fissure. This part of area 17 becomes thin as it represents the extreme periphery of the visual field, and it is adjoined by area prostriata (Allman and Kaas, 1971; Rosa et al., 1997), which is characterized by a less pronounced lamination pattern in Nissl preparations (Allman and Kaas, 1971). Area prostriata (Sanides, 1972; Yu et al., 2012) is a small limbic area adjacent to retrosplenial cortex.
The middle temporal visual area, MT (Allman and Kaas, 1971) is a subdivision of visual cortex that has been found in all primates studied to date. MT is distinctive in that this area in the upper temporal lobe shares architectonic features with primary sensory areas of cortex (Bourne and Rosa, 2006). In the mouse lemur, evidence for MT was obtained from sections processed for VGluT2, which are densely expressed in layer 4 (Figure 6), as in primary sensory areas. Other evidence came from higher expressions of VGluT2 and CO processed sections of flattened cortex and VGluT2 processed sections cut in the sagittal plane. The adjoining middle superior temporal area, MST, has similar architecture (Wong and Kaas, 2010), so it can be difficult to separate the two areas. The architectonic features of MT in mouse lemurs may reflect only MT, or MT plus MST (Figure 1).
Figure 6.
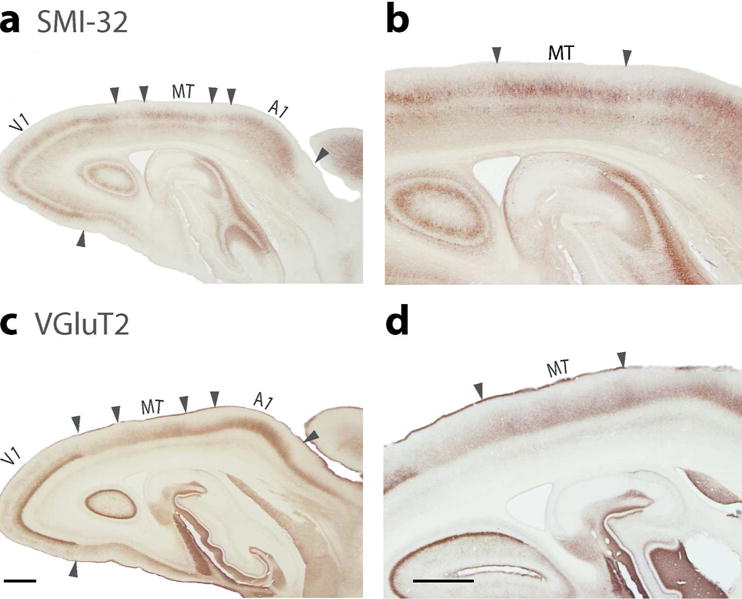
Coronal brain sections through occipital temporal cortex processed for SMI-32 (a, b) or VGluT2 (c, d). (a) MT is seen in the section processed for SMI-32, between area 17 and the auditory cortex. SMI-32 marks pyramidal neurons of layers 3 and 5. (b) Higher magnification of MT (c) The presumptive area MT is characterized by the expression of VGluT2 in layer 4. Area 17 (V1) is also defined by dense VGluT2 expression in layer 4 along with auditory cortex (A1). (d) Higher magnification of the part of the section showing MT. Scale bar in a = 1 mm, in b = 0.5 mm.
Somatosensory and Motor cortex
The somatosensory cortex of mouse lemurs includes a primary area, S1 or area 3b, a dysgranular strip of narrow cortex along the rostral borders of S1, likely area 3a, and cortex along the caudal border of S1 that is likely area 1 (Figure 1 a). Cortex extending from lateral S1 into the rostral bank of the lateral fissure has less pronounced features of sensory cortex, and this cortex includes the expected locations of the second somatosensory area, S2, and the parietal ventral area, PV (see Wong and Kaas 2010, for galagos). S1 (3b) in mouse lemurs can be identified in Nissl or NeuN preparations by a layer 4 that is densely packed with neurons (Figure 6), as in other primates, including other strepsirrhines (Wong and Kaas, 2010). Layer 3 in area 3b is also somewhat densely packed with neurons and therefore is not so sharply demarcated from layer 4 (Figure 6 b). The pattern of VGluT2 expression reveals one of the features of S1 (3b) that is especially predominant in mouse lemurs. As seen in Figure 7 A, the expression of VGluT2 is dense in layer 4 of area 3 b, and this expression extends somewhat up into layer 3. Most notably, VGluT2 expression is interrupted in coronal sections by VGluT2 poor gaps. In other primates, such gaps are less frequent and narrow, and they reflect discontinuities in the representations of the body, such as between the hand and face, or around the teeth and tongue representations (e.g. Jain et al., 2001). The gaps in layer 4 are also apparent in sections stained for PV and CO, and in the labeling for SMI-32 in layer 3 and 5 (Figure 8 c, d and b). Thus, the gaps include other layers in addition to layer 4. Along the caudal border of S1, cortex in the expected regions of S2 and PV has a well-developed layer 4 and other features of sensory cortex, although less pronounced than in S1 (3b). More medial cortex along the caudal border of S1 (3b), presumptive area 1, also has less pronounced sensory features (not shown).
Figure 7.
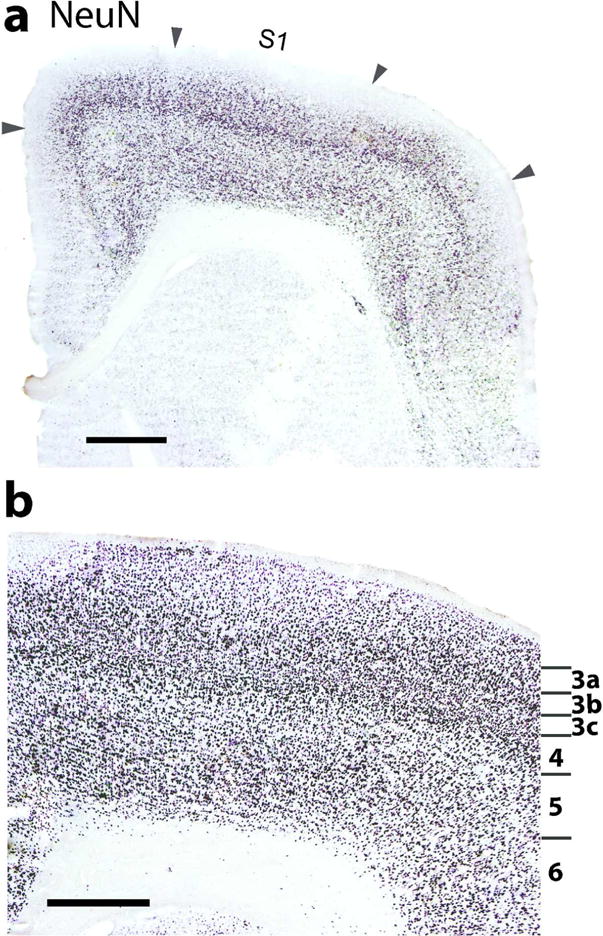
A coronal section of S1 cortex processed for neuron cell bodies with NeuN. (a) At a lower magnification, S1 (area 3b) appears to be patchy (arrowheads) due to disjunctions in the S1 representation (likely representing foot and hand). (b) A view of S1 at a higher magnification marking layers 3 through 6. Layers 3 and 4 are densely packed with neurons. Also note the sublaminar pattern in layer 3. Scale bar in a = 1 mm, in b = 0.5 mm.
