Abstract
We previously reported that low-dose leptin infusions into the third or fourth ventricle that do not affect energy balance when given independently cause rapid weight loss when given simultaneously. Therefore, we tested whether hindbrain leptin enhances the response to forebrain leptin or whether forebrain leptin enhances the response to hindbrain leptin. Rats received fourth-ventricle infusions of saline or 0.01, 0.1, 0.3, or 0.6 μg leptin/day for 13 days. On days 9 and 13, 0.1 μg leptin was injected into the third ventricle. The injection inhibited food intake for 36 h in saline-infused rats but for 60 h in those infused with 0.6 μg leptin/day. Leptin injection increased intrascapular brown fat temperature in leptin-infused, but not saline-infused, rats. In a separate experiment, rats received third-ventricle infusions of saline or 0.005, 0.01, 0.05, or 0.1 μg leptin/day and fourth-ventricle injections of 1.0 μg leptin on days 9 and 13. Leptin injection inhibited food intake, respiratory exchange ratio, and 14-h food intake in rats infused with saline or the two lowest doses of leptin. There was no effect with higher-dose leptin infusions because food intake, body fat, and lean mass were already inhibited. These data suggest that activation of leptin receptors in the hindbrain enhances the response to third-ventricle leptin, whereas activation of forebrain leptin receptors does not enhance the response to fourth-ventricle leptin, consistent with our previous finding that weight loss in rats treated with fourth-ventricle leptin is associated with indirect activation of hypothalamic STAT3.
Keywords: food intake, weight gain, body composition, respiratory exchange ratio, intrascapular brown fat temperature
previous studies have shown that low-dose third- or fourth-ventricle infusions of leptin that do not cause a significant change in food intake or body composition of rats when given independently will cause a rapid and substantial inhibition of food intake and loss of body fat and lean body mass when given simultaneously (4). The weight loss is associated with increased phosphorylation of signal transducer and activator of transcription 3 (STAT3) in hypothalamic nuclei that are known to express leptin receptors (5). In addition, we have shown that weight loss in rats receiving fourth-ventricle infusions of progressively increasing doses of leptin correlates with phosphorylation of STAT3 (p-STAT3) in these hypothalamic nuclei (13). We have interpreted these data as evidence that activation of receptors in both the forebrain and hindbrain is required for a leptin-induced loss of weight and that when leptin concentration is increased in the hindbrain, this results in a lowering of the threshold for activation of forebrain leptin receptors. This mechanism could potentially increase the precision of control of energy balance in a normal weight, leptin-responsive animal by increasing hypothalamic sensitivity to small changes in central leptin that would occur when peripheral leptin concentrations rise because of enlargement of body fat stores.
We and others have reported that single injections (20, 26) or continuous infusions of leptin into either the third (27) or the fourth ventricle (13) inhibit food intake and body weight of rats; however, activation of leptin receptors distant from the site of injection or infusion was not evaluated. Leptin injected into the third ventricle has the potential to diffuse through the brain ventricles and activate receptors in the fourth ventricle (9, 27), and it has been reported that leptin injection (24) or infusion (13) into the fourth ventricle increases p-STAT3 in hypothalamic nuclei. Thus leptin receptors in both the hypothalamus and hindbrain are activated when leptin induces a state of negative energy balance.
In our previous experiments we found that infusions of 0.6 μg leptin/day into the fourth ventricle increase the level of p-STAT3 in hypothalamic nuclei that are known to express leptin receptors. When the rats receive a simultaneous subthreshold infusion of leptin (0.1 μg leptin/day) in the third ventricle, there is further stimulation of hypothalamic p-STAT3, and the animals reduce their food intake and lose weight (5). These data suggest that low levels of leptin in the forebrain and hindbrain act synergistically to produce a state of negative energy balance. The objective of the studies described here was to test whether leptin in the hindbrain enhances the response to leptin in the forebrain or, conversely, whether leptin in the forebrain enhances the response to leptin in the hindbrain. The first experiment was a dose-response study to identify appropriate concentrations of leptin to use for acute, near-threshold-level injections into either the third or the fourth ventricle. The second experiment tested whether infusions of low doses of leptin into the fourth ventricle exaggerated the energetic response to acute injections of leptin into the third ventricle, whereas the third experiment tested whether low-dose infusions of leptin into the third ventricle exaggerated the energetic response to leptin injections into the fourth ventricle.
METHODS
All animals used in these experiments were male Sprague-Dawley rats (Envigo, Tampa, FL) weighing 275–320 g at the start of an experiment. They were housed individually either in hanging wire mesh cages or in calorimetry shoe box cages. Rats in the wire cages had a Nylabone (Nylabone Products, Neptune, NJ) for enrichment. All animals had free access to water and rodent chow (Harlan Teklad Rodent Diet 8604) throughout the experiment, unless indicated otherwise. Body weights were recorded daily. The animal room was maintained at 68–70°F with lights on for 12 h a day from 6:00 AM. All animal procedures were approved by the Institutional Animal Care and Use Committee of Augusta University and were consistent with the Guide for the Care and Use of Laboratory Animals (19).
Experiment 1: dose-response third- or fourth-ventricle leptin injections.
Fourteen rats were fitted with a third-ventricle guide cannula (Plastics One, Roanoke, VA) on the midline, 2.8 mm posterior and −8.3 mm ventrodorsal relative to the bregma. Fourteen rats were fitted with a fourth-ventricle guide cannula on the midline, 2.5 mm anterior and −5.2 mm ventrodorsal from the occipital suture. The rats were anesthetized with ketamine (90 mg/kg) and xylazine (10 mg/kg) during surgery and received 2 mg/kg Ketofen (ketoprofen; Fort Dodge Animal Health, Fort Dodge, IA) analgesic immediately before surgery and again 24 h after surgery. Five days after surgery, third-ventricle cannula placement was tested by injecting 10 μg angiotensin II in 2 μl of sterile saline and monitoring drinking behavior during the 5 min after injection. The injections were delivered from Hamilton syringes over 1 min using an infusion pump (PHD 2000 Infusion pump; Harvard Apparatus, Holliston, MA). All of the rats drank voraciously after the injection. Placement of the fourth-ventricle cannula was confirmed by measuring blood glucose before and 30 min after an injection of 210 μg 5-thio-d-glucose (Sigma-Aldrich, St. Louis, MO) (22). Only those rats in which blood glucose doubled were included in the experiment. Two rats did not meet this criterion.
The rats were housed in individual wire mesh cages, and the experiment was initiated 5 days after testing cannula placement. On each test day, food was removed from the cages at 8:00 AM. Starting at 5:00 PM, the rats were weighed again, and each rat received a 2-μl icv injection of leptin (recombinant rat leptin; R&D Systems, Minneapolis, MN) diluted in 0.9% saline. Third-ventricle injections were 0, 0.1, 0.3, 0.5, or 1.0 μg leptin. Fourth-ventricle injections were 0, 1.0, 1.5, 2.0, or 3.0 μg leptin. Food was returned to the cage at 6:00 PM, and food intakes and body weights were recorded 14, 24, 38, and 62 h after injection. Food was placed on the cage floor, and intake was corrected for spillage. The rats were tested a total of five times with a minimum of 3 days between each test. Each rat was tested with each dose of leptin in random order, and there were at least two rats treated with each dose during each test. Food intake and change in body weight for third- or for fourth-ventricle injections were compared at each time point using one-way ANOVA and post hoc one-tailed paired t-tests between the control (0 μg leptin) and each dose of leptin. Differences were considered significant at P < 0.05. This was a lenient analysis of the data, but because the goal was to identify a threshold dose of leptin, it ensured that we could detect even small differences between treatments.
Experiment 2: energetic response to third-ventricle injections of leptin in rats receiving fourth-ventricle infusions of leptin.
