Abstract
Objective
Identify factors associated with benefit of middle ear implants (MEIs) as compared to conventional hearing aids (HAs).
Study design
Independent review of audiological data from a multicenter prospective FDA clinical trial. Pre-operative and post-operative earphone, unaided/aided/implanted pure-tone thresholds and word recognition scores were evaluated.
Results
Ninety-one subjects were included in this study. Mean word recognition was better with MEIs than with HAs (81.8 ±12.0% vs. 77.6 ±14.6%, p=0.035). Word recognition with MEIs showed a low positive correlation with word recognition measured with earphones (r=0.25, p=0.016) and a moderate positive correlation with aided word recognition (r=0.42, p<0.001). Earphone word recognition alone was not predictive of MEI benefit over HA benefit (r=0.09, p=0.41), but differences between scores with earphone and HAs (earphone-aided differences, EAD) were (r=0.62, p<0.011). As compared to those with –EADs, subjects with +EADs showed greater improvement in word recognition from unaided to implanted and from HAs to implanted (p<0.0001). Using the 95% CI for word recognition scores, 16 subjects showed significantly higher scores with the MEI than with HAs; of which, 14 had +EAD.
Conclusion
Word recognition benefit derived from conventional HAs and MEIs from this large, multi-center FDA trial provides further evidence of the importance of aided word recognition in clinical decision making, such as determining candidacy for and success with MEIs.
Keywords: middle ear implant, hearing aid, hearing, sensorineural hearing loss, word recognition
Introduction
Sensorineural hearing loss (SNHL) is the most common sensory deficit, affecting up to 42% of adults older than 65 years in the United States1 and 538 million adults worldwide.2 Despite significant advancements in hearing-assistive technologies, SNHL continues to have a major impact on the lives of those affected. Although most patients with mild to moderate SNHL can benefit from conventional hearing aids (HAs), as many as 60% of patients with moderate to severe SNHL cannot effectively use HAs due to complaints of occlusion, distortion, or feedback.3,4 These patients may be in the difficult position of not communicating optimally with HAs and not meeting hearing loss criteria for cochlear implantation. Active middle ear implants (MEIs) have been developed to potentially fill this gap in patient needs.
Currently available evidence suggests that speech recognition ability with MEIs is comparable to scores with HAs, whereas the subjective experience is improved with MEIs. 5 However, the higher financial costs and the need for a surgical procedure make it important to identify patients who are likely to have significantly greater benefit with MEIs than with HAs. Unfortunately, few data are available from the standard clinical audiologic test battery to predict this difference in communication benefit.
The standard audiologic test battery includes measures of patients' pure-tone thresholds and word recognition scores measured at high speech levels in quiet under earphones. Unfortunately, these results provide little predictive value of HA benefit for individual patients.6 Results of McRackan et al.6 from a multicenter FDA clinical trial demonstrate the value to clinical decision making of unaided and aided word recognition scores as predictors of communication abilities with hearing aids, rather than word recognition measured under earphones. In addition, the “earphone-to-aided difference” (EAD), was defined as the word recognition score measured under earphones minus the aided word recognition score. Positive EAD (+EAD) was recognized as a marker for patients with poorer hearing and poorer aided word recognition, but relatively high word recognition scores measured under earphones due to higher presentation levels. In the current study, data from the same FDA clinical trial were used to assess pure-tone thresholds, unaided and aided measures of word recognition, and EAD as predictors of patients' benefit from MEIs and HAs.
Methods
Data for these analyses were obtained from the multicenter Phase III FDA clinical trial for the Soundtec Direct Drive Hearing System, which is now known as the Maxum® Hearing Implant (Ototronix, Houston, TX). All subject information was de-identified prior to acquisition and Ototronix personnel did not participate in any experimental planning, analysis of data, or review of publication drafts. Subjects (n=95) were 21 to 80-year-old fluent English speakers with >2 year history of bilateral symmetrical SNHL without fluctuation. The specific inclusion criteria for pure-tone average (PTA; average of air-conduction thresholds at 1000, 2000, and 4000 Hz), pure-tone thresholds, bone conduction thresholds, word recognition scores, and exclusion criteria have been previously described.6 Subjects were required to have at least 6 months of HA use prior to enrollment. HAs for all subjects were fit by study audiologists according to NAL-R7 gain targets prior to testing. Individuals who met study inclusion criteria but whose HA settings were not consistent with NAL-R targets had the necessary adjustments made by study audiologists during their first visit. Candidates were then required to use their newly programmed HAs at least 30 days prior to enrollment.