Figure 8.
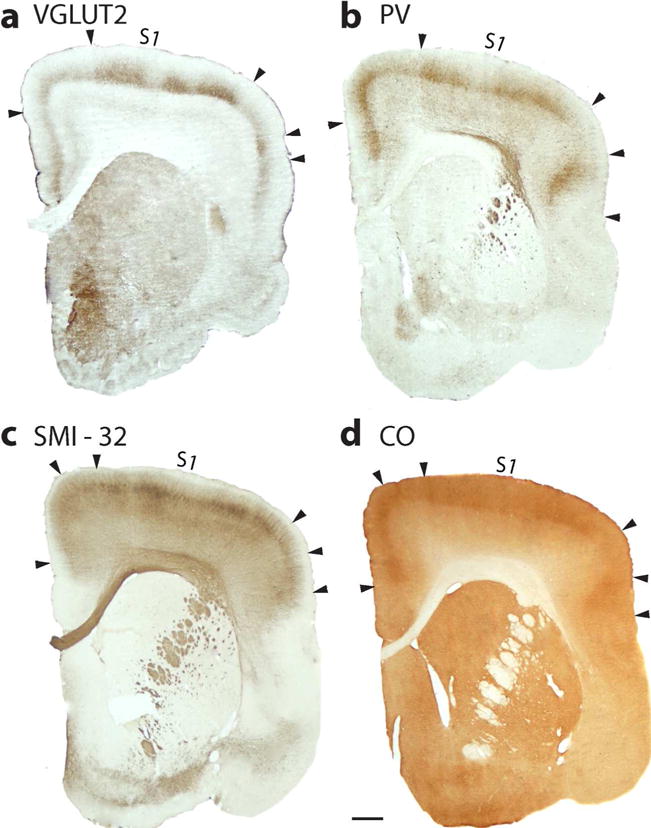
S1 in especially more frontal sections appears to be patchy, due to disjunctions in the somatotopy. (a) Coronal section through frontal cortex processed for VGluT2. Dense patches of label (marked by arrowheads) in layer 4 and extending into layer 3, characterize parts of S1 (3b) likely representing the foot and hand. A lateral patch (arrow heads) may correspond to mouth representation. (b) PV is expressed densely in layer 4 and somewhat in layer 3 of S1. (c) Section processed for SMI-32 has dense patches of labeled neurons, mainly in layer 3 of S1, but also in layer 5. The dorsolateral patches likely represent the foot and hand of S1, while a medial patch may represent the tail and hand limb. A most lateral patch could be S1 teeth or tongue cortex. (d) The S1 patches are also apparent in a sections processed for CO. Scale bar = 1 mm.
Cortex just rostral to area 3b includes the dysgranular area 3a and then the agranular area 4 or primary motor cortex (Figure 1). The mediolateral extents of these areas have a caudorostral tilt so that all three areas are present in favourable coronal brain sections (Figure 9). In Nissl preparations, area 3b clearly has a more pronounced layer 4 of densely packed cells than presumptive area 3a. In M1, a layer 4 is not distinguished from layer 3. VGluT2 is densely expressed in layer 4 of area 3b, and less so in area 3a, as area 3b receives dense activating inputs from the ventroposterior nucleus of the thalamus, and area 3a receives sensory inputs from the ventroposterior superior nucleus in primates (Kaas, 2011). The reduced VGluT2 staining in M1 likely reflects activating inputs from the motor thalamus. As a clear layer 4 is not usually recognized in agranular M1, we attribute the VGluT2 labeling to the deeper part of layer 3. Alternatively, this zone of presumptive thalamus inputs could be considered as an atypical layer 4 (Garcia-Cohezas and Barbas, 2014).
Figure 9.

Primary Motor cortex, M1, is located rostromedial to S1 and 3a (Figure 1). Coronal sections processed for Nissl (A, B) and VGluT2 (c, d) show the relative locations and architectonic features of these areas as shown at higher magnification (b, d). In Nissl preparations, layer 4 of small cells is well developed in area 3b, reduced in dysgranular 3a, and greatly reduced or absent in agranular M1. In VGluT2 preparations, layer 4 of area 3a is less darkly stained, and the layer 3–4 region of M1 is lightly stained Arrow heads mark boundaries. Scale bar in a and c = 1 mm, in b and d = 0.5 mm.
Auditory cortex
In primates, auditory cortex consists of two or more areas with histological characteristics of primary sensory cortex. These core areas are located in temporal cortex along the dorsal end of the lateral sulcus, extending somewhat into the caudal bank of the central sulcus (Kaas and Hackett 2000; Kaas, 2011). The most dorsal and caudal of these areas is A1, and the adjoining rostral core area is area R (for rostral). A small rostral temporal area, RT, is sometimes included in the core, and a most caudal area, CM, has some features of core cortex, but is not included in the core. In the mouse lemurs, the region we define as primary auditory cortex is mainly A1, although some or much of area R may be included (Figure 1). In VGluT2 preparations A1 is characterized by a densely labeled layer 4 (Figure 10 A). Medial and lateral to A1, the medial and lateral belt areas of secondary auditory cortex express lower levels of VGluT2. Sections processed for PV show A1 as an area with a densely labeled layer 4, with the PV label extending into lower layer 3 (Figure 10 c). The sections reacted for CO also have a more densely labeled layer 4, with label extending into layer 3 (Figure 10 e). In sections processed for NeuN, layer 4 is densely packed with neurons, as is layer 3, so there is only slight contrast between the somewhat denser layer 4 and layer 3 (Figure 10 d). In belt cortex, just lateral to A1, layer 4 is narrower and layer 3c is less packed with neurons, appearing as a lighter band. In sections processed for SMI-32, the dark layer 3 band of labeled pyramidal neurons and dendrites is broader and darker in A1 than in the adjoining lateral medial belt areas.
Figure 10.
