Abstract
Pharmacological activation of the glucagon-like peptide-1 receptor (GLP-1R) in the ventromedial hypothalamus (VMH) reduces food intake. Here, we assessed whether suppression of food intake by GLP-1R agonists (GLP-1RA) in this region is dependent on AMP-activated protein kinase (AMPK) and mammalian target of rapamycin (mTOR). We found that pharmacological inhibition of glycolysis, and thus activation of AMPK, in the VMH attenuates the anorectic effect of the GLP-1R agonist exendin-4 (Ex4), indicating that glucose metabolism and inhibition of AMPK are both required for this effect. Furthermore, we found that Ex4-mediated anorexia in the VMH involved mTOR but not acetyl-CoA carboxylase, two downstream targets of AMPK. We support this by showing that Ex4 activates mTOR signaling in the VMH and Chinese hamster ovary (CHO)-K1 cells. In contrast to the clear acute pharmacological impact of the these receptors on food intake, knockdown of the VMH Glp1r conferred no changes in energy balance in either chow- or high-fat-diet-fed mice, and the acute anorectic and glucose tolerance effects of peripherally dosed GLP-1RA were preserved. These results show that the VMH GLP-1R regulates food intake by engaging key nutrient sensors but is dispensable for the effects of GLP-1RA on nutrient homeostasis.
Keywords: GLP-1R, hypothalamus, food intake, nutrient sensor, nutrient homeostasis
glucagon-like peptide-1 (GLP-1) is a gut-secreted peptide that augments insulin secretion via a pancreatic GLP-1 receptor GLP-1R (1). GLP-1R agonists (GLP-1RA) also reduce food intake and body weight via actions on brain regions, including the hypothalamus (7) and brain stem (24). We (7) have recently reported that GLP-1R in the hypothalamic paraventricular nucleus (PVN) and proopiomelanocortin neurons of the arcuate nucleus (ARC) are sufficient but not necessary for the effects of GLP-1RA on energy balance, suggesting potential contributions from other hypothalamic nuclei.
There is a growing body of literature suggesting that GLP-1RA regulate energy homeostasis via the ventromedial hypothalamus (VMH). Peripherally injected GLP-1 increases neuronal activity in the VMH (9, 22). Moreover, targeted delivery of GLP-1 to the VMH in rats (27) or liraglutide in mice (5) suppresses food intake. VMH-targeted liraglutide stimulates brown adipose tissue (BAT) thermogenesis and white adipose tissue (WAT) browning (5), suggesting that VMH GLP-1R signaling also regulates energy expenditure (EE). The molecular mechanisms mediating these effects of VMH GLP-1R signaling on energy balance are unknown.
The VMH is a satiety center (2, 4) populated by both glucose-excited and glucose-inhibited neurons (23). Glucose-inhibited neurons are activated by a decrease in glucose and subsequent activation of the nutrient sensor AMP-activated protein kinase (AMPK; Ref. 20). We have previously shown a role for intracellular glucose metabolism and AMPK in the regulation of food intake by the GLP-1R agonist exendin-4 (Ex4). Specifically, the ability of an intracerebroventricular (ICV) administration of Ex4 to reduce food intake is attenuated by ICV pretreatments with the glycolytic inhibitor 2-deoxyglucose (2-DG) or the AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR; Ref. 6). The anorectic effect of third cerebroventricularly administered GLP-1 is also blocked by third cerebroventricular injection of 2-DG or AICAR in rats (26). These findings suggest that GLP-1RA reduce food intake via stimulation of intracellular glucose metabolism and inhibition of AMPK. Given the key role for intracellular glucose metabolism and AMPK in the regulation of VMH neuronal activity, the present study tested the hypothesis that these mechanisms mediate the anorectic effects of GLP-1RA in this hypothalamic nucleus. We demonstrate that GLP-1R activation in the VMH reduces food intake via activation of glucose metabolism and inhibition of AMPK. We also show that VMH-targeted inhibition of another nutrient sensor and AMPK target protein, mammalian target of rapamycin (mTOR; Ref. 33), attenuates the anorectic effect of Ex4. This is supported by our findings that Ex4 activates mTOR signaling in VMH brain slices and in GLP-1R-expressing Chinese hamster ovary (CHO)-K1 cells and that inhibition of mTOR in the VMH with rapamycin blocks the anorectic effect of Ex4. Taken together, these data identify nutrient-sensing signaling via AMPK and mTOR as mediators of the anorectic effects of Ex4 in the VMH. Surprisingly, VMH Glp1r knockdown had no effect on energy balance parameters in mice fed either a chow or high-fat diet (HFD). In sum, these results show that the VMH Glp1r engages nutrient-sensing mechanisms to regulate food intake, but it is not necessary for the maintenance of overall energy balance. This highlights the complexity of brain GLP-1R action and supports our (7) previous findings that GLP-1R signaling in hypothalamic nuclei is sufficient but not necessary for the maintenance of energy homeostasis.
METHODS
Animals and housing.
All experiments were performed in male mice. For gain-of-function experiments, 15- to 20-wk-old C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) maintained on a chow diet (Harlan Teklad 2016) were used. Mice lacking Glp1r expression in the VMH (GLP-1RKDΔNr5aCre) were generated by breeding floxed Glp1r mice (GLP-1Rf/f; Ref. 31) with nuclear receptor 5A1 (Nr5a)-Cre mice (The Jackson Laboratory) as previously described (15). Chow diet experiments were performed in 3- to 4-mo-old animals. Following energy balance assessment, mice were placed on a 60% HFD (D12492; Research Diets, New Brunswick, NJ) for 12 wk. Animals were maintained on a 12:12-h light-dark cycle in facilities at Sanford Burnham Prebys Medical Discovery Institute at Lake Nona (SBP). Protocols were approved by the SBP Institutional Animal Care and Use Committee.
Cannula implantation.
Cannulae were implanted as previously described (6). Bilateral guide cannulae (Plastics One, Roanoke, VA) targeted the VMH (1.45 mm caudal, 0.30 mm from midline, 5.40 mm ventral) or cortex (0.46 mm caudal, 2.00 mm from midline, 1.75 mm ventral). Proper coordinates were verified histologically following injection of 1% Evans blue dye. Mice recovered for 7 days before experimentation.
Validation of Glp1r knockdown.
Glp1r knockdown in the VMH of GLP-1RKDΔNr5aCre mice was confirmed by RNA in situ hybridization as described previously (7). Glp1r expression was also assessed in the PVN and ARC. In additional experiments, 18-h food intake and changes in body weight were measured following VMH-targeted delivery of Ex4 (0.00175 µg; R&D Systems, Minneapolis, MN) or artificial cerebrospinal fluid (ACSF; 100 nl bilateral) vehicle (Harvard Apparatus, Holliston, MA) in 5-h-fasted GLP-1Rf/f and GLP-1RKDΔNr5aCre mice just before the onset of the dark cycle at 1800.
Body composition analysis and assessment of energy balance.
Lean, fat, and fluid mass were measured in 5-h-fasted mice using a LF90II Time-Domain Nuclear Magnetic Resonance (TD-NMR; Bruker, Billerica, MA). Body weights were recorded weekly over the 12-wk HFD feeding. Energy balance was assessed using a Comprehensive Laboratory Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH) as previously described (7).
Food intake and EE responses to VMH- and peripherally administered GLP-1RA.
Following cannulation, recovery and acclimation, 18-h food intake and EE were measured after VMH-targeted delivery of 0.0025, 0.005, or 0.025 µg/100 nl GLP-1 or Ex4 (R&D Systems) or ACSF (100 nl bilateral) vehicle (Harvard Apparatus) in 5-h-fasted mice just before the onset of the dark cycle at 1800. In separate experiments, mice received injections of Ex4 (0.00175 µg/100 nl) counterbalanced with injections of the AMPK activator AICAR (0.25 µg/100 nl), glycolytic inhibitor 2-DG (10 mM), acetyl-CoA inhibitor (0.25 µg/100 nl; PF-05175157), mTOR inhibitor rapamycin (0.45 µg/100 nl), or ACSF vehicle. We observed that the anorectic effect of Ex4 was much more pronounced when it was administered as part of a counterbalanced, pretreatment dosing regimen compared with the dose-response experiments, which involved only a single Ex4 injection with no counterbalanced pretreatment. Therefore, a lower dose of Ex4 was chosen for these experiments. For peripheral administration, 16-h food intake was measured in mice treated with Ex4 [3 µg/kg body wt ip (30)] or saline vehicle.
