Abstract
African-American (AA) women with high-grade serous ovarian carcinoma (HGSOC) have worse outcomes compared to women of European descent. Although the discrepancy is partially attributed to differences in access to care, the tumor immune microenvironment may also contribute. Expression of targetable immune regulatory molecules such as PD-L1 and IDO is of particular interest as it may help guide therapy in this population. Using cases from the largest study of AA women with ovarian cancer, the African American Cancer Epidemiology Study, we characterized PD-L1 and IDO expression in 112 HGSOC. Immunohistochemistry for PD-L1, IDO, CD8, FOX3p, and CD68 was performed. PD-L1 and IDO were scored as the percentage of positive tumor cells and tumor-associated immune cells. CD8 and FOX3p counts were averaged across 10 high-power fields. Cox proportional hazards regression was used to evaluate the association between PD-L1 and IDO expression and survival. Tumor cells were positive for PD-L1 and IDO in 29% and 58% of cases, respectively. The majority showed <10% staining, and no cases exceeded 25% positivity. The majority of PD-L1-positive cases co-expressed IDO. PD-L1 and IDO expression was associated with higher CD8 and FOX3p counts (p<0.05). No association was observed between PD-L1 and IDO and survival. In summary, expression of PD-L1 and IDO is seen in a subset of HGSOC from AA women and is correlated with elevated lymphocyte infiltration. While PD-L1 and IDO co-expression suggests a role for dual immunotherapy, diffuse expression of PD-L1 and IDO is rare, invoking caution regarding the potential for immunotherapeutic response.
BACKGROUND
Ovarian cancer is the 5th deadliest malignancy among women, with an estimated 14,080 deaths in 2017.1,2 The vast majority of deaths are due to high-grade serous ovarian carcinoma (HGSOC). Survival has increased only modestly despite advances in treatment, with a relative 45% 5-year survival among women of European descent diagnosed in 2005–2011 compared to 36% in those diagnosed in 1975–1977.2 Survival rates remain even lower among African-American (AA) women, with 38% survival for those diagnosed in 2005–2011.2,3 This disparity can be partially attributed to differences in treatment access and quality of care, but these variables do not fully account for the outcome discrepancy.4–6
Improving the prognosis for AA women with ovarian cancer requires a multifocal effort, including not only careful epidemiologic characterization, but also directed study of tumor biology. Immune context is increasingly understood to contribute to tumor behavior.7,8,9 It may be that racial survival discrepancies in HGSOC could be partially attributable to differences in the immune milieu. AAs have been shown to have elevated inflammatory biomarkers relative to individuals of European descent,10,11 and differences in inflammatory markers have been linked to altered cancer outcomes.12,13 Genetic variability in inflammatory genes has also been shown to impact ovarian cancer risk.14–16
Understanding the immune context of tumors is also important given the recent rise of immunotherapy.17–21 Immune checkpoint blockades have proven effective, particularly in the context of an elevated inflammatory milieu.17,20,22 Targets include programmed cell death-1 (PD-1) and its partner, programmed cell death ligand-1 (PD-L1/CD274), and evidence suggests that inhibiting this axis could be useful in ovarian cancer treatment.23–27 Another mechanism of immunotherapy is through enzymatic interference. Indoleamine 2,3 dioxygenase (IDO) is an immune modulatory enzyme of interest for ovarian cancer therapy because it is expressed in over half of ovarian carcinomas, has been correlated with adverse outcomes, and has clinically available antagonists.28–30
Immune regulatory molecule expression has not been well investigated in ovarian carcinomas from AA women due to the paucity of studies containing a sufficient proportion of these patients. The African American Cancer Epidemiology Study (AACES) is a multi-center population-based case-control study of ovarian cancer in AA women and represents the largest available cohort of this patient population.31 This study population therefore represents a unique opportunity to evaluate clinically actionable components of the immune microenvironment of women with HGSOC who are underrepresented in existing literature. We herein complete a directed assessment of PD-L1 and IDO expression alone and in the context of CD8+ cytotoxic T cell and FOX3p+ regulatory T cell infiltrates in HGSOCs from AA women enrolled in the AACES study in order to 1) understand how tumoral immune evasion might contribute to poor prognosis in AA women with this cancer and 2) address potential immunotherapeutic vulnerability in this population.
METHODS
Study Population
Cases were selected from AACES, the largest population based case-control study of AA women with epithelial ovarian cancer. Study enrollment procedures and methods have been discussed elsewhere.31 Briefly, newly diagnosed cases of invasive epithelial ovarian cancer were identified between December 1, 2010 and December 31, 2015 using a rapid case ascertainment approach at cancer registries and gynecologic oncology departments and hospitals in 11 geographic locations (Alabama, Georgia, Illinois, Louisiana, Michigan, North Carolina, New Jersey, Ohio, South Carolina, Tennessee, and Texas). Patients were eligible for the study if they were 20–79 years of age, self-reported AA race, resided in one of the 11 geographic locations, and were able to complete a baseline telephone interview in English. A total of 601 cases were enrolled in AACES.
We requested formalin-fixed paraffin-embedded (FFPE) tumor blocks for the primary ovarian carcinoma from the diagnosing facility, and for each tissue block received, twenty-five sections were cut, with one section hematoxylin and eosin (H&E) stained. All H&E slides and original pathology reports were centrally reviewed by expert pathologists at Duke University (R.B.) and the University of Virginia (UVA) (A.M.) to confirm diagnosis and stage. Although still ongoing, as of August 2017, the pathology review had been completed for 445 cases and of those, 294 are HGSOCs. For the purposes of this analysis, we selected 124 cases with sufficient tissue for immunohistochemical (IHC) staining; cases were selected chiefly based on the availability of an adequate number of unstained sections for complete analysis.
Vital status was ascertained through an annual systematic collection of follow-up data from cancer registries as well as annual contact with AACES participants. Follow-up time was calculated as the number of days from the date of diagnosis to the date of death or the date last known to be alive.
Immunohistochemistry
All IHC staining was performed at the UVA Biorepository and Tissue Research Facility. Whole section slides of FFPE tumor were stained on a Dako instrument with PD-L1 (Spring Biosciences, SP142, 1:200 dilution) and IDO (Sigma Prestige, HPA 023072, 1:2,000 dilution) antibodies. This PD-L1 clone has been previously validated in this laboratory on a large array of non-small cell lung cancer against results obtained on the Dako 22C3 antibody using the Dako platform with satisfactory results (e.g. no discrepant cases at clinically relevant thresholds). Although clinical standards for IDO expression do not exist, this IDO clone has performed reliably in our research setting in a variety of tissue types. Placental tissue was used as a positive control, with circumferential villous staining without significant background stromal staining required for validity for the PD-L1 immunostain and villous vascular staining required for the IDO immunostain.
