Abstract
Background
Beta-blockers are antihypertensive drugs and have shown potential in cancer prognosis. However, this benefit has not been well defined due to inconsistent results from the published studies.
Methods
To investigate the association between administration of beta-blocker and cancer prognosis, we performed a meta-analysis. A literature search of PubMed, Embase, Cochrane Library, and Web of Science was conducted to identify all relevant studies published up to September 1, 2017. Thirty-six studies involving 319,006 patients were included. Hazard ratios were pooled using a random-effects model. Subgroup analyses were conducted by stratifying ethnicity, duration of drug use, cancer stage, sample size, beta-blocker type, chronological order of drug use, and different types of cancers.
Results
Overall, there was no evidence to suggest an association between beta-blocker use and overall survival (HR=0.94, 95% CI: 0.87–1.03), all-cause mortality (HR=0.99, 95% CI: 0.94–1.05), disease-free survival (HR=0.59, 95% CI: 0.30–1.17), progression-free survival (HR=0.90, 95% CI: 0.79–1.02), and recurrence-free survival (HR=0.99, 95% CI: 0.76–1.28), as well. In contrast, beta-blocker use was significantly associated with better cancer-specific survival (CSS) (HR=0.78, 95% CI: 0.65–0.95). Subgroup analysis generally supported main results. But there is still heterogeneity among cancer types that beta-blocker use is associated with improved survival among patients with ovarian cancer, pancreatic cancer, and melanoma.
Conclusion
The present meta-analysis generally demonstrates no association between beta-blocker use and cancer prognosis except for CSS in all population groups examined. High-quality studies should be conducted to confirm this conclusion in future.
Keywords: cancer, prognosis, beta-blocker, meta-analysis
Introduction
Cancer is the main disease that endangers human life worldwide. The incidence of cancer remains grim that 1.7 million new cancer cases and 0.6 million cancer deaths are projected to occur in USA in 2017.1 Since cancer often leads to poor survival and a marked decline in quality of life, effective and safe therapies for prolonging cancer survival are urgently needed.
Beta-blockers have been considered as a safe cardiovascular treatment for decades.2 At present, the beta-adrenergic receptor downstream signaling pathway is certified as an important regulator of progression and metastasis of some important tumors,3 making beta-blockers a new alternative for cancer adjuvant chemotherapy.4 So far, a growing number of studies have supported the use of beta-blockers in prolonging survival of cancer patients,8–30 but several studies have put forward controversial conclusions.31–43
The purpose of this study was to use meta-analysis to quantitatively and comprehensively summarize the evidence for the relationship between beta-blocker exposure and survival outcomes of various cancers.
Materials and methods
Search strategy
Under the guidance of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA), we conducted this meta-analysis. To identify the studies of interest, we systematically searched PubMed (Supplementary material online file), Embase, Cochrane Library, and Web of Science for research reports published up to September 1, 2017. Search terms included: {Adrenergic beta-Antagonist(s), beta-blocker(s), atenolol, bisoprolol, carvedilol, metoprolol, propranolol, sotalol, timolol, arotinolol, betaxolol, bevan-tolol, carteolol or celiprolol} combined with {cancer(s), carcinoma(s), malignancy(ies), neoplasm(s) or tumour(s)} and {prognosis, survival or mortality}. We scanned the titles and abstracts of the studies identified in the initial search, excluding those apparently unrelated. The full text of the remaining articles was read to determine the studies that can be included. In addition, we have further studied the reference lists of articles for additional studies.
Inclusion and exclusion criteria
Our inclusion criteria were: 1) case–control or cohort studies or randomized controlled trials (RCTs); 2) patients with cancer; 3) reported at least 20 patients; 4) evaluated the therapeutic value of beta-blockers in cancer prognosis; 5) compared beta-blocker users with non-users in patients; 6) reported survival outcomes like overall survival (OS), all-cause mortality, cancer-specific survival (CSS), disease-free survival (DFS), progression-free survival (PFS), and recurrence-free survival (RFS); 7) reported HR with 95% CI for survival of comparison between exposure group and control group or HR could be obtained from other sufficient information.
Articles were excluded from the analyses for any of the following reasons: 1) reviews, commentaries, experimental laboratory articles, animal studies, or letters; 2) repeated publications; 3) impossible to calculate HR with 95% CI for survival from the paper.
Data extraction
The following information was extracted from each study: 1) publication data: first author’s name, publication year, and geographical location of the study; 2) study design; 3) number and characteristics of participants; 4) types of beta-blockers used; 5) HR estimates with their 95% CIs and control for multiple factors by matching or adjustments. If the HR and 95% CI could not be obtained directly, they were estimated from Kaplan–Meier curves.5
Quality assessment
Quality of the included studies was assessed using the Newcastle–Ottawa Quality Assessment Scale (NOS). Studies of medium quality scored 6–7 points. This assessment was completed by two investigators (ZN and XQ) independently, and any disagreements were solved by a revaluation of the original article with a third author (XH).
Statistical analysis
For the meta-analysis, we calculated pooled HRs with 95% CI for all the studies. We used the Cochran’s Q-test to examine whether the results of the studies were homogeneous. The P-value <0.10 for Q-test indicated heterogeneity. Quantity of I2 was also calculated to describe the percentage variation across studies due to heterogeneity. We regarded an I2 value >50% as indicative of significant heterogeneity. A fixed-effects model (inverse variance method) was used to calculate pooled results when no heterogeneity existed among the included studies; otherwise, a random-effects model (DerSimonian and Laird method) was used with the weights inversely proportional to the variance of hazard ratio of each trial.6,7 To identify potential sources of between-study heterogeneity, subgroup analyses were conducted by stratifying ethnicity, duration of drug use, cancer stage, sample size, beta-blocker type, chronological order of drug use, and different types of cancers. We conducted sensitivity analysis to determine the relative effect of a particular study on the meta-analysis model. To assess the influence of potential causes, meta-regression models were fitted separately for each cause except for beta-blocker therapy. The Begg’s adjusted rank correlation test and the Egger’s regression asymmetry tests were used to evaluate the effects of publication bias. All analyses were conducted using Stata 12.0 software (Markummitchell, Torrance, CA, USA), and we read Kaplan–Meier curves with Engauge Digitizer version 9.8.
Results
Study search and characteristics
The flow of literature selection applying the systematic search and selection strategies to identify qualified reports is shown in Figure 1. Six hundred and thirty studies were initially identified by the search. Of these, we retrieved 49 potential studies by filtering the titles and abstracts. Due to insufficient information (12 studies) or including the same patients (one study), 13 studies were excluded after further comprehensive review. Two studies were conducted in the same institute, but as the sample patients were at different stages and were treated differently, we considered them to be different cohorts.8,9 Finally, a total of 36 studies were included in the pooled analyses.
Figure 1.
PRISMA flowchart of article selection for this meta-analysis.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1 showed the characteristics of the 36 studies. The articles were published from 2011 to 2017, which included 319,006 patients. Of them, 35 studies utilized cohort design8–10,12–43 and one study used case–control design.11 Besides, there were 22 hospital-based studies9–11,14–16,18,19,21,23,24,26–31,33–35,40,41 and 14 population-based studies.8,12,13,17,20,22,25,32,36–39,42,43 Overall, all the 36 studies reported the prognostic value of beta-blockers in the survival of cancer patients.
Table 1.