A total of 48 rats weighing ~280 g were housed in wire mesh cages with food offered from the food hopper. After 1 wk of adaptation the rats were fitted with a fourth-ventricle infusion cannula on the midline, 2.5 mm anterior and −6.5 mm ventrodorsal to the occipital suture, and a third-ventricle guide cannula using the coordinates described above. They were allowed 3–5 days to recover from surgery, placement of the injection cannula was confirmed as described above, and then the rats were transferred to calorimetry cages (TSE LabMaster, Metabolic Research Platform; TSE Systems International, Chesterfield, MO). The experiment was completed with 4 cohorts of rats because we have only 12 calorimetry cages. Oxygen consumption, carbon dioxide production, and activity were sampled for 3 min from each cage every 39 min. Values measured during the last of the 3 min were used to calculate energy expenditure expressed both as kilocalories per hour per rat and per unit metabolic body weight (kcal·h−1·body wt−0.75) and respiratory exchange ratio (RER) as an index of macronutrient oxidation. Total activity was measured by InfraMot, food intake was recorded every 39 min, and body weight was recorded manually at 7:30 AM each morning when food hoppers and water bottles were refilled and cage bedding was changed as necessary. Daily calorimetry measures were initiated at 8:00 AM and stopped at 7:20 AM the next day so that only one cycle of measurement was lost each day. After 3–5 days’ adaptation to the calorimeter, the rats were divided into five weight-matched groups, and an Alzet miniosmotic pump (model 2004; Durect, Cupertino, CA) with an infusion rate of 0.25 µl/h was attached to the infusion cannula and delivered 0, 0.01, 0.1, 0.3, or 0.6 μg leptin per 24 h. An iButton (Embedded Data Systems, Lawrenceburg, KY) was placed under the intrascapular white fat, on top of intrascapular brown fat (IBAT), and the pump was placed subcutaneously to the left of the iButton. Temperature was recorded every 30 min until the end of the experiment. On day 9 of infusion, a time when food intake had stabilized, food was removed from the cages at 7:30 AM. Starting at 5:00 PM, each rat was weighed and received a third-ventricle injection of 0 or 0.1 μg leptin in a 2-μl volume of sterile saline delivered over a period of 1 min. Food was returned to the cage at 6:00 PM, calorimetry measures continued, and the rats were weighed 14, 24, 38, 48, and 62 h after the injection. On day 13 the same protocol was followed, and the rats that had been injected with leptin on day 9 were injected with saline and vice versa. On day 16, food was removed from the cages at 7:30 AM. Starting at 9:30 AM, the rats were euthanized by decapitation. Trunk blood was collected for measurement of serum leptin [rat leptin radioimmunoassay (RIA); Millipore], insulin (rat insulin RIA; Millipore), and glucose (glucose assay kit GAGO20; Sigma-Aldrich). The brain was rapidly removed, and tissue blocks containing the hypothalamus or nucleus of the solitary tract (NTS) were dissected as described previously (16), snap frozen, and used to measure p-STAT3 and suppressor of cytokine signaling 3 (SOCS3) by Western blotting (28). Inguinal, epididymal, retroperitoneal, and mesenteric fat depots were dissected and weighed. The carcass including fat depots, but excluding the gastrointestinal tract, was analyzed for composition as described previously (10). Because it was not possible to test the placement of the infusion cannula, carcass fat content at the end of the experiment was used as a surrogate measure that could potentially account for a misplaced cannula that damaged tissue during the 16 days of infusion or for pumps that were not delivering leptin as expected. Three rats (1 infused with saline, 1 with 0.01 μg leptin/day, and 1 with 0.1 μg leptin/day) that had a carcass fat content >2 SD from the mean for their infusion group were removed from the experiment.
Experiment 3: energetic response to fourth-ventricle injections of leptin in rats receiving third-ventricle infusions of leptin.
The design for this experiment was identical to that of experiment 2 except that rats were fitted with a third-ventricle infusion cannula on the midline, 2.5 mm posterior and −9.0 mm ventrodorsal to the bregma, and a fourth-ventricle guide cannula using the coordinates described for experiment 1. Placement of the injection cannula was tested as described for experiment 1. The miniosmotic infusion pumps delivered 0, 0.005, 0.01, 0.05, or 0.1 μg leptin/day, and the fourth-ventricle injections on days 9 and 13 were either saline or 1.0 μg leptin. Body fat content at the end of the experiment was used as a surrogate measure for effective placement of the infusion cannula. Four rats were removed from the study on the basis of body fat mass (1 infused with saline, 1 with 0.005 μg leptin/day, 1 with 0.01 μg leptin/day, and 1 with 0.05 μg leptin/day).
Statistical analysis.
Analysis of data in experiment 1 is described above. The effects of constant leptin infusion in experiments 2 or 3 were compared by repeated-measures ANOVA. Single end point measures, such as body composition, were compared by one-way ANOVA. Responses to saline or leptin injection were compared by two-way repeated-measures ANOVA using rat number as a covariate. Post hoc comparisons were made by Duncan’s multiple-range test. Differences at specific time points during the response to leptin or saline injection within an infusion group were identified using paired t-tests. P < 0.05 was considered statistically significant. Statistical comparisons were made using Statistica version 9.0 (StatSoft, Tulsa, OK). Because of the large amount of data collected during the 60 h after each test injection, the averages (RER and IBAT temperature) or sums (food intake and energy expenditure) for 6-h intervals were compared rather than testing the data collected every 39 min.
RESULTS
Experiment 1: dose-response third- or fourth-ventricle leptin injections.
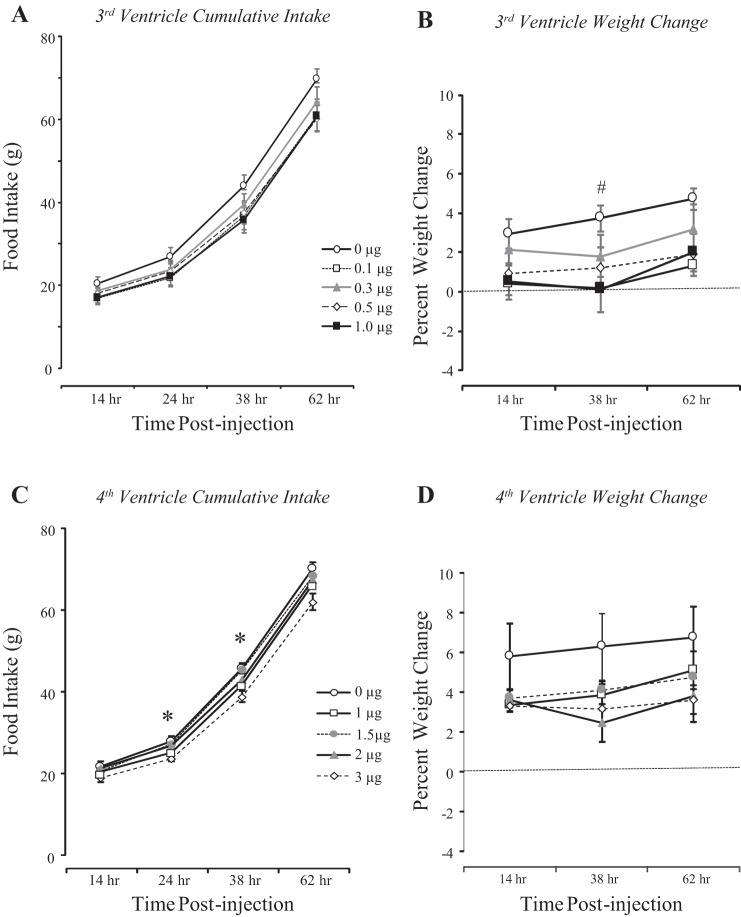
There was no time point at which food intake was different between groups of rats receiving third-ventricle injections of saline or leptin. This was true for both intervaled food intake (data not shown) and cumulative intake (Fig. 1A) during the experimental period. Weight change, rather than body weight, after injection was compared to reduce variability caused by the rats gaining weight continuously during the experimental period. There was a significant effect of leptin on weight change 38 h after injection. Doses of 0.1, 0.5, and 1.0 μg leptin inhibited weight gain, but 0.3 μg did not reach significance (Fig. 1B: P < 0.07).
Fig. 1.
Cumulative food intake and weight change of rats receiving an injection of leptin into the third (A and B) or the fourth ventricle (C and D) at 0 h in experiment 1. Data are means ± SE for groups of 12 (4th ventricle) or 14 (3rd ventricle) rats. *Significant difference (P < 0.5) between rats receiving fourth-ventricle injections of saline and those injected with 3 μg leptin. #Significant difference (P < 0.05) between rats receiving a third-ventricle injection of saline and those injected with 0.1 or 1.0 μg leptin.
Injections of 3 μg leptin into the fourth ventricle inhibited intervaled food intake between 14 and 24 h (data not shown) after injection and cumulative intake at 24 and 38 h after injection (Fig. 1C). There was no effect of any of the other doses of leptin on food intake at any time point. There was no significant inhibition of weight gain by any dose of leptin at any time point (Fig. 1D).
On the basis of these results, we elected to use injections of 0.1 μg leptin into the third ventricle in experiment 2 and 1 μg leptin into the fourth ventricle in experiment 3.
Experiment 2: energetic response to third-ventricle injections of leptin in rats receiving fourth-ventricle infusions of leptin.
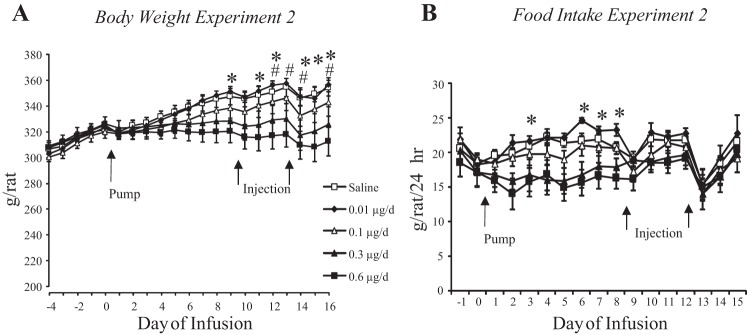
There was a dose-dependent effect of infusing leptin into the fourth ventricle on body weight of the rats (Fig. 2A: leptin, P < 0.05; day, P < 0.001; interaction, P < 0.001). None of the rats lost weight, but the highest dose of 0.6 μg leptin/day inhibited weight gain, so that by the end of the experiment these rats weighed significantly less than those infused with saline or 0.01 µg leptin/day. Similarly, there was a dose-response effect of leptin on daily food intake [Fig. 2B: leptin, P < 0.0001; day, P < 0.001; interaction, not significant (NS)]. Rats infused with 0.6 μg leptin/day ate less than those infused with 0.01 μg leptin/day during the first part of the experiment, but by day 9 there was no longer any difference between any of the groups. RER during the light and dark period both changed with time during the experiment, but there was no effect of leptin infusion on RER (data not shown: leptin, NS; day, P < 0.001; interaction, NS). Energy expenditure of saline-infused rats was greater than that of rats infused with 0.3 or 0.6 μg leptin/day during the dark period only on days 1 and 2 of infusion (data not shown: leptin, P < 0.05; time, P < 0.001; interaction, NS). There was no effect of leptin on expenditure during the light period. Activity during the light phase was higher in rats infused with 0.6 μg leptin/day than saline-infused rats on days 2 and 3 of infusion (leptin, P < 0.03; time, P < 0.001; interaction, NS), but there was no effect of leptin during the dark period (data not shown). There was no effect of leptin infusion on IBAT temperature during either the light or dark periods (data not shown).