PD-L1 was considered positive when circumferential membranous staining was identified. IDO was considered positive when staining was identified in the cytoplasmic compartment. PD-L1 and IDO were scored separately in tumor cells and in tumor-associated immune cells (chiefly lymphocytes and macrophages) and were given a score from 0–5 for each compartment. The 5 scoring categories were generated to include all clinically meaningful cut-offs for all immunotherapy drugs (0: 0%, 1: 1–5%, 2: 6–10%, 3: 11–25%, 4: 26–50%, 5: >50%). These stains were interpreted in concert with a separate slide stained for CD68 (KP1, company: Novus Biologicals, 1:200 dilution) to ensure that tumor vs. macrophage staining was interpreted appropriately.
Dual staining was also performed for the lymphocyte markers CD8 (Dako, C8/144B, 1:100 dilution) and FOX3p (Abcam, clone 236A/E7, 1:50 dilution) to characterize the intratumoral cytotoxic and regulatory T cell populations, respectively. Intratumoral lymphocytes were defined as immunopositive cells touching tumor cells; therefore, peritumoral immune cells located in the stroma but not directly in contact with the tumor were not enumerated. This method was chosen to control for the fact that not all tumor sections contained stroma; some consisted exclusively of tumor cells. CD8 and FOX3p counts were performed manually and averaged across 10 high power fields measuring 0.55mm in diameter (10× 22mm ocular, 40× objective). The initial field selected for each case was chosen based on the area of highest lymphocyte density, after which fields were selected randomly.
Statistics
The dataset was restricted to include only those cases with data for the immune modulatory markers (PD-L1 and IDO) as well as the lymphocyte makers (CD8 and Fox3p)., N=112. CD8 and Fox3p counts were evaluated categorically using the median count as the cut-point (median value for CD8: 12.95 and Fox3p: 3.20). Due to the small sample size, the scoring categories of PD-L1 and IDO expression were collapsed into 2 levels: positive vs. no staining. We used Chi square tests to evaluate whether PD-L1 and IDO expression differed by counts of CD8 and Fox3p and whether PD-L1 and IDO positivity differed according to demographic and clinical characteristics of AACES patients.
Cox-proportional hazards regression models were used to estimate hazard ratios (HR) and 95% confidence intervals (CI) for the relationship between PD-L1 and IDO expression and overall survival. The following a priori confounders were adjusted for in all models: age at diagnosis (continuous, years), stage (stage I-II and III-IV), region of residence (Northeast: Michigan, Illinois, New Jersey and Ohio, Southeast: Georgia, Tennessee, North Carolina and South Carolina, South: Alabama, Texas and Louisiana), and residual disease (optimal debulking: <1 cm residual tumor diameter after cytoreductive surgery or CA-125 < 35 after adjuvant chemotherapy, suboptimal debulking: ≥1 cm residual tumor diameter after cytoreductive surgery or CA-125 ≥ 35 after adjuvant chemotherapy). We also repeated these analyses stratified by high and low CD8 and Fox3p counts to evaluate whether the effects of PD-L1 and IDO on outcome are dependent on the presence of tumor infiltrating lymphocytes. SAS Version 9.4 (Cary, North Carolina) was used to complete all analyses.
RESULTS
A total of 112 women with high-grade serous ovarian carcinomas who were enrolled in AACES were included (Table 1). The mean age at diagnosis was 59.7 ± 10.0 years, and the majority were residents of the Southeast region of the U.S. at the time of diagnosis (69% in Georgia, Tennessee, North Carolina or South Carolina) and were diagnosed with late stage disease (83% Stage III-IV). Treatment data were available for 104 cases; the majority of women underwent an optimal debulking surgery followed by adjuvant chemotherapy. Approximately 42% of the women were deceased and the mean survival duration was 2.9 ± 1.5 years.
Table 1.
Patient Characteristics (N=112)
| Patient Characteristics | n (%) |
|---|---|
| Age at diagnosis, mean (SD) | 59.7 (10.0) |
| Region of residencea | |
| Northeast | 28 (25) |
| Southeast | 77 (69) |
| South | 7 (6) |
| Stage | |
| I–II | 19 (17) |
| III–IV | 92 (83) |
| Unknown | 1 |
| Neoadjuvant chemotherapy | |
| Yes | 15 (16) |
| No | 79 (84) |
| Unknown | 18 |
| Adjuvant chemotherapy | |
| Yes | 81 (93) |
| No | 6 (7) |
| Unknown | 25 |
| Residual diseaseb | |
| Optimal debulking | 57 (61) |
| Suboptimal debulking | 36 (39) |
| Unknown | 19 |
| Survival duration (years), mean (SD) | 2.9 (1.5) |
| Vital Status | |
| Deceased | 47 (42) |
| Alive | 65 (58) |
SD: standard deviation.
The Northeast region includes Michigan, Illinois, New Jersey and Ohio. The Southeast region includes Georgia, Tennessee, North Carolina and South Carolina. The South region includes Alabama, Texas and Louisiana.
Residual disease is defined as optimal if <1cm tumor diameter after cytoreductive surgery or a CA-125 <35 after the last cycle of the first round of adjuvant chemotherapy.
PD-L1 Expression
PD-L1 expression was uncommon in tumor cells. The majority of cases (71%) were entirely negative for PD-L1 within the tumor cells, while 29% of cases showed patchy positivity involving 1–10% of cells (Figure 1, Table 2). No cases exceeded 10% staining in tumor cells. Immunopositivity was often concentrated at the infiltrating edge of the tumor and/or in most robust in areas of high lymphocyte infiltration, while other cases showed only rare scattered positive cells (Figure 2–3). Tumor-associated immune staining for PD-L1 was more common, with 73% of cases showing some positivity in tumor-associated lymphocytes and/or macrophages, and 24% displaying >10% positivity. (Figure 4, Table 2) Immune cell staining was most commonly seen in macrophages that were either infiltrating or immediately juxtaposed to the tumor, including but not limited to congregations of macrophages in necrotic areas. (Figure 2–3)
Figure 1.