Characteristics of studies included for meta-analysis
| Reference | Study | Country | Duration | Sample size | Median age (years) | Study design | Cancer type | Stage | Surgery | Beta-blocker type | No. of patients
|
Exposure category | Follow-up time (months) | Treatment | HR | 95% CI | Survival outcome | Multivariable analysis | Adjusted for | Study quality (NOS score) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure | Control | ||||||||||||||||||||
| 8 | Grytli et al (2013) | Norway | 2004–2009 | 655 | 72 | PB cohort | Prostate cancer | I/II 60.1%, III/IV 39.9% | NR | Mixed: beta selective (80.2%); non-selective (19.8%) | 80 | 575 | Pre-diagnostic beta-blocker use | 122 | ADT or not | 0.88 | 0.56–1.38 | OS | Yes | Age at diagnosis, metastasis at diagnosis, and level of education | 8 |
| 0.79 | 0.68–0.91 | CSS | |||||||||||||||||||
| 9 | Grytli et al (2014) | Norway | 2000–2011 | 3,561 | 76.3 | HB cohort | Prostate cancer | ≤T2A 14.9%; T2b–T2c 18.5%; ≥T3a 66.6% | NR | Mixed: beta 1 selective (77.9%); non-selective (3.0%); alpha and beta mixed (4.5%) | 1,115 | 2,446 | Pre-diagnostic beta-blocker use | 39 | RT or radical prostatectomy | 0.96 | 0.87–1.05 | OS | Yes | Age, prostate-specific antigen level, Gleason score, clinical T stage, presence and type of metastases, performance status, and androgen deprivation, therapy initiated within 6 months after diagnosis | 7 |
| 0.97 | 0.72–1.31 | CSS | |||||||||||||||||||
| 10 | Al-Niaimi et al (2016) | USA | 2000–2010 | 185 | 66.2 63.8 | HB cohort | Ovarian cancer | I/II 26%, III/IV 74% | Yes | NR | 70 | 115 | Post-diagnostic beta-blocker use (time-dependent) | 91 | CT | 0.68 | 0.46–0.99 | OS | Yes | Age, stage, grade, cytoreduction status, BMI, and presence or absence of diabetes | 7 |
| 11 | Aydiner et al (2013) | Turkey | 2003–2011 | 107 | 61 (42–81) | HB case–control | Non-small-cell lung cancer | NR | Mixed | Mixed | 35 | 72 | Post-diagnostic beta-blocker use (time-fixed) | 17.8 (1–102) | CT | 0.69 | 0.36–1.34 | OS | Yes | Age, sex, performance status, histologic subtype, smoking status, presence of comorbidities (COPD, IHD, HT, and DM) | 7 |
| 12 | Barron et al (2011) | Ireland/USA | 2001–2006 | 4,808 | 69.1 71 | PB cohort | Breast cancer | I/II 75.6%, III/IV 24.4% | NR | Beta non-selective | 70 | 4,738 | Pre-diagnostic beta-blocker use | 42 43.2 32.4 36 |
CT or not | 0.19 | 0.06–0.60 | OS | Yes | Age, stage, grade, and comorbidity | 7 |
| 12 | Barron et al (2011) | Ireland/USA | 2001–2006 | 5,263 | 69.1 71 | PB cohort | Breast cancer | I/II 75.6%, III/IV 24.4% | NR | Beta selective | 525 | 4,738 | Pre-diagnostic beta-blocker use | 42 43.2 32.4 36 |
CT or not | 1.08 | 0.84–1.40 | OS | Yes | Age, stage, grade, and comorbidity | 7 |
| 12 | Barron et al (2011) | Ireland/USA | 2001–2006 | 5,801 | 69.1 71 | PB cohort | Breast cancer | I/II 75.6%, III/IV 24.4% | NR | Mixed: beta selective (88%); non-selective (12%) | 595 | 4,738 | Pre-diagnostic beta-blocker use | 42 43.2 32.4 36 |
CT or not | 1.08 | 0.84–1.39 | CSS | Yes | Age, stage, grade, and comorbidity | 7 |
| 13 | Beg et al (2017) | USA | 2006–2009 | 13,702 | 76 | PB cohort | Pancreatic adenocarcinoma | I/II 38.1%, III/IV 61.9% | Mixed 69.3% | NR | 5,209 | 8,493 | NR | NR | NR | 0.9 | 0.85–0.95 | OS | Yes | Age, sex, race, stage at diagnosis, site of cancer, and Charlson comorbidity index | 8 |
| 14 | Bir et al (2015) | USA | 2001–2013 | 225 | 57.34± 10.98 | HB cohort | Metastatic brain tumors | NR | Yes | Beta 1 selective | 40 | 185 | NR | 10.57 | GKRS | 1.08 | 0.65–1.79 | OS | Yes | MBT kind, metastasis, tumor recurrence, tumor response, GKRS, prognostic factor | 7 |
| 15 | De Giorgi et al (2013) | Italy | 1993–2009 | 741 | 64 53 | HB cohort | Thick melanoma | NR | Mixed | Mixed: beta 1 selective (73%); non-selective (27%) | 79 | 662 | Post-diagnostic beta-blocker use (time-dependent) | 50.4 | NR | 0.03 | 0.01–0.17 | DFS | Yes | Age, Breslow thickness, and ulceration | 7 |
| 0.04 | 0.00–0.38 | OS | |||||||||||||||||||
| 16 | Diaz et al (2012) | USA | 1996–2006 | 248 | 67 | HB cohort | Ovarian cancer | III/IV 100% | Yes | Mixed: beta 1 selective (75%); non-selective (13%); mixed alpha and beta adrenergic antagonist (13%) | 23 | 225 | NR | NR | CT | 0.56 | 0.43–1.26 | OS | Yes | Age, stage, grade, and cytoreduction status | 6 |
| 17 | Ganz et al (2011) | USA | 1997–2002 | 1,779 | NR | PB cohort | Breast cancer | I/II 96.9%, III/IV 3.1% | NR | Mixed: beta selective (86%); non-selective (14%) | 204 | 1,372 | NR | 98.4 | CT, RT, both or none | 1.04 | 0.72–1.51 | OS | Yes | Age at diagnosis, race, stage of disease, pre-diagnosis BMI, adjuvant treatment, hormone receptor status, tamoxifen use, and self-reported hypertension and diabetes | 8 |
| 0.86 | 0.57–1.32 | RFS | |||||||||||||||||||
| 0.76 | 0.44–1.33 | CSS | |||||||||||||||||||
| 18 | Giampieri et al (2015) | Italy | 2010–2013 | 235 | NR | HB cohort | Colorectal cancer | NR | NR | NR | 29 | 206 | Pre-diagnostic beta-blocker use | NR | CT or with bevacizumab | 1.51 | 0.88–2.31 | OS | Yes | Age, sex, and site of metastases, previous adjuvant chemotherapy, and ECOG performance status | 7 |
| 1.19 | 0.81–1.72 | PFS | |||||||||||||||||||
| 19 | Hwa et al (2017) | USA | 1995–2010 | 1,971 | 64 | HB cohort | Myeloma | I/II 75%, III/IV 25% | Mixed | Mixed | 549 | 1,733 | Post-diagnostic beta-blocker use (time-fixed) | 74.3 | CT | 0.67 | 0.55–0.81 | OS | Yes | Demographics, disease characteristics, diagnosis year, and various chemotherapies | 7 |
| 0.53 | 0.42–0.67 | CSS | |||||||||||||||||||
| 20 | Jansen et al (2014) | Germany | 2003–2007 | 1,975 | 68 | PB cohort | Colorectal cancer | I/II 55% III/IV 45% | Mixed 97.3% | Mixed: beta selective (86%); non-selective (14%) | 509 | 1,311 | Pre-diagnostic beta-blocker use | 60 | CT or RT | 0.99 | 0.79–1.22 | OS | Yes | Age at diagnosis, sex, Union for International Cancer Control (UICC) stage (I–IV), surgery, chemotherapy, radiotherapy, body mass index, hypertension, CVD (including heart failure, myocardial infarction, stroke, and cardiac circulatory disorder), diabetes, regular use of nonsteroidal anti-inflammatory drugs (NSAIDs) including aspirin, regular use of statins, use of hormone replacement therapy (HRT), lifetime pack-years of active smoking, physical activity (quartiles of lifetime metabolic equivalents [METs] in hours per week), and participation in health check-up | 8 |
| 0.93 | 0.71–1.21 | CSS | |||||||||||||||||||
| 21 | Kim et al (2017) | Korea | 2001–2012 | 1,274 | 61 (20–87) | HB cohort | Head and neck squamous cell carcinoma (HNSCC) | I/II 41.4% III/IV 58.6% | Mixed 69.2% | Mixed: beta 1 selective (84%); non-selective (16%) | 114 | 1,160 | Post-diagnostic beta-blocker use (time-fixed) | 98 | Primary curative surgery, RT, CRT with or without IC, or a combination of these treatments | 1.33 | 0.93–1.91 | DFS | Yes | Age, sex, BMI, CCI, smoking, alcohol, tumor site, tumor classification T3–4, nodal classification N1–3, overall TNM stage III–IV, primary treatment, second primary cancer, hypertension | 6 |
| 1.49 | 0.99–2.22 | CSS | |||||||||||||||||||
| 1.54 | 1.17–2.05 | OS | |||||||||||||||||||
| 22 | Lemeshow et al (2011) | Denmark | Since 1943 | 4,179 | 66 | PB cohort | Melanoma | I/II 63.8%, III/IV 36.2% | Mixed | Mixed | 372 | 3,807 | Pre-diagnostic beta-blocker use | 58.8 | NR | 0.81 | 0.67–0.97 | OS | Yes | Age and comorbidity index score | 7 |
| 0.87 | 0.64–1.2 | CSS | |||||||||||||||||||
