Summary
Purpose:
B cells participate in diverse retinal immunopathologies. Endothelial adhesion molecules and chemokines direct leukocyte trafficking. We examined the involvement of three molecular signals in retinal transendothelial migration of human B cells: ICAM-1, VCAM-1 and CXCL13.
Methods:
Peripheral blood B cells were isolated by negative selection. Migration was studied in transwells populated with human retinal endothelial monolayers, using antibody to block ICAM-1 or VCAM-1. Retinal expression of CXCL13 was investigated.
Results:
B cells crossed retinal endothelium. ICAM-1 blockade significantly reduced migration when results for all subjects were combined, and for a majority when results were analyzed by individual. This effect was irrespective of the presence or absence of CXCL13, although CXCL13 increased migration. CXCL13 was detected in neural retina and retinal pigment epithelium. Endothelial cells of some retinal vessels presented CXCL13 protein.
Conclusions:
ICAM-1 blockade may be an effective treatment in some patients with retinal diseases that involve B cells.
Keywords: human, retina, B cell, ICAM-1, CXCL13
Introduction
The retina is the compartment of the central nervous system (CNS) that contains the photoreceptors and other neurons responsible for the initiation of visual processing.(1) Although the retina enjoys immune privilege, lymphocytes may enter this tissue under certain circumstances. In particular, B cells – or B lymphocytes – are key players in several diseases that are based within the retina. Malignant B cells infiltrate the retina and the adjacent vitreous cavity in diffuse large B cell vitreoretinal lymphoma.(2) Terminally differentiated plasma cells produce antibody within the eye during retinal infections, the most common of which is ocular toxoplasmosis.(3) B cells join T cells and other leukocyte subsets in the retina in several forms of non-infectious uveitis;(4–6) their pathogenic roles in this inflammatory disease are not well understood, but the B cell-targeted drug, rituximab, is reported to be an effective treatment.(7) Additionally, and extrapolating from studies of other parts of the CNS,(8) B cells also may enter the retina in health, to participate in physiological immune surveillance.
The molecular signals controlling B cell entry into the retina from the bloodstream have not been considered previously, although T cell trafficking to the retina has been the subject of multiple investigations.(9–13) Passage of leukocytes across the wall of a blood vessel, from the circulation to the tissue, is strictly controlled at a molecular level by the vascular endothelial cells that line the vessel.(14) Proteins expressed by the endothelium, and recognized by leukocyte ligands, coordinate the stages of leukocyte migration that include: rolling, firm adhesion, spreading and crawling, and transmigration. Common families of adhesion molecules and chemokines are involved in transendothelial migration of leukocyte subsets at any body site, but the participation of individual family members depends on the subset of migrating leukocytes and the specific molecular phenotype of the local vascular endothelial cell population.(15)
We investigated the involvement of three molecular signals in the entry of B cells into the human retina: intercellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and CXCL13 or B cell-attracting chemokine-1. We selected the immunoglobulin (Ig) superfamily members, ICAM-1 and VCAM-1, for interrogation, because these cell adhesion molecules have been implicated across multiple stages of leukocyte migration,(14) and both are highly expressed by human retinal endothelial cells.(16) We chose to study the possible influence of CXCL13, as this chemokine is potent and relatively selective for B cells.(17) Clarifying the molecular signals that facilitate B cell migration into the human retina is informative for both normal ocular immunology and the pathogenesis of diverse retinal diseases. Such investigation also may suggest new drug targets for eye diseases that involve pathogenic B cells.
Materials and Methods
Primary antibodies and chemokines
Goat polyclonal anti-human CXCL3 antibody, mouse anti-human ICAM-1 antibody (clone BBIG-I1, isotype IgG1), mouse anti-human VCAM-1 antibody (clone BBIG-V1, isotype IgG1) and mouse IgG (clone 11711, isotype IgG1) were obtained from R&D Systems (Minneapolis, MN). Goat Ig was obtained from Vector Laboratories (Burlingame, CA). Mouse anti-human CD31 antibody (clone WM59, isotype IgG1ĸ), Horizon V500 (V500)-tagged mouse anti-human CD19 antibody (clone HIB19, isotype IgG1ĸ), phycoerythrin (PE)-tagged mouse anti-human CD27 antibody (clone M-T271, isotype IgG1ĸ), allophycocyanin (APC)-tagged mouse anti-human CD38 antibody (clone HIT2, isotype IgG1ĸ) and Alexa Fluor 488-conjugated rat anti-human CXCR5 (clone RF8B2, isotype IgG2bĸ) were bought from BD Biosciences-Pharmingen (San Diego, CA). eFluor (eF) 450-tagged mouse anti-human CD20 antibody (clone 2H7, isotype Mouse IgG2bĸ) was bought from eBioscience (San Diego, CA). Human recombinant CXCL13 was sourced from R&D Systems.