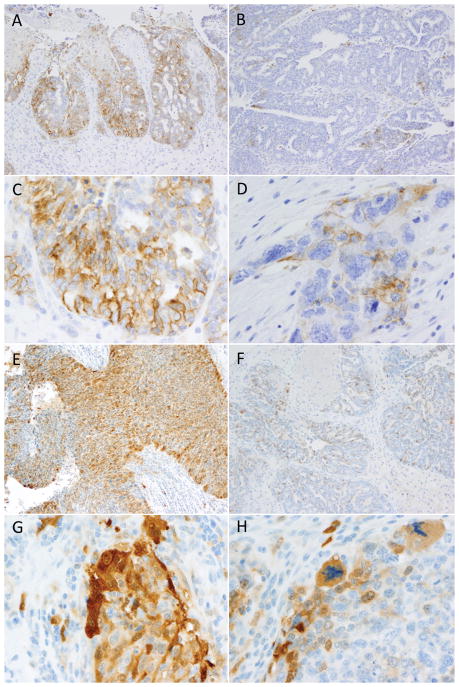
Examples of PD-L1 and IDO tumoral expression patterns in high grade serous ovarian carcinomas. Tumoral PD-L1 expression was seen in a 29% of high-grade serous ovarian carcinomas and never exceeded 10% overall positivity. The case illustrated in plate A had 10% positivity which was concentrated in the focus pictured here; the remainder of the tumor was predominantly PD-L1-negative. In contrast, most positive cases showed a pattern similar to the case illustrated in plate B, with only scattered patches of expression. Crisp membranous staining was required for positivity, as is illustrated in C-D, as was the exclusion of macrophage staining based on comparison with the CD68 stain. IDO expression was more common with 58% of cases showing tumoral staining. Occasional cases showed up to 25% positivity, as pictured in plate E; however, as was observed with PD-L1, such strongly positive swathes of tumor were admixed with larger negative areas. The most common pattern of expression was patchy positivity as pictured in plat e F. Clear cytoplasmic staining as depicted in G–H was required for diagnosis, as was exclusion of macrophages using the CD68 comparison slide.
Table 2.
Distribution of Immune Markers (N=112)
| Immune Markers | n (%) |
|---|---|
| PD-L1 Tumor | |
| 0% | 80 (71) |
| 1–5% | 22 (20) |
| 6–10% | 10 (9) |
| 11–25% | 0 (0) |
| 26–50% | 0 (0) |
| >50% | 0 (0) |
| PD-L1 Immune | |
| 0% | 30 (27) |
| 1–5% | 31 (28) |
| 6–10% | 24 (21) |
| 11–25% | 18 (16) |
| 26–50% | 9 (8) |
| >50% | 0 (0) |
| IDO Tumor | |
| 0% | 47 (42) |
| 1–5% | 43 (38) |
| 6–10% | 15 (13) |
| 11–25% | 7 (6) |
| 26–50% | 0 (0) |
| >50% | 0 (0) |
| IDO Immune | |
| 0% | 51 (46) |
| 1–5% | 53 (47) |
| 6–10% | 6 (5) |
| 11–25% | 1 (1) |
| 26–50% | 1 (1) |
| >50% | 0 (0) |
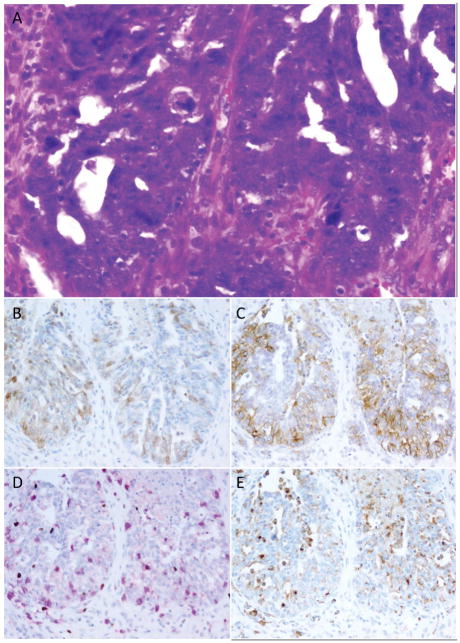
Figure 2. Relationship between PD-L1, IDO, and tumor-associated lymphocytes and macrophages (Example 1).
High-grade serous ovarian carcinomas often showed immune modulatory molecule expression in areas of increased lymphocytic infiltration. This case (A) shows patchy IDO (B) and stronger PD-L1 (C) expression in an area of high density tumor-infiltrating lymphocytes (D; pink=CD8, brown=FOX3p). Some IDO and PD-L1-positive cells correlated with the distribution of CD68-positive tumor-associated macrophages (E) however some tumoral staining was also present for both markers.
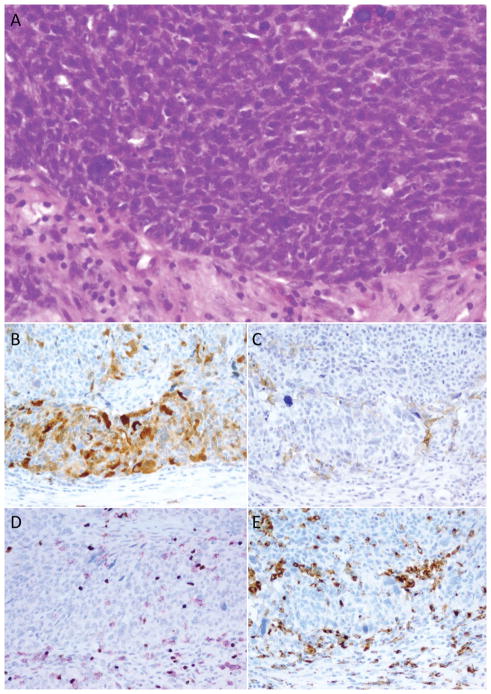
Figure 3. Relationship between PD-L1, IDO, and tumor-associated lymphocytes and macrophages (Example 2).
Some high-grade serous ovarian carcinomas showed bands of immune modulatory molecule expression at the tumor periphery. This case (A) showed a dense border of tumoral positivity for IDO (A) at the tumor’s infiltrating edge, where both cytotoxic and regulatory T cells were congregated (C). In contrast, PD-L1 staining (B) was limited to scattered intratumoral cells favored to be macrophages based on their overlap with CD68 (D) positivity.
Figure 4.