| 23 | Melhem-Bertrandt et al (2011) | USA | 1995–2007 | 1,413 | 57 49 | HB cohort | Breast cancer | I/II 55.6%, III/IV 44.4% | Yes | Mixed: beta selective (89%); non-selective (11%) | 102 | 1,311 | Post-diagnostic beta-blocker use (time-fixed) | 58.8 | Anthracylines and taxane-based neoadjuvant CT | 0.3 | 0.10–0.87 | RFS | Yes | Age, race, stage, grade, receptor status, lymphovascular invasion, body mass index, diabetes, hypertension, and angiotensin-converting enzyme inhibitor use | 7 |
| 0.76 | 0.44–1.33 | CSS | |||||||||||||||||||
| 0.35 | 0.12–1.00 | OS | |||||||||||||||||||
| 24 | Springate et al (2015) | NR | 1997–2006 | 11,302 | NR | HB cohort | Mixed cancer | NR | NR | Mixed | 4,030 | 7,272 | Pre-diagnostic beta-blocker use | 29 30 | NR | 1.03 | 0.93–1.14 | OS | No | No | 7 |
| 24 | Springate et al (2015) | NR | 1997–2006 | 6,274 | NR | HB cohort | Mixed cancer | NR | NR | Mixed | 1,406 | 4,868 | Pre-diagnostic beta-blocker use | 29 30 | NR | 1.18 | 1.04–1.33 | OS | No | No | 7 |
| 25 | Udumyan et al (2017) | Swedish | 2006–2009 | 2,394 | 70.9 67.1 | PB cohort | Pancreatic adenocarcinoma | I/II 21%, III/IV 79% | NR | Mixed: beta 1 selective (89%); non-selective (11%) | 522 | 1,872 | Pre-diagnostic beta-blocker use | 5 | NR | 0.79 | 0.70–0.90 | OS | Yes | Sociodemographic factors, tumor characteristics, comorbidity score, and other medications | 8 |
| 0.77 | 0.69–0.87 | CSS | |||||||||||||||||||
| 26 | Wang et al (2013) | USA | 1998–2010 | 722 | 65 (34–95) | HB cohort | Non-small-cell lung cancer | I/II 6.2%, III 93.8% | Mixed | Mixed: beta selective (86%); non-selective (14%) | 155 | 567 | Post-diagnostic beta-blocker use (time-fixed) | 44 (1–155) | Definitive RT | 0.91 | 0.64–1.31 | PFS | Yes | Age, Karnofsky performance score, clinical stage, tumor histology, use of concurrent chemotherapy, radiation dose, GTV, hypertension, chronic obstructive pulmonary disease, and aspirin consumption | 7 |
| 0.67 | 0.50–0.91 | DMFS | |||||||||||||||||||
| 0.74 | 0.58–0.95 | DFS | |||||||||||||||||||
| 0.78 | 0.63–0.97 | OS | |||||||||||||||||||
| 27 | Watkins et al (2015) | USA | 2000–2010 | 1,425 | 61.6 68 | HB cohort | Ovarian cancer | I/II 10%, III/IV 90% | Yes | Mixed: beta selective (72.1%); non-selective (27.9%) | 269 | 1,156 | Post-diagnostic beta-blocker use (time-fixed) | NR | CT | 0.26 | 0.19–0.37 | OS | No | No | 6 |
| 0.24 | 0.17–0.34 | CSS | |||||||||||||||||||
| 28 | Yusuf et al (2012) | USA | 2000–2006 | 456 | 67 | HB cohort | Mixed cancer | NR | NR | NR | 211 | 245 | NR | 1.2 | Chest RT or CT | 0.64 | 0.51–0.81 | OS | Yes | Age, cancer status, cancer type, previous chemotherapy, chest radiotherapy, hyperlipidemia | 6 |
| 29 | Botteri et al (2013) | Italy | 1997–2006 | 800 | 62 59 | HB cohort | Breast cancer | I/II 86%, III/IV 14% | Yes | Mixed: beta 1 selective (84.1%); non-selective (4%); alpha and beta mixed (11.9%) | 74 | 726 | Pre-diagnostic beta-blocker use | 72 67.2 | Adjuvant CT and RT | 0.42 | 0.18–0.97 | CSS | Yes | Age, tumor stage, and treatment, peritumoral vascular invasion and use of other antihypertensive drugs, antithrombotics, and statins | 7 |
| 30 | Spera et al (2017) | Canada | NR | 1,144 | 60 53 | HB cohort | Breast cancer | NR | Yes | Mixed | 153 | 991 | Pre/post-diagnostic beta-blocker use (time-dependent) | 25.1 | CT | 0.81 | 0.66–0.99 | PFS | Yes | Treatment arm (RAM vs PBO), HHRR status, geographic region, THE | 7 |
| 1.05 | 0.85–1.29 | OS | |||||||||||||||||||
| 31 | Johannesdottir et al (2013) | Denmark | 1999–2010 | 6,253 | 65 | HB cohort | Ovarian cancer | NR | Mixed | NR | 87 | 6,166 | Pre-diagnostic beta-blocker use | 30.6 | HRT | 1.18 | 0.90–1.55 | OS | Yes | Age, comorbidity level, prior use of diuretics, year of diagnosis, aspirin, and statins | 7 |
| 31 | Johannesdottir et al (2013) | Denmark | 1999–2010 | 6,539 | 65 | HB cohort | Ovarian cancer | NR | Mixed | NR | 373 | 6,166 | Pre-diagnostic beta-blocker use | 30.6 | HRT | 1.17 | 1.02–1.34 | OS | Yes | Age, comorbidity level, prior use of diuretics, year of diagnosis, aspirin, and statins | 7 |
| 32 | Assayag et al (2014) | Canada/UK | 1998–2012 | 6,270 | 72.3 | PB cohort | Prostate cancer | NR | Yes | Mixed: beta selective (59.4%); non-selective (40.6%) | 673 | 1,088 | Post-diagnostic beta-blocker use (time-dependent) | 45.6 | Prostatectomy, RT, ADT, and CT | 0.97 | 0.8–1.16 | OS | No | No | 7 |
| 0.97 | 0.72–1.31 | CSS | |||||||||||||||||||
| 33 | Cata et al (2014) | USA | NR | 391 | NR | HB cohort | Non-small-cell lung cancer | I/II 75.2%, III 24.8% | Yes | Beta 1 selective | 149 | 242 | NR | NR | NR | 1.304 | 0.973–1.747 | RFS | Yes | Age, stage of disease, BMI, ASA physical status, smoking status, CAD, postoperative radiation treatment, type of surgery, and perioperative blood transfusions | 7 |
| 1.335 | 0.966–1.846 | OS | |||||||||||||||||||
| 33 | Cata et al (2014) | USA | NR | 286 | NR | HB cohort | Non-small-cell lung cancer | I/II 75.2%, III 24.8% | Yes | Beta non-selective | 44 | 242 | NR | NR | NR | 0.989 | 0.639–1.532 | RFS | Yes | Age, stage of disease, BMI, ASA physical status, smoking status, CAD, postoperative radiation treatment, type of surgery, and perioperative blood transfusions | 7 |
| 34 | Heitz et al (2013) | Germany/Canada | NR | 381 | 60 | HB cohort | Ovarian cancer | I/II 6.5%, III/IV 93.5% | Yes | Mixed: beta selective (84%); non-selective (16%) | 1.108 | 0.678–1.812 | OS | ||||||||
| 38 | 343 | Post-diagnostic beta-blocker use (time-fixed) | 17 | CT | 0.92 | 0.65–1.31 | PFS | Yes | Age, stage, grade, and cytoreduction status | 7 | |||||||||||
| 35 | Heitz et al (2017) | Germany | 1999–2014 | 801 | 58 (19–90) | HB cohort | Ovarian cancer | I/II 43.3%, III/IV 56.7% | Yes | Beta 1 selective | 0.74 | 0.49–1.11 | OS | ||||||||
| 141 | 660 | NR | 40 | CT | 0.94 | 0.69–1.29 | OS | Yes | Age, ECOG, ASA, Charlton comorbidity score (metric), tumor residuals, histology, body mass index, and FIGO stage | 7 | |||||||||||
| 36 | Holmes et al (2013) | Canada | 2004–2008 | 2,433 | 68.3 | PB cohort | Breast cancer | NR | NR | Mixed | 0.95 | 0.72–1.27 | PFS | ||||||||
| 36 | Holmes et al (2013) | Canada | 2004–2008 | 2,016 | 68.3 | PB cohort | Colorectal cancer | NR | NR | Mixed | 123 | 2,310 | Pre-diagnostic beta-blocker use | NR | NR | 1.1 | 0.92–1.32 | OS | No | No | 6 |
| 36 | Holmes et al (2013) | Canada | 2004–2008 | 2,125 | 68.3 | PB cohort | Lung cancer | NR | NR | Mixed | 152 | 1,864 | Pre-diagnostic beta-blocker use | NR | NR | 1.05 | 0.93–1.18 | OS | No | No | 6 |
| 36 | Holmes et al (2013) | Canada | 2004–2008 | 1,868 | 68.3 | PB cohort | Prostate cancer | NR | NR | Mixed | 196 | 1,929 | Pre-diagnostic beta-blocker use | NR | NR | 1.01 | 0.93–1.11 | OS | No | No | 6 |
| 37 | Jansen et al (2017) | The Netherlands | 1998–2011 | 2,530 | 73 68 | PB cohort | Colorectal cancer | I/II 55.7%, III/IV 44.3% | Mixed 89.8% | Mixed: beta selective (55%); non-selective (45%) | 163 | 1,705 | Pre-diagnostic beta-blocker use | NR | NR | 1.18 | 0.99–1.40 | OS | No | No | 6 |
| 37 | Jansen (2017) | The Netherlands | 1998–2011 | 1,374 | 73 68 | PB cohort | Colorectal cance | I/II 55.7%, III/IV 44.3% | Mixed 89.8% | Mixed: beta selective (66%); non-selective (34%) | 1456 | 1,074 | Pre-diagnostic beta-blocker use | 79.2 | NR | 1.07 | 0.96–1.19 | OS | Yes | Age at diagnosis, sex, year of diagnosis, socioeconomic status based on the place of residence, Union for International Cancer Control (UICC) stage (I, II, III, IV), cancer site (colon, rectum/rectosigmoid), surgery, chemotherapy, radiotherapy, cancer, cardiovascular disease, cerebrovascular disease, diabetes, hypertension, time-dependent use of NSAIDs, statins and diabetes medication after diagnosis and number of distinct ATC classes prescribed during 4 months prior to diagnosis (0, 1–3, 4–5, 6+ distinct ATC classes [first letter of the ATC] dispensed during 4 months prior to diagnosis) | 7 |