Human tissues and cells
Human neural retina or retinal pigment epithelium was isolated from posterior eyecups of cadaver donors. After removal of iris and vitreous, neural retina was dissected free and cut into strips. Strips were immersed in RNAlater RNA Stabilization Reagent (Qiagen, Hilden, Germany) and stored at −80 °C ahead of RNA extraction, or fixed in 10% neutral buffered formalin overnight, followed by a 70% ethanol hold, prior to paraffin embedding. After removal of neural retina, retinal pigment epithelium was washed with phosphate buffered saline (PBS) and detached from sclera along with adherent choroid. Epithelium was removed from choroid in PBS using a sapphire blade and pelleted by centrifugation. Isolates were treated with RNAlater RNA Stabilization Reagent and/or transferred to Buffer RLT (Qiagen) with 0.55 mM β-mercaptoethanol (Sigma-Aldrich, St. Louis, MO) and frozen at −80 °C prior to RNA extraction.
Endothelial cells were isolated from human retina, as we have previously described.(18) In brief, the retinae were dissected from both eyes of cadaver donors, and digested with 0.2 mg/ml Dispase and 0.25–1 mg/ml type II collagenase (both from ThermoFisher Scientific-GIBCO, Grand Island, NY). After 7 to 10 days of culture in MCDB-131 medium (Sigma-Aldrich) supplemented with 2% fetal bovine serum (FBS) (HyClone-GE Healthcare Life Sciences, Logan, UT) and endothelial growth factors (EGM-2 SingleQuots supplement, omitting FBS, hydrocortisone and gentamicin; Clonetics-Lonza, Walkersville, MD) at 37 °C with 5% CO2, retinal endothelial cells were purified using magnetic Dynabeads (ThermoFisher Scientific-Invitrogen Dynal, Oslo, Norway) coated with mouse anti-human CD31 antibody, and grown in modified MCDB-131 medium with 10% FBS. Subculturing of retinal endothelial cells was performed with 0.05% trypsin (ThermoFisher Scientific-GIBCO). Retinal endothelial cells used in the transendothelial migration studies were transduced with the mouse recombinant amphotropic retrovirus, LXSN16E6E7,(19) to generate sufficient cells for those studies. As we have previously reported,(18) the transduced cells retain their endothelial phenotype, including expression of endothelial markers and formation of capillary-like tubes on basement membrane substitute.
Sections of human lymph node mounted on glass slides were purchased from Abcam (Cambridge, United Kingdom). Human spleen RNA was purchased from ThermoFisher Scientific-Ambion (Carlsbad, CA). Leukocytes were collected from peripheral blood of healthy adults, and B cells were isolated by negative selection using a kit from Miltenyi Biotec (Auburn, CA). Ahead of use in transendothelial migration assays, the B cells were cultured overnight in complete RPMI medium (ThermoFisher Scientific-GIBCO) supplemented with 10% FBS, 2 mM L-glutamine, 100 U/ml-100 μg/ml penicillin-streptomycin (both from ThermoFisher Scientific-GIBCO) and 10 mM HEPES (Thermofisher Scientific-Fisher Scientific, Fair Lawn, NJ) at 37 °C and 5% CO2 in 6 cm diameter dishes at 2 × 106 cells/ml (maximum of 5 ml/dish).
Human subjects research was approved by the Oregon Health & Science University Institutional Review Board (Portland, OR) or the Southern Adelaide Clinical Human Research Ethics Committee (Adelaide, Australia). Human cadaver donor eyes were obtained from Lions VisionGift (Portland, OR) or the Eye Bank of South Australia (Adelaide, Australia). Human cadaver eye donors ranged from 35 to 74 years at death, and had no past history of eye diseases. Time from death to processing of the retina averaged 19 hours.
Immunophenotyping of B cells
Expression of cell surface molecules was determined by staining cells with the following anti-human antibodies diluted in PBS with 1% FBS and 0.1% sodium azide on ice for 30 minutes: V500-tagged mouse anti-CD19 antibody (1.25 μg/μl); eF 450-tagged mouse anti-CD20 antibody (1.25 μg/μl); PE-tagged mouse anti-CD27 antibody (0.625 μg/μl); and APC-tagged mouse anti-CD38 antibody (0.3125 μg/μl); and Alexa Fluor 488-conjugated rat anti-CXCR5 (1.25 μg/μl). After washing with buffer, the B cells were acquired on the BD LSR II (Becton-Dickinson, San Jose, CA). Cells were gated on the lymphocyte population based on size and granularity. Data were analyzed using FCS Express v3 (De Novo Software, Los Angeles, CA).