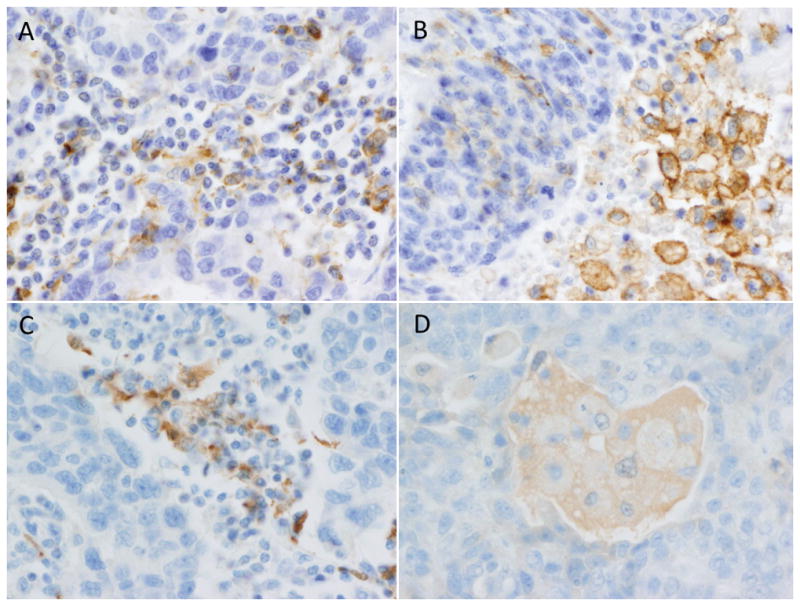
Examples of PD-L1 and IDO expression in tumor-associated immune cells. PD-L1 was frequently positive in tumor-associated immune cells with 73% showing some reactivity in tumor-associated lymphocytes (A) and/or macrophages (B). Macrophage staining was seen in cells peppered throughout the tumor, in bands at the infiltrating edge of the tumor, and in pools of necrosis adjacent to tumor (as pictured in B). Immune cell staining was less common for IDO with 54% of cases showing some staining and only rare cases showing diffuse staining. Lymphocyte staining was often identified in clusters located at the tumor periphery (C). When macrophage expression was present it was typically very faint and blush-like (D).
IDO Expression
Tumor cell staining for IDO was more common, although diffuse expression remained rare: tumor cell staining for IDO was identified in 58% of cases, with most showing <10% staining. (Figure 1, Table 2) A small subset (6%) demonstrated positivity in 11–25% of tumor cells, and no cases exceeded the 25% threshold. IDO positivity was enhanced at the infiltrating edge of tumors and in regions of increased lymphocyte density. (Figure 2–3) Tumor-associated immune staining for IDO was identified in 54% of cases. (Figure 4) Most IDO positives had only focal patchy staining involving ≤10% of lymphocytes and macrophages, while 2 cases had staining in 11–50% of the immune compartment. (Table 2, Figure 2–4)
PD-L1 and IDO Co-Expression
PD-L1 and IDO were often expressed in concert. Approximately 78% of cases with positive PD-L1 staining in tumor cells also expressed IDO tumor staining and 38% of IDO-positive cases also expressed PD-L1. In the immune compartment, 66% of PD-L1-positive cases also expressed IDO and 89% of IDO-positive cases expressed PD-L1 (data not shown).
CD8+/FOX3p+ Tumor-Associated Lymphocytes
CD8-positive cytotoxic T cells and FOX3p-positive regulatory T cells showed a range of distributions. (Figure 5) The median count of CD8-positive cytotoxic T cells per HPF was 13.0, with a range from 0–203.9. (Table 2) Fewer regulatory FOX3p-positive cells were present (median 3.2/HPF, range 0–39). High CD8 counts were observed more frequently for tumors with positive PD-L1 and IDO staining for both the tumor and immune cell compartments (p< 0.05) (Table 3). However, these differences were more striking among the immune compartment: among women with positive PD-L1 immune staining, 60% had high CD8 counts versus 23% for women with negative PD-L1 immune staining (p=0.0006). Similarly, for IDO, 66% of women with positive immune staining had high CD8 counts compared to 31% with negative staining (p=0.0003). Similar trends were observed for Fox3p staining where tumors with positive staining for PD-L1 and IDO had higher Fox3p counts. However, these differences were only significant for women with PD-L1 expression in the immune compartment (p=0.01).
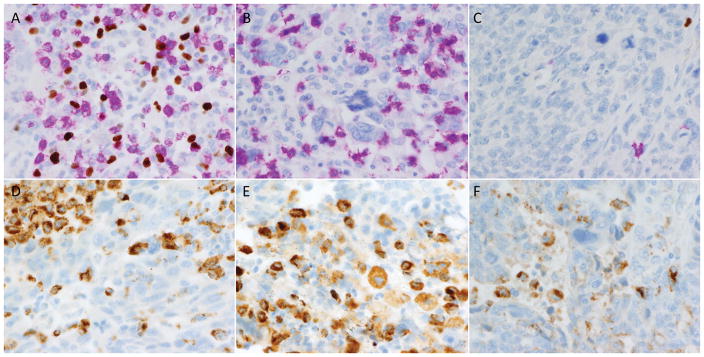
Figure 5.
Examples of tumor-associated lymphocytes and macrophages. Some tumors showed robust infiltrates of both CD8+ (pink) and FOX3p+ (brown) cells (A), while others showed predominantly CD8+ infiltrates (B). Still others showed only sparse lymphocytic inflammation (C). CD68 immunostaining was performed to highlight macrophages in order to facilitate mapping of immune modulatory molecule expression to either tumor cells or macrophages. Plates D–F demonstrate the extent to which CD68-positive macrophages can be subtly admixed among tumor cells, which can complicate assessment of tumoral expression for immune modulatory molecule markers.
Table 3.
Co-Expression of CD8 and Fox3p with PD-L1 and IDO (N=112)
| PD-L1 Tumor Staining | p-value | PD-L1 Immune Staining | p-value | IDO Tumor Staining | p-value | IDO Immune Staining | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||
| Negative | Positive | Negative | Positive | Negative | Positive | Negative | Positive | |||||
|
|
|
|
|
|||||||||
| n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | |||||
| CD8 | ||||||||||||
| Low (<12.95) | 45 (56) | 11 (34) | 0.04 | 23 (77) | 33 (40) | 0.0006 | 30 (64) | 26 (40) | 0.01 | 35 (69) | 21 (34) | 0.0003 |
| High (≥12.95) | 35 (44) | 21 (66) | 7 (23) | 49 (60) | 17 (36) | 39 (60) | 16 (31) | 40 (66) | ||||
| Fox3p | ||||||||||||
| Low (<3.2) | 44 (55) | 12 (38) | 0.09 | 21 (70) | 35 (43) | 0.01 | 28 (60) | 28 (43) | 0.08 | 30 (59) | 26 (43) | 0.09 |
| High (≥3.2) | 36 (45) | 20 (63) | 9 (30) | 47 (57) | 19 (40) | 37 (57) | 21 (41) | 35 (57) | ||||
PD-L1/IDO Expression and Survival
Supplementary Table 1 provides the distribution of participant characteristics by PD-L1 and IDO staining. There were no statistically significant differences in the positivity of these markers by most demographic and clinical characteristics, p>0.05. However, we observed notable differences in outcome, where women with positive PD-L1 and IDO tumor staining were significantly more likely to be alive than women with no PD-L1 and IDO tumor staining (75% vs. 51% and 67% vs. 45%, respectively). This trend was also observed for staining in the immune cell compartment, but the differences were not statistically significant. Similarly, a slightly better mean survival time was observed for women with positive staining, yet not statistically significant.