Fig. 2.
Daily body weight (A) and food intake (B) of rats receiving fourth-ventricle infusions of leptin. Pumps were attached to the cannulas on day 0, and the rats also received third-ventricle injections of 0.1 μg leptin on days 9 and 13 of infusion. *Significant difference between rats receiving infusions of 0.6 μg leptin/day and those infused with 0.01 μg leptin/day. #Significant difference between rats infused with 0.06 μg leptin/day and those infused with saline.
At the end of the experiment, rats infused with 0.6 μg leptin/day had a reduced carcass weight compared with saline-infused controls, and the weight difference was due to a reduction in both fat and lean tissue (Table 1). The rats infused with 0.3 μg leptin/day also had less fat than the controls, but lean body mass was not significantly different from that of controls. The weight of all fat depots was reduced by ~50% in rats infused with the two highest doses of leptin. There were no differences in serum leptin, insulin, or glucose (data not shown). Hypothalamic p-STAT3 was doubled in rats infused with 0.1, 0.3, or 0.6 μg leptin/day compared with saline-infused rats, but these differences did not reach statistical significance (P < 0.1). There was no effect of fourth-ventricle leptin infusion on hypothalamic SOCS3 or on brain stem p-STAT3 or SOCS3 (Table 2).
Table 1.
Body composition of rats in experiment 2 receiving fourth-ventricle infusions of leptin
| Leptin |
|||||
|---|---|---|---|---|---|
| Saline | 0.01 μg | 0.1 μg | 0.3 μg | 0.6 μg | |
| Fat depots | |||||
| Inguinal, g | 5.3 ± 0.3a | 4.7 ± 0.3a,b | 4.4 ± 0.5a,b | 2.8 ± 0.8b | 2.8 ± 0.5b |
| Epididymal, g | 3.4 ± 0.2a | 3.0 ± 0.2a,b | 2.9 ± 0.3a,b | 1.8 ± 0.5b | 1.6 ± 0.4b,c |
| Retroperitoneal, g | 1.5 ± 0.2a | 1.3 ± 0.1a,b | 1.3 ± 0.2a,b | 0.7 ± 0.3a,b | 0.6 ± 0.2b |
| Mesenteric, g | 1.6 ± 0.2a | 1.4 ± 0.2a,b | 1.3 ± 0.3a,b | 0.8 ± 0.3a,b | 0.7 ± 0.1b |
| Carcass composition | |||||
| Weight, g | 310 ± 6a | 307 ± 5a,b | 298 ± 8a,b | 282 ± 11a,b | 269 ± 9b |
| Fat, g | 24.5 ± 1.7a | 21.4 ± 0.7a,c | 18.5 ± 1.8a,b,c | 11.9 ± 3.5b,c | 11.4 ± 2.5b |
| Lean, g | 274 ± 5a | 272 ± 4a,b | 267 ± 7a,b | 257 ± 8a,b | 247 ± 7b |
| Ash, g | 11.9 ± 0.5 | 13.6 ± 0.8 | 12.2 ± 0.3 | 13.3 ± 0.7 | 11.3 ± 0.4 |
Values are means ± SE for groups of 7–9 rats euthanized after 16 days of fourth-ventricle infusions of different doses of leptin in experiment 2. Values for a specific measure that do not share a common superscripted letter are significantly different at P < 0.05.
Table 2.
Hypothalamic and brain stem p-STAT3 and SOCS3 in experiments 2 and 3
|
Experiment 2: Fourth-Ventricle Leptin Infusion |
|||||
|---|---|---|---|---|---|
| Saline | 0.01 μg Leptin | 0.1 μg Leptin | 0.3 μg Leptin | 0.6 μg Leptin | |
| Hypothalamic p-STAT3/total STAT3 | 0.63 ± 0.16 | 0.63 ± 0.08 | 1.85 ± 0.92 | 1.22 ± 0.37 | 1.64 ± 0.44 |
| Hypothalamic SOCS3/actin | 3.2 ± 0.4 | 4.2 ± 0.6 | 3.3 ± 1.1 | 3.7 ± 0.4 | 3.1 ± 0.5 |
| BS p-STAT3/total STAT3 | 0.44 ± 0.15 | 0.80 ± 0.10 | 0.62 ± 0.19 | 0.54 ± 0.13 | 0.53 ± 0.11 |
| BS SOCS3/actin | 0.15 ± 0.06 | 0.19 ± 0.05 | 0.23 ± 0.03 | 0.22 ± 0.02 | 0.20 ± 0.02 |
| Experiment 3: Third-Ventricle Leptin Infusion | |||||
| Saline | 0.005 μg Leptin | 0.01 μg Leptin | 0.05 μg Leptin | 0.1 μg Leptin | |
| Hypothalamic p-STAT3/total STAT3 | 0.52 ± 0.15 | 0.45 ± 0.09 | 0.90 ± 0.11 | 1.01 ± 0.23 | 0.99 ± 0.17 |
| Hypothalamic SOCS3/actin | 0.47 ± 0.18a | 0.26 ± 0.11a,b | 0.15 ± 0.12a,b | 0.07 ± 0.01b | 0.04 ± 0.01b |
| BS p-STAT3/total STAT3 | 0.64 ± 0.13 | 0.74 ± 0.19 | 0.62 ± 0.16 | 0.53 ± 0.13 | 0.42 ± 0.10 |
| BS SOCS3/actin | 0.52 ± 0.13 | 0.51 ± 0.12 | 0.51 ± 0.07 | 0.64 ± 0.04 | 0.73 ± 0.12 |
Values are means ± SE for groups of 7–9 rats euthanized after 16 days of third- or fourth-ventricle infusions of different doses of leptin in experiments 2 and 3. Data are arbitrary units from Western blot analysis of tissue samples and cannot be directly compared between experiments or between hypothalamus and brain stem because samples from each brain area for each experiment were run at different times. BS, brain stem. Values for a specific measure that do not share a common superscripted letter are significantly different at P < 0.05.
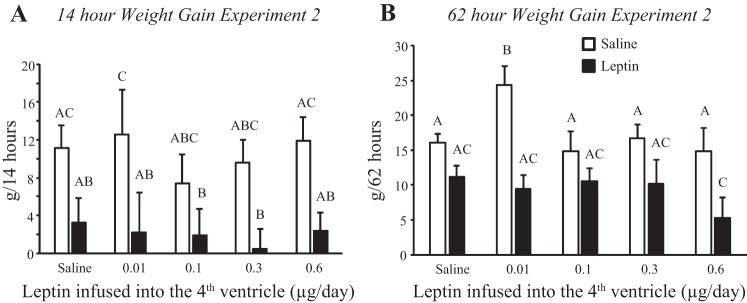
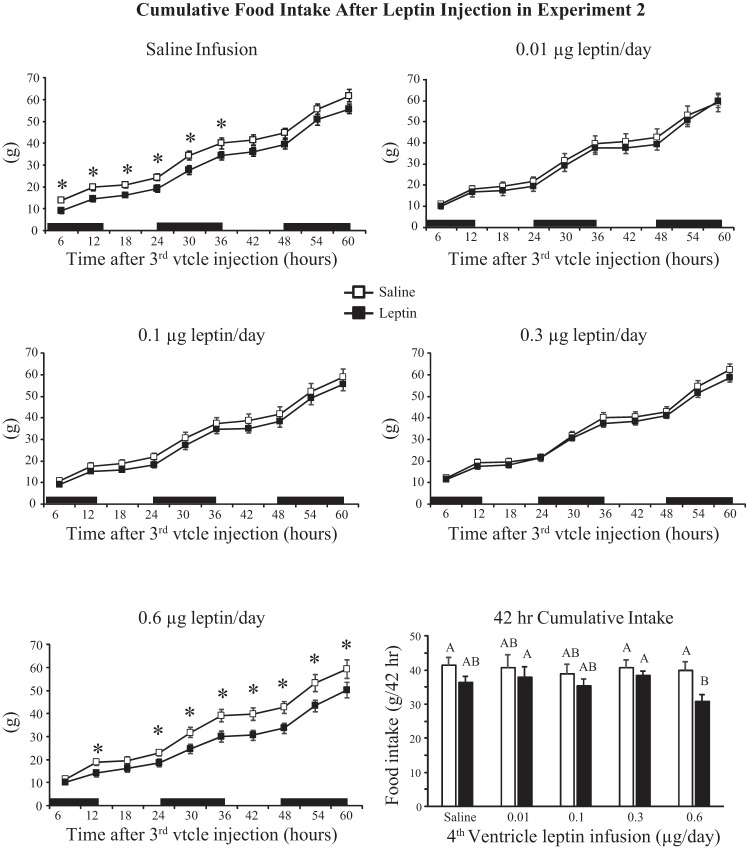
Injection of 0.1 μg leptin into the third ventricle of rats receiving fourth-ventricle infusions inhibited weight gain (Fig. 3A: infusion, NS; injection, P < 0.001; time, P < 0.001; injection × time, P < 0.01). Fourteen hours postinjection this reached significance only for rats infused with 0.01 μg leptin/day and was due to increased weight gain following saline injection, rather than exaggerated weight loss after leptin injection. The difference in weight gain at 62 h was significant for rats infused with 0.01 or 0.6 μg leptin/day. (Fig. 3B: infusion, NS; injection, P < 0.001; time, P < 0.001; infusion × time, P < 0.01). In rats infused with 0.01 μg leptin/day the significance was due to increased weight gain following saline injection, but in rats infused with 0.6 μg leptin/day it was due to an exaggerated inhibition of gain by leptin. There was no effect of leptin injection on food intake of rats infused with 0.01, 0.1, or 0.3 μg leptin/day, but intake was inhibited for 36 h in saline-infused rats (Fig. 4) and for at least 60 h in rats infused with 0.6 μg leptin/day (Fig. 4: infusion, NS; injection, P < 0.001; time, P < 0.001; infusion × time, P < 0.001; injection × time, P < 0.001). Comparison of intakes at 42 h, which was the first time point at which leptin inhibited food intake in only the 0.6 μg leptin/day rats, showed that the significance was due to a lower intake following leptin injection, rather than an increase in intake following saline injection (Fig. 4).