| 38 | Livingstone et al (2013) | Germany/The Netherlands | 709 | 67 59 | PB cohort | Melanoma | NR | Mixed | Mixed: beta 1 selective (84%); non-selective (16%) | 919 | 455 | Post-diagnostic beta blocker use (time-dependent) | 79.2 | NR | 1.1 | 0.98–1.23 | OS | Yes | Age at diagnosis, sex, year of diagnosis, socio-economic status based on the place of residence, Union Internationale Contre le Cancer (UICC) stage (I, II, III, IV), cancer site (colon, rectum/rectosigmoid), surgery, chemotherapy, radiotherapy, previous cancer, cardiovascular disease, cerebrovascular disease, diabetes, hypertension, time- dependent use of NSAIDs, statins and diabetes medication after diagnosis and number of distinct ATC classes prescribed during four months prior to diagnosis (0, 1–3, 4–5, 6+ distinct ATC classes [first letter of the ATC] dispensed during four months prior to diagnosis) | 7 | |
| 120 | 589 | Post-diagnostic beta-blocker use (time-dependent) | 39 | NR | 0.82 | 0.55–1.24 | OS | No | No | 6 | |||||||||||
| 39 | Musselman et al (2014) | Canada | 2002–2010 | 66,889 | NR | PB cohort | Breast cancer | NR | Yes | NR | 4,372 | 7,013 | NR | 57.6 6 30.5, 43.1 6 28.7, and 53.4 6 31.0 | NR | 0.99 | 0.87–1.13 | OS | No | No | 6 |
| 39 | Musselman et al (2014) | Canada | 2002–2010 | 66,890 | NR | PB cohort | Lung cancer | NR | Yes | NR | 1,901 | 2,314 | NR | 57.6 6 30.5, 43.1 6 28.7, and 53.4 6 31.0 | NR | 1.06 | 0.91–1.24 | OS | No | No | 6 |
| 39 | Musselman et al (2014) | Canada | 2002–2010 | 66,891 | NR | PB cohort | Colorectal cancer | NR | Yes | NR | 22,170 | 30,118 | NR | 57.6 6 30.5, 43.1 6 28.7, and 53.4 6 31.0 | NR | 1.06 | 0.99–1.02 | OS | No | No | 6 |
| 40 | Parker et al (2017) | USA | 2000–2010 | 913 | 65 67 | HB cohort | Renal cell carcinoma | I/II 51.6%, III/IV 48.4% | Yes | Mixed: beta 1 selective (90%); non-selective (4%); alpha and beta mixed (6%) | 104 | 809 | Pre-diagnostic beta-blocker use | 98.4 | NR | 0.83 | 0.59–1.16 | OS | Yes | Age at surgery, sex, onstitutional symptoms, smoking history, eGFR category, ECOG performance status, Charlson score, type of surgery, tumor size, 2010 pT classification, grade, coagulative tumor necrosis | 7 |
| 0.78 | 0.43–1.41 | CSS | |||||||||||||||||||
| 41 | Sakellakis et al (2014) | Greece | 1983–2013 | 610 | 63 55 | HB cohort | Breast cancer | I/II 73.6%, III/IV 26.4% | Yes | Mixed | 47 | 430 | Post-diagnostic beta-blocker use (time-dependent) | 24 48 | CT | 0.849 | 0.537–1.343 | DFS | No | No | 6 |
| 42 | Shah et al (2011) | UK | 1997–2009 | 3,462 | HR | PB cohort | Mixed cancer | NR | NR | Mixed: beta selective (83%); non-selective (17%) | 1,406 | 2,056 | Pre-diagnostic beta-blocker use | NR | NR | 1.18 | 1.04–1.33 | OS | No | No | 6 |
| 43 | Weberpals et al (2017) | Holland | 1998–2011 | 2,221 | 70.4 | PB cohort | Lung cancer | I/II 24.1%, III/IV 75.9% | Mixed 17.4% | Mixed: beta selective (88%); non-selective (12%) | 1,107 | 1,114 | Pre-diagnostic beta-blocker use | 78 | NR | 1 | 0.92–1.08 | OS | Yes | Comorbidities, time-varying treatment, and distinct numbers of medications used | 7 |
| 43 | Weberpals et al (2017) | Holland | 1998–2011 | 2,221 | 70.4 | PB cohort | Lung cancer | I/II 24.1%, III/IV 75.10% | Mixed 17.5% | Mixed: beta selective (88%); non-selective (13%) | 1,224 | 997 | Post-diagnostic beta-blocker use (time-dependent) | 78 | NR | 1.03 | 0.94–1.11 | OS | Yes | Comorbidities, time-varying treatment, and distinct numbers of medications used | 7 |
Abbreviations: NR, not reported; PB, population-based; HB, hospital-based; RT, radiation therapy; CT, chemotherapy; ADT, androgen deprivation therapy; CRT, concurrent chemoradiotherapy; IC, induction chemotherapy; GKRS, gamma knife radiosurgery; HRT, hormone replacement therapy; OS, overall survival; CSS, cancer-specific survival; DFS, disease-free survival; PFS, progression-free survival; RFS, recurrence-free survival; NOS, Newcastle–Ottawa Quality Assessment Scale; BMI, body mass index; IHD, ischemic heart disease; HT, hypertension; MBT, Metastatic brain tumors; ECOG, electrocorticogram; CVD, cardiovascular disease; GTN,GTV, gross tumor volume; RAM, Ramucirumab; PBO, Placebo; HHRR, hormonal receptor; THE, treatment emergent hypertension; ASA, American Standards Association; CAD, coronary artery disease; DM, diabetes mellitus; FIGO, International Federation of Gynecology and Obstetrics; eGFR, epidermal growth factor receptor; ATC, Anatomical Therapeutic Chemical; CCI, Charlson comorbidity index; DMFS, distant metastasis-free survivall; pT, primary tumour.
Quality assessment
While there was small variation in the methodological quality of the included studies, all 36 included studies were judged as moderate to relative high quality according to the NOS assessment tool, with scores of 6 (11 studies), 7 (20 studies), and 8 (five studies, Table S1).
Beta-blockers and survival of cancer
Meta-analysis of overall survival
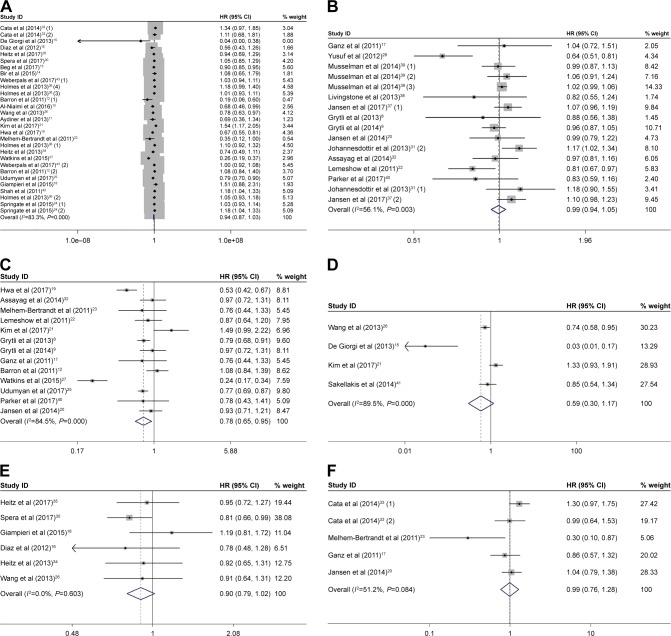
As displayed in Figure 2A, the forest plot showed that beta-blocker use was not associated with OS. The pooled HR was 0.94 (95% CI: 0.87–1.03, P=0.172) from 22 observational studies. Considering the high heterogeneity (I2=83.3%, P<0.001), we used random-effects model to pool the studies.
Figure 2.
Forest plots showing the effects of beta-blocker use on OS (A), all-cause mortality (B), CSS (C), DFS (D), PFS (E), and RFS (F).
Notes: Weights are from random-effects analysis. The numbers in parentheses indicate the different included studies in the same year.
Abbreviations: OS, overall survival; CSS, cancer-specific survival; DFS, disease-free survival; PFS, progression-free survival; RFS, recurrence-free survival.
Meta-analysis of all-cause mortality
Twelve studies focused on beta-blocker use and all-cause mortality. A random-effects model was used and the combined HR of 0.99 (95% CI: 0.94–1.05, P=0.807, Figure 2B) showed that beta-blocker use was also not correlated with all-cause mortality.
Meta-analysis of cancer-specific survival
Thirteen studies presented the data concerning the association between beta-blocker use and CSS (Figure 2C). We calculated that beta-blocker use was significantly correlated with long CSS, with a pooled HR of 0.78 (95% CI: 0.65–0.95, P=0.012) by using a random-effects model.
Meta-analysis of disease-free survival
Four studies reported the data on beta-blocker use and DFS outcome. The pooled HR was 0.59 (95% CI: 0.30–1.17, P=0.134, Figure 2D) with significant heterogeneity between studies (I2=89.5%, P<0.001), which demonstrated that beta-blocker use was also prominently not related to DFS.
Meta-analysis of progression-free survival
The data on beta-blocker use and PFS outcome was presented in six studies. Meta-analysis adopting the fixed-effects model revealed that beta-blocker use was not associated with PFS (HR=0.90, 95% CI: 0.79–1.02, P=0.087, Figure 2E) and exhibited no heterogeneity (I2=0.00%, P=0.603).
Meta-analysis of recurrence-free survival
Four studies provided sufficient data on beta-blocker use and RFS outcome. The pooled HR was 0.99 (95% CI: 0.76–1.28, P=0.944, Figure 2F) by a random-effects model. Beta-blocker use was also significantly not related to RFS.
Subgroup analysis
To deeply explore the relationship between beta-blocker use and OS, we performed subgroup analysis based on ethnicity, duration of drug use, cancer stage, sample size, beta-blocker type, chronological order of drug use, and different types of cancers. The median values of original data from included studies in “duration of drug use” and “sample size” were chosen as cut-off values to divide our subgroups. The results are summarized in Table 2, with the corresponding forest plots presented in Figure S1.