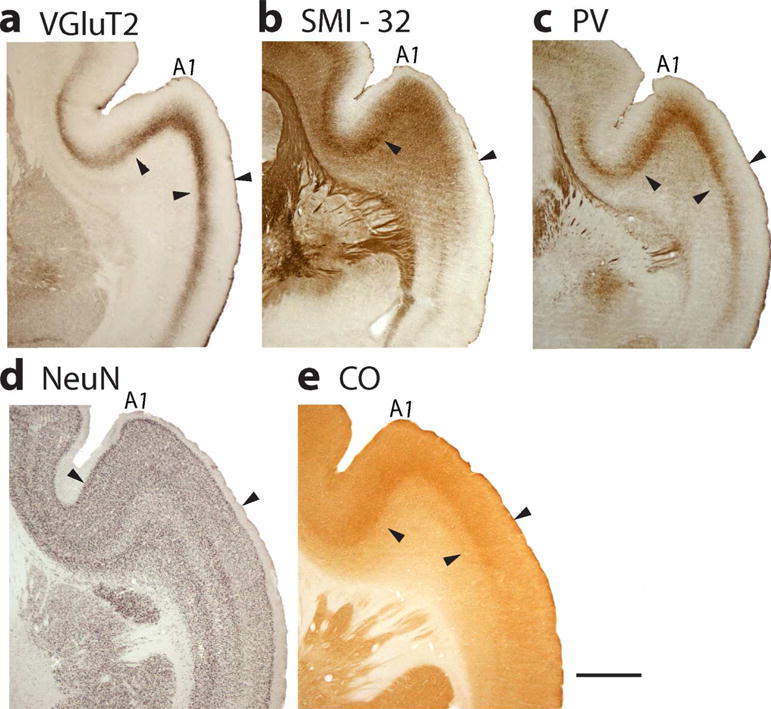
Primary auditory cortex, A1, is located in the dorsal temporal lobe where it extends into the lateral sulcus. Features that characterize primary sensory cortex in A1 are shown in coronal sections processed for (a) VGluT2, (b) SMI-32, (c) PV, (d) NeuN, and (e) CO. Scale bar = 1 mm.
DISCUSSION
Here we describe the histological features of sensory cortex in the mouse lemur, Microcebus murinus. This enables us to compare present results and conclusions with those of two excellent previous studies of the architecture of neocortex in Microcebus murinus, which were completed before the current range of effective histochemical procedures were available. We also relate present interpretations to the histological studies of sensory cortex in other primates, especially the extensively studied strepsirrhine primate, the African galagos, as well as the smaller New World marmoset monkeys, and the nocturnal New World owl monkeys. We start with a brief review of the phylogenetic relations of the primates under consideration.
Primate phylogeny
While the times of the divergences of different branches of the primate radiation have been difficult to resolve, there is widespread agreement concerning the relationships of extant primates, and the overall pattern of their evolution (see Perelman et al., 2011 for details and references). Early primates include the now extinct plesiadaptiforms and the euprimate ancestors of present day primates. The early euprimates split into strepsirrhine and haplorrhine lines as early as 80–90 Mya (but perhaps more recently), and the early strepsirrhines diverged into the lemuriformes and the lorisiformes 70–90 Mya. The lorisiformes include the pottos, lorises, and galagos. The lemuriformes survive as 97 or more species derived from a few members of a single species that reached and colonized Madagascar (Yoder et al., 1996). The many extant species of mouse lemurs are closely related. Surviving haplorrhine primates include the early branch that led to present day New World (platyrrhine) monkeys and Old World (haplorrhine) monkeys which gave rise to apes and humans.
Relation of present results to previous studies of mouse lemur cortex
Our present depiction of the extents and locations of sensory and motor areas of neocortex are reasonably consistent with the earlier observations of Zilles et al. (1979) and the much earlier observations of Le Gros Clark (1971) (Figure 11). Clark, more than anyone, called attention to the mouse lemur’s brain as a key to understanding early stages of primate evolution (Le Gros Clark, 1958). Given that Clark’s 1931 study was limited to cytoarchitecture, and occurred well before a modern understanding of cortical organization emerged from studies of brain connections and physiological mapping, Clark’s conclusions were impressive. Zilles’s study was also based on cytoarchitecture, but there was already physiological evidence for visual and other sensory and motor areas in primates to guide interpretations. Thus, Zilles et al (1979) included an area MT, previously described from microelectrode mapping of its representation of the contralateral visual hemifield and its myeloarchitecture and cytoarchitecture in owl monkeys (Allman and Kaas, 1971) and then in galagos (Allman et al., 1973). The existence of V1 and V2 as representations corresponding to areas 17 and 18 was already well established in primates (Allman and Kaas 1971; 1974), as was some impression of where primary auditory, somatosensory and motor areas are located. Yet, the Zilles et al (1979) study preceded the identification of primary somatosensory or auditory cortex by microelectrode mapping methods in any strepsirrhine primate (Sur et al., 1980; Brugge 1982). It was not yet clear that the primary somatosensory cortex (S1) of non-primates corresponds to only area 3b of primates (Kaas, 1982), and not areas 1–3 as Brodmann (1909) suggested for some non-primates, and even some primates (marmosets). In mouse lemurs, both Clark and Zilles identified a primary region that is similar in location to our S1, but our S1 extends further medial and onto the cortex of the medial wall, and extends into more of ventrolateral cortex. S1 is known to occupy these regions in galagos (Sur et al 1980; Kaas et al., 2006) and other primates (Iyengar et al., 2007; Manger et al., 1996). The locations of primary motor cortex (area 4) also differ somewhat from our description, perhaps because the histological features of M1 tend to become more muted where the face and mouth are represented in lateral M1 in primates (for galagos, see Wu et al., 2000). According to Zilles et al.(1979), primary auditory cortex extends further ventrally in the temporal lobe than shown here, which now seems unlikely following studies of auditory cortex in galagos (Brugge, 1982) and other primates (Kaas, 2011). The cortical area Te1 of primary auditory cortex in Zilles et al., (1979) probably includes area R, as well as RT, and perhaps more. Area 22 of Clark (1931) probably includes A1, R, RT and visual areas MT and MST, as all these areas have architectonic features of sensory cortex.
Figure 11.
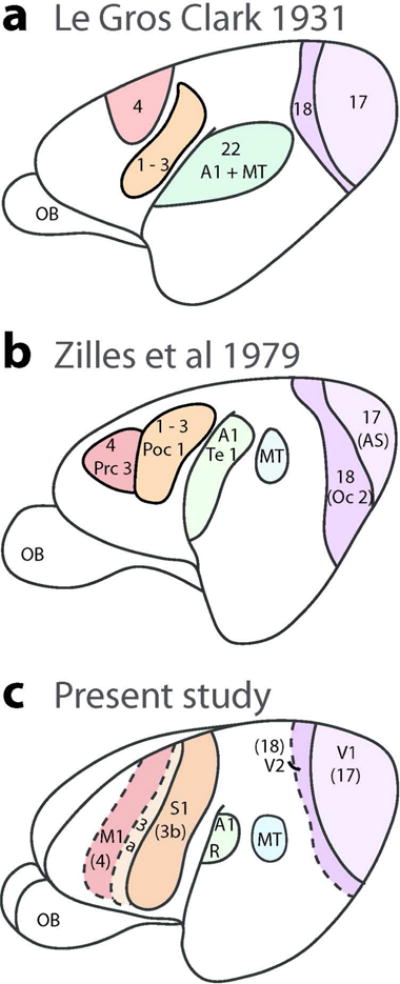
Comparisons of illustrated summary diagrams from earlier architecture studies in mouse lemurs (a and b) and present results (c). The earlier studies included more architectonics fields but the redrawn diagrams shown here only include proposed sensory and motor fields relevant to present results. Scale bar = 1 mm.