B cell transendothelial migration assay
Human retinal endothelial cells were suspended in modified MCDB-131 medium with 10% FBS, seeded at 30,000 cells on polyethylene terephthalate transwell membranes (0.3 cm2 diameter, 3 micron pore size; BD Falcon Labware, Franklin Lakes, NJ), positioned in wells of 24-well plates, and incubated at 37 °C and 5% CO2. The transwell membranes were pre-coated with bovine type I collagen (50 μg/ml, Santa Cruz Biotechnology, Santa Cruz, CA) for 2 hours at 37 °C. At 4 to 5 days after seeding of the endothelial monolayers, allogeneic B cells, suspended in modified MCDB-131 medium with 10% FBS, were added to upper chambers of transwells at 0.5 × 106 cells/transwell. Lower chambers were filled with medium alone. Anti-ICAM-1 antibody (10 μg/ml, blocking activity(20)) or anti-VCAM-1 antibody (30 μg/ml, blocking activity(20)) – or mouse IgG1 directed against irrelevant antigen (matched concentration) – was added to the upper chambers one hour prior to the addition of B cells. In some experiments CXCL13 (100 ng/ml) was added to lower chambers concurrently with the B cells. Transwells were incubated for 18 hours at 37 °C and 5% CO2, after which time migrated B cells were recovered from the lower chambers and counted on a hematocytometer. Conditions were performed in triplicate. For all assays, integrity of the endothelial monolayers was confirmed by tracking diffusion of 1 mg/ml Texas Red-conjugated dextran (molecular weight 70,000; ThermoFisher Scientific-Molecular Probes, Eugene, OR) across the monolayers.
RNA isolation and reverse transcription-polymerase chain reaction
Total RNA was isolated from neural retina or retinal pigment epithelium using the RNeasy Mini Kit (Qiagen), according to the manufacturer’s instructions and including the optional on-column DNase treatment. Reverse transcription was performed with iScript Reverse Transcription Supermix for RT-qPCR (Bio-Rad, Hercules, CA) with 100 ng RNA yielding 20 μL cDNA for eyes and 100 μL cDNA for spleen. Polymerase chain reaction was performed with 5 μL cDNA, 0.4 U HotStarTaq DNA polymerase (Qiagen), 1x CoralLoad PCR Buffer (Qiagen), 1.5 mM magnesium chloride, 1 μM each forward and reverse primers (CXCL13: forward 5’-CAGAATGAAGTTCATCTCGACATC-3’ and reverse 5’-ACAACCATTCCCACGGGGCAA-3’, 184 bp product; cyclophilin A: forward 5’-GAGCACTGGAGAGAAAGGATTT-3’ and reverse 5’-GGTGATCTTCTTGCTGGTCTT-3’, 355 bp product) (Sigma-Aldrich) and 0.2 mM deoxynucleotide triphosphates (Qiagen). Amplification consisted of: a pre-cycling hold at 95 °C for 5 minutes; 40 cycles of denaturation for 30 seconds at 95 °C; annealing for 30 seconds at 55 °C; extension for 1 minute at 72 °C; and a post-cycling hold at 72 °C for 5 minutes. Products of the PCR were resolved on a 3% agarose gel with SYBR Safe DNA Gel Stain (ThermoFisher Scientific-Invitrogen, Carlsbad, CA).
Immunohistochemistry
Paraffin sections of neural retina were cut 5 microns thick and mounted on StarFrost Advanced Adhesive slides (Knittel Glass, Brunswick, Germany). Slides were heated at 60 °C for 1 hour to ensure adhesion of retina and stored at room temperature ahead of staining. Human retinal and lymph node sections were deparaffinized in xylene, and rehydrated through graded ethanol solutions to water. Sections were boiled for 10 minutes in 10 mM citrate buffer with 0.05% Tween-20 (Sigma-Aldrich) at pH 6.0, and washed in a buffer of 50 mM Tris and 0.15 M sodium chloride at pH 7.5 (TBS). Non-specific binding was blocked for 1 hour with 2% v/v rabbit serum in TBS with 0.1% bovine serum albumin and 0.375% Triton-X 100 (all from Sigma-Aldrich). Serial sections were incubated overnight at 4 °C with goat anti-human CXCL13 antibody or control goat Ig, diluted to 2.5 μg/ml in blocking solution. Following washing with TBS, they were incubated for 1 hour at room temperature with biotinylated rabbit anti-goat Ig (Vector Laboratories), diluted 1:200 in blocking solution, and washed again with TBS. Sections were treated with streptavidin-biotin complex conjugated to alkaline phosphatase (Vector Laboratories), following the manufacturer’s protocol, and washed with TBS. They were incubated with HIGHDEF red IHC chromogen (Enzo Life Sciences, Farmingdale, NY) to visualize antibody complexes, and counterstained with a hematoxylin.
Statistical analysis
Data for test and control conditions obtained in transendothelial migration assays were compared by one-tailed paired or unpaired Student t-tests, using GraphPad Prism versions 4.0 or 6.0 (GraphPad Software, La Jolla, CA). In all analyses, a significant difference was defined as one yielding a p-value of 0.05 or less.
Results
Migration of human B cells across simulated retinal endothelium
In separate experiments, peripheral blood B cell isolates from individual adult human subjects were migrated through transwells divided by type I collagen-supported human retinal endothelial cell monolayers. To examine the involvement of Ig superfamily adhesion molecules, ICAM-1 and VCAM-1, in transendothelial migration, specific antibody or isotype-matched negative control IgG was added to the upper chambers of the transwells prior to the addition of B cells. For all experiments, integrity of the retinal endothelial monolayers was confirmed by limited diffusion of high molecular weight dextran. When results for all human subjects were considered together (Figure 1A and B), ICAM-1 and VCAM-1 blockade each significantly reduced human B cell migration across the simulated retinal endothelium. When results for individual human subjects were considered separately (Figure 1C and D), B cell migration was significantly reduced by anti-ICAM-1 antibody in 7 of 13 donors (53.8%) and by anti-VCAM-1 antibody in 2 of 10 (20.0%) donors. These results indicate a role for both ICAM-1 and VCAM-1 in the migration of human B cells across retinal endothelium, and suggest that ICAM-1 exerts more influence over this process across individuals.