Glucose tolerance tests.
Glucose tolerance tests were performed on 5-h-fasted mice. In chow-fed mice, 2 g/kg body wt glucose was administered orally or intraperitoneally. In HFD-fed animals, 1 g/kg body wt glucose was administered orally. Glucose tolerance tests were also performed in animals that received a VMH-targeted injection of Ex4 (0.025 µg/100 nl, bilateral) concurrent with an intraperitoneal glucose dose at time 0. Blood glucose was measured at the indicated time points. Glucose tolerance was calculated as area under the curve above baseline.
Body weight response to peripheral administration of liraglutide.
Liraglutide [200 µg/kg body wt sc bid (28)] or isotonic saline was administered for 14 days in chow- and HFD-fed mice, and body weight was measured daily as well as during a 7-day recovery period over which animals received no injection.
AMPK and mTOR responses to Ex4.
PathHunter CHO-K1 cells overexpressing the GLP-1R (CHO-K1-GLP-1R; DiscoveRx) were maintained at 37°C (5% CO2, humidified) in Ham’s F-12 media (10 mM glucose, 1 mM pyruvate, 1 mM glutamine; SH30026.01; HyClone, Rockford, IL), 10% fetal bovine serum (FBS) and 10 ml/l penicillin-streptomycin (HyClone). Cells were passaged every 48–72 h. Media were supplemented with Geneticin G418 Sulfate antibiotic (0.8 mg/ml) to preserve selection of GLP-1R-expressing cells. Five hundred thousand cells per well (passages 4–6) were seeded in six-well tissue culture plates. The next day, media were replaced with serum-free media for 80 min followed by incubation with 1× PBS or Ex4 (20 pM) for 60 min. Cells were washed with 1× PBS, snap-frozen, and kept at −80°C until analysis. VMH brain punches were obtained from 5-h-fasted C57BL/6 mice instrumented with VMH cannulae. Mice received a VMH-targeted injection of Ex4 (0.00175 µg/100 nl) or ACSF at 1800 and were euthanized for brain harvest at the indicated time points. Brains were immediately frozen on dry ice and subsequently embedded in optimal cutting temperature compound. Coronally oriented brains were mounted onto a cryostat (Leica), and VMH samples were collected directly into lysis buffer (cat. no. 9700; PathScan Akt Signaling Antibody Array Kit; Cell Signaling Technology) using a tissue puncher. VMH punches from 3 to 4 mice were pooled for each biological sample. Processing of cells and tissue and analysis of protein phosphorylation were carried out using a PathScan Akt Signaling Antibody Array Kit per manufacturer’s instructions. Arrays were imaged with an Odyssey Imaging System and analyzed using Image Studio software (LI-COR). In separate immunohistochemistry experiments, changes in mTOR signaling in response to VMH-targeted Ex4 were assessed. GLP-1Rf/f and GLP-1RKDΔNr5aCre mice received Ex4 (0.00175 µg) or ACSF vehicle (100 nl bilateral) targeted to the VMH, and brains were harvested 2 h later. Formalin-fixed paraffin sections (5 µm) were stained (BOND RX; Leica) with a phosphorylated p70S6 kinase (p70S6K) antibody (1:200; Ser424; Thermo Fisher Scientific). Heat retrieval was performed using Epitope Retrieval Solution 1 (citrate buffer, pH 6) for 20 min. Antibody incubation was performed at room temperature for 1 h, and labeling was visualized with a Leica Bond Polymer Refine Red detection kit (DS9800). Slides were counterstained with hematoxylin, dehydrated, coverslipped, and scanned using an Aperio system (Leica).
Statistical analyses.
Data are presented as means ± SE. Differences between groups were determined by one-way or two-way ANOVA followed by Tukey multiple comparisons post hoc test or by two-tailed t-test as appropriate. Statistical significance was set to P < 0.05.
RESULTS
VMH-targeted Ex4 but not GLP-1 dose-dependently suppresses food intake and EE.
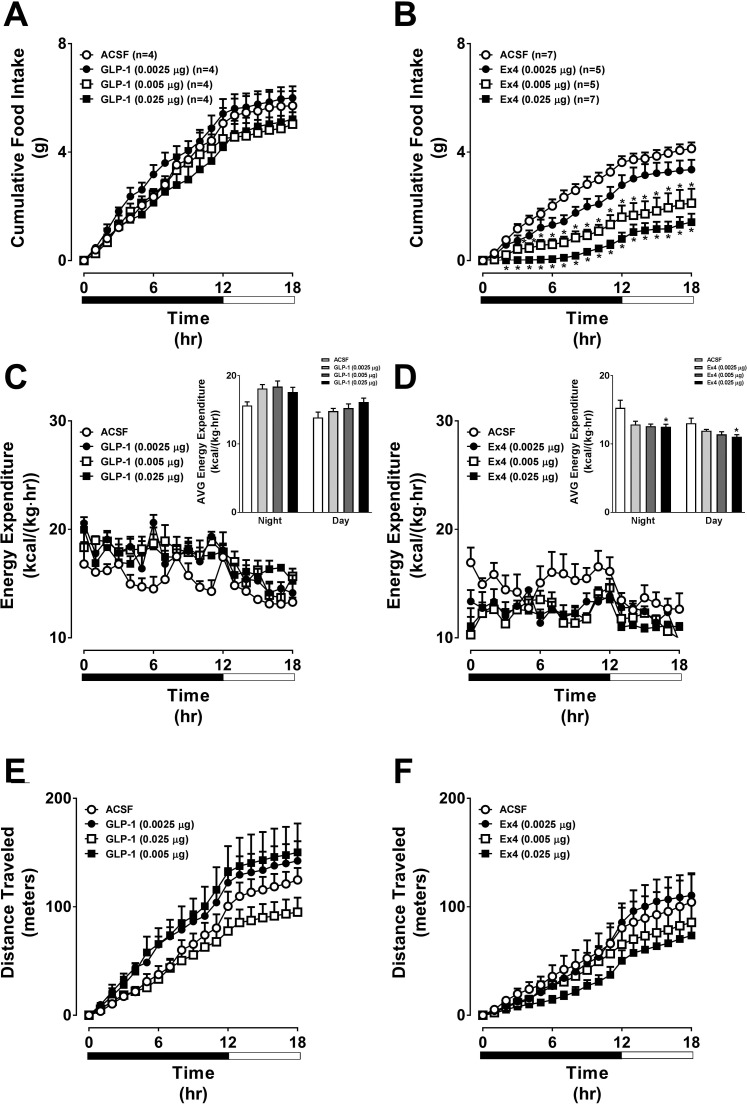
We first tested whether the VMH is functionally responsive to GLP-1RA. VMH-targeted GLP-1 had no effect on 18-h food intake, EE, or locomotor activity (Fig. 1, A, C, and E). There was a trend for GLP-1 to increase EE (Fig. 1C). In contrast, VMH-targeted Ex4 dose-dependently reduced 18-h food intake and EE but had no effect on locomotor activity (Fig. 1, B, D, and F).
Fig. 1.
Direct injection of Ex4 into the VMH dose-dependently suppresses food intake and energy expenditure, whereas GLP-1 has no effect. The values are means ± SE and represent the time course of cumulative 18-h food intake, 18-h energy expenditure, and cumulative 18-h locomotor activity in chow-fed, male C57BL/6J mice receiving GLP-1 (A, C, and E), Ex4 (0.0025, 0.005, or 0.025 µg; B, D, and F), or artificial cerebrospinal fluid (ACSF; 100 nl bilateral). n = 4–7 Mice/group. Insets for C and D represent the average (AVG) energy expenditure during the night vs. day periods. *P < 0.05 vs. ACSF. A, B, E, and F, 2-way (2W-) ANOVA; C and D, 1-way (1W-) ANOVA.
The VMH GLP-1R reduces food intake by stimulating intracellular glucose metabolism and inhibiting AMPK.
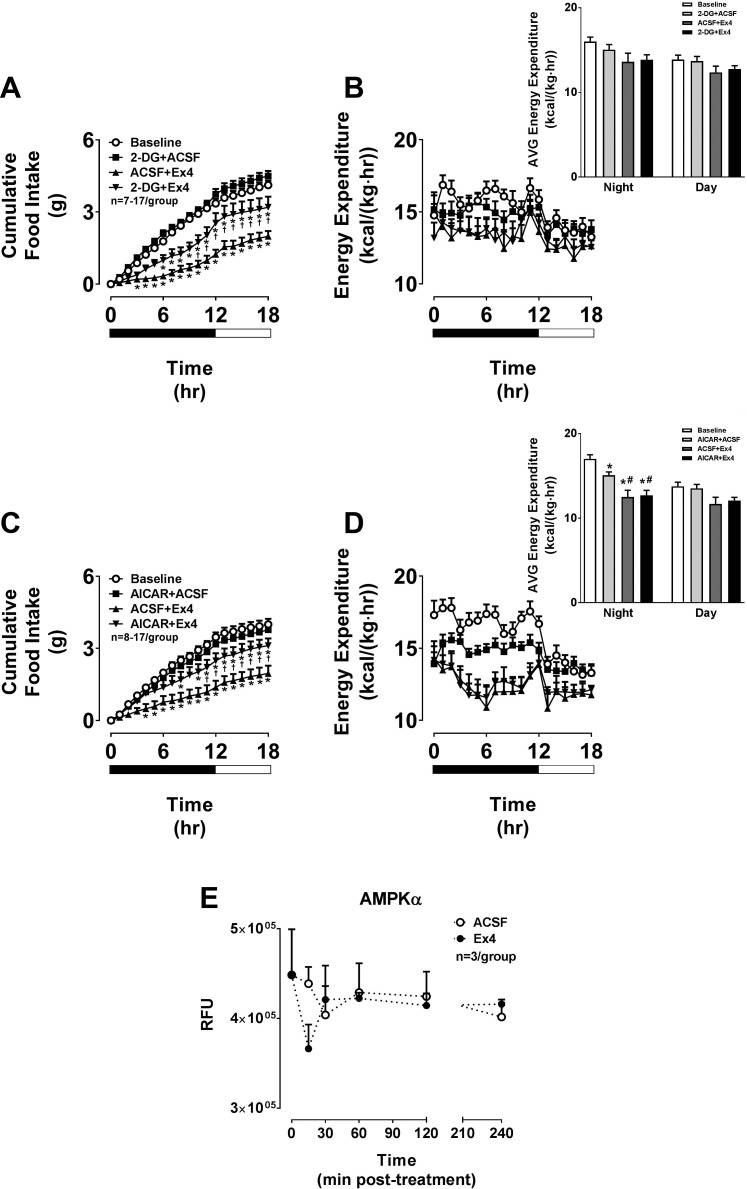
We next assessed the molecular mechanisms underlying the Ex4-induced anorectic effect via the VMH. We have shown that pretreatments with the glycolytic inhibitor 2-DG or the AMPK activator AICAR attenuate the anorectic effect of GLP-1R agonists when administered as ICV injections (6, 26). In the present study, we targeted these agents specifically to the VMH. VMH-targeted 2-DG or AICAR significantly attenuated the suppression of food intake by Ex4 (Fig. 2, A and C). Importantly, each pretreatment compound was administered at a dose that itself had no impact on food intake. VMH-targeted Ex4 also reduced EE, but this was not attenuated by any of the pretreatments (Fig. 2, B and D). These results localize to the VMH our previous observations with ICV injections (6, 26) and indicate that VMH GLP-1R activation specifically reduces food intake via stimulation of intracellular glucose metabolism and inhibition of AMPK. We further support this by showing a tendency for a transient reduction of AMPK phosphorylation in VMH micropunches obtained from mice following VMH-targeted Ex4 treatment (Fig. 2E).
Fig. 2.
The anorectic effect of Ex4 in the VMH involves stimulation of glycolysis and inhibition of AMPK. The values are means ± SE and represent cumulative 18-h food intake in chow-fed, male C57BL/6J mice receiving Ex4 (0.00175 µg) either with or without a 15-min pretreatment (pt) with the glycolytic inhibitor 2-deoxyglucose (2-DG; 10 mM; A), AMPK activator 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR; 0.25 µg; C), or vehicle (ACSF; 100 nl bilateral) in the VMH. B and D: 18-h energy expenditures for the corresponding time periods are also shown. Insets for B and D represent the average energy expenditure during the night vs. day periods. n = 7–17 Mice/group. *P < 0.05 vs. baseline. †P < 0.05 pt + Ex4 vs. ACSF + Ex4. #P < 0.05 vs. pt + ACSF. A and C, 2W-ANOVA; B and D, 1W-ANOVA. E: the values are means ± SE and represent the time course of phosphorylated AMPKα levels in VMH brain punches collected from chow-fed, male C57BL/6J mice receiving VMH-targeted Ex4 (0.00175 µg) or ACSF vehicle and brains harvested at 0, 15, 30, 60, 120, and 240 min postadministration. n = 3/Group. E, 2W-ANOVA. RFU, relative fluorescence units.
The VMH GLP-1R reduces food intake by stimulating mTOR.
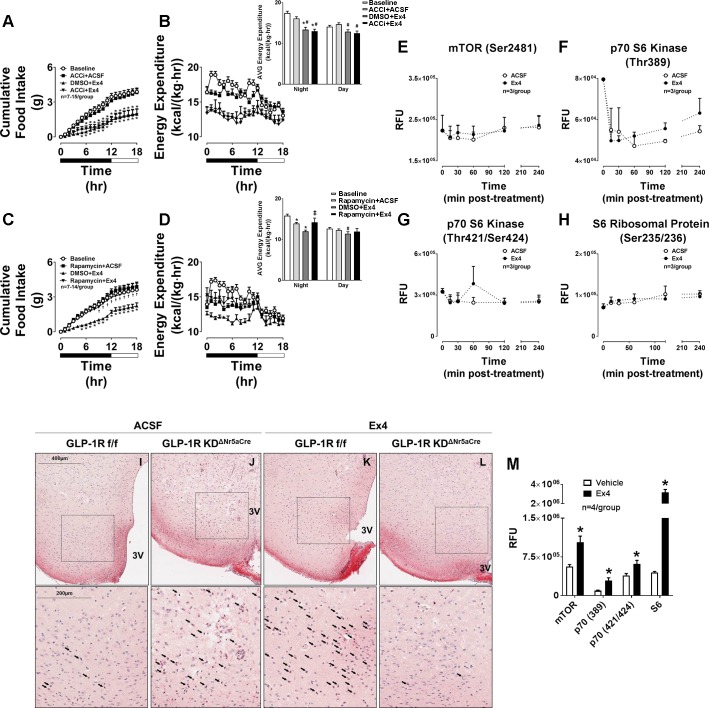
AMPK phosphorylates and inhibits acetyl-CoA carboxylase (ACC; Ref. 19). Inhibition of AMPK by Ex4 should, therefore, activate ACC. Stimulation of hypothalamic ACC has been shown to reduce food intake (11, 21). We tested whether inhibition of ACC in the VMH attenuates the anorectic effect of Ex4. Surprisingly, pretreatment with the ACC inhibitor PF-05175157 targeted to the VMH did not affect Ex4-mediated reductions in food intake and EE (Fig. 3, A and B). This suggests that ACC is not the AMPK target protein mediating the anorectic effects of Ex4 in the VMH. We next assessed whether another AMPK-regulated protein, mTOR, plays a role in the food-intake-suppressive effect of the VMH GLP-1R. Since AMPK inhibits mTOR (14), Ex4-mediated inhibition of AMPK should stimulate mTOR. Targeted activation of hypothalamic mTOR reduces food intake (10, 32), thus suggesting that Ex4 suppresses feeding via mTOR activation. We demonstrate that the mTOR inhibitor rapamycin completely blocked the anorectic effect of Ex4 when targeted to the VMH (Fig. 3C). Rapamycin pretreatment also blocked the ability of Ex4 to reduce EE (Fig. 3D). Taken together, these results demonstrate that the VMH GLP-1R reduces food intake and EE in response to Ex4 via stimulation of mTOR.
Fig. 3.
The anorectic effect of Ex4 in the VMH does not involve stimulation of ACC but rather activation of mTOR. The values are means ± SE and represent cumulative 18-h food intake in chow-fed, male C57BL/6J mice receiving Ex4 (0.00175 µg) either with or without a 15-min pretreatment with the ACC inhibitor (ACCi) PF 05175157 (0.25 µg; A), the mTOR inhibitor rapamycin (0.45 µg; C), or vehicle (DMSO; 100 nl bilateral) in the VMH. B and D: 18-h energy expenditures for the corresponding time periods are also shown. Insets for B and D represent the average energy expenditure during the night vs. day periods. n = 7–15 Mice/group. *P < 0.05 vs. baseline. †P < 0.05 pt + Ex4 vs. DMSO + Ex4. #P < 0.05 vs. pt + ACSF. ‡P < 0.05 vs. DMSO + Ex4. A and C, 2W-ANOVA; B and D, 1W-ANOVA. The values are means ± SE and represent the time course of phosphorylated mTOR (Ser2481; E), p70 S6 kinase (Thr389; F), p70 S6 kinase (Thr421/Ser424; G), and S6 ribosomal protein (Ser235/236; H) in VMH brain punches collected from chow-fed, male C57BL/6J mice receiving VMH-targeted Ex4 (0.00175 µg) or ACSF vehicle and brains harvested at 0, 15, 30, 60, 120, and 240 min postadministration. n = 3/Group. E–H, 2W-ANOVA. I–L: representative histology images showing phosphorylated p70S6K protein expression in VMH sections of chow-fed, male GLP-1RKDΔNr5aCre mice (J and L) compared with GLP-1Rf/f controls (I and K) receiving either Ex4 (0.00175 µg; K and L) or ACSF (100 nl, bilateral; I and J) 2 h prior. Protein expression is indicated as a red-purple chromogenic signal. Images are shown at both 200 and 400 µm (magnified area designated by the boxed area). Arrows designate positively stained regions. 3V, 3rd ventricle. M: the values are means ± SE and represent the levels of phosphorylated mTOR and its downstream targets p70S6K and S6 following treatment with Ex4 or PBS vehicle in CHO-K1-GLP-1R cells. n = 4/Group. *P < 0.05 vs. vehicle. I, 2-tailed t-test.
We next assessed the effect of VMH-targeted Ex4 on mTOR signaling in VMH micropunches. Surprisingly, VMH-targeted Ex4 did not enhance mTOR phosphorylation at the activating serine 2481 site (Fig. 3E). Furthermore, phosphorylation of the downstream mTOR targets p70S6 kinase (p70S6K) and S6 ribosomal protein (Fig. 3, F–H) was also not increased following VMH-targeted Ex4. Thus, although mTOR inhibition with rapamycin blocks the anorectic effect of VMH-targeted Ex4, these data suggest that there is no direct activation of mTOR signaling in the VMH by Ex4. One potential explanation is that the Glp1r is sparsely expressed in the VMH (Fig. 4). Changes in mTOR signaling in the subpopulation of Glp1r-expressing VMH neurons could be masked by the majority of non-Glp1r-expressing neurons, which might also explain the tendency for a transient effect of Ex4 to inhibit AMPK phosphorylation (Fig. 2E). We, therefore, assessed mTOR signaling in VMH brain slices via immunohistochemistry. We show that delivery of Ex4 to the VMH increases p70S6K phosphorylation in this region in control GLP-1R floxed (GLP-1Rf/f) mice (Fig. 3K) compared with ACSF vehicle (Fig. 3I), and this response was completely absent in VMH Glp1r knockout (GLP-1RKDΔNr5aCre) mice (Fig. 3, J and L). Validation of Glp1r knockdown in GLP-1RKDΔNr5aCre mice is described in the section below. As shown in Fig. 3M, mTOR, p70S6K, and S6 ribosomal phosphorylation was robustly enhanced by Ex4 in CHO-K1-GLP-1R cells, demonstrating that GLP-1R activation directly stimulates mTOR signaling.
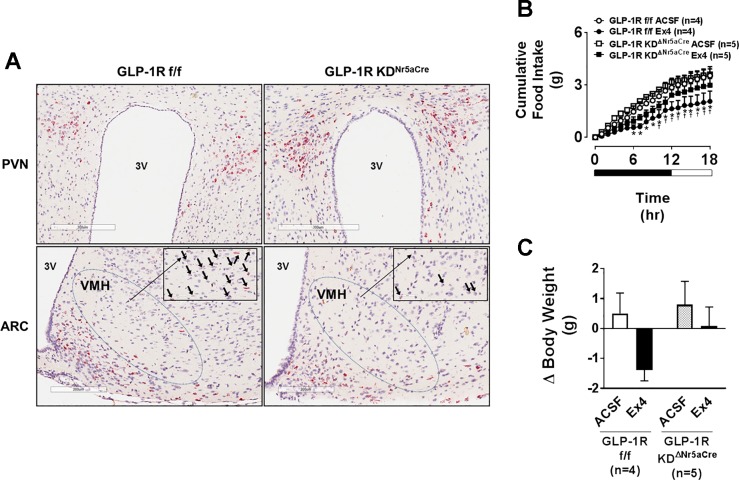
Fig. 4.
Nr5a-driven Cre recombinase site-selectively knocks down the Glp1r in the VMH. A: representative histology images showing Glp1r RNA expression in PVN, ARC, and VMH sections of chow-fed, male GLP-1RKDΔNr5aCre mice compared with GLP-1Rf/f controls. Glp1r RNA expression is indicated as a red chromogenic signal. Images are shown at both 200 and 300 µm (magnified area designated by the boxed area). Arrows designate positively stained regions. B: the values are means ± SE and represent cumulative 18-h food intake in chow-fed, male GLP-1Rf/f or GLP-1RKDΔNr5aCre mice receiving Ex4 (0.00175 µg) or ACSF vehicle (100 nl bilateral). *P < 0.05 vs. GLP-1RKDΔNr5aCre Ex4. †P < 0.05 vs. GLP-1Rf/f ACSF. B, 2W-ANOVA. C: the values are means ± SE and represent the 18-h change in body weight in response to Ex4 or ACSF in the same animals for which data are shown in B. C, 1W-ANOVA.
Disruption of VMH Glp1r expression does not impact body weight and composition or energy balance.
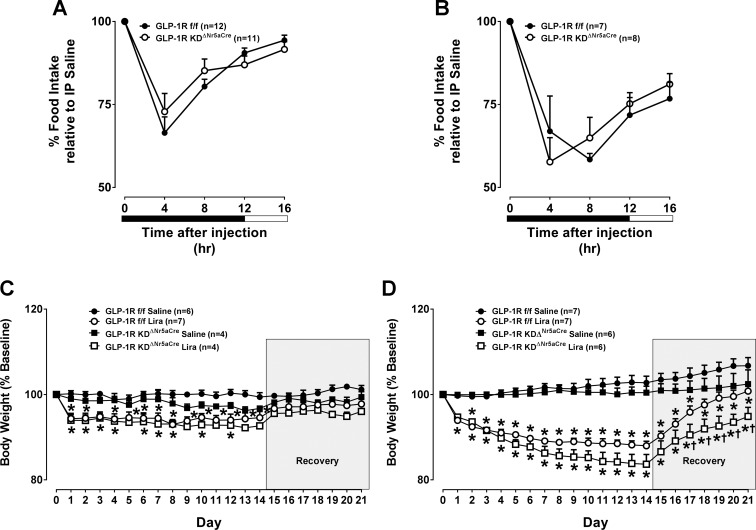
To test whether the VMH GLP-1R is necessary for chronic maintenance of energy balance, we generated GLP-1RKDΔNr5aCre mice. In situ hybridization analyses show a small population of Glp1r-expressing cells in the VMH that is reduced in GLP-1RKDΔNr5aCre mice (Fig. 4A). Knockdown of the Glp1r in GLP-1RKDΔNr5aCre mice was restricted to the VMH compared with the PVN and ARC (Fig. 4A). As further validation of the GLP-1RKDΔNr5aCre model, the anorectic (Fig. 4B) and body-weight-lowering (Fig. 4C) effects of VMH-targeted Ex4 were attenuated in GLP-1RKDΔNr5aCre mice.
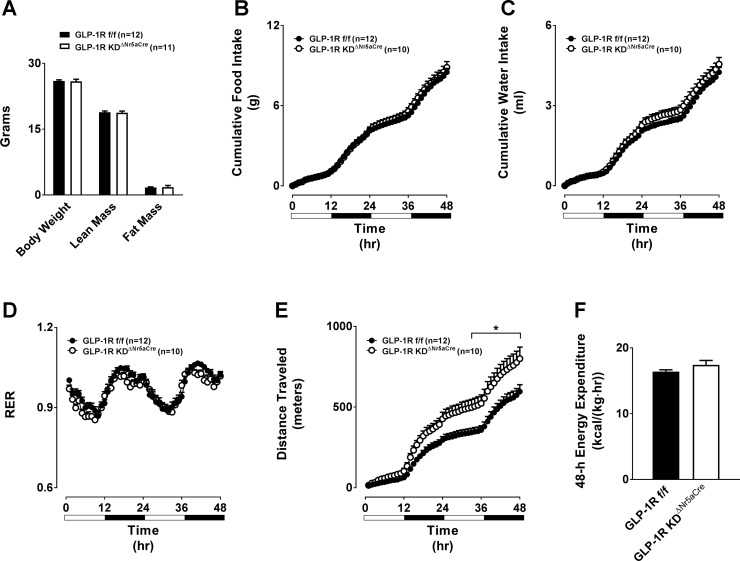
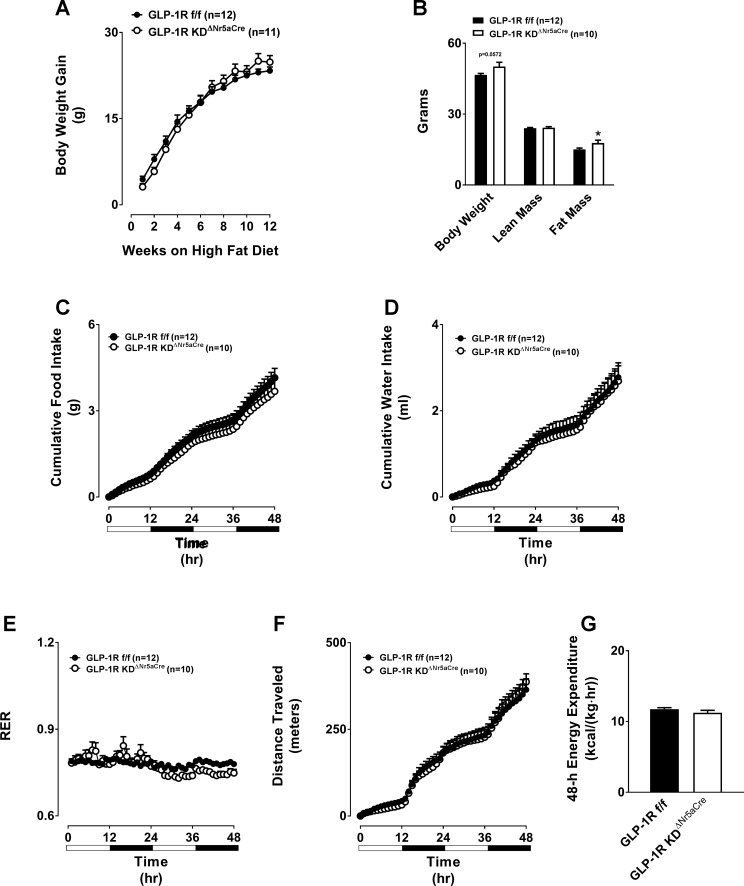
Total, lean, and fat mass were unaltered in chow-fed GLP-1RKDΔNr5aCre mice (Fig. 5A). Loss of VMH Glp1r expression did not affect 48-h food intake, water intake, or respiratory exchange ratio (Fig. 5, B–D). Although GLP-1RKDΔNr5aCre mice displayed slightly elevated locomotor activity, this was not associated with changes in EE (Fig. 5, E and F). We next assessed each of these parameters following chronic HFD feeding. HFD-induced weight gain was identical between GLP-1RKDΔNr5aCre and control mice (Fig. 6A). However, there was a tendency (P = 0.0572) for absolute body mass to be higher in GLP-1RKDΔNr5aCre mice due to a significant increase in fat mass (Fig. 6B). Loss of the VMH Glp1r conferred no changes in 48-h food intake, water intake, respiratory exchange ratio, locomotor activity, or EE in HFD-fed mice (Fig. 6, C–G).
Fig. 5.
Chow-fed GLP-1RKDΔNr5aCre mice exhibit normal energy balance compared with GLP-1Rf/f controls. The values are means ± SE and represent 5-h-fasted body composition (body weight, lean mass, and fat mass; A), cumulative 48-h food intake (B), cumulative 48-h water intake (C), 48-h respiratory exchange ratio (RER; D), cumulative 48-h locomotor activity (E), and 48-h energy expenditure (F) in chow-fed, male GLP-1RKDΔNr5aCre mice (n = 11) compared with GLP-1Rf/f controls (n = 12). *P < 0.05 vs. GLP-1Rf/f. A and F, 2-tailed t-test. B–E, 2W-ANOVA.
Fig. 6.
Disruption of the GLP-1R in GLP-1RKDΔNr5aCre mice does not alter HFD-induced weight gain or overall energy balance, but it does impact body composition. The values are means ± SE and represent HFD-induced body weight gain (A), 5-h-fasted body composition (body weight, lean mass, and fat mass; B), cumulative 48-h food intake (C), cumulative 48-h water intake (D), 48-h RER (E), cumulative 48-h locomotor activity (F), and 48-h energy expenditure (G) in HFD-fed, male GLP-1RKDΔNr5aCre mice (n = 11) compared with GLP-1Rf/f controls (n = 12). *P < 0.05 vs. GLP-1Rf/f. A and C–F, 2W-ANOVA. B and G, 2-tailed t-test.
VMH Glp1r knockdown mice exhibit normal anorectic response to peripheral administration of GLP-1RA.
We next tested the hypothesis that loss of VMH Glp1r expression blunts the anorectic effect of peripherally dosed GLP-1RA. Acute suppression of food intake by intraperitoneal Ex4 administration was unaffected in chow- and HFD-fed GLP-1RKDΔNr5aCre mice (Fig. 7, A and B). We also show that the body-weight-reducing effect of a chronic dosing regimen of liraglutide was unaffected in both chow- and HFD-fed GLP-1RKDΔNr5aCre mice (Fig. 7, C and D).
Fig. 7.
Disruption of the VMH GLP-1R does not blunt the food-intake-suppressive effect of peripherally dosed Ex4 or the body-weight-lowering effect of chronic liraglutide treatment. The values are means ± SE and represent 16-h food intake following treatment with Ex4 (3 µg/kg ip) in chow-fed, male GLP-1RKDΔNr5aCre mice (n = 11) compared with GLP-1Rf/f controls (n = 12; A) or HFD-fed, male GLP-1RKDΔNr5aCre mice (n = 8) compared with GLP-1Rf/f controls (n = 7; B). Data are shown at 4-h intervals and expressed as a percentage of the food-intake response observed following treatment with vehicle. A and B, 2W-ANOVA. IP, intraperitoneal. C and D: the values are means ± SE and represent daily body weight over the course of 21 days in chow- (n = 4; C) or HFD-fed, male GLP-1RKDΔNr5aCre mice (n = 6; D) compared with GLP-1Rf/f controls (n = 6–7) treated with liraglutide (Lira). Data are expressed as a percentage of baseline (i.e., before liraglutide treatment) body weight. On days 0–13, morning body weight was measured, and animals received a twice-daily injection of liraglutide (200 µg/kg body wt sc) or vehicle. On recovery days 14–21, only morning body weight was measured. *P < 0.05 vs. saline. †P < 0.05 vs. GLP-1Rf/f. C and D, 2W-ANOVA.
Glucose tolerance is improved by pharmacological activation of the VMH GLP-1R, but loss of VMH Glp1r expression does not impair glucose tolerance.
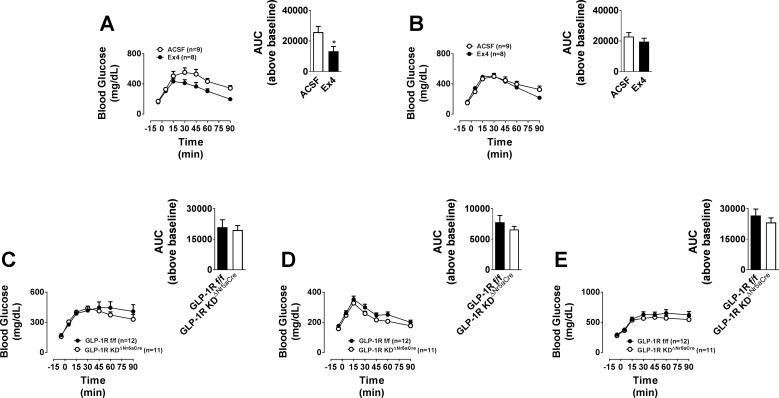
Brain GLP-1R signaling modulates peripheral glucose production and/or use (8, 25). We assessed the effect of targeted delivery of Ex4 to the VMH on glucose tolerance. An anorectic dose of Ex4 (0.025 µg) improved glucose tolerance when targeted to the VMH but not to the cortex (Fig. 8, A and B). However, intraperitoneal and oral glucose tolerances were unaffected in chow-fed GLP-1RKDΔNr5aCre mice (Fig. 8, C and D). Similarly, oral glucose tolerance was unaffected by the loss of VMH Glp1r expression in HFD-fed mice (Fig. 8E).
Fig. 8.
Direct injection of Ex4 into the VMH robustly improves glucose tolerance, whereas disruption of the VMH GLP-1R in GLP-1RKDΔNr5aCre mice does not affect glucose tolerance. The values are means ± SE and represent glucose excursion in chow-fed, male C57BL/6J mice following a gavage of glucose (2 g/kg body wt ip) and treatment with Ex4 (0.025 µg) or ACSF (100 nl) in the VMH (n = 8–9; A) or cortex (n = 8–9; B) at time 0. Inset: area under the curve (AUC) above baseline for each group. *P < 0.05 vs. ACSF. All insets, 2-tailed t-test. The values are means ± SE and represent glucose excursion in chow- or HFD-fed, male GLP-1RKDΔNr5aCre mice (n = 12) compared with GLP-1Rf/f controls (n = 11). C: chow diet, intraperitoneal glucose tolerance test. D: chow diet, oral glucose tolerance test (OGTT). E: HFD, OGTT. Insets represent the AUC above baseline for each group.
DISCUSSION
The ability of GLP-1RA to reduce food intake via the central nervous system (CNS) has been recognized for two decades, yet the precise regions and mechanisms mediating this effect remain unknown. The present study demonstrates that targeted delivery of Ex4 to the VMH reduces food intake via stimulation of intracellular glucose metabolism, inhibition of AMPK, and activation of mTOR. These findings demonstrate that VMH GLP-1R signaling engages key nutrient-sensing mechanisms that coordinate the response to increased nutrient availability. Surprisingly, VMH Glp1r deletion yielded no effects on energy balance. This parallels our (7) previous observation that disruption of Glp1r expression in proopiomelanocortin neurons or the PVN does not affect energy balance. These results further highlight the likelihood that GLP-1RA reduce food intake via multiple CNS regions.
Administration of GLP-1RA to the VMH reduces food intake in rats (5). We confirm these findings in mice by demonstrating that delivery of Ex4 to the VMH suppresses food intake and lowers body weight. Surprisingly, VMH-targeted GLP-1 did not suppress food intake. Although it is unknown whether the GLP-1-degrading enzyme dipeptidyl peptidase IV is expressed in the VMH, it is possible that the lack of a GLP-1 effect is attributable to dipeptidyl peptidase IV-mediated degradation of GLP-1, whereas Ex4 is resistant to such degradation (1). Unlike Ex4, VMH-targeted GLP-1 tended to increase EE. This is consistent with observations that VMH-targeted liraglutide reduces body weight in rats independently of an effect on food intake (5). This is likely due to increased BAT thermogenesis and WAT browning and thus increased EE (5). When administered into the CNS, Ex4 is more potent at reducing food intake than GLP-1 (3) or liraglutide (29). It is possible that the more pronounced reduction in food intake elicited by Ex4 also drives down EE, whereas the more subtle effects of GLP-1 and liraglutide on food intake allow for an increase in EE to be observed. We can also not exclude the possibility of ligand-specific effects on EE. Nevertheless, these observations propose that pharmacological VMH GLP-1R activation reduces body weight via regulation of food intake and/or EE.
We next explored the molecular mechanisms mediating the anorectic effect of VMH-targeted Ex4. Minokoshi et al. (18) demonstrated a key role for hypothalamic AMPK in the regulation of food intake. Hypothalamic expression of a dominant-negative AMPK isoform reduced food intake and body weight, whereas constitutively active AMPK increased both (18). We (6) have shown that hypothalamic AMPK activation and food intake are elevated in whole body GLP-1R knockout mice, suggesting that hypothalamic GLP-1R activation reduces food intake by inhibiting AMPK. Supporting this, we reported that the anorectic effect of ICV-delivered GLP-1RA is blunted by pretreatment with AMPK activators in mice and rats (6, 26). We, therefore, tested the hypothesis that inhibition of AMPK specifically in the VMH mediates the anorectic effect of VMH-targeted Ex4. Similar to our observations using ICV delivery, we show that the anorectic response following VMH delivery of Ex4 is attenuated when AMPK is activated by AICAR. We also show that VMH-targeted Ex4 reduces AMPK phosphorylation, albeit transiently. Interestingly, the ability of VMH-targeted liraglutide to stimulate BAT activity, WAT browning, and EE in rats also requires inhibition of AMPK (5). Contrasting this, our experiments in mice show that VMH-targeted Ex4 decreased EE, and this was not attenuated by AICAR. This raises the possibility that different GLP-1RA (e.g., liraglutide vs. Ex4) engage distinct mechanisms to regulate food intake and EE, thus highlighting the complexity of the molecular events mediating the overall negative energy balance effects of GLP-1RA.
Similar to our (6, 26) previous findings using ICV injections, Ex4-mediated reduction of food intake was significantly attenuated by VMH-targeted delivery of 2-DG, suggesting that intracellular glucose metabolism in the VMH is required for the anorectic effect of Ex4. This provides one potential mechanism for the inhibition of AMPK by GLP-1RA since increased intracellular glucose metabolism reduces AMPK activity (33). Indeed, we (6) have previously shown that glucose is required for the ability of Ex4 to inhibit AMPK in hypothalamic GT1–7 cells, and this is likely attributed to increased intracellular glucose metabolism in response to Ex4. Furthermore, Hurtado-Carneiro et al. (13) showed that low glucose concentrations increased AMPK activity in the VMH and that this was reversed by GLP-1 treatment in rat hypothalamic slices. Moreover, Ex4 reversed fasting-induced elevation of VMH AMPK activity (13), further suggesting a role for VMH GLP-1R signaling in sensing nutrient status via AMPK. Inhibition of VMH AMPK following GLP-1R activation would be expected to stimulate ACC, and stimulation of hypothalamic ACC reduces food intake (11, 21). Surprisingly, inhibition of ACC in the VMH had no effect on the reduction of food intake by Ex4, suggesting that ACC is not the AMPK target protein mediating the anorectic effects of VMH GLP-1R signaling. We, therefore, explored the role of another protein downstream of AMPK, mTOR, as the mediator of Ex4 effects on food intake. Similar to ACC, inhibition of AMPK by Ex4 would be expected to stimulate mTOR, and increased hypothalamic mTOR is associated with reduced food intake (10, 32). We demonstrate that inhibition of VMH mTOR with rapamycin completely blocks the anorectic effect of VMH-targeted Ex4. This suggests that VMH GLP-1R activation stimulates mTOR signaling, resulting in decreased food intake. These observations support previous findings showing that refeeding-induced reductions in p70S6K activity in the VMH are reversed by Ex4 (13). Furthermore, we show that Ex4 robustly increases the phosphorylation and, hence, activation of mTOR signaling proteins p70S6K and S6 ribosomal protein in CHO-K1-GLP-1R cells. Ex4 also robustly increases the phosphorylation of p70S6K in VMH brain slices. Taken together, these findings strongly implicate mTOR activation as a principal signaling event required for GLP-1R-mediated regulation of energy balance. Future experiments will address the molecular mechanisms linking GLP-1R activation with mTOR stimulation. Recent experiments by Liu and colleagues (16) showed that mTOR is phosphorylated and activated by PKA, the canonical signaling protein downstream of the GLP-1R. We have also alluded to the possibility that stimulation of mTOR is directly due to inhibition of AMPK by Ex4. AMPK and mTOR activity is also coordinated by the nutrient sensor PAS kinase (PASK), a target of GLP-1R signaling (12). PASK is expressed in the VMH, where its gene expression is decreased in response to glucose, and silencing of PASK impairs both glucose- and GLP-1-induced changes in AMPK and mTOR activity (12).
Our results showing reduced food intake in response to VMH-targeted Ex4 do not reflect the physiological, long-term regulation of feeding by the VMH GLP-1R. To address this, we assessed energy balance parameters in VMH Glp1r knockdown mice. As validation of the model, we show reduced Glp1r expression in these mice. Furthermore, the reductions in food intake, body weight, and stimulation of mTOR signaling in response to VMH-targeted Ex4 are attenuated in VMH Glp1r knockdown mice. Surprisingly, we report that loss of VMH Glp1r expression does not affect overall energy balance or responsiveness to peripheral administration of clinically relevant GLP-1RA. Similarly, we observe that VMH-targeted Ex4 enhances glucose tolerance, yet loss of VMH Glp1r expression does not affect glucose tolerance. We have made similar observations in hypothalamic as well as PVN- and ARC-specific Glp1r knockdown mice (7). These findings demonstrate that classic GLP-1RA-responsive hypothalamic neurons are sufficient but not necessary for the anorectic and glucoregulatory effects of GLP-1RA. This is perhaps not surprising given the fact that the Glp1r is expressed in multiple hypothalamic nuclei, including nuclei receiving direct inputs from GLP-1-positive neurons from the hindbrain such as the arcuate and paraventricular nuclei. The Glp1r is also in nonhomeostatic, food-intake-regulatory regions, including the central amygdala, dorsal striatum, and lateral septum (17). We can, therefore, not exclude the possibility that the loss of Glp1r expression in one region, such as the VMH, is compensated for by GLP-1R signaling in one or multiple other brain regions.
In sum, these experiments demonstrate that pharmacological activation of the VMH GLP-1R reduces food intake by engaging key nutrient-sensing molecules. We identify inhibition of AMPK and stimulation of mTOR as molecular events that mediate the anorectic effect of VMH-targeted Ex4. Although Glp1r expression in the VMH, a prominent satiety center, is sufficient for the anorectic effects of pharmacological GLP-1RA, it is not necessary for the effects of GLP-1RA on energy balance and glucose homeostasis. This highlights the importance of combining targeted pharmacological and genetic approaches to unravel the complex mechanisms by which GLP-1RA maintain nutrient homeostasis.
GRANTS
This work was supported by American Diabetes Association Grant 1-14-CD to J. E. Ayala and National Institute of Diabetes and Digestive and Kidney Diseases Grants 4-R01-DK-097361 to J. E. Ayala, 1-R01-DK-107652 to R. J. Seeley, and 7-R01-DK-082480 to D. A. Sandoval.
DISCLOSURES
The authors report no conflict of interest. R. J. Seeley has received research support from Ethicon Endo-Surgery/Johnson & Johnson, Novo Nordisk, Janssen/Johnson & Johnson, MedImmune, Boehringer Ingelheim, and Sanofi. R. J. Seeley has served on scientific advisory boards for Ethicon Endo-Surgery/Johnson & Johnson, Orexigen Therapeutics, Novo Nordisk, Daiichi Sankyo, Janssen/Johnson & Johnson, Novartis, Paul Hastings Law Firm, Zafgen, Takeda, and Boehringer Ingelheim. D. A. Sandoval has received research support from Ethicon Endo-Surgery/Johnson & Johnson, Novo Nordisk, and Sanofi.
AUTHOR CONTRIBUTIONS
M.A.B., J.D.B., and Julio E. Ayala conceived and designed research; M.A.B., J.D.B., and Jennifer E. Ayala performed experiments; M.A.B., J.D.B., Jennifer E. Ayala, and Julio E. Ayala analyzed data; M.A.B., J.D.B., Jennifer E. Ayala, and Julio E. Ayala interpreted results of experiments; M.A.B. prepared figures; M.A.B. and Julio E. Ayala drafted manuscript; M.A.B., J.D.B., Jennifer E. Ayala, D.A. Sandoval, R.J.S., and Julio E. Ayala edited and revised manuscript; M.A.B., J.D.B., Jennifer E. Ayala, D.A. Stoffers, D.A. Sandoval, R.J.S., and Julio E. Ayala approved final version of manuscript.
ACKNOWLEDGMENTS
We recognize John Shelley (SBP) for assistance with the RNA in situ hybridization and immunohistochemistry experiments.
REFERENCES
- 1.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 132: 2131–2157, 2007. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 2.Balagura S, Devenport LD. Feeding patterns of normal and ventromedial hypothalamic lesioned male and female rats. J Comp Physiol Psychol 71: 357–364, 1970. doi: 10.1037/h0029118. [DOI] [PubMed] [Google Scholar]
- 3.Barrera JG, D’Alessio DA, Drucker DJ, Woods SC, Seeley RJ. Differences in the central anorectic effects of glucagon-like peptide-1 and exendin-4 in rats. Diabetes 58: 2820–2827, 2009. doi: 10.2337/db09-0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker EE, Kissileff HR. Inhibitory controls of feeding by the ventromedial hypothalamus. Am J Physiol 226: 383–396, 1974. [DOI] [PubMed] [Google Scholar]
- 5.Beiroa D, Imbernon M, Gallego R, Senra A, Herranz D, Villarroya F, Serrano M, Fernø J, Salvador J, Escalada J, Dieguez C, Lopez M, Frühbeck G, Nogueiras R. GLP-1 agonism stimulates brown adipose tissue thermogenesis and browning through hypothalamic AMPK. Diabetes 63: 3346–3358, 2014. doi: 10.2337/db14-0302. [DOI] [PubMed] [Google Scholar]
- 6.Burmeister MA, Ayala J, Drucker DJ, Ayala JE. Central glucagon-like peptide 1 receptor-induced anorexia requires glucose metabolism-mediated suppression of AMPK and is impaired by central fructose. Am J Physiol Endocrinol Metab 304: E677–E685, 2013. doi: 10.1152/ajpendo.00446.2012. [DOI] [PubMed] [Google Scholar]
- 7.Burmeister MA, Ayala JE, Smouse H, Landivar-Rocha A, Brown JD, Drucker DJ, Stoffers DA, Sandoval DA, Seeley RJ, Ayala JE. The hypothalamic glucagon-like peptide 1 receptor is sufficient but not necessary for the regulation of energy balance and glucose homeostasis in mice. Diabetes 66: 372–384, 2017. doi: 10.2337/db16-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burmeister MA, Ferre T, Ayala JE, King EM, Holt RM, Ayala JE. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am J Physiol Endocrinol Metab 302: E334–E343, 2012. doi: 10.1152/ajpendo.00409.2011. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhri OB, Parkinson JR, Kuo YT, Druce MR, Herlihy AH, Bell JD, Dhillo WS, Stanley SA, Ghatei MA, Bloom SR. Differential hypothalamic neuronal activation following peripheral injection of GLP-1 and oxyntomodulin in mice detected by manganese-enhanced magnetic resonance imaging. Biochem Biophys Res Commun 350: 298–306, 2006. doi: 10.1016/j.bbrc.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ. Hypothalamic mTOR signaling regulates food intake. Science 312: 927–930, 2006. doi: 10.1126/science.1124147. [DOI] [PubMed] [Google Scholar]
- 11.Gao S, Kinzig KP, Aja S, Scott KA, Keung W, Kelly S, Strynadka K, Chohnan S, Smith WW, Tamashiro KL, Ladenheim EE, Ronnett GV, Tu Y, Birnbaum MJ, Lopaschuk GD, Moran TH. Leptin activates hypothalamic acetyl-CoA carboxylase to inhibit food intake. Proc Natl Acad Sci USA 104: 17358–17363, 2007. doi: 10.1073/pnas.0708385104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hurtado-Carneiro V, Roncero I, Blazquez E, Alvarez E, Sanz C. PAS kinase as a nutrient sensor in neuroblastoma and hypothalamic cells required for the normal expression and activity of other cellular nutrient and energy sensors. Mol Neurobiol 48: 904–920, 2013. doi: 10.1007/s12035-013-8476-9. [DOI] [PubMed] [Google Scholar]
- 13.Hurtado-Carneiro V, Sanz C, Roncero I, Vazquez P, Blazquez E, Alvarez E. Glucagon-like peptide 1 (GLP-1) can reverse AMP-activated protein kinase (AMPK) and S6 kinase (P70S6K) activities induced by fluctuations in glucose levels in hypothalamic areas involved in feeding behaviour. Mol Neurobiol 45: 348–361, 2012. doi: 10.1007/s12035-012-8239-z. [DOI] [PubMed] [Google Scholar]
- 14.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003. doi: 10.1016/S0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- 15.Kim KW, Zhao L, Donato J Jr, Kohno D, Xu Y, Elias CF, Lee C, Parker KL, Elmquist JK. Steroidogenic factor 1 directs programs regulating diet-induced thermogenesis and leptin action in the ventral medial hypothalamic nucleus. Proc Natl Acad Sci USA 108: 10673–10678, 2011. doi: 10.1073/pnas.1102364108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu D, Bordicchia M, Zhang C, Fang H, Wei W, Li JL, Guilherme A, Guntur K, Czech MP, Collins S. Activation of mTORC1 is essential for β-adrenergic stimulation of adipose browning. J Clin Invest 126: 1704–1716, 2016. doi: 10.1172/JCI83532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Merchenthaler I, Lane M, Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. J Comp Neurol 403: 261–280, 1999. doi:. [DOI] [PubMed] [Google Scholar]
- 18.Minokoshi Y, Alquier T, Furukawa N, Kim YB, Lee A, Xue B, Mu J, Foufelle F, Ferré P, Birnbaum MJ, Stuck BJ, Kahn BB. AMP-kinase regulates food intake by responding to hormonal and nutrient signals in the hypothalamus. Nature 428: 569–574, 2004. doi: 10.1038/nature02440. [DOI] [PubMed] [Google Scholar]
- 19.Munday MR, Carling D, Hardie DG. Negative interactions between phosphorylation of acetyl-CoA carboxylase by the cyclic AMP-dependent and AMP-activated protein kinases. FEBS Lett 235: 144–148, 1988. doi: 10.1016/0014-5793(88)81251-1. [DOI] [PubMed] [Google Scholar]
- 20.Murphy BA, Fakira KA, Song Z, Beuve A, Routh VH. AMP-activated protein kinase and nitric oxide regulate the glucose sensitivity of ventromedial hypothalamic glucose-inhibited neurons. Am J Physiol Cell Physiol 297: C750–C758, 2009. doi: 10.1152/ajpcell.00127.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh W, Abu-Elheiga L, Kordari P, Gu Z, Shaikenov T, Chirala SS, Wakil SJ. Glucose and fat metabolism in adipose tissue of acetyl-CoA carboxylase 2 knockout mice. Proc Natl Acad Sci USA 102: 1384–1389, 2005. doi: 10.1073/pnas.0409451102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Parkinson JR, Chaudhri OB, Kuo YT, Field BC, Herlihy AH, Dhillo WS, Ghatei MA, Bloom SR, Bell JD. Differential patterns of neuronal activation in the brainstem and hypothalamus following peripheral injection of GLP-1, oxyntomodulin and lithium chloride in mice detected by manganese-enhanced magnetic resonance imaging (MEMRI). Neuroimage 44: 1022–1031, 2009. doi: 10.1016/j.neuroimage.2008.09.047. [DOI] [PubMed] [Google Scholar]
- 23.Routh VH. Glucose sensing neurons in the ventromedial hypothalamus. Sensors (Basel) 10: 9002–9025, 2010. doi: 10.3390/s101009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rupprecht LE, Mietlicki-Baase EG, Zimmer DJ, McGrath LE, Olivos DR, Hayes MR. Hindbrain GLP-1 receptor-mediated suppression of food intake requires a PI3K-dependent decrease in phosphorylation of membrane-bound Akt. Am J Physiol Endocrinol Metab 305: E751–E759, 2013. doi: 10.1152/ajpendo.00367.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandoval D. CNS GLP-1 regulation of peripheral glucose homeostasis. Physiol Behav 94: 670–674, 2008. doi: 10.1016/j.physbeh.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandoval D, Barrera JG, Stefater MA, Sisley S, Woods SC, D’Alessio DD, Seeley RJ. The anorectic effect of GLP-1 in rats is nutrient dependent. PLoS One 7: e51870, 2012. doi: 10.1371/journal.pone.0051870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schick RR, Zimmermann JP, vorm Walde T, Schusdziarra V. Glucagon-like peptide 1-(7–36) amide acts at lateral and medial hypothalamic sites to suppress feeding in rats. Am J Physiol Regul Integr Comp Physiol 284: R1427–R1435, 2003. doi: 10.1152/ajpregu.00479.2002. [DOI] [PubMed] [Google Scholar]
- 28.Secher A, Jelsing J, Baquero AF, Hecksher-Sørensen J, Cowley MA, Dalbøge LS, Hansen G, Grove KL, Pyke C, Raun K, Schäffer L, Tang-Christensen M, Verma S, Witgen BM, Vrang N, Bjerre Knudsen L. The arcuate nucleus mediates GLP-1 receptor agonist liraglutide-dependent weight loss. J Clin Invest 124: 4473–4488, 2014. doi: 10.1172/JCI75276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sisley S, Smith K, Sandoval DA, Seeley RJ. Differences in acute anorectic effects of long-acting GLP-1 receptor agonists in rats. Peptides 58: 1–6, 2014. doi: 10.1016/j.peptides.2014.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Talsania T, Anini Y, Siu S, Drucker DJ, Brubaker PL. Peripheral exendin-4 and peptide YY3–36 synergistically reduce food intake through different mechanisms in mice. Endocrinology 146: 3748–3756, 2005. doi: 10.1210/en.2005-0473. [DOI] [PubMed] [Google Scholar]
- 31.Wilson-Pérez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D, Drucker DJ, Pérez-Tilve D, Seeley RJ. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide 1 receptor deficiency. Diabetes 62: 2380–2385, 2013. doi: 10.2337/db12-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Woods SC, Seeley RJ, Cota D. Regulation of food intake through hypothalamic signaling networks involving mTOR. Annu Rev Nutr 28: 295–311, 2008. doi: 10.1146/annurev.nutr.28.061807.155505. [DOI] [PubMed] [Google Scholar]
- 33.Xue B, Kahn BB. AMPK integrates nutrient and hormonal signals to regulate food intake and energy balance through effects in the hypothalamus and peripheral tissues. J Physiol 574: 73–83, 2006. doi: 10.1113/jphysiol.2006.113217. [DOI] [PMC free article] [PubMed] [Google Scholar]