Maxum® Hearing Implant
The Maxum® hearing implant is a semi-implantable hearing device that utilizes electromagnetic energy for amplification. An ear mold placed in the external ear canal contains a microphone that detects sound, a processor that amplifies and converts sound energy into an electrical signal, and an electromagnetic coil that receives the signal and generates an electromagnetic field in the middle ear space. A magnet is surgically attached to the stapes and vibrates synchronously with the original input, transmitting enhanced oscillations onto the oval window.8,9 All patients received unilateral implants. All postoperative outcomes were reported 20 weeks after surgery.
Outcome Measures
Pure-tone thresholds were measured with earphones and warble-tone thresholds were measured in the sound field at 250, 500, 1000, 2000, 3000, 4000, and 6000 Hz under four conditions: earphones (pre- and postoperatively), unaided in the sound field (pre- and postoperatively), aided with HAs in the sound field (pre-operatively only), and implanted with the MEI in the sound field (Table 1). For the remainder of this report in reference to pure-tone or warble-tone thresholds, the terms earphone, unaided, aided, and implanted will refer to these four conditions, respectively (see Table 2). For all sound field testing the unimplanted ear was occluded.
Table 1. Description of the testing conditions.
| Condition | Methods | Speech level |
|---|---|---|
| Earphone | Unaided with either insert earphone or supra-aural headphones | 40 dB above SRT |
| Unaided | Sound field without hearing aids | 63 dB SPL |
| Aided | Sound field with hearing aids | 63 dB SPL |
| Implanted | Sound field with unilateral middle-ear implant | 63 dB SPL |
Table 2. Conditions (columns) in which a given outcome was measured.
| Preoperative | Postoperative | ||||||
|---|---|---|---|---|---|---|---|
| Outcome Measure | Earphone | Unaided | Aided | Earphone | Unaided | Aided | Implanted |
| Pure-Tone Thresholds | X | X | |||||
| Warble-Tone Thresholds | X | X | X | x | |||
| Word Recognition Score | X | X | X | X | X | x | |
For the current study using the FDA clinical trial data, the primary outcome measure was the word recognition score in quiet using 50-word lists from the Northwestern University Test Number 6 (NU-6).10 Word recognition was measured in four conditions: earphone (with either supra-aural headphones or insert earphones) with words presented at 40 dB above the speech reception threshold (SRT) (40 dB SL), unaided in the sound field with words presented at 63 dB SPL, aided with HAs in the sound field with words presented at 63 dB SPL, and aided with the MEI in the sound field with words presented at 63 dB SPL. For the remainder of this report in reference to word recognition scores, the terms earphone, unaided, aided, and implanted will refer to these four conditions, respectively.
Associative and Correlative Queries
The following preoperative factors were evaluated: SRT, PTA (average pure-tone thresholds at 500, 1000, 2000, and 3000 Hz), sex, age at implantation, duration of hearing loss, duration of HA use, and earphone, unaided, and aided word recognition scores.
Subjects were also assigned to two groups based on EAD (+EAD group had higher word recognition scores in the earphone condition than in the aided condition and –EAD group had word recognition scores in the earphone condition that were equal to or lower than in the aided condition). Word recognition was compared between +EAD and –EAD groups for the following conditions: (1) unaided vs. aided, (2) unaided vs. implanted, and (3) aided vs. implanted.
Statistical Analyses
Data analyses were performed with SPSS 24.0 (SPSS, Inc, Chicago, Illinois) and SigmaPlot 12.5 (Systat Software Inc., San Jose, California). Audiologic information and demographic variables were described with summary statistics. Continuous variables were summarized by the mean ±1 standard deviation (SD) and range where appropriate. Nominal variables were summarized by frequency and percentage. Comparisons of continuous variables between groups were performed with independent t-tests or a Mann–Whitney rank-sum tests with Bonferroni correction. Finally, a correlation model was used to determine associations among all variables (SRT, earphone PTA, sex, age at implantation, duration of hearing loss, duration of HA use, preoperative earphone, unaided, aided, implanted, word recognition, and EAD). After Bonferroni correction, a p value of <0.003 was considered statistically significant for subject baseline characteristics. A p-value of <0.05 was considered statistically significant for all other statistical tests.