Figure 1.
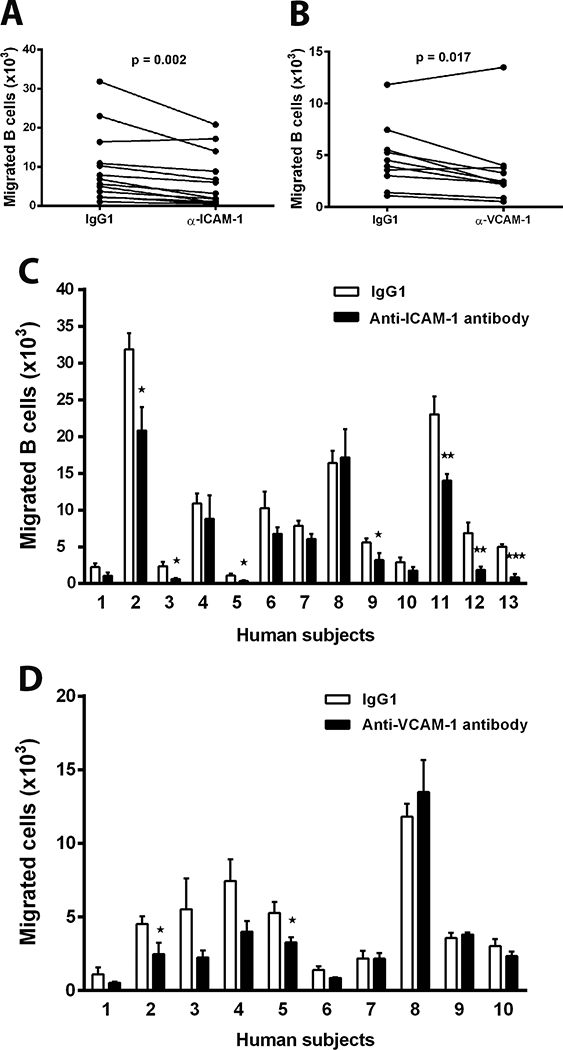
Migration of human B cells across simulated retinal endothelium. B cell isolates from individual adult human subjects were migrated across a human retinal endothelial cell monolayer in a transwell for 18 hours (0.5 × 106 cells/transwell). Blocking antibody directed against ICAM-1 [A & C] or blocking antibody directed against VCAM-1 [B & D] – or isotype-matched negative control IgG – was added to the upper chambers of the transwells prior to addition of the B cell isolates. In graphs [A] and [B], each pair of circles joined by a line represents the mean number of migrated B cells in the presence of control antibody or blocking antibody for individual human subjects. n = 3 transwells/condition. Data were analyzed by one-tailed paired Student’s t-test. In graphs [C] and [D], bars represent mean number of migrated B cells – with error bars showing standard error of the mean – in the presence of control antibody or blocking antibody for numbered individual human subjects. n = 3 transwells/condition. Data were analyzed by one-tailed unpaired Student’s t-test. *=p≤0.05; **=p⩽0.01; ***=p⩽0.001.
Expression of CXCL13 in human retina
CXCL13 is potently chemotactic and relatively selective for B cells.(17) It is highly expressed by follicular dendritic cells in follicles of lymphoid organs, including spleen and lymph nodes.(21) Presence of CXCL13 transcript in human retina of 8 human cadaver eye donors was examined by RT-PCR. The neural retina – a complex tissue containing three orders of neurons, glial cells, myeloid-derived cells and blood vessels – and the retinal pigment epithelium – the single cell layer that separates neural retina from underlying choroid – were examined separately. CXCL13 transcript was identified in the neural retina (Figure 2A; reference gene product shown in Figure 2B) and the retinal pigment epithelium (Figure 2C; reference gene product shown in Figure 2D) of all human donor eyes, as well as positive control human spleen.
Figure 2.
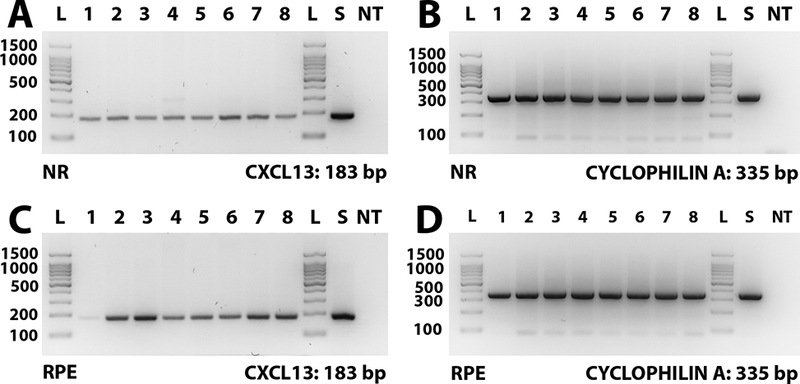
Agarose gels demonstrating CXCL13 and cyclophilin A (reference gene) RT-PCR products from neural retina (NR) [A & B] and retinal pigment epithelium (RPE) [C & D] isolated from eyes of 8 human cadaver donors, as well as human spleen. Expected product sizes: CXCL13 = 184 bp; cyclophilin A = 335 bp. Lane labels: L = DNA ladder; numbers = human eyes (with each number indicating product from the same individual donor); S = human spleen; NT = no cDNA template.