Fig. 3.
Weight gain of rats in experiment 2 following a third-ventricle injection of saline or 0.1 μg leptin. A: 14-h weight gain. B: 62-h weight gain. Data are means + SE for groups of 6–10 rats. Values for a specific parameter that do not share a common letter are significantly different at P < 0.05.
Fig. 4.
Cumulative food intake of rats in experiment 2 during the 60 h following a third-ventricle injection of either saline or 0.1 μg leptin. *Significant inhibition of intake following leptin injection. Values for intake at 42 h that do not share a common letter are significantly different at P < 0.05. Data are means ± SE for groups of 6–10 rats. Black bars indicate the dark phase of the light cycle; vtcle, ventricle.
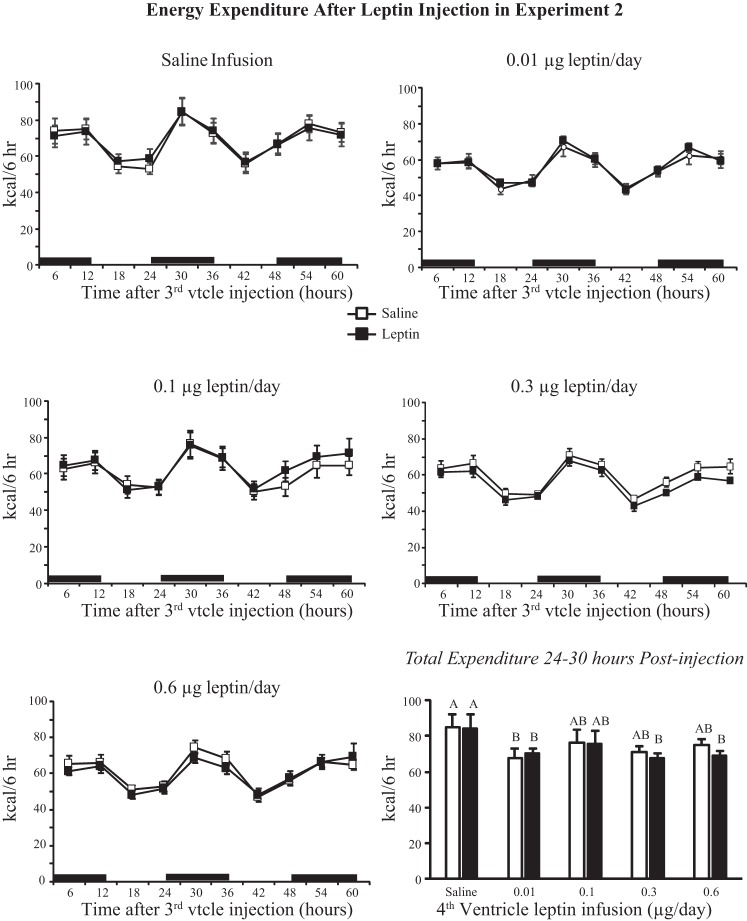
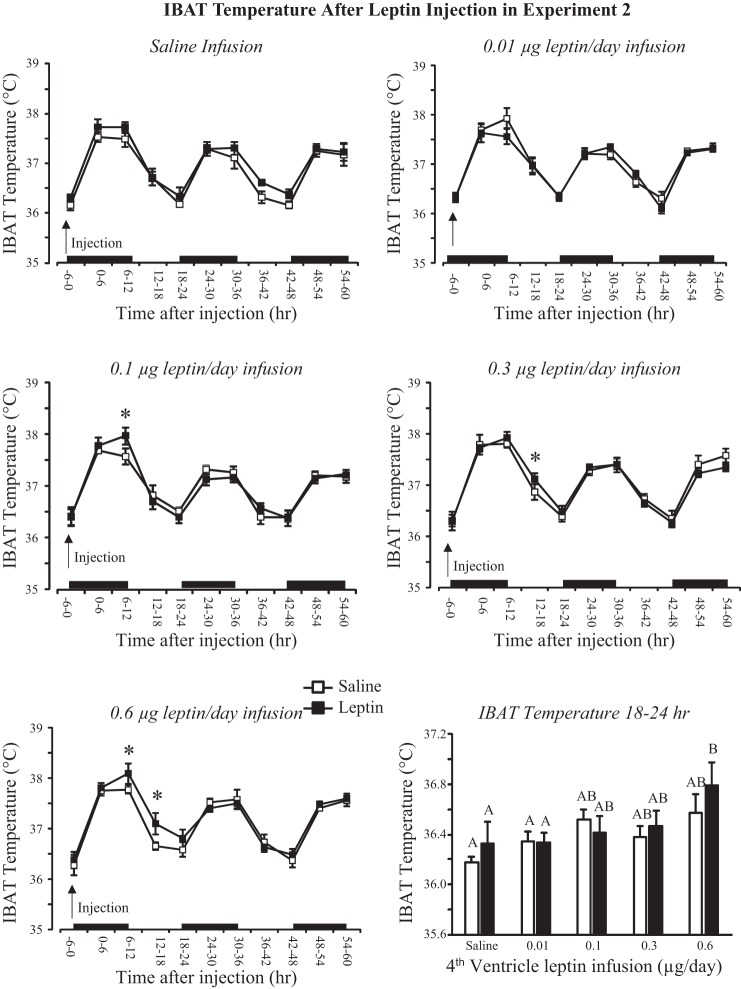
There was no effect of leptin injection on expenditure in any treatment group (Fig. 5). Energy expenditure of all rats infused with leptin tended to be lower than that of saline-infused rats (infusion, P < 0.01; injection, NS; time, P < 0.001). This is illustrated in Fig. 5, which shows expenditure of rats for the 6-h time interval 24–30 h after injection. Rats infused with 0.3 or 0.6 μg leptin/day and injected with leptin and rats infused with 0.1 μg leptin/day and injected with saline or leptin had a lower expenditure than that of saline-infused rats. There was no effect of leptin infusion or injection on RER (data not shown). IBAT temperature was increased by leptin infusion (infusion, P < 0.03; injection, NS; time, P < 0.001). There was no effect of third-ventricle leptin injection on IBAT temperature in saline- or 0.01 μg leptin/day-infused animals. There was a small but significant effect of leptin on IBAT temperature during the 24 h after injection in the other treatment groups, and this effect was greatest in rats infused with 0.6 μg leptin/day (Fig. 6). Comparison of average IBAT temperature during the 6-h interval starting 18 h after injection shows that there was trend for IBAT temperature to be higher in leptin-infused than saline-infused rats, but the difference was significant only for rats infused with 0.6 μg leptin/day and injected with leptin (Fig. 6).
Fig. 5.
Energy expenditure of rats in experiment 2 during the 60 h following a third-ventricle injection of either saline or 0.1 μg leptin. There was no effect of leptin injection on expenditure of any treatment group. Energy expenditure 24–30 h after injection illustrates the effect of fourth-ventricle leptin infusion on expenditure. Values for a specific parameter that do not share a common letter are significantly different at P < 0.05. Data are means ± SE for groups of 6–10 rats.
Fig. 6.
Intrascapular brown fat (IBAT) temperature of rats in experiment 2 during the 60 h following a third-ventricle injection of either saline or 0.1 μg leptin. *Significant elevation in temperature following leptin injection. Average temperatures between 18 and 24 h that do not share a common letter are significantly different at P < 0.05. Data are means ± SE for groups of 6–10 rats. Black bars indicate the dark phase of the light cycle.