In multivariable models, adjusting for age at diagnosis, region, stage, residual disease, we restricted the sample to only those women with data on these covariates (N=93). PD-L1 and IDO staining in the tumor and immune compartment were not associated with overall survival (Table 4). However, PD-L1 and IDO tumor staining was suggestive of improved patient outcome, although not statistically significant (HR=0.61, 95% CI=0.26, 1.41 and HR=0.79, 95% CI=0.41–1.51, respectively). Among women with high CD8 counts, a significantly lower risk of mortality was observed for women with positive PD-L1 tumor staining (HR=0.19, 95% CI=0.04–0.94) but not among women with low CD8 counts (HR=0.81, 95% CI=0.26–2.51). No other statistically significant differences in the relationship between PD-L1 and IDO and overall survival were observed by CD8 and Fox3p counts. These results are based on small sample sizes and should be interpreted with caution.
Table 4.
Estimated hazard ratios and 95% confidence intervals for the relationship between PD-L1 and IDO and overall survival (N=93)
| Total | Low CD8 | High CD8 | Low Fox3p | High Fox3p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Cases (Deaths) | HR (95% CI)a | Cases (Deaths) | HR (95% CI)a | Cases (Deaths) | HR (95% CI)a | Cases (Deaths) | HR (95% CI)a | Cases (Deaths) | HR (95% CI)a | |
| PD-L1 Tumor | ||||||||||
| Negative | 66 (33) | 1.00 (Referent) | 36 (16) | 1.00 (Referent) | 30 (17) | 1.00 (Referent) | 35 (16) | 1.00 (Referent) | 31 (17) | 1.00 (Referent) |
| Positive | 27 (7) | 0.61 (0.26–1.41) | 9 (4) | 0.81 (0.26–2.51) | 18 (3) | 0.19 (0.04–0.94) | 10 (3) | 0.45 (0.12–1.60) | 17 (4) | 0.89 (0.26–3.02) |
| PD-L1 Immune | ||||||||||
| Negative | 23 (11) | 1.00 (Referent) | 17 (8) | 1.00 (Referent) | 6 (3) | 1.00 (Referent) | 15 (7) | 1.00 (Referent) | 8 (4) | 1.00 (Referent) |
| Positive | 70 (29) | 1.43 (0.68–3.00) | 28 (12) | 1.33 (0.50–3.53) | 42 (17) | 0.90 (0.23–3.44) | 30 (12) | 1.42 (0.49–4.14) | 40 (17) | 1.22 (0.40–3.67) |
| IDO Tumor | ||||||||||
| Negative | 36 (20) | 1.00 (Referent) | 23 (12) | 1.00 (Referent) | 13 (8) | 1.00 (Referent) | 20 (11) | 1.00 (Referent) | 16 (9) | 1.00 (Referent) |
| Positive | 57 (20) | 0.79 (0.41–1.51) | 22 (8) | 0.83 (0.32–2.16) | 35 (12) | 0.56 (0.21–1.48) | 25 (8) | 0.70 (0.25–1.94) | 32 (12) | 0.67 (0.26–1.74) |
| IDO Immune | ||||||||||
| Negative | 41 (19) | 1.00 (Referent) | 27 (14) | 1.00 (Referent) | 14 (5) | 1.00 (Referent) | 23 (11) | 1.00 (Referent) | 18 (8) | 1.00 (Referent) |
| Positive | 52 (21) | 1.11 (0.57–2.17) | 18 (6) | 0.86 (0.32–2.32) | 34 (15) | 1.03 (0.34–3.13) | 22 (8) | 0.94 (0.35–2.50) | 30 (13) | 0.99 (0.36–2.70) |
Adjusted for age at diagnosis, stage, region of residence, and residual disease.
DISCUSSION
Cancer is increasingly understood to be a disease both of neoplastic cells and of the tumor environment. Existing evidence suggests that there may be significant racial differences in both systemic and intratumoral inflammation, however data are limited given the poor representation of minority populations in the medical literature.10–16 Data on racial differences in the inflammatory context of ovarian carcinomas are particularly sparse, yet are of considerable interest given the persistent survival differences between ovarian cancer patients of African versus European descent. Potential differences in the immune microenvironment are of particular clinical interest given the emergence of therapeutic targets for a variety of immune modulatory molecules including PD-L1 and IDO. We herein characterize PD-L1 and IDO expression in HGSOCs in AA women from the AACES study to assess potential immunotherapeutic vulnerability in this population.3–6
Prior work has suggested a role for PD-1/PD-L1 inhibition in ovarian cancer treatment. PD-L1 expression by monocytes in blood and ascites fluid has been correlated with worsened outcomes in ovarian carcinoma patients23,24 and can also be identified in peritumoral lymphocytes and macrophages as well as directly on tumor cells.25–27 Tumoral and peri-tumoral immune PD-L1 positivity is also correlated with increased tumor infiltrating lymphocytes and BRCA1/2 mutations in ovarian cancer,25,26 suggesting that more immunogenic tumors may have increased vulnerability to PD-1/PD-L1 inhibition. As has been documented in other studies of HGSOC,25–27 tumor cell staining for PD-L1 was seen in a minority of cases in this series of AA women. When present, tumoral expression of PD-L1 was typically concentrated at the infiltrating edge in areas of greatest lymphocyte infiltration, indicative of an adaptive immune response. It is notable that, at 29% positive, the proportion of cases with PD-L1 expression seen here is considerably higher than was observed in a prior study from this institution, which showed tumor cell staining in only 8% of cancers,27 and in a prior study from Webb and colleagues,26 which showed tumor staining in 13% of tumors. Importantly, the prior study consisted almost exclusively of women of European descent and the methodology for both the original UVA study and the current UVA-ACCES study was identical, including the utilization of CD68 in attempt to ensure that tumor-associated macrophages were not erroneously scored as tumor cells. Further studies including a comparison group of women of European descent are needed to ascertain whether PD-L1 expression is indeed more common in tumors from AA women, thereby suggesting that there may be increased vulnerability to immunotherapy targeting the PD-1/PD-L1 axis within the population.