Immunohistochemistry was used to localize CXCL13 expression in the neural retina. Specific antibody identified CXCL13-expressing cells in lymphoid follicles of positive control human lymph node (Figure 3A; negative control serial section shown in Figure 3B). Ganglion cells and photoreceptors in neural retina stained positively for CXCL13 (Figure 3C, E and G; negative control serial sections shown in Figure 3D, F and H). Endothelium of some, but not all, blood vessels of the neural retina also expressed CXCL13 (Figure 3E and G; negative control serial sections shown in Figure 3F and H). Vascular endothelium may express chemokines by transcytosis from the tissue,(22) as well as intracellular synthesis. Therefore presence of CXCL13 transcript in retinal endothelial isolates from 5 human cadaver eye donors was evaluated by RT-PCR. CXCL13 transcript was not detected in any endothelial isolate, in contrast to cyclophilin A reference gene (Figure 3I), suggesting that human retinal endothelial cells express CXCL13 protein by transcytosis.
Figure 3.
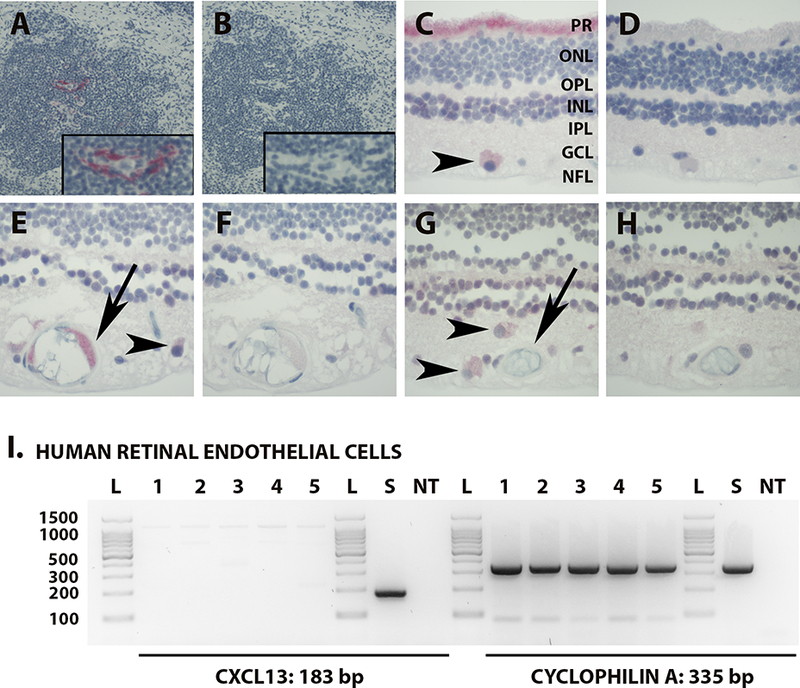
[A & B] Photomicrograph of immunostained human lymph node showing expression of CXCL13 centrally within the follicle of the tonsil (magnified insert) [A]; serial section immunostained with negative control IgG has no corresponding positive staining [B]. Fast Red with hematoxylin counterstain. Original magnification: 100X. [C - H]. Photomicrographs of human neural retina showing photoreceptor processes [C, ‘PR’] and ganglion cells [C, E & G, arrow heads] staining positively for CXCL13, and vascular endothelium staining positively [E, arrow] or negatively [G, arrow] for CXCL13; matching serial sections immunostained with negative control IgG have no corresponding positive staining [D, F & H]. Fast Red with hematoxylin counterstain. Original magnification: 400X. Abbreviations: PR = photoreceptor layer; ONL = outer nuclear layer; OPL = outer plexiform layer; INL = inner nuclear layer; IPL = inner plexiform layer; GCL = ganglion cell layer; NFL = nerve fiber layer. [I] Agarose gels demonstrating CXCL13 and cyclophilin A (reference gene) RT-PCR products from human retinal endothelial cells isolated from eyes of 5 human cadaver donors, as well as human spleen. Expected product sizes: CXCL13 = 184 bp; cyclophilin A = 335 bp. Lane labels: L = DNA ladder; numbers = human eyes (with each number indicating product from the same individual donor); S = human spleen; NT = no cDNA template.