Results
A total of 95 individuals met inclusion and exclusion criteria and were enrolled in the FDA multi-center trial. Data from one subject were excluded from the current analyses due to word recognition scores in the aided condition that were more than 3 SDs below the mean. Data from an additional three subjects were excluded due to lack of postoperative MEI word recognition scores, leaving 91 subjects. Subject characteristics are described in Table 3.
Table 3.
Subject characteristics for all subjects and for +EAD and –EAD groups, including counts (percent), means ±1 SD, and p values. EAD indicates earphone to aided difference; HA, hearing aid.
| All | +EAD | -EAD | p value | |
|---|---|---|---|---|
| n | 91 | 45 (49.5%) | 46 (50.5%) | - |
| Male | 59 (64.8%) | 27 (45.8%) | 32 (54.2%) | 0.33Ø |
| Female | 32 (36.0%) | 18 (56.3%) | 14 (43.8%) | |
| Age at implantation (yr) | 65.1 ±11.6 | 66.9 ±9.7 | 63.4 ±13.1 | 0.15 |
| Duration of hearing loss (yr) | 15.0 ±10.4 | 14.6 ±10.7 | 15.4 ±10.3 | 0.71 |
| Duration of HA use (yr) | 7.2 ±6.1 | 7.2 ±6.5 | 7.2 ±5.7 | 1.0 |
chi-square analysis of relationship between sex and EAD group.
Mean (±SD) word recognition scores in the preoperative unaided condition was 42.2 ±28.1% (Table 4). As expected, word recognition was higher in the implanted condition than in the unaided condition for 89 of 91 subjects (98.0%). This MEI benefit (implanted vs. unaided) ranged from 2% to 90% (mean improvement 40.8 ±25.7%). One subject showed no benefit and word recognition after implantation for one subject decreased by 24%. Unfortunately, operative and post-operative clinical history was not available for the patient with the large decrease in word recognition. This subject's earphone PTA increased by only 5 dB after surgery, but their word recognition score measured under earphones decreased from 74 to 22%.
Table 4.
Hearing outcomes for all subjects and for +EAD and –EAD groups, including counts (percent), means ±SD, and p values. EAD indicates earphone to aided difference; PTA, pure-tone average; SRT, speech reception threshold.
| All | +EAD | -EAD | p value | |
|---|---|---|---|---|
| n | 91 | 45 (49.4%) | 46 (50.5%) | - |
| Preoperative Earphone PTA (dB HL) | 46.1 ± 6.9 | 47.7 ±6.5 | 44.5 ±7.0 | 0.025 |
| Preoperative Unaided PTA (dB HL) | 45.8 ±8.3 | 48.9 ±7.3 | 42.7 ±8.1 | <0.001 |
| Aided PTA (dB HL) | 28.9 ±5.7 | 31.6 ±5.1 | 23.6 ±5.0 | <0.001 |
| Implanted PTA (dB HL) | 24.9 ±5.6 | 26.3 ±5.0 | 23.6 ±5.9 | 0.025 |
| Preoperative Earphone SRT (dB HL) | 38.5 ±12.2 | 42.1 ±11.0 | 34.9 ±12.4 | 0.004 |
| Implanted SRT (dB HL) | 42.3 ±13.4 | 46.2 ±12.8 | 38.5 ±12.9 | <0.001 |
| Word recognition earphone (%) | 81.5 ±10.7 | 86.0 ±8.8 | 77.1 ±10.7 | <0.001 |
| Word recognition unaided (%) | 42.2 ±28.1 | 30.0 ±24.2 | 54.1 ±26.6 | <0.001 |
| Word recognition aided (%) | 77.6 ±14.6 | 68.6 ±14.2 | 86.4 ±8.3 | <0.001 |
| Word recognition implanted (%) | 81.0 ±12.0 | 79.0 ±12.0 | 84.5 ±11.5 | 0.028 |
| EAD (%) | 3.9 ±17.1 | 17.4 ±12.6 | -9.3 ±8.2 | <0.001 |
Very similar results were observed with HAs. Aided word recognition was higher than the unaided condition for 87 of 91 subjects (95.6%), with HA benefit ranging from 2 to 76% (mean improvement 37.0 ±20.1%). Three subjects showed no improvement and word recognition for one subject decreased by 2%.