The layers and modules of area 17 in mouse lemurs
Our present architectonic results from area 17 of mouse lemurs shed further light on two issues of contention. Our results demonstrate for the first time that area 17 of mouse lemurs have the modular subunit of blobs and non-blobs surrounds. Blobs were first recognized in area 17 by Margaret Wong-Riley (1979) when she initiated a series of studies using cytochrome oxidase to describe cortical areas (see Horton, 1984). The dark ovals of CO expression in layer 3 of area 17 of some monkeys were called puffs and patches, but finally the term “blobs” won out (Casagrande and Kaas, 1999). Area 17 of primates receives M, P and K cell inputs from the magnocellular, parvocellular and koniocellular layers of the lateral geniculate (LGN) nucleus. These inputs are very much like the X, Y and W cell inputs of other mammals, and they probably reflect homologous subsystems (Casagrande and Kaas, 1994). The K neurons of the LGN terminate in the layer 3 blobs of area 17 but not in the non-blob surrounds (Lachica and Casagrande, 1992; Ding and Casagrande, 1997; 1998). The functions of the K subsystem remain under investigation but in diurnal primates with trichromatic vision, the blobs are implicated in blue cone color vision (Casagrande 1994). However, blobs are prominent in those nocturnal primates that have been studied, and early primates were likely nocturnal (Allman, 1988). Thus, blobs more broadly appear to be concerned with stimulus contrast. In diurnal primates, K cell subsystem may be sensitive to differences in color and in lightning (Shostak et al., 2002). If the restriction of K cell inputs to blobs in area 17 evolved in early primates, or their immediate ancestors, then blobs are likely present in all primates. Although blobs have not always been found in strepsirrhine primates (McGuiness et al., 1986), this could reflect the condition of postmortum brains from primates that died a natural death. Other strepsirrhine primates where blobs have been demonstrated include the dwarf lemur, Cheirogaleus medius, (Preuss and Kaas, 1996), which diverged from the mouse lemur line some 30 mya (Horvath and Willard, 2007). Blobs have also been described in galagos (Condo and Casagrande, 1990) and the loris, Nycticebus coucang (Preuss et al., 1993). Finally, present day tarsiers, as the earliest surviving other branch of the haplorrhine line of primate evolution, also have blobs in area 17 (Collins et al., 2005). Thus, our identification of blobs in mouse lemur brain here adds to the already impressive assay of comparative evidence that blobs evolved in early primates, or in their immediate ancestors, and have been retained in all extant primates.
Another issue is over the identities of layers in area 17 of primates. Brodmann (1909) established the now dominant view that layer 4 of primates has three prominent sublayers, 4a, 4b, and 4c. Hassler (1966) considered a greater range of primate species and concluded that sublayers 4a and 4b of Brodmann (1909) are actually sublayers of layer 3, and termed them 3b and 3c, as in the present study. The issue is important if one is comparing the features of area 17 layers across species, or across cortical areas. Brodmann (1909) concluded that layers 4a, 4b, and 4c of area 17 merged to form a single, homogenous layer 4 in area 18, while Hassler (1966) argued from his material that only Brodmann’s 4c merged with layer 4 of area 18, and that layer 4a terminated at the 17–18 border while layer 4b of area 17 merged with layer 3 of area 18. This issue has been more specifically investigated in a comparative study using VGluT2 immunoreactivity as a label in area 17 of seven primate species with results that support the conclusions of Hassler (Balaram and Kaas, 2014). Hassler (1966) also noted that layer 4 of tarsiers, has three sublayers, cell dense sublayers 4a and 4c, separated by a cell poor middle layer, 4b (all within Brodmann’s 4 c). In area 17 of mouse lemurs, we see that layer 4, as defined by Hassler, merges with layer 4 of area 18 (Figure 4 a). Moreover, this layer 4 of area 17 has two main sublayers, a narrow cell dense deep sublayer (4b), and an outer cell dense sublayer (4a) separated by a narrow cell-poor septum. These 4a and 4b sublayers correspond to those of 4c alpha and 4c beta of Brodmann’s layer 4. We use the terms 4a, septum, and 4b in mouse lemurs to facilitate comparisons with the common use of 4c beta and 4c alpha subdivisions of 4c of Brodmann in other primates. The septum of mouse lemurs becomes much less apparent as layer 4 of area 17 merges to form layer 4 of area 18 at the 17/18 border. The superficial and deep sublayers of layer 4 are innervated by axons from the magnocellular geniculate layers or the parvocellular geniculate layers, respectively (Casagrande and Kaas, 1994), and the cell poor middle sublayer of 4, when present, appears as a “no man’s land” between the two inputs. In VGluT2 preparations of area 17 in mouse lemurs, layer 4, as presently defined, has a densely labeled inner sublayer and a less densely labeled outer sublayer, suggesting that the innervation by the parvocellular LGN layers is denser than the innervation by the magnocellular LGN layers. These sublaminar differences in VGluT2 expression in mouse lemurs also exist in layer 4 of area 17 in other primates (Balaram and Kaas, 2014). PV preparations also reveal the two cell-dense sublayers of layer 4. PV expression in area 17 varies with species and age, but the expression of PV in adult marmosets (Spatz et al., 1994) and galagos (Wong and Kaas, 2010) is also darker in the inner than compared to the outer layer 4. In mouse lemurs, SMI 32 labels pyramidal cells in layer 3c and layer 5 while leaving layer 4 almost free of label (Figure 3). In marmosets, SMI-32 preparations also identified layer 4 of area 17, as presently defined, as a band nearly free of SMI- 32 that extends to form layer 4 of area 18 (Baldauf, 2005). Layer 5 and inner layer 3 (identified in marmosets as 4b) of area 17 were densely labeled, as in mouse lemurs, and these dense bands extended into layers 3c and 5 of area 18. In a similar manner, layers 3c (identified as 4b) and 5 were densely labeled by SMI-32 in macaques, while layer 4 (identified as 4C) was free of SMI- 32 (Hof and Morrison, 1995). Overall, the histological results from area 17 of mouse lemurs are consistent with the conclusion of Hassler (1966) that Brodmann (1909) misidentified sublayer of layer 3 in primates as sublayers of layer 4 (e.g., that 4a and 4b are 3b and 3c).