The proportion of cases with PD-L1-positive immune cells (74%) was identical to what was observed in the prior UVA study.27 The presence of prominent PD-L1 staining within the immune compartment is intriguing, particularly since most of this staining centered on macrophages. Evidence from murine models has shown that tumor-associated macrophage expression of PD-L1 correlates negatively with tumor phagocytosis, and that blocking PD-1/PD-L1 in vivo reduces tumor growth in a macrophage-dependent fashion.32 This suggests that PD-1/PD-L1 blockades may be of clinical utility even when tumor cell staining is not identified. However, preliminary evidence on response to anti-PD-1/PD-L1 therapy has been relatively lackluster in HGSOC despite high rates of PD-L1-positive macrophages. In the KEYNOTE-028 trial, a nonrandomized, multicohort phase Ib trial of pembrolizumab in patients with PD-L1-positive solid tumors, 11% of ovarian carcinomas showed complete or partial response (ClinicalTrials.gov identifier: NCT02054806).33 Similarly, in a phase II trial of nivolumab in platinum-resistant cancers showed a best overall response rate of 15% (UMIN Clinical Trials Registry identifier: UMIN000005714).21,34 Given that PD-L1 positive tumor-associated macrophages are so common in serous ovarian cancer—but that treatment anti-PD-1/PD-L1 response is relatively uncommon—the predictive utility of PD-L1-positive macrophages remains dubious in this tumor type. That said, response rates are equivalent to what is seen with other chemotherapeutic agents in the platinum-resistant/refractory setting, and given their improved tolerability relative to existing options anti-PD-1/PD-L1 drugs may still play an important role in this context.
IDO expression has not been well characterized in ovarian carcinomas, and no previous studies have focused on AA women. IDO impairs T cell function and survival through two mechanisms: starvation (via tryptophan depletion), and toxicity (through the generation of the toxic metabolite kynurenine. IDO has also been implicated in the peritoneal dissemination of ovarian cancer cells30 and mouse models of ovarian carcinoma have shown that IDO inhibition with the small molecule IDO inhibitor 1-methyl-tryptophan (1-MT) can augment the efficacy to cytotoxic chemotherapies.28 IDO is of particular interest in HGSOC given limited responses to PD-1/PD-L1 inhibitors. We found tumoral IDO expression in 58% of HGOSC from AA women, including 78% of all PD-L1-positive cancers. The high rates of PD-L1 and IDO co-expression suggest that IDO may represent a mechanism of anti-PD-1/anti-PD-L1 resistance, and that dual antagonistic therapy may be of benefit in this setting. As with PD-L1, tumoral IDO expression was typically highest at the infiltrative margin of the tumor, particularly in areas of heightened lymphocyte infiltration.
The observed rates of IDO positivity parallel prior work in this tumor type, which have demonstrated IDO expression in more than half of ovarian cancers.28 However, ascertaining whether prior reports specifically refer to tumor cell vs. immune cell staining is difficult in the absence of an associated CD68 stain given the ease with which macrophages can be mistaken for tumor cells.27 It may be that true tumor cell staining is indeed elevated in AA patients once potential false positive staining in tumor-associated macrophages is taken into account. Studies using identical methodologies are therefore needed to determine whether racial differences exist in tumoral IDO expression. Furthermore, the significance of IDO positivity within the immune compartment is unclear. Presumably, IDO produced by either the tumor or by associated lymphocytes and macrophages should have the same effect on dampening the immune response, but this has not been demonstrated. Investigations detailing role of immune cell IDO expression in predicting response to anti-IDO therapy will therefore also be of interest. At present, there are limited data on the role of therapies targeting IDO in this tumor type. The KEYNOTE-037 clinical trial (ClinicalTrials.gov identifier: NCT02178722) is currently enrolling subjects to assess the safety, tolerability, and efficacy of pembrolizumab in combination with the IDO inhibitor epacodostat in patients with ovarian cancer as well as a variety of other malignancies, however IDO expression is not a criterion for enrollment and preliminary response data are pending.
CD8-positive cytotoxic T cell infiltrates and associated FOX3p-positive regulatory T cells demonstrated a range of densities among HGSOCs in the AA population. Prior studies enumerating CD8-positive cells in racially unselected HGSOCs have shown that higher numbers (mean: 5.75/HPF) correlates with a good outcome, whereas lower numbers (mean: 0.12/HPF) correlate with poor outcome.35 It is notable that average CD8-positive cells per HPF were higher in our study at 20.5/HPF, suggesting increased tumor-associated lymphocytes in the AA population. However, drawing direct comparisons is difficult given variabilities in methodologies across studies.
Cytotoxic CD8-positive lymphocyte counts provided some prognostic information in the context of PD-L1 positivity, with a significantly reduced rate of mortality among women with PD-L1 staining and high CD8 counts; however, these findings were based on a relatively small sample size and further confirmation of these findings in a large sample is warranted. PD-L1 and IDO expression did not independently correlate with outcome in this study population. The absence of prognostic significance to these variables is not entirely surprising given the complexity of the immune interplay and the heterogeneity of data from other tumor types. Although, for instance, cytotoxic T cell infiltrates and PD-L1 expression sometimes correlate with better outcome, they also often correlate with higher tumor grade and other negative prognostic variables.25,36–38 Furthermore, the examined markers are just a subset of many immune modulatory molecules in play in the tumor microenvironment. It is possible that a more complete characterization of the immune milieu including analysis of other checkpoint inhibitors and lymphocyte activators/suppressors might have prognostic power. Largest studies comparing patients with and without neoadjuvant therapy would also be warranted, as preliminary data here suggests that prior therapy does not increase immune modulatory molecule expression.
Another limitation to note is the absence of multiplex staining, which makes it challenging to definitively determine whether immune modulatory molecule expression is centered on tumor cells or associated immune cells. This can be particularly problematic as tumor-associated macrophages may be quiet common in HGSOC and frequently approach the size of tumor cells. While the inclusion of a separately stained CD68 slide helped prevent egregious misinterpretation macrophages as tumor cells, this doesn’t entirely eliminate this problem as it requires switching slides and is subject to cutting in/out artifacts. The problem is compounded by the fact that true tumoral staining seems to concentrate at the infiltrating edge of tumor nests and this area also often shows a high density of macrophages. Furthermore, IHC interpretation was performed by a single pathologist so this study is not powered to address interpretive reproducibility.
A final limitation worth emphasizing is the lack of information on BRCA mutation status. Existing evidence from Strickland et al. has shown that BRCA-mutated serous ovarian carcinomas have a higher neoantigen load than BRCA-wildtype cancers, and that these tumors increased CD8+ lymphocytic infiltrates and elevated PD-L1 positivity.25 Indeed, CD8+ counts approached 40/HPF in BRCA-mutated cancers from that series, indicating that mutational status is a significant contributor to immunogenicity. Data from our prior series also showed increased PD-L1 expression in BRCA-mutated cases (50% vs. 11.8% of cases with no mutation) however statistical significance was limited by the small number of patients with BRCA mutation testing results. 27 However, AA women have not been shown to have significantly higher rates of deleterious BRCA mutations relative to other racial groups39 therefore BRCA status is unlikely to disproportionally account for immune activation in tumors from this population.