Mean word recognition in the implanted condition (81.8 ±12.0%) was significantly higher than in the aided condition (77.6 ±14.6%, p=0.035). In Figure 1, word recognition scores in the implanted condition are plotted against word recognition scores in the unaided condition (Fig. 1A) and in the aided condition (Fig. 1B) with 95% confidence intervals (CI) for the NU-6 word recognition materials.11 Word recognition in the implanted condition showed low positive correlations with word recognition in earphone (r=0.25, p=0.016, not shown) and unaided (r=0.33, p=0.001; Fig. 1A) conditions, and showed moderately positive correlations with word recognition in the aided condition (r=0.42, p<0.001; Fig. 1B). Although mean scores in the implanted condition were found to be significantly higher than in the aided condition and these scores were significantly positively correlated, a majority of score differences fall within the 95% CI (Fig. 1B).
Figure 1.
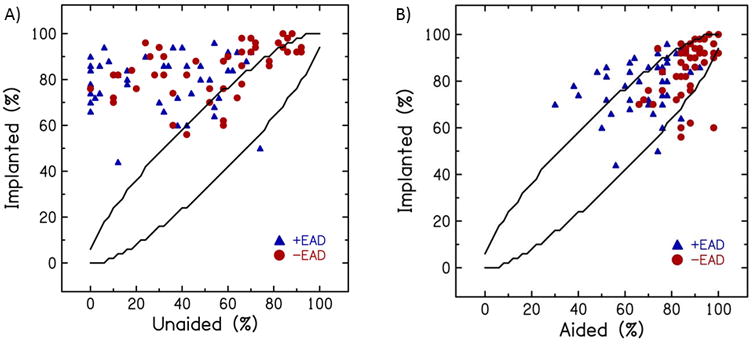
Word recognition scores in the implanted condition plotted against word recognition scores in the unaided (A) and aided conditions (B). Lines indicate 95% confidence intervals.
Implanted word recognition showed a moderate negative correlation with earphone PTA (r=-0.35, p=0.001, not shown) and SRT (r=-0.36, p=0.001, not shown), consistent with poorer word recognition for subjects with more hearing loss. No statistically significant correlations were found between implanted word recognition and age at implantation, sex, duration of hearing loss, duration of HA use, or duration of current HA use (all p>0.05).
Differences between implanted and aided word recognition were negatively correlated with unaided (r= -0.41, p<0.001) and aided (r= -0.66, p<0.001) word recognition. That is, with poorer unaided and aided word recognition, the improvement in word recognition provided by the implant vs. HAs increased. In contrast, earphone word recognition scores were not predictive of MEI benefit over HAs (r=0.09, p=0.41). Pre-operative earphone SRT, PTA, age at implantation, sex, duration of hearing loss, and duration of HA use showed no significant associations with MEI-HA word recognition differences (all p>0.05).
Using the upper 95% CI, word recognition in the implanted condition was significantly better than in the aided condition for 16 of 91 subjects, 14 of which were in the +EAD group. These 16 subjects also had poorer thresholds and word recognition than the remaining 75 subjects, as indicated by their significantly higher unaided SRTs (46.2 dB HL vs. 36.8 dB HL; p=0.03), poorer unaided word recognition (17.6% vs. 48.5%; p<0.001), and poorer aided word recognition (58.7% vs. 81.6%; p<0.001). There was no difference in pre-operative earphone PTAs between subjects whose scores fell within or higher than the upper CI (p=0.11).
Differences between implanted and aided word recognition were positively correlated with EAD (r= 0.62, p<0.001) (Fig. 2). That is, with larger EADs (earphone-aided differences), the improvement in word recognition provided by the implant vs. HAs increased. Table 3 displays subject characteristics and Table 4 displays hearing outcomes for all subjects and for +EAD and -EAD groups. No significant differences were observed between groups for age at implantation, duration of hearing loss and duration of HA use. For subjects in the –EAD group (a marker for better hearing and better aided word recognition), mean MEI benefit (MEI vs. unaided) was 30.4 ±23.4%, which is not statistically different from the benefit with HAs (35.4 ±21.1%, p=0.23). For subjects in the +EAD group (a marker for poorer hearing and poorer aided word recognition), mean MEI benefit (implant vs. unaided) was 49.0 ±26.7%, which is larger than the benefit with HAs (38.5 ±21.1%, p=0.039). As displayed in Figure 3, the +EAD group had a larger difference in word recognition from unaided to MEI than the –EAD group (+49.0% vs. +30.4%; p<0.001) and a larger difference from HAs to MEI (+10.4% vs. -1.9%; p<0.001); no EAD group difference was observed for the difference between the unaided and aided condition (+38.5% vs. +32.3%; p=0.16).