In contrast to layer 4, the three sublayers in layer 3 in area 17 of seven primate species are not as obvious in mouse lemurs (Balaram and Kaas, 2014; Collins et al., 2005). However, there is a hint of a cell-sparse layer 3c in parts of layer 3 near the 17/18 border (Figure 4 a).
MT exists in mouse lemurs
In the present study, we provide histological evidence for the existence of the middle temporal visual area, MT, in mouse lemur brains. MT was most clearly evident in VGluT2 and SMI-32 preparations, where VGluT2 was expressed densely in layer 4 of MT and SMI-32 was expressed densely in layers 3c and 5, in comparisons with adjoining cortex. As shown in Figure 11, Zilles et al., (1979) had previously identified MT in Nissl stained sections in mouse lemurs, while Frahm et al., 1998 identified MT in myelin-stained sections from mouse lemurs (not shown) and 26 other primate species. The dense myelination of MT led to its early discovery (Allman and Kaas, 1973). Our present results clearly support their previous evidence for MT in this smallest of primates, where the size of MT is roughly 10% of V1 and 1.8% of neocortex (Frahm et al., 1998). In large brained monkeys, MT is somewhat larger relative to V1, but smaller relative to neocortex.
The extent and discontinuities in S1 (3b)
We found primary somatosensory cortex of mouse lemurs to be more extensive than in previous descriptions based solely on Nissl preparations (Figure 11). In order to use a consistent architectonic term for this area in primates and other mammals, we refer to S1 as area 3b, instead of the 1–3 designation variously used by Brodmann (1909) in rabbits and elsewhere. In the depiction of the architectonic areas of a large lemur brain, Brodmann (1909), referred to the S1 region as area 1, while noting that this area 1 becomes S1 (3b) of monkeys. This variation in terminology reflects variations in the architectonic distinctiveness of S1 (3b), and confuses issues of homology. S1 has a distinctive functional organization across mammals, and its dense direct inputs from the somatosensory thalamus clearly identify it as homologous across species (Kaas, 1983). Thus, a consistent architectonic term is preferable. While Brodmann (1909) extended “S1” of the lemur brain onto the medial wall of the cerebral hemisphere, as in the present study, the area was not extended as far laterally as shown here. Studies of the somatotopy of the S1 representation in galagos and monkeys show that S1 (3b) extends ventrally in the frontal cortex, and that this portion of the area represents parts of the face, tongue, and teeth (Jain et al., 2001; Kaas et al., 2006). A lateral portion of S1 is characterized by a narrow septum of cell poor cortex that separates the hand and face representations, while other more ventral cell poor regions separate parts of the face representation, and representations of the teeth and tongue. Histological gaps in S1 (3b) of mouse lemurs (Figure 7), likely present discontinuities in the S1 representation, as they do in galagos and monkeys. Similar partial disruptions of the granular S1 representation by dysgranular cortex are also seen in rodents (Krubitzer et al., 1986; Dawson and Killackey, 1987). We assume that the cortex along the caudal border of S1 (3b) is the area 1 representation, which is likewise characterized by direct inputs from S1 (3b) and less pronounced histological features of sensory cortex (Kaas, 2004). All or most mammals have a strip of cortex along the caudal border of S1 that receives S1 projections, so this presumptive area 1 may be one of the older areas of mammalian cortex (Kaas, 2017). A narrow strip of dysgranular cortex separates S1 from primary motor cortex, M1, in mouse lemurs, as in other primates, including galagos (Wong and Kaas, 2010). This narrow strip of cortex is area 3a of primates and an area 3a likely exists in all or most mammals (Kaas, 2017).
Motor cortex
The full extent of primary motor cortex, M1 was difficult to delimit in the present study, as one of the features of M1, the large layer 5 pyramidal neurons, varies across the representation. These neurons are larger when they are most medial in M1 and project the longest distance in the spinal cord. In the small mouse lemurs, even the longest projections are short, and large pyramidal neurons were not evident. However, the extent of M1 (Figure 10 c) is consistent with the expected extent from M1 as identified by microstimulation in galagos (Wu et al, 2000). Both Le Gros Clark (1931) and Zilles et al (1979) identified an area 4 rostral to somatosensory cortex that overlaps our present M1 (Figure 11). Because of the distribution of large pyramidal neurons is variable across M1 in primates, a full correspondence between architectonic area 4 and M1 as determined by microstimulation, does not always occur (Stepniewska et al, 1993).
Auditory cortex
Auditory cortex in primates has been less intensively studied than the visual and somatosensory areas, but now there are commonly accepted models of auditory cortex organization in monkeys (e.g., Kaas and Hackett, 2000). These models include a core of primary areas, with a surrounding belt of eight secondary areas and parabelt regions of at least two areas. We are far from understanding how this model applies to strepsirrhine primates but here we have been able to identify a primary region in mouse lemurs that corresponds to A1, or A1 plus R, as in galagos (Brugge, 1981; Wong and Kaas, 2010).
What does the mouse lemur brain tell us about the evolution of neocortex?
Two trends characterize the evolution of brains from early mammals to humans: brains and especially neocortex got bigger, and there was an increase in the number of cortical areas (Kaas and Preuss, 2014, Kaas 2017). Early mammals were small, and had small brains with a neocortex that was nevertheless divided into as many as 20 cortical areas. Sensory representations took up much of the space. Early primates were not that much bigger, but their brains were already larger compared to body size, and much of this increase was in neocortex. Judging from galagos, (Wong and Kaas, 2010), early primates may have had as many as 50 cortical areas. Estimates for macaque monkeys are 180 or more (e.g. Van Essen et al., 2012a), and humans even higher (Van Essen et al., 2012b). In early mammals with few cortical areas, laminar and areal differences were less pronounced, while cellular, laminar and modular distinctions increased as the number of areas increased in present day primates with much larger brains. Although only a few cortical areas have been defined in mouse lemur cortex in the present study, proportionately more of the cortex in this species appears to be devoted to primary sensory and motor areas compared to most other anthropoid primates, and perhaps more than even the more comprehensively studied galagos (see Frahm et al., 1984, for area 17). Thus, mouse lemurs are likely to have fewer cortical areas than most or all monkeys, and be more similar to the more closely related galagos. Given their small brains, mouse lemur may have fewer cortical areas than any extant primates. As cortical areas must be of a certain size to contain enough neurons to mediate their basic functions, sizes of neocortex constrain the numbers and functions of cortical areas (Kaas, 2000; Catania et al., 1999, Cooper et al., 1993). Overall, mouse lemur brains are expected to closely resemble those of early primates.
Acknowledgments
This paper is dedicated to the memory of Vivien Casagrande who contributed greatly to the understandings of cortical and thalamic organization and function in her nearly 40 years at Vanderbilt University.