Impact of CXCL13 on human B cell transendothelial migration
Recognizing that retinal endothelial cells do not synthesize CXCL13, assays of B cell migration across human retinal endothelium with ICAM-1 or VCAM-1 blockade were also performed with chemokine added to lower chambers of the transwells. Expression of the CXCL13 receptor, CXCR5, on human peripheral B cell subsets was confirmed by flow cytometry. B cells isolated by negative selection from human peripheral blood included 86% ± 3% CD19+ cells, which represented a balance of immature B cells (defined as CD20+CD27-CD38+), naïve B cells (defined as CD20+CD27-CD38-) and memory B cells (defined as CD20+CD27+CD38-), with a small percentage of plasma cells (defined as CD20-CD27+CD38+) (Figure 4A). A proportion of B cells within each subpopulation expressed CXCR5 (Figure 4B and C). In the migration assay, the number of B cells that moved across simulated human retinal endothelium increased in the presence of CXCL13 under control conditions (Figure 4D). When results for all human subjects were considered together, ICAM-1 blockade, but not VCAM-1 blockade, significantly reduced B cell migration. When results for individual human subjects were considered separately, a significant reduction in B cell migration was observed with antibody-mediated blockade for ICAM-1 in 6 of 10 donors (60%), and with antibody-mediated blockade for VCAM-1 in 1 of 7 (14%) donors. These results suggest that, in the presence – as in the absence – of CXCL13, ICAM-1 exerts more influence over B cell migration across human retinal endothelium across individuals than VCAM-1.
Figure 4.
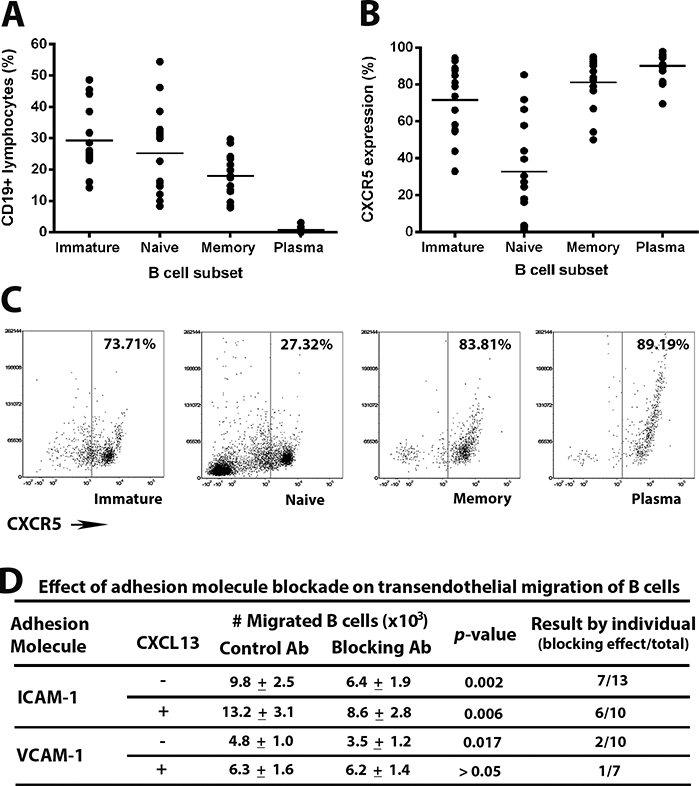
[A-C] Expression of CXCR5 on human peripheral blood B cells of adult human subjects. CD19+ B cells, enriched from peripheral blood leukocytes by negative selection, were classified as immature, naïve, memory and plasma cells on the basis of CD20, CD27 and CD38 expression [A], and percentage of cells expressing CXCR5 was determined for each subset [B]. Circles represent results for individual subjects and crossbars represent mean. Flow dot plots present CXCR5 expression on B cell subsets from one representative human subject [C]. [D] Effect of antibody (Ab)-mediated adhesion molecule blockade on human B cell migration across simulated retinal endothelium, as described in the Figure 1 legend. Migration was studied without or with CXCL13 added to the lower chambers of transwells. Number of migrated B cells is expressed as mean ± standard error of the mean, and compared by one-tailed paired Student’s t-test for each set of conditions. Effect of blockade for individual human subjects is presented separately, as number of subjects whose B cells migrated in significantly reduced numbers in the presence of adhesion molecule blockade, over total number of human subjects.
Discussion
In the human, B cells access the retina to participate in diverse diseases that often impair vision, including vitreoretinal B cell lymphoma, retinal infections and non-infectious uveitis. B cells also may enter the eye in small numbers in health, for the purposes of immune surveillance. The molecular mechanisms of B cell entry into the retina have not been reported previously, but understanding this process at the molecular level will expand knowledge of disease pathogenesis and also may provide leads for the development of new therapeutics. We investigated the involvement of three molecular signals in the entry of B cells into the human retina. Our work demonstrates: 1. the ability of human peripheral blood B cells to cross simulated retinal endothelium; 2. the expression of the B cell chemokine, CXCL13, in intact human retina; and importantly 3. the participation of cell adhesion molecule, ICAM-1, in the migration of human B cells across retinal endothelium in the presence or absence of CXCL13.