Table 2.
Summary of the subgroup analysis results of beta-blocker use and OS
| Variables | Number of studies | Number of patients | Model | Outcome (OS)
|
Heterogeneity
|
||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | I2 (%) | P-value | ||||
| Ethnicity | |||||||
| Non-Europeans | 16 | 30,607 | R | 0.90 (0.78–1.02) | 0.106 | 87.2 | <0.001 |
| Europeans | 8 | 12,182 | R | 1.00 (0.89–1.12) | 0.958 | 72.2 | 0.001 |
| Duration of drug use | |||||||
| >2 years | 6 | 8,899 | F | 1.03 (0.93–1.14) | 0.617 | 0.0 | 0.576 |
| <2 years | 6 | 10,812 | R | 1.01 (0.91–1.11) | 0.897 | 54.7 | 0.051 |
| Cancer stage | |||||||
| I/II | 11 | 2,870 | F | 0.97 (0.89–1.06) | 0.507 | 15.6 | 0.295 |
| III/IV | 13 | 4,835 | R | 1.04 (0.94–1.14) | 0.468 | 59.1 | 0.003 |
| Sample size | |||||||
| >1,500 | 15 | 65,834 | R | 1.01 (0.94–1.08) | 0.783 | 76.7 | <0.001 |
| <1,500 | 18 | 11,839 | R | 0.81 (0.66–1.00) | 0.053 | 83.5 | <0.001 |
| Beta-blocker type | |||||||
| Non-selective | 12 | 17,714 | R | 1.04 (0.89–1.22) | 0.596 | 75.7 | <0.001 |
| Selective | 10 | 17,714 | R | 0.93 (0.83–1.05) | 0.243 | 83.5 | <0.001 |
| Chronological order of drug use | |||||||
| Pre-diagnostic beta-blocker use | 13 | 55,710 | R | 1.03 (0.95–1.11) | 0.493 | 74.7 | <0.001 |
| Post-diagnostic beta-blocker use (time-fixed) | 7 | 6,372 | R | 0.65 (0.43–0.99) | 0.046 | 91.0 | <0.001 |
| Post-diagnostic beta blocker use (time-dependent) | 2 | 2,406 | R | 0.87 (0.59–1.30) | 0.508 | 76.8 | 0.038 |
| Cancer type | |||||||
| Lung cancer | 7 | 10,189 | F | 1.01 (0.96–1.05) | 0.818 | 40.1 | 0.124 |
| Melanoma | 2 | 4,910 | F | 0.81 (0.67–0.97) | 0.026 | 0.0 | 0.892 |
| Mixed cancer | 4 | 21,494 | R | 1.00 (0.83–1.21) | 0.974 | 87.7 | <0.001 |
| Colorectal cancer | 2 | 4,202 | R | 1.16 (0.84–1.61) | 0.353 | 51.3 | 0.152 |
| Ovarian cancer | 5 | 3,140 | R | 0.59 (0.36–0.96) | 0.034 | 88.0 | <0.001 |
| Breast cancer | 6 | 16,637 | R | 0.97 (0.78–1.21) | 0.783 | 61.20 | 0.024 |
| Pancreatic cancer | 2 | 16,096 | R | 0.85 (0.75–0.97) | 0.014 | 71.10 | 0.063 |
Abbreviations: F, fixed-effects model; R, random-effects model; OS, overall survival.
The subgroups of sample size and ethnicity demonstrated no significant effect of beta-blocker use on OS. Similarly, beta-blocker showed no obvious impact on OS for patients with duration of drug use more than 2 years (HR=1.03, 95% CI: 0.93–1.14, P=0.617) or patients with duration of drug use less than 2 years (HR=1.01, 95% CI: 0.91–1.11, P=0.897). Additionally, the subgroup analysis indicated that the administration of beta-blockers had no relationship with longer OS when the meta-analysis was restricted to patients with cancer in I/II stage (HR=0.97, 95% CI: 0.89–1.06, P=0.507) or cancer in III/IV stage (HR=1.04, 95% CI: 0.94–1.14, P=0.468). In addition, the studies using selective beta-blocker (HR=0.93, 95% CI: 0.83–1.05, P=0.243) and non-selective beta-blocker (HR=1.04, 95% CI: 0.89–1.22, P=0.596) were found to have no effect on OS. However, beta-blocker showed a more positive effect on OS for patients with time-fixed post-diagnostic beta-blocker use (HR=0.65, 95% CI: 0.43–0.99, P=0.046) than pre-diagnostic beta-blocker use (HR=1.03, 95% CI: 0.95–1.11, P=0.493) and time-dependent post-diagnostic beta-blocker use (HR=0.87, 95% CI: 0.59–1.30, P=0.508).
Analysis according to cancer type showed predominantly longer OS in ovarian cancer (HR=0.59, 95% CI: 0.36–0.96, P=0.034), pancreatic cancer (HR=0.85, 95% CI: 0.75–0.97, P=0.014), and melanoma (HR=0.81, 95% CI: 0.67–0.97, P=0.026), but no effects on lung cancer (HR=1, 95% CI: 0.96–1.05, P=0.818), breast cancer (HR=0.97, 95% CI: 0.78–1.21, P=0.783), colorectal cancer (HR=1.16; 95% CI: 0.84–1.61, P=0.353), and mixed cancer (HR=1.00; 95% CI: 0.83–1.21, P=0.974). Owing to the small numbers of studies and lack of information, subgroup analyses were not performed on other survival outcomes.
Sensitivity analysis
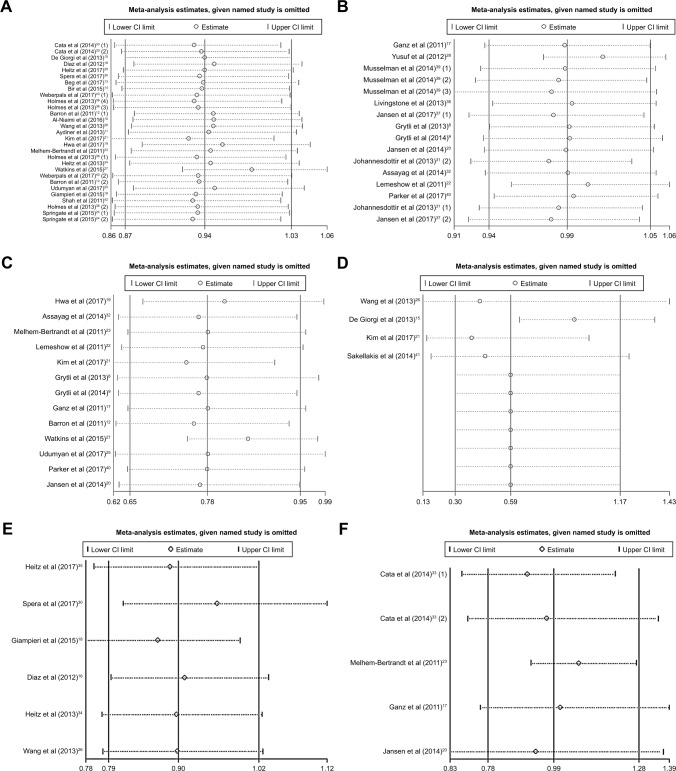
Sensitivity analysis was conducted on different survival outcomes. The meta-analyses of beta-blockers and survival were performed by removing a single study in turn. After removing the study results, the comprehensive estimation direction and amplitude of OS, all-cause mortality, CSS, DFS, PFS, and RFS were not significantly changed, indicating that the reliability of the meta-analysis was good and the results were not affected by any research (Figure 3). In addition, sensitivity analyses were also conducted in those studies whose HR and 95% CI values were presented in original articles (not calculated from the Kaplan–Meier plots) (Figure S2) and whose NOS score was ≥7 (Figure S3). These factors did not affect the main results.
Figure 3.
Sensitivity analysis of beta-blocker use on OS (A), all-cause mortality (B), CSS (C), DFS (D), PFS (E), and RFS (F).
Abbreviations: OS, overall survival; CSS, cancer-specific survival; DFS, disease-free survival; PFS, progression-free survival; RFS, recurrence-free survival.