This research was supported by National Institute of Health, EY002686 (JHK), EY025422 (JHK), LABEX CORTEX (ANR-11-LABX-0042) of Université de Lyon (ANR-11-IDEX-0007) operated by the French National Research Agency (ANR) (H.K.), ANR-14-CE13-0033, ARCHI-CORE (H.K.), ANR-15-CE32-0016, CORNET (H.K.)., the CNRS (PF), MNHN (PF) and GIS IBISA (PF)
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Role of authors
M. P. Saraf processed brain sections, analyzed areal boundaries, reconstructed brain views and prepared figure illustrations. P. Balaram processed brain sections. Fabien Pifferi provided all animals used in this study. H. Kennedy, J. H Kaas and P. Balaram planned the study. All authors contributed to text.
References
- Allman JM, Kaas JH. Representation of the visual field in striate and adjoining cortex of the owl monkey (Aotus trivirgatus) Brain Research. 1971;35(1):89–106. doi: 10.1016/0006-8993(71)90596-8. [DOI] [PubMed] [Google Scholar]
- Allman JM, Kaas JH, Lane RH. The middle temporal visual area (MT) in the bush baby, Galago senegalensis. Brain Res. 1973;57:197–202. doi: 10.1016/0006-8993(73)90576-3. [DOI] [PubMed] [Google Scholar]
- Allman J. Variations in visual cortex organization in primates. Neurobiology of Neocortex. 1988:249–40. [Google Scholar]
- Azevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Herculano Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled up primate brain. Journal of Comparative Neurology. 2009;513(5):532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Balaram P, Kaas JH. Towards a unified scheme of cortical lamination for primary visual cortex across primates: insights from NeuN and VGLUT2 immunoreactivity. Frontiers in neuroanatomy. 2014;8:81. doi: 10.3389/fnana.2014.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaram P, Hackett TA, Kaas JH. Differential expression of vesicular glutamate transporters 1 and 2 may identify distinct modes of glutamatergic transmission in the macaque visual system. Journal of Chemical Neuroanatomy. 2013;50:21–38. doi: 10.1016/j.jchemneu.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaram P, Young NA, Kaas JH. Histological features of layers and sublayers in cortical visual areas V1 and V2 of chimpanzees, macaque monkeys, and humans. Eye and Brain. 2014;2014(6 Suppl 1):5. doi: 10.2147/EB.S51814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldauf ZB. SMI-32 parcellates the visual cortical areas of the marmoset. Neuroscience Letters. 2005;383(1):109–114. doi: 10.1016/j.neulet.2005.03.055. [DOI] [PubMed] [Google Scholar]
- Bloch JI, Boyer DM. Grasping primate origins. Science. 2002;298(5598):1606–1610. doi: 10.1126/science.1078249. [DOI] [PubMed] [Google Scholar]
- Bourne JA, Rosa MG. Hierarchical development of the primate visual cortex, as revealed by neurofilament immunoreactivity: early maturation of the middle temporal area (MT) Cereb Cortex. 2006;16:405–414. doi: 10.1093/cercor/bhi119. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. J.A Barth; Leipzig: 1909. [Google Scholar]
- Brugge JF. auditory areas in primates. In: Woolsey CW, editor. Cortical sensory organization, Vol.3, multiple auditory areas. Humana Press; Clitton NJ: 1982. pp. 59–70. [Google Scholar]
- Casagrande VA. A third visual pathway to primate V1. Trends in Neurosciences. 1994;17:305–310. doi: 10.1016/0166-2236(94)90065-5. [DOI] [PubMed] [Google Scholar]
- Bullier J, Kennedy H. Projection of the lateral geniculate nucleus onto cortical area V2 in the macaque monkey. Experimental Brain Research. 1983;53(1):168–172. doi: 10.1007/BF00239409. [DOI] [PubMed] [Google Scholar]
- Casagrande VA, Kaas JH. The afferent, intrinsic, and efferent connections of primary visual cortex in primates. In: Peters Alan, Rockland Kathlene., editors. cerebral cortex. Vol. 10. Plenurm Press; New York: 1994. pp. 201–259. [Google Scholar]
- Catania KC, Lyon DC, Mock OB, Kaas JH. Cortical organization in shrews: evidence from five species. Journal of Comparative Neurology. 1999;410(1):55–72. [PubMed] [Google Scholar]
- Cerkevich CM, Qi HX, Kaas JH. Thalamic input to representations of the teeth, tongue, and face in somatosensory area 3b of macaque monkeys. Journal of Comparative Neurology. 2013;521(17):3954–3971. doi: 10.1002/cne.23386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CE, Hendrickson A, Kaas JH. Overview of the visual system of Tarsius. The Anatomical Record. 2005;287(1):1013–1025. doi: 10.1002/ar.a.20263. [DOI] [PubMed] [Google Scholar]
- Condo GJ, Casagrande VA. Organization of cytochrome oxidase staining in the visual cortex of nocturnal primates (Galago crassicaudatus and Galago senegalensis): I. Adult patterns. Journal of Comparative Neurology. 1990;293(4):632–645. doi: 10.1002/cne.902930408. [DOI] [PubMed] [Google Scholar]
- Cooper HM, Kennedy H, Magnin M, Vital Durand F. Thalamic projections to area 17 in a prosimian primate, Microcebus murinus. Journal of Comparative Neurology. 1979;187(1):145–167. doi: 10.1002/cne.901870109. [DOI] [PubMed] [Google Scholar]
- Dawson DR, Killackey HP. The organization and mutability of the forepaw and hindpaw representations in the somatosensory cortex of the neonatal rat. Journal of Comparative Neurology. 1987;256:246–256. doi: 10.1002/cne.902560205. [DOI] [PubMed] [Google Scholar]
- Ding Y, Casagrande VA. The distribution and morphology of LGN K pathway axons within the layers and CO blobs of owl monkey V1. Visual Neuroscience. 1997;14(4):691–704. doi: 10.1017/s0952523800012657. [DOI] [PubMed] [Google Scholar]
- Ding Y, Casagrande VA. Synaptic and neurochemical characterization of parallel pathways to the cytochrome oxidase blobs of primate visual cortex. Journal of Comparative Neurology. 1998;391(4):429–443. doi: 10.1002/(sici)1096-9861(19980222)391:4<429::aid-cne2>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Dunn RH, Rose KD, Rana RS, Kumar K, Sahni A, Smith T. New euprimate postcrania from the early Eocene of Gujarat, India, and the strepsirrhine–haplorhine divergence. Journal of Human Evolution. 2016;99:25–51. doi: 10.1016/j.jhevol.2016.06.006. [DOI] [PubMed] [Google Scholar]
- Frahm HD, Stephan H, Baron G. Comparison of brain structure volumes in Insectivora and primates. V. Area striata (AS) Journal fur Hirnforschung. 1984;25:537–557. [PubMed] [Google Scholar]
- Frahm HD, Zilles K, Schleicher A, Stephan H. The size of the middle temporal area in primates. Journal fur Hirnforschung. 1998;39(1):45–54. [PubMed] [Google Scholar]