Tissue-specific combinations of endothelial adhesion molecules and chemokines direct the migration of leukocyte subpopulations across the vascular endothelium. Immunoglobulin superfamily members, ICAM-1 and VCAM-1, are expressed at relatively high levels by human retinal endothelial cells;(16) they participate in multiple stages of leukocyte trafficking from the circulation to the tissue, including slow rolling over, arrest on and spreading across the endothelium, and in the case of ICAM-1, transendothelial migration.(14) Using antibody-mediated adhesion molecule blockade, we identified a potential role for both ICAM-1 and VCAM-1 in promoting human B cell movement across the retinal endothelium. However, in analyzing our data by individual human subject, it was apparent that ICAM-1 exerted more influence over this process than VCAM-1. Interestingly, Alter et al.(23) were able to significantly inhibit the movement of human CD19+ B cells across temporal lobe endothelial monolayers using antibody targeting ICAM-1, but not antibody targeting VCAM-1, in a transwell system similar to the one we employed.
CXCL13 is a potent B cell chemokine that was described as a homeostatic lymphoid chemokine originally,(21) but later was identified in pathological settings, particularly lymphoma and chronic inflammatory diseases.(24–26) We observed CXCL13 expression by the endothelium of blood vessels in intact normal human retina. This pattern of expression suggests the possibility that CXCL13 might encourage entry of appropriately activated B cells into the eye. Two observations are pertinent: CXCL13 transcript was not detected in non-diseased retinal endothelial cell isolates; and CXCL13 protein was not identified in the endothelium of all retinal blood vessels of the intact retina. These observations imply that expression of the chemokine by retinal endothelium occurs by transcytosis, not synthesis, which is well described for other endothelia.(22) Indeed, we previously reported this phenomenon for brain endothelium in primary central nervous system lymphoma.(27) Thus, any CXCL13 expression by the endothelium of a retinal blood vessel would depend on expression within the region of tissue through which the vessel courses.
We also noted expression of CXCL13 by retinal neurons and the retinal pigment epithelium. CXCL13 expression by retinal pigment epithelium may be particularly relevant to the location of vitreoretinal B cell lymphoma within the eye; malignant B cells typically associate with the epithelium in this tumor.(28) While identification of CXCL13 in non-diseased human neural retina and retinal pigment epithelium is a new finding, the recent publication by Yuen et al.(29) corroborates our observation in studies of myelination in the Fischer 344 rat retina. Chan et al.(30) previously detected CXCL13 transcript and protein in retinal pigment epithelium of three human eyes with vitreoretinal B cell lymphoma, but not in epithelium of one non-diseased eye that was studied in parallel. Individual variation in the retinal level of CXCL13 expression or methodological differences are possible explanations for our contrasting findings.
As a research study conducted in a human system, our investigation of the molecular signals involved in human B cell migration into the retina is limited by its in vitro nature. On the other hand, differences in B cell immunology and immunopathology of humans and mice are well recognized,(31) and the use of human retinal endothelial cells and human B cells gives our findings direct relevance to clinical diseases. Our results suggest ICAM-1 blockade may be an effective treatment in some patients with retinal diseases that are mediated by pathogenic B cells, including vitreoretinal B cell lymphoma and/or some forms of non-infectious uveitis.
Acknowledgements
FUNDING
This work was supported by grants from the National Eye Institute/National Institutes of Health (R21 EY022009) and the Australian Research Council (FT130101648).
Abbreviations:
- CNS
central nervous system
- ICAM-1
intercellular adhesion molecule-1
- VCAM-1
vascular cell adhesion molecule-1
- Ig
immunoglobulin
- V500
Horizon V500
- PE
phycoerthrin
- APC
allophycocyanin
- eF
eFluor
- PBS
phosphate buffered saline
- FBS
fetal bovine serum
- TBS
Tris-buffered saline
- RT
reverse transcription
- PCR
polymerase chain reaction
Footnotes
Declaration of Interest
The authors report no conflicts of interest.