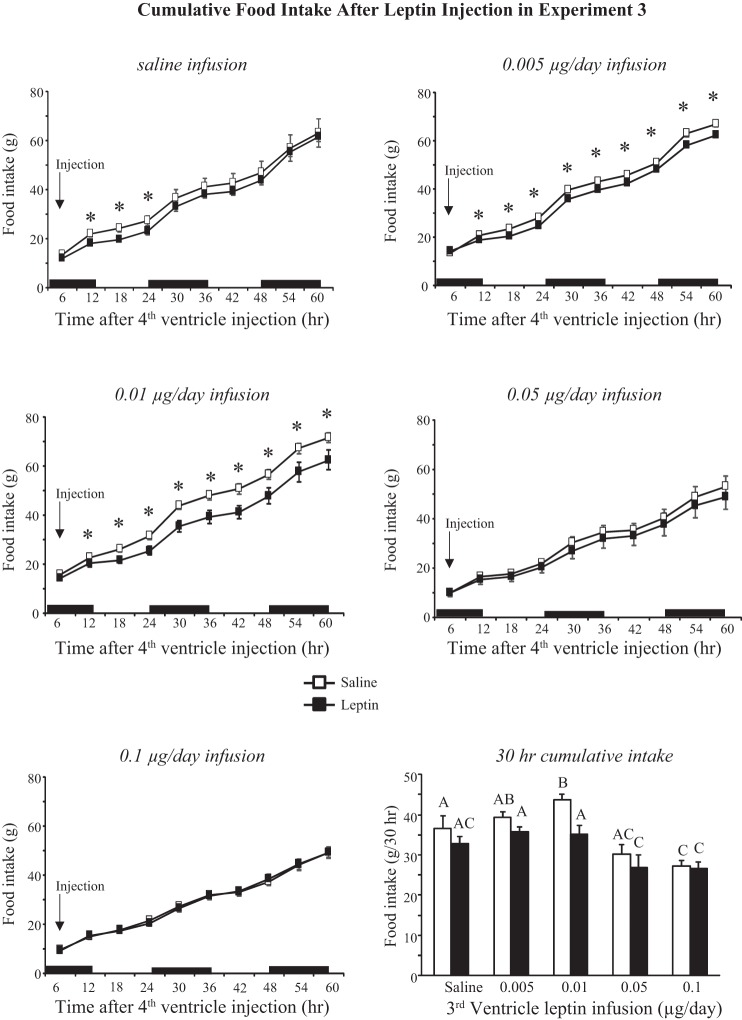
Experiment 3: energetic response to fourth-ventricle injections of leptin in rats receiving third-ventricle infusions of leptin.
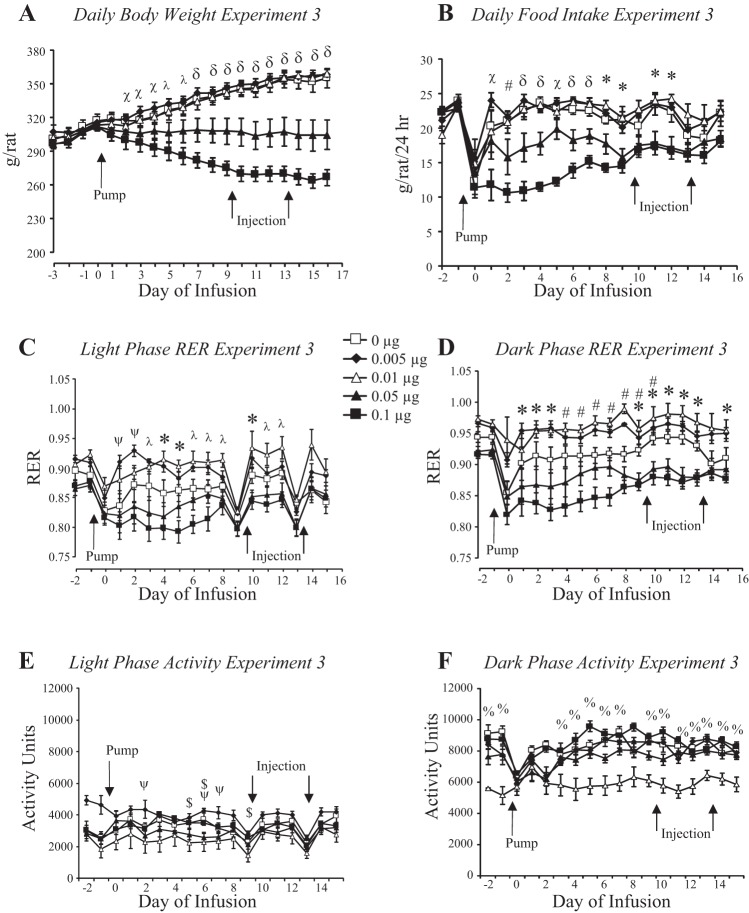
There was a dose-response relationship between third-ventricle infusion and daily body weight of the rats (Fig. 7A: leptin, P < 0.001; day, P < 0.001; interaction, P < 0.001). Rats infused with saline or 0.005 or 0.01 μg leptin/day gained weight during the experimental period, whereas rats infused with 0.05 μg leptin/day stopped gaining weight and those infused with 0.1 μg leptin/day lost weight. The weight of rats infused with 0.05 μg leptin/day was significantly different from controls from day 7 and for those infused with 0.1 μg leptin from day 2. The initial weight loss was associated with an inhibition of 24-h food intake (Fig. 7B: leptin, P < 0.001; day, P < 0.001; interaction, P < 0.001), which was inhibited in the 0.1 μg-infused rats from day 1 and in the 0.05 μg-infused rats from day 3. By the end of the experiment the intake of both of these groups was the same as that of controls but less than that of rats infused with 0.005 or 0.01 μg leptin/day. RER of rats infused with 0.1 μg leptin was lower than that of other groups on the days when food intake was inhibited (leptin, P < 0.001; day, P < 0.001; interaction, NS). The differences were greater during the dark (Fig. 7D) than the light phase (Fig. 7C), consistent with rats consuming a majority of their food at night. The RER of rats infused with 0.005 or 0.01 μg leptin/day tended to be higher than that of other groups. This was not due to diet composition because all of the rats were offered the same diet. There was no difference in energy expenditure during the light period. Analysis of variance identified an interaction between leptin infusion and day of infusion on energy expenditure during the dark period (leptin, NS; day, P < 0.001; interaction, P < 0.001), but post hoc analysis did not find any difference between groups on any specific day (data not shown). There was a significant interaction between leptin infusion and day of infusion on activity during the light period (leptin, NS; day, P < 0.001; interaction, P < 0.006). Post hoc analysis identified a significantly greater activity in rats infused with 0.1 μg leptin/day compared with those infused with 0.005 μg leptin/day on only a few days of the experiment. During the dark phase the activity of rats infused with 0.01 μg leptin/day was consistently lower than that of other groups even before the infusion started (Fig. 7F: leptin, P < 0.001; day, P < 0.001; interaction, P < 0.01).
Fig. 7.
Daily body weight (A), food intake (B), respiratory exchange ratio (RER; C and D), and activity (E and F) of rats in experiment 3 that received third-ventricle infusions of increasing doses of leptin. Pumps were attached to the third-ventricle cannulas on day 0, and rats received fourth-ventricle injections of 1.0 μg leptin or saline on days 9 and 13 of infusion. Data are means ± SE for groups of seven to nine rats. *Significant difference between 0.1 and 0.05 μg/day groups and 0.005 and 0.01 μg leptin/day rats. #Significant difference between 0.1 μg leptin/day and saline-infused groups. λSignificant difference between rats infused with 0.1 μg leptin/day and those infused with 0.05 and 0.01 μg leptin/day. ψSignificant difference between rats infused with 0.005 μg leptin/day and those infused with 0.05 and 0.1 μg leptin/day. δRats infused with 0.1 or 0.05 μg leptin/day were different from all other groups. χSignificant difference between rats infused with 0.1 μg leptin/day and those infused with 0, 0.005, and 0.01 μg leptin/day. $Significant difference between rats infused with 0.01 μg leptin/day and those infused with 0.005 and 0.1 μg leptin/day or saline. %Significant difference between rats infused with 0.01 μg leptin/day and all other groups.
At the end of the experiment, the carcass weights of rats infused with 0.05 or 0.1 μg leptin/day were significantly different from the other groups (Table 3). The rats lost both fat and lean tissue. Loss of fat was reflected in all of the fat depots measured. Serum leptin was significantly reduced in the rats that had lost body fat, but there were no differences in serum insulin or blood glucose levels (Table 3). Hypothalamic p-STAT3 was doubled in rats infused with 0.01, 0.05, or 0.1 μg leptin/day compared with saline-infused rats, but this did not reach statistical significance (P < 0.09). Hypothalamic SOCS3 was significantly (P < 0.05) decreased in rats infused with 0.05 or 0.1 μg leptin/day compared with saline-infused rats (Table 2).
Table 3.
Body composition of rats in experiment 3 receiving third-ventricle infusions of leptin
| Leptin |
|||||
|---|---|---|---|---|---|
| Saline | 0.005 μg | 0.01 μg | 0.05 μg | 0.1 μg | |
| Fat depots | |||||
| Inguinal, g | 4.79 ± 0.35a | 5.00 ± 0.20a | 5.49 ± 0.24a | 2.06 ± 0.62b | 1.04 ± 0.04b |
| Epididymal, g | 3.03 ± 0.19a | 3.28 ± 0.22a | 3.16 ± 0.18a | 1.35 ± 0.43b | 0.28 ± 0.08b |
| Retroperitoneal, g | 1.32 ± 0.18a | 1.58 ± 0.12a | 1.53 ± 0.14a | 0.42 ± 0.16b | 0.25 ± 0.03b |
| Mesenteric, g | 1.26 ± 0.20a | 1.59 ± 0.12a | 1.53 ± 0.14a | 0.42 ± 0.16b | 0.25 ± 0.28b |
| Carcass composition | |||||
| Weight, g | 308 ± 7a | 315 ± 5a | 312 ± 5a | 263 ± 14b | 228 ± 6b |
| Fat, g | 19.1 ± 1.9a | 21.4 ± 0.9a | 22.0 ± 1.2a | 7.7 ± 2.6b | 2.5 ± 0.1b |
| Lean, g | 275 ± 6a | 279 ± 5a | 277 ± 5a | 246 ± 11b | 217 ± 6b |
| Ash, g | 13.1 ± 0.8 | 14.8 ± 1.2 | 13.1 ± 0.6 | 10.0 ± 0.7 | 8.4 ± 0.9 |
| Serum assays | |||||
| Leptin, ng/ml | 2.2 ± 0.4a | 2.2 ± 0.4a | 1.9 ± 0.2a | 0.7 ± 0.2b | 0.3 ± 0.1b |
| Glucose, mg/dl | 200 ± 7 | 215 ± 15 | 198 ± 23 | 158 ± 14 | 193 ± 8 |
| Insulin, ng/ml | 0.82 ± 0.28 | 1.69 ± 0.48 | 1.94 ± 0.26 | 0.90 ± 0.30 | 1.41 ± 0.51 |
Values are means ± SE for groups of 7–9 rats euthanized after 16 days of third-ventricle infusions of different doses of leptin in experiment 3. Values for a specific measure that do not share a common superscripted letter are significantly different at P < 0.05.