- Gebo DL. A shrew sized origin for primates. American Journal of Physical Anthropology. 2004;125(S39):40–62. doi: 10.1002/ajpa.20154. [DOI] [PubMed] [Google Scholar]
- Hassler R. Comparative anatomy of the central visual systems in day-and night active primates. In: Hassler R, Stephen S, editors. Evolution of the Forebrain. Thieme; Stuttgart: 1966. pp. 419–434. [Google Scholar]
- Herculano-Houzel S. The Human Advantage: A New Understanding of how Our Brain Became Remarkable. MIT Press; Cambridge, Mass: 2016. [Google Scholar]
- Herculano-Houzel S, Collins CE, Wong P, Kaas JH. Cellular scaling rules for primate brains. Proceedings of the National Academy of Sciences. 2007;104(9):3562–3567. doi: 10.1073/pnas.0611396104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. Neurofilament protein defines regional patterns of cortical organization in the macaque monkey visual system: a quantitative immunohistochemical analysis. Journal of Comparative Neurology. 1995;352(2):161–186. doi: 10.1002/cne.903520202. [DOI] [PubMed] [Google Scholar]
- Homman Ludiye J, Manger PR, Bourne JA. Immunohistochemical parcellation of the ferret (Mustela putorius) visual cortex reveals substantial homology with the cat (Felis catus) Journal of Comparative Neurology. 2010;518(21):4439–4462. doi: 10.1002/cne.22465. [DOI] [PubMed] [Google Scholar]
- Horton JC. Cytochrome oxidase patches: a new cytoarchitectonic feature of monkey visual cortex. Philos Trans R Soc Lond (Biol) 1984;304:199–253. doi: 10.1098/rstb.1984.0021. [DOI] [PubMed] [Google Scholar]
- Horvát S, Gămănuț R, Ercsey-Ravasz M, Magrou L, Gămănuț B, Van Essen DC, Kennedy H. Spatial embedding and wiring cost constrain the functional layout of the cortical network of rodents and primates. PLoS Biology. 2016;14(7):e1002512. doi: 10.1371/journal.pbio.1002512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath JE, Willard HF. Primate comparative genomics: lemur biology and evolution. Trends in Genetics. 2007;23(4):173–182. doi: 10.1016/j.tig.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Horvath JE, Weisrock DW, Embry SL, Fiorentino I, Balhoff JP, Kappeler P, Yoder AD. Development and application of a phylogenomic toolkit: resolving the evolutionary history of Madagascar’s lemurs. Genome Research. 2008;18(3):489–499. doi: 10.1101/gr.7265208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar S, Qi HX, Jain N, Kaas JH. Cortical and thalamic connections of the representations of the teeth and tongue in somatosensory cortex of new world monkeys. Journal of Comparative Neurology. 2007;501(1):95–120. doi: 10.1002/cne.21232. [DOI] [PubMed] [Google Scholar]
- Jain N, Qi HX, Catania KC, Kaas JH. Anatomic correlates of the face and oral cavity representations in the somatosensory cortical area 3b of monkeys. Journal of Comparative Neurology. 2001;429(3):455–468. doi: 10.1002/1096-9861(20010115)429:3<455::aid-cne7>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Somatosensory system. In: Mai JK, Paxinos G, editors. The Human Nervous System. Third. Oxford: Elsevier; 2011. pp. 1074–1109. [Google Scholar]
- Kaas JH. Evolution of nervous systems. 2nd. Oxford: Elsevier; 2017. The organization of neocortex in early mammals; pp. 87–101. Vol. 2, Suzana Herculano-Houzel, vol. edition. The nervous systems of early mammals and their evolution. [Google Scholar]
- Kaas JH. What, if anything, is SI? Organization of first somatosensory area of cortex. Physiological Reviews. 1983;63(1):206–231. doi: 10.1152/physrev.1983.63.1.206. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Why is brain size so important: Design problems and solutions as neocortex gets bigger or smaller Brain and Mind. 2000;1(1):7–23. [Google Scholar]
- Kaas JH. The evolution of auditory cortex: the core areas. In: Winer JA, Schreiner CE, editors. The Auditory Cortex. Springer; New York: 2011. pp. 407–427. [Google Scholar]
- Kaas JH, Hackett TA. Subdivisions of auditory cortex and processing streams in primates. Proceedings of the National Academy of Sciences. 2000;97(22):117911799. doi: 10.1073/pnas.97.22.11793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaas JH, Preuss TM. Human Brain Evolution. In: Squire LR, editor. Fundamental Neurosci. 4. Oxford: Elsevier; 2014. pp. 901–918. Chpt.42. [Google Scholar]
- Kaas JH, Qi HX, Iyengar S. Cortical network for representing the teeth and tongue in primates. The Anatomical Record. 2006;288(2):182–190. doi: 10.1002/ar.a.20267. [DOI] [PubMed] [Google Scholar]
- Kappeler PM. Lemur origins: rafting by groups of hibernators? Folia Primatologica. 2000;71(6):422–425. doi: 10.1159/000052741. [DOI] [PubMed] [Google Scholar]
- Kaskan PM, Franco ECS, Yamada ES, de Lima Silveira LC, Darlington RB, Finlay BL. Peripheral variability and central constancy in mammalian visual system evolution. Proceedings of the Royal Society of London B: Biological 636 Sciences. 2005;272(1558):91–100. doi: 10.1098/rspb.2004.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk EC, Daghighi P, Macrini TE, Bhullar BAS, Rowe TB. Cranial anatomy of the Duchesnean primate Rooneyia viejaensis: new insights from high resolution computed tomography. Journal of Human Evolution. 2014;74:82–95. doi: 10.1016/j.jhevol.2014.03.007. [DOI] [PubMed] [Google Scholar]
- Krubitzer LA, Sesma MA, Kaas JH. Microelectrode maps, myeloarchitecture, and cortical connections of three somatotopically organized representations of the body surface in the parietal cortex of squirrels. Journal of Comparative Neurology. 1986;250(4):403–430. doi: 10.1002/cne.902500402. [DOI] [PubMed] [Google Scholar]
- Lachica EA, Casagrande VA. The morphology of the collicular and retinal axons ending on small relay (W-like) cells of the primate lateral geniculate nucleus. Visual Neuroscience. 1999;10:403–418. doi: 10.1017/s0952523800004648. [DOI] [PubMed] [Google Scholar]
- Le Gros Clark WE. The Antecendants of Man. 3rd. Quandrangle books; Chicago: 1971. [Google Scholar]
- Le Gros Clark WE. The Brain of Microcebus murinus. Journal of Zoology. 1931;101(2):463–486. [Google Scholar]
- Lin CS, Wagor E, Kaas JH. Projections from the pulvinar to the middle 646 temporal visual area (MT) in the owl monkey, Aotus trivirgatus. Brain Research. 1974;76(1):647 145–149. doi: 10.1016/0006-8993(74)90520-4. [DOI] [PubMed] [Google Scholar]