Publication bias
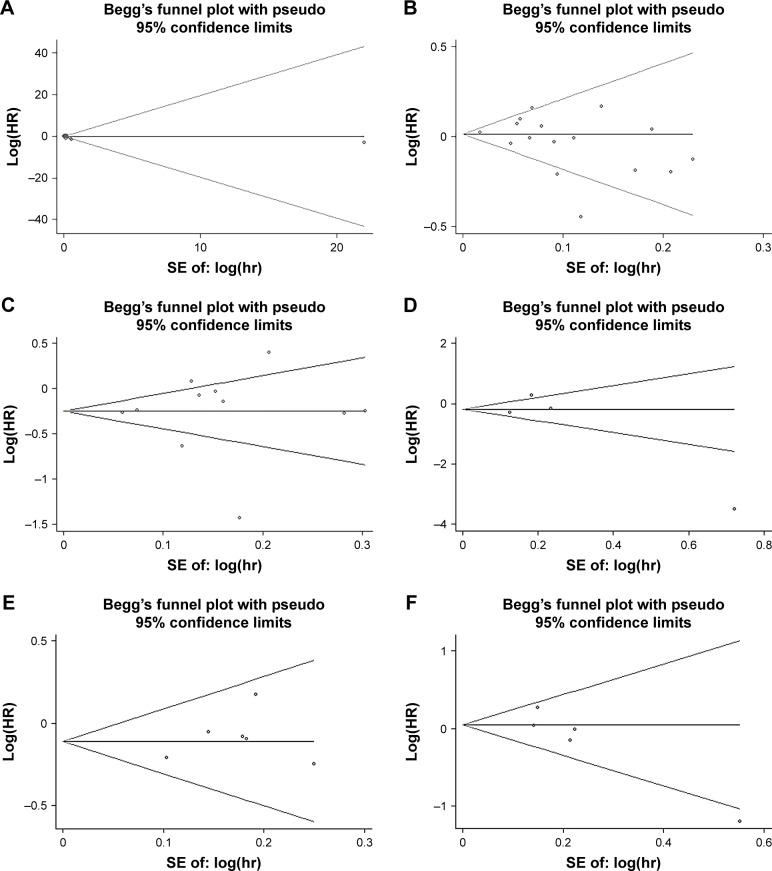
The funnel plot revealed no evidence of publication bias in the meta-analysis of beta-blocker use and OS (Figure 4A, Egger’s test: P-value =0.358; Begg’s test: P-value =0.115). There was no potential publication bias on beta-blocker use and all-cause mortality as well (Figure 4B, Egger’s test: P-value =0.261; Begg’s test: P-value =0.260). Besides, there was also no potential publication bias on beta-blocker use, CSS, DFS, PFS, and RFS of cancer patients (Figure 4C–F).
Figure 4.
Funnel plot of Begg’s test of beta blocker use on OS (A), all-cause mortality (B), CSS (C), DFS (D), PFS (E), and RFS (F).
Abbreviations: OS, overall survival; CSS, cancer-specific survival; DFS, disease-free survival; PFS, progression-free survival; RFS, recurrence-free survival; SE, standard error.
Meta-regression
The meta-regression analysis was performed to investigate the effects of various cohort study characteristics on the study estimates of the HRs. We grouped the studies according to specific characteristics, the size of sample, the sex of patients, the cancer sites, study duration, and study quality. There was no inverse association between sample size (P=0.892), sex of the patients (P=0.135), cancer sites (P=0.364), study duration (P=0.076), and study quality (P=0.571). Because of the lack of information, meta-regression was not performed on other survival outcomes.
Discussion
This meta-analysis summarizes 36 currently published studies examining the association between beta-blocker use and prognosis of cancer across a wide range of geographic regions and cancer types. Overall, the administration of beta-blocker was not associated with OS, all-cause mortality, DFS, PFS and RFS of cancer patients. However, beta-blocker use was significantly correlated with long CSS (HR=0.78, 95% CI: 0.65–0.95). Since the patients included in the clinical trials differed in stages, therapies, and so on, the heterogeneity was inescapable. Then we conducted subgroup analysis. Among the cancer types, positive associations between beta-blocker use and cancer prognosis were observed in breast cancer, pancreatic cancer, and melanoma, but could not be detected in lung cancer, ovarian cancer, colorectal cancer, and mixed cancer. Interestingly, beta-blocker use is associated with improved survival only among patients with ovarian cancer, pancreatic cancer, and melanoma. However, the results should be interpreted carefully because the number of studies on these three cancers was small. In addition, the results showed that beta-blockers prolonged OS for patients with time-fixed post-diagnostic beta-blocker use. Generally, the subgroups of cancer stage, beta-blocker type, cumulative beta-blocker use, sample size, and ethnicity demonstrated no significant effect of beta-blocker on longer OS. Hence, we did not find a beneficial effect of beta-blocker use on cancer survival.
To our knowledge, this meta-analysis is the fourth one to be conducted on beta-blocker use and prognosis in various cancers. Indeed, this analysis objectively confirmed the latest development in this topic. All the previous three articles drew a conclusion that beta-blocker use could prolong the survival of cancer patients,44–46 but our current analysis showed an opposite conclusion that there is generally no relationship between beta-blocker use and cancer prognosis.
We then hypothesize some possible reasons for this conclusion. Preclinical studies have suggested that β-blockers play an anti-cancer role in multiple kinds of cancers by targeting at β-adrenergic signaling pathway.47,48 β-blockers can inhibit multiple processes of tumor progression and metastasis, including the inhibition of tumor cell proliferation, migration, invasion, as well as resistance to tumor angiogenesis and metastasis.3 Although the basic research may be effective, it is not recommended for speculating on the clinical survival of cancer patients due to the current evidence of evidence-based medicine. Beta-blocker is not a necessary medication for general adjuvant chemotherapy in cancer patients.49
Since cardiovascular diseases are common in the population, cancer patients frequently receive cardiovascular medications, including beta-blockers,2 but beta-blockers might not be recommended for chemotherapy in the absence of other indications. Further studies should be done to investigate the relationship between cancer survival and beta-blocker use in cancer patients without cardiovascular disease. Additionally, different effects in different cancers might have contributed to the lack of a discernible relationship between beta-blockers and OS of various cancers in the current studies. To find out the actual concrete relationship between the two, further analysis can be confined to beta-blocker use and one specific cancer based on a large enough population. Besides, beta-blockers themselves might have some undefined side effects on other organ systems, which might lead to cancer progression.50
However, there are still several limitations in this study. First, the studies included in this analysis were all cohort studies or case–control studies, as there were no RCTs yet investigating this topic. Second, while sensitivity analysis supported the stability of our results and a relatively large number of studies were included, we should still carefully interpret the results. The heterogeneity found in the study may be attributed to the multivariable influence factors in some studies. Third, the power of Begg’s and Egger’s tests to detect bias will be low with small number of studies, and when the between-study heterogeneity is large, none of the bias detection tests work well. Fourth, the dose–response analyses were not carried out due to a limited amount of literature.
Despite the limitations, there are several strengths in our study compared with previous meta-analyses. First, our current analysis showed a completely different main conclusion from the previous meta-analyses that there was no relationship between beta-blocker use and cancer prognosis. Second, we separated all-cause mortality from OS to make the analysis more precise. Third, we included 36 studies involving 319,006 patients, which was a larger number of patients than previous meta-analyses. Fourth, we discussed almost all variables that could describe the outcome of survival, including OS, all-cause mortality, CSS, DFS, PFS, and RFS.
Conclusion
The beta-blocker administration is not associated with cancer prognosis except for the positive effect on long CSS. Moreover, there are apparent protective effects of beta-blocker use in ovarian cancer, pancreatic cancer, and melanoma. We need more high-quality studies, such as RCTs, to confirm this conclusion in the future.
Supplementary materials
Subgroup analysis on beta-blocker use and OS in patients with non-Europeans (A), Europeans (B); duration of drug use >2 years (C), duration of drug use <2 years (D); Stage I/II (E), Stage III/IV (F); sample size >80 (G), sample size <80 (H); non-selective beta-blocker (I), selective blocker-type (J); pre-diagnostic beta-blocker use (K), post-diagnostic beta-blocker use (time-fixed) (L), post-diagnostic beta-blocker use (time-dependent) (M); lung cancer (N), melanoma (O), mixed cancer (P), colorectal cancer (Q), ovarian cancer (R), breast cancer (S), and pancreatic cancer (T).
Note: Weights are from random-effects analysis. The numbers in parentheses indicate the different included studies in the same year.
Sensitivity analysis of beta-blocker use on OS (A), all-cause mortality (B), CSS (C), DFS (D), PFS (E), and RFS (F) in studies except the studies obtaining estimates from KM plots.
Abbreviations: OS, overall survival; CSS, cancer-specific survival; DFS, disease-free survival; PFS, progression-free survival; RFS, recurrence-free survival; KM, kaplanmeier.
Sensitivity analysis of beta-blocker use on OS (A), all-cause mortality (B), CSS (C), PFS (D), and RFS (E) in high-quality studies (NOS score ≥7).
Abbreviations: OS, overall survival; CSS, cancer-specific survival; PFS, progression-free survival; RFS, recurrence-free survival; NOS, Newcastle–Ottawa Quality Assessment Scale.
Table S1.
Quality assessment of the included studies
| Subjects | Items | Standards | Reference no.