Figure 2.
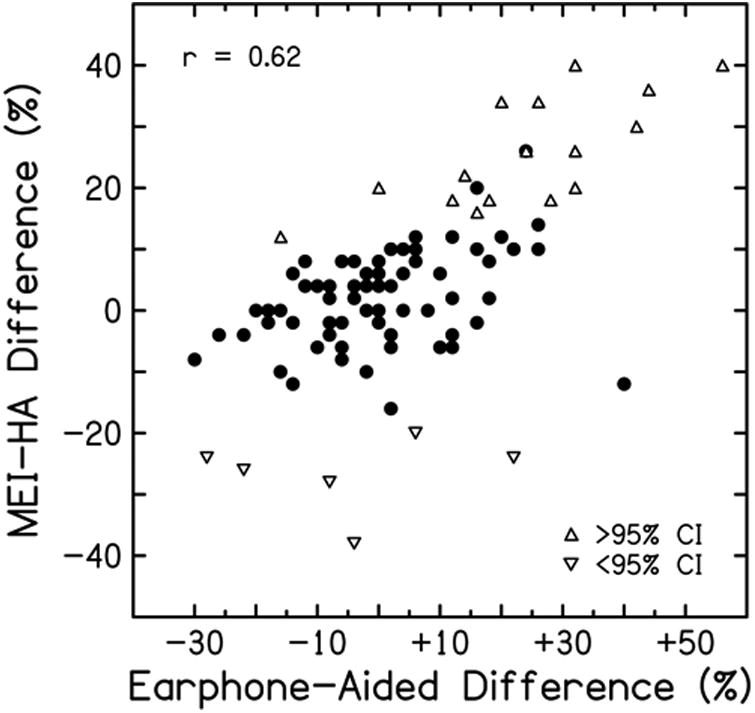
Differences between word recognition scores with a middle-ear implant and with hearing aids (MEI-HAs) plotted against earphone-aided differences (see text). Triangles symbols identify subjects whose word recognition scores with MEIs was significantly better than score with HAs (upward triangles) or significantly worse than scores with HAs, (downwards triangles) using 95% confidence intervals (CI) for word recognition scores.
Figure 3.
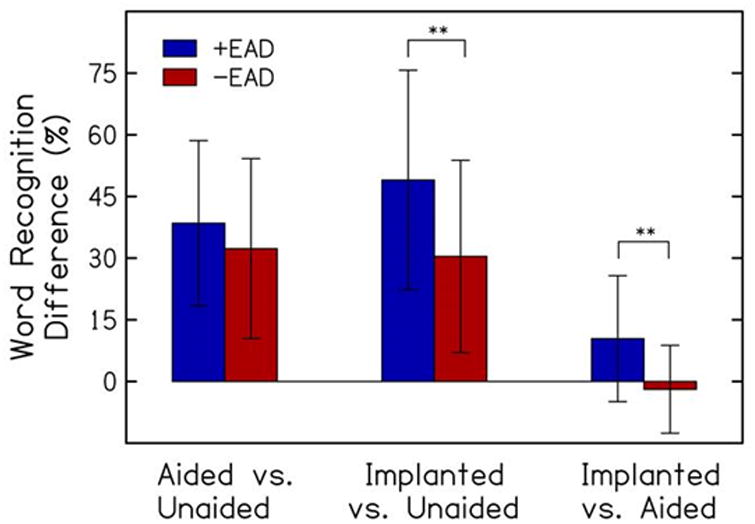
Comparisons of subjects by EAD (earphone-aided difference), including mean (±SD) change in word recognition score from unaided to aided, unaided to implanted, and aided to implanted conditions(**p<0.001).