- Morecraft RJ, Rockland KS, Van Hoesen GW. Localization of area prostriata and its projection to the cingulate motor cortex in the rhesus monkey. Cerebral Cortex. 10(2):192–203. doi: 10.1093/cercor/10.2.192. (200) [DOI] [PubMed] [Google Scholar]
- Frahm HD, Stephan H, Baron G. Comparison of brain structure volumes in insectivora and primates: V. Area striata (AS) Journal fur Hirnforschung. 1984;25(5):537–557. [PubMed] [Google Scholar]
- Mittermeier RA. Lemurs of Madagascar: Conservation International Tropical Field Guide Series. Conservation International; Washington, DC: 2006. [Google Scholar]
- Paxinos G, Watson C, Peptrides M, Rosa M, Tokuno H. The marmoset brain in stereotaxic coorinates. Elsevier; Oxford: 2012. [Google Scholar]
- Perelman P, Johnson WE, Roos C, Seuánez HN, Horvath JE, Moreira MA, Schneider MPC. A molecular phylogeny of living primates. PLoS Genetics. 2011;7(3):e1001342. doi: 10.1371/journal.pgen.1001342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM, Kaas JH. Cytochrome oxidase ‘blobs’ and other characteristics of primary visual cortex in a lemuroid primate, Cheirogaleus medius. Brain, Behavior 661 and Evolution. 1996;47(2):103–112. doi: 10.1159/000113231. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Beck PD, Kaas JH. Areal, modular, and connectional organization of visual cortex in a prosimian primate, the slow loris (Nycticebus coucang) Brain, Behavior and Evolution. 1993;42(6):321–335. doi: 10.1159/000114169. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Casagrande VA, Preuss T, Kaas JH. Visual field representation in striate and prestriate cortices of a prosimian primate (Galago garnetti) Journal of Neurophysiology. 1997;77(6):3193–3217. doi: 10.1152/jn.1997.77.6.3193. [DOI] [PubMed] [Google Scholar]
- Sanides FRIEDRICH. Representation in the cerebral cortex and its areal lamination patterns. The structure and function of nervous tissue. 1972;5:329–453. [Google Scholar]
- Sherman SM, Guillery RW. Distinct functions for direct and transthalamic corticocortical connections. Journal of neurophysiology. 2011;106(3):1068–1077. doi: 10.1152/jn.00429.2011. [DOI] [PubMed] [Google Scholar]
- Shostak Y, Ding Y, Mavity-Hudson J, Casagrande VA. Cortical synaptic arrangements of the third visual pathway in three primate species: Macaca mulatta, Saimiri sciureus, and Aotus trivirgatus. Journal of Neuroscience. 2002;22(7):2885–2893. doi: 10.1523/JNEUROSCI.22-07-02885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silcox MT, Benham AE, Bloch JI. Endocasts of Microsyops (Microsyopidae, Primates) and the evolution of the brain in primitive primates. Journal of human evolution. 2010;58(6):505–521. doi: 10.1016/j.jhevol.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Silcox MT, Dalmyn CK, Bloch JI. Virtual endocast of Ignacius graybullianus (Paromomyidae, Primates) and brain evolution in early primates. Proceedings of the National Academy of Sciences. 2009;106(27):10987–10992. doi: 10.1073/pnas.0812140106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spatz WB, Illing RB, Weisenhorn DM. Distribution of cytochrome oxidaseand parvalbumin in the primary visual cortex of the adult and neonate monkey, Callithrix jacchus. Journal of Comparative Neurology. 1994;339(4):519–534. doi: 10.1002/cne.903390405. [DOI] [PubMed] [Google Scholar]
- Stepniewska I, Collins CE, Kaas JH. Reappraisal of DL/V4 boundaries based on connectivity patterns of dorsolateral visual cortex in macaques. Cerebral Cortex. 2004;15(6):809–822. doi: 10.1093/cercor/bhh182. [DOI] [PubMed] [Google Scholar]
- Sur M, Nelson RJ, Kaas JH. Representation of the body surface in somatic koniocortex in the prosimian Galago. Journal of Comparative Neurology. 1980;189(2):381–40. doi: 10.1002/cne.901890211. [DOI] [PubMed] [Google Scholar]
- Sussman RW, Tab Rasmussen D, Raven PH. Rethinking primate origins again. American Journal of Primatology. 2013;75(2):95–106. doi: 10.1002/ajp.22096. [DOI] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J. Cortical parcellations of the macaque monkey analyzed on surface-based atlases. Cerebral Cortex. 2011;22(10):2227–2240. doi: 10.1093/cercor/bhr290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Essen DC, Glasser MF, Dierker DL, Harwell J, Coalson T. Parcellations and hemispheric asymmetries of human cerebral cortex analyzed on surface-based atlases. Cerebral Cortex. 2011;22(10):2241–2262. doi: 10.1093/cercor/bhr291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley M. Changes in the visual system of monocularly sutured system or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Research. 1979;171:111–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- Wong P, Kaas JH. Architectonic subdivisions of neocortex in the gray squirrel (Sciurus carolinensis) The Anatomical Record. 2008;291(10):1301–1333. doi: 10.1002/ar.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Kaas JH. Architectonic subdivisions of neocortex in the tree shrew (Tupaia belangeri) The Anatomical Record. 2009;292(7):994–1027. doi: 10.1002/ar.20916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Kaas JH. Architectonic subdivisions of neocortex in the Galago (Otolemur garnetti) The Anatomical Record. 2010;293(6):1033–1069. doi: 10.1002/ar.21109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CWH, Bichot NP, Kaas JH. Converging evidence from microstimulation, architecture, and connections for multiple motor areas in the frontal and cingulate cortex of prosimian primates. Journal of Comparative Neurology. 2000;423(1):140–177. doi: 10.1002/1096-9861(20000717)423:1<140::aid-cne12>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Yoder AD, Campbell CR, Blanco MB, dos Reis M, Ganzhorn JU, Goodman SM, Ralison JM. Geogenetic patterns in mouse lemurs (genus Microcebus) reveal the ghosts of Madagascar’s forests past. Proceedings of the National Academy of Sciences. 2016:8049–8056. doi: 10.1073/pnas.1601081113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HH, Chaplin TA, Davies AJ, Verma R, Rosa MG. A specialized area in limbic cortex for fast analysis of peripheral vision. Current Biology. 2012;22(14):13511357. doi: 10.1016/j.cub.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Zilles K, Rehkämper G, Stephan H, Schleicher A. A quantitative approach to cytoarchitectonics. Anatomy and Embryology. 1979;157(1):81–103. doi: 10.1007/BF00315642. [DOI] [PubMed] [Google Scholar]