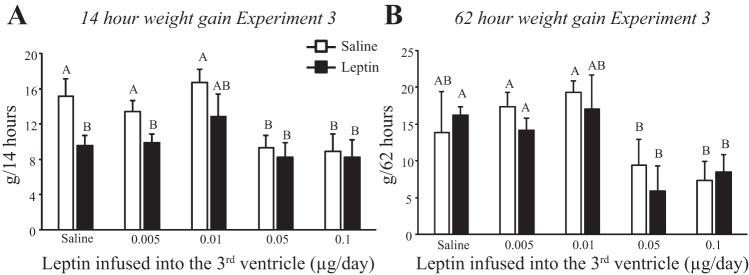
Injection of 1 μg leptin into the fourth ventricle inhibited weight gain for 14 h in rats receiving third-ventricle infusions of saline or 0.005 μg leptin/day. Weight gain was already inhibited in rats infused with the two highest doses of leptin, and leptin injection did not suppress it any further. There was no effect of leptin on weight gain of any of the rats at later time points; Fig. 8B shows data from 62 h postinjection as an example.
Fig. 8.
Weight gain of rats in experiment 3 following a fourth-ventricle injection of saline or 1.0 μg leptin. A: 14-h weight gain. B: 62-h weight gain. Data are means + SE for groups of 7–9 rats. Values for weight gain at either 14 or 62 h after injection that do not share a common letter are significantly different at P < 0.05.
Comparison of cumulative food intake during the 60 h following fourth-ventricle injection of 1 μg leptin or saline showed significant effects of both leptin infusion and injection (infusion, P < 0.0001; injection, P < 0.001; time, P < 0.0001; infusion × time, P < 0.0001; injection × time, P < 0.00001; infusion × injection × time, P < 0.04). Cumulative intake, compared by paired t-test, was inhibited in saline-infused rats from 12 to 24 h postinjection and in those infused with 0.005 or 0.01 μg leptin/day from 12 to 60 h postinjection (Fig. 9). This exaggerated effect of leptin was due to higher intakes in the control condition of saline injection, rather than a greater suppression of intake by leptin, and this is illustrated by comparison of cumulative intakes 30 h after injection (Fig. 9). In saline-injected rats the 30-h intake of rats infused with 0.01 μg leptin/day was significantly higher than that of rats infused with saline. This difference was initiated within minutes after the injection because the 0.01 μg leptin/day rats ate 44% more food than any other group during the first 39 min after injection (data not shown). Food intake of rats infused with the two highest doses of leptin and injected with saline was already lower than that of leptin-injected rats in the other treatment groups, and leptin injection did not reduce it any further (Fig. 9).
Fig. 9.
Cumulative food intake of rats in experiment 3 during the 60 h following a fourth-ventricle injection of either saline or 1.0 μg leptin. *Significant inhibition of intake following leptin injection. Values for intake at 30 h that do not share a common letter are significantly different at P < 0.05. Data are means ± SE for groups of 7–9 rats. Black bars indicate the dark phase of the light cycle.
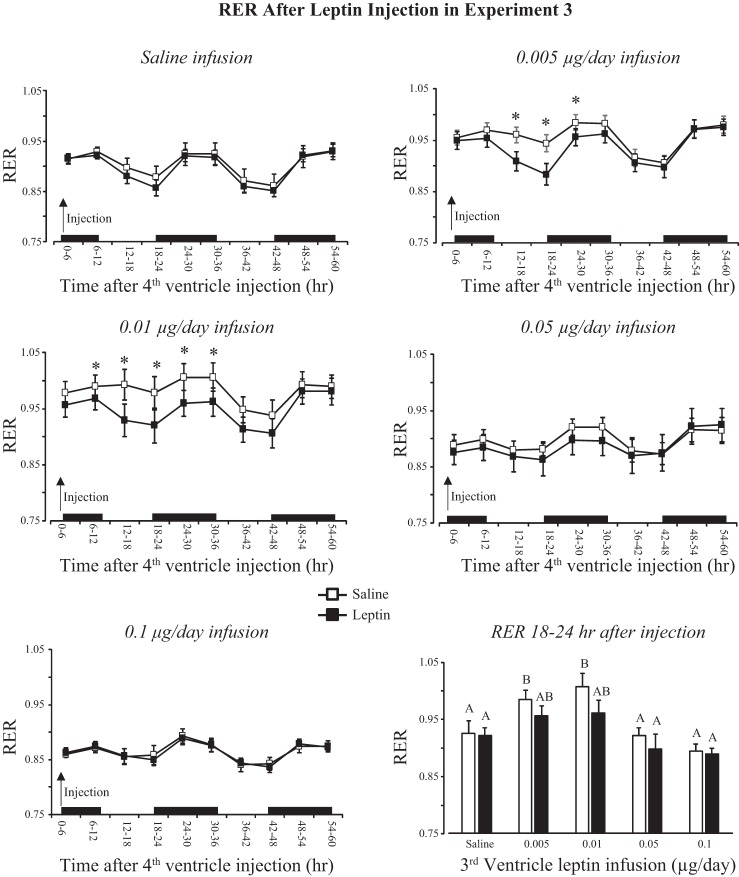
RER measured after injection was consistent with the data on food intake; basal RER was higher in rats infused with 0.005 or 0.01 μg leptin/day than any other group. Leptin injection inhibited RER only in these groups, and the inhibition was most obvious during the light period that started 12 h after the injection (Fig. 10: infusion, P < 0.001; injection, P < 0.02; time, P < 0.001; infusion × time, P < 0.001; injection × time, P < 0.001). Average RER during the 6 h between 12:00 and 6:00 PM on the day after injection is plotted in Fig. 10 and shows that RERs of rats infused with 0.005 or 0.01 μg leptin/day were higher than those of other groups (infusion, P < 0.001; injection, P < 0.007; injection × infusion, NS). There were no effects of leptin infusion or leptin injection on energy expenditure or IBAT temperature during the 60 h following injection (data not shown). Leptin injection did not have any effect on activity of any group of rats, but activity of rats infused with 0.01 μg leptin/day was lower than that of the other groups of rats during the dark period (data not shown: infusion, P < 0.001; injection, NS; time, P < 0.001; infusion × time, P < 0.001). This was consistent with the daily measures of activity shown in Fig. 7.
Fig. 10.
RER of rats in experiment 3 during the 60 h following a fourth-ventricle injection of either saline or 1.0 μg leptin. *Significant differences in RER following saline or leptin injection. Average values for RER during 18 and 24 h postinjection that do not share a common letter are significantly different at P < 0.05. Data are means ± SE for groups of 7–9 rats. Black bars indicate the dark phase of the light cycle.
DISCUSSION
The objective of the experiments described here was to determine whether leptin in the hindbrain facilitated leptin response in the forebrain or whether leptin in the forebrain facilitated the response to leptin in the hindbrain. The effects of leptin injection in leptin-infused rats were small, but this was due to the deliberate use of threshold doses to maximize the opportunity to detect an enhanced response. The doses of leptin used for infusion were based on our previous reports that simultaneous fourth-ventricle infusion of 0.6 μg leptin/day and third-ventricle infusion of 0.1 μg leptin/day inhibited food intake and caused weight loss (4, 5). These doses were the highest used in experiments 2 and 3, respectively, and lower-dose infusions were included to determine whether there was a lower threshold for an interaction between the two sites. The results of experiments 2 and 3 suggest that fourth-ventricle infusions of leptin enhance the response to a third-ventricle injection, whereas third-ventricle infusions of leptin do not change the response to fourth-ventricle injections.
In experiment 2, fourth-ventricle leptin infusion exaggerated the effect of third-ventricle leptin injection on food intake by extending the relative hypophagia from 36 h in saline-infused rats to at least 60 h in the 0.6 μg leptin/day-infused animals. Infusion of 0.1 μg leptin/day or more also caused a small increase in IBAT temperature 6–18 h after injection with the greatest effect in rats infused with 0.6 μg leptin/day. It is worth noting that this enhancement was also characterized by an extension of the duration of stimulation of IBAT temperature. This suggests that either leptin is cleared more slowly from the cerebrospinal fluid (CSF) during the postinjection period or that fourth-ventricle leptin infusion allows the forebrain to continue to respond to leptin as the concentration gradually declines in the postinjection period. The second option would imply that fourth-ventricle leptin infusions lower the threshold of response to leptin in the third ventricle and would be consistent with previous reports that leptin injections into the fourth ventricle or directly into the NTS lead to activation of p-STAT3 in the hypothalamus (13, 24). A third alternative is that leptin injected into the third ventricle diffuses into the fourth ventricle and the resultant rise in fourth-ventricle leptin concentration is responsible for the enhanced response to leptin injection, but this would be expected to exaggerate, not extend, the changes that follow leptin injection. In a dose-response study we previously found that hypophagia and weight loss occurred in rats receiving fourth-ventricle infusions of 0.9 μg or more of leptin per day. These effects were associated with a significant increase in expression of p-STAT3 in the hypothalamus even though the leptin was administered in the fourth ventricle. Injection of 0.1 μg leptin in the third ventricle of rats infused with 0.6 μg leptin/day in this study could not have raised fourth-ventricle leptin concentrations to the same level as in rats infused with 0.9 μg leptin/day. If, however, fourth-ventricle leptin lowers the threshold for response to leptin in the forebrain, then that would explain why this low-dose third-ventricle injection of leptin had a greater effect on food intake in rats infused with 0.6 μg leptin/day than those infused with saline. In the experiments described here, p-STAT3 doubled in the hypothalamus of rats receiving the higher-dose fourth-ventricle leptin infusions, which is also consistent with fourth-ventricle leptin lowering the threshold for activation of hypothalamic leptin receptors and resulting in activation of p-STAT3 by basal endogenous concentrations of leptin.