References
- 1.Grossniklaus HE, Geisert EE, Nickerson JM. Introduction to the Retina. Prog Mol Biol Transl Sci. 2015;134: 383–96. [DOI] [PubMed] [Google Scholar]
- 2.Chan CC, Rubenstein JL, Coupland SE, et al. Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. Oncologist. 2011;16(11):1589–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fekkar A, Bodaghi B, Touafek F, et al. Comparison of immunoblotting, calculation of the Goldmann-Witmer coefficient, and real-time PCR using aqueous humor samples for diagnosis of ocular toxoplasmosis. J Clin Microbiol. 2008;46(6):1965–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan CC, Palestine AG, Kuwabara T, et al. Immunopathologic study of Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol. 1988;105(6):607–11. [DOI] [PubMed] [Google Scholar]
- 5.Lubin JR, Albert DM, Weinstein M. Sixty-five years of sympathetic ophthalmia. A clinicopathologic review of 105 cases (1913−−1978). Ophthalmology. 1980;87(2):109–21. [DOI] [PubMed] [Google Scholar]
- 6.Palestine AG, Nussenblatt RB, Chan CC, et al. Histopathology of the subretinal fibrosis and uveitis syndrome. Ophthalmology. 1985;92(6):838–44. [DOI] [PubMed] [Google Scholar]
- 7.Davatchi F, Shams H, Rezaipoor M et al. Rituximab in intractable ocular lesions of Behcet’s disease; randomized single-blind control study (pilot study). Int J Rheum Dis. 2010;13(3):246–52. [DOI] [PubMed] [Google Scholar]
- 8.Loeffler C, Dietz K, Schleich A, et al. Immune surveillance of the normal human CNS takes place in dependence of the locoregional blood-brain barrier configuration and is mainly performed by CD3(+)/CD8(+) lymphocytes. Neuropathology. 2011;31(3):230–8. [DOI] [PubMed] [Google Scholar]
- 9.Crane IJ, Xu H, Wallace C, et al. Involvement of CCR5 in the passage of Th1-type cells across the blood-retina barrier in experimental autoimmune uveitis. J Leukoc Biol. 2006;79(3):435–43. [DOI] [PubMed] [Google Scholar]
- 10.Liversidge J, Sewell HF, Forrester JV. Interactions between lymphocytes and cells of the blood-retina barrier: mechanisms of T lymphocyte adhesion to human retinal capillary endothelial cells and retinal pigment epithelial cells in vitro. Immunology. 1990; 71(3):390–6. [PMC free article] [PubMed] [Google Scholar]
- 11.Xu H, Manivannan A, Jiang HR, et al. Recruitment of IFN-gamma-producing (Th1-like) cells into the inflamed retina in vivo is preferentially regulated by P-selectin glycoprotein ligand 1:P/E-selectin interactions. J Immunol. 2004;172(5):3215–24. [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Manivannan A, Liversidge J, et al. Requirements for passage of T lymphocytes across non-inflamed retinal microvessels. J Neuroimmunol. 2003;142(1–2):47–57. [DOI] [PubMed] [Google Scholar]
- 13.Bharadwaj AS, Schewitz-Bowers LP, Wei L, et al. Intercellular adhesion molecule 1 mediates migration of Th1 and Th17 cells across human retinal vascular endothelium. Invest Ophthalmol Vis Sci. 2013;54(10):6917–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nourshargh S, Alon R. Leukocyte migration into inflamed tissues. Immunity. 2014;41(5):694–707. [DOI] [PubMed] [Google Scholar]
- 15.Nolan DJ, Ginsberg M, Israely E, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Dev Cell 2013;26(2):204–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith JR, Choi D, Chipps TJ, et al. Unique gene expression profiles of donor-matched human retinal and choroidal vascular endothelial cells. Invest Ophthalmol Vis Sci. 2007;48(6):2676–84. [DOI] [PubMed] [Google Scholar]
- 17.Legler DF, Loetscher M, Roos RS, et al. B cell-attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187(4):655–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bharadwaj AS, Appukuttan B, Wilmarth PA, et al. Role of the retinal vascular endothelial cell in ocular disease. Prog Retin Eye Res. 2013;32:102–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Halbert CL, Demers GW, Galloway DA. The E7 gene of human papillomavirus type 16 is sufficient for immortalization of human epithelial cells. J Virol. 1991;65(1):473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furtado JM, Bharadwaj AS, Ashander LM, et al. Migration of Toxoplasma gondii-infected dendritic cells across human retinal vascular endothelium. Invest Ophthalmol Vis Sci. 2012;53(11):6856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286(5447):2098–102. [DOI] [PubMed] [Google Scholar]
- 22.Middleton J, Patterson AM, Gardner L, et al. Leukocyte extravasation: chemokine transport and presentation by the endothelium. Blood. 2002;100(12):3853–60. [DOI] [PubMed] [Google Scholar]
- 23.Alter A, Duddy M, Hebert S, et al. Determinants of human B cell migration across brain endothelial cells. J Immunol. 2003;170(9):4497–505. [DOI] [PubMed] [Google Scholar]
- 24.De Roos AJ, Mirick DK, Edlefsen KL, et al. Markers of B-cell activation in relation to risk of non-Hodgkin lymphoma. Cancer Res. 2012;72(18):4733–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rubenstein JL, Wong VS, Kadoch C, et al. CXCL13 plus interleukin 10 is highly specific for the diagnosis of CNS lymphoma. Blood. 2013;121(23):4740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6(3):205–17. [DOI] [PubMed] [Google Scholar]
- 27.Smith JR, Braziel RM, Paoletti S, et al. Expression of B-cell-attracting chemokine 1 (CXCL13) by malignant lymphocytes and vascular endothelium in primary central nervous system lymphoma. Blood. 2003;101(3):815–21. [DOI] [PubMed] [Google Scholar]
- 28.Chan CC. Molecular pathology of primary intraocular lymphoma. Trans Am Ophthalmol Soc. 2003;101:275–92. [PMC free article] [PubMed] [Google Scholar]
- 29.Yuen TJ, Johnson KR, Miron VE, et al. Identification of endothelin 2 as an inflammatory factor that promotes central nervous system remyelination. Brain. 2013;136(Pt4):1035–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chan CC, Shen D, Hackett JJ, et al. Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003;110(2):421–6. [DOI] [PubMed] [Google Scholar]
- 31.Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731–8. [DOI] [PubMed] [Google Scholar]