Discussion
Due to increased costs and the required surgical intervention, there is a need to identify patients who are likely to have significantly greater benefit with MEIs than with HAs. Outcomes using the various MEIs have been widely reported,8,12-16 but there are little data to pre-operatively identify factors that predict significant advantage of MEIs over HAs for an individual patient. Standard audiological test batteries include measures of word recognition ability under earphones (headphones or inserts). Aided word recognition testing is typically reserved for patients who are being considered for cochlear implantation. The authors have previously shown that word recognition measured under earphones at high levels do not predict aided word recognition ability and recommended that aided word recognition be used to measure and monitor HA benefit.6 The current analysis shows similar results for MEIs with low correlation between earphone and MEI word recognition scores.
The results with MEIs support an additional utility of aided word recognition. Word recognition scores with the MEI compared to HAs were positively correlated with the EAD (r= 0.62) and negatively correlated with aided word recognition (r=-0.65). That is, subjects whose aided word recognition was poorer than that measured under earphones were also those who achieved the greatest improvement with the MEI relative to HAs. Further, of the 25 subjects with EAD values >+12%, 22 (88.0%) had better word recognition with the MEI than with HAs. In addition, of the 16 subjects whose MEI-HA score differences exceeded the 95% CI, 14 had +EADs (mean EAD = 24.8). This suggests that a large +EAD may serve as a marker for patients who are likely to have significantly greater benefit with MEIs than with HAs.
Compared to the current study, Dyer et al.17 recently found a much stronger correlation between patient earphone and Maxum implant word recognition scores (r=0.86). There are several potential reasons for this difference between studies. First, the two studies differ in their overall design. Dyer et al. is a retrospective analysis of a single institution's small cohort (n=10), whereas the current study is evaluating data from a large, multi-institutional, prospective FDA clinical trial with strict enrollment criteria. Second, patients' baseline earphone word recognition differs between the studies. Two subjects in Dyer et al. had earphone word recognition scores less than 60%, whereas 60% was the minimal value allowed for inclusion in the FDA clinical trial. The impact of these differences is currently unknown, but it is likely that these two study populations are not homogenous. No additional audiological data were available in Dyer et al. to allow further comparison.
One limitation of the current study is that all speech recognition testing was performed in quiet, which may not adequately represent patients' real-world communication experiences. The utility of measures of speech recognition in noise to predict MEI-HA benefit is beyond the scope of the current study and will be included in future analyses of data from the FDA clinical trial. The main limitation of the current study is that participants enrolled in the FDA clinical trial were interested in MEIs, possibly due to dissatisfaction with their HAs; this could introduce selection bias. Nevertheless, the patients enrolled in the clinical trial demonstrated significant improvement with HAs as compared to the unaided condition (assessed by word recognition), and for most subjects word recognition scores were equivalent to scores predicted based on hearing loss and speech levels.6 In addition, for a retrospective analysis of prospectively collected data, conclusions are limited by the data that were collected at the time of FDA clinical trial. Finally, the clinical trial was limited to one MEI model in a middle-aged to older adult population, so conclusions cannot necessarily be generalized to other MEIs or younger or older patients.
Conclusions
Because middle ear implantation carries with it considerable financial costs and a risks inherent in a middle ear surgical procedure, it is important to identify prior to implantation patients who are likely to have significantly greater benefit with MEIs than with conventional HAs. These subjects had poorer aided thresholds and poorer aided word recognition as compared to word recognition measured under earphones using typical clinical procedures. These results suggest that the EAD (earphone-aided difference) may help guide the selection of surgical candidates and highlights the importance of measuring and monitoring aided word recognition in routine audiologic evaluations.
Acknowledgments
This publication was supported by a K12 award through the South Carolina Clinical & Translational Research (SCTR) Institute, with an academic home at the Medical University of South Carolina, NIH/NCATS Grant Number UL1TR001450, NIH funding from NIH/NIDCD R01 DC000184 and NIH/NIDCD P50 DC000422, and a grant from the Doris Duke Foundation.
Footnotes
The authors have no conflicts of interest to declare.
The authors have no financial disclosures to make.