By contrast, third-ventricle leptin infusion blunted the difference between fourth-ventricle saline and leptin injections because baseline food intake and weight change of rats receiving the higher-dose infusions were already reduced to those found in leptin-injected, saline-infused rats. There was no additive effect of third-ventricle leptin infusion and fourth-ventricle leptin injection, which implies that the sensitivity and responsiveness to leptin in the fourth ventricle were unchanged by the third-ventricle infusions. Because CSF drains from the third ventricle into the fourth ventricle, it is possible that the reduced food intake and weight loss in rats receiving the highest doses of third-ventricle leptin were due to the combined effects of leptin in the forebrain and hindbrain, consistent with our hypothesis that weight loss occurs only when leptin receptors are activated in both of these areas of the brain. If the hindbrain receptors were already activated, then this also would explain why there was no additive effect of third-ventricle leptin infusion and injection of a threshold dose of leptin into the fourth ventricle. Western blots did not show an increase in brain stem p-STAT3 of rats receiving third-ventricle infusions of leptin, but there also was no detectable increase in brain stem p-STAT3 of rats receiving fourth-ventricle leptin infusions. This is consistent with previous results (4, 5), and it is possible that leptin is activating a different signaling pathway in the hindbrain. ERK activation has been measured in other studies, but there was no measurable response to fourth-ventricle leptin infusions (4, 13). The Western blots did show that infusing 0.01 μg (or more) of leptin per day into the third ventricle doubled hypothalamic p-STAT3. Although this difference was not significant, the two highest doses of leptin also inhibited SOCS3 expression, which may have facilitated leptin activity as these were the two groups of rats that lost substantial amounts of body fat.
We have previously reported that rats receiving either third-ventricle infusions of 0.1 μg leptin or fourth-ventricle infusions of 0.6 μg leptin do not reduce their food intake or lose weight but that simultaneous infusions cause a substantial hypophagia and weight loss. In both experiments 2 and 3, rats infused with the two highest doses of leptin lost both fat and lean tissue. The difference between these and previous experiments may be explained by the rats in previous studies receiving simultaneous infusions of PBS. Therefore, if leptin diffused into the fourth ventricle of rats that were receiving third-ventricle infusion of leptin, the final concentration would have been diluted by the PBS infusion into the fourth ventricle. Similarly, infusion of PBS into the third ventricle of rats receiving fourth-ventricle infusions of leptin would have diluted endogenous leptin, reducing the likelihood of activation of hypothalamic leptin receptors. Even if this were the case, we previously found that independent infusion of 0.1 μg leptin/day into the third ventricle or 0.6 μg leptin/day into the fourth ventricle each increased p-STAT3 in areas of the hypothalamus that express leptin receptors and that the level of activation increased significantly when the infusions were applied simultaneously (5). The only group of rats in which a leptin infusion enhanced the response to a leptin injection were those receiving fourth-ventricle infusions of 0.6 μg leptin/day. These rats had less fat and lean tissue than the other groups in experiment 2, and it may be argued that the change in body composition was responsible for the extended response to leptin. However, rats receiving fourth-ventricle infusions of 0.3 μg leptin/day had less body fat than their saline-infused controls, and those receiving third-ventricle infusions of 0.05 or 0.1 μg leptin/day had less fat and lean tissue than their saline-infused controls. None of these groups showed an exaggerated response to the acute leptin injections, supporting the conclusion that the enhanced response in 0.6 μg leptin/day rats was a specific effect of the leptin infusion.
In both experiments 2 and 3 the combination of leptin in the third and fourth ventricle, whether by injection or infusion, inhibited food intake. In experiment 3, this was accompanied by a drop in RER, but there was no effect of leptin on activity or energy expenditure. In experiment 2, fourth-ventricle infusion plus third-ventricle injection increased IBAT temperature but had no effect on energy expenditure, RER, or activity. The increase in IBAT temperature is consistent with previous reports that leptin injections increase body temperature through activation of hindbrain melanocortin receptors (26). We did not monitor site-specific leptin receptor activation and assumed that similar sites would have been activated in the two experiments. The difference in response, however, implies that either different sites were activated in different conditions or that the relative concentration of leptin at any particular site is important in determining whole animal response to leptin. Energy expenditure was not changed by leptin injection in either study, consistent with previous observations in leptin-infused rats (4) and in weight-reduced human subjects (23) that leptin maintains energy expenditure even in conditions of negative energy balance when energy efficiency would be a more appropriate response.
It is clear from the experiments described here in addition to many in the literature (3, 13, 20, 21, 26) that there is a large difference in hypothalamic vs. hindbrain leptin sensitivity. The 10-fold lower sensitivity in the hindbrain could be interpreted as it having a less important role in mediating the effects on energy balance; however, it is possible that the difference in sensitivity is associated with the different functions of the brain sites that express leptin receptors. It has been shown that hindbrain leptin exaggerates the response to peripheral satiety signals such as CCK (6, 17) and gastric distention (15). Therefore, in this context, the transient rise in leptin that follows a meal (25) may be considered to function as a short-term satiety signal. By contrast, the experiments described here are testing the role of hindbrain leptin in determining energy balance. We hypothesize that a sustained rise in hindbrain leptin activity would lower the threshold for activation of hypothalamic leptin receptors. Thus a small increase in hypothalamic leptin would result in a relatively large inhibition of food intake, increasing the precision of control of energy balance. In previous studies we found a limited number of hypothalamic areas that responded to fourth-ventricle leptin including the arcuate, ventromedial, and dorsomedial nuclei. These hypothalamic nuclei have an intact blood-brain barrier, and leptin entry is determined by transport mechanisms (1). Arcuate neurons that express leptin receptors send projections into the median eminence (7) and may be expected to be the most sensitive to changes in circulating concentrations of leptin, but they are also the first to develop leptin resistance (18). Correction of positive energy balance in response to a small change in central leptin concentrations may help protect against excess adiposity and development of leptin resistance in arcuate neurons (18).
In a previous study, we demonstrated that activation of hypothalamic nuclei in rats receiving simultaneous infusions of low doses of leptin into both the third and fourth ventricle was limited to those that showed increased levels of p-STAT3 (5). Therefore, it appears to be a mechanism designed to specifically increase leptin responsiveness. There are several possible mechanisms by which hindbrain leptin may influence hypothalamic leptin sensitivity. The first is direct diffusion of leptin from the hindbrain to raise local leptin concentrations in the forebrain. CSF cannot diffuse directly from the fourth to the third ventricle (2), but leptin could be transported to more rostral areas of the brain in subarachnoid space (9). This possibility was addressed by Ruiter et al. (24), who reported that fourth-ventricle or NTS injections of leptin increased p-STAT3 in hypothalamic nuclei of rats. They determined that injections of fluorogold into either the fourth ventricle or NTS were carried into the subarachnoid space but did not reach the brain areas that showed increased levels of p-STAT3. A third possibility, which needs to be explored, is that NTS neurons that express leptin receptors project to the hypothalamic sites and activate or release inhibition on leptin-responsive hypothalamic cells.
Although fourth-ventricle injection of 1 μg leptin did not have any effect on food intake in experiment 1, it did inhibit food intake of rats receiving third-ventricle infusion of saline or the lowest doses of leptin in experiment 3. This difference in sensitivity may have been due to the difference in housing conditions. In experiment 1 the rats were housed in hanging wire mesh cages and were likely subjected to a greater thermogenic stress than those housed in enclosed calorimetry cages. The increased requirement for heat production may have overridden the mild hypophagia caused by leptin injection. In addition, in experiment 1, food intake was measured every 12 h by weighing food remaining in the cage and spillage under the cage, which would have been less precise than the calorimeter recording the weight of a hanging hopper every 39 min. It may also have been due to the unexpected observation that rats receiving third-ventricle infusions of 0.01 μg leptin/day ate more following a fourth-ventricle saline injection than other groups of rats in experiment 3. Although these rats ate more following saline injection, their response to leptin injection was the same as that of the saline-infused and 0.005 μg leptin/day groups, which resulted in an exaggeration of the difference in food intake between saline-and leptin-injected conditions. This was also reflected in RER, which reached a value of 1.0 in the 30 h following saline injection, indicative of a state of positive energy balance (8). The increase in intake following saline injection was not apparent as an increase in daily intake, and these rats ate the same amount as saline-infused controls during the experimental period. It also was not the result of poor weight matching at the start of the experiment, although the animals had a low level of activity compared with other groups of rats, even before the start of leptin infusion. This low level of activity did not impact long-term energy balance because body fat was not different from that of saline-infused animals at the end of the experiment.