In summary, we herein characterize expression of the targetable immune regulatory molecules PD-L1 and IDO in HGSOCs from AA women. We demonstrate that PD-L1 and IDO are seen in a subset of these cancers, with higher tumoral PD-L1 expression relative to existing data from racially unselected cohorts. The majority of PD-L1-positive cases also express IDO, suggesting a role for dual immunotherapy. However, diffuse expression of PD-L1 and IDO remains rare, invoking some caution regarding the potential for immunotherapy in this tumor type should high expression levels prove necessary for treatment response. Clinical trials are needed to determine biomarker thresholds predictive of response to immune modulatory molecule antagonism in HGSOCs, as are comparison studies with European Americans to assess racial differences in immune modulatory molecule expression and in the overarching tumoral microenvironment.
Supplementary Material
Distribution of patient characteristics by PD-L1 and IDO staining
SD: standard deviation.
Acknowledgments
We would like to thank Rex Bentley, MD at Duke University Medical Center for directing and performing the centralized review of these tumors. We would also like to acknowledge the AACES interviewers, Christine Bard, LaTonda Briggs, Whitney Franz (North Carolina) and Robin Gold (Detroit). We also acknowledge the individuals responsible for facilitating case ascertainment across the ten sites including: Christie McCullum-Hill (Alabama); the Metropolitan Detroit Cancer Surveillance System staff (Detroit); Rana Bayakly, Vicki Bennett, Judy Andrews, and Debbie Chambers (Georgia); the Louisiana Tumor Registry; Lisa Paddock and Manisha Narang (New Jersey); Diana Slone, Yingli Wolinsky, Steven Waggoner, Anne Heugel, Nancy Fusco, Kelly Ferguson, Peter Rose, Deb Strater, Taryn Ferber, Donna White, Lynn Borzi, Eric Jenison, Nairmeen Haller, Debbie Thomas, Vivian von Gruenigen, Michele McCarroll, Joyce Neading, John Geisler, Stephanie Smiddy, David Cohn, Michele Vaughan, Luis Vaccarello, Elayna Freese, James Pavelka, Pam Plummer, William Nahhas, Ellen Cato, John Moroney, Mark Wysong, Tonia Combs, Marci Bowling, Brandon Fletcher, (Ohio); Susan Bolick, Donna Acosta, Catherine Flanagan (South Carolina); Martin Whiteside (Tennessee) and Georgina Armstrong and the Texas Registry, Cancer Epidemiology and Surveillance Branch, Department of State Health Services.
FUNDING
This study was supported the National Cancer Institute (R01CA142081) and resources within the Cancer Control and Population Health program at the University of Virginia Cancer Center. Additional support was provided by the Metropolitan Detroit Cancer Surveillance System with funding from the National Cancer Institute, National Institute of Health, and the Department of Health and Human Services (Contract HHSN261201000028C), and the Epidemiology Research Core, supported in part by the National Cancer Institute (P30CA22453) to the Karmanos Cancer Institute, Wayne State University School of Medicine. The New Jersey State Cancer Registry, Cancer Epidemiology Services, New Jersey Department of Health, is funded by the Surveillance, Epidemiology and End Results (SEER) Program of the National Cancer Institute under contract HHSN261201300021I, the National Program of Cancer Registries (NPCR), Centers for Disease Control and Prevention under grant 5U58DP003931-02 as well as the State of New Jersey and the Rutgers Cancer Institute of New Jersey.
Footnotes
CONFLICT OF INTEREST STATEMENT
The authors have no relevant conflicts of interest to report. Funding support is detailed below.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349. [DOI] [PubMed] [Google Scholar]
- 3.DeSantis CE, Siegel RL, Sauer AG, et al. Cancer statistics for african americans, 2016: Progress and opportunities in reducing racial disparities. CA Cancer J Clin. 2016;66(4):290–308. doi: 10.3322/caac.21340. [DOI] [PubMed] [Google Scholar]
- 4.Bristow RE, Powell MA, Al-Hammadi N, et al. Disparities in ovarian cancer care quality and survival according to race and socioeconomic status. J Natl Cancer Inst. 2013;105(11):823–832. doi: 10.1093/jnci/djt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terplan M, Schluterman N, McNamara EJ, Tracy JK, Temkin SM. Have racial disparities in ovarian cancer increased over time? an analysis of SEER data. Gynecol Oncol. 2012;125(1):19–24. doi: 10.1016/j.ygyno.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 6.Bandera EV, Lee VS, Rodriguez-Rodriguez L, Powell CB, Kushi LH. Racial/ethnic disparities in ovarian cancer treatment and survival. Clin Cancer Res. 2016;22(23):5909–5914. doi: 10.1158/1078-0432.CCR-16-1119. doi:1078-0432.CCR-16-1119. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–907. doi: 10.1038/nature06309. nature06309 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Becht E, Giraldo NA, Germain C, et al. Immune contexture, immunoscore, and malignant cell molecular subgroups for prognostic and theranostic classifications of cancers. Adv Immunol. 2016;130:95–190. doi: 10.1016/bs.ai.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. 313/5795/1960 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Paalani M, Lee JW, Haddad E, Tonstad S. Determinants of inflammatory markers in a bi-ethnic population. Ethn Dis. 2011;21(2):142–149. [PMC free article] [PubMed] [Google Scholar]
- 11.Albert MA, Glynn RJ, Buring J, Ridker PM. C-reactive protein levels among women of various ethnic groups living in the united states (from the women’s health study) Am J Cardiol. 2004;93(10):1238–1242. doi: 10.1016/j.amjcard.2004.01.067. [DOI] [PubMed] [Google Scholar]
- 12.Enewold L, Mechanic LE, Bowman ED, et al. Serum concentrations of cytokines and lung cancer survival in african americans and caucasians. Cancer Epidemiol Biomarkers Prev. 2009;18(1):215–222. doi: 10.1158/1055-9965.EPI-08-0705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallace TA, Prueitt RL, Yi M, et al. Tumor immunobiological differences in prostate cancer between african-american and european-american men. Cancer Res. 2008;68(3):927–936. doi: 10.1158/0008-5472.CAN-07-2608. [DOI] [PubMed] [Google Scholar]