References
- 1.Nash SD, Cruickshanks KJ, Klein R, et al. The prevalence of hearing impairment and associated risk factors: the Beaver Dam Offspring Study. Arch Otolaryngol Head Neck Surg. 2011;137(5):432–439. doi: 10.1001/archoto.2011.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stevens G, Flaxman S, Brunskill E, et al. Global and regional hearing impairment prevalence: an analysis of 42 studies in 29 countries. Eur J Public Health. 2013;23(1):146–152. doi: 10.1093/eurpub/ckr176. [DOI] [PubMed] [Google Scholar]
- 3.Tysome JR, Moorthy R, Lee A, Jiang D, O'Connor AF. Systematic review of middle ear implants: do they improve hearing as much as conventional hearing AIDS? Otol Neurotol. 2010;31(9):1369–1375. doi: 10.1097/MAO.0b013e3181db716c. [DOI] [PubMed] [Google Scholar]
- 4.Boeheim K, Pok SM, Schloegel M, Filzmoser P. Active middle ear implant compared with open-fit hearing aid in sloping high-frequency sensorineural hearing loss. Otol Neurotol. 2010;31(3):424–429. doi: 10.1097/MAO.0b013e3181cabd42. [DOI] [PubMed] [Google Scholar]
- 5.Kahue CN, Carlson ML, Daugherty JA, Haynes DS, Glasscock ME. Middle ear implants for rehabilitation of sensorineural hearing loss: a systematic review of FDA approved devices. Otol Neurotol. 2014;35(7):1228–1237. doi: 10.1097/MAO.0000000000000341. [DOI] [PubMed] [Google Scholar]
- 6.McRackan TR, Ahlstrom JB, Clinkscales WB, Meyer TA, Dubno JR. Clinical Implications of Word Recognition Differences in Earphone and Aided Conditions. Otol Neurotol. 2016;37(10):1475–1481. doi: 10.1097/MAO.0000000000001205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrne D, Dillon H. The National Acoustic Laboratories' (NAL) new procedure for selecting the gain and frequency response of a hearing aid. Ear Hear. 1986;7(4):257–265. doi: 10.1097/00003446-198608000-00007. [DOI] [PubMed] [Google Scholar]
- 8.Pelosi S, Carlson ML, Glasscock ME. Implantable hearing devices: the Ototronix MAXUM system. Otolaryngol Clin North Am. 2014;47(6):953–965. doi: 10.1016/j.otc.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Dyer K. The SOUNDTEC direct system: surgical technique. Cochlear Implants Int. 2005;6(1):69–72. doi: 10.1179/cim.2005.6.Supplement-1.69. [DOI] [PubMed] [Google Scholar]
- 10.Tillman TW, Carhart R. Tech Rep SAM-TR. 1966. An expanded test for speech discrimination utilizing CNC monosyllabic words. Northwestern University Auditory Test No. 6. SAM-TR-66-55; pp. 1–12. [DOI] [PubMed] [Google Scholar]
- 11.Thornton AR, Raffin MJ. Speech-discrimination scores modeled as a binomial variable. J Speech Hear Res. 1978;21(3):507–518. doi: 10.1044/jshr.2103.507. [DOI] [PubMed] [Google Scholar]
- 12.Lee HJ, Lee JM, Choi JY, Jung J. Evaluation of Maximal Speech Intelligibility With Vibrant Soundbridge in Patients With Sensorineural Hearing Loss. Otol Neurotol. 2017;38(9):1246–1250. doi: 10.1097/MAO.0000000000001537. [DOI] [PubMed] [Google Scholar]
- 13.Iwasaki S, Usami SI, Takahashi H, et al. Round Window Application of an Active Middle Ear Implant: A Comparison With Hearing Aid Usage in Japan. Otol Neurotol. 2017;38(6):e145–e151. doi: 10.1097/MAO.0000000000001438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kraus EM, Shohet JA, Catalano PJ. Envoy Esteem Totally Implantable Hearing System: phase 2 trial, 1-year hearing results. Otolaryngol Head Neck Surg. 2011;145(1):100–109. doi: 10.1177/0194599811401709. [DOI] [PubMed] [Google Scholar]
- 15.Hough JV, Matthews P, Wood MW, Dyer RK. Middle ear electromagnetic semi-implantable hearing device: results of the phase II SOUNDTEC direct system clinical trial. Otol Neurotol. 2002;23(6):895–903. doi: 10.1097/00129492-200211000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Silverstein H, Atkins J, Thompson JH, Gilman N. Experience with the SOUNDTEC implantable hearing aid. Otol Neurotol. 2005;26(2):211–217. doi: 10.1097/00129492-200503000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Dyer RK, Spearman M, Spearman B, McCraney A. Evaluating speech perception of the MAXUM middle ear implant versus speech perception under inserts. Laryngoscope. 2017 doi: 10.1002/lary.26605. [DOI] [PubMed] [Google Scholar]