The mechanism by which leptin infusion increased baseline food intake has not been explored, but we have reported leptin-induced weight gain in other experimental conditions (11, 12, 14). Leptin infusion increases body fat in chronically decerebrate rats (12), in which the hindbrain is neurally isolated from the forebrain, supporting the notion that weight loss only occurs when leptin activity in both areas is integrated through a neural mechanism. Previously, in response to observations that selective activation of hindbrain leptin receptors was anabolic but that the effect was easily abolished if leptin receptors in additional central or peripheral sites were simultaneously active, we proposed that it represented a response in which small elevations of leptin in the hindbrain exerted a protective effect on lean mass when forebrain leptin levels were at nonstimulated levels (11). The results from experiment 3 would not fit with this hypothesis because weight gain occurred in food-deprived rats receiving third-ventricle infusions of 0.01 μg leptin/day. It may be that this dose was low enough to produce isolated activation of hypothalamic receptors and that exclusive activation of leptin receptors in any one of multiple sites promotes weight gain. Although this represents an experimental phenomenon because in normal conditions, receptors at multiple sites are activated in synchrony, it does support our hypothesis that weight loss occurs only when receptors at multiple sites are activated.
In summary, the results of experiments described here indicate that chronic activation of hindbrain leptin receptors exaggerates the hypophagic effect of leptin injected into the third ventricle by extending the duration rather than the amplitude of response. These results are consistent with our previous reports that simultaneous infusions of subthreshold doses of leptin into the third and fourth ventricle cause a significant inhibition of food intake and weight loss (5). In addition, we have shown that doses of leptin infused into the fourth ventricle that cause weight loss are associated with a simultaneous activation of STAT3 in areas of the hypothalamus that express leptin receptors (13). Future studies will test whether the synergistic effect of hindbrain leptin on hypothalamic leptin responsiveness is mediated by a humoral or a neural mechanism.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases 53903.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.B.H. conceived and designed research; R.B.H. performed experiments; R.B.H. analyzed data; R.B.H. interpreted results of experiments; R.B.H. prepared figures; R.B.H. drafted manuscript; R.B.H. edited and revised manuscript; R.B.H. approved final version of manuscript.
REFERENCES
- 1.Banks WA, Clever CM, Farrell CL. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am J Physiol Endocrinol Metab : E1158–E1165, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Daniels D, Marshall A. Evaluating the potential for rostral diffusion in the cerebral ventricles using angiotensin II-induced drinking in rats. Brain Res : 62–67, 2012. doi: 10.1016/j.brainres.2012.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Jonghe BC, Hayes MR, Zimmer DJ, Kanoski SE, Grill HJ, Bence KK. Food intake reductions and increases in energetic responses by hindbrain leptin and melanotan II are enhanced in mice with POMC-specific PTP1B deficiency. Am J Physiol Endocrinol Metab : E644–E651, 2012. doi: 10.1152/ajpendo.00009.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Desai BN, Harris RB. Integrated effects of leptin in the forebrain and hindbrain of male rats. Endocrinology : 2663–2675, 2013. doi: 10.1210/en.2013-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai BN, Harris RB. Leptin in the hindbrain facilitates phosphorylation of STAT3 in the hypothalamus. Am J Physiol Endocrinol Metab : E351–E361, 2015. doi: 10.1152/ajpendo.00501.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Emond M, Ladenheim EE, Schwartz GJ, Moran TH. Leptin amplifies the feeding inhibition and neural activation arising from a gastric nutrient preload. Physiol Behav : 123–128, 2001. doi: 10.1016/S0031-9384(00)00393-0. [DOI] [PubMed] [Google Scholar]
- 7.Faouzi M, Leshan R, Björnholm M, Hennessey T, Jones J, Münzberg H. Differential accessibility of circulating leptin to individual hypothalamic sites. Endocrinology : 5414–5423, 2007. doi: 10.1210/en.2007-0655. [DOI] [PubMed] [Google Scholar]
- 8.Flatt JP. Body composition, respiratory quotient, and weight maintenance. Am J Clin Nutr , Suppl: 1107S–1117S, 1995. [DOI] [PubMed] [Google Scholar]
- 9.Ghersi-Egea JF, Finnegan W, Chen JL, Fenstermacher JD. Rapid distribution of intraventricularly administered sucrose into cerebrospinal fluid cisterns via subarachnoid velae in rat. Neuroscience : 1271–1288, 1996. doi: 10.1016/0306-4522(96)00281-3. [DOI] [PubMed] [Google Scholar]
- 10.Harris RB. Growth measurements in Sprague-Dawley rats fed diets of very low fat concentration. J Nutr : 1075–1080, 1991. [DOI] [PubMed] [Google Scholar]
- 11.Harris RB. Leptin-induced increase in body fat content of rats. Am J Physiol Endocrinol Metab : E267–E281, 2013. doi: 10.1152/ajpendo.00251.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris RB, Bartness TJ, Grill HJ. Leptin responsiveness in chronically decerebrate rats. Endocrinology : 4623–4633, 2007. doi: 10.1210/en.2006-1565. [DOI] [PubMed] [Google Scholar]
- 13.Harris RB, Desai BN. Fourth-ventricle leptin infusions dose-dependently activate hypothalamic signal transducer and activator of transcription 3. Am J Physiol Endocrinol Metab : E939–E948, 2016. doi: 10.1152/ajpendo.00343.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harris RB, Mitchell TD, Kelso EW, Flatt WP. Changes in environmental temperature influence leptin responsiveness in low- and high-fat-fed mice. Am J Physiol Regul Integr Comp Physiol : R106–R115, 2007. doi: 10.1152/ajpregu.00848.2006. [DOI] [PubMed] [Google Scholar]
- 15.Hayes MR, Covasa M. Gastric distension enhances CCK-induced Fos-like immunoreactivity in the dorsal hindbrain by activating 5-HT3 receptors. Brain Res : 120–130, 2006. doi: 10.1016/j.brainres.2006.03.018. [DOI] [PubMed] [Google Scholar]
- 16.Kasser TR, Harris RB, Martin RJ. Level of satiety: fatty acid and glucose metabolism in three brain sites associated with feeding. Am J Physiol Regul Integr Comp Physiol : R447–R452, 1985. [DOI] [PubMed] [Google Scholar]
- 17.Matson CA, Reid DF, Ritter RC. Daily CCK injection enhances reduction of body weight by chronic intracerebroventricular leptin infusion. Am J Physiol Regul Integr Comp Physiol : R1368–R1373, 2002. doi: 10.1152/ajpregu.00080.2001. [DOI] [PubMed] [Google Scholar]
- 18.Münzberg H, Flier JS, Bjørbaek C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology : 4880–4889, 2004. doi: 10.1210/en.2004-0726. [DOI] [PubMed] [Google Scholar]
- 19.National Research Council Guide for the Care and Use of Laboratory Animals. Washington, DC: National Academy Press, 1996. [Google Scholar]
- 20.Penn DM, Jordan LC, Kelso EW, Davenport JE, Harris RB. Effects of central or peripheral leptin administration on norepinephrine turnover in defined fat depots. Am J Physiol Regul Integr Comp Physiol : R1613–R1621, 2006. doi: 10.1152/ajpregu.00368.2006. [DOI] [PubMed] [Google Scholar]
- 21.Pocai A, Morgan K, Buettner C, Gutierrez-Juarez R, Obici S, Rossetti L. Central leptin acutely reverses diet-induced hepatic insulin resistance. Diabetes : 3182–3189, 2005. doi: 10.2337/diabetes.54.11.3182. [DOI] [PubMed] [Google Scholar]
- 22.Ritter RC, Slusser P. 5-Thio-d-glucose causes increased feeding and hyperglycemia in the rat. Am J Physiol Endocrinol Metab : E141–E144, 1980. [DOI] [PubMed] [Google Scholar]
- 23.Rosenbaum M, Goldsmith R, Bloomfield D, Magnano A, Weimer L, Heymsfield S, Gallagher D, Mayer L, Murphy E, Leibel RL. Low-dose leptin reverses skeletal muscle, autonomic, and neuroendocrine adaptations to maintenance of reduced weight. J Clin Invest : 3579–3586, 2005. doi: 10.1172/JCI25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ruiter M, Duffy P, Simasko S, Ritter RC. Increased hypothalamic signal transducer and activator of transcription 3 phosphorylation after hindbrain leptin injection. Endocrinology : 1509–1519, 2010. doi: 10.1210/en.2009-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schoeller DA, Cella LK, Sinha MK, Caro JF. Entrainment of the diurnal rhythm of plasma leptin to meal timing. J Clin Invest : 1882–1887, 1997. doi: 10.1172/JCI119717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skibicka KP, Grill HJ. Hindbrain leptin stimulation induces anorexia and hyperthermia mediated by hindbrain melanocortin receptors. Endocrinology : 1705–1711, 2009. doi: 10.1210/en.2008-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaill MI, Desai BN, Harris RB. Blockade of the cerebral aqueduct in rats provides evidence of antagonistic leptin responses in the forebrain and hindbrain. Am J Physiol Endocrinol Metab : E414–E423, 2014. doi: 10.1152/ajpendo.00661.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zimmerman AD, Harris RB. In vivo and in vitro evidence that chronic activation of the hexosamine biosynthetic pathway interferes with leptin-dependent STAT3 phosphorylation. Am J Physiol Regul Integr Comp Physiol : R543–R555, 2015. doi: 10.1152/ajpregu.00347.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]