- 14.White KL, Schildkraut JM, Palmieri RT, et al. Ovarian cancer risk associated with inherited inflammation-related variants. Cancer Res. 2012;72(5):1064–1069. doi: 10.1158/0008-5472.CAN-11-3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hung RJ, Ulrich CM, Goode EL, et al. Cross cancer genomic investigation of inflammation pathway for five common cancers: Lung, ovary, prostate, breast, and colorectal cancer. J Natl Cancer Inst. 2015;107(11) doi: 10.1093/jnci/djv246. Print 2015 Nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charbonneau B, Block MS, Bamlet WR, et al. Risk of ovarian cancer and the NF-kappaB pathway: Genetic association with IL1A and TNFSF10. Cancer Res. 2014;74(3):852–861. doi: 10.1158/0008-5472.CAN-13-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mittica G, Genta S, Aglietta M, Valabrega G. Immune checkpoint inhibitors: A new opportunity in the treatment of ovarian cancer? Int J Mol Sci. 2016;17(7) doi: 10.3390/ijms17071169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reiss KA, Forde PM, Brahmer JR. Harnessing the power of the immune system via blockade of PD-1 and PD-L1: A promising new anticancer strategy. Immunotherapy. 2014;6(4):459–475. doi: 10.2217/imt.14.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Taube JM, Klein A, Brahmer JR, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20(19):5064–5074. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zamarin D, Jazaeri AA. Leveraging immunotherapy for the treatment of gynecologic cancers in the era of precision medicine. Gynecol Oncol. 2016;141(1):86–94. doi: 10.1016/j.ygyno.2015.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamanishi J, Mandai M, Konishi I. Immune checkpoint inhibition in ovarian cancer. Int Immunol. 2016;28(7):339–348. doi: 10.1093/intimm/dxw020. [DOI] [PubMed] [Google Scholar]
- 22.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol. 2006;90:297–339. doi: 10.1016/S0065-2776(06)90008-X. S0065-2776(06)90008-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu QX, Huang Q, Shen Y, Zhu YB, Zhang XG. The increase of circulating PD-L1-expressing CD68(+) macrophage in ovarian cancer. Tumour Biol. 2016;37(4):5031–5037. doi: 10.1007/s13277-015-4066-y. [DOI] [PubMed] [Google Scholar]
- 24.Maine CJ, Aziz NH, Chatterjee J, et al. Programmed death ligand-1 over-expression correlates with malignancy and contributes to immune regulation in ovarian cancer. Cancer Immunol Immunother. 2014;63(3):215–224. doi: 10.1007/s00262-013-1503-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strickland KC, Howitt BE, Shukla SA, et al. Association and prognostic significance of BRCA1/2-mutation status with neoantigen load, number of tumor-infiltrating lymphocytes and expression of PD-1/PD-L1 in high grade serous ovarian cancer. Oncotarget. 2016;7(12):13587–13598. doi: 10.18632/oncotarget.7277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Webb JR, Milne K, Kroeger DR, Nelson BH. PD-L1 expression is associated with tumor-infiltrating T cells and favorable prognosis in high-grade serous ovarian cancer. Gynecol Oncol. 2016;141(2):293–302. doi: 10.1016/j.ygyno.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 27.Gottlieb CE, Mills AM, Cross JV, Ring KL. Tumor-associated macrophage expression of PD-L1 in implants of high grade serous ovarian carcinoma: A comparison of matched primary and metastatic tumors. Gynecol Oncol. 2017 doi: 10.1016/j.ygyno.2016.12.021. S0090-8258(16)31685-7 [pii] [DOI] [PubMed] [Google Scholar]
- 28.Inaba T, Ino K, Kajiyama H, et al. Role of the immunosuppressive enzyme indoleamine 2,3-dioxygenase in the progression of ovarian carcinoma. Gynecol Oncol. 2009;115(2):185–192. doi: 10.1016/j.ygyno.2009.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Takao M, Okamoto A, Nikaido T, et al. Increased synthesis of indoleamine-2,3-dioxygenase protein is positively associated with impaired survival in patients with serous-type, but not with other types of, ovarian cancer. Oncol Rep. 2007;17(6):1333–1339. [PubMed] [Google Scholar]
- 30.Nonaka H, Saga Y, Fujiwara H, et al. Indoleamine 2,3-dioxygenase promotes peritoneal dissemination of ovarian cancer through inhibition of natural killercell function and angiogenesis promotion. Int J Oncol. 2011;38(1):113–120. [PubMed] [Google Scholar]
- 31.Schildkraut JM, Alberg AJ, Bandera EV, et al. A multi-center population-based case-control study of ovarian cancer in african-american women: The african american cancer epidemiology study (AACES) BMC Cancer. 2014;14 doi: 10.1186/1471-2407-14-688. 688-2407-14-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gordon SR, Maute RL, Dulken BW, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545(7655):495–499. doi: 10.1038/nature22396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varga A, Piha-Paul S, Ott PA, et al. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase ib study. JCO. 2015;33(15):5510–5510. doi: 10.1200/jco.2015.33.15_suppl.5510. http://ascopubs.org/doi/abs/10.1200/jco.2015.33.15_suppl.5510. [DOI] [Google Scholar]
- 34.Hamanishi J, Mandai M, Ikeda T, et al. Safety and antitumor activity of anti-PD-1 antibody, nivolumab, in patients with platinum-resistant ovarian cancer. J Clin Oncol. 2015;33(34):4015–4022. doi: 10.1200/JCO.2015.62.3397. [DOI] [PubMed] [Google Scholar]
- 35.Preston CC, Maurer MJ, Oberg AL, et al. The ratios of CD8+ T cells to CD4+CD25+ FOXP3+ and FOXP3− T cells correlate with poor clinical outcome in human serous ovarian cancer. PLoS One. 2013;8(11):e80063. doi: 10.1371/journal.pone.0080063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hermans C, Anz D, Engel J, Kirchner T, Endres S, Mayr D. Analysis of FoxP3+ T-regulatory cells and CD8+ T-cells in ovarian carcinoma: Location and tumor infiltration patterns are key prognostic markers. PLoS One. 2014;9(11):e111757. doi: 10.1371/journal.pone.0111757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suemori T, Susumu N, Iwata T, et al. Intratumoral CD8+ lymphocyte infiltration as a prognostic factor and its relationship with cyclooxygenase 2 expression and microsatellite instability in endometrial cancer. Int J Gynecol Cancer. 2015;25(7):1165–1172. doi: 10.1097/IGC.0000000000000482. [DOI] [PubMed] [Google Scholar]
- 38.Kondratiev S, Sabo E, Yakirevich E, Lavie O, Resnick MB. Intratumoral CD8+ T lymphocytes as a prognostic factor of survival in endometrial carcinoma. Clin Cancer Res. 2004;10(13):4450–4456. doi: 10.1158/1078-0432.CCR-0732-3. [DOI] [PubMed] [Google Scholar]
- 39.Hall MJ, Reid JE, Burbidge LA, et al. BRCA1 and BRCA2 mutations in women of different ethnicities undergoing testing for hereditary breast-ovarian cancer. Cancer. 2009;115(10):2222–2233. doi: 10.1002/cncr.24200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of patient characteristics by PD-L1 and IDO staining
SD: standard deviation.