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | |||
| Score
| ||||||||||||||||||||||||||||||||||||||
| Grytli et al (2013) |
Grytli et al (2014) |
Al-Niaimi et al (2016) |
Aydiner et al (2013) |
Barron et al (2011) |
Beg et al (2017) |
Bir et al (2015) |
De Giorgi et al (2013) |
Diaz et al (2012) |
Ganz et al (2011) |
Giampieri et al (2015) |
Hwa et al (2017) |
Jansen et al (2014) |
Kim et al (2017) |
Lemeshow et al (2011) |
Melhem-Bertrandt et al (2011) |
Springate et al (2015) |
Udumyan et al (2017) |
Wang et al (2013) |
Watkins et al (2015) |
Yusuf et al (2012) |
Botteri et al (2013) |
Spera et al (2017) |
Johannesdottir et al (2013) |
Assayag et al (2014) |
Cata et al (2014) |
Heitz et al (2013) |
Heitz et al (2017) |
Holmes et al (2013) |
Jansen et al (2017) |
Livingstone et al (2013) |
Musselman et al (2014) |
Parker et al (2017) |
Sakellakis et al (2014) |
Shah et al (2011) |
Weberpals et al (2017) |
|||
| Selection | 1. Is the case definition adequate? | 1. Yes, with independent validation* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2. Yes, eg, record linkage or based on self-reports | ||||||||||||||||||||||||||||||||||||||
| 3. No description | ||||||||||||||||||||||||||||||||||||||
| 2. Representativeness of the cases | 1. Consecutive or obviously representative series of cases* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2. Potential for selection biases or not stated | ||||||||||||||||||||||||||||||||||||||
| 3. Selection of controls | 1. Community controls* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||||||||
| 2. Hospital controls | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||
| 3. No description | ||||||||||||||||||||||||||||||||||||||
| 4. Definition of controls | 1. No history of disease (end point)* | |||||||||||||||||||||||||||||||||||||
| 2. No description of source | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Comparability | Comparability ofcases and controls on the basis of the design or analysis | 1. Study controls for the most important factor* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2. Study controls for any additional factor (this criteria could be modified to indicate specific control for a second important factor*) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | ||
| Exposure | 1. Ascertainment of exposure | 1. Secure record (eg, surgical records)* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||
| 2. Structured interview where blind to case/control status* | ||||||||||||||||||||||||||||||||||||||
| 3. Interview not blinded to case/control status | ||||||||||||||||||||||||||||||||||||||
| 4. Written self-report or medical record only | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||||||||||||
| 5. No description | 0 | |||||||||||||||||||||||||||||||||||||
| 2. Same method of ascertainment for cases and controls | 1. Yes* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2. No | ||||||||||||||||||||||||||||||||||||||
| 3. Nonresponse rate | 1. Same rate for both groups* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||
| 2. Nonrespondents described | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
| 3. Rate different and no designation | ||||||||||||||||||||||||||||||||||||||
| 8 | 7 | 7 | 7 | 8 | 8 | 7 | 7 | 6 | 7 | 7 | 7 | 8 | 6 | 7 | 7 | 7 | 8 | 7 | 6 | 6 | 7 | 7 | 6 | 7 | 7 | 7 | 7 | 6 | 7 | 6 | 6 | 7 | 6 | 6 | 7 | |||
Note:
Indicates 1 score.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Wiysonge CS, Bradley HA, Volmink J, et al. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2017;1:CD002003. doi: 10.1002/14651858.CD002003.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Creed SJ, Le CP, Hassan M, et al. Beta2-adrenoceptor signaling regulates invadopodia formation to enhance tumor cell invasion. Breast Cancer Res. 2015;17:145. doi: 10.1186/s13058-015-0655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagaraja AS, Sadaoui NC, Lutgendorf SK, Ramondetta LM, Sood AK. Beta-blockers: a new role in cancer chemotherapy? Expert Opin Investig Drugs. 2013;22:1359–1363. doi: 10.1517/13543784.2013.825250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med. 1998;17:2815–2834. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 7.Harris R, Bradburn M, Deeks J, et al. METAN: stata module for fixed and random effects meta-analysis. Statistical Software Components. 2010;8(1):3–28. [Google Scholar]
- 8.Grytli HH, Fagerland MW, Fossa SD, Tasken KA, Haheim LL. Use of beta-blockers is associated with prostate cancer-specific survival in prostate cancer patients on androgen deprivation therapy. Prostate. 2013;73:250–260. doi: 10.1002/pros.22564. [DOI] [PubMed] [Google Scholar]
- 9.Grytli HH, Fagerland MW, Fossa SD, Tasken KA. Association between use of beta-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol. 2014;65:635–641. doi: 10.1016/j.eururo.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Al-Niaimi A, Dickson EL, Albertin C, et al. The impact of perioperative beta blocker use on patient outcomes after primary cytoreductive surgery in high-grade epithelial ovarian carcinoma. Gynecol Oncol. 2016;143:521–525. doi: 10.1016/j.ygyno.2016.09.019. [DOI] [PubMed] [Google Scholar]
- 11.Aydiner A, Ciftci R, Karabulut S, Kilic L. Does beta-blocker therapy improve the survival of patients with metastatic non-small cell lung cancer? Asian Pac J Cancer Prev. 2013;14:6109–6114. doi: 10.7314/apjcp.2013.14.10.6109. [DOI] [PubMed] [Google Scholar]
- 12.Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population-based study. J Clin Oncol. 2011;29:2635–2644. doi: 10.1200/JCO.2010.33.5422. [DOI] [PubMed] [Google Scholar]
- 13.Beg MS, Gupta A, Sher D, et al. Impact of concurrent medication use on pancreatic cancer survival-SEER-medicare analysis. Am J Clin Oncol. 2017 Jan;:10. doi: 10.1097/COC.0000000000000359. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bir SC, Kalakoti P, Ahmed O, Bollam P, Nanda A. Elucidating the role of incidental use of beta-blockers in patients with metastatic brain tumors in controlling tumor progression and survivability. Neurol India. 2015;63:19–23. doi: 10.4103/0028-3886.152625. [DOI] [PubMed] [Google Scholar]
- 15.De Giorgi V, Gandini S, Grazzini M, Bollam P, Nanda A. Effect of beta-blockers and other antihypertensive drugs on the risk of melanoma recurrence and death. Mayo Clin Proc. 2013;88:1196–1203. doi: 10.1016/j.mayocp.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Diaz ES, Karlan BY, Li AJ. Impact of beta blockers on epithelial ovarian cancer survival. Gynecol Oncol. 2012;127:375–378. doi: 10.1016/j.ygyno.2012.07.102. [DOI] [PubMed] [Google Scholar]
- 17.Ganz PA, Habel LA, Weltzien EK, Caan BJ, Cole SW. Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res Treat. 2011;129:549–556. doi: 10.1007/s10549-011-1505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giampieri R, Scartozzi M, Del Prete M, et al. Prognostic value for incidental antihypertensive therapy with beta-blockers in metastatic colorectal cancer. Medicine (Baltimore) 2015;94:e719. doi: 10.1097/MD.0000000000000719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwa YL, Shi Q, Kumar SK, et al. Beta-blockers improve survival outcomes in patients with multiple myeloma: a retrospective evaluation. Am J Hematol. 2017;92:50–55. doi: 10.1002/ajh.24582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jansen L, Hoffmeister M, Arndt V, Chang-Claude J, Brenner H. Stage-specific associations between beta blocker use and prognosis after colorectal cancer. Cancer. 2014;120:1178–1186. doi: 10.1002/cncr.28546. [DOI] [PubMed] [Google Scholar]
- 21.Kim SA, Moon H, Roh JL, et al. Postdiagnostic use of beta-blockers and other antihypertensive drugs and the risk of recurrence and mortality in head and neck cancer patients: an observational study of 10,414 person-years of follow-up. Clin Transl Oncol. 2017;19:826–833. doi: 10.1007/s12094-016-1608-8. [DOI] [PubMed] [Google Scholar]
- 22.Lemeshow S, Sorensen HT, Phillips G, et al. Beta-blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomarkers Prev. 2011;20:2273–2279. doi: 10.1158/1055-9965.EPI-11-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2645–2652. doi: 10.1200/JCO.2010.33.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Springate DA, Ashcroft DM, Kontopantelis E, Doran T, Ryan R, Reeves D. Can analyses of electronic patient records be independently and externally validated? Study 2 – the effect of beta-adrenoceptor blocker therapy on cancer survival: a retrospective cohort study. BMJ Open. 2015;5:e007299. doi: 10.1136/bmjopen-2014-007299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Udumyan R, Montgomery S, Fang F, et al. Beta-blocker drug use and survival among patients with pancreatic adenocarcinoma. Cancer Res. 2017;77:3700–3707. doi: 10.1158/0008-5472.CAN-17-0108. [DOI] [PubMed] [Google Scholar]
- 26.Wang HM, Liao ZX, Komaki R, et al. Improved survival outcomes with the incidental use of beta-blockers among patients with non-small-cell lung cancer treated with definitive radiation therapy. Ann Oncol. 2013;24:1312–1319. doi: 10.1093/annonc/mds616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Watkins JL, Thaker PH, Nick AM, et al. Clinical impact of selective and nonselective beta-blockers on survival in patients with ovarian cancer. Cancer. 2015;121:3444–3451. doi: 10.1002/cncr.29392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yusuf SW, Daraban N, Abbasi N, Lei X, Durand JB, Daher IN. Treatment and outcomes of acute coronary syndrome in the cancer population. Clin Cardiol. 2012;35:443–450. doi: 10.1002/clc.22007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Botteri E, Munzone E, Rotmensz N, et al. Therapeutic effect of b-blockers in triple-negative breast cancer postmenopausal women. Breast Cancer Res Treat. 2013;140:567–575. doi: 10.1007/s10549-013-2654-3. [DOI] [PubMed] [Google Scholar]
- 30.Spera G, Fresco1 R, Fung H, et al. Beta blockers and improved progression free survival in patients with advanced HER2 negative breast cancer: a retrospective analysis of the ROSE/TRIO-012 study. Ann Oncol. 2017;28(8):1836–1841. doi: 10.1093/annonc/mdx264. [DOI] [PubMed] [Google Scholar]
- 31.Johannesdottir SA, Schmidt M, Phillips G, et al. Use of ß-blockers and mortality following ovarian cancer diagnosis: a population-based cohort study. BMC Cancer. 2013;13:85. doi: 10.1186/1471-2407-13-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Assayag J, Pollak MN, Azoulay L. Post-diagnostic use of beta-blockers and the risk of death in patients with prostate cancer. Eur J Cancer. 2014;50:2838–2845. doi: 10.1016/j.ejca.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Cata JP, Villarreal J, Keerty D, et al. Perioperative beta-blocker use and survival in lung cancer patients. J Clin Anesth. 2014;26:106–117. doi: 10.1016/j.jclinane.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Heitz F, du Bois A, Harter P, et al. Impact of beta blocker medication in patients with platinum sensitive recurrent ovarian cancer-a combined analysis of 2 prospective multicenter trials by the AGO Study Group, NCIC-CTG and EORTC-GCG. Gynecol Oncol. 2013;129:463–466. doi: 10.1016/j.ygyno.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Heitz F, Hengsbach A, Harter P, et al. Intake of selective beta blockers has no impact on survival in patients with epithelial ovarian cancer. Gynecol Oncol. 2017;144:181–186. doi: 10.1016/j.ygyno.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 36.Holmes S, Griffith EJ, Musto G, Minuk GY. Antihypertensive medications and survival in patients with cancer: a population-based retrospective cohort study. Cancer Epidemiol. 2013;37:881–885. doi: 10.1016/j.canep.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 37.Jansen L, Weberpals J, Kuiper JG, et al. Pre- and post-diagnostic beta-blocker use and prognosis after colorectal cancer: results from a population-based study. Int J Cancer. 2017;141:62–71. doi: 10.1002/ijc.30717. [DOI] [PubMed] [Google Scholar]
- 38.Livingstone E, Hollestein LM, van Herk-Sukel MP, et al. beta-Blocker use and all-cause mortality of melanoma patients: results from a population-based Dutch cohort study. Eur J Cancer. 2013;49:3863–3871. doi: 10.1016/j.ejca.2013.07.141. [DOI] [PubMed] [Google Scholar]
- 39.Musselman RP, Li W, Gomes T, et al. Association between beta blocker usage and cancer survival in a large, matched population study among hypertensive patients. J Surg Res. 2014;186:639–640. [Google Scholar]
- 40.Parker WP, Lohse CM, Zaid HB, et al. Evaluation of beta-blockers and survival among hypertensive patients with renal cell carcinoma. Urol Oncol. 2017;35:36.e1–36.e6. doi: 10.1016/j.urolonc.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Sakellakis M, Kostaki A, Starakis I, Koutras A. Beta-blocker use and risk of recurrence in patients with early breast cancer. Chemotherapy. 2014;60:288–289. doi: 10.1159/000371871. [DOI] [PubMed] [Google Scholar]
- 42.Shah SM, Carey IM, Owen CG, Harris T, Dewilde S, Cook DG. Does beta-adrenoceptor blocker therapy improve cancer survival? Findings from a population-based retrospective cohort study. Br J Clin Pharmacol. 2011;72:157–161. doi: 10.1111/j.1365-2125.2011.03980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weberpals J, Jansen L, Haefeli WE, et al. Pre- and post-diagnostic beta-blocker use and lung cancer survival: a population-based cohort study. Sci Rep. 2017;7:2911. doi: 10.1038/s41598-017-02913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choi CH, Song TJ, Kim TH, et al. Meta-analysis of the effects of beta blocker on survival time in cancer patients. J Cancer Res Clin Oncol. 2014;140:1179–1188. doi: 10.1007/s00432-014-1658-7. [DOI] [PubMed] [Google Scholar]
- 45.Weberpals J, Jansen L, Carr PR, Hoffmeister M, Brenner H. Beta blockers and cancer prognosis – the role of immortal time bias: a systematic review and meta-analysis. Cancer Treat Rev. 2016;47:1–11. doi: 10.1016/j.ctrv.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 46.Zhong S, Yu D, Zhang X, et al. Beta-blocker use and mortality in cancer patients: systematic review and meta-analysis of observational studies. Eur J Cancer Prev. 2016;25:440–448. doi: 10.1097/CEJ.0000000000000192. [DOI] [PubMed] [Google Scholar]
- 47.Jean Wrobel L, Bod L, Lengagne R, Kato M, Prévost-Blondel A, Le Gal FA. Propranolol induces a favourable shift of anti-tumor immunity in a murine spontaneous model of melanoma. Oncotarget. 2016;7:77825–77837. doi: 10.18632/oncotarget.12833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Partecke LI, Speerforck S, Kading A, et al. Chronic stress increases experimental pancreatic cancer growth, reduces survival and can be antagonised by beta-adrenergic receptor blockade. Pancreatology. 2016;16:423–433. doi: 10.1016/j.pan.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 49.Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 50.Barron AJ, Zaman N, Cole GD, Wensel R, Okonko DO, Francis DP. Systematic review of genuine versus spurious side-effects of beta-blockers in heart failure using placebo control: recommendations for patient information. Int J Cardiol. 2013;168(4):3572–3579. doi: 10.1016/j.ijcard.2013.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Subgroup analysis on beta-blocker use and OS in patients with non-Europeans (A), Europeans (B); duration of drug use >2 years (C), duration of drug use <2 years (D); Stage I/II (E), Stage III/IV (F); sample size >80 (G), sample size <80 (H); non-selective beta-blocker (I), selective blocker-type (J); pre-diagnostic beta-blocker use (K), post-diagnostic beta-blocker use (time-fixed) (L), post-diagnostic beta-blocker use (time-dependent) (M); lung cancer (N), melanoma (O), mixed cancer (P), colorectal cancer (Q), ovarian cancer (R), breast cancer (S), and pancreatic cancer (T).
Note: Weights are from random-effects analysis. The numbers in parentheses indicate the different included studies in the same year.
Sensitivity analysis of beta-blocker use on OS (A), all-cause mortality (B), CSS (C), DFS (D), PFS (E), and RFS (F) in studies except the studies obtaining estimates from KM plots.
Abbreviations: OS, overall survival; CSS, cancer-specific survival; DFS, disease-free survival; PFS, progression-free survival; RFS, recurrence-free survival; KM, kaplanmeier.
Sensitivity analysis of beta-blocker use on OS (A), all-cause mortality (B), CSS (C), PFS (D), and RFS (E) in high-quality studies (NOS score ≥7).
Abbreviations: OS, overall survival; CSS, cancer-specific survival; PFS, progression-free survival; RFS, recurrence-free survival; NOS, Newcastle–Ottawa Quality Assessment Scale.
Table S1.
Quality assessment of the included studies
| Subjects | Items | Standards | Reference no.
|
|||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 | |||
| Score
| ||||||||||||||||||||||||||||||||||||||
| Grytli et al (2013) |
Grytli et al (2014) |
Al-Niaimi et al (2016) |
Aydiner et al (2013) |
Barron et al (2011) |
Beg et al (2017) |
Bir et al (2015) |
De Giorgi et al (2013) |
Diaz et al (2012) |
Ganz et al (2011) |
Giampieri et al (2015) |
Hwa et al (2017) |
Jansen et al (2014) |
Kim et al (2017) |
Lemeshow et al (2011) |
Melhem-Bertrandt et al (2011) |
Springate et al (2015) |
Udumyan et al (2017) |
Wang et al (2013) |
Watkins et al (2015) |
Yusuf et al (2012) |
Botteri et al (2013) |
Spera et al (2017) |
Johannesdottir et al (2013) |
Assayag et al (2014) |
Cata et al (2014) |
Heitz et al (2013) |
Heitz et al (2017) |
Holmes et al (2013) |
Jansen et al (2017) |
Livingstone et al (2013) |
Musselman et al (2014) |
Parker et al (2017) |
Sakellakis et al (2014) |
Shah et al (2011) |
Weberpals et al (2017) |
|||
| Selection | 1. Is the case definition adequate? | 1. Yes, with independent validation* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2. Yes, eg, record linkage or based on self-reports | ||||||||||||||||||||||||||||||||||||||
| 3. No description | ||||||||||||||||||||||||||||||||||||||
| 2. Representativeness of the cases | 1. Consecutive or obviously representative series of cases* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2. Potential for selection biases or not stated | ||||||||||||||||||||||||||||||||||||||
| 3. Selection of controls | 1. Community controls* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||||||||||
| 2. Hospital controls | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||
| 3. No description | ||||||||||||||||||||||||||||||||||||||
| 4. Definition of controls | 1. No history of disease (end point)* | |||||||||||||||||||||||||||||||||||||
| 2. No description of source | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Comparability | Comparability ofcases and controls on the basis of the design or analysis | 1. Study controls for the most important factor* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 2. Study controls for any additional factor (this criteria could be modified to indicate specific control for a second important factor*) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 1 | ||
| Exposure | 1. Ascertainment of exposure | 1. Secure record (eg, surgical records)* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||||||||||
| 2. Structured interview where blind to case/control status* | ||||||||||||||||||||||||||||||||||||||
| 3. Interview not blinded to case/control status | ||||||||||||||||||||||||||||||||||||||
| 4. Written self-report or medical record only | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||||||||||||||||
| 5. No description | 0 | |||||||||||||||||||||||||||||||||||||
| 2. Same method of ascertainment for cases and controls | 1. Yes* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |
| 2. No | ||||||||||||||||||||||||||||||||||||||
| 3. Nonresponse rate | 1. Same rate for both groups* | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||||||
| 2. Nonrespondents described | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||||||||||||||||||||||||||
| 3. Rate different and no designation | ||||||||||||||||||||||||||||||||||||||
| 8 | 7 | 7 | 7 | 8 | 8 | 7 | 7 | 6 | 7 | 7 | 7 | 8 | 6 | 7 | 7 | 7 | 8 | 7 | 6 | 6 | 7 | 7 | 6 | 7 | 7 | 7 | 7 | 6 | 7 | 6 | 6 | 7 | 6 | 6 | 7 | |||
Note:
Indicates 1